Natural Photosensitizers in Clinical Trials
Abstract
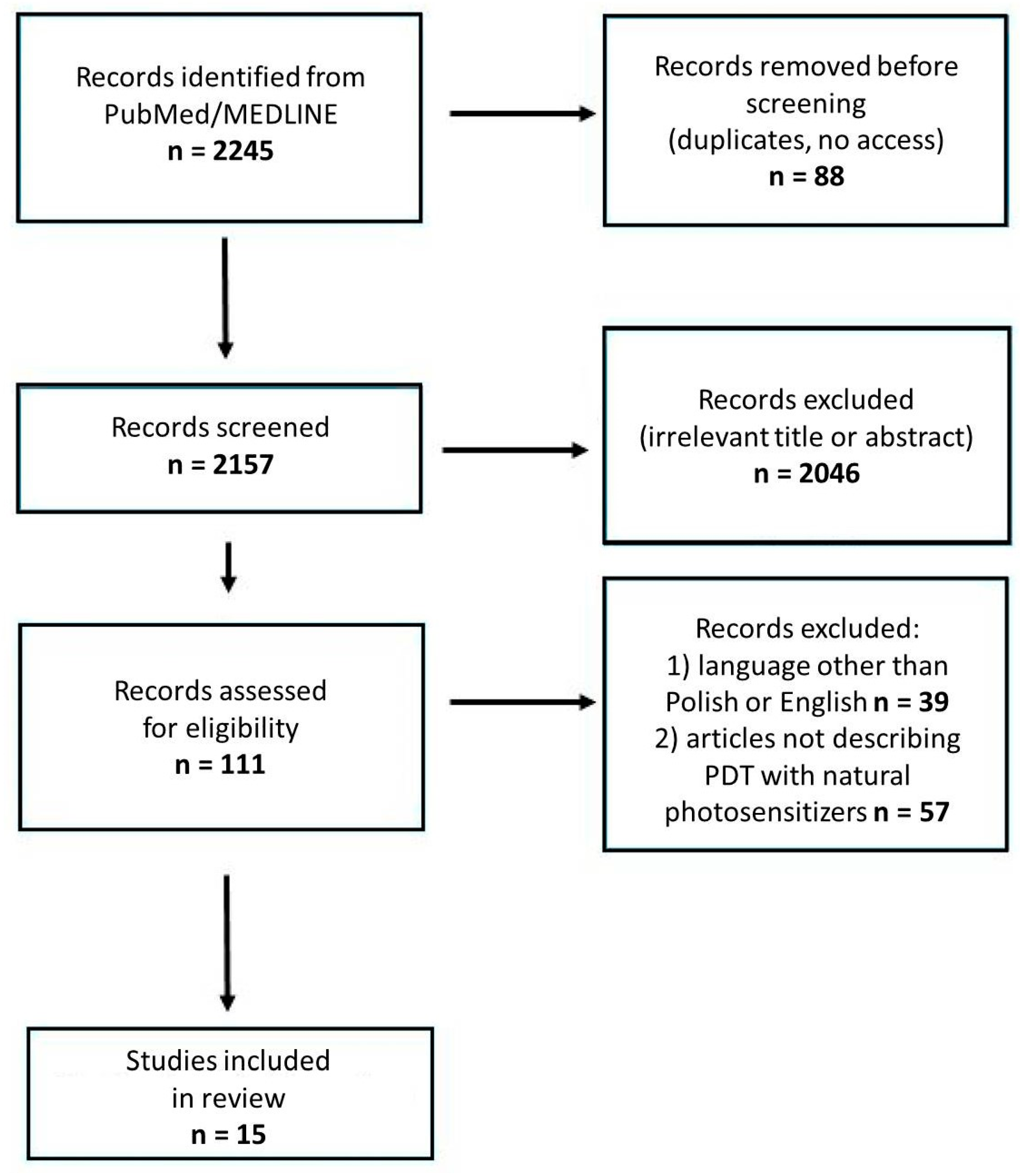
1. Introduction
2. Methods
3. Natural Photosensitizers in Clinical Trials
3.1. Curcumine
Curcumin in Clinical Trials of Photodynamic Therapy
3.2. Hypericin
Hypericin in Clinical Trials of Photodynamic Therapy
3.3. Riboflavin
Riboflavin in Clinical Trials of Photodynamic Therapy
3.4. Phycocyanin
Phycocyanin in Clinical Trials of Photodynamic Therapy
3.5. Anthraquinones
Anthraquinones in Clinical Trials of Photodynamic Therapy
4. Natural Photosensitizers Pending Clinical Trials
4.1. Furanocoumarins
4.2. Pheophorbide a
4.3. Alkaloids
4.4. Chlorophyllin
4.5. Hypocrellin
4.6. Cercosporin
4.7. Toliporphins
5. Reported Toxicity and Adverse Side Effects of Natural Photosensitizers
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Oscar, R. Uber die wirkung fluoreszierender stoffe auf infusorien. Zeitschr. Biol. 1900, 39, 524–546. [Google Scholar]
- Cengel, K.A.; Simone, C.B., 2nd; Glatstein, E. PDT: What’s Past Is Prologue. Cancer Res. 2016, 76, 2497–2499. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The use of photodynamic therapy in medical practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Photodynamic Therapy: Critical PDT Theory. Photochem. Photobiol. 2023, 99, 199–203. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Naurecka, M.L.; Sierakowski, B.M.; Kwaśny, M. Spectroscopic properties of second generation photosensitizers for photo-diagnostics and photo-dynamic therapy. In Laser Technology 2016: Progress and Applications of Lasers; SPIE: Bellingham, WA, USA, 2016; Volume 10159. [Google Scholar]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Woźnicki, P.; Dynarowicz, K.; Aebisher, D. Photosensitizers for Photodynamic Therapy of Brain Cancers-A Review. Brain Sci. 2023, 13, 1299. [Google Scholar] [CrossRef]
- Meerovich, G.A.; Akhlyustina, E.V.; Tiganova, I.G.; Lukyanets, E.A.; Makarova, E.A.; Tolordava, E.R.; Yuzhakova, O.A.; Romanishkin, I.D.; Philipova, N.I.; Zhizhimova, Y.S.; et al. Novel Polycationic Photosensitizers for Antibacterial Photodynamic Therapy. Adv. Exp. Med. Biol. 2020, 1282, 1–19. [Google Scholar] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Chandran, R.; Abrahamse, H. Photodynamic therapy of cervical cancer by eradication of cervical cancer cells and cervical cancer stem cells. Oncotarget 2019, 10, 4380–4396. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H. New Generation of Photosensitizers Based on Inorganic Nanomaterials. Methods Mol. Biol. 2022, 2451, 213–244. [Google Scholar] [PubMed]
- Fu, P.P.; Chiang, H.M.; Xia, Q.; Chen, T.; Chen, B.H.; Yin, J.J.; Wen, K.C.; Lin, G.; Yu, H. Quality assurance and safety of herbal dietary supplements. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 91–119. [Google Scholar] [CrossRef]
- Xiao, Q.; Wu, J.; Pang, X.; Jiang, Y.; Wang, P.; Leung, A.W.; Gao, L.; Jiang, S.; Xu, C. Discovery and Development of Natural Products and their Derivatives as Photosensitizers for Photodynamic Therapy. Curr. Med. Chem. 2018, 25, 839–860. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-activated green drugs: How we can use them in photodynamic therapy and mass-produce them with biotechnological tools. Phytomed. Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Li, S.; Meng, X.; Peng, B.; Huang, J.; Liu, J.; Xiao, H.; Ma, L.; Liu, Y.; Tang, J. Cell membrane-based biomimetic technology for cancer phototherapy: Mechanisms, recent advances and perspectives. Acta Biomater. 2024, 174, 26–48. [Google Scholar] [CrossRef]
