Abstract
Additive manufacturing resins used in dental prosthetics may retain uncured monomers post-polymerization, posing potential long-term patient exposure risks. Understanding the biological safety of these materials is crucial, particularly for 3D-printed acrylic-based prosthodontic devices such as occlusal nightguards, complete and partial dentures, and temporary fixed prostheses. This paper reviews the literature evaluating the cytotoxicity of such materials. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, we conducted a scoping review using the MESH keywords related to population (P), intervention (I), comparison (C), and outcome (O) across databases, including OVID Medline, EMBASE, and SCOPUS. Our search, limited to peer-reviewed English language articles from 2015 to 2023, resulted in 22 papers. These studies, utilizing digital light processing (DLP) or stereolithography (SLA) printing methods, varied in examining different 3D-printed materials, as well as washing and post-curing protocols. The primary experimental cells used were human gingival fibroblasts (HGF) and mouse fibroblasts (L929). There are no statistical differences in biocompatibility regarding different commercially available resins, washing solutions, or methods. Improvements in cell viability were related to an increase in washing time, as well as post-curing time. After the polishing procedure, 3D resin-based printed occlusal devices perform similarly to milled and conventionally processed ones. Our findings underline the importance of appropriate washing and post-curing protocols in minimizing the cytotoxic risks associated with these 3D-printed resin-based devices.
1. Introduction
The use of additive manufacturing (AM), or 3D printing, has expanded significantly within dentistry, particularly in the fabrication of prosthodontic devices such as occlusal nightguards, complete and partial dentures, and temporary fixed prostheses. This innovative technology offers the advantage of rapid workflow, customization to patient-specific needs, and the potential for cost reduction (Figure 1). In restorative dentistry, materials used in additive manufacturing are called printable biomaterials and are available as acrylates, resin-based composites, and, more recently, ceramic-infiltrated composites [].

Figure 1.
Three-dimensional printing workflow diagram demonstrating the steps in fabricating a dental device.
The additive methods most frequently applied in dentistry are stereolithography (SLA) and digital light processing (DLP) technology. Each process uses light to activate the polymerization of the printable resin. While SLA uses UV light, DLP uses a high-power LED to start the chain reaction of the monomers [].
The introduction of 3D-printed materials into clinical practice raises concerns about the biological safety of these materials, especially regarding the release of residual monomers from the polymerized products []. Residual monomers, primarily from acrylic-based resins, can be cytotoxic. Since prosthodontic devices are intended for long-term use, often in direct contact with sensitive oral tissues, releasing these monomers poses a health concern [].
Consequently, evaluating the cytotoxic effects of these materials is essential to ensure the safety and efficacy of 3D-printed dental prostheses. This review aims to systematically evaluate the cytotoxicity of 3D-printed materials used in dental prosthetics, focusing on identifying and synthesizing existing research findings while highlighting areas where further study is needed. This paper seeks to contribute to the safe and effective integration of 3D printing technologies in dental care by comprehensively analyzing the cytotoxic risks and mitigation strategies associated with these innovative materials.
As with directly photocured resin-based composites, 3D printable resins are not fully converted to the polymeric chain after exposure to light. This deficiency in the degree of conversion must be overcome by a post-curing process, usually, a light oven that uses extra-time light exposure and heat to improve the degree of conversion of these materials (Figure 2). However, complete conversion is rarely achieved, and the free unreacted monomers represent a well-known risk to biological properties [].
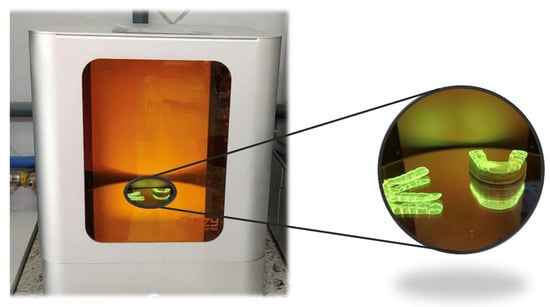
Figure 2.
A dental device in a UV light post-curing oven.
