Abstract
Intestinal digestion and absorption are complex processes; thus, it is a challenge to imitate them realistically. There are numerous approaches available, with different disadvantages and advantages. The simplest methods to mimic absorption are the non-cell-based transport models but these lack important characteristics of enterocytes of the intestine. Therefore, the most often used method is to measure absorption through viable mammalian cells (most commonly Caco-2 cells, cultured on membrane insert plates), which not only assures the incorporation of brush border enzymes (responsible for the final digestion of peptides and disaccharides), it also simulates the absorption process. This means that influx/efflux transporter-facilitated transport, carrier-mediated transport, endocytosis, and transcytosis is also imitated besides passive diffusion. Still, these also lack the complexity of intestinal epithelium. Organoids or ex vivo models are a better approach if we want to attain precision but the highest accuracy can be achieved with microfluidic systems (gut-on-a-chip models). We propose that more research is necessary, and food absorption should also be studied on gut-on-a-chips, especially with fragmented organoids. Our review supports the choices of a proper intestinal epithelium model, which may have a key role in functional food development, nutrition studies, and toxicity assessment.
1. Introduction
In order to determine the nutritional quality of a foodstuff, besides composition data, it is important to determine nutrient bioavailability [1]. Bioavailability is the quantity of the ingested component that becomes available at the site of action, after the following phases: digestion, absorption by intestinal cells, transport into the circulation, and transport from the circulation to the site of action [1]. Bioavailability is determined through in vivo assessments [1]. Due to ethical and economic reasons, the number of in vivo assays should be reduced as much as possible; thus to serve this purpose, in vitro digestion studies were designed. Although numerous dynamic in vitro digestion systems are available [2], the static COST Infogest in vitro digestion protocol [3] is still one of the most popular methods to mimic food digestion, as it is time effective, less costly and less labor-intensive [4]. This method is standardized, the protocol is reproducible, and suitable for carrying out easy inter-laboratory comparisons of the results. This protocol simulates the oral, gastric, and intestinal phase of digestion, and is used to measure the components released from the matrix and is accessible (bioaccessibility) but does not model absorption (bioavailability).
In vivo, the intestinal epithelium regulates the absorption of nutrients and electrolytes, acts as a physical barrier, and blocks pathogens, toxic metabolites, and allergenic compounds [5]. The small intestine, due to the presence of integral membrane proteins called brush border membrane enzymes (peptidases, alkaline phosphatase, disaccharidases (maltase, sucrase-isomaltase, lactase)), also participates in the degradation of macronutrients [6,7]. The absorption through the intestinal epithelium is a decisive component of bioavailability. Furthermore, not only the intestinal epithelium itself but also the intestinal epithelium, the gut microbiota, and their interactions play a key role in the bioavailability of food ingredients. Thus, the reproduction of the intestinal epithelium becomes important for digestive studies.
The intestinal epithelium is a complex tissue with multiple cell types (Section 1.1). Intestinal epithelium in vitro models should contain the different cell types in correct proportion and three-dimensional orientation. This is useful as the interaction between different cells can be studied [8].
So far, there is no standardized protocol for the in vitro investigation of the absorption of food components through the intestinal barrier [8]. Numerous methods exist and the goal of the study should be exactly determined before choosing the proper method [9]. This review describes the most important information on methods and gives suggestions which may be applied to investigate the absorption of food components for the required purpose. Digestion–absorption is a very complex biochemical process system and the accurate reproduction of their specific physiological conditions is very difficult. It is important to mention that although methods have disadvantages as well as advantages, they are nevertheless of great help in understanding absorption processes.
1.1. Description of Intestinal Epithelium
The gut epithelium is made of numerous cell types (enterocytes/colonocytes, goblet cells, stem cells, enteroendocrine cells, Tuft cells, M cells, and Paneth cells) (Figure 1) [10]. While enterocytes are present everywhere, goblet cells are on the villi, and Paneth cells can be found in the crypts [11].
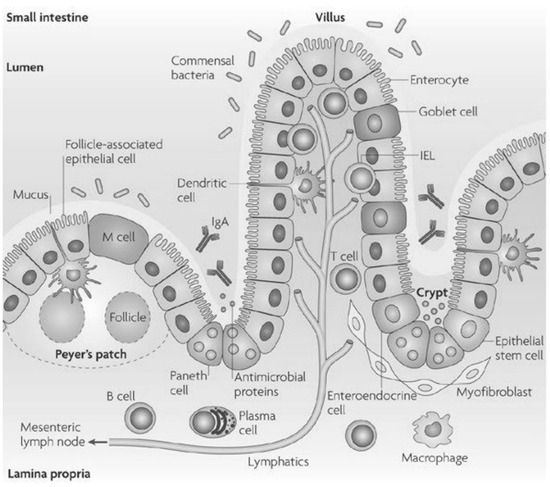
Figure 1.
The small intestinal epithelium cells and their localization [6].
Junctional complexes (intercellular attachment structures) connect the epithelial cells that form the intestinal barrier. They limit the passive movement of food components across the epithelium. Out of these, the tight junctions regulate paracellular passive diffusion of ions and small molecules, and exclude large molecules [6,12].
Enterocytes, with long, densely packed microvilli, are responsible for absorption of nutrients, like carbohydrates, peptides, amino acids, and lipids [6]. They also absorb ions and water, release antimicrobial proteins like β-defensins and cathelicidins, and express brush border enzymes [6].
Goblet cells secrete mucin fibers and antimicrobial peptides [6].
At the base of the crypts, quickly renewing epithelial stem cells can be found [8].
Paneth cells have a role in maintaining the proper stem cell niche, by presenting ligands and secreting growth factors, such as Wnt ligands [13,14]. They also secrete antimicrobial peptides, which are synthesized by ribosomes [14,15].
Enteroendocrine cells regulate appetite and gut motility by secretion of numerous hormones [8].
Tuft cells are chemosensory epithelial cells that secrete effector molecules with immunomodulatory functions [16]. They have a role in the protection against protozoa and helminths [8].
M cells (“microfold cells”) present the antigens from the lumen to the immune cells [8]. The proportion of M cells in the gastrointestinal tract is low (<1%). They can only be found in the small intestine, above the Peyer’s patches [8].
All differentiated cells of the epithelium originate from stem cells [8]. The progenitors of stem cells migrate upwards from the crypts [17]. The migration and differentiation of cells on the crypt/villus axis are maintained by biochemical (e.g., growth factors) and biophysical gradients (e.g., extracellular matrix properties) [8,18].
As digestive enzymes hydrolyze foodstuffs in the intestinal lumen, bioaccessible molecules are released and are transferred into the bloodstream by passive or active transport through the intestinal epithelium [19]. Passive transport is manifested in transcellular or paracellular diffusion, while active transport can be realized by influx/efflux transporter-facilitated transport, carrier-mediated transport, endocytosis, and transcytosis (Figure 2) [12]. All cell types are capable of these absorption processes but enterocytes express a selection of genes that maximize the absorption of food components [20].
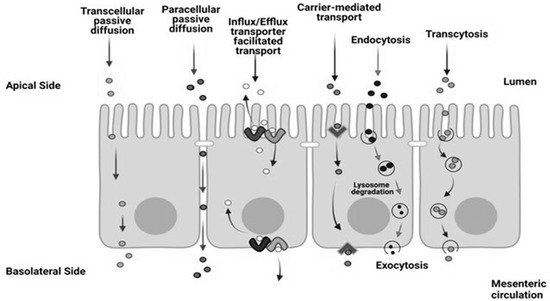
Figure 2.
Transport processes in the intestinal epithelium [21].
The small intestine is covered by one mucus layer. The mucus is a hydrated, complex heterogeneous mixture of mucin fibers, lipids, proteins, carbohydrates, cell debris and bacteria [22]. The mucus layer covers and protects the surface of the intestine (e.g., from digestive enzymes, bacteria) [8]. As it influences the solubility on the surface of the cells, it has a significant impact on the absorption of different compounds [12,23]. In the mucus, the main type of mucin present is mucin 2 (Muc2) [8].
The large intestine differs from the small intestine in several aspects. It also contains crypts but no villi are present. The colonocytes of the large intestine are similar to enterocytes but they do not express brush border enzymes and have short, irregular microvilli [6]. They absorb water, electrolytes, and bacterial metabolites [6]. The large intestine has two mucus layers: a sterile, inner, strongly adherent layer and an outer, looser one, which forms a specific environmental condition for the growth of bacteria [8,10]. In the case of the large intestine, a very steep oxygen gradient can be noticed. In the proximity of the large intestine lumen, the environment is almost oxygen deprived, which enables the presence of diverse anaerobic (obligate and facultative) bacteria. At the same time, the intestinal stem cells are under normoxic (normal oxygen levels) conditions [8]. The epithelial cells adapted to the hypoxic environment, by using as an energy source the butyric acid, produced by bacteria [8]. The intestinal tissues contain other cells besides epithelial cells (immune cells, endothelial cells, and neurons).
