Effect of Ozonized Water against Pathogenic Bacteria and Filamentous Fungi on Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.1.1. Bacteria
- Listeria monocytogenes (Murray et al.) Pirie ATCC 7644, isolated from a source not specified by the ATCC website.
- Listeria monocytogenes Scott A (Murray et al.) Pirie ATCC 49594, derived from an existing strain.
- Salmonella enterica subsp. enterica serotype Senftenberg NCTC 9959 (=ATCC 43845 = Col Sal 385/57 = DSM 10062 = JT 493; 775/W), isolated from a source not specified by the ATCC website.
- Salmonella enterica subsp. enterica serotype Typhimurium ATCC 14028 (=CIP 104115 = DSM 19587 = CDC 6516-60 = NCTC 12023) isolated from the pooled heart and liver tissue of four-week-old chickens.
2.1.2. Filamentous Fungi
- Penicillium nordicum SSICA 1169, isolated from dry-cured meat production environments in Italy;
- Penicillium nordicum SSICA B4798, isolated from a fermented meat product in Italy;
- Hyphopichia burtonii SSICA 175717, isolated from spoiled sandwich bread in Italy;
- Hyphopichia burtonii SSICA 251105, isolated from a spreadable fat used to cover dry-cured hams during seasoning in Italy;
- Aspergillus brasiliensis ATCC 16404, isolated from blueberries in North Carolina (USA).
2.2. Preparation of the Microbial Suspensions
2.2.1. Bacteria
2.2.2. Filamentous Fungi
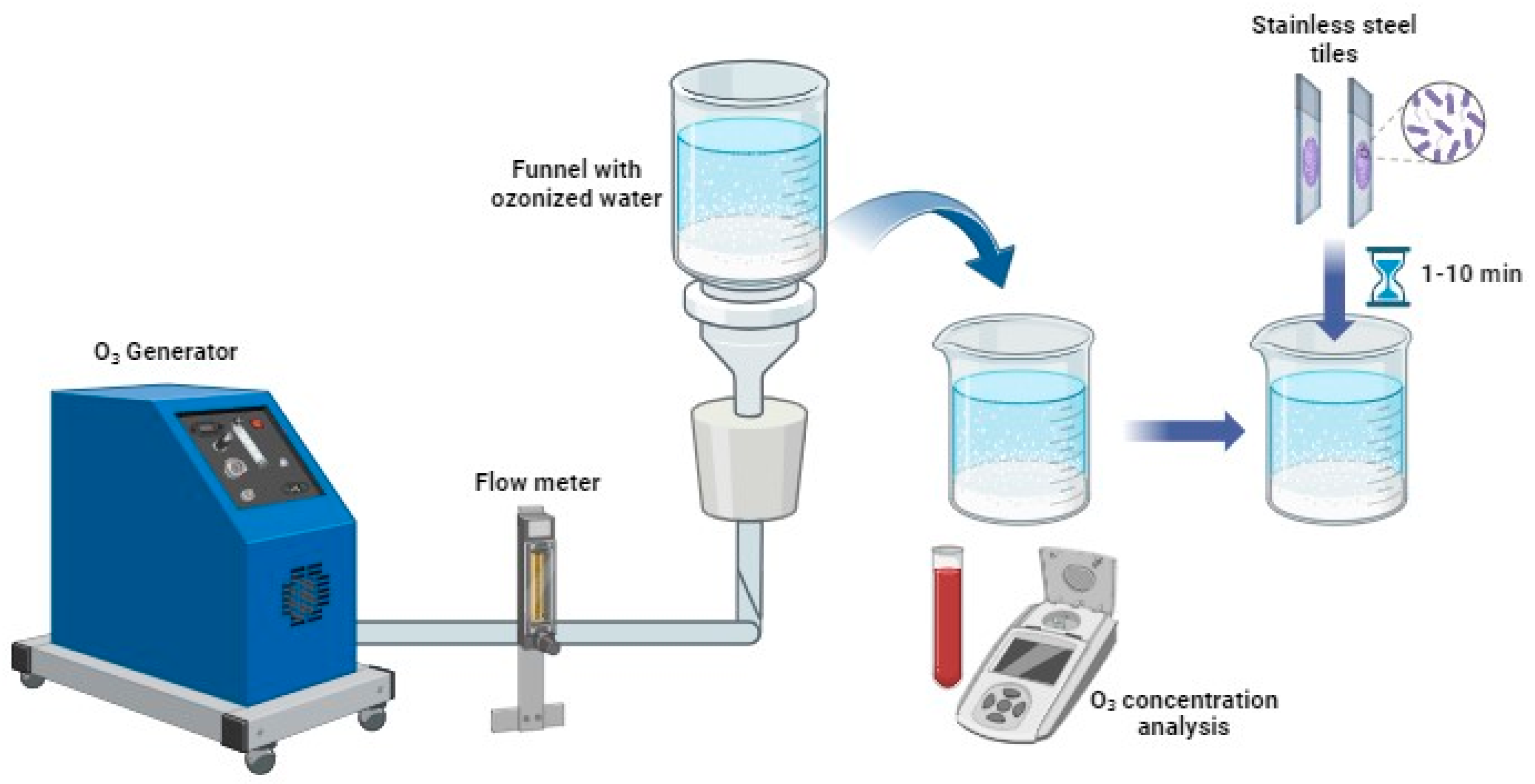
2.3. Ozonized Water Treatments
2.3.1. Bacteria
2.3.2. Filamentous Fungi
2.4. Statistical Analysis
3. Results and Discussion
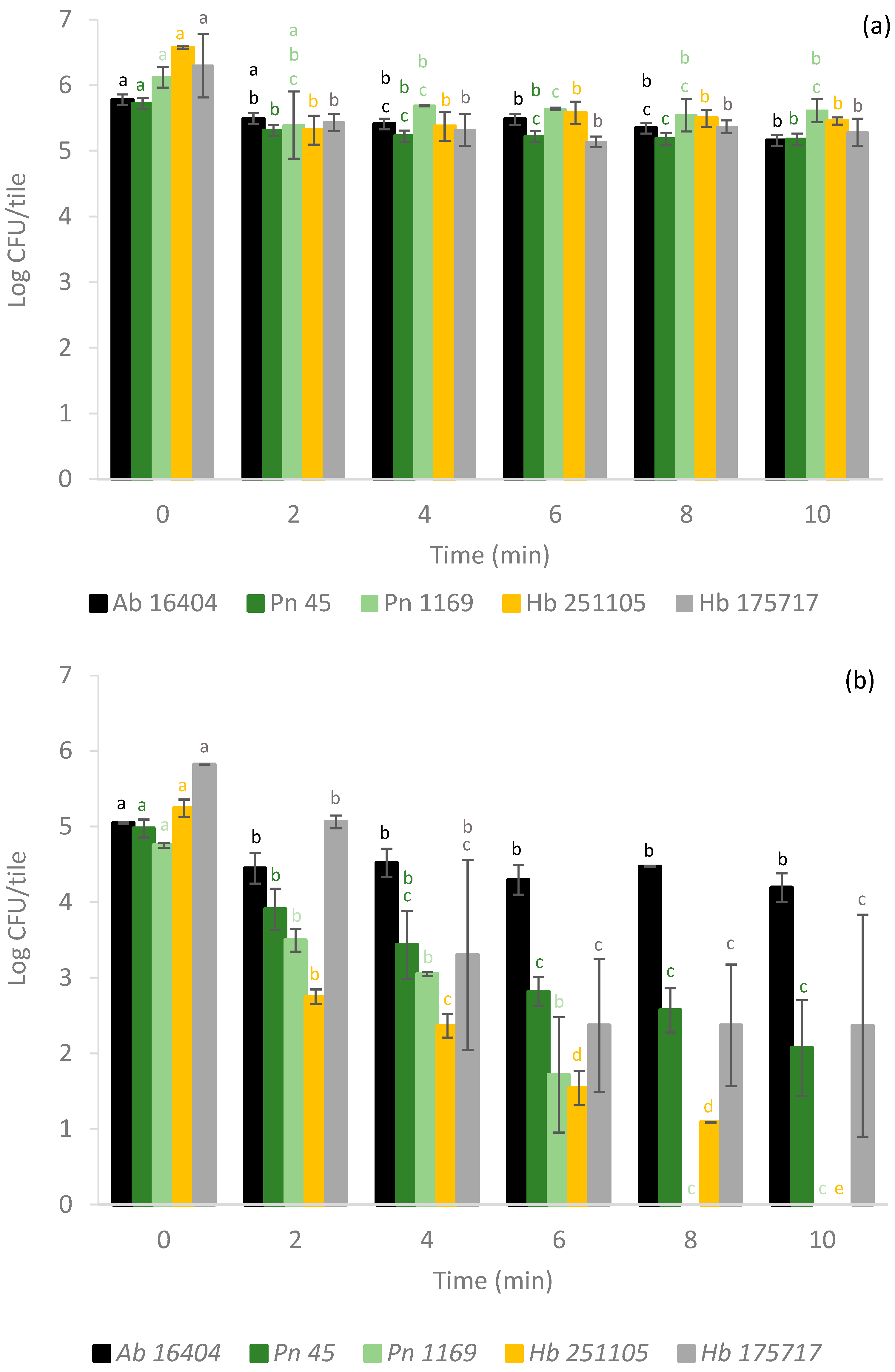
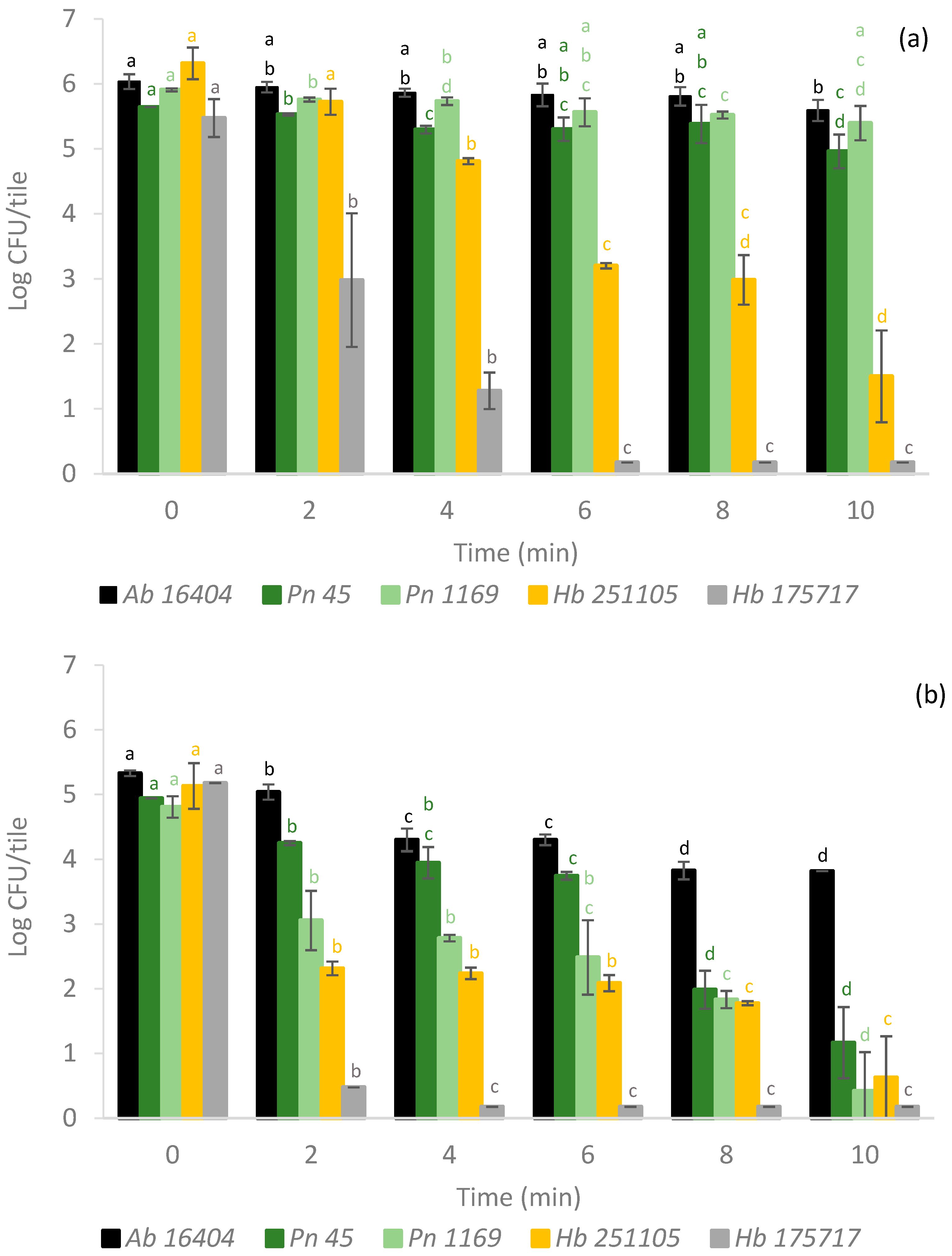
3.1. Bacteria
3.2. Filamentous Fungi
