Reviewing the Phenomenon of Antimicrobial Resistance in Hospital and Municipal Wastewaters: The Crisis, the Challenges and Mitigation Methods
Abstract
:Featured Application
Abstract
1. Introduction
2. Methods for AMR Detection
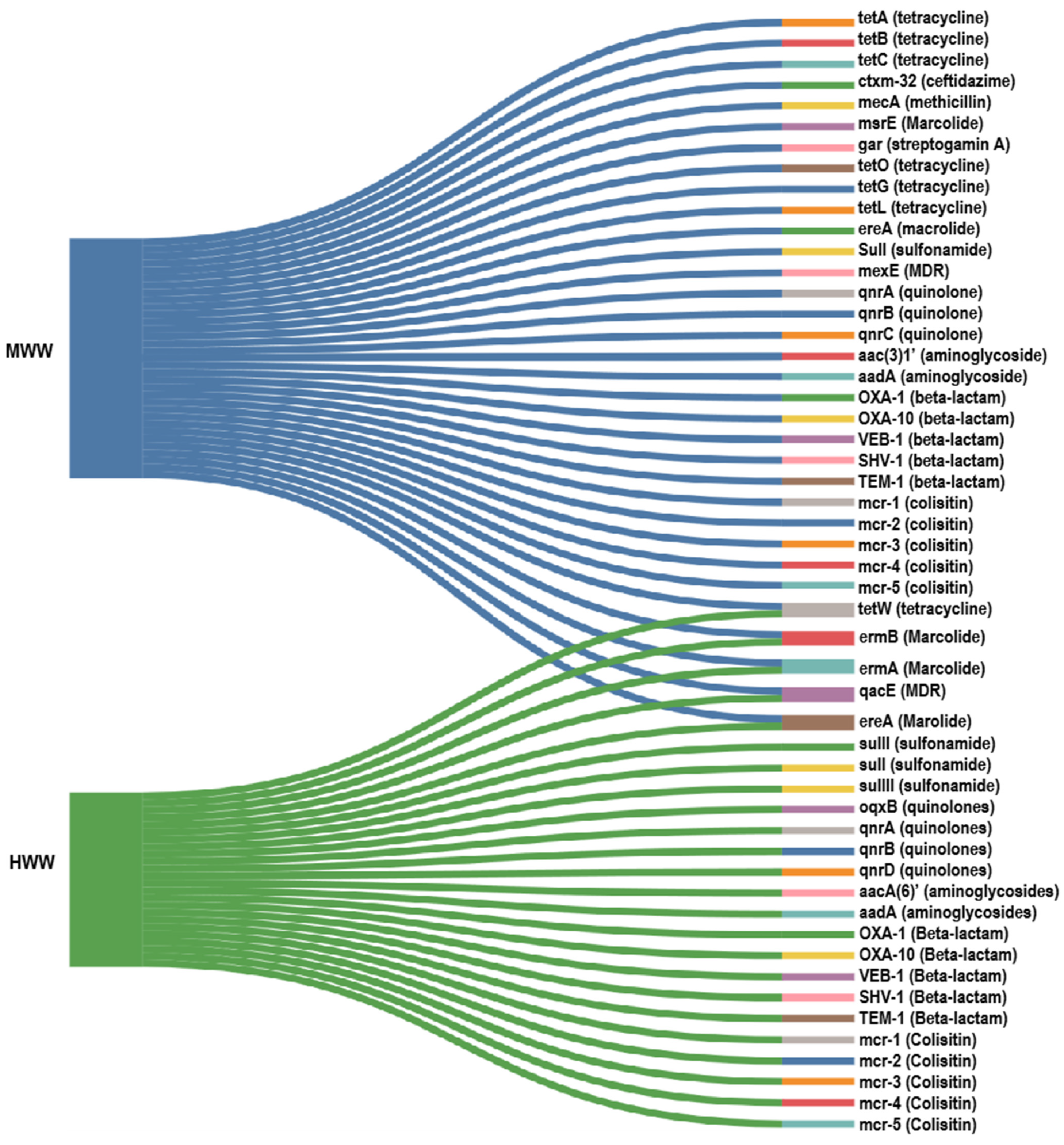
3. Antibiotic Resistance in Hospital and Municipal Wastewater
3.1. Hospital Wastewater
3.2. Regional Variation of ARGs in HWWs
3.3. Municipal Wastewater
| S. No | Samples | Concentrations of ARGs | Resistance against Antibiotics | ARGs | References |
|---|---|---|---|---|---|
| 1 | MWW | 3.35 × 107 CFU/100 mL of ARB | Gentamicin, chloramphenicol and ceftidazime | AAC(3)-1, cmlA1, ctx-m-32 | [11] |
| 2 | MWW | Sulfonamide: 9.73 × 1010 copies/kizumL Tetracycline 1.81 × 1011 copies/mL | Sulfonamide and tetracycline | SulI, sulII, tet A. tetB, tetC, tetG, tetL, tetM, tetO, tetW and tetX | [78] |
| 3 | MWW | Beta-lactam and quinolone | qnrA, qnrB, qnrVC, qnrC, qnrS, qnrD, incU, aac(6′)-IB, blaoxa | [66] | |
| 3 | MWW | - | Sulfonamide, aminoglycoside and marcolide | blaGES, qacE, sul1, mph (E), blaOXA, blaGES and msrE | [79] |
| 4 | MWW | Upto 1 × 10−3 copies of ARG /16SrRNA gene | Linezolid, Colisitin, aminoglycoside and sulfonamide | gar, sul4, mcr(1–5) genes | [67] |
| 5 | MWW | Tetracycline and marcolide | Tet40, tet37, tetQ, tet36, tetW, tetO, emrB, emrF, ereB, ereA and ermX | [15] | |
| 6 | MWW | - | Beta-lactam, marcolides, Fluroquinolones, tetracycline and sulfonamides | qnrS, teteW, sul1, blaTEM and ermB | [22] |
| 7 | MWW | Tet genes 0.82 × 10−2 to 1.57 × 10−2 level of abundance | Sulfonamides and tetracycline | Sul1, sul2, tetM, tetO, tetQ and tetW | [74] |
| Sul genes: 2.82 × 10−3 to 4.76 × 10−2 level of abudance | |||||
| 8 | MWW | −8 log copies/16SrRNA gene | Sulfonamides, beta-lactam methicillin, tetracycline and marcolide | mecA | [14] |
| −7.35 log copies/16SrRNA gene | Sul2 | ||||
| −7 log copies/16SrRNA gene | Sul1 | ||||
| −7 log copies/16SrRNA gene | ermB | ||||
| −7.75 log copies/16SrRNA gene | blaTEM | ||||
| −7.4 log copies/16SrRNA gene | tetO | ||||
| −7.5 log copies/16SrRNA gene | blaCTX-M | ||||
| 9 | MWW | - | MDR, tetracycline, aminoglycoside, beta-lactam, MLS and amphenicol | aadA, cmxA, blaoxa, quacEdelta1, ermF and tetQ | [17] |
| 10 | MWW | 1.106 copies per 16S rRNA gene | Beta-lactam, sufonamide, tetracycline, chloramphenicol and aminoglycoside | blaOXA, sul1, tetM, ermFB, aadA, sul2 and tetX | [52] |
| 11 | MWW | Approximately 105 to 109 | Tetracycline and sulfonamide | tetO, tetW and sulI | [56] |
| 12 | MWW | 5.2 × 108 genes/ml | Beta-lactam and carbapenems | BlaCTX-M, blaNDM, blaOXA-48 and blaVIM | [76] |
| 13 | MWW | 1.59–145.57 ppm | Aminoglycosides, bacitracin, beta-lactams, MDR, tetracycline, sulfonamide and MLS | TetQ, TetW, TetM, aadA, blaOXA and ermF | [55] |
| 14 | MWW | MDR, MLS, beta-lactam, tetracycline and aminoglycosides | mexF, mexL and Rm3 | [53] |
| S. No | Samples | Abundances of ARGs | Resistance against Antibiotics | Most Abundant ARGs | References |
|---|---|---|---|---|---|
| 1 | HWW | (2.793 ± 0.55) × 104 | Tetracycline, marcolide, suflonamide, quinolones and multidrug resistant | qnrA | [20] |
| (4.845 ± 0.71) × 104 | tetM | ||||
| (3.057 ± 1.075) × 107 | tetO | ||||
| (3.07 ± 0.94) × 104 | ereA | ||||
| (1.93 ± 0.33) | oqxB | ||||
| (2.73 ± 0.56) × 106 | ermB | ||||
| (3.81 ± 0.80) × 107. | sulI | ||||
| (4.42 ± 0.29) × 106 | sulII | ||||
| (4.49 ± 0.24) × 104 | sulIII | ||||
| (3.073 ± 0.34) × 106 | tetX | ||||
| (2.34 ± 0.97) × 103 | qnrD | ||||
| 1.88 | ermA | ||||
| 2 | HWW | Mean relative abundances of all ARGs = 1501 | Multidrug, aminoglycoside, cephalosporin, macrolide, penam, tetracycline, and fluoroquinolone | blaGES-5, mef A and aac, mel, AAC-A (6)lb9′, aadA11, sulI, tet36, msrE, LCR1 and mexW | [60] |
| 3 | HWW | MDR, MLS, bacitacin, tetacycline and beta-lactam | [61] | ||
| 4 | HWW | - | Extended spectrum beta-lactam and ciprofloxacin | qepA blaCTX-M and blaTEM | [59] |
| 5 | GHWW | Tetracycline, penicillin, tobramycin and sisomycin | N.Ds | [62] | |
| THWW | Tetracycline, marcolide and nacitracin | ||||
| SHWW | Bacitracin, tetracycline, sulfonamide and cephalosporin | ||||
| 6 | HWW | 101–103 order | Extended spectrum beta-lactam | blaTEM, blaSHV, and blaCTX-M | [51] |
| 7 | HWW | Beta-lactam = 4.87 × 106 copies/mL Quinolone genes 5.38 × 104 copies/mL | Quinolone and beta-lactam resistance | blaOXA-1, blaOXA-10, blaTEM-1, blaDHA-1, blaSHV-1, blaGES-1, qnrA, qnrS, qnrD, and qepA | [58] |
| 8 | HWW | 100% of E. coli | Beta-lactam, sulfonamide and quinolone | blaSHV | [80] |
| 50% of E. coli | blaCTX | ||||
| 100% of E. coli | blaTEM | ||||
| 60% of E. coli | blaVIM | ||||
| 100% of E. coli | SulI | ||||
| 50% of E. coli | QnrS | ||||
| 9 | HWW | 1.883 ± 0.451 ARG/16S rRNA, | Tetracycline, aminoglycoside, beta-lactam and multidrug | tet 39, aph(3″)-I, sulI, aadA | [63] |
| HWW (Eye Hospital) | 1.614 ± 0.177 ARG/16S rRNA | Sulfanomide, aminoglycoside, multidrug and beta-lactam | Sul1, aadA, qacEΔ1 and bacA | [63] | |
| 10 | HWW | - | Aminoglycoside, beta-lactam, sulfonamide, marcolide | aads, ErmF, msrE, Bla-VEB | [48] |
| 11 | HWW (Dental) | - | MDR, aminoglycoside, tetracycline and flouroquinolone | tetE, MexE, TEM (84 subtypes), tet 39, tet 41, AAC-3 (2 subtypes), AAC-2, VanB, and dfrC | [65] |
| 12 | HWW | - | Beta-lactam, marcolides, tetracycline, aminoglycoside and methicillin | blaTEM, blaKPC, mph(A), mel tetD, tetM, tetA, strB and mecA | [68] |