1.2. Challenges Regarding the Design of Intestinal Epithelium Models Used for Absorption Studies
It is probably impossible to imitate all aspects of the small and large intestinal digestion phase in a manner that it reproduces the in vivo state perfectly [24]. A major obstacle is the precise imitation of the intestinal epithelium. For example, it is difficult to reproduce the complex histological organization, the presence of the microbiota, and the effect gut motility in a single model [24].
The gut bacteria participate in the differentiation of stem cells to enterocytes, the motility of the gastrointestinal tract, the metabolism of foodstuffs and drugs, the degradation of dietary toxins and carcinogens, the synthesis of essential vitamins (K, B12, folic acid, B1, B6) and short chain fatty acids, and bile acid circulation [25,26]. They also synthesize enzymes, which can degrade dietary polysaccharides to simpler carbohydrates [26].
The presence of stem cell niche, the simultaneous cell differentiation, migration, and the shedding of dead cells are prerequisites of tissue homeostasis in the intestine [27], which is also difficult to adjust for in vitro analysis.
Moreover, the gut motility should also be simulated but this also causes difficulties. The gut motility influences the mixture and propagation of gut content [28]. The distortion that can be noticed during peristaltic expansion and contraction, and the flow/shear stress caused by digesta movement influences the epithelium, by inducing the division and differentiation of epithelial cells [29]. Peristaltic movement also inhibits the overgrowth of bacteria in vivo [30]. According to Li et al. [31], it is difficult, if not impossible, to reproduce in vitro the peristaltic movements of the gastrointestinal tract. The contractions of the small intestine are highly influenced by the time that has passed since the last meal, while large intestine motility is determined by circadian rhythm [8].
During the investigation of the absorption of food components, several different approaches can be applied. In the case of some high-throughput experiments, non-cell-based transport models or brush border membrane vesicles (Section 2.2) can be used [1,32].
Still, the complex processes that determine the absorption rate of food components through the intestinal barrier [22], can only be precisely simulated with models that include mammalian cells. For example, the active transport of D-glucose, in the case of dialysis, is only reproduced as passive diffusion, which is only determined by the concentration gradient [33]. Due to the complexity of the intestinal tissue, numerous approaches (the application of different cell lines or stem cells, various cell culture techniques) were developed to imitate the epithelium. The simpler methods mainly focus on the establishment of proper epithelial barrier integrity and the development of a mucus layer on the surface of cells. The imitation of the physiological density of mucus is also important, since it may affect absorption [8]. Usually, the digesta cannot be added directly to the surface of mammalian cell cultures. It can diminish the viability of cells, due to the osmotic shock it may induce, or due to the presence of bile acids, digestive enzymes, and enzyme inhibitors [3].
2. Technological Approaches of Intestinal Epithelium Models
Intestinal epithelium models can be useful tools at the beginning of nutritional studies and during the development of functional foods, because they enable cost-effective screening of samples [22]. They also facilitate the investigation of the cause–effect relationship between different factors, because they provide a well-controlled and characterized experimental setup.
During the investigation of the absorption of food components, several different approaches can be applied (Figure 3). In cases of some high-throughput experiments, non-cell-based transport models, brush border membrane vesicles, or dialysis can be used for fast estimation of the passive absorption of food components [1,22,32]. Still, the complex processes that determine the absorption rate of food components through the intestinal barrier [22] can only be precisely simulated with models that include mammalian cells. Due to the complexity of the intestinal tissue, numerous approaches (the application of different cell lines or stem cells, various cell culture techniques) were developed to imitate the epithelium. The simpler methods mainly focus on the establishment of proper epithelial barrier integrity, and the development of mucus layer on the surface of cells.
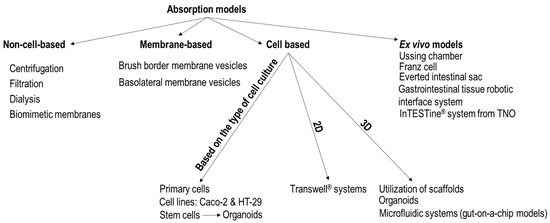
Figure 3.
Approaches for the investigation of the absorption of food components. In Transwell® systems, primary cells, cell lines, or fragmented organoids derived from stem cells can be cultured.
Intestine-on-a-chip models with immortalized cell lines have been used to study nutrient absorption [17]. Organoids, microfluidic systems, and ex vivo models can also be used for absorption studies. In numerous cases, these more complex models are chosen because of their increased precision, even if they are more expensive and more laborious than the simple Transwell systems.
2.1. Non-Cell-Based Transport Models
Non-cell-based transport models are useful when the goal is to screen time-efficiently as many samples as possible. These models may apply solubility (centrifugation or filtration) or dialyzability measurements [1,33,34]. Numerous widely used in vitro digestion models, for example, the TIM systems, use dialysis to determine the intestinal permeability [33]. If a proper pore size (8000 Da MWCO) of the dialysis membrane is chosen, coupled with simulation of the motility of the small intestine, the human intestinal effective permeability of D-glucose can be predicted with the respective method [33]. Still, the dialysis membrane cannot reproduce active transport [35].
Another approach may be the application of biomimetic membranes. These are filters or membranes that contain phospholipids or liposomes [22,35].
There are several types of biomimetic membranes available:
- parallel artificial membrane permeability (PAMPA): a filter is infused with an organic solvent that contains phospholipids, in a manner that it imitates the lipid composition of the intestinal membrane [22].
- vesicle-based permeation assay (PVPA): liposomes are deposited in the pores and on the surface of the filter [22].
- PermeaPad®: two cellulose-hydrate membranes act as support layers in a sandwich-like structure, where the middle layer is made of dry phospholipids. This structure hinders the erosion of the middle layer and prevents the leakage of lipids into the solutions used during the experiment [22].
They are only appropriate to evaluate the passive transcellular absorption of hydrophobic components, they do not reproduce active transport or paracellular permeability [12,35].
2.2. Membrane-Based Transport Models
Brush border or basolateral membrane vesicles do not contain intact cells but they are prepared from mammalian intestinal epithelial cells. They are purified fractions of the apical or the basolateral membranes of the intestine [22].
The purified fraction of apical membranes is extracted from inverted intestine homogenate [22]. Schmitz et al. [36] and Kessler et al. [37] describe methods for the purification of brush border membranes. According to Kessler et al. [37], several approaches exist to purify brush border membranes from intestine, for example:
- (i)
- “Tris disruption procedure, followed by density gradient centrifugation”;
- (ii)
- the hypotonic EDTA procedure which can be complemented with density gradient centrifugations;
- (iii)
- the Ca2+ precipitation protocol. In the presence of Ca2+, endoplasmic reticulum and mitochondria are converted to larger particles due to aggregation. These larger particles can be easily separated by centrifugation at 2000× g. Following this, the brush border fragments are separated by centrifugation of the supernatant at 20,000× g [38];
- (iv)
- “isotonic homogenization and density gradient centrifugation”;
- (v)
- “isolation of enterocytes, homogenization and density gradient centrifugation or free flow electrophoresis”.
To obtain basolateral membrane vesicles [39], the mucosa of healthy, human jejunum tissue is scraped with a metal spatula. After homogenization of the mucosal scraping in an appropriate buffer, the homogenate was centrifuged at 2500× g for 20 min. The supernatant was centrifuged at 22,000× g for 25 min. After the removal of the supernatant, a whitish fluffy layer remained at the top of the solid yellow pellet. This layer was resuspended in a buffer and was homogenized. The homogenate was mixed with 15% (wt/wt) Percoll and centrifuged at 48,000× g for 60 min. On the top of the Percoll pellet, basolateral membrane vesicles appeared as a distinct whitish layer. The vesicles were removed, resuspended in another buffer (called transport buffer) and centrifuged at 48,000× g for 20 min. This washing step was repeated twice. The retrieved basolateral membrane vesicles can be used for transport studies after resuspension in the transport buffer. These models are not appropriate for the evaluation of paracellular transport. The information they provide regarding transcellular transport is also partial since they do not incorporate the apical and basolateral membranes at the same time [22].