- Pashootan, P.; Saadati, F.; Fahimi, H.; Rahmati, M.; Strippoli, R.; Zarrabi, A.; Cordani, M.; Moosavi, M.A. Metal-based nanoparticles in cancer therapy: Exploring photodynamic therapy and its interplay with regulated cell death pathways. Int. J. Pharm. 2024, 649, 123622. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Zhou, X.; Ying, X.; Wu, L.; Liu, L.; Wang, Y.; He, Y.; Han, M. Research Progress of Natural Product Photosensitizers in Photodynamic Therapy. Planta Med. 2024, 90, 368–379. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Wolnicka-Glubisz, A.; Wisniewska-Becker, A. Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective. Antioxidants 2023, 12, 1725. [Google Scholar] [CrossRef]
- Law, S.K.; Leung, A.W.N.; Xu, C. Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2-A Review. Pharmaceuticals 2023, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Schöne, L.; Ayoub, A.M.; Librizzi, D.; Amin, M.U.; Engelhardt, K.; Yousefi, B.H.; Bender, L.; Schaefer, J.; Preis, E.; et al. Modern Photodynamic Glioblastoma Therapy Using Curcumin- or Parietin-Loaded Lipid Nanoparticles in a CAM Model Study. ACS Appl. Bio Mater. 2023, 6, 5502–5514. [Google Scholar] [CrossRef] [PubMed]
- Roschenko, V.; Ayoub, A.M.; Engelhardt, K.; Schäfer, J.; Amin, M.U.; Preis, E.; Mandic, R.; Bakowsky, U. Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas. Pharmaceutics 2023, 15, 2412. [Google Scholar] [CrossRef] [PubMed]
- Labban, N.; Taweel, S.M.A.; ALRabiah, M.A.; Alfouzan, A.F.; Alshiddi, I.F.; Assery, M.K. Efficacy of Rose Bengal and Curcumin mediated photodynamic therapy for the treatment of denture stomatitis in patients with habitual cigarette smoking: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2021, 35, 102380. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.P.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef]
- Ricci Donato, H.A.; Pratavieira, S.; Grecco, C.; Brugnera-Júnior, A.; Bagnato, V.S.; Kurachi, C. Clinical Comparison of Two Photosensitizers for Oral Cavity Decontamination. Photomed. Laser Surg. 2017, 35, 105–110. [Google Scholar] [CrossRef]
- Panhóca, V.H.; Esteban Florez, F.L.; Corrêa, T.Q.; Paolillo, F.R.; de Souza, C.W.; Bagnato, V.S. Oral Decontamination of Orthodontic Patients Using Photodynamic Therapy Mediated by Blue-Light Irradiation and Curcumin Associated with Sodium Dodecyl Sulfate. Photomed. Laser Surg. 2016, 34, 411–417. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Bonadiman, A.C.; Borges Lemos, A.L.D.A.; Annicchino, B.M.; Segatti, B.; Pucca, D.S.; Dutra, P.T.; de Carvalho ESilva, R.M.; Leal, F. Photobiomodulation Therapy in Cancer Patients with Mucositis: A Clinical Evaluation. Photobiomodul. Photomed. Laser Surg. 2019, 37, 142–150. [Google Scholar] [CrossRef]
- Saini, M.; Barakat, A.; Qamar, Z.; Shenoy, M.; Alotaibi, R.J.; Alotaibi, A.M.; Noushad, M.; Niazi, F. Use of photosensitizers activated by photodynamic therapy on the canal disinfection of radicular dentin bonded to Dimethacrylate-based glass fiber post: An assessment of pushout bond strength. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7850–7857. [Google Scholar]