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ölmez, H.; Kretzschmar, U. Potential Alternative Disinfection Methods for Organic Fresh-Cut Industry for Minimizing Water Consumption and Environmental Impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Richardson, S.D.; Thruston, A.D., Jr.; Caughran, T.V.; Chen, P.H.; Collette, T.W.; Schenck, K.M.; Lykins, B.W., Jr.; Rav-Acha, C.; Glezer, V. Identification of New Drinking Water Disinfection by—Products from Ozone, Chlorine Dioxide, Chloramine, and Chlorine. Water Air Soil. Pollut. 2000, 123, 95–102. [Google Scholar] [CrossRef]
- Suslow, T. Ozone Applications for Postharvest Disinfection of Edible Horticultural Crops; UCANR Publications: Irvine, CA, USA, 2004. [Google Scholar] [CrossRef]
- Martinelli, M.; Giovannangeli, F.; Rotunno, S.; Trombetta, C.M.; Montomoli, E. Water and Air Ozone Treatment as an Alternative Technology. J. Prev. Med. Hyg. 2017, 58, E48. [Google Scholar] [CrossRef]
- Bryce, E.A.; Dorovoni-Zis, K.; Trudeau, D.; Sinclair, M.; Roberts, F.J. Disinfection of Hospital Laundry Using Ozone Microbiological Evaluation. Infect. Control Hosp. Epidemiol. 2000, 21, 248. [Google Scholar] [CrossRef]
- Epelle, E.I.; Emmerson, A.; Nekrasova, M.; Macfarlane, A.; Cusack, M.; Burns, A.; Mackay, W.; Yaseen, M. Microbial Inactivation: Gaseous or Aqueous Ozonation? Ind. Eng. Chem. Res. 2022, 61, 9600–9610. [Google Scholar] [CrossRef]
- Nahim-Granados, S.; Rivas-Ibáñez, G.; Antonio Sánchez Pérez, J.; Oller, I.; Malato, S.; Polo-López, M.I. Synthetic Fresh-Cut Wastewater Disinfection and Decontamination by Ozonation at Pilot Scale. Water Res. 2020, 170, 115304. [Google Scholar] [CrossRef]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254 Nm Radiation for the Removal of Microorganisms and Antibiotic Resistance Genes from Urban Wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef]
- Bialka, K.L.; Demirci, A. Decontamination of Escherichia Coli O157:H7 and Salmonella Enterica on Blueberries Using Ozone and Pulsed UV-Light. J. Food Sci. 2007, 72, M391–M396. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Margosan, D.M.; Mlikota Gabler, F. Impact of Ozonated Water on the Quality and Shelf-Life of Fresh Citrus Fruit, Stone Fruit, and Table Grapes. Ozone Sci. Eng. 2002, 24, 343–356. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Santos-Pedro, D.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of Aqueous Ozone, Blanching and Combined Treatments on Microbial Load of Red Bell Peppers, Strawberries and Watercress. J. Food Eng. 2011, 105, 277–282. [Google Scholar] [CrossRef]
- Zorlugenç, B.; Kiroǧlu Zorlugenç, F.; Öztekin, S.; Evliya, I.B. The Influence of Gaseous Ozone and Ozonated Water on Microbial Flora and Degradation of Aflatoxin B1 in Dried Figs. Food Chem. Toxicol. 2008, 46, 3593–3597. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Silva, O.; Morales-Valle, H.; Venâncio, A. Potential of Aqueous Ozone to Control Aflatoxigenic Fungi in Brazil Nuts. ISRN Biotechnol. 2013, 2013, 859830. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.; Plessas, S.; Ceciu, S.; Lazar, V.; Mantzourani, I.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Evaluation of Ozone Efficacy on the Reduction of Microbial Population of Fresh Cut Lettuce (Lactuca Sativa) and Green Bell Pepper (Capsicum Annuum). Food Control 2013, 30, 491–496. [Google Scholar] [CrossRef]
- Bhilwadikar, T.; Pounraj, S.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Decontamination of Microorganisms and Pesticides from Fresh Fruits and Vegetables: A Comprehensive Review from Common Household Processes to Modern Techniques. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1003–1038. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.A.; Marquez-Gonzalez, M.; Cabrera-Diaz, E.; Lucia, L.M.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C.; Castillo, A. Comparison of Multiple Chemical Sanitizers for Reducing Salmonella and Escherichia Coli O157:H7 on Spinach (Spinacia Oleracea) Leaves. Food Res. Int. 2012, 45, 1123–1128. [Google Scholar] [CrossRef]