| 13 | HWW | Tetracycline 1.81 × 1011 copies/mL Sulfonamide: 9.73 × 1010 copies/mL | Tetracycline and sulfonamide | SulI, sulII, tet A. tetB, tetC, tetG, tetL, tetM, tetO, tetW and tetX | [78] |
| 14 | HWW MWW | - | Beta-lactam, marcolides, Fluroquinolones, tetracycline and sulfonamides | qnrS, teteW, sul1, blaTEM and ermB | [22] |
| 15 | HWW | HWW 4.19 × 107 CFU/100 mL of ARB | Gentamicin, chloramphenicol and ceftidazime | AAC(3)-1, cmlA1, ctx-m-32 | [11] |
| 16 | HWW | Quinolone resistance and beta-lactamases | qnrA, qnrB, qnrVC, qnrC, qnrS, qnrD, incU, aac(6′)-IB, blaoxa | [66] | |
| 17 | HWW | Upto 1 × 10−3 copies of ARG/16SrRNA gene | Linezolid, Colisitin, and sulfonamide | gar, sul4 and mcr(1–5) genes | [67] |
3.4. Regional Variation of ARGs in MWW
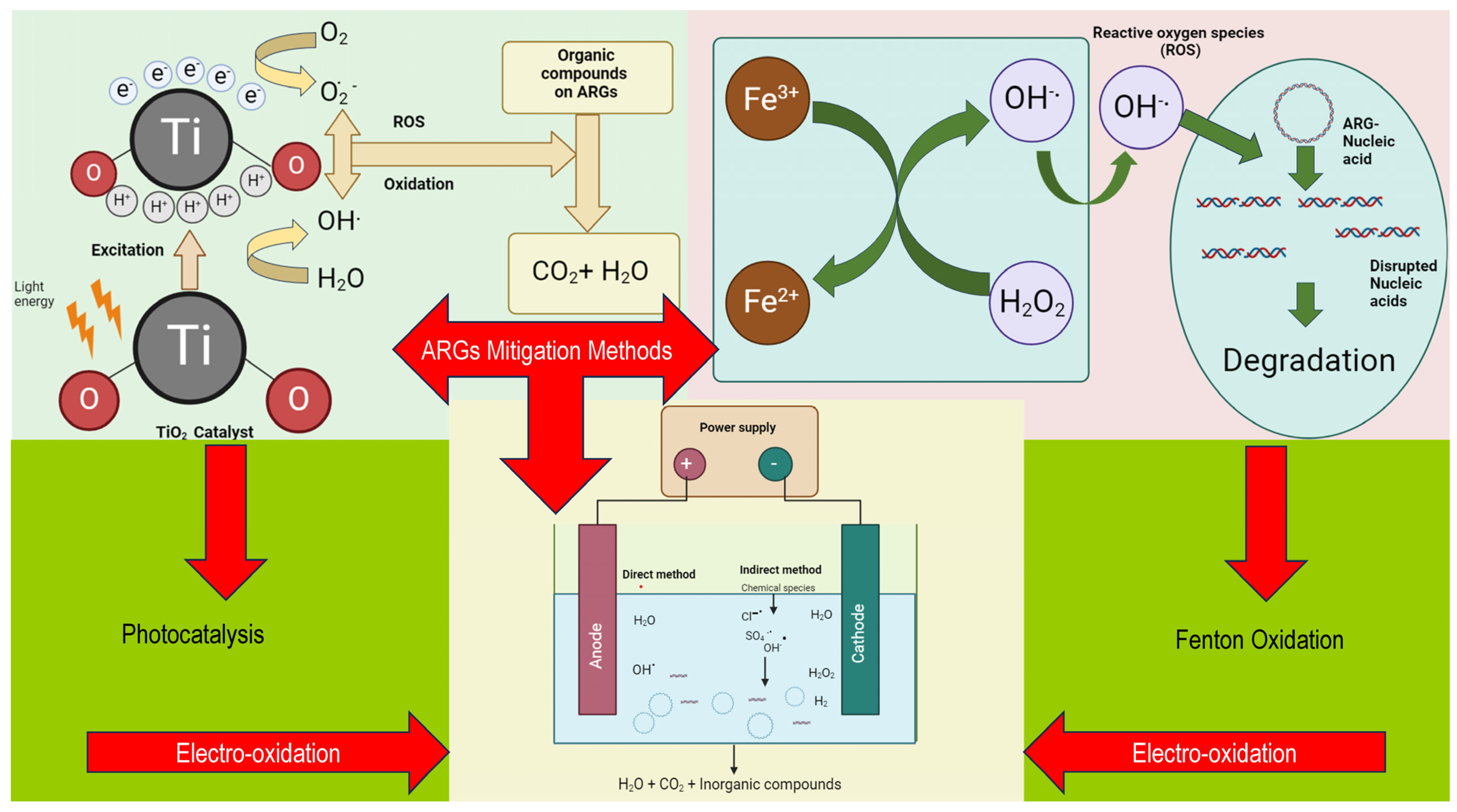
4. Mitigation Methods for Disinfecting AMR Pathogens and Genes in Wastewater Treatment
| S. No | Sample Type | Target ARGs | Method of Wastewater Treatment | Reduction in ARGs | References |
|---|---|---|---|---|---|
| 1 | Urban MWW | blaTEM, qnrS and sulI | Photocatalytic ozonation (TiO2 rings and LED light) | Reduced to 10 gene copies/mL | [109] |
| 2 | MWW | mecA | Photocatalysis (TiO2/H2O2 based and under UV radiation) | 5.8 log | [102] |
| ampC | 4.7 log | ||||
| 3 | WWTP | Sul1 | Photocatalytic ultrafilteration | ~98% | [101] |
| Sul2 | |||||
| floR | |||||
| 4 | Wastewater | ampC | Photocatalysis (Graphene based TiO2/Solar radiation) | Complete removal | [100] |
| 5 | WWTP effluents | tetA | Photocatalysis (Ag/AgBr/g-C3N4 nano composites/Visible light) | 49% | [96] |
| tetM | 86% | ||||
| tetQ | 69% | ||||
| 6 | Domestic WW | i (sulI, sulII and tetQ) | Photocatalysis (with constructed wetlands) | 1.27–1.72 log | [97] |
| e (sulI, sulII and tetQ) | 0.23–0.65 log | ||||
| 7 | WWTP | sul1, tetX and tetG | Fenton oxidation (UV/H2O2) | 2.58 to 3.79 logs | [116] |
| 8 | Urban MWW | blaOXA, blaCTX-M, sul1 and tetM | Solar Fenton process | Reduced to ~10 copies/mL | [106] |
| 9 | Wastewater effluents | Intracellular tetA | Photo-Fenton process | 3.79log | [105] |
| Intracellular blaTEM-1 | 2.19log | ||||
| Extracellular tetA and Extracellular blaTEM-1 | ~6log | ||||
| 10 | HWW | qnrA, qnrB, qnrS, qnrD, aac(6′)-Ib-cr, qepA | Electro-peroxone and sequencing batch reactor treatment | - | [117] |
| 11 | MWW | tetA and Sul1 | Electrochemical oxidation (Blue TiO2 nanotube/under UV lights) | 2.1−2.3 for long sequence target 1.3−1.8 for short sequence target | [114] |
| 12 | MWW | tetG , floR and sul1 | Electro-oxidation using a Magneli phase Ti4O7 anode | 99% | [104] |
| 13 | MWW | sulI and sulII | EMBF bioreactor | (2/3)rd compared with conventional biofilm membrane | [103] |
| 14 | Wastewater | sulI | Electro-oxidation | 4.8 log | [118] |
| tetA | 1.8 log |
4.1. Photocatalysis
4.2. Fenton
4.3. Electro-Oxidation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| ARG | Antibiotic resistance genes |
| CTX-M | Cefotaximase-Munich |
| eARG | Extracellular antibiotic resistance genes |
| ESBL | Extended spectrum beta-lactamases |
| HWW | Hospital wastewater |
| GHWW | General hospital wastewater |
| iARG | Intracellular antibiotic resistance genes |
| LPS | Lipopolysaccharide |
| LPS-MIP | Lipopolysaccharide imprinted polymer film |
| MDR | Multidrug resistance |
| MWW | Municipal wastewater |
| nZVI | Nanoscale zero-valent iron |
| OXA | Oxacillinases |
| PCR | Polymerase chain reactor |
| qPCR | Quantitative polymerase chain reactor |
| ROS | Reactive oxygen species |
| RT-PCR | Real time polymerase chain reactor |
| SHV | Sulf-hydryl variable site |
| SHWW | Stemmatological hospital wastewater |
| TCM | Traditional Chinese medicine hospitals |
| TEM | Temoneira |
| THWW | TCM hospital wastewater |
| WW | Wastewater |
| WWT | Wastewater treatment |
| WWTP | Wastewater treatment plants |
References
- World Health Organisation. Antimicrobial Resistance; World Health Organisation: Geneva, Switzerland, 2022. [Google Scholar]
- Manikandan, M.; Chun, S.; Kazibwe, Z.; Gopal, J.; Singh, U.B.; Oh, J.-W. Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions. Int. J. Environ. Res. Public. Health 2020, 17, 5053. [Google Scholar] [CrossRef]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic Resistance Genes Identified in Wastewater Treatment Plant Systems–A Review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- Preethy, R.; Mohanram, K.; Aruna, D. A Trend of Antibiotic Resistance Pattern of Klebsiella pneumoniae Isolates from Sputum. Saudi J. Pathol. Microbiol. 2019, 4, 466–469. [Google Scholar]
- Yalavarthi, S.; Nallaswamy, D.; Jain, A.R. Resistance to Oral Pathogens among Dentures Wearers. J. Pure Appl. Microbiol. 2018, 12, 961–967. [Google Scholar] [CrossRef]
- Ranganathan, A.; Carmelin, D.S.; Muthusamy, R. Rifampicin Resistance Pattern of Mycobacterium Tuberculosis Infection in Tertiary Care Hospital Settings. Cureus 2024, 16, e55755. [Google Scholar] [CrossRef]
- Pepper, I.L.; Brooks, J.P.; Gerba, C.P. Antibiotic Resistant Bacteria in Municipal Wastes: Is There Reason for Concern? Environ. Sci. Technol. 2018, 52, 3949–3959. [Google Scholar] [CrossRef]
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of Antibiotics in Hospital, Residential, and Dairy Effluent, Municipal Wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring Release of Pharmaceutical Compounds: Occurrence and Environmental Risk Assessment of Two WWTP Effluents and Their Receiving Bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M. Effects of Advanced Treatment Systems on the Removal of Antibiotic Resistance Genes in Wastewater Treatment Plants from Hangzhou, China. Environ. Sci. Technol. 2013, 47, 8157–8163. [Google Scholar] [CrossRef]