- Zhang, Y.; Wang, D.; Liao, C.; Liu, X.; Zhang, L.; Wang, P.; Wang, X. Curcumin-mediated photodynamic therapy for mild to moderate Acne: A self-controlled split-face randomized study. Photodiagn. Photodyn. Ther. 2024, 45, 103887. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Zhang, Z.; Fu, J.; You, L.; He, Y.; Hao, Y.; Gu, Z.; Yu, Z.; Qu, C.; et al. Hypericin-mediated photodynamic therapy for the treatment of cancer: A review. J. Pharm. Pharmacol. 2021, 73, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Kim, S.Y. Hypericin, a Naphthodianthrone Derivative, Prevents Methylglyoxal-Induced Human Endothelial Cell Dysfunction. Biomol. Ther. 2017, 25, 158–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Domka, W.; Bartusik-Aebisher, D.; Rudy, I.; Dynarowicz, K.; Pięta, K.; Aebisher, D. Photodynamic therapy in brain cancer: Mechanisms, clinical and preclinical studies and therapeutic challenges. Front. Chem. 2023, 11, 1250621. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Song, J.; Tang, Z.; Wei, S.; Chen, L.; Zhou, R. Hypericin-mediated photodynamic therapy inhibits growth of colorectal cancer cells via inducing S phase cell cycle arrest and apoptosis. Eur. J. Pharmacol. 2021, 900, 174071. [Google Scholar] [CrossRef] [PubMed]
- Huntosova, V.; Stroffekova, K. Hypericin in the Dark: Foe or Ally in Photodynamic Therapy? Cancers 2016, 8, 93. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef]
- Krammer, B.; Verwanger, T. Molecular response to hypericin-induced photodamage. Curr. Med. Chem. 2012, 19, 793–798. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.W.; Seok, K.H.; Hwang, C.W.; Ahn, J.C.; Jin, J.O.; Kang, H.W. Hypericin-assisted photodynamic therapy against anaplastic thyroid cancer. Photodiagn. Photodyn. Ther. 2018, 24, 15–21. [Google Scholar] [CrossRef]
- Stupáková, V.; Varinská, L.; Mirossay, A.; Sarisský, M.; Mojzis, J.; Dankovcík, R.; Urdzík, P.; Ostró, A.; Mirossay, L. Photodynamic effect of hypericin in primary cultures of human umbilical endothelial cells and glioma cell lines. Phytother. Res. 2009, 23, 827–832. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Yang, S.; Wang, C.; Fu, X.; Wang, J.; Mao, Y.; Zhang, J.; Li, Y. Cellular and molecular mechanisms of photodynamic hypericin therapy for nasopharyngeal carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 847–853. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Cheng, W.; Wang, Y.; Yi, K.; Wang, Z.; Zhang, Y.; Shao, L.; Zhao, T. Hypericin-photodynamic therapy inhibits the growth of adult T-cell leukemia cells through induction of apoptosis and suppression of viral transcription. Retrovirology 2019, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, D.; Zhuang, Z.; Jin, K.; Zheng, L.; Yang, Q.; Guo, K. Hypericin-mediated photodynamic therapy induces apoptosis in K562 human leukemia cells through JNK pathway modulation. Mol. Med. Rep. 2015, 12, 6475–6482. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Mangold, A.R.; DeSimone, J.A.; Wong, H.K.; Seminario-Vidal, L.; Guitart, J.; Appel, J.; Geskin, L.; Lain, E.; Korman, N.J.; et al. Efficacy and Safety of Topical Hypericin Photodynamic Therapy for Early-Stage Cutaneous T-Cell Lymphoma (Mycosis fungoides): The FLASH Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 1031–1039. [Google Scholar] [CrossRef]