2.3. Cell Cultures Used in Epithelium Models
The main categories of cell cultures that can be used in intestinal epithelium protocols are primary cells, cell lines, and stem cells [5]. Primary cells are directly obtained from the tissue of origin and they cannot be sub-cultured indefinitely. In contrast, cell lines that are immortalized cells can be cultured for long periods. The immortalized cells are either of cancerous origin or have been genetically modified to proliferate indefinitely [40]. The biochemical and physiological characteristics of primary cell cultures are closer to the conditions that can be noticed in vivo since these cells do not incorporate genetic modifications [23,41]. The acquirement of these cells is more problematic than the maintenance of the immortalized cell lines [6]. Another disadvantage is that exogenous growth factors are necessary to maintain these cell cultures [23,42]. Besides this, it is still a challenge to develop a reproducible protocol for the long-term sustainment of primary cell cultures that originate from the intestinal tissue, as primary cells, in vitro, are susceptible to apoptosis [5,29]. Only a few studies describe treatment of primary cells with digested foods [7]. The main disadvantage of using cancerous cell lines is that their phenotype is different from the epithelial cells of the small or large intestine. Diverse culture conditions may also cause experimental variability [43]. This complicates the extrapolation of the results regarding the in vivo situation [27]. The traditional and most used in vitro model of the gastrointestinal microenvironment is the culture of Caco-2 and HT-29 cell lines in Transwell systems [28,44,45].
The Caco-2 cells are human colorectal adenocarcinoma cells, which differentiate into polarized epithelial cells, and are organized spontaneously in crypt and villi-like structures [23,46,47]. Besides its cancerous origin, this cell line has limitations. It is hard to control its differentiation, its transporters, and the diameter of its tight junctions are different than in the case of the intestinal epithelium [47]. The intestinal epithelium is covered with a mucus layer, which has not only a protective role but also affects the absorption rate and host–microbiota interaction. An important deficiency of Caco-2 cell cultures is that they do not secrete mucin glycoproteins that form the mucus layer [48]. In opposition, if Caco-2 cells are cultured under shear stress, in a gut-on-a-chip model (Section 2.3.5), the presence of MUC2 could be detected.
The HT-29 culture also originates from human colorectal adenocarcinoma cells but in contrast to Caco-2 cells, it also contains mucin-secreting goblet cells [10]. If Caco-2 and HT29-MTX (HT-29 cells stimulated with methotrexate) are mixed, co-cultures secrete the gastric mucin called (MUC)5AC instead of the typical intestinal MUC2 [6,49]. Furthermore, the HT29-MTX cells divide significantly faster than Caco-2 cells [10,30]. So, the absorptive: goblet cells ratio changes with the aging of the culture, in a manner that will not correspond to the in vivo state [10,30].
TC7, NCM460, T84, SK-CO15, and HCT-8 are other human cancerous cell lines that are often used as epithelium models [6,7,22,50]. The latter four can be used as models of the large intestine epithelium [6,7]. Models based on tumor cells do not incorporate all intestinal epithelial cell types. The expression of receptors, transporters, and enzymes is not the same as in case of in vivo conditions [8].
Noncancerous cell lines of animal origin like, MDCK (Madin–Darby canine kidney), IEC-18 (established from rat small intestine), and IPEC-J2 (isolated from piglet small intestine) can be also utilized as in vitro absorption models [22,51,52]. The phenotype of these is more similar to primary cells [9]. Out of the animal-derived cell lines, the IPEC-J2 culture is most similar to the human epithelium [1,22].
Another approach to creating intestinal epithelium models is the utilization of stem cells. These can be the best choice, as they can be propagated in the long term, and they can differentiate into multiple cell types [5]. A disadvantage is that the acquirement and maintenance of stem cells is laborious [5].
The self-renewing 3D cultures originating from embryonic, induced pluripotent and multipotent stem cells are called organoids [47,53]. The structure, metabolism, and phenotype of the organoids are similar to the characteristics of tissue of origin [27]. Enteroids are prepared from the small intestine, while colonoids from the colon [28]. Compared to 2D cell cultures, the arrangement of cell junctions and the extracellular matrix are more similar to the in vivo situation, and more importantly, the expression of brush border proteins is better [9].
Another example of a 3D model is the Epi-IntestinalTM developed by MatTek Corporation [12]. Epi-IntestinalTM is a reconstructed 3D human gut tissue model derived from primary intestinal cells that can be very useful in the investigation of nutrient processing [12]. Unlike organoids, this organotypic microtissue has an accessible luminal surface [12].
To avoid microinjection, the organoids may be fragmented and cultured as a monolayer in 2D [28,41]. These cultures also organize themselves in stem cell zones and differentiated cell areas, just as the original tissue [14]. When they are maintained on membrane inserts plates, the system is appropriate for studying nutrient absorption. However, in this case, only a thin mucus layer is formed [49]. According to Ashammakhi et al. [23], cells originating from the intestinal organoids are also able to reproduce the physiological responses effectively. In contrast, according to Pimenta et al. [54] and Zhao et al. [55], the generation of intestinal organoids from pluripotent stem cells may lead to the development of a tissue with fetal properties.
In cases of nutrient absorption studies, the presence of endothelial cells can be important, as vasculature is fundamental in nutrient and waste transport [54].
The reproducibility of models that apply cell cultures can be diminished due to the utilization of animal serum during their maintenance [53].
It is very interesting that the digesta obtained by the Infogest protocol is toxic to Caco-2 cell monolayers [7]. The digestive enzymes may be toxic and may cause detachment of cell layers, and bile salts are not only toxic to cells but can also alter osmolality in a harmful manner [7]. Metabolites generated from in vitro food digestion may also have deleterious effect on mammalian cells [7]. Although it was proven that the digesta imitates physiologically relevant osmolality and concentrations of bile acids and enzymes, a detoxification of digesta is necessary [7]. Kondrashina et al. [7] recommend different detoxification protocols, which should be chosen based on the purpose of the research. These will also alter the digesta, diminishing the precision of the imitation of the in vivo digesta. Due to the above-mentioned setbacks, it is important to apply a negative control during the experiments, which contains all digestive juices in the digesta but the investigated food is not included [7].
2.3.1. Transwell® Systems
The commercially available Transwell® systems can imitate the epithelial barrier to some extent. These are plastic supports with a permeable, porous membrane that can be placed in the wells of a microtiter plate. The epithelial cells can be cultured on the membrane, which is coated with extracellular matrix [28]. The support separates two compartments: the upper one represents the intestinal lumen, while the lower one the blood vessels [29].
On the membrane of Transwell systems, a polarized epithelium monolayer can be grown due to the tight junctions formed between the cells [30]. The Caco-2 cells that are cultured on the Transwell membranes also express brush border enzymes [22]. This is an important advantage since in the standardized international INFOGEST method [3] brush border enzymes are not yet included. Thus, when the absorption of the digesta is investigated with this model, the effect of brush border enzymes can also be evaluated, although their activities are lower than in the case of enterocytes [7]. Cells from enzymatically fragmented organoids can also be seeded on Transwell supports, which facilitate access to the apical side of the enterocytes [17].
The models that apply Transwell® systems are simple and can be standardized easily but they oversimplify the physiological processes [29]. The intestinal epithelium cells that are cultured on Transwell systems do not form villi-like structures, and do not differentiate into the epithelial cell-types that are characteristic of the normal intestine [56].
The permeability of the static Caco-2 cell layer is much lower than in vivo, because the shear stress that is caused by the flow of digesta and the peristaltic motility is not reproduced in the Transwell system, so the water layer above the cells is not stirred [30]. In vivo, the fluid flow also ensures nutrient supply and removal of the components of the digesta that are not absorbed [57].
2.3.2. Verification of Cell-Based Absorption Models
It is very important that before and during absorption experiments the quality of the intestinal monolayer is checked. Cell viability can be determined by LDH (lactate dehydrogenase) assay or MTT (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. Membrane permeability can be checked with Trypan blue, cell proliferation with 5-bromo-2′-deoxyuridine (BrdU), while apoptosis with annexin or catalase [7]. Redox damage can be established by measuring reactive oxygen species or glutathione [7].
It is essential to determine barrier integrity before and after the experiment, to be sure that the absorption measurements are accurate. Thomas et al. [58] describe transepithelial electrical resistance measurements (TEER), dextran permeability, and cellular junctional complex imaging as proper methods to measure barrier integrity. Out of these, TEER is the most commonly used. TEER measures “the resistance of electrical current passing through a cellular monolayer”, thus representing the permeability of ions and barrier integrity (Figure 4) [58]. Phase contrast and fluorescence confocal microscopy can also be applied to evaluate the morphology of the cells [59].