The extensive range of available 3D printing polymer materials from different manufacturers, each using a slightly different manufacturing and curing protocol, might lead to different outcomes regarding these materials’ mechanical and biological properties []. Three-dimensional printing materials can be indicated for surgical guides or custom trays to be used quickly (Figure 3). Other appliances, such as interocclusal nightguard devices and complete and partial dentures, may be produced using these materials, and long-term use occurs []. While mechanical properties have been widely investigated and documented [], and data from these experiments are more easily translated to clinical practice, the biological behavior of 3D printing materials has a complex methodology, which may confuse the dentist about their biological safety [,].

Figure 3.
Examples of materials used in 3D-printed dental devices.
Some published papers present data on the biocompatibility of printable resins for temporary and permanent prosthetic devices. Different methodologies can assess the biological response, such as inflammatory response, oxidative stress, biofilm interaction, cytotoxicity, genotoxicity, cell adhesion, cell proliferation, and cell viability [,]. Considering the amount of data produced, the complexity of methodology and data interpretation, and the need for further understanding by the general dentist regarding the cytotoxicity outcomes of printable materials, this paper aims to review the literature on the cytotoxicity evaluation of 3D-printed materials used in long-term acrylic-based prosthodontic devices, such as occlusal nightguards, complete and partial dentures, and temporary fixed prostheses.
2. Materials and Methods
A systematically conducted scoping review was utilized to summarize the currently available research on the cytotoxicity of 3D printable acrylic resins. The workflow followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement to identify, screen, elect, and include scientific papers in this review. MESH keywords were inserted into the databases to perform the search. The keywords selection obeyed the P (population), I (intervention), C (comparison), and O (outcome) question system to associate them with the advanced search option of each database portal. Table 1 describes the PICO keywords association.

Table 1.
MESH Keyword.
The scoping review search was conducted at the OVID Medline, EMBASE, and SCOPUS databases to compile papers evaluating topics related to the cytotoxicity of 3D printable resins. English-language peer-reviewed journal papers were selected from 2015 to 2023. Papers, such as dentures and occlusal nightguards, were included when investigating 3D-printed acrylic resin designs for long-term clinical use. Papers were excluded if they did not focus on 3D-printed acrylic materials used for prosthetic devices, if they studied temporary rehabilitation materials, if they investigated experimental non-commercially available materials, resin-based composite materials, ceramic and metallic materials, materials designed for biodegradable scaffolds or implant dentistry. These papers were compiled into Covidence for further review and selection, and a librarian removed duplicate entries.
Two independent reviewers used the Covidence software (www.covidence.org accessed on 14 July 2023) to screen the titles and abstracts to filter out papers relevant to the topic, applying inclusion and exclusion criteria. To ensure consistency, both reviewers evaluated all article abstracts and voted “yes”, “no”, or “maybe” for the relevance of the articles, with ties broken by a third investigator. The reviewers performed another screen, now evaluating the full text, and finalized the selection of these papers, resulting in 24 papers included in this scoping review. Figure 4 states the PRISMA workflow from identification to the inclusion of papers.
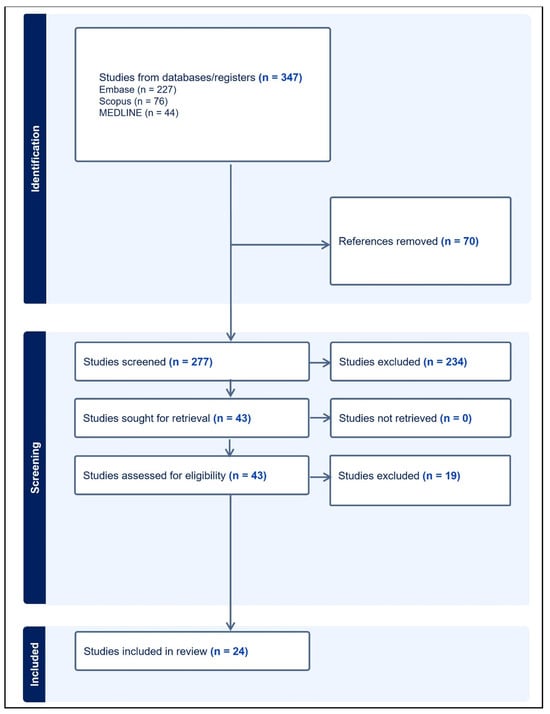
Figure 4.