- Kacerovská, D.; Pizinger, K.; Majer, F.; Smíd, F. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract--a pilot study. Photochem. Photobiol. 2008, 84, 779–785. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, M.; Na, J.I.; Huh, C.H.; Shin, J.W. A Randomized Split-Face Study of Photodynamic Therapy With St. John’s Wort and Indole-3-Acetic Acid for the Treatment of Acne. Dermatol. Surg. 2023, 49, 483–488. [Google Scholar] [CrossRef]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef]
- Hassan, I.; Chibber, S.; Naseem, I. Vitamin B2: A promising adjuvant in cisplatin based chemoradiotherapy by cellular redox management. Food Chem. Toxicol. 2013, 59, 715–723. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. [Google Scholar] [CrossRef]
- Rivas Aiello, M.B.; Castrogiovanni, D.; Parisi, J.; Azcárate, J.C.; García Einschlag, F.S.; Gensch, T.; Bosio, G.N.; Mártire, D.O. Photodynamic Therapy in HeLa Cells Incubated with Riboflavin and Pectin-coated Silver Nanoparticles. Photochem. Photobiol. 2018, 94, 1159–1166. [Google Scholar] [CrossRef]
- Makdoumi, K.; Hedin, M.; Bäckman, A. Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes in vitro. Lasers Med. Sci. 2019, 34, 1799–1805. [Google Scholar] [CrossRef]
- Alqerban, A. Effectiveness of Riboflavin and Rose Bengal Photosensitizer Modified Adhesive Resin for Orthodontic Bonding. Pharmaceuticals 2021, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Al Deeb, L.; Almohareb, T.; Al Ahdal, K.; Maawadh, A.; Alshamrani, A.S.; Alrahlah, A. The impact of PEEK pretreatment using H2SO4, riboflavin, and aluminum trioxide on the extrusion bond strength to canal dentin luted with Polymethyl methacrylate and resin-based composite cement. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9639–9647. [Google Scholar]
- Alkhudhairy, F.; Aljamhan, A.S. Surface conditioning of PEEK post using Nd: YVO4 laser, photodynamic therapy, and sulfuric acid on the pushout bond strength to canal dentin. Photodiagn. Photodyn. Ther. 2023, 42, 103601. [Google Scholar] [CrossRef]
- AlGhamdi, A.S.; Alsalhi, H.; Almutairi, N.; Alotaibi, B.; Barakat, A.A.; Khanam, H.K.; ElGendy, F.; Alawfi, A.A. Push out bond strength of fiber post to radicular dentin using Q-mix, lemon/garlic extract, and riboflavin activated by photodynamic therapy as a final canal irrigant. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3793–3798. [Google Scholar]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. Nutr. 2017, 57, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, X.; Wang, Y.; Zhou, T.; Bai, Y.; Li, Y.C.; Huang, B. Photosensitization of phycocyanin extracted from Microcystis in human hepatocellular carcinoma cells: Implication of mitochondria-dependent apoptosis. J. Photochem. Photobiol. 2012, 117, 70–79. [Google Scholar] [CrossRef]
- Bannu, S.M.; Lomada, D.; Gulla, S.; Chandrasekhar, T.; Reddanna, P.; Reddy, M.C. Potential Therapeutic Applications of C-Phycocyanin. Curr. Drug Metab. 2019, 20, 967–976. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Manivasagan, P.; Santha Moorthy, M.; Bui, N.Q.; Jang, B.; Phan, T.T.V.; Jung, W.K.; Kim, Y.M.; Lee, K.D.; Oh, J. Photo-based PDT/PTT dual model killing and imaging of cancer cells using phycocyanin-polypyrrole nanoparticles. Eur. J. Pharm. Biopharm. 2018, 123, 20–30. [Google Scholar] [CrossRef]
