Multi-Graph Assessment of Temporal and Extratemporal Lobe Epilepsy in Resting-State fMRI
Abstract
1. Introduction
- Explore the brain reorganization characteristics and their differences between epileptic patients and healthy controls.
- Reveal the hub regions, utilizing different brain connectivity methods and assessing the centrality measures to quantify the importance of a node.
- Uncover the regional alterations in hub probability, identifying the ROIs that play a critical role in both healthy individuals and TLE and/or ETLE patients.
2. Materials and Methods
2.1. Subjects
2.2. Data Acquisition
2.3. Preprocessing
2.4. Segmentation and Time Series Extraction
2.5. Brain Connectivity Estimation
2.6. Global Network Analysis
2.7. Hub-Related Analysis
2.7.1. Probability Distribution in Regions of Interest
2.7.2. Probability Distribution in Brain Sectors
3. Results
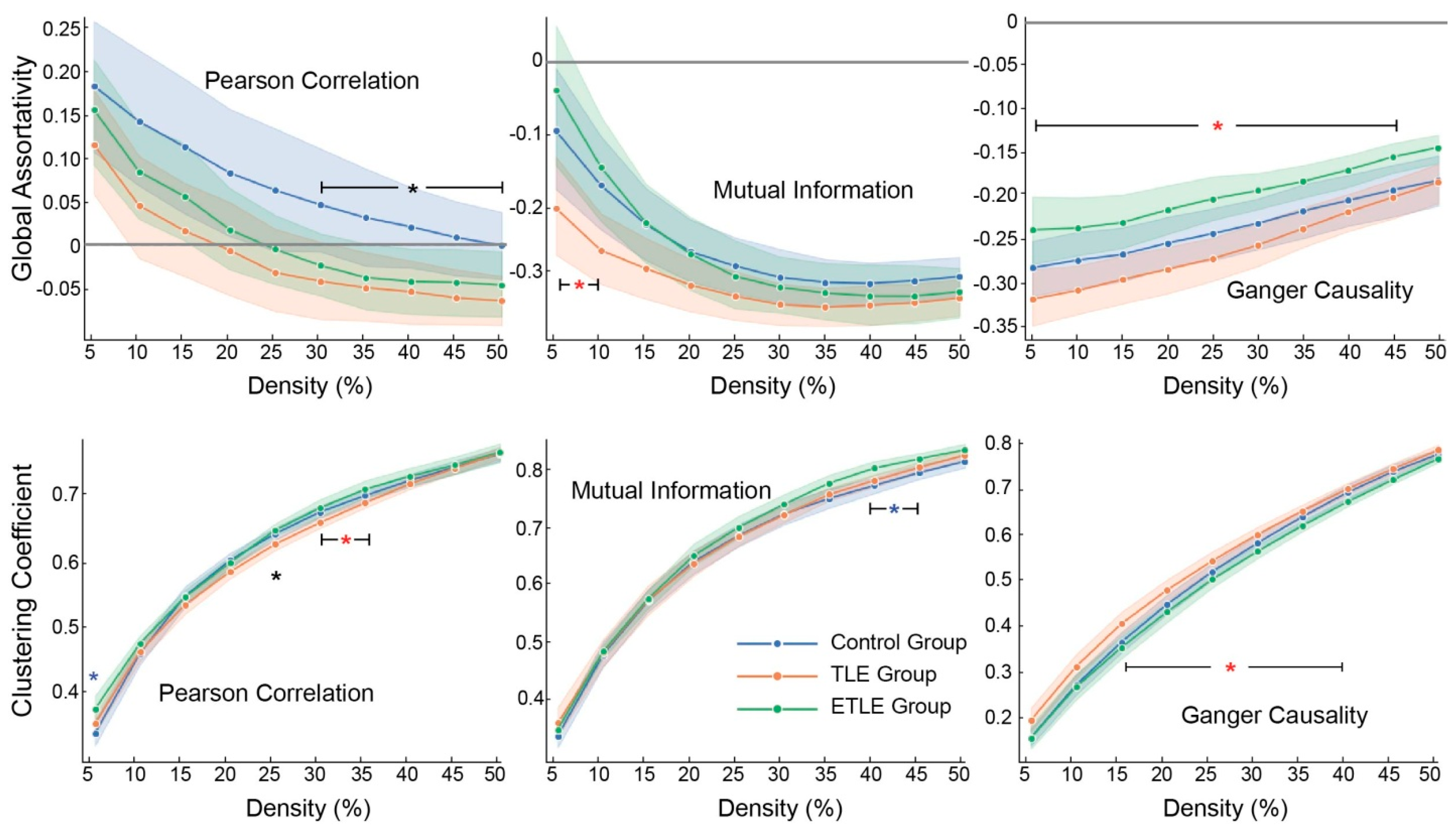
3.1. Topological Characteristics
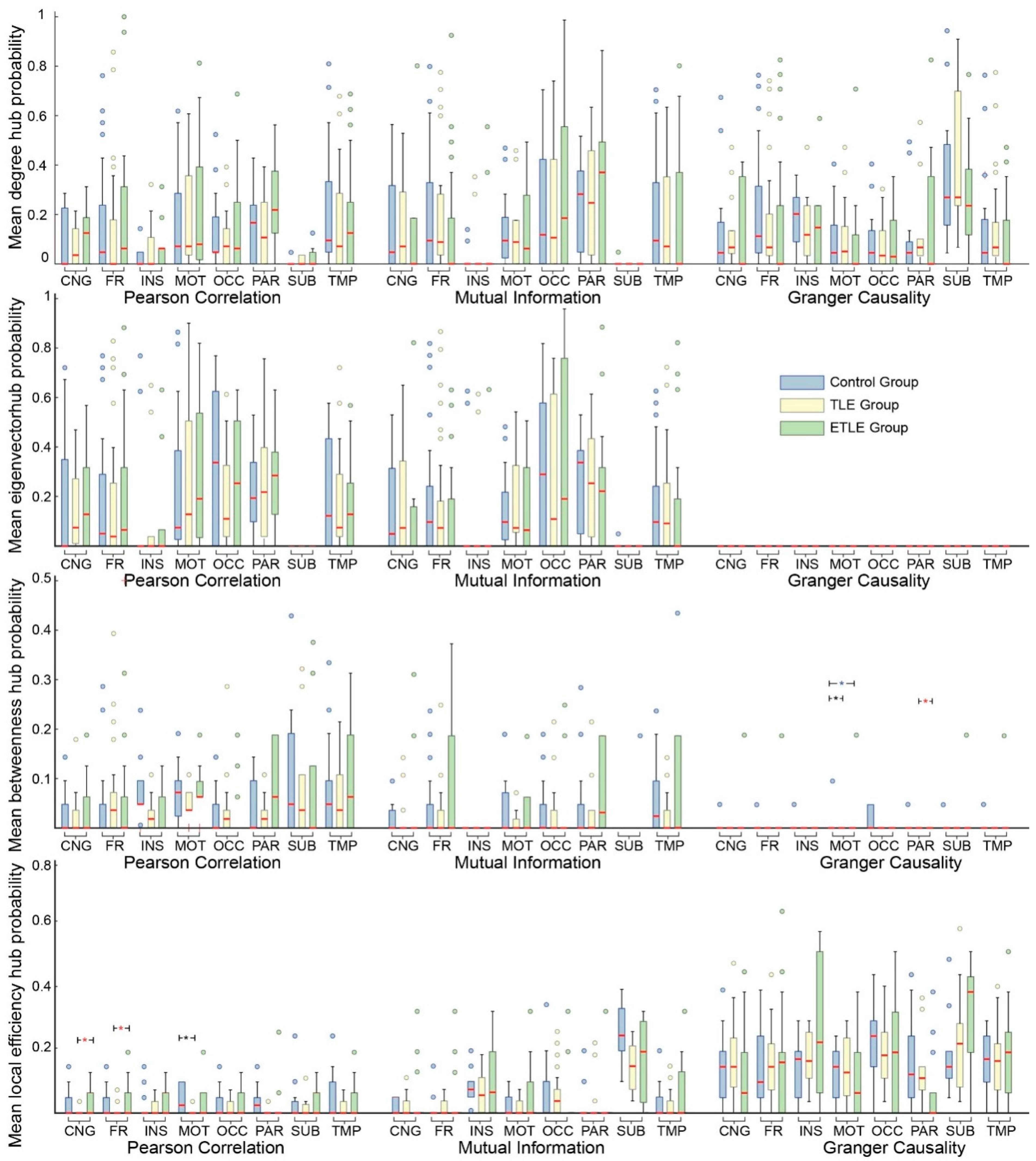
3.2. Hub Probabilities in Regions of Interest
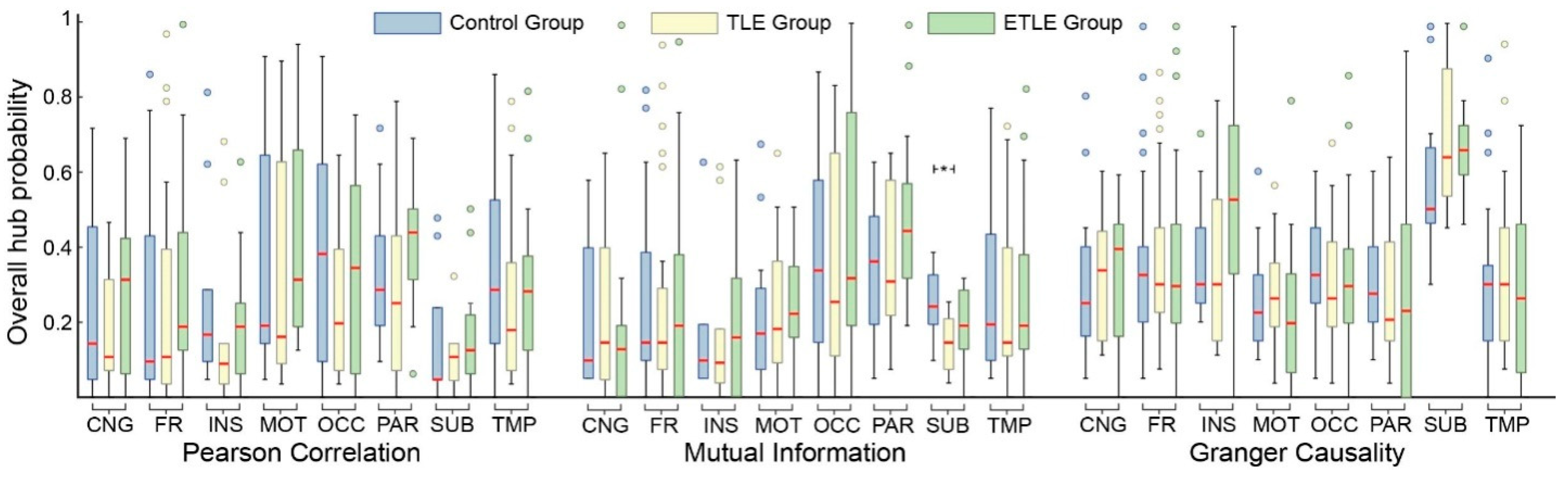
3.3. Hub Probabilities in Brain Sectors
4. Discussion
4.1. Brain Reorganization
4.2. Key Region Mapping
4.3. Network Analysis Comparisons
4.4. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Index | Brainsector | ROI | Abbreviation |
|---|---|---|---|
| 1 | CNG | Anterior part of the cingulate gyrus and sulcus | ACC |
| 2 | CNG | Middle anterior part of the cingulate gyrus and sulcus | aMCC |
| 3 | CNG | Middle posterior part of the cingulate gyrus and sulcus | pMCC |
| 4 | FR | Fronto-marginal gyrus of Wernicke and sulcus | frontomarg GS |
| 5 | OCC | Inferior occipital gyrus and sulcus | IOG |
| 6 | MOT | Paracentral lobule and sulcus | ParaCG |
| 7 | MOT | Subcentral gyrus (central operculum) and sulci | SubCG |
| 8 | FR | Transverse frontopolar gyri and sulci | frontopoltransv GS |
| 9 | CNG | Posterior-dorsal part of the cingulate gyrus | dPCC |
| 10 | CNG | Posterior-ventral part of the cingulate gyrus (isthmus of the cingulate gyrus) | vPCC |
| 11 | OCC | Cuneus | CUN |
| 12 | FR | Opercular part of the inferior frontal gyrus | IFG Operc |
| 13 | FR | Orbital part of the inferior frontal gyrus | IFG orb |
| 14 | FR | Triangular part of the inferior frontal gyrus | IFG Triang |
| 15 | FR | Middle frontal gyrus | MFG |
| 16 | FR | Superior frontal gyrus | SFG |
| 17 | INS | Long insular gyrus and central sulcus of the insula | long insul G |
| 18 | INS | Short insular gyri | insular short |
| 19 | OCC | Middle occipital gyrus (lateral occipital gyrus) | MOG |
| 20 | OCC | Superior occipital gyrus | SOG |