- Aali, R.; Nikaeen, M.; Khanahmad, H.; Hassanzadeh, A. Monitoring and Comparison of Antibiotic Resistant Bacteria and Their Resistance Genes in Municipal and Hospital Wastewaters. Int. J. Prev. Med. 2014, 5, 887–894. [Google Scholar]
- Liu, Y.; Dyall-Smith, M.; Marenda, M.; Hu, H.W.; Browning, G.; Billman-Jacobe, H. Antibiotic Resistance Genes in Antibiotic-Free Chicken Farms. Antibiotics 2020, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Yu, S.; Rysz, M.; Luo, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and Proliferation of Antibiotic Resistance Genes in Two Municipal Wastewater Treatment Plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Neudorf, K.D.; Huang, Y.N.; Ragush, C.M.; Yost, C.K.; Jamieson, R.C.; Truelstrup Hansen, L. Antibiotic Resistance Genes in Municipal Wastewater Treatment Systems and Receiving Waters in Arctic Canada. Sci. Total Environ. 2017, 598, 1085–1094. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Y.; Chan, C.-L.; On, H.; Wai, H.K.-F.; Shekhawat, S.S.; Gupta, A.B.; Varshney, A.K.; Chuanchuen, R.; Zhou, X.; et al. Metagenomic Survey Reveals More Diverse and Abundant Antibiotic Resistance Genes in Municipal Wastewater Than Hospital Wastewater. Front. Microbiol. 2021, 12, 712843. [Google Scholar] [CrossRef]
- Le, T.-H.; Ng, C.; Tran, N.H.; Chen, H.; Gin, K.Y.-H. Removal of Antibiotic Residues, Antibiotic Resistant Bacteria and Antibiotic Resistance Genes in Municipal Wastewater by Membrane Bioreactor Systems. Water Res. 2018, 145, 498–508. [Google Scholar] [CrossRef]
- Pärnänen, K.M.M.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic Resistance in European Wastewater Treatment Plants Mirrors the Pattern of Clinical Antibiotic Resistance Prevalence. Sci. Adv. 2019, 5, eaau9124. [Google Scholar] [CrossRef]
- Cai, M.; Wang, Z.; Gu, H.; Dong, H.; Zhang, X.; Cui, N.; Zhou, L.; Chen, G.; Zou, G. Occurrence and Temporal Variation of Antibiotics and Antibiotic Resistance Genes in Hospital Inpatient Department Wastewater: Impacts of Daily Schedule of Inpatients and Wastewater Treatment Process. Chemosphere 2022, 292, 133405. [Google Scholar] [CrossRef]
- Thakali, O.; Malla, B.; Tandukar, S.; Sthapit, N.; Raya, S.; Furukawa, T.; Sei, K.; Sherchand, J.B.; Haramoto, E. Release of Antibiotic-Resistance Genes from Hospitals and a Wastewater Treatment Plant in the Kathmandu Valley, Nepal. Water 2021, 13, 2733. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and Diversity of Antibiotic Resistance in Untreated Hospital Wastewater. Sci. Total Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef]
- Szekeres, E.; Baricz, A.; Chiriac, C.M.; Farkas, A.; Opris, O.; Soran, M.-L.; Andrei, A.-S.; Rudi, K.; Balcázar, J.L.; Dragos, N.; et al. Abundance of Antibiotics, Antibiotic Resistance Genes and Bacterial Community Composition in Wastewater Effluents from Different Romanian Hospitals. Environ. Pollut. 2017, 225, 304–315. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kannan, E.P.; Gopal, J.; Muthu, M. Analytical Techniques for Assessing Antimicrobial Resistance: Conventional Solutions, Contemporary Problems and Futuristic Outlooks. TrAC Trends Anal. Chem. 2024, 178, 117843. [Google Scholar] [CrossRef]
- Tang, X.; Shen, Y.; Song, X.; Benghezal, M.; Marshall, B.J.; Tang, H.; Li, H. Reassessment of the Broth Microdilution Method for Susceptibility Testing of Helicobacter Pylori. J. Infect. Dis. 2022, 226, S486–S492. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.V.; Bari, A.K.; Kokare, R.; Poojary, A. Comparison of in Vitro Fosfomycin Susceptibility Testing Methods with Agar Dilution for Carbapenem Resistant Klebsiella pneumoniae and Escherichia coli. Indian. J. Med. Microbiol. 2023, 42, 39–45. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; D’Antonio, D.; Catamo, G.; Ballone, E.; Piccolomini, R. Comparison of Etest, Agar Dilution, Broth Microdilution and Disk Diffusion Methods for Testing in Vitro Activity of Levofloxacin against Staphylococcus spp. Isolated from Neutropenic Cancer Patients. Int. J. Antimicrob. Agents 2002, 19, 147–154. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, F.; Wang, Z.; Chen, H.; Wang, X.; Zhang, Y.; Li, S.; Wang, H. Evaluation of the Etest and Disk Diffusion Method for Detection of the Activity of Ceftazidime-Avibactam against Enterobacterales and Pseudomonas Aeruginosa in China. BMC Microbiol. 2020, 20, 187. [Google Scholar] [CrossRef]
- Raphael, B.H.; Pham, C.D.; Sharpe, S.; Mauk, K.; Harvey, A.; Khubbar, M.; Triplett, L.; Soge, O.O.; Denny, M.; Palavecino, E.L.; et al. Implementation and Evaluation of Gradient Strip Antimicrobial Susceptibility Testing in US Public Health Laboratories to Respond to Resistant Gonorrhea. Sex. Transm. Dis. 2021, 48, S157–S160. [Google Scholar] [CrossRef]
- Galhano, B.S.P.; Ferrari, R.G.; Panzenhagen, P.; de Jesus, A.C.S.; Conte-Junior, C.A. Antimicrobial Resistance Gene Detection Methods for Bacteria in Animal-Based Foods: A Brief Review of Highlights and Advantages. Microorganisms 2021, 9, 923. [Google Scholar] [CrossRef]
- Pournajaf, A.; Ardebili, A.; Goudarzi, L.; Khodabandeh, M.; Narimani, T.; Abbaszadeh, H. PCR-Based Identification of Methicillin–Resistant Staphylococcus Aureus Strains and Their Antibiotic Resistance Profiles. Asian Pac. J. Trop. Biomed. 2014, 4, S293–S297. [Google Scholar] [CrossRef]