- Liang, H.; Sun, Y.; Li, C.; Lin, H.; Huang, Q.; Li, C. Facile synthesis of phycocyanin/polydopamine hierarchical nanocomposites for synergizing PTT/PDT against cancer. RSC Adv. 2022, 12, 34815–34821. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, X.; Huang, W.; Li, C.; Wang, X.; Huang, B. Photodynamic effect and mechanism study of selenium-enriched phycocyanin from Spirulina platensis against liver tumours. J. Photochem. Photobiol. B 2018, 180, 89–97. [Google Scholar] [CrossRef]
- Memon, J.; Shabbir, T.; Ishrat, M.; Aslam, H.; Khowaja, A.A.; Leemani, M.J. Caries affected disinfection using Phycocyanin activated by PDT, Holy Basil, and Ti-sapphire laser on adhesive bond strength, microleakage, and bond failure. Photodiagn. Photodyn. Ther. 2023, 43, 103691. [Google Scholar] [CrossRef] [PubMed]
- El Mourad, A.M.; Al-Shamrani, A.S. Phycocyanin-loaded silver nanoparticles activated with photodynamic therapy and Nd: YAG laser for caries-affected dentin disinfection: Impact on Streptococcus mutans survival rate and shear bond strength to the tooth-colored restorative material. Photodiagn. Photodyn. Ther. 2024, 47, 104108. [Google Scholar] [CrossRef]
- Hashemikamangar, S.S.; Alsaedi, R.J.F.; Chiniforush, N.; Motevaselian, F. Effect of antimicrobial photodynamic therapy with different photosensitizers and adhesion protocol on the bond strength of resin composite to sound dentin. Clin. Oral Investig. 2022, 26, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Perlak, M.; Ziółkowski, P.; Woźniak, M. A promising natural anthraquinones mediated by photodynamic therapy for anti-cancer therapy. Phytomedicine 2023, 119, 155035. [Google Scholar] [CrossRef]
- Comini, L.R.; Fernandez, I.M.; Rumie Vittar, N.B.; Núñez Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Photodynamic activity of anthraquinones isolated from Heterophyllaea pustulata Hook f. (Rubiaceae) on MCF-7c3 breast cancer cells. Phytomedicine 2011, 18, 1093–1095. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in anticancer therapy—For and against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Hübinger, L.; Runge, R.; Rosenberg, T.; Freudenberg, R.; Kotzerke, J.; Brogsitter, C. Psoralen as a Photosensitizers for Photodynamic Therapy by Means of In Vitro Cherenkov Light. Int. J. Mol. Sci. 2022, 23, 15233. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Emodin—A natural anthraquinone derivative with diverse pharmacological activities. Phytochemistry 2021, 190, 112854. [Google Scholar] [CrossRef]
- Nowak-Perlak, M.; Bromke, M.A.; Ziółkowski, P.; Woźniak, M. The Comparison of the Efficiency of Emodin and Aloe-Emodin in Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 6276. [Google Scholar] [CrossRef]
- Yaghobee, S.; Pourhajibagher, M.; Bahrami, R.; Isaabadi, M. Nano-emodin mediated photodynamic therapy for wound healing of donor site after free gingival graft: A parallel clinical trial. Photodiagn. Photodyn. Ther. 2024, 45, 103958. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef] [PubMed]