| 21 | OCC | Lateral occipito-temporal gyrus (fusiform gyrus) | Fusiform G |
| 22 | OCC | Lingual gyrus, lingual part of the medial occipito-temporal gyrus | LING |
| 23 | TMP | Parahippocampal gyrus, parahippocampal part of the medial occipito-temporal gyrus | Parahip G |
| 24 | FR | Orbital gyri | orb G |
| 25 | PAR | Superior parietal lobule | SPG |
| 26 | PAR | Angular gyrus | ANG |
| 27 | PAR | Supramarginal gyrus | IPG supramar |
| 28 | MOT | Postcentral gyrus | PostCG |
| 29 | MOT | Precentral gyrus | PreCG |
| 30 | PAR | Precuneus | PCUN |
| 31 | FR | Straight gyrus, Gyrus rectus | Rectus |
| 32 | FR | Subcallosal area, subcallosal gyrus | subcallosal |
| 33 | TMP | Inferior temporal gyrus | ITG |
| 34 | TMP | Middle temporal gyrus | MTG |
| 35 | TMP | Anterior transverse temporal gyrus of Heschl | STG ant transv |
| 36 | TMP | Lateral aspect of the superior temporal gyrus | STG lat |
| 37 | TMP | Planum polare of the superior temporal gyrus | STG plan polar |
| 38 | TMP | Temporal plane of the superior temporal gyrus | STG plan tempo |
| 39 | FR | Horizontal ramus of the anterior segment of the lateral sulcus | latFis ant Hor |
| 40 | FR | Vertical ramus of the anterior segment of the lateral sulcus | latFis ant Vert |
| 41 | TMP | Posterior ramus of the lateral sulcus | latFis post |
| 42 | OCC | Occipital pole | occ Pole |
| 43 | TMP | Temporal pole | temp Pole |
| 44 | OCC | Calcarine sulcus | Calcarine |
| 45 | MOT | Central sulcus | CS |
| 46 | CNG | Marginal branch of the cingulate sulcus | cingulmarginalis |
| 47 | INS | Anterior segment of the circular sulcus of the insula | circ ins ant |
| 48 | INS | Inferior segment of the circular sulcus of the insula | circ ins inf |
| 49 | INS | Superior segment of the circular sulcus of the insula | circ ins sup |
| 50 | TMP | Anterior transverse collateral sulcus | collattransv ant |
| 51 | TMP | Posterior transverse collateral sulcus | collattransv post |
| 52 | FR | Inferior frontal sulcus | IFS |
| 53 | FR | Middle frontal sulcus | MFS |
| 54 | FR | Superior frontal sulcus | SFS |
| 55 | PAR | Sulcus intermedius primus of Jensen | inter prim Jensen |
| 56 | PAR | Intraparietal sulcus and transverse parietal sulci | |
| 57 | OCC | Anterior occipital sulcus and preoccipital notch | occ ant S |
| 58 | OCC | Middle occipital sulcus and lunatus sulcus | occ midLunatus |
| 59 | OCC | Superior occipital sulcus and transverse occipital sulcus | occ suptransv |
| 60 | TMP | Lateral occipito-temporal sulcus | oct lat S |
| 61 | OCC | Medial occipito-temporal sulcus (collateral sulcus) and lingual sulcus | oct medLING |