- Cano, C.; Meneses, Y.; Chaves, B.D. Ozone-Based Interventions To Improve the Microbiological Safety and Quality of Poultry Carcasses and Parts: A Review. J. Food Prot. 2019, 82, 940–947. [Google Scholar] [CrossRef]
- Gorman, B.M.; Sofos, J.N.; Morgan, J.B.; Schmidt, G.R.; Smith, G.C. Evaluation of Hand-Trimming, Various Sanitizing Agents, and Hot Water Spray-Washing as Decontamination Interventions for Beef Brisket Adipose Tissue. J. Food Prot. 1995, 58, 899–907. [Google Scholar] [CrossRef]
- Watanabe, M.; Masaki, H.; Mori, T.; Tsuchiya, T.; Konuma, H.; Hara-Kudo, Y.; Takatori, K. Inactivation Effects of UV Irradiation and Ozone Treatment on the Yeast and the Mold in Mineral Water. J. Food Prot. 2010, 73, 1537–1542. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, X.; Cao, R.; Wan, Q.; Xu, H.; Wang, J.; Lin, Y.; Huang, T.; Wen, G. Synergistic Effect of Ozone and Chlorine on Inactivating Fungal Spores: Influencing Factors and Mechanisms. J. Hazard. Mater. 2021, 420, 126610. [Google Scholar] [CrossRef]
- Tadepalli, S.; Bridges, D.F.; Anderson, R.; Zhang, R.; Wu, V.C.H. Synergistic Effect of Sequential Wash Treatment with Two Different Low-Dosage Antimicrobial Washes in Combination with Frozen Storage Increases Salmonella Typhimurium and Listeria Monocytogenes Reduction on Wild Blueberries. Food Control 2019, 102, 87–93. [Google Scholar] [CrossRef]
- Britton, H.C.; Draper, M.; Talmadge, J.E. Antimicrobial Efficacy of Aqueous Ozone in Combination with Short Chain Fatty Acid Buffers. Infect. Prev. Pract. 2020, 2, 100032. [Google Scholar] [CrossRef] [PubMed]
- Crapo, C.; Himelbloom, B.; Vitt, S.; Pedersen, L. Ozone Efficacy as a Bactericide in Seafood Processing. J. Aquat. Food Prod. Technol. 2004, 13, 111–123. [Google Scholar] [CrossRef]