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Caffieri, S.; Di Lisa, F.; Bolesani, F.; Facco, M.; Semenzato, G.; Dall’Acqua, F.; Canton, M. The mitochondrial effects of novel apoptogenic molecules generated by psoralen photolysis as a crucial mechanism in PUVA therapy. Blood 2007, 109, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Caffieri, S. Furocoumarin photolysis: Chemical and biological aspects. Photochem. Photobiol. Sci. 2002, 1, 149–157. [Google Scholar] [CrossRef]
- Akita, Y.; Watanabe, D.; Yanagishita, T.; Kuhara, T.; Kawamura, C.; Masuda, Y.; Kawada, M.; Nakaseko, H.; Tamada, Y.; Matsumoto, Y. The effect of psoralen plus ultraviolet A in vitro in HUT-78 enhances by 5-aminolevulinic acid. Photodermatol. Photoimmunol. Photomed. 2007, 23, 95–97. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.K.; Park, I.K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Ping, Q.; Yeo, Y. Quantitative Assessment of Nanoparticle Biodistribution by Fluorescence Imaging, Revisited. ACS Nano 2018, 12, 6458–6468. [Google Scholar] [CrossRef]
- Xodo, L.E.; Rapozzi, V.; Zacchigna, M.; Drioli, S.; Zorzet, S. The chlorophyll catabolite pheophorbide a as a photosensitizer for the photodynamic therapy. Curr. Med. Chem. 2012, 19, 799–807. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Liu, L.Y.; Man, X.X.; Yao, H.X.; Tan, Y.Y. Effects of pheophorbide a-mediated photodynamic therapy on proliferation and metastasis of human prostate cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5571–5579. [Google Scholar] [PubMed]
- Szafraniec, M.J.; Toporkiewicz, M.; Gamian, A. Zinc-Substituted Pheophorbide A Is a Safe and Efficient Antivascular Photodynamic Agent. Pharmaceuticals 2022, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Liu, X.Z.; Zhang, D.M.; Fong, W.P.; Fung, K.P. Pheophorbide a based photodynamic therapy induces apoptosis via mitochondrial-mediated pathway in human uterine carcinosarcoma. Cancer Biol. Ther. 2009, 8, 533–539. [Google Scholar] [CrossRef]
- Cho, M.; Park, G.M.; Kim, S.N.; Amna, T.; Lee, S.; Shin, W.S. Glioblastoma-specific anticancer activity of pheophorbide a from the edible red seaweed Grateloupia elliptica. J. Microbiol. Biotechnol. 2014, 24, 346–353. [Google Scholar] [CrossRef]
- Tang, P.M.; Chan, J.Y.; Au, S.W.; Kong, S.K.; Tsui, S.K.; Waye, M.M.; Mak, T.C.; Fong, W.P.; Fung, K.P. Pheophorbide a, an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol. Ther. 2006, 5, 1111–1116. [Google Scholar] [CrossRef]
- Hoi, S.W.; Wong, H.M.; Chan, J.Y.; Yue, G.G.; Tse, G.M.; Law, B.K.; Fong, W.P.; Fung, K.P. Photodynamic therapy of Pheophorbide a inhibits the proliferation of human breast tumour via both caspase-dependent and -independent apoptotic pathways in in vitro and in vivo models. Phytother. Res. 2012, 26, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Kwon, S.M.; Kim, Y.C.; Ahn, S.G.; Yoon, J.H. Pheophorbide a-mediated photodynamic therapy induces apoptotic cell death in murine oral squamous cell carcinoma in vitro and in vivo. Oncol. Rep. 2012, 27, 1772–1778. [Google Scholar]
- Isabel, D.-P. Biosynthesis of alkaloids in Amaryllidaceae plants: A review. Phytochem. Rev. 2021, 20, 409–431. [Google Scholar]