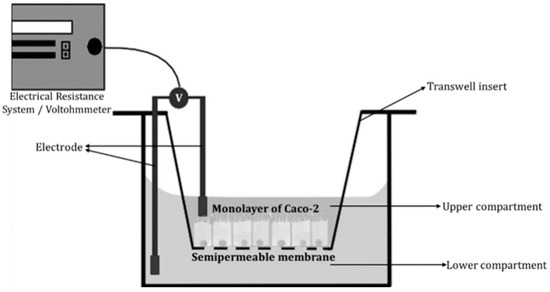
Figure 4.
Schematic of transepithelial electrical resistance (TEER) measurements [58].
2.3.3. Utilization of Scaffolds
The standardization of 3D cell cultures is a challenge [53]. The reproduction of the 3D structure of the tissue improves the physiological behavior of intestinal cells [24].
Some protocols use scaffolds for the preparation of 3D models. In most cases, the scaffolds reproduce (or induce) the structure of the intestinal villi/crypts or the tube-like shape of the gut [29]. The scaffold can be made of synthetic or semi-synthetic substances (such as polyethylene glycol or hyaluronan hydrogels) or extracts of animal origin (collagen, extracellular matrix (ECM)) [60]. The determination of the most important characteristics of synthetic scaffold matrixes and the standardization of the scaffolds are important research goals because the matrix properties (biochemical characteristics, rigidity, architecture) influence cell behavior [8,17]. Using micro-engineered surfaces may also be an option [54].
Costello et al. [61] built a bioreactor by inserting porous villous scaffolds made of poly-ethylene-co-vinyl-acetate (PEVA) in a fluidic device. They cultured Caco-2 cells on these scaffolds. The Caco-2 cells actively transported more glucose in the presence of flow. Compared to membrane inserts plates, culturing Caco-2 on villous scaffold reduces TEER and increases brush border enzyme expression [61].
In cases of shaped 3D intestinal systems, it is a challenge to synchronize the proper environment for the complex necessities (e.g., surface mechanics, adhesion, porosity) of primary cells with the growth and differentiation factors in a manner that will facilitate a cell-type distribution that is similar to the in vivo condition [8].
2.3.4. Organoid Cultures
Organoids are cell-based in vitro models that mimic many characteristics of the corresponding in vivo tissue [55]. During the generation of an intestinal organoid, tissues from the small or large intestine are fragmented. The fragments are treated with EDTA, which chelates the Ca2+ and disrupts cell–cell adhesion. This way, primary intestinal crypts are obtained, which can be used for the generation of organoid cultures [55]. Cells are seeded into biologically derived matrices (e.g., Matrigel, collagen) or into synthetic hydrogels [55]. The cells of epithelial organoids are arranged in a single layer and form a sphere [8]. Besides the predominant absorptive enterocytes, other specialized cells are also present, like goblet, Paneth, Tuft and enteroendocrine cells [27]. Furthermore, the differentiation of M cells from the epithelial precursor cells can also be induced [27].
The proliferative regions, which are enriched with stem cells, form protrusions oriented in the outward direction of organoids [8]. Generally, in intestinal organoids, crypt and villus-like parts can be distinguished but the apical side of the epithelium is facing inward [17].
In vivo, the self-renewing capacity of the intestinal epithelium (turnover rate 4–5 days) may compensate for the extensive cell death caused by the digesta and the mechanical abrasion [14]. In organoids, new progenitor cells that differentiate into different intestinal cells types are continuously generated. It is possible that this regeneration could compensate for the toxicity of the digesta.
By sustaining the self-renewing capacity of the stem cells, organoids imitate the structure and functions of the tissues, while keeping the cell-type diversity of the tissue [62]. Still, unpredictable growth can occur even in the case of the same stem cell lines [47]. This may diminish reproducibility [63]. The absorption and metabolism are higher in organoids than in 2D cell cultures [63].
Organoids are lacking some components of the whole intestine, for example, immune cells, neurons, and blood vessels covered with endothelium [28,42,45]. The morphology of the organoids significantly restricts the accomplishment of different experiments.
In the case of organoids, the apical part of the epithelium faces the inside of the organoids. This hinders the investigation of the effect of bacteria and food components on organoids and the evaluation of transport processes [42,62]. In contrast with the in vivo organization, in this case, the regions that are similar to the villus do not form protrusions, while crypts extend outwards (Figure 5). In the case of normal gastrointestinal organization, the apical part of the epithelium is oriented in the direction of the lumen, where it comes into contact with the digesta and microbiota.
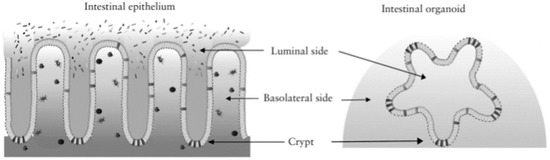
Figure 5.
The structure of organoids compared to the intestinal epithelium [64].
It was proven that these “mini-guts” reproduce intestinal absorption effectively [17]. Due to the presence of goblet cells, a small amount of mucus is also secreted to the lumen together with the cell debris [8,27,28].
In vivo, the substances secreted by goblet, enteroendocrine, and Paneth cells, and the dead cells are removed by peristaltic motility and the flow of the digesta, while in the case of organoids, these accumulate in the central area [42]. The imitation of the in vivo physical forces that affect the intestinal epithelium (shear stress generated by fluid flow and distortion caused by peristaltic movements) is impossible because the lumen of organoids is inaccessible [17,28].
The investigated food components and bacteria can be introduced to the luminal side of organoids by microinjection but this is a technically challenging and time-consuming task, which may cause damage to the organoid structure [17,47].
Another approach to make the apical side more accessible is to reverse the epithelial polarity of organoids [41,64]. For this, the chelator EDTA is used to bind cations, which otherwise induce the polymerization of the extracellular matrix protein laminin [64]. Then, organoids are cultured in suspension, in low-attachment plates, and 3 days later, their polarity will be reversed [64]. As one side of the cell layer is still inaccessible, measuring the integrity of the intestinal barrier is still impossible [65]. Organoids can also be cultured on hydrogel scaffolds that imitate the 3D organization of the epithelium [65].
2.3.5. Microfluidic Systems That May Be Used in Absorption Studies
Microfluidic systems (also called organ-on-a-chip models) are micrometer-sized chambers, where cells can be cultured in continuous contact with flowing culture media [14]. In gut-on-a-chips, the differentiation of epithelial cells is influenced by the reproduction of flow and peristaltic motility. The intestinal epithelium may be more robust when it is cultured under continuous flow [66].
The configuration of the microfluidic systems that reproduce the gut may be determined by the research goal, as the consideration of different factors is necessary in the case of absorption– or host–microbiota interaction studies.
Imura et al. [67] built a microfluidic system for the evaluation of absorption. They used a microchannel separated by a membrane on which Caco-2 cells were maintained. The medium was pumped to the lower and upper compartments at a flow rate of 1 µL/min. The validity of the system was proven by tests with cyclophosphamide (CP, a reagent used for chemotherapy) and Lucifer yellow (LY, a fluorescent dye). In control experiments without Caco-2 cells, both CP and LY could be transferred through the membrane. When Caco-2 cells formed a confluent layer, only CP could permeate from the upper compartment to the lower one. These results are in accordance with the in vivo results, as CP can be absorbed in vivo and the intestinal wall is impermeable for LY.
Gut-on-a-chips usually contain two microchannels: the upper microchannel represents the lumen, while the lower one, where endothelial cells are cultured, simulates blood vessels (Figure 6) [58]. In the case of more complex microfluidic systems, more microchannels are applied, separated from each other by a porous semipermeable membrane [14]. Epithelial cell lines or fragmented organoids can be cultured on the membrane.
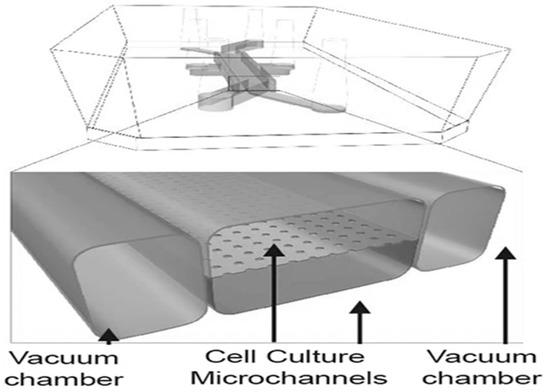
Figure 6.
The best-known method to simulate peristaltic motility in a gut-on-a-chip [42].