- El Seedy, F.R.; Samy, A.A.; Salam, H.S.H.; Khairy, E.A.; Koraney, A.A. Polymerase Chain Reaction Detection of Genes Responsible for Multiple Antibiotic Resistance Staphylococcus Aureus Isolated from Food of Animal Origin in Egypt. Vet. World 2017, 10, 1205–1211. [Google Scholar] [CrossRef]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial Resistance Genes in ESBL-Producing Escherichia coli Isolates from Animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Gwida, M.; Awad, A.; El-Ashker, M.; Hotzel, H.; Monecke, S.; Ehricht, R.; Müller, E.; Reißig, A.; Barth, S.A.; Berens, C.; et al. Microarray-Based Detection of Resistance and Virulence Factors in Commensal Escherichia coli from Livestock and Farmers in Egypt. Vet. Microbiol. 2020, 240, 108539. [Google Scholar] [CrossRef]
- Anjum, M.F.; Zankari, E.; Hasman, H. Molecular Methods for Detection of Antimicrobial Resistance. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Bell, A.G.; Thornber, K.; Chaput, D.L.; Hasan, N.A.; Mehedi, A.; Haque, M.M.; Cable, J.; Temperton, B.; Tyler, C.R. Metagenomic Assessment of the Diversity and Ubiquity of Antimicrobial Resistance Genes in Bangladeshi Aquaculture Ponds. Aquac. Rep. 2023, 29, 101462. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; Li, R.; Zhang, Y.; Wang, Z. Metagenomics-Based Antibiotic Resistance Genes Diversity and Prevalence Risk Revealed by Pathogenic Bacterial Host in Taihu Lake, China. Environ. Geochem. Health 2022, 44, 2531–2543. [Google Scholar] [CrossRef] [PubMed]
- Quillaguamán, J.; Guzmán, D.; Campero, M.; Hoepfner, C.; Relos, L.; Mendieta, D.; Higdon, S.M.; Eid, D.; Fernández, C.E. The Microbiome of a Polluted Urban Lake Harbors Pathogens with Diverse Antimicrobial Resistance and Virulence Genes. Environ. Pollut. 2021, 273, 116488. [Google Scholar] [CrossRef]
- Pillay, S.; Calderón-Franco, D.; Urhan, A.; Abeel, T. Metagenomic-Based Surveillance Systems for Antibiotic Resistance in Non-Clinical Settings. Front. Microbiol. 2022, 13, 1066995. [Google Scholar] [CrossRef]
- Burrer, A.; Findeisen, P.; Jäger, E.; Ghebremedhin, B.; Grundt, A.; Ahmad-Nejad, P.; Miethke, T.; Neumaier, M. Rapid Detection of Cefotaxime-Resistant Escherichia coli by LC–MS. Int. J. Med. Microbiol. 2015, 305, 860–864. [Google Scholar] [CrossRef]
- Grundt, A.; Findeisen, P.; Miethke, T.; Jäger, E.; Ahmad-Nejad, P.; Neumaier, M. Rapid Detection of Ampicillin Resistance in Escherichia coli by Quantitative Mass Spectrometry. J. Clin. Microbiol. 2012, 50, 1727–1729. [Google Scholar] [CrossRef]
- Filipiak, W.; Żuchowska, K.; Marszałek, M.; Depka, D.; Bogiel, T.; Warmuzińska, N.; Bojko, B. GC-MS Profiling of Volatile Metabolites Produced by Klebsiella pneumoniae. Front. Mol. Biosci. 2022, 9, 1019290. [Google Scholar] [CrossRef]
- Boots, A.W.; Smolinska, A.; van Berkel, J.J.B.N.; Fijten, R.R.R.; Stobberingh, E.E.; Boumans, M.L.L.; Moonen, E.J.; Wouters, E.F.M.; Dallinga, J.W.; Van Schooten, F.J. Identification of Microorganisms Based on Headspace Analysis of Volatile Organic Compounds by Gas Chromatography–Mass Spectrometry. J. Breath. Res. 2014, 8, 27106. [Google Scholar] [CrossRef]
- Florio, W.; Baldeschi, L.; Rizzato, C.; Tavanti, A.; Ghelardi, E.; Lupetti, A. Detection of Antibiotic-Resistance by MALDI-TOF Mass Spectrometry: An Expanding Area. Front. Cell Infect. Microbiol. 2020, 10, 572909. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Zhao, Z.; Cao, Y.; Li, B. Hospital Wastewater as a Reservoir for Antibiotic Resistance Genes: A Meta-Analysis. Front. Public. Health 2020, 8, 574968. [Google Scholar] [CrossRef]
- Bardhan, T.; Chakraborty, M.; Bhattacharjee, B. Prevalence of Colistin-Resistant, Carbapenem-Hydrolyzing Proteobacteria in Hospital Water Bodies and Out-Falls of West Bengal, India. Int. J. Environ. Res. Public. Health 2020, 17, 1007. [Google Scholar] [CrossRef]
- Moges, F.; Endris, M.; Belyhun, Y.; Worku, W. Isolation and Characterization of Multiple Drug Resistance Bacterial Pathogens from Waste Water in Hospital and Non-Hospital Environments, Northwest Ethiopia. BMC Res. Notes 2014, 7, 215. [Google Scholar] [CrossRef]
- Talat, A.; Blake, K.S.; Dantas, G.; Khan, A.U. Metagenomic Insight into Microbiome and Antibiotic Resistance Genes of High Clinical Concern in Urban and Rural Hospital Wastewater of Northern India Origin: A Major Reservoir of Antimicrobial Resistance. Microbiol. Spectr. 2023, 11, e04102-22. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Kara, E.M.; Celik, B.O.; Vergili, I.; Kaya, Y.; Altinkum, S.M.; Bagdatli, Y.; Yilmaz, G. Detailed Characterization, Antibiotic Resistance and Seasonal Variation of Hospital Wastewater. Environ. Sci. Pollut. Res. 2021, 28, 16380–16393. [Google Scholar] [CrossRef]
- Rozman, U.; Duh, D.; Cimerman, M.; Turk, S.Š. Hospital Wastewater Effluent: Hot Spot for Antibiotic Resistant Bacteria. J. Water Sanit. Hyg. Dev. 2020, 10, 171–178. [Google Scholar] [CrossRef]
- Walia, S.; Murleedharn, C.; Band, J.; Kanwar, M.; Kumar, A. Quantitation of Antibiotic Resistance Genes Pollution in Hospital Waste Water Effluent and Urban Clinton River Water, Michigan, USA. Curr. Med. Res. Pract. 2016, 6, 149–151. [Google Scholar] [CrossRef]