| 62 | FR | Orbital sulci (H-shaped sulci) | orb H shaped |
| 63 | FR | Lateral orbital sulcus | orb lat |
| 64 | FR | Medial orbital sulcus (olfactory sulcus) | orb med olfact |
| 65 | OCC | Parieto-occipital sulcus | par occ S |
| 66 | CNG | Pericallosal sulcus | Pericallosal |
| 67 | MOT | Postcentral sulcus | PostCS |
| 68 | MOT | Inferior part of the precentral sulcus | PreCS inf part |
| 69 | MOT | Superior part of the precentral sulcus | PreCS sup part |
| 70 | FR | Suborbital sulcus | Suborb |
| 71 | PAR | Subparietal sulcus | Subpar |
| 72 | TMP | Inferior temporal sulcus | ITS |
| 73 | TMP | Superior temporal sulcus (parallel sulcus) | STS |
| 74 | TMP | Transverse temporal sulcus | temp trasnv |
| 75 | SUB | Amygdala | Amygdala |
| 76 | SUB | Caudate | Caudate |
| 77 | SUB | Hippocampus | Hippocampus |
| 78 | SUB | Pallidum | Pallidum |
| 79 | SUB | Putamen | Putamen |
| 80 | SUB | Thalamus | Thalamus |
Appendix B
Appendix C
| ROI | pDR,G | pBR,G | pEFR,G | pEVR,G | pR,G | Brain Sector |
|---|---|---|---|---|---|---|
| Healthy Controls | ||||||
| R SFG | 76.2% | 23.8% | 0 | 66.7% | 85.7% | FR |
| R LING | 52.4% | 14.3% | 0 | 76.2% | 90.5% | OCT |
| R PreCG | 61.9% | 4.8% | 0 | 85.7% | 90.5% | S/M |
| R MTG | 81% | 4.8% | 0 | 52.4% | 85.7% | TEMP |
| TLE group | ||||||
| L SFG | 78.6% | 21.4% | 0 | 57.1% | 82.1% | FR |
| R SFG | 85.7% | 39.3% | 0 | 71.4% | 96.4% | FR |
| L SPG | 35.7% | 0 | 0 | 82.1% | 82.1% | PAR |
| R SPG | 25% | 4.6% | 0 | 75% | 78.6% | PAR |
| L PreCG | 60.7% | 3.6% | 0 | 89.3% | 89.3% | S/M |
| R PreCG | 46.4% | 0 | 0 | 82.1% | 85.7% | S/M |
| L IPG—Supramar | 39.3% | 10.7% | 0 | 60.7% | 75% | PAR |
| R PostCG | 25% | 0 | 3.6% | 75% | 78.6% | S/M |
| R PCUN | 46.4% | 7.1% | 0 | 64.3% | 75% | PAR |
| R MTG | 67.9% | 10.7% | 0 | 42.9% | 71.4% | TEMP |
| R STS | 60.7% | 14.3% | 0 | 71.4% | 78.6% | TEMP |
| ETLE group | ||||||
| L SFG | 93.75% | 50% | 0 | 62.5% | 100% | FR |
| R SFG | 100% | 31.25% | 0 | 87.5% | 100% | FR |
| L PreCG | 81.25% | 6.25% | 0 | 75% | 93.75% | S/M |
| R PreCG | 56.25% | 6.25% | 0 | 81.25% | 81.25% | S/M |
| R PCUN | 81.25% | 6.25% | 0 | 75% | 87.5% | PAR |
| R STG-lateral | 62.5% | 25% | 0 | 31.25% | 81.25% | TEMP |
| R STS | 68.75% | 18.75% | 0 | 56.25% | 81.25% | TEMP |
| ROI | pDR,G | pBR,G | pEFR,G | pEVR,G | pR,G | Brain Sector |
|---|---|---|---|---|---|---|
| Healthy Controls | ||||||
| L SOG | 61.9% | 14.3% | 4.8% | 66.7% | 71.4% | OCC |
| R SOG | 71.4% | 9.5% | 0 | 81% | 85.7% | OCC |
| L SPG | 57.1% | 9.5% | 0 | 76.2% | 76.2% | PAR |