- Greene, A.K.; Few, B.K.; Serafini, J.C. A Comparison of Ozonation and Chlorination for the Disinfection of Stainless Steel Surfaces. J. Dairy Sci. 1993, 76, 3617–3620. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Maifreni, M.; Baggio, A.; Innocente, N. Inactivation of Foodborne Bacteria Biofilms by Aqueous and Gaseous Ozone. Front. Microbiol. 2018, 9, 406181. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Dong, Y.; Chen, X.; Xu, X.; Wang, H. Modeling the Elimination of Mature Biofilms Formed by Staphylococcus Aureus and Salmonella Spp. Using Combined Ultrasound and Disinfectants. Ultrason. Sonochem. 2020, 69, 105269. [Google Scholar] [CrossRef]
- Wirtanen, G.; Langsrud, S.; Salo, S.; Olofson, U.; Alnås, H.; Neuman, M.; Homleid, J.P.; Mattila-Sandholm, T. Evaluation of Sanitation Procedures for Use in Dairies; VTT Publications 481; Julkaisija Utgivare Publisher: Helsinki, Finland, 2002. [Google Scholar]
- Khadre, M.A.; Yousef, A.E. Decontamination of a multilaminated aseptic food packaging material and stainless steel by ozone. J. Food Saf. 2001, 21, 1–13. [Google Scholar] [CrossRef]
- Botti, L.C.M.; Bócoli, P.F.J.; Leber, A.S.M.L.; Parada; Tribst, A.A.L.; Cristianini, M. Sanitation of Plastic Bottles Using Ozonated Water. Int. Food Res. J. 2014, 21, 1375–1379. [Google Scholar]
- Korany, A.M.; Hua, Z.; Green, T.; Hanrahan, I.; El-Shinawy, S.H.; El-Kholy, A.; Hassan, G.; Zhu, M.J. Efficacy of Ozonated Water, Chlorine, Chlorine Dioxide, Quaternary Ammonium Compounds and Peroxyacetic Acid against Listeria Monocytogenesbiofilm on Polystyrene Surfaces. Front. Microbiol. 2018, 9, 411379. [Google Scholar] [CrossRef]
- Cabo, M.L.; Herrera, J.J.; Crespo, M.D.; Pastoriza, L. Comparison among the Effectiveness of Ozone, Nisin and Benzalkonium Chloride for the Elimination of Planktonic Cells and Biofilms of Staphylococcus Aureus CECT4459 on Polypropylene. Food Control 2009, 20, 521–525. [Google Scholar] [CrossRef]