The membrane and the chambers can be covered with natural polymers, which can support the adhesion, migration, or specific function of the cells [23]. The development of intestinal villi in some microfluidic systems (e.g., [24]) may be facilitated by the presence of scaffolds (e.g., from collagen) [23]. Another approach is based on the fact that the Caco-2 cells and the organoids can form villi-like structures spontaneously due to mechanical signals [57]. On the side and the top of these villi-like structures, distinct types of differentiated epithelial cells (absorptive, goblet, enteroendocrine, Paneth cells) are present, while the proliferative cells are at the bottom of the crypts [57,68].
The integrity of the epithelial barrier, the tight junctions, and the mucus production are improved in the case of microfluidic systems, in comparison to static Caco-2 Transwell cultures [57].
The microfluidic systems can reproduce the chemical, hydrodynamic, and mechanical gradients that are characteristic of the investigated organ [69].
The flow can be established by different methods, like syringes, pumps, or gravity [43]. It removes dead cells and enhances access to nutrients [43]. It also induces the formation of villi-like structures and microvilli and mucin secretion [69]. Flow shear stress also induces a better reproduction of the in vivo circumstances [9]. So, according to Valiei et al. [69], a proper model of the human intestine cannot be realized without reproduction of the fluid flow.
In several cases, a pneumatic cyclic vacuum suction is applied in the chambers that surround the microchannels, which causes pulsatile flow and periodically stretches the membrane (Figure 6) [17]. The manipulation of the membrane induces cell distortion, which simulates the effect of peristalsis [8,47]. The high-throughput screening with these chips is hindered by the complexity of the system that is needed for its operation [5].
To imitate peristaltic motion, peristaltic liquid pumps can also be used instead of gas-actuated channels [69]. When fluid flow and cyclic mechanical force was applied together, the epithelial permeability measurements correlated better with in vivo data, and mucin production was similar to in vivo conditions [69]. It also optimized the formation of villi-like structures [69].
Gut-on-a-chip models usually simulate flow in one direction, while in vivo, besides peristalsis, segmentation is also present, which also moves flow in the opposite direction [58,61]. Microfluidic systems are more precise than organoids and 2D models due to the establishment of proper hydrodynamics, mechanical stimulation, and chemical (e.g., oxygen) gradients [5,69].
The oxygen gradient (hypoxic in the lumen, normoxic at the surface of the cells) is also important when integration of microbiota is necessary because of the research topic.
The alterations in the simulated intestine can be continuously monitored in the case of many organ-on-a-chip models. These microfluidic systems can be made of transparent materials, and/or may contain sensors or real-time analytical instruments [23]. Through integrated sensors, for example, barrier integrity or oxygen concentration can be continuously monitored [70].
While in the case of 3D organoids, the inner side is not accessible for verification of barrier integrity, the culture of fragmented organoids on gut-on-a-chip models enables the evaluation of intestinal barrier effectiveness [42]. Induced pluripotent stem cell-derived organoids and also organoids derived from human biopsies have been cultured in gut-on-a-chip models [43].
The small scale of microfluidic systems entails several disadvantages [29]. For example, the utilization of gut-on-a-chip models requires experience and is also laborious and costly [12,41].
Several different approaches are applied to simulate and evaluate host–microbiota interaction. The “HuMix” device by Greenhalgh et al. [71] and the “HMI” module by Marzorati et al. [72] separate the microbiota from the Caco-2 cells by a membrane and an artificial mucus layer [65]. The so-called “Intestine Chip” [73] and the “Anoxic-Oxic Interface-on-a-Chip” [74] simulate the in vivo circumstances more precisely because the mucus layer that separates the intestinal cells from the microbiota is secreted by the intestinal cells. The mucus acts as a lubricating barrier that protects the intestinal cells from the harmful components in the lumen [17]. It also influences absorption [11], so it can be a decisive part of an intestinal in vitro absorption model. The “Intestine Chip” is presented in Figure 7, which is based on [65]. The sizes of the elements in Figure 7 are not proportional. Jalili-Firoozinezhad et al. [73] designed the “Intestine Chip” both with Caco-2 cells and with intestinal epithelial cells isolated from organoids. In the case of Caco-2 cell cultures, oxygen sensors were also included in the system. When culturing Caco-2 cells, immortalized endothelial cells were also cultured in the bottom channel. Shin et al. [74] cultured Caco-2 cells in the “Anoxic-Oxic Interface-on-a-Chip”. The main difference between the “Intestine Chip” and the “Anoxic-Oxic Interface-on-a-Chip” is the strategy applied for oxygen modulation [65]. In both cases, aerobic cell medium is perfused through the lower channel. In the case of the “Intestine Chip”, the chip is placed in an anaerobic box, while in the top channel of “Anoxic-Oxic Interface-on-a-Chip” anoxic medium (obtained by mixing the medium with 6.5 mM sodium sulfide) is perfused [65]. In the case of the “Intestine Chip”, endothelial cells can also be included in the bottom channel.
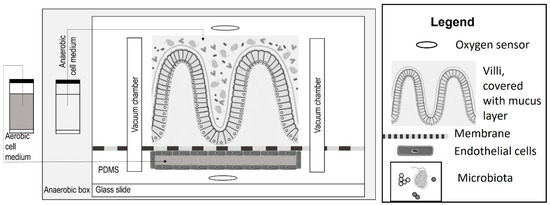
Figure 7.
The schematic organization of the “Intestine Chip” [73], based on [65].
As the gut bacteria may produce metabolites from some food components (e.g., polyphenols), it also may be an advantage that these gut-on-a-chip models (Figure 6 and Figure 7) also include the microbiota. Microbiota and their metabolites affect each cell type differently [64].
Xavier et al. [66] prepared a modular microfluidic-based platform to imitate the gastrointestinal tract. The first module was the digestion-chip, which enables the digestion of small volumes with the COST Infogest protocol. The second module consisted of a gut-on-a-chip system, where Caco-2/HT29-MTX co-cultures were maintained under continuous flow. According to our knowledge, Xavier et al. [66] were the first ones who combined the COST Infogest protocol with an absorption model that is made of a gut-on-a-chip system. The integrity of the intestinal barrier was proven to be effective with Lucifer yellow permeability assessment.
When Xavier et al. [66] maintained Caco-2/HT29-MTX co-cultures in a gut-on-a-chip system, under continuous flow, the application of a serine protease inhibitor (Pefabloc® SC) and 1:6 dilution of digesta was enough to reduce cytotoxicity of digesta [66]. de Haan et al. [75] diluted the digesta 8 times in a flow-through Transwell insert. They have shown that concentration below 62.5% of digesta is not toxic to Caco-2/HT29-MTX-E12 co-culture after incubation for 24 h. In accordance with this, Kalungwana et al. [76] only diluted the digesta 3 times to avoid the cytotoxicity of bile salts but they only incubated the sample with the digesta for 4 h, while Xavier et al. [66] investigated the effect of digesta on cell viability for 24 h. While this 3-fold dilution is the best result so far, the method still needs improvement.
The culture of organoids in microfluidic devices may have the potential of recreating the organ-level physiology [12]. As organoids maintain their self-renewal capacity, the application of organoids in the gut-on-a-chip system may compensate for the cell death induced by the toxicity of the digesta.
2.4. Ex Vivo Models
Ex vivo refers to experiments conducted in or on tissue from an organism in an external environment with minimal alteration of natural conditions. Ex vivo models replicate the structure and physiology of the intestine more precisely since they apply tissues excised from animals or humans but unfortunately, the viability of these samples is very short (usually less than 1 day, in some cases, less than an hour) [22,28].
The interaction of different cell types can be better evaluated with ex vivo models than with microfluidic systems. In the case of microfluidic systems, besides epithelial cells, endothelial and/or immune cells [77] can be integrated in the chips but replicating the exact histological organization is difficult, if not impossible. In cases of tissues maintained ex vivo, the endothelial, immune cells, and neurons are already present in the right proportion.
The ex vivo absorption studies often use tissues of animal origin (e.g., rat, pig) to estimate the transport processes of the human intestine [22]. The human intestine tissue removed during surgeries provides a better representation of the human physiology. However, the inter-individual variability impedes the collection of systematic data regarding absorption in the intestine, while the utilization of animal tissues can be standardized [22].
It is hard to draw relevant, unequivocal conclusions regarding the human physiological processes from results that are obtained from different mammalian tissues. These experiments can be contradictory [41]. The application of ex vivo models is also restricted by the limited accessibility of human tissue [47].
The application of ex vivo models is quite easy and cheap [22].
The functioning of the Franz cell and the Ussing chamber (Figure 8) is based on the same principle: the intestinal tissue is mounted between two compartments, separating apical and basolateral sides [22,47]. In the case of the Ussing chamber, the orientation of the intestinal tissue is vertical, while in the case of the Franz cells it is horizontal [22].