- Ng, C.; Tan, B.; Jiang, X.-T.; Gu, X.; Chen, H.; Schmitz, B.W.; Haller, L.; Charles, F.R.; Zhang, T.; Gin, K. Metagenomic and Resistome Analysis of a Full-Scale Municipal Wastewater Treatment Plant in Singapore Containing Membrane Bioreactors. Front. Microbiol. 2019, 10, 172. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shin, H.; Han, D.; Hur, H.-G.; Unno, T. Metagenomic Analysis Reveals the Prevalence and Persistence of Antibiotic- and Heavy Metal-Resistance Genes in Wastewater Treatment Plant. J. Microbiol. 2018, 56, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Yang, J.; Kim, S.; Park, J.; Kim, M.; Park, W. Occurrence of Antibiotic Resistance Genes and Multidrug-Resistant Bacteria during Wastewater Treatment Processes. Sci. Total Environ. 2022, 811, 152331. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, G. Investigation of the Prevalence of Antibiotic Resistance Genes According to the Wastewater Treatment Scale Using Metagenomic Analysis. Antibiotics 2021, 10, 188. [Google Scholar] [CrossRef]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of Antibiotic Resistant Bacteria and Genes in the Effluent and Biosolids of Five Wastewater Utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef]
- Volkmann, H.; Schwartz, T.; Bischoff, P.; Kirchen, S.; Obst, U. Detection of Clinically Relevant Antibiotic-Resistance Genes in Municipal Wastewater Using Real-Time PCR (TaqMan). J. Microbiol. Methods 2004, 56, 277–286. [Google Scholar] [CrossRef]
- Yao, S.; Ye, J.; Yang, Q.; Hu, Y.; Zhang, T.; Jiang, L.; Munezero, S.; Lin, K.; Cui, C. Occurrence and Removal of Antibiotics, Antibiotic Resistance Genes, and Bacterial Communities in Hospital Wastewater. Environ. Sci. Pollut. Res. 2021, 28, 57321–57333. [Google Scholar]
- Lien, L.; Lan, P.; Chuc, N.; Hoa, N.; Nhung, P.; Thoa, N.; Diwan, V.; Tamhankar, A.; Stålsby Lundborg, C. Antibiotic Resistance and Antibiotic Resistance Genes in Escherichia coli Isolates from Hospital Wastewater in Vietnam. Int. J. Environ. Res. Public. Health 2017, 14, 699. [Google Scholar] [CrossRef]
- Petrovich, M.L.; Zilberman, A.; Kaplan, A.; Eliraz, G.R.; Wang, Y.; Langenfeld, K.; Duhaime, M.; Wigginton, K.; Poretsky, R.; Avisar, D.; et al. Microbial and Viral Communities and Their Antibiotic Resistance Genes Throughout a Hospital Wastewater Treatment System. Front. Microbiol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Srinivasan, S.; Shanmugam, G.; Ahn, Y.H. Shotgun Metagenomic Analysis Reveals the Prevalence of Antibiotic Resistance Genes and Mobile Genetic Elements in Full Scale Hospital Wastewater Treatment Plants. J. Environ. Manag. 2021, 296, 113270. [Google Scholar] [CrossRef]
- Guo, X.; Tang, N.; Lei, H.; Fang, Q.; Liu, L.; Zhou, Q.; Song, C. Metagenomic Analysis of Antibiotic Resistance Genes in Untreated Wastewater From Three Different Hospitals. Front. Microbiol. 2021, 12, 709051. [Google Scholar] [CrossRef]
- Ma, X.; Dong, X.; Cai, J.; Fu, C.; Yang, J.; Liu, Y.; Zhang, Y.; Wan, T.; Lin, S.; Lou, Y.; et al. Metagenomic Analysis Reveals Changes in Bacterial Communities and Antibiotic Resistance Genes in an Eye Specialty Hospital and a General Hospital before and after Wastewater Treatment. Front. Microbiol. 2022, 13, 848167. [Google Scholar] [CrossRef]
- Wang, C.; Mantilla-Calderon, D.; Xiong, Y.; Alkahtani, M.; Bashawri, Y.M.; Al Qarni, H.; Hong, P.-Y. Investigation of Antibiotic Resistome in Hospital Wastewater during the COVID-19 Pandemic: Is the Initial Phase of the Pandemic Contributing to Antimicrobial Resistance? Environ. Sci. Technol. 2022, 56, 15007–15018. [Google Scholar] [CrossRef]
- Jiao, X.; Guo, W.; Li, X.; Yao, F.; Zeng, M.; Yuan, Y.; Guo, X.; Wang, M.; Xie, Q.D.; Cai, L.; et al. New Insight into the Microbiome, Resistome, and Mobilome on the Dental Waste Water in the Context of Heavy Metal Environment. Front. Microbiol. 2023, 14, 1106157. [Google Scholar] [CrossRef]
- Varela, A.R.; Nunes, O.C.; Manaia, C.M. Quinolone Resistant Aeromonas Spp. as Carriers and Potential Tracers of Acquired Antibiotic Resistance in Hospital and Municipal Wastewater. Sci. Total Environ. 2016, 542, 665–671. [Google Scholar] [CrossRef]
- Hutinel, M.; Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance Genes of Emerging Concern in Municipal and Hospital Wastewater from a Major Swedish City. Sci. Total Environ. 2022, 812, 151433. [Google Scholar] [CrossRef]
- Batista, M.P.B.; Cavalcante, F.S.; Cassini, S.T.A.; Schuenck, R.P. Diversity of Bacteria Carrying Antibiotic Resistance Genes in Hospital Raw Sewage in Southeastern Brazil. Water Sci. Technol. 2023, 87, 239–250. [Google Scholar] [CrossRef]
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital Wastewater as a Source of Environmental Contamination: An Overview of Management Practices, Environmental Risks, and Treatment Processes. J. Water Process Eng. 2021, 41, 101990. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Hassoun-Kheir, N.; Stabholz, Y.; Kreft, J.-U.; de la Cruz, R.; Romalde, J.L.; Nesme, J.; Sørensen, S.J.; Smets, B.F.; Graham, D.; Paul, M. Comparison of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes Abundance in Hospital and Community Wastewater: A Systematic Review. Sci. Total Environ. 2020, 743, 140804. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, X.; Zheng, Y.; Xiao, Y. Use and Prescription of Antibiotics in Primary Health Care Settings in China. JAMA Internal Med. 2014, 174, 1914. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M. Occurrence and Removal of Antibiotic Resistance Genes in Municipal Wastewater and Rural Domestic Sewage Treatment Systems in Eastern China. Environ. Int. 2013, 55, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, X.; Huang, H.; Zhang, J. Prevalence of Antibiotic Resistance Genes and Their Association with Antibiotics in a Wastewater Treatment Plant: Process Distribution and Analysis. Water 2019, 11, 2495. [Google Scholar] [CrossRef]