- Naitou, S.; Takahara, H. Recent Developments in Food and Agricultural Uses of Ozone as an Antimicrobial Agent-Food Packaging Film Sterilizing Machine Using Ozone. Ozone Sci. Eng. 2008, 30, 81–87. [Google Scholar] [CrossRef]
- Shelobolina, E.S.; Walker, D.K.; Parker, A.E.; Lust, D.V.; Schultz, J.M.; Dickerman, G.E. Inactivation of Pseudomonas Aeruginosa Biofilms Formed under High Shear Stress on Various Hydrophilic and Hydrophobic Surfaces by a Continuous Flow of Ozonated Water. Biofouling 2018, 34, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Belloli, M.; Cigarini, M.; Milesi, G.; Mutti, P.; Berni, E. Effectiveness of Two UV-C Light-Emitting Diodes (LED) Systems in Inactivating Fungal Conidia on Polyethylene Terephthalate. Innov. Food Sci. Emerg. Technol. 2022, 79, 103050. [Google Scholar] [CrossRef]
- Berni, E.; Belloli, M.; Cigarini, M.; Brindani, D.; Cardoso, C.C.; Mutti, P.; Imperiale, D. Sanitizing of Stainless Steel Surfaces in the Food Industry: Effect of Gaseous Ozone against Pathogens and Filamentous Fungi. J. Food Saf. 2024, 44, e13106. [Google Scholar] [CrossRef]
- EN 13697:2015+A1; Chemical Disinfectants and Antiseptics—Quantitative Nonporous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity of Chemical Disinfectants Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements without Mechanical Action (Phase 2, Step 2). UNI—Ente Nazionale di Normazione: Milan, Italy, 2019.
- EN 1650; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Fungicidal or Yeasticidal Activity of Chemical Disinfectants and Antiseptics Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements (Phase 2, Step 1). UNI—Ente Nazionale di Normazione: Milan, Italy, 2019.
- EN 17272; Chemical Disinfectants and Antiseptics—Methods of Airborne Room Disinfection by Automated Process—Determination of Bactericidal, Mycobactericidal, Sporicidal, Fungicidal, Yeasticidal, Virucidal and Phagocidal Activities. UNI—Ente Nazionale di Normazione: Milan, Italy, 2020.