- Tarasiuk, A.; Pawlik, L.; Fichna, J. Berberyna jako potencjalny terapeutyk w leczeniu ostrego zapalenia trzustki [Berberine as a potential therapeutic agent in the treatment of acute pancreatitis]. Postepy Biochem. 2019, 65, 224–230. [Google Scholar]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Floriano, B.F.; Carvalho, T.; Lopes, T.Z.; Takahashi, L.A.U.; Rahal, P.; Tedesco, A.C.; Calmon, M.F. Effect of berberine nanoemulsion Photodynamic therapy on cervical carcinoma cell line. Photodiagn. Photodyn. Ther. 2021, 33, 102174. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.M.; Lopes, T.Z.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of berberine associated with photodynamic therapy in cell lines. Photodiagn. Photodyn. Ther. 2020, 32, 102045. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Q.; Song, C.; Fang, J.; Yang, Y.; Liang, X.; Huang, X.; Liu, J. Berberine-photodynamic therapy sensitizes melanoma cells to cisplatin-induced apoptosis through ROS-mediated P38 MAPK pathways. Toxicol. Appl. Pharmacol. 2021, 418, 115484. [Google Scholar] [CrossRef] [PubMed]
- An, Y.W.; Jin, H.T.; Yuan, B.; Wang, J.C.; Wang, C.; Liu, H.Q. Research progress of berberine mediated photodynamic therapy. Oncol. Lett. 2021, 21, 359. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.Z.; de Moraes, F.R.; Tedesco, A.C.; Arni, R.K.; Rahal, P.; Calmon, M.F. Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed. Pharmacother. 2020, 123, 109794. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Huang, X.; Yang, Y.; Wang, X.; Liang, X.; Liu, J. Berberine-photodynamic induced apoptosis by activating endoplasmic reticulum stress-autophagy pathway involving CHOP in human malignant melanoma cells. Biochem. Biophys. Res. Commun. 2021, 552, 183–190. [Google Scholar] [CrossRef]
- Comincini, S.; Manai, F.; Sorrenti, M.; Perteghella, S.; D’Amato, C.; Miele, D.; Catenacci, L.; Bonferoni, M.C. Development of Berberine-Loaded Nanoparticles for Astrocytoma Cells Administration and Photodynamic Therapy Stimulation. Pharmaceutics 2023, 15, 1078. [Google Scholar] [CrossRef]
- Carriero, F.; Martinelli, C.; Gabriele, F.; Barbieri, G.; Zanoletti, L.; Milanesi, G.; Casali, C.; Azzalin, A.; Manai, F.; Paolillo, M.; et al. Berberine Photo-Activation Potentiates Cytotoxicity in Human Astrocytoma Cells through Apoptosis Induction. J. Pers. Med. 2021, 11, 942. [Google Scholar] [CrossRef]
- Gomaa, I.; Ali, S.E.; El-Tayeb, T.A.; Abdel-kader, M.H. Chlorophyll derivative mediated PDT versus methotrexate: An in vitro study using MCF-7 cells. Photodiagn. Photodyn. Ther. 2012, 9, 362–368. [Google Scholar] [CrossRef]
- Nagini, S.; Palitti, F.; Natarajan, A.T. Chemopreventive potential of chlorophyllin: A review of the mechanisms of action and molecular targets. Nutr. Cancer 2015, 67, 203–211. [Google Scholar] [CrossRef]
- Lihuan, D.; Jingcun, Z.; Ning, J.; Guozeng, W.; Yiwei, C.; Wei, L.; Jing, Q.; Yuanfang, Z.; Gang, C. Photodynamic therapy with the novel photosensitizer chlorophyllin f induces apoptosis and autophagy in human bladder cancer cells. Lasers Surg. Med. 2014, 46, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Song, Z.; Ma, Z.; Zhang, Y.; Xu, G.; Chen, G. Chlorophyllin e6-mediated photodynamic therapy inhibits proliferation and induces apoptosis in human bladder cancer cells. Oncol. Rep. 2019, 41, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, Z.; Li, W.; Jia, G.; Lu, J.; Fang, J.; Chen, G. Chlorophyllin e4 is a novel photosensitizer against human bladder cancer cells. Oncol. Rep. 2012, 27, 1455–1460. [Google Scholar] [PubMed]