- Makowska, N.; Bresa, K.; Koczura, R.; Philips, A.; Nowis, K.; Mokracka, J. Urban Wastewater as a Conduit for Pathogenic Gram-Positive Bacteria and Genes Encoding Resistance to β-Lactams and Glycopeptides. Sci. Total Environ. 2021, 765, 144176. [Google Scholar] [CrossRef]
- Su, H.; Hu, X.; Xu, W.; Xu, Y.; Wen, G.; Cao, Y. Diversity, Abundances and Distribution of Antibiotic Resistance Genes and Virulence Factors in the South China Sea Revealed by Metagenomic Sequencing. Sci. Total Environ. 2022, 814, 152803. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W.; Xu, L.; Jiao, Y.; Baig, S.A.; Chen, H. Occurrence and Removal of Antibiotics and the Corresponding Resistance Genes in Wastewater Treatment Plants: Effluents’ Influence to Downstream Water Environment. Environ. Sci. Pollut. Res. 2016, 23, 6826–6835. [Google Scholar] [CrossRef]
- Sekizuka, T.; Itokawa, K.; Tanaka, R.; Hashino, M.; Yatsu, K.; Kuroda, M. Metagenomic Analysis of Urban Wastewater Treatment Plant Effluents in Tokyo. Infect. Drug Resist. 2022, 15, 4763–4777. [Google Scholar] [CrossRef]
- Asghari, F.B.; Dehghani, M.H.; Dehghanzadeh, R.; Farajzadeh, D.; Yaghmaeian, K.; Mahvi, A.H.; Rajabi, A. Antibiotic Resistance and Antibiotic-Resistance Genes of Pseudomonas spp. and Escherichia coli Isolated from Untreated Hospital Wastewater. Water Sci. Technol. 2021, 84, 172–181. [Google Scholar] [CrossRef]
- Pallares-Vega, R.; Blaak, H.; van der Plaats, R.; de Roda Husman, A.M.; Hernandez Leal, L.; van Loosdrecht, M.C.M.; Weissbrodt, D.G.; Schmitt, H. Determinants of Presence and Removal of Antibiotic Resistance Genes during WWTP Treatment: A Cross-Sectional Study. Water Res. 2019, 161, 319–328. [Google Scholar] [CrossRef]
- Arzate, S.; Pfister, S.; Oberschelp, C.; Sánchez-Pérez, J.A. Environmental Impacts of an Advanced Oxidation Process as Tertiary Treatment in a Wastewater Treatment Plant. Sci. Total Environ. 2019, 694, 133572. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment With CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Bampos, G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes 2022, 10, 2304. [Google Scholar] [CrossRef]
- Yeoh, J.X.; Jamil, S.N.A.; Syukri, F.; Koyama, M.; Nourouzi Mobarekeh, M. Comparison between Conventional Treatment Processes and Advanced Oxidation Processes in Treating Slaughterhouse Wastewater: A Review. Water 2022, 14, 3778. [Google Scholar] [CrossRef]
- Michael, S.G.; Michael-Kordatou, I.; Nahim-Granados, S.; Polo-López, M.I.; Rocha, J.; Martínez-Piernas, A.B.; Fernández-Ibáñez, P.; Agüera, A.; Manaia, C.M.; Fatta-Kassinos, D. Investigating the Impact of UV-C/H2O2 and Sunlight/H2O2 on the Removal of Antibiotics, Antibiotic Resistance Determinants and Toxicity Present in Urban Wastewater. Chem. Eng. J. 2020, 388, 124383. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Vélez-Peña, E.; Osorio-Vargas, P.; Jiménez, J.N.; Salazar-Ospina, L.; Guaca-González, Y.M.; Torres-Palma, R.A. Inactivation of Carbapenem-Resistant Klebsiella pneumoniae by Photo-Fenton: Residual Effect, Gene Evolution and Modifications with Citric Acid and Persulfate. Water Res. 2019, 161, 354–363. [Google Scholar] [CrossRef]
- McKinney, C.W.; Pruden, A. Ultraviolet Disinfection of Antibiotic Resistant Bacteria and Their Antibiotic Resistance Genes in Water and Wastewater. Environ. Sci. Technol. 2012, 46, 13393–13400. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, M.; Zhang, S.; Yu, X. Can Chlorination Co-Select Antibiotic-Resistance Genes? Chemosphere 2016, 156, 412–419. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, H.; Geng, J.; Zhang, Y.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of Antibiotic Resistance Genes in Municipal Wastewater by Chlorination, Ultraviolet, and Ozonation Disinfection. Environ. Sci. Pollut. Res. 2015, 22, 7037–7044. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous Ozonation of Urban Wastewater: Removal of Antibiotics, Antibiotic-Resistant Escherichia coli and Antibiotic Resistance Genes and Phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254nm Radiation for the Removal of Microorganisms and Antibiotic Resistance Genes from Urban Wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Khiadani, M.; Kakhki, S.; Kholghi, V.; Naderi, K.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci. Total Environ. 2022, 811, 151404. [Google Scholar] [PubMed]
- Zhang, J.; Yang, M.; Zhong, H.; Liu, M.; Sui, Q.; Zheng, L.; Tong, J.; Wei, Y. Deciphering the Factors Influencing the Discrepant Fate of Antibiotic Resistance Genes in Sludge and Water Phases during Municipal Wastewater Treatment. Bioresour. Technol. 2018, 265, 310–319. [Google Scholar] [CrossRef]
- Li, Z.-H.; Yuan, L.; Gao, S.-X.; Wang, L.; Sheng, G.-P. Mitigated Membrane Fouling and Enhanced Removal of Extracellular Antibiotic Resistance Genes from Wastewater Effluent via an Integrated Pre-Coagulation and Microfiltration Process. Water Res. 2019, 159, 145–152. [Google Scholar] [CrossRef]
- Yu, P.; Zhou, X.; Li, Z.; Yan, Y. Inactivation and Change of Tetracycline-Resistant Escherichia coli in Secondary Effluent by Visible Light-Driven Photocatalytic Process Using Ag/AgBr/g-C3N4. Sci. Total Environ. 2020, 705, 135639. [Google Scholar] [CrossRef]
- Chen, P.; Yu, X.; Zhang, J. Photocatalysis Enhanced Constructed Wetlands Effectively Remove Antibiotic Resistance Genes from Domestic Wastewater. Chemosphere 2023, 325, 138330. [Google Scholar] [CrossRef] [PubMed]