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a Freeware Tool to Assess Non-Log-Linear Microbial Survivor Curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef]
- John, D.; Ramaswamy, H.S. Comparison of Pulsed Light Inactivation Kinetics and Modeling of Escherichia Coli (ATCC-29055), Clostridium Sporogenes (ATCC-7955) and Geobacillus Stearothermophilus (ATCC-10149). Curr. Res. Food Sci. 2020, 3, 82–91. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On Calculating Sterility in Thermal Preservation Methods: Application of the Weibull Frequency Distribution Model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural Model Requirements to Describe Microbial Inactivation during a Mild Heat Treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Coroller, L.; Leguerinel, I.; Mettler, E.; Savy, N.; Mafart, P. General Model, Based on Two Mixed Weibull Distributions of Bacterial Resistance, for Describing Various Shapes of Inactivation Curves. Appl. Environ. Microbiol. 2006, 72, 6493–6502. [Google Scholar] [CrossRef]
- Bigelow, W.D.; Esty, J.R. The Thermal Death Point in Relation to Time of Typical Thermophilic Organisms. J. Infect. Dis. 1920, 27, 602–617. [Google Scholar] [CrossRef]
- Cerf, O.; METRO, F. Tailing of Survival Curves of Bacillus Licheniformis Spores Treated with Hydrogen Peroxide. J. Appl. Bacteriol. 1977, 42, 405–415. [Google Scholar] [CrossRef]
- Racchi, I.; Scaramuzza, N.; Hidalgo, A.; Cigarini, M.; Berni, E. Sterilization of Food Packaging by UV-C Irradiation: Is Aspergillus Brasiliensis ATCC 16404 the Best Target Microorganism for Industrial Bio-Validations? Int. J. Food Microbiol. 2021, 357, 109383. [Google Scholar] [CrossRef] [PubMed]
- Baysal, A.H.; Molva, C.; Unluturk, S. UV-C Light Inactivation and Modeling Kinetics of Alicyclobacillus Acidoterrestris Spores in White Grape and Apple Juices. Int. J. Food Microbiol. 2013, 166, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation Credit of UV Radiation for Viruses, Bacteria and Protozoan (Oo)Cysts in Water: A Review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Oppezzo, O.J.; Abrevaya, X.C.; Giacobone, A.F.F. An Alternative Interpretation for Tailing in Survival Curves for Bacteria Exposed to Germicidal Radiation. Photochem. Photobiol. 2024, 100, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Fisher, C.W.; Moltz, A.G.; Martin, S.E. Elimination of Listeria Monocytogenes Biofilms by Ozone, Chlorine, and Hydrogen Peroxide. J. Food Prot. 2005, 68, 494–498. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.; van Veluw, G.J.; Wang, F.; Müller, W.H.; Dijksterhuis, J.; Wösten, H.A.B. Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef]
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.; Lisboa, H.F.; Gonçalves, R.C. Production of Melanin Pigment by Fungi and Its Biotechnological Applications. Melanin 2017, 4, 47–75. [Google Scholar] [CrossRef]
- De Obeso Fernandez Del Valle, A.; Scheckhuber, C.Q. Superoxide Dismutases in Eukaryotic Microorganisms: Four Case Studies. Antioxidants 2022, 11, 188. [Google Scholar] [CrossRef]
- Fisher, C.W.; Lee, D.; Dodge, B.A.; Hamman, K.M.; Robbins, J.B.; Martin, S.E. Influence of Catalase and Superoxide Dismutase on Ozone Inactivation of Listeria Monocytogenes. Appl. Environ. Microbiol. 2000, 66, 1405–1409. [Google Scholar] [CrossRef]
- Hageskal, G.; Tryland, I.; Liltved, H.; Skaar, I. No Simple Solution to Waterborne Fungi: Various Responses to Water Disinfection Methods. Water Supply 2012, 12, 220–226. [Google Scholar] [CrossRef]