| R SPG | 66.7% | 9.5% | 0 | 81% | 81% | PAR |
| L IOG | 66.7% | 19% | 0 | 71.4% | 76.2% | OCC |
| L Jensen | 81% | 19% | 0 | 38.1% | 81% | PAR |
| L occ suptransv | 57.1% | 4.8% | 0 | 71.4% | 71.4% | OCC |
| L temp transverse | 71.4% | 23.8% | 0 | 28.6% | 76.2% | TEMP |
| R col transv post | 66.7% | 0 | 4.8% | 61.9% | 71.4% | TEMP |
| R occ midLunatus | 61.9% | 19% | 0 | 66.7% | 71.4% | OCC |
| TLE group | ||||||
| L SOG | 71.4% | 7.1% | 3.6% | 75% | 82.1% | OCC |
| R SOG | 75% | 21.4% | 3.6% | 75% | 78.6% | OCC |
| L SPG | 71.4% | 3.6% | 0 | 85.7% | 92.9% | PAR |
| R SPG | 78.6% | 7.1% | 0 | 78.6% | 82.1% | PAR |
| L LING | 50% | 3.6% | 0 | 71.4% | 71.4% | OCT |
| L Jensen | 71.4% | 25% | 0 | 46.4% | 71.4% | PAR |
| R IOG | 60.7% | 10.7% | 0 | 64.3% | 71.4% | OCC |
| R occ Pole | 64.3% | 14.3% | 0 | 71.4% | 71.4% | OCC |
| ETLE group | ||||||
| L SOG | 56.25% | 0 | 0 | 93.75% | 93.75% | OCC |
| R SOG | 100% | 25% | 0 | 100% | 100% | OCC |
| L CUN | 81.25% | 18.75% | 0 | 100% | 100% | OCC |
| L LING | 81.25% | 0 | 0 | 100% | 100% | OCT |
| L Jensen | 93.75% | 56.25% | 0 | 25% | 93.75% | PAR |
| R IPG—Ang | 62.5% | 56.25% | 0 | 6.25% | 100% | PAR |
| R PostCG | 87.5% | 6.25% | 0 | 87.5% | 87.5% | S/M |
| ROI | pDR,G | pBR,G | pEVR,G | pR,G | Brain Sector |
| Healthy Controls | |||||
| L Orb med Olf | 81% | 0 | 9.5% | 95.2% | ORB |
| R Orb med Olf | 76.2% | 0 | 4.8% | 81% | ORB |
| L Pallidum | 100% | 0 | 0 | 100% | SUB |
| R Pallidum | 85.7% | 4.8% | 4.8% | 90.5% | SUB |
| L subcallosal | 76.2% | 0 | 4.8% | 81% | FR |
| L circ insula-ant | 71.4% | 0 | 4.8% | 76.2% | INS |
| L Amygdala | 81% | 0 | 4.8% | 85.7% | FR |
| TLE group | |||||
| L Amygdala | 82.1% | 0 | 7.1% | 89.3% | SUB |
| R Amygdala | 82.1% | 0 | 7.1% | 89.3% | SUB |
| L Pallidum | 96.4% | 0 | 3.6% | 100% | SUB |
| R Pallidum | 96.4% | 0 | 3.6% | 100% | SUB |
| L col transv-ant | 50% | 0 | 25% | 75% | TEMP |
| R subcallosal | 78.6% | 0 | 3.6% | 82.1% | FR |
| R Lat-FiSant-Hor | 67.9% | 0 | 7.1% | 75% | FR |
| R Lat-FiSant-Vert | 75% | 0 | 0 | 75% | PAR |
| R Orb med Olf | 64.3% | 0 | 10.7% | 75% | ORB |
| ETLE group | |||||
| L subcallosal | 62.5% | 0 | 18.75% | 81.25% | FR |
| R subcallosal | 75% | 0 | 18.75% | 93.75% | FR |
| L Orb med Olf | 81.25% | 18.75% | 0 | 81.25% | ORB |
| R Orb med Olf | 87.5% | 0 | 0 | 87.5% | ORB |
| R rectus | 87.5% | 0 | 0 | 87.5% | FR |
| R col transv-ant | 62.5% | 0 | 31.25% | 93.75% | TEMP |
| R pericallosal | 0 | 0 | 81.25% | 81.25% | CING |
| R Pallidum | 81.25% | 0 | 18.75% | 100% | SUB |
References
- Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 26 July 2024).