- Felis, E.; Buta-Hubeny, M.; Zieliński, W.; Hubeny, J.; Harnisz, M.; Bajkacz, S.; Korzeniewska, E. Solar-Light Driven Photodegradation of Antimicrobials, Their Transformation by-Products and Antibiotic Resistance Determinants in Treated Wastewater. Sci. Total Environ. 2022, 836, 155447. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, D.; Yu, P.; Sun, R.; Javed, H.; Wu, G.; Alvarez, P.J.J. Selective Adsorption and Photocatalytic Degradation of Extracellular Antibiotic Resistance Genes by Molecularly-Imprinted Graphitic Carbon Nitride. Environ. Sci. Technol. 2020, 54, 4621–4630. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of Antibiotics, Antibiotic-Resistant Bacteria and Their Associated Genes by Graphene-Based TiO2 Composite Photocatalysts under Solar Radiation in Urban Wastewaters. Appl. Catal. B 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Ren, S.; Boo, C.; Guo, N.; Wang, S.; Elimelech, M.; Wang, Y. Photocatalytic Reactive Ultrafiltration Membrane for Removal of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2018, 52, 8666–8673. [Google Scholar] [CrossRef]
- Guo, C.; Wang, K.; Hou, S.; Wan, L.; Lv, J.; Zhang, Y.; Qu, X.; Chen, S.; Xu, J. H2O2 and/or TiO2 Photocatalysis under UV Irradiation for the Removal of Antibiotic Resistant Bacteria and Their Antibiotic Resistance Genes. J. Hazard. Mater. 2017, 323, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, R.; Yang, B.; Chen, M.; Wang, X.; Wang, Z. An Electrochemical Membrane Biofilm Reactor for Removing Sulfonamides from Wastewater and Suppressing Antibiotic Resistance Development: Performance and Mechanisms. J. Hazard. Mater. 2021, 404, 124198. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, H.; Liu, S.; Xiao, L. Removal of Pathogen and Antibiotic Resistance Genes from Waste Activated Sludge by Different Pre-Treatment Approaches. Sci. Total Environ. 2021, 763, 143014. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.; Zhong, J.; Yuan, Z.; Guo, J. Simultaneous Removal of Antibiotic Resistant Bacteria, Antibiotic Resistance Genes, and Micropollutants by a Modified Photo-Fenton Process. Water Res. 2021, 197, 117075. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.G.; Michael-Kordatou, I.; Beretsou, V.G.; Jäger, T.; Michael, C.; Schwartz, T.; Fatta-Kassinos, D. Solar Photo-Fenton Oxidation Followed by Adsorption on Activated Carbon for the Minimisation of Antibiotic Resistance Determinants and Toxicity Present in Urban Wastewater. Appl. Catal. B 2019, 244, 871–880. [Google Scholar] [CrossRef]
- Ahmed, Y.; Lu, J.; Yuan, Z.; Bond, P.L.; Guo, J. Efficient Inactivation of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes by Photo-Fenton Process under Visible LED Light and Neutral pH. Water Res. 2020, 179, 115878. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Martínez-Pachón, D.; Moncayo-Lasso, A.; Ibáñez, M.; Hernández, F.; Torres-Palma, R.A. Degradation of Seventeen Contaminants of Emerging Concern in Municipal Wastewater Effluents by Sonochemical Advanced Oxidation Processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Pereira, M.F.R.; Castro-Silva, S.; Segundo, M.A.; et al. Photocatalytic Ozonation of Urban Wastewater and Surface Water Using Immobilized TiO2 with LEDs: Micropollutants, Antibiotic Resistance Genes and Estrogenic Activity. Water Res. 2016, 94, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An Overview of the Application of Fenton Oxidation to Industrial Wastewaters Treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Alexander, J.; Schwartz, T.; Fatta-Kassinos, D. Investigation of the Potential of a Membrane BioReactor Followed by Solar Fenton Oxidation to Remove Antibiotic-Related Microcontaminants. Chem. Eng. J. 2017, 310, 491–502. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical Advanced Oxidation Processes for Wastewater Treatment: Advances in Formation and Detection of Reactive Species and Mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Quispe, E.; Yang, H.; Sanfiorenzo, C.; Rogers, S.W.; Wang, K.; Yang, Y.; Hoffmann, M.R. Removal of Antibiotic Resistant Bacteria and Genes by UV-Assisted Electrochemical Oxidation on Degenerative TiO2 Nanotube Arrays. ACS ES T Eng. 2021, 1, 612–622. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Removal of Antibiotic Resistance Genes (ARGs) in Various Wastewater Treatment Processes: An Overview. Crit. Rev. Environ. Sci. Technol. 2022, 52, 571–630. [Google Scholar]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Xu, K.; Ding, L. Reduction of Antibiotic Resistance Genes in Municipal Wastewater Effluent by Advanced Oxidation Processes. Sci. Total Environ. 2016, 550, 184–191. [Google Scholar] [CrossRef]
- Zheng, H.-S.; Guo, W.-Q.; Wu, Q.-L.; Ren, N.-Q.; Chang, J.-S. Electro-Peroxone Pretreatment for Enhanced Simulated Hospital Wastewater Treatment and Antibiotic Resistance Genes Reduction. Environ. Int. 2018, 115, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Wang, Y.; Hou, X.; Ma, D.; Zhang, Y.; Li, Q.; Gao, B. Removing Antibiotic Resistance Genes through the Synergistic Effect of Hydroxyl Radical and Hypochlorite Radical Generated in a CeO2@CNT Electrified Membrane–NaClO System. Chem. Eng. J. 2023, 471, 144645. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Gmurek, M.; Mazierski, P.; Zaleska-Medynska, A.; Klimczuk, T.; Quinta-Ferreira, R.M.; Martins, R.C. TiO2 Nanotube Arrays-Based Reactor for Photocatalytic Oxidation of Parabens Mixtures in Ultrapure Water: Effects of Photocatalyst Properties, Operational Parameters and Light Source. Sci. Total Environ. 2019, 689, 79–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Xiong, Z.; Wang, W.; Zheng, D.; He, T.; Liu, Y.; Ran, Y.; Deng, L.; Lai, B. Removal of Antibiotic Resistance Genes from Post-Treated Swine Wastewater by MFe/NCu System. Chem. Eng. J. 2020, 400, 125953. [Google Scholar] [CrossRef]