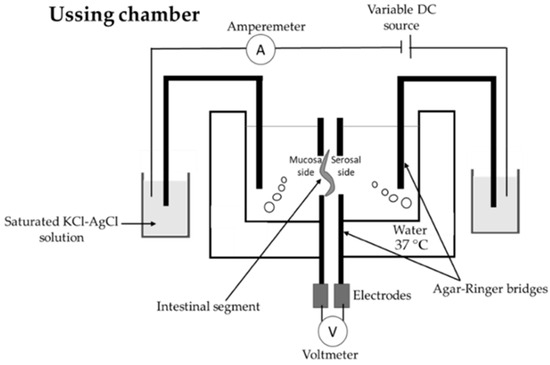
Figure 8.
Illustration of the Ussing chamber [47].
The Ussing chamber measures voltage, transepithelial resistance, and short-circuit current with electrodes [10,22]. “Short-circuit current reflects electrogenic ionic flux across the epithelium” [22]. This allows the continuous surveillance of the integrity and viability of the investigated tissue/cell layer [22]. Kalungwana et al. [76] used the Ussing chamber with mouse intestinal tissue and Caco-2 cell cultures to evaluate the absorption of dietary carotenoids. They concluded that the utilization of the Ussing chamber model with mouse intestinal tissue after in vitro digestion with Infogest protocol can be an effective method to determine carotenoid bioavailability.
In the case of Franz cells, the contents of the compartment that is in contact with the basal side of the epithelium (the compartment that is opposite to the chamber where the food/digesta is added) is continuously stirred, which may cause enhanced permeability compared to the Ussing chamber [22].
von Erlach et al. [78] recently developed the gastrointestinal tissue robotic interface system (GI-TRIS), which enables long-term maintenance of the tissue [22]. In this case, the porcine intestinal tissue is incubated in a 96-well plate, enabling high-throughput screening, i.e., more samples and/or more parallel experiments can be performed [22,78]. The GI-TRIS was developed in two different set-ups. The first design can be combined with commercially available multiwell plates, while the other one can be used for direct measurements in a spectrophotometer without disassembly and is also compatible with a robotic system [78].
In contrast to Caco-2 Transwell transport assays, where the digesta is toxic to cells, native intestinal fluid can be applied in GI-TRIS [78].
The InTESTine® system from TNO is also appropriate for high-throughput screening as it contains 24 or 96 chambers. In each chamber, fresh, human, or porcine intestine tissue is mounted horizontally between two compartments, creating an apical and a basolateral side [47].
The “everted intestinal sac” model is also used in the research laboratory (Figure 9). In this case, sections of the intestine are turned inside out with a glass rod. Then, the intestinal sac is created by sealing the ends of the 3–4 cm long sections with moistened threads [47,79,80]. Turning the intestine inside out can damage the tissue and it can also cause accidental removal of the mucus layer. The stress may also cause mucus overproduction [22]. If the intestine sections are not turned inside out, the tissue damage can be avoided, the experiments become simpler, and a smaller quantity of the investigated compound is needed. In this case, continuous sampling also becomes possible without disturbing the integrity of the intestinal tissue [22].
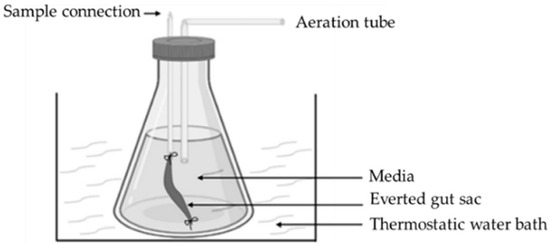
Figure 9.
Illustration of everted gut sac [47].
2.5. Advantages and Disadvantages of Models of the Intestinal Barrier
Since there are numerous approaches to imitate intestinal absorption, it is important to weigh the advantages and disadvantages of each model to choose the protocol that answers the research question, with a minimal investment of resources. The advantages and disadvantages of the most relevant intestinal models are summarized in Table 1.

Table 1.
Summary of the advantages and disadvantages of in vitro and ex vivo models of the intestinal epithelium.
3. Conclusions
A proper absorption model should be chosen according to the precision and feasibility that is necessary for obtaining relevant results [65]. The control and interpretation of simpler systems (non-cell-based transport-, 2D, ex vivo models) is easier but they may lack details that can be essential in the absorptive process.
Organoids sustain the self-renewing capacity of stem cells, maintain the cell-type diversity of the tissue, and imitate the structure and function of tissues [62]. However, experiments with 3D organoids are hindered by the inaccessibility of the inner side of the organoid.
According to our knowledge, absorption experiments with digested food on gut-on-a-chip models are rare. Still, studies about drug absorption [12,22] already recommend the utilization of gut-on-a-chip models. The application of fragmented organoids in gut-on-a-chips would make possible a more precise estimation of the absorption of food components from digested food.
This review describes the most relevant methods that can be applied for the simulation of the absorption of food in the intestinal tract. Until now, there are no standardized methods to imitate intestinal absorption of food components. It was proven that the standardized COST Infogest protocol is an effective and cheap method to evaluate food digestion in the upper gastrointestinal tract. As it lacks brush border enzymes and the intestinal tissue that regulates absorption, it should be complemented with a standardized protocol for the imitation of the intestinal barrier. It is possible that it is unachievable to apply a single model for each research question and several standardized protocols have to be developed. For example, several foodstuffs can be metabolized by the intestinal microbiota. In these cases, more complex models are needed that enable the cultivation of anaerobic intestinal microbiota in the presence of aerobic intestinal cells.
This review not only could be a starting point in the standardization of absorption protocols, it also can promote the reduction of in vivo experiments necessary for the evaluation of foodstuffs. We presented numerous in vitro methods that do not need animal tissue at all for the investigation of absorption. Ex vivo models need animal sacrifice but in numerous cases, the tissue of one animal can be used for multiple experiments.
Choosing the proper in vitro or ex vivo absorption model allows reproducible transport, nutrition, and toxicity studies with the replacement or reduction of animal experiments.