Flow chart describing the extraction and selection process of relevant papers.
Both reviewers extracted data from the selected papers, considering the following criteria: author, year of paper publication, 3D-printed materials evaluated, 3D-printer device, post-processing method performed, variables tested in the experiment, cytotoxicity assay and cell lineage used, the mains results, and conclusion. All data were extracted independently, and the results from each reviewer were discussed with a third researcher when needed and merged into Table 2.

Table 2.
Extracting datasheet.
3. Results
Twenty-one papers ranging from 2021 to 2023 were finally selected and had their information and data extracted. The main characteristics of each study, as well as the outcomes related to our research question, are presented in Table 2. The study assessed various 3D-printed dental resins and their biocompatibility, focusing also on the effects of post-processing methods on cell viability. The results evidenced that the post-curing impacts the biocompatibility of such materials. For instance, uncured resins consistently demonstrated high cytotoxicity, with cell viability reduced by 50–65% depending on the method of testing and resin type []. Post-curing for at least 5 min notably improved cell viability, with further increases in post-curing time enhancing biocompatibility []. However, varying post-curing times and methods showed differential effects across different studies. For example, resins post-cured for 20 min in UV light chambers exhibited varying levels of cytotoxicity, with some showing improved biocompatibility compared to others [].
Additionally, the type of post-processing equipment and protocols influenced the cytotoxicity outcomes. Different UV light chambers and washing protocols resulted in variations in cell viability, indicating that optimal post-processing conditions are critical for reducing cytotoxic effects [,]. Materials with extended post-curing times and more thorough washing procedures generally demonstrated lower cytotoxicity, although certain resins like Freeprint Splint showed higher toxicity even with extensive post-processing [].
4. Discussion
Overall, the studies reviewed here indicated that acrylic resins used in prosthodontic appliances can exhibit varying levels of cytotoxicity, with significant differences observed across different resin formulations. Residual monomers, such as methyl methacrylate (MMA), are a primary source of cytotoxicity, as these compounds are released over time and negatively affect cellular viability []. Moreover, we observed that the cytotoxic response seems to be influenced by factors such as the curing process, resin composition, and storage conditions.
Cytotoxicity analysis of dental materials is of utmost importance as it ensures the safety and biocompatibility of these materials when in contact with human tissues. By evaluating the potentially toxic effects of dental materials on cells, such as pulp cells or gingival fibroblasts, cytotoxicity analysis provides valuable insights into their potential adverse effects on oral tissues. This analysis aids in identifying any potential risks or side effects associated with dental materials, allowing dentists and dental professionals to make informed decisions when selecting suitable materials for various dental procedures. Ultimately, cytotoxicity analysis plays a crucial role in promoting patient safety and optimizing the long-term success of dental treatments.
According to International Standard Organization (ISO) 10993-5 [], the in vitro cytotoxicity test plays a vital role in assessing the biocompatibility of biomedical materials. It serves as the first step in determining the safety of these materials before further tests, including those involving laboratory animals. In vitro techniques offer numerous advantages compared to in vivo methods. These include controlling experimental variables, obtaining relevant data more efficiently, and often having shorter testing periods. Although there is a challenge in extrapolating in vitro data to clinical applications of biomaterials, this can be addressed using suitable reference materials currently utilized in clinical settings. Overall, by establishing the non-toxicity of a material through in vitro cytotoxicity analysis, researchers can proceed with confidence in studying its overall biocompatibility, which will include other materials’ biological properties.