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel Jr, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Barry, S.J.E.; Bamagous, G.A.; Norrie, J.D.; Kwan, P. Patterns of Treatment Response in Newly Diagnosed Epilepsy. Neurology 2012, 78, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Strzelczyk, A.; Aledo-Serrano, A.; Coppola, A.; Didelot, A.; Bates, E.; Sainz-Fuertes, R.; Lawthom, C. The Impact of Epilepsy on Quality of Life: Findings from a European Survey. Epilepsy Behav. 2023, 142, 109179. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Adali, N. Cognitive Disorders, Depression and Anxiety in Temporal Lobe Epilepsy: An Overview. J. Biosci. Med. 2024, 12, 77–93. [Google Scholar] [CrossRef]
- Jain, P.; Smith, M.L.; Speechley, K.; Ferro, M.; Connolly, M.; Ramachandrannair, R.; Almubarak, S.; Andrade, A.; Widjaja, E.; Team, P.S. Seizure Freedom Improves Health-Related Quality of Life after Epilepsy Surgery in Children. Dev. Med. Child Neurol. 2020, 62, 600–608. [Google Scholar] [CrossRef]
- Swarup, O.; Waxmann, A.; Chu, J.; Vogrin, S.; Lai, A.; Laing, J.; Barker, J.; Seiderer, L.; Ignatiadis, S.; Plummer, C.; et al. Long-Term Mood, Quality of Life, and Seizure Freedom in Intracranial EEG Epilepsy Surgery. Epilepsy Behav. 2021, 123, 108241. [Google Scholar] [CrossRef]
- Lehnertz, K.; Bröhl, T.; Wrede, R. von Epileptic-Network-Based Prediction and Control of Seizures in Humans. Neurobiol. Dis. 2023, 181, 106098. [Google Scholar] [CrossRef]
- Gleichgerrcht, E.; Keller, S.S.; Drane, D.L.; Munsell, B.C.; Davis, K.A.; Kaestner, E.; Weber, B.; Krantz, S.; Vandergrift, W.A.; Edwards, J.C.; et al. Temporal Lobe Epilepsy Surgical Outcomes Can Be Inferred Based on Structural Connectome Hubs: A Machine Learning Study. Ann. Neurol. 2020, 88, 970–983. [Google Scholar] [CrossRef]
- De Palma, L.; Benedictis, A.D.; Specchio, N.; Marras, C.E. Epileptogenic Network Formation. Neurosurg. Clin. 2020, 31, 335–344. [Google Scholar] [CrossRef]
- Cohen, M.S.; Bookheimer, S.Y. Localization of Brain Function Using Magnetic Resonance Imaging. Trends Neurosci. 1994, 17, 268–277. [Google Scholar] [CrossRef]
- Feng, X.; Piper, R.J.; Prentice, F.; Clayden, J.D.; Baldeweg, T. Functional Brain Connectivity in Children with Focal Epilepsy: A Systematic Review of Functional MRI Studies. Seizure Eur. J. Epilepsy 2024, 117, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Friston, K. Structural and Functional Brain Networks: From Connections to Cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S.J. Functional Connectivity: The Principal-Component Analysis of Large (PET) Data Sets. J. Cereb. Blood Flow Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the Brain Network: A Review on Resting-State fMRI Functional Connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.S. Neural Networks in Human Epilepsy: Evidence of and Implications for Treatment. Epilepsia 2002, 43, 219–227. [Google Scholar] [CrossRef]
- Boerwinkle, V.L.; Mirea, L.; Gaillard, W.D.; Sussman, B.L.; Larocque, D.; Bonnell, A.; Ronecker, J.S.; Troester, M.M.; Kerrigan, J.F.; Foldes, S.T.; et al. Resting-State Functional MRI Connectivity Impact on Epilepsy Surgery Plan and Surgical Candidacy: Prospective Clinical Work. J. Neurosurg. Pediatr. 2020, 25, 574–581. [Google Scholar] [CrossRef]
- Foit, N.A.; Bernasconi, A.; Bernasconi, N. Functional Networks in Epilepsy Presurgical Evaluation. Neurosurg. Clin. 2020, 31, 395–405. [Google Scholar] [CrossRef]
- Bernhardt, B.C.; Bonilha, L.; Gross, D.W. Network Analysis for a Network Disorder: The Emerging Role of Graph Theory in the Study of Epilepsy. Epilepsy Behav. 2015, 50, 162–170. [Google Scholar] [CrossRef]
- Farahani, F.V.; Karwowski, W.; Lighthall, N.R. Application of Graph Theory for Identifying Connectivity Patterns in Human Brain Networks: A Systematic Review. Front. Neurosci. 2019, 13, 585. [Google Scholar] [CrossRef]
- Larivière, S.; Bernasconi, A.; Bernasconi, N.; Bernhardt, B.C. Connectome Biomarkers of Drug-Resistant Epilepsy. Epilepsia 2021, 62, 6–24. [Google Scholar] [CrossRef]
- Doucet, G.E.; Rider, R.; Taylor, N.; Skidmore, C.; Sharan, A.; Sperling, M.; Tracy, J.I. Presurgery Resting-State Local Graph-Theory Measures Predict Neurocognitive Outcomes after Brain Surgery in Temporal Lobe Epilepsy. Epilepsia 2015, 56, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, B.C.; Chen, Z.; He, Y.; Evans, A.C.; Bernasconi, N. Graph-Theoretical Analysis Reveals Disrupted Small-World Organization of Cortical Thickness Correlation Networks in Temporal Lobe Epilepsy. Cereb. Cortex 2011, 21, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Yang, C.; Lu, M.; Ren, J.; Li, Z.; Zhang, Y. Abnormal White Matter Structural Network Topological Property in Patients with Temporal Lobe Epilepsy. CNS Neurosci. Ther. 2024, 30, e14414. [Google Scholar] [CrossRef] [PubMed]
- Van Diessen, E.; Zweiphenning, W.J.E.M.; Jansen, F.E.; Stam, C.J.; Braun, K.P.J.; Otte, W.M. Brain Network Organization in Focal Epilepsy: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e114606. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, X.; Song, C.; Han, S.; Li, W.; Wang, K.; Mao, X.; Zhang, Y.; Cheng, J. Altered Topological Properties and Their Relationship to Cognitive Functions in Unilateral Temporal Lobe Epilepsy. Epilepsy Behav. 2023, 144, 109247. [Google Scholar] [CrossRef]
- Haneef, Z.; Chiang, S. Clinical Correlates of Graph Theory Findings in Temporal Lobe Epilepsy. Seizure 2014, 23, 809–818. [Google Scholar] [CrossRef]
- Vlooswijk, M.C.G.; Vaessen, M.J.; Jansen, J.F.A.; de Krom, M.C.F.T.M.; Majoie, H.J.M.; Hofman, P.A.M.; Aldenkamp, A.P.; Backes, W.H. Loss of Network Efficiency Associated with Cognitive Decline in Chronic Epilepsy. Neurology 2011, 77, 938–944. [Google Scholar] [CrossRef]