Author Contributions
Conceptualization, O.A.; writing—original draft preparation, O.A.; writing—review and editing, K.T.; project administration, I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- Antal, O.; Némethné Szerdahelyi, E.; Takács, K. In vitro humán emésztési modellek alkalmazása a táplálkozástudomány területén. (Appl. Vitr. Hum. Dig. Models Int He Field Nutr. Sci.) Élelmiszervizsgálati Közlemények J. Food Investig. 2020, 66, 31–57. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Tormási, J.; Abrankó, L. Lipid digestibility of sour cream and its analogue during in vitro digestion simulation and co-consumption with cooked pasta. Int. J. Food Sci. Technol. 2023, 58, 6330–6341. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, X.; Shang, Y.; Ding, Y. Microfluidic intestine-on-a-chip: Current progress and further perspectives of probiotic-foodborne pathogen interactions. Trends Food Sci. Technol. 2023, 134, 207–221. [Google Scholar] [CrossRef]
- Haddad, M.J.; Sztupecki, W.; Delayre-Orthez, C.; Rhazi, L.; Barbezier, N.; Depeint, F.; Anton, P.M. Complexification of in vitro models of intestinal barriers, a true challenge for a more accurate alternative approach. Int. J. Mol. Sci. 2023, 244, 3595. [Google Scholar] [CrossRef]
- Kondrashina, A.; Arranz, E.; Cilla, A.; Faria, M.A.; Santos-Hernández, M.; Miralles, B.; Hashemi, N.; Rasmussen, M.K.; Young, J.F.; Barberá, R.; et al. Coupling in vitro food digestion with in vitro epithelial absorption; recommendations for biocompatibility. Crit. Rev. Food Sci. Nutr. 2023, 26, 1–19. [Google Scholar] [CrossRef]
- Dutton, J.S.; Hinman, S.S.; Kim, R.; Wang, Y.; Allbritton, N.L. Primary cell-derived intestinal models: Recapitul. Physiol. Trends Biotechnol. 2019, 377, 744–760. [Google Scholar] [CrossRef]
- Grzymajlo, K. The game for three: Salmonella-host-microbiota interaction models. Front. Microbiol. 2022, 13, 854112. [Google Scholar] [CrossRef]
- Pearce, S.C.; Coia, H.G.; Karl, J.P.; Pantoja-Feliciano, I.G.; Zachos, N.C.; Racicot, K. Intestinal in vitro and ex vivo models to study host-microbiome interactions and acute stressors. Front. Physiol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Lechanteur, A.; das Neves, J.; Sarmento, B. The role of mucus in cell-based models used to screen mucosal drug delivery. Advandced Drug Deliv. Rev. 2018, 124, 50–63. [Google Scholar] [CrossRef]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control Release 2021, 335, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Suzuki, K.; Sifuentes-Dominguez, L.; Miyata, N.; Song, J.; Lopez, A.; Starokadomskyy, P.; Gopal, P.; Dozmorov, I.; Tan, S.; et al. Paneth cell-derived growth factors support tumorigenesis in the small intestine. Life Sci. Alliance. 2020, 43, e202000934. [Google Scholar] [CrossRef] [PubMed]
- Creff, J.; Malaquin, L.; Besson, A. In vitro models of intestinal epithelium: Toward bioengineered systems. J. Tissue Eng. 2021, 12, 2041731420985202. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.; Salzman, N. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft cells and their role in intestinal diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef]
- Steinway, S.N.; Saleh, J.; Koo, B.K.; Delacour, D.; Kim, D.H. Human microphysiological models of intestinal tissue and gut microbiome. Front. Bioeng. Biotechnol. 2020, 8, 725. [Google Scholar] [CrossRef]
- Siwczak, F.; Loffet, E.; Kaminska, M.; Koceva, H.; Mahe, M.M.; Mosig, A.S. Intestinal stem cell-on-chip to study human host-microbiota interaction. Front. Immunol. 2021, 12, 798552. [Google Scholar] [CrossRef]
- Tormási, J.; Abrankó, L. Impact of grape seed powder and black tea brew on lipid digestion-An in vitro co-digestion study with real foods. Nutrients 2023, 1510, 2395. [Google Scholar] [CrossRef]
- Tümer, E.; Bröer, A.; Balkrishna, S.; Jülich, T.; Bröer, S. Enterocyte-specific regulation of the apical nutrient transporter SLC6A19 B0AT1 by transcriptional and epigenetic networks. J. Biol. Chem. 2013, 28847, 33813–33823. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Martinez, M.N.; Dao, K.; Gabriel, V.; Zdyrski, C.; Jergens, A.E.; Atherly, T.; Iennarella-Servantez, C.A.; Burns, L.E.; Schrunk, D.; et al. Canine intestinal organoids as a novel in vitro model of intestinal drug permeability: A proof-of-concept study. Cells 2023, 129, 1269. [Google Scholar] [CrossRef]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. An overview of in vitro, ex vivo and in vivo models for studying the transport of drugs across intestinal barriers. Adv. Drug Deliv. Rev. 2021, 175, 113795. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Nasiri, R.; Barros, N.R.; Tebon, P.; Thakor, J.; Goudie, M.; Shamloo, A.; Martin, M.G.; Khademhosseini, A. Gut-on-a-chip: Current progress and future opportunities. Biomaterials 2020, 255, 120196. [Google Scholar]
- Shim, K.Y.; Lee, D.; Han, J.; Nguyen, N.T.; Park, S.; Sung, J.H. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed. Microdevices 2017, 192, 37. [Google Scholar] [CrossRef] [PubMed]
- Gościniak, A.; Eder, P.; Walkowiak, J.; Cielecka-Piontek, J. Artificial gastrointestinal models for nutraceuticals research-achievements and challenges: A practical review. Nutrients 2022, 1413, 2560. [Google Scholar] [CrossRef]
- Biagini, F.; Calvigioni, M.; Lapomarda, A.; Vecchione, A.; Magliaro, C.; De Maria, C.; Montemurro, F.; Celandroni, F.; Mazzantini, D.; Mattioli-Belmonte, M.; et al. A novel 3D in vitro model of the human gut microbiota. Sci. Rep. 2020, 10, 21499. [Google Scholar] [CrossRef]
- Hentschel, V.; Seufferlein, T.; Armacki, M. Intestinal organoids in coculture: Redefining the boundaries of gut mucosa ex vivo modeling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 3216, G693–G704. [Google Scholar] [CrossRef]
- O’Farrell, C.; Stamatopoulos, K.; Simmons, M.; Batchelor, H. In vitro models to evaluate ingestible devices: Present status and current trends. Adv. Drug Deliv. Rev. 2021, 178, 113924. [Google Scholar] [CrossRef]
- Costa, J.; Ahluwalia, A. Advances and current challenges in intestinal in vitro model engineering: A Digest. Front. Bioeng. Biotechnol. 2019, 7, 144. [Google Scholar] [CrossRef]
- Kang, T.H.; Kim, H.J. Farewell to animal testing: Innovations on human intestinal microphysiological systems. Micromachines 2016, 77, 107. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, H.J.; Xiu, H.; Akoh, C.C.; Kong, F. Predicting intestinal effective permeability of different transport mechanisms: Comparing ex vivo porcine and in vitro dialysis models. J. Food Eng. 2023, 338, 111256. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Sanchis, V.; Ramos, A.J. Mycotoxin bioaccessibility/absorption assessment using in vitro digestion models: A review. World Mycotoxin J. 2013, 62, 167–184. [Google Scholar] [CrossRef]
- Lefebvre, D.E.; Venema, K.; Gombau, L.; Valerio, L.G., Jr.; Raju, J.; Bondy, G.S.; Bouwmeester, H.; Singh, R.P.; Clippinger, A.J.; Collnot, E.M.; et al. Utility of models of the gastrointestinal tract for assessment of the digestion and absorption of engineered nanomaterials released from food matrices. Nanotoxicology 2015, 94, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Preiser, H.; Maestracci, D.; Ghosh, B.K.; Cerda, J.J.; Crane, R.K. Purification of the human intestinal brush border membrane. Biochim. Biophys. Acta BBA-Biomembr. 1973, 3231, 98–112. [Google Scholar] [CrossRef]
- Kessler, M.; Acuto, O.; Storelli, C.; Murer, H.; Müller, M.; Semenza, G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim. Biophys. Acta BBA 1978, 5061, 136–154. [Google Scholar] [CrossRef]
- Patel, G.; Misra, A. 10—Oral Delivery of Proteins and Peptides: Concepts and Applications. In Challenges in Delivery of Therapeutic Genomics and Proteomics, 1st ed.; Misra, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 481–529. [Google Scholar]
- Said, H.M.; Redha, R.; Nylander, W. Biotin transport in basolateral membrane vesicles of human intestine. Gastroenterology 1988, 945, 1157–1163. [Google Scholar] [CrossRef]
- Carter, M.; Essner, R.; Goldstein, N.; Iyer, M. Guide to Research Techniques in Neuroscience, 3rd ed.; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Anjum, M.; Laitila, A.; Ouwehand, A.C.; Forssten, S.D. Current perspectives on gastrointestinal models to assess probiotic-pathogen interactions. Front. Microbiol. 2022, 13, 831455. [Google Scholar] [CrossRef]
- Kasendra, M.; Tovaglieri, A.; Sontheimer-Phelps, A.; Jalili-Firoozinezhad, S.; Bein, A.; Chalkiadaki, A.; Scholl, W.; Zhang, C.; Rickner, H.; Richmond, C.A.; et al. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci. Rep. 2018, 81, 2871. [Google Scholar] [CrossRef]
- Morelli, M.; Kurek, D.; Ng, C.P.; Queiroz, K. Gut-on-a-chip models: Current and future perspectives for host-microbial interactions research. Biomedicines 2023, 112, 619. [Google Scholar] [CrossRef]
- Corning Incorporated. Transwell®, SnapwellTM, Netwell, and Falcon® Permeable Supports. Available online: https://www.corning.com/au/en/products/life-sciences/products/permeable-supports/transwell-snapwell-netwell-falcon-permeable-supports.html (accessed on 12 March 2024).