ISO 10993-5 also establishes methodologies and cell types for cytotoxicity analysis. In this review, we observed that most of the studies used different methods and cell types compared to the suggested by ISO. According to ISO 10993-5, if viability drops below 70%, the material is considered to have cytotoxic potential. ISO suggests the MTT test, which uses the reagent (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) to indicate cell metabolism. Many studies included in this review applied analyses based on this cell behavior, which differs from cell viability. Despite being widely applied, the MTT assay has some disadvantages compared to viability tests such as the sulforhodamine B (SRB) assay, which are also found in the studies along with this review. The SRB method calculates cell enumeration based on protein content, minimizing the interference of endogenous and exogenous substances, which strongly influence the MTT outcomes. For this reason, despite not being the suggested ISO method, the SRB test has presented superior predictive power than the MTT assay and is encouraged to be performed.
Microscopy analyses with Live/Dead kit were performed in some of the selected studies. This methodology is a valuable tool for assessing the cytotoxicity of materials due to its ability to visualize the cell damage directly. One major advantage of live/dead microscopy is its real-time assessment, enabling the observation of cellular responses immediately after exposure to a material. This provides valuable information regarding the speed and extent of cell death or damage. However, despite the name, live/dead kits do not necessarily stain live and dead cells.
In contrast, they stain cells with or without membrane damage. Another drawback is that live/dead microscopy can only depict the current status of cells and does not provide information about the underlying mechanisms or long-term effects of cellular damage. For these reasons, it is recommended that other complementary tests are performed to gain a more comprehensive understanding of cytotoxic effects.
The safety of 3D-printed acrylic resins highly depends on their degree of conversion. The incomplete polymerization may lead to a higher concentration of free monomers known to cause cytotoxicity []. Previous in vitro studies comparing heat-polymerized acrylic resins to auto-polymerized resins also found that the latter tend to exhibit higher cytotoxicity due to incomplete polymerization, leading to increased monomer release [,]. The duration of resin exposure to biological tissues also plays a critical role in cytotoxicity, with prolonged exposure correlating with heightened adverse effects on cell cultures []. To reduce the number of uncured monomers from 3D-printed devices, washing protocols are essential [,]. Usually, the rinsing liquid is a 90% or higher concentration of ethylic or isopropyl alcohol (IPA) [,], or tripropylene glycol monomethyl ether (TPM) [,]. There are no statistical differences in biocompatibility regarding the washing solutions []. The washing method was also investigated, and no differences in cytotoxicity were found between the rotatory wash and the ultrasonic bath []. The washing time presents conflicting data. While some studies do not present differences in washing time biocompatibility results, ranging from 5 min to 1 h [,], others demonstrate a significant improvement in cell viability related to an increase in washing time, reaching their best results at 20 min [,].
Surface treatment of resin-based 3D-printed dental devices is recommended to reduce surface roughness, which can minimize biofilm formation and enhance the longevity of prosthetic appliances. For a long time, it has been known that the surface roughness of materials plays a critical role in bacterial adhesion, and smoother surfaces exhibit a significantly lower risk of biofilm development []. A novel approach involves applying unpolymerized 3D printing resin as a coating agent to the device’s surface, which can be particularly advantageous when physical modifications, such as abrasive polishing, are not desired—for example, in the intaglio surface of dentures. This technique has been shown to improve surface smoothness and mechanical properties without altering the physical structure of the prosthetic. Additionally, in vitro studies using the L929 mouse fibroblast line have demonstrated that this method does not induce significant cytotoxic effects, as determined by LDL assay (ISO 10993-5 standards) [,].
Special attention must be paid to the post-curing treatment, which is essential to properly polymerize the coating resin []. On the other hand, when no coating is applied, mechanical polishing of 3D-printed devices with low-grit silicon carbide instruments is essential to reduce their cytotoxic effects. After a proper polishing procedure, 3D resin-based printed occlusal devices perform similarly regarding cytotoxic effects in human gingival fibroblast cell assays compared to milled and conventionally processed ones [].
Post-curing protocols vary in the literature. The time of post-curing is the most studied variable, ranging from 1 min to 120 min [,,,]. An increase in post-curing time enhanced cell viability and biocompatibility in human periodontal ligament fibroblasts [] and human gingival fibroblasts [,]. Data show that a post-curing time of around 20 to 30 min is necessary to reduce the cytotoxicity of 3D printable resins to clinically acceptable levels [,,,]. The temperature of the post-curing protocol ranges from 40 °C to 80 °C [].