- Stam, C.J. Hub Overload and Failure as a Final Common Pathway in Neurological Brain Network Disorders. Netw. Neurosci. 2024, 8, 1–23. [Google Scholar] [CrossRef]
- Crossley, N.A.; Mechelli, A.; Scott, J.; Carletti, F.; Fox, P.T.; McGuire, P.; Bullmore, E.T. The Hubs of the Human Connectome Are Generally Implicated in the Anatomy of Brain Disorders. Brain 2014, 137, 2382–2395. [Google Scholar] [CrossRef]
- Mazrooyisebdani, M.; Nair, V.A.; Garcia-Ramos, C.; Mohanty, R.; Meyerand, E.; Hermann, B.; Prabhakaran, V.; Ahmed, R. Graph Theory Analysis of Functional Connectivity Combined with Machine Learning Approaches Demonstrates Widespread Network Differences and Predicts Clinical Variables in Temporal Lobe Epilepsy. Brain Connect. 2020, 10, 39–50. [Google Scholar] [CrossRef]
- Royer, J.; Bernhardt, B.C.; Larivière, S.; Gleichgerrcht, E.; Vorderwülbecke, B.J.; Vulliémoz, S.; Bonilha, L. Epilepsy and Brain Network Hubs. Epilepsia 2022, 63, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Gkiatis, K.; Garganis, K.; Benjamin, C.F.; Karanasiou, I.; Kondylidis, N.; Harushukuri, J.; Matsopoulos, G.K. Standardization of Presurgical Language fMRI in Greek Population: Mapping of Six Critical Regions. Brain Behav. 2022, 12, e2609. [Google Scholar] [CrossRef] [PubMed]
- Dachena, C.; Casu, S.; Fanti, A.; Lodi, M.B.; Mazzarella, G. Combined Use of MRI, fMRIand Cognitive Data for Alzheimer’s Disease: Preliminary Results. Appl. Sci. 2019, 9, 3156. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Greve, D.N.; Fischl, B. Accurate and Robust Brain Image Alignment Using Boundary-Based Registration. NeuroImage 2009, 48, 63–72. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Beckmann, C.F.; Smith, S.M. Probabilistic Independent Component Analysis for Functional Magnetic Resonance Imaging. IEEE Trans. Med. Imaging 2004, 23, 137–152. [Google Scholar] [CrossRef]
- Gkiatis, K.; Garganis, K.; Karanasiou, I.; Chatzisotiriou, A.; Zountsas, B.; Kondylidis, N.; Matsopoulos, G.K. Independent Component Analysis: A Reliable Alternative to General Linear Model for Task-Based fMRI. Front. Psychiatry 2023, 14, 1214067. [Google Scholar] [CrossRef]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic Parcellation of Human Cortical Gyri and Sulci Using Standard Anatomical Nomenclature. Neuroimage 2010, 53, 1–15. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson Correlation Coefficient. In Noise Reduction in Speech Processing; Cohen, I., Huang, Y., Chen, J., Benesty, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. ISBN 978-3-642-00296-0. [Google Scholar]
- Kraskov, A.; Stögbauer, H.; Grassberger, P. Estimating Mutual Information. Phys. Rev. E 2004, 69, 066138. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.W.J. Investigating Causal Relations by Econometric Models and Cross-Spectral Methods. Econometrica 1969, 37, 424–438. [Google Scholar] [CrossRef]
- Garrison, K.A.; Scheinost, D.; Finn, E.S.; Shen, X.; Constable, R.T. The (in)Stability of Functional Brain Network Measures across Thresholds. NeuroImage 2015, 118, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E.J. Assortative Mixing in Networks. Phys. Rev. Lett. 2002, 89, 208701. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective Dynamics of ‘Small-World’ Networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Nesterov, A.I. On Clustering Coefficients in Complex Networks 2024. arXiv 2024, arXiv:2401.02999. [Google Scholar]
- Freeman, L.C. A Set of Measures of Centrality Based on Betweenness. Sociometry 1977, 40, 35–41. [Google Scholar] [CrossRef]
- Brandes, U. A Faster Algorithm for Betweenness Centrality. J. Math. Sociol. 2001, 25, 163–177. [Google Scholar] [CrossRef]
- Newman, M.E.J. Finding Community Structure in Networks Using the Eigenvectors of Matrices. Phys. Rev. E 2006, 74, 036104. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, Z.; Pan, Z.; Mantini, D.; Ding, J.; Duan, X.; Luo, C.; Lu, G.; Chen, H. Altered Functional Connectivity and Small-World in Mesial Temporal Lobe Epilepsy. PLoS ONE 2010, 5, e8525. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.L.; Moussa, M.N.; Paolini, B.; Lyday, R.G.; Burdette, J.H.; Laurienti, P.J. Defining Nodes in Complex Brain Networks. Front. Comput. Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Vaessen, M.J.; Braakman, H.M.H.; Heerink, J.S.; Jansen, J.F.A.; Debeij-van Hall, M.H.J.A.; Hofman, P.A.M.; Aldenkamp, A.P.; Backes, W.H. Abnormal Modular Organization of Functional Networks in Cognitively Impaired Children with Frontal Lobe Epilepsy. Cereb. Cortex 2013, 23, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Omidvarnia, A.H.; Walz, J.M.; Jackson, G.D. Increased Segregation of Brain Networks in Focal Epilepsy: An fMRI Graph Theory Finding. NeuroImage Clin. 2015, 8, 536–542. [Google Scholar] [CrossRef]
- Larivière, S.; Weng, Y.; Vos De Wael, R.; Royer, J.; Frauscher, B.; Wang, Z.; Bernasconi, A.; Bernasconi, N.; Schrader, D.V.; Zhang, Z.; et al. Functional Connectome Contractions in Temporal Lobe Epilepsy: Microstructural Underpinnings and Predictors of Surgical Outcome. Epilepsia 2020, 61, 1221–1233. [Google Scholar] [CrossRef]
- Prando, G.; Zorzi, M.; Bertoldo, A.; Corbetta, M.; Zorzi, M.; Chiuso, A. Sparse DCM for Whole-Brain Effective Connectivity from Resting-State fMRI Data. NeuroImage 2020, 208, 116367. [Google Scholar] [CrossRef]
- Al Musawi, A.F.; Roy, S.; Ghosh, P. Examining Indicators of Complex Network Vulnerability across Diverse Attack Scenarios. Sci. Rep. 2023, 13, 18208. [Google Scholar] [CrossRef]
- Englot, D.J.; Konrad, P.E.; Morgan, V.L. Regional and Global Connectivity Disturbances in Focal Epilepsy, Related Neurocognitive Sequelae, and Potential Mechanistic Underpinnings. Epilepsia 2016, 57, 1546–1557. [Google Scholar] [CrossRef]