- Bracamontes-Ruelas, A.R.; Ordaz-Díaz, L.A.; Bailón-Salas, A.M.; Ríos-Saucedo, J.C.; Reyes-Vidal, Y.; Reynoso-Cuevas, L. Emerging Pollutants in Wastewater, Advanced Oxidation Processes as an Alternative Treatment and Perspectives. Processes 2022, 10, 1041. [Google Scholar] [CrossRef]
- Wang, B.; Shi, H.; Habteselassie, M.Y.; Deng, X.; Teng, Y.; Wang, Y.; Huang, Q. Simultaneous Removal of Multidrug-Resistant Salmonella Enterica Serotype Typhimurium, Antibiotics and Antibiotic Resistance Genes from Water by Electrooxidation on a Magnéli Phase Ti4O7 Anode. Chem. Eng. J. 2021, 407, 127134. [Google Scholar]
- Maillard, J.-Y.; Hartemann, P. Silver as an Antimicrobial: Facts and Gaps in Knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef]
- Calderón-Franco, D.; Apoorva, S.; Medema, G.; van Loosdrecht, M.C.; Weissbrodt, D.G. Upgrading residues from wastewater and drinking water treatment plants as low-cost adsorbents to remove extracellular DNA and microorganisms carrying antibiotic resistance genes from treated effluents. Sci. Total Environ. 2021, 778, 146364. [Google Scholar] [PubMed]
- Ngigi, A.N.; Ok, Y.S.; Thiele-Bruhn, S. Biochar affects the dissipation of antibiotics and abundance of antibiotic resistance genes in pig manure. Bioresour. Technol. 2020, 315, 123782. [Google Scholar]
- Anthony, E.T.; Ojemaye, M.O.; Okoh, A.I.; Okoh, O.O. Potentials of low-cost methods for the removal of antibiotic-resistant bacteria and their genes in low budget communities: A review. J. Water Process Eng. 2021, 40, 101919. [Google Scholar]
- Paruch, L.; Paruch, A.M.; Iordache, T.-V.; Olaru, A.G.; Sarbu, A. Mitigating Antibiotic Resistance Genes in Wastewater by Sequential Treatment with Novel Nanomaterials. Polymers 2021, 13, 1593. [Google Scholar] [CrossRef]
- He, F.; Li, Z.; Shi, S.; Xu, W.; Sheng, H.; Gu, Y.; Jiang, Y.; Xi, B. Dechlorination of Excess Trichloroethene by Bimetallic and Sulfidated Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2018, 52, 8627–8637. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Shi, L.; Xue, X.; Kang, M.; Zheng, X. The Mechanism for Bacteriophage F2 Removal by Nanoscale Zero-Valent Iron. Water Res. 2016, 105, 429–435. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Duan, W.; Zhang, W.; Zhao, Y.; Liu, J. Inactivation of Sulfonamide Antibiotic Resistant Bacteria and Control of Intracellular Antibiotic Resistance Transmission Risk by Sulfide-Modified Nanoscale Zero-Valent Iron. J. Hazard. Mater. 2020, 400, 123226. [Google Scholar] [CrossRef]
- Zhang, W.-Z.; Gao, J.-F.; Duan, W.-J.; Zhang, D.; Jia, J.-X.; Wang, Y.-W. Sulfidated Nanoscale Zero-Valent Iron Is an Efficient Material for the Removal and Regrowth Inhibition of Antibiotic Resistance Genes. Environ. Pollut. 2020, 263, 114508. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Yao, M. Use of Zero-Valent Iron Nanoparticles in Inactivating Microbes. Water Res. 2009, 43, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Mao, Y.; Ding, L. Carbon Nanotubes as Antimicrobial Agents for Water Disinfection and Pathogen Control. J. Water Health 2018, 16, 171–180. [Google Scholar] [PubMed]
- Chun, S.; Muthu, M.; Gansukh, E.; Thalappil, P.; Gopal, J. The ethanopharmacological aspect of carbon nanodots in turmeric smoke. Sci. Rep. 2016, 6, 35586. [Google Scholar]
- Dar, K.K.; Shao, S.; Tan, T.; Lv, Y. Molecularly Imprinted Polymers for the Selective Recognition of Microorganisms. Biotechnol. Adv. 2020, 45, 107640. [Google Scholar] [CrossRef]
- Long, Y.; Li, Z.; Bi, Q.; Deng, C.; Chen, Z.; Bhattachayya, S.; Li, C. Novel Polymeric Nanoparticles Targeting the Lipopolysaccharides of Pseudomonas aeruginosa. Int. J. Pharm. 2016, 502, 232–241. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable ecofriendly phytoextract mediated one pot green recovery of chitosan. Sci. Rep. 2019, 9, 13832. [Google Scholar]
- Gavrila, A.-M.; Zaharia, A.; Paruch, L.; Perrin, F.X.; Sarbu, A.; Olaru, A.G.; Paruch, A.M.; Iordache, T.-V. Molecularly Imprinted Films and Quaternary Ammonium-Functionalized Microparticles Working in Tandem against Pathogenic Bacteria in Wastewaters. J. Hazard. Mater. 2020, 399, 123026. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, N.; Kannan, E.P.; Hakami, O.; Alamri, A.A.; Gopal, J.; Muthu, M. Reviewing the Phenomenon of Antimicrobial Resistance in Hospital and Municipal Wastewaters: The Crisis, the Challenges and Mitigation Methods. Appl. Sci. 2024, 14, 8358. https://doi.org/10.3390/app14188358
Hasan N, Kannan EP, Hakami O, Alamri AA, Gopal J, Muthu M. Reviewing the Phenomenon of Antimicrobial Resistance in Hospital and Municipal Wastewaters: The Crisis, the Challenges and Mitigation Methods. Applied Sciences. 2024; 14(18):8358. https://doi.org/10.3390/app14188358
Chicago/Turabian StyleHasan, Nazim, Embar Prasanna Kannan, Othman Hakami, Abdullah Ali Alamri, Judy Gopal, and Manikandan Muthu. 2024. "Reviewing the Phenomenon of Antimicrobial Resistance in Hospital and Municipal Wastewaters: The Crisis, the Challenges and Mitigation Methods" Applied Sciences 14, no. 18: 8358. https://doi.org/10.3390/app14188358