Data from the literature explained that the brand of the post-curing oven is not the most important issue to consider regarding the cytotoxicity of 3D print resins []. One may achieve excellent biocompatibility outcomes by associating high temperatures, such as 80 °C, a long post-curing period, such as 30 min, and high UV-light intensity []. These combined factors must be present in the post-curing process to promote a higher degree of conversion of the 3D-printed device, which is associated with better outcomes of cytotoxicity [].
Different commercially available resins did not present statistical differences regarding cytotoxicity for human periodontal ligament fibroblasts when following the manufacturer’s instructions []. When compared to conventional materials, 3D-printed materials did not show significant differences to acrylic resins on human gingival fibroblast [,]. However, samples milled from resin blocks are less cytotoxic than printed ones [], while conventional light-cured resin-based composites presented similar cytotoxic behavior to 3D printable resins [,]. After printing, washing, and post-curing a resin-based material, one may increase its biocompatibility by immersing the printed resin in 100 °C water for 1 to 5 min without affecting its mechanical or optical properties [].
A limitation of this review is that it does not perform a statistical analysis, such as a systematic review with meta-analysis. Therefore, we cannot ensure what factors, overall, in the literature, significantly impact the outcomes. Future research could organize the extracted data to evaluate the statistical significance of different protocols on the cells’ viability.
5. Conclusions
This review highlights key practices for improving the biocompatibility of 3D-printed dental resins. Within the limitation of this review, it seems that washing devices for at least 20 min effectively reduces cytotoxicity by removing residual monomers and that the post-curing is crucial to ensure fewer possible cytotoxic effects. High-intensity UV-light post-curing for 20–30 min seems to significantly decrease cytotoxicity. Overall, these findings underline the importance of both post-curing and washing protocols in enhancing the biocompatibility of 3D-printed dental resins and emphasize the need for standardized post-processing of these materials.
Author Contributions
G.A.A., N.A.A., B.H., I.M.G., R.Z. and M.A.M. contributed significantly to produce this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, G.; Wu, L.; Hu, J.; Zhou, X.; He, F.; Wan, L.; Pan, S.-T. Main Applications and Recent Research Progresses of Additive Manufacturing in Dentistry. BioMed Res. Int. 2022, 2022, e5530188. [Google Scholar] [CrossRef]
- Rekow, E.D. Digital dentistry: The new state of the art—Is it disruptive or destructive? Dent. Mater. 2020, 36, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Conejo, J. The Current State of Chairside Digital Dentistry and Materials. Dent. Clin. N. Am. 2019, 63, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, L.; Güth, J.-F.; Schweiger, J.; Kollmuss, M.; Reichl, F.-X.; Edelhoff, D.; Högg, C. Elution behavior of a 3D-printed, milled and conventional resin-based occlusal splint material. Dent. Mater. 2021, 37, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.M.; Immich, F.; De Araujo, T.S.; Lund, R.G.; Da Silva, A.F.; Piva, E.; Da Rosa, W.L.D.O. Photosensitive resins used in additive manufacturing for oral application in dentistry: A scoping review from lab to clinic. J. Mech. Behav. Biomed. Mater. 2023, 141, 105732. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sayed, M.E.; Shetty, M.; Alqahtani, S.M.; Al Wadei, M.H.D.; Gupta, S.G.; Othman, A.A.A.; Alshehri, A.H.; Alqarni, H.; Mobarki, A.H.; et al. Physical and Mechanical Properties of 3D-Printed Provisional Crowns and Fixed Dental Prosthesis Resins Compared to CAD/CAM Milled and Conventional Provisional Resins: A Systematic Review and Meta-Analysis. Polymers 2022, 14, 2691. [Google Scholar] [CrossRef] [PubMed]