- Heo, S.Y.; Lee, Y.; Kim, T.H.; Heo, S.J.; Shin, H.; Lee, J.; Yi, M.; Kang, H.W.; Jung, W.K. Anti-Cancer Effect of Chlorophyllin-Assisted Photodynamic Therapy to Induce Apoptosis through Oxidative Stress on Human Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 11565. [Google Scholar] [CrossRef]
- Fang, L.Z.; Qing, C.; Shao, H.J.; Yang, Y.D.; Dong, Z.J.; Wang, F.; Zhao, W.; Yang, W.Q.; Liu, J.K.; Hypocrellin, D. A cytotoxic fungal pigment from fruiting bodies of the ascomycete Shiraia bambusicola. J. Antibiot. 2006, 59, 351–354. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, W.; Wu, J.; Zheng, X.; Ge, J.; Ren, H.; Zhang, W.; Lee, C.S.; Wang, P. Ultrasound-Enhanced Self-Exciting Photodynamic Therapy Based on Hypocrellin B. Chem. Asian J. 2021, 16, 1221–1224. [Google Scholar] [CrossRef]
- Qi, S.; Guo, L.; Yan, S.; Lee, R.J.; Yu, S.; Chen, S. Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta Pharm. Sin. B 2019, 9, 279–293. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.; Wei, G. Effects of photodynamic therapy using Red LED-light combined with hypocrellin B on apoptotic signaling in cutaneous squamous cell carcinoma A431 cells. Photodiagn. Photodyn. Ther. 2023, 43, 103683. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Effect of photodynamic therapy with hypocrellin B on apoptosis, adhesion, and migration of cancer cells. Int. J. Radiat. Biol. 2014, 90, 575–579. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, X.; Leung, A.W.; Xiang, J.; Xu, C. Apoptosis of breast cancer cells induced by hypocrellin B under light-emitting diode irradiation. Photodiagn. Photodyn. Ther. 2012, 9, 337–343. [Google Scholar] [CrossRef]
- Ji, Y.Y.; Ma, Y.J.; Wang, J.W. Cytoprotective role of nitric oxide in HepG2 cell apoptosis induced by hypocrellin B photodynamic treatment. J. Photochem. Photobiol. B 2016, 163, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Tian, Y.; Shi, Y.; Guo, G.; Tong, Y.; Wang, G. Antifibrotic effects of Hypocrellin A combined with LED red light irradiation on keloid fibroblasts by counteracting the TGF-β/Smad/autophagy/apoptosis signalling pathway. Photodiagn. Photodyn. Ther. 2021, 34, 102202. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Li, S.; Jiao, Y.; Qi, T.; Wei, G.; Han, G. Effects of Photodynamic Therapy Using Yellow LED-light with Concomitant Hypocrellin B on Apoptotic Signaling in Keloid Fibroblasts. Int. J. Biol. Sci. 2017, 13, 319–326. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Inactivation of Staphylococcus aureus by photodynamic action of hypocrellin B. Photodiagn. Photodyn. Ther. 2013, 10, 600–606. [Google Scholar] [CrossRef]
- Deininger, M.H.; Weinschenk, T.; Morgalla, M.H.; Meyermann, R.; Schluesener, H.J. Release of regulators of angiogenesis following Hypocrellin-A and -B photodynamic therapy of human brain tumor cells. Biochem. Biophys Res. Commun. 2002, 298, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, W.; Wu, J.; Zheng, X.; Ge, J.; Ren, H.; Zhang, W.; Lee, C.S.; Wang, P. Near-Infrared Hypocrellin