- Platforma Medyczna, P.; Dariusz Jerzy, B.; Ewa, B.; Bożena, B.; Magdalena, C.; Beata, S.-L.; Stefan, T.; Białoszewski, D.; Bocian, E.; Bukowska, B.; et al. Antimicrobial Activity of Ozonated Water. Med. Sci. Monit 2010, 16, 71–75. [Google Scholar]
- Restaino, L.; Frampton, E.W.; Hemphill, J.B.; Palnikar, P. Efficacy of Ozonated Water against Various Food-Related Microorganisms. Appl. Environ. Microbiol. 1995, 61, 3471–3475. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Chmielewski, R.; Keswani, J.; Law, S.E.; Frank, J.F. Inactivation of Aflatoxigenic Aspergilli by Treatment with Ozone. Lett. Appl. Microbiol. 1999, 29, 202–205. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Tiwari, B.K.; Cullen, P.J.; Rice, R.G. Ozone in Food Processing; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]

| Ozone Concentration (mg L−1) | Strain | Inactivation Curve | Inactivation Model | |||
|---|---|---|---|---|---|---|
| Parameter | RMSE (-) | R2 (-) | 1D-Value (min) | |||
| 1.0 | Ls | δ | 0.1404 | 0.97 | 5.20 | [41] |
| 3.0 | Kmax | 0.2867 | 0.96 | 0.44 | [42] | |
| 6.0 | Kmax | 0.2604 | 0.99 | 0.17 | [42] | |
| 1.0 | Lm | δ | 0.1910 | 0.98 | 0.81 | [41] |
| 3.0 | Kmax | 0.0520 | 1.00 | 0.27 | [42] | |
| 6.0 | Kmax | 0.1735 | 1.00 | 0.10 | [42] | |
| 1.0 | Se | δ | 0.2390 | 0.97 | 0.82 | [41] |
| 3.0 | δ | 0.1701 | 0.99 | 0.26 | [41] | |
| 6.0 | Kmax | 0.2217 | 0.99 | 0.22 | [42] | |
| 1.0 | St | δ | 0.0300 | 1.00 | 7.27 | [41] |
| 3.0 | δ | 0.1241 | 0.98 | 1.70 | [41] | |
| 6.0 | δ | 0.0926 | 0.99 | 1.68 | [41] | |
| Inoculum | Strain | Inactivation Curve | Inactivation Model | |||
|---|---|---|---|---|---|---|
| Parameter | RMSE (-) | R2 (-) | 1D-Value (min) | |||
| multi-layer | Ab | nd | nd | nd | nd | - |
| Hb1 | nd | nd | nd | nd | - | |
| Hb2 | nd | nd | nd | nd | - | |
| Pn1 | nd | nd | nd | nd | - | |
| Pn2 | nd | nd | nd | nd | - | |
| single-layer | Ab | nd | nd | nd | nd | - |
| Hb1 | Kmax | 1.1924 | 0.54 | 1.47 | [42] | |
| Hb2 | δ | 0.3046 | 0.98 | 0.52 | [43] | |
| Pn1 | Kmax | 0.4085 | 0.95 | 1.77 | [44] | |
| Pn2 | Kmax | 0.3567 | 0.89 | 3.66 | [44] | |
| Inoculum | Strain | Inactivation Curve | Inactivation Model | |||
|---|---|---|---|---|---|---|
| Parameter | RMSE (-) | R2 (-) | 1D-Value (min) | |||
| multi-layer | Ab | nd | nd | nd | nd | - |
| Hb1 | δ | 0.5613 | 0.96 | 0.48 | [43] | |
| Hb2 | δ | 0.3724 | 0.97 | 3.86 | [43] | |
| Pn1 | nd | nd | nd | nd | - | |
| Pn2 | nd | nd | nd | nd | - | |
| single-layer | Ab | δ | 0.1842 | 0.92 | 5.00 | [41] |
| Hb1 | Kmax | 0.0949 | 1.00 | 0.18 | [45] | |
| Hb2 | δ | 0.4658 | 0.91 | 0.09 | [41] | |
| Pn1 | δ | 0.5224 | 0.89 | 1.62 | [41] | |
| Pn2 | δ | 0.3875 | 0.94 | 4.89 | [41] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berni, E.; Moroni, C.; Cigarini, M.; Brindani, D.; Catelani Cardoso, C.; Imperiale, D. Effect of Ozonized Water against Pathogenic Bacteria and Filamentous Fungi on Stainless Steel. Appl. Sci. 2024, 14, 8392. https://doi.org/10.3390/app14188392
Berni E, Moroni C, Cigarini M, Brindani D, Catelani Cardoso C, Imperiale D. Effect of Ozonized Water against Pathogenic Bacteria and Filamentous Fungi on Stainless Steel. Applied Sciences. 2024; 14(18):8392. https://doi.org/10.3390/app14188392
Chicago/Turabian StyleBerni, Elettra, Chiara Moroni, Massimo Cigarini, Demetrio Brindani, Claudia Catelani Cardoso, and Davide Imperiale. 2024. "Effect of Ozonized Water against Pathogenic Bacteria and Filamentous Fungi on Stainless Steel" Applied Sciences 14, no. 18: 8392. https://doi.org/10.3390/app14188392