- Wuersching, S.N.; Hickel, R.; Edelhoff, D.; Kollmuss, M. Initial biocompatibility of novel resins for 3D printed fixed dental prostheses. Dent. Mater. 2022, 38, 1587–1597. [Google Scholar] [CrossRef]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Shrestha, B.; Patel, J.; Shih, B.; Shearston, K.; Ngo, H.; Fawzy, A. Effect of post-curing light exposure time on the physico–mechanical properties and cytotoxicity of 3D-printed denture base material. Dent. Mater. 2022, 38, 57–67. [Google Scholar] [CrossRef]
- Alamo, L.; Cassiano, F.B.; Bordini, E.A.F.; Stuani, V.T.; Pacheco, L.E.; Gallinari, M.D.O.; Souza Costa, C.A.; Mondelli, R.F.L.; Soares, D.G. An organotypic model of oral mucosa cells for the biological assessment of 3D-printed resins for interim restorations. J. Prosthet. Dent. 2022, 132, 251–259. [Google Scholar] [CrossRef]
- Atria, P.J.; Bordin, D.; Marti, F.; Nayak, V.V.; Conejo, J.; Benalcázar Jalkh, E.; Witek, L.; Sampaio, C.S. 3D-printed resins for provisional dental restorations: Comparison of mechanical and biological properties. J. Esthet. Restor. Dent. 2022, 34, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaikhan, E.; Gu, H.; Hwangbo, N.-K.; Lim, J.-H.; Shim, J.-S.; Lee, K.-W.; Kim, J.-E. Influence of different postcuring parameters on mechanical properties and biocompatibility of 3D printed crown and bridge resin for temporary restorations. J. Mech. Behav. Biomed. Mater. 2022, 128, 105127. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaikhan, E.; Lim, J.-H.; Shin, S.-H.; Park, K.-H.; Park, Y.-B.; Lee, J.-H.; Kim, J.-E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Bieger, V.; Thieringer, F.M.; Fischer, J.; Rohr, N. Fibroblast behavior on conventionally processed, milled, and printed occlusal device materials with different surface treatments. J. Prosthet. Dent. 2023, 129, 939–945. [Google Scholar] [CrossRef]
- Britto, V.T.; Cantelli, V.; Collares, F.M.; Bertol, C.D.; Della Bona, Á. Biomechanical properties of a 3D printing polymer for provisional restorations and artificial teeth. Dent. Mater. 2022, 38, 1956–1962. [Google Scholar] [CrossRef]
- Burgers, R.; Schubert, A.; Muller, J.; Krohn, S.; Rodiger, M.; Leha, A.; Wassmann, T. Cytotoxicity of 3D-printed, milled, and conventional oral splint resins to L929 cells and human gingival fibroblasts. Clin. Exp. Dent. Res. 2022, 8, 650–657. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, D.-H.; Huang, S.-C.; Lin, Y.-M. Comparison of flexural properties and cytotoxicity of interim materials printed from mono-LCD and DLP 3D printers. J. Prosthet. Dent. 2021, 126, 703–708. [Google Scholar] [CrossRef]
- Dai, J.; Luo, K.; Spintzyk, S.; Unkovskiy, A.; Li, P.; Xu, S.; Fernandez, P. Post-processing of DLP-printed denture base polymer: Impact of a protective coating on the surface characteristics, flexural properties, cytotoxicity, and microbial adhesion. Dent. Mater. 2022, 38, 2062–2072. Available online: https://www.sciencedirect.com/science/article/pii/S0109564122003128 (accessed on 19 September 2023). [CrossRef]
- Guerrero-Gironés, J.; López-García, S.; Pecci-Lloret, M.R.; Pecci-Lloret, M.P.; Rodríguez Lozano, F.J.; García-Bernal, D. In vitro biocompatibility testing of 3D printing and conventional resins for occlusal devices. J. Dent. 2022, 123, 104163. [Google Scholar] [CrossRef]