- Park, K.M.; Cho, K.H.; Lee, H.-J.; Heo, K.; Lee, B.I.; Kim, S.E. Predicting the Antiepileptic Drug Response by Brain Connectivity in Newly Diagnosed Focal Epilepsy. J. Neurol. 2020, 267, 1179–1187. [Google Scholar] [CrossRef]
- Yu, Y.; Qiu, M.; Zou, W.; Zhao, Y.; Tang, Y.; Tian, J.; Chen, X.; Qiu, W. Impaired Rich-Club Connectivity in Childhood Absence Epilepsy. Front. Neurol. 2023, 14, 1135305. [Google Scholar] [CrossRef]
- Oldham, S.; Fornito, A. The Development of Brain Network Hubs. Dev. Cogn. Neurosci. 2019, 36, 100607. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.P.; Sporns, O. Network Hubs in the Human Brain. Trends Cogn. Sci. 2013, 17, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, W.; Li, Y.; Liu, P.; Zhang, Y.; Gong, Q.; Zhou, D.; An, D. Aberrant Structural Rich Club Organization in Temporal Lobe Epilepsy with Focal to Bilateral Tonic–Clonic Seizures. NeuroImage Clin. 2023, 40, 103536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhuang, B.; Wang, M.; Zhu, J.; Chen, T.; Yang, Y.; Shi, H.; Zhu, X.; Ma, L. Delineating Abnormal Individual Structural Covariance Brain Network Organization in Pediatric Epilepsy with Unilateral Resection of Visual Cortex. Epilepsy Behav. Rep. 2024, 27, 100676. [Google Scholar] [CrossRef] [PubMed]
- Galovic, M.; van Dooren, V.Q.H.; Postma, T.S.; Vos, S.B.; Caciagli, L.; Borzì, G.; Cueva Rosillo, J.; Vuong, K.A.; de Tisi, J.; Nachev, P.; et al. Progressive Cortical Thinning in Patients With Focal Epilepsy. JAMA Neurol. 2019, 76, 1230–1239. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, B.; Tan, Z.; Hu, W.; Sang, L.; Zhang, C.; Wang, X.; Wang, Y.; Liu, C.; Mo, J.; et al. Intrinsic Brain Activity Changes in Temporal Lobe Epilepsy Patients Revealed by Regional Homogeneity Analysis. Seizure 2020, 81, 117–122. [Google Scholar] [CrossRef]
- Ke, M.; Hou, Y.; Zhang, L.; Liu, G. Brain Functional Network Changes in Patients with Juvenile Myoclonic Epilepsy: A Study Based on Graph Theory and Granger Causality Analysis. Front. Neurosci. 2024, 18, 1363255. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, S.; Xu, Y.; Liu, Z.; Wen, X.; Hu, X.; Zhang, R.; Li, M.; Wang, W.; Huang, R. Graph Theoretical Analysis Reveals Disrupted Topological Properties of Whole Brain Functional Networks in Temporal Lobe Epilepsy. Clin. Neurophysiol. 2014, 125, 1744–1756. [Google Scholar] [CrossRef]
- Bell, B.; Lin, J.J.; Seidenberg, M.; Hermann, B. The Neurobiology of Cognitive Disorders in Temporal Lobe Epilepsy. Nat. Rev. Neurol. 2011, 7, 154–164. [Google Scholar] [CrossRef]
- McCormick, C.; Protzner, A.B.; Barnett, A.J.; Cohn, M.; Valiante, T.A.; McAndrews, M.P. Linking DMN Connectivity to Episodic Memory Capacity: What Can We Learn from Patients with Medial Temporal Lobe Damage? NeuroImage Clin. 2014, 5, 188–196. [Google Scholar] [CrossRef]
- Seghier, M.L. Multiple Functions of the Angular Gyrus at High Temporal Resolution. Brain Struct. Funct. 2023, 228, 7–46. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Bertolero, M.A.; Liu, W.B.; D’Esposito, M. The Human Thalamus Is an Integrative Hub for Functional Brain Networks. J. Neurosci. 2017, 37, 5594–5607. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, H.; Varghese, G.I.; Purcaro, M.J.; Motelow, J.E.; Enev, M.; McNally, K.A.; Levin, A.R.; Hirsch, L.J.; Tikofsky, R.; Zubal, I.G.; et al. Cortical and Subcortical Networks in Human Secondarily Generalized Tonic–Clonic Seizures. Brain 2009, 132, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Brodovskaya, A.; Kapur, J. Circuits Generating Secondarily Generalized Seizures. Epilepsy Behav. 2019, 101, 106474. [Google Scholar] [CrossRef] [PubMed]
- Labate, A.; Cerasa, A.; Gambardella, A.; Aguglia, U.; Quattrone, A. Hippocampal and Thalamic Atrophy in Mild Temporal Lobe Epilepsy: A VBM Study. Neurology 2008, 71, 1094–1101. [Google Scholar] [CrossRef]
- He, X.; Doucet, G.E.; Pustina, D.; Sperling, M.R.; Sharan, A.D.; Tracy, J.I. Presurgical Thalamic “Hubness” Predicts Surgical Outcome in Temporal Lobe Epilepsy. Neurology 2017, 88, 2285–2293. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, B.I.; Shin, K.J.; Ha, S.Y.; Park, J.; Kim, S.E.; Kim, S.E. Pivotal Role of Subcortical Structures as a Network Hub in Focal Epilepsy: Evidence from Graph Theoretical Analysis Based on Diffusion-Tensor Imaging. J. Clin. Neurol. 2019, 15, 68–76. [Google Scholar] [CrossRef]
- Chen, M.; Guo, D.; Li, M.; Ma, T.; Wu, S.; Ma, J.; Cui, Y.; Xia, Y.; Xu, P.; Yao, D. Critical Roles of the Direct GABAergic Pallido-Cortical Pathway in Controlling Absence Seizures. PLOS Comput. Biol. 2015, 11, e1004539. [Google Scholar] [CrossRef]
- Moazeni, O.; Northoff, G.; Batouli, S.A.H. The Subcortical Brain Regions Influence the Cortical Areas during Resting-State: An fMRI Study. Front. Hum. Neurosci. 2024, 18, 1363125. [Google Scholar] [CrossRef]
- Rodriguez-Sabate, C.; Gonzalez, A.; Perez-Darias, J.C.; Morales, I.; Sole-Sabater, M.; Rodriguez, M. Causality Methods to Study the Functional Connectivity in Brain Networks: The Basal Ganglia—Thalamus Causal Interactions. Brain Imaging Behav. 2024, 18, 1–18. [Google Scholar] [CrossRef]
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 100, 1181–1228. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.P.; Sporns, O. Rich-Club Organization of the Human Connectome. J. Neurosci. 2011, 31, 15775–15786. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Z.; Beaulieu, C.; Gross, D.W. Disrupted Anatomic White Matter Network in Left Mesial Temporal Lobe Epilepsy. Epilepsia 2014, 55, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Mijalkov, M.; Volpe, G.; Pereira, J.B. Directed Brain Connectivity Identifies Widespread Functional Network Abnormalities in Parkinson’s Disease. Cereb. Cortex 2022, 32, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.; Diaconis, P. On the Histogram as a Density Estimator:L 2 Theory. Zeitschrift für Wahrscheinlichkeitstheorie und Verwandte Gebiete 1981, 57, 453–476. [Google Scholar] [CrossRef]