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic organ-on-a-chip models of human intestine. Cell. Mol. Gastroenterol. Hepatol. 2018, 54, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Roupar, D.; Berni, P.; Martins, J.T.; Caetano, A.C.; Teixeira, J.A.; Clarisse Nobre, C. Bioengineering approaches to simulate human colon microbiome ecosystem. Trends Food Sci. Technol. 2021, 112, 808–822. [Google Scholar] [CrossRef]
- Rahman, S.; Ghiboub, M.; Donkers, J.M.; van de Steeg, E.; van Tol, E.A.F.; Hakvoort, T.B.M.; de Jonge, W.J. The Progress of intestinal epithelial models from cell lines to gut-on-chip. Int. J. Mol. Sci. 2021, 2224, 13472. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Failla, M.L. Intestinal cell models for investigating the uptake, metabolism and absorption of dietary nutrients and bioactive compounds. Curr. Opin. Food Sci. 2021, 41, 169–179. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Chou, D.B.; Tovaglieri, A.; Ferrante, T.C.; Duckworth, T.; Fadel, C.; Frismantas, V.; Sutherland, A.D.; Jalili-Firoozinezhad, S.; Kasendra, M.; et al. Human colonon-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Langerholc, T.; Maragkoudakis, P.A.; Wollgast, J.; Gradisnik, L.; Cencic, A. Novel and established intestinal cell line models—An indispensable tool in food science and nutrition. Trends Food Sci. Technol. 2011, 22, S11–S20. [Google Scholar] [CrossRef]
- Cencič, A.; Langerholc, T. Functional cell models of the gut and their applications in food microbiology—A review. Int. J. Food Microbiol. 2010, 141, S4–S14. [Google Scholar] [CrossRef]
- Jensen, H.H.; Holst, M.R.; Login, F.H.; Morgen, J.J.; Nejsum, L.N. Ectopic expression of aquaporin-5 in noncancerous epithelial MDCK cells changes cellular morphology and actin fiber formation without inducing epithelial-to-mesenchymal transition. Am. J. Physiol. Cell Physiol. 2018, 3146, C654–C661. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative human three-dimensional tissue-engineered models as an alternative to animal testing. Bioengineering 2020, 73, 115. [Google Scholar] [CrossRef]
- Pimenta, J.; Ribeiro, R.; Almeida, R.; Costa, P.F.; da Silva, M.A.; Pereira, B. Organ-on-chip approaches for intestinal 3D in vitro modeling. Cell. Mol. Gastroenterol. Hepatol. 2022, 132, 351–367. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Prim. 2022, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef] [PubMed]
- Donkers, J.M.; Amirabadi, H.E.; van de Steeg, E. Intestine-on-a-chip: Next level in vitro research model of the human intestine. Curr. Opin. Toxicol. 2021, 25, 6–14. [Google Scholar] [CrossRef]
- Thomas, D.P.; Zhang, J.; Nguyen, N.T.; Ta, H.T. Microfluidic gut-on-a-chip: Fundamentals and challenges. Biosensors 2023, 131, 136. [Google Scholar] [CrossRef]
- Zhang, D.; Qiao, L. Intestine-on-a-chip for intestinal disease study and pharmacological research. View 2023, 4, 20220037. [Google Scholar] [CrossRef]
- Barrila, J.; Radtke, A.L.; Crabbé, A.; Sarker, S.F.; Herbst-Kralovetz, M.M.; Ott, C.M.; Nickerson, C.A. Organotypic 3D cell culture models: Using the rotating wall vessel to study host-pathogen interactions. Nat. Rev. Microbiol. 2010, 8, 791–801. [Google Scholar] [CrossRef]
- Costello, C.M.; Phillipsen, M.B.; Hartmanis, L.M.; Kwasnica, M.A.; Chen, V.; Hackam, D.; Chang, M.W.; Bentley, W.E.; March, J.C. Microscale bioreactors for in situ characterization of GI epithelial cell physiology. Sci. Rep. 2017, 7, 12515. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Chen, Y.G. Generation of 3D human gastrointestinal organoids: Principle and applications. Cell Regen. 2020, 91, 6. [Google Scholar] [CrossRef]
- Stanton, J.E.; Grabrucker, A.M. The use of organoids in food research. Curr. Opin. Food Sci. 2023, 49, 100977. [Google Scholar] [CrossRef]
- Poletti, M.; Arnauts, K.; Ferrante, M.; Korcsmaros, T. Organoid-based models to study the role of host-microbiota interactions in IBD. J. Crohn’s Colitis 2021, 157, 1222–1235. [Google Scholar] [CrossRef]
- Bossink, E.G.B.M.; Segerink, L.I.; Odijk, M. Organ-on-chip technology for aerobic intestinal host—anaerobic microbiota research. Organs–A-Chip 2022, 4, 100013. [Google Scholar] [CrossRef]
- Xavier, M.; Rodrigues, P.M.; Neto, M.D.; Guedes, M.I.; Calero, V.; Pastrana, L.; Gonçalves, C. From mouth to gut: Microfluidic in vitro simulation of human gastro-intestinal digestion and intestinal permeability. Analyst 2023, 14814, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Asano, Y.; Sato, K.; Yoshimura, E. A microfluidic system to evaluate intestinal absorption. Anal. Sci. 2009, 25, 1403–1407. [Google Scholar] [CrossRef]
- Workman, M.J.; Gleeson, J.P.; Troisi, E.J.; Estrada, H.Q.; Kerns, S.J.; Hinojosa, C.D.; Hamilton, G.A.; Targan, S.R.; Svendsen, C.N.; Barrett, R.J. Enhanced utilization of induced pluripotent stem cell-derived human intestinal organoids using microengineered chips. Cell. Mol. Gastroenterol. Hepatol. 2017, 54, 669–677.e2. [Google Scholar] [CrossRef] [PubMed]
- Valiei, A.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Gut-on-a-chip models for dissecting the gut microbiology and physiology. APL Bioeng. 2023, 71, 011502. [Google Scholar] [CrossRef]
- Marrero, D.; Pujol-Vila, F.; Vera, D.; Gabriel, G.; Illa, X.; Elizalde-Torrent, A.; Alvarez, M.; Villa, R. Gut-on-a-chip: Mimicking and monitoring the human intestine. Biosens. Bioelectron. 2021, 181, 113156. [Google Scholar] [CrossRef]
- Greenhalgh, K.; Ramiro-Garcia, J.; Heinken, A.; Ullmann, P.; Bintener, T.; Pacheco, M.P.; Baginska, J.; Shah, P.; Frachet, A.; Halder, R.; et al. Integrated in vitro and in silico modeling delineates the molecular effects of a synbiotic regimen on colorectal-cancer-derived cells. Cell Rep. 2019, 275, 1621–1632.e9. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, M.; Vanhoecke, B.; De Ryck, T.; Sadaghian Sadabad, M.; Pinheiro, I.; Possemiers, S.; Van den Abbeele, P.; Derycke, L.; Bracke, M.; Pieters, J.; et al. The HMI™ module: A new tool to study the Host-Microbiota Interaction in the human gastrointestinal tract in vitro. BMC Microbiol. 2014, 14, 133. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Shin, W.; Wu, A.; Massidda, M.W.; Foster, C.; Thomas, N.; Lee, D.W.; Koh, H.; Ju, Y.; Kim, J.; Kim, H.J. A robust longitudinal co-culture of obligate anaerobic gut microbiome with human intestinal epithelium in an anoxic-oxic interface-on-a-chip. Front. Bioeng. Biotechnol. 2019, 7, 13. [Google Scholar] [CrossRef]
- de Haan, P.; Santbergen, M.J.C.; van der Zande, M.; Bouwmeester, H.; Nielen, M.W.F.; Verpoorte, E. A versatile, compartmentalised gut-on-a-chip system for pharmacological and toxicological analyses. Sci. Rep. 2021, 111, 4920. [Google Scholar] [CrossRef] [PubMed]
- Kalungwana, N.; Marshall, L.; Mackie, A.; Boesch, C. An ex vivo intestinal absorption model is more effective than an in vitro cell model to characterise absorption of dietary carotenoids following simulated gastrointestinal digestion. Food Res. Int. 2023, 166, 112558. [Google Scholar] [CrossRef] [PubMed]
- Gijzen, L.; Marescotti, D.; Raineri, E.; Nicolas, A.; Lanz, H.L.; Guerrera, D.; van Vught, R.; Joore, J.; Vulto, P.; Peitsch, M.C.; et al. An intestine-on-a-chip model of plug-and-play modularity to study inflammatory processes. SLAS Technol. 2020, 256, 585–597. [Google Scholar] [CrossRef] [PubMed]
- von Erlach, T.; Saxton, S.; Shi, Y.; Minahan, D.; Reker, D.; Javid, F.; Lee, Y.L.; Schoellhammer, C.; Esfandiary, T.; Cleveland, C.; et al. Robotically handled whole-tissue culture system for the screening of oral drug formulations. Nat. Biomed. Eng. 2020, 45, 544–559. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Butt, A.G. Glucose transport into everted sacs of the small intestine of mice. Adv. Physiol. Educ. 2013, 374, 415–426. [Google Scholar] [CrossRef]
- van Krimpen, M.M.; Hulst, M.M.; van der Meulen, J.; Schokker, D.; Savelkoul, H.F.J.; Tijhaar, E.J.; Rutten, V.P.M.G. Nutritional Intervention in Animals: Benchmarking of Strategies, Monitoring Biomarkers and Immune Competence. Available online: https://edepot.wur.nl/320444 (accessed on 27 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).