- Hwangbo, N.-K.; Nam, N.-E.; Choi, J.-H.; Kim, J.-E. Effects of the Washing Time and Washing Solution on the Biocompatibility and Mechanical Properties of 3D Printed Dental Resin Materials. Polymers 2021, 13, 4410. [Google Scholar] [CrossRef]
- Jin, G.; Gu, H.; Jang, M.; Bayarsaikhan, E.; Lim, J.-H.; Shim, J.-S.; Lee, K.-W.; Kim, J.-E. Influence of postwashing process on the elution of residual monomers, degree of conversion, and mechanical properties of a 3D printed crown and bridge materials. Dent. Mater. 2022, 38, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kwon, J.-S.; Park, J.-M.; Lo Russo, L.; Shim, J.-S. Effects of postpolymerization conditions on the physical properties, cytotoxicity, and dimensional accuracy of a 3D-printed dental restorative material. J. Prosthet. Dent. 2022, 132, 241–250. [Google Scholar] [CrossRef]
- Li, P.; Lambart, A.-L.; Stawarczyk, B.; Reymus, M.; Spintzyk, S. Postpolymerization of a 3D-printed denture base polymer: Impact of post-curing methods on surface characteristics, flexural strength, and cytotoxicity. J. Dent. 2021, 115, 103856. [Google Scholar] [CrossRef]
- Nam, N.-E.; Hwangbo, N.-K.; Jin, G.; Shim, J.-S.; Kim, J.-E. Effects of heat-treatment methods on cytocompatibility and mechanical properties of dental products 3D-printed using photopolymerized resin. J. Prosthodont. Res. 2023, 67, 121–131. [Google Scholar] [CrossRef]
- Oh, R.; Lim, J.-H.; Lee, C.-G.; Lee, K.-W.; Kim, S.-Y.; Kim, J.-E. Effects of washing solution temperature on the biocompatibility and mechanical properties of 3D-Printed dental resin material. J. Mech. Behav. Biomed. Mater. 2023, 143, 105906. [Google Scholar] [CrossRef]
- Srinivasan, M.; Kalberer, N.; Kamnoedboon, P.; Mekki, M.; Durual, S.; Özcan, M.; Müller, F. CAD-CAM complete denture resins: An evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 2021, 114, 103785. [Google Scholar] [CrossRef] [PubMed]
- Wulff, J.; Schweikl, H.; Rosentritt, M. Cytotoxicity of printed resin-based splint materials. J. Dent. 2022, 120, 104097. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xepapadeas, A.B.; Koos, B.; Geis-Gerstorfer, J.; Li, P.; Spintzyk, S. Effect of post-rinsing time on the mechanical strength and cytotoxicity of a 3D printed orthodontic splint material. Dent. Mater. 2021, 37, e314–e327. [Google Scholar] [CrossRef]
- Jorge, J.H.; Giampaolo, E.T.; Machado, A.L.; Vergani, C.E. Cytotoxicity of denture base acrylic resins: A literature review. J. Prosthet. Dent. 2003, 90, 190–193. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 9 September 2024).
- Bhargav, A.; Sanjairaj, V.; Rosa, V.; Feng, L.W.; Fuh YH, J. Applications of additive manufacturing in dentistry: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2058–2064. [Google Scholar] [CrossRef]
- Huang, F.M.; Tai, K.W.; Hu, C.C.; Chang, Y.C. Cytotoxic effects of denture base materials on a permanent human oral epithelial cell line and on primary human oral fibroblasts in vitro. Int. J. Prosthodont. 2001, 14, 439–443. [Google Scholar] [PubMed]
- Raszewski, Z. Influence of polymerization method on the cytotoxicity of three different denture base acrylic resins polymerized in different methods. Saudi J. Biol. Sci. 2020, 27, 2612–2616. [Google Scholar] [CrossRef] [PubMed]
- Maktabi, H.; Ibrahim, M.S.; Balhaddad, A.A.; Alkhubaizi, Q.; Garcia, I.M.; Collares, F.M.; Strassler, H.; Fugolin, A.P.P.; Pfeifer, C.S.; Melo, M.A.S. Improper Light Curing of Bulkfill Composite Drives Surface Changes and Increases S. mutans Biofilm Growth as a Pathway for Higher Risk of Recurrent Caries around Restorations. Dent. J. 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).