- McHugh, M.L. The Chi-Square Test of Independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef]




| Characteristics | |
|---|---|
| Number of Individuals | 21 |
| Female, N (%) | 12 (57.1) |
| Age, mean in years (std, range) | 31.6 (7.4, 18–44) |
| Handedness: left, N (%) | 0 (0) |
| Handedness: right, N (%) | 20 (100) |
| Language Lateralization: left, N (%) | 19 (95) |
| Language Lateralization: right, N (%) | 1 (5) |
| Characteristics | TLE | ETLE |
|---|---|---|
| Number of Individuals | 28 | 16 |
| Age, mean in years (range) | 28.4 ± 8.9 (14–41) *, ** | 21.3 ± 6.4 (13–40) ** |
| Age of seizure onset, | 17.1 ± 12.1 (0.2–39) ** | 2.5–39 |
| Affected Hemisphere | ||
| Left, N (%) | 19 (67.9) | 11 (68.75) |
| Right, N (%) | 7 (25) | 5 (31.25) |
| Bilateral, N (%) | 2 (7.1) | 0 (0) |
| Pathology | ||
| Grade I astrocytoma | 3 (10.7) | 2 (12.5) |
| Ganglioglioma | 2 (7.1) | 0 (0) |
| Gliosis | 0 (0) | 1 (6.25) |
| MTS | 5 (17.9) | 0 (0) |
| Meningioma | 0 (0) | 1 (6.25) |
| DNET | 3 (10.7) | 1 (6.25) |
| FCD | 3 (10.7) | 3 (18.75) |
| unknown | 12 (42.9) | 5 (31.25) |
| Features | Description |
|---|---|
| Global assortativity | Assesses the similarity of the nodes connected in the graph, concerning their degree centrality. High positive values of assortativity indicate that the nodes tend to connect with nodes with similar centrality degrees. Negative values indicate that the hubs of the network tend to connect with low-degree nodes [46]. |
| Global efficiency | Measures how easily information can travel between any pair of nodes. High values indicate a more integrated and well-connected network. Lower global efficiency suggests a network where information transfer is less efficient, indicating potential disruptions or fragmentation in network connectivity [47]. Mathematically, it can be formed as follows:
|
| Mean clustering coefficient | Quantifies the degree to which nodes tend to cluster together. High mean clustering coefficient values suggest that there are many localized clusters of interconnected brain regions, which might be indicative of specialized functional modules within the brain [48]. Low values of the mean clustering coefficient indicate that the graph cannot be divided into clusters, and most of its nodes participate in closed triangles [49]. The mean clustering coefficient can be calculated as the average of the nodal clustering coefficient from all the nodes in the graph: |
| Number of components | Evaluates the maximal connected subgraphs within the entire network. A graph with only one component is fully connected, while a graph with multiple components is disconnected or has distinct clusters. |
| Measures | Description |
| Degree centrality | Measures the number of edges starting from the region. For the directed graph of Granger causality, we used the sum of the in-degree (number of incoming edges) and out-degree (number of outgoing edges) of each node. Nodes with higher degree centrality have a higher number of connections within the network and are considered to be more influential [50]. |
| Betweenness centrality | Quantifies the ability of a node to act as a bridge along the shortest paths between pairs of other nodes. Nodes with high betweenness centrality act as intermediaries that facilitate the flow of information or resources between other nodes [51]. |
| Local efficiency | Evaluates the network’s ability to maintain communication despite potential node failures. Nodes with high local efficiency have neighbors that can communicate with each other via multiple paths, ensuring robust communication pathways within local clusters or neighborhoods. [47]. |
| Eigenvector centrality | Assesses the significance of a node within a network based on its connections to other highly central nodes. Nodes with high eigenvector centrality are deemed influential due to their direct connections with high centrality score nodes [52]. |
| Global Topological Features | HC vs. TLE | HC vs. ETLE | TLE vs. ETLE | Metric | Density Level |
| Global assortativity | ↑ | Pearson correlation | 30–50% | ||
| ↓ | Mutual information | 5–10% | |||
| ↓ | Granger causality | 5–45% | |||
| Global efficiency | ↑ | Pearson correlation | 45–50% | ||
| ↓ | Pearson correlation | 30–50% | |||
| ↑ | Granger causality | 5% | |||
| Global clustering coefficient | ↓ | Pearson correlation | 5% | ||
| ↑ | Pearson correlation | 25% | |||
| ↓ | Pearson correlation | 30–35% | |||
| ↓ | Mutual information | 40–45% | |||
| ↑ | Granger causality | 15–40% | |||
| ↓ | Pearson correlation | 5% | |||
| ↑ | Pearson correlation | 25% | |||
| Number of components | ↓ | Pearson correlation | 40–50% | ||
| ↑ | Pearson correlation | 30–50% | |||
| ↑ | Granger causality | 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoiridou, D.; Gkiatis, K.; Kakkos, I.; Garganis, K.; Matsopoulos, G.K. Multi-Graph Assessment of Temporal and Extratemporal Lobe Epilepsy in Resting-State fMRI. Appl. Sci. 2024, 14, 8336. https://doi.org/10.3390/app14188336
Amoiridou D, Gkiatis K, Kakkos I, Garganis K, Matsopoulos GK. Multi-Graph Assessment of Temporal and Extratemporal Lobe Epilepsy in Resting-State fMRI. Applied Sciences. 2024; 14(18):8336. https://doi.org/10.3390/app14188336
Chicago/Turabian StyleAmoiridou, Dimitra, Kostakis Gkiatis, Ioannis Kakkos, Kyriakos Garganis, and George K. Matsopoulos. 2024. "Multi-Graph Assessment of Temporal and Extratemporal Lobe Epilepsy in Resting-State fMRI" Applied Sciences 14, no. 18: 8336. https://doi.org/10.3390/app14188336
APA StyleAmoiridou, D., Gkiatis, K., Kakkos, I., Garganis, K., & Matsopoulos, G. K. (2024). Multi-Graph Assessment of Temporal and Extratemporal Lobe Epilepsy in Resting-State fMRI. Applied Sciences, 14(18), 8336. https://doi.org/10.3390/app14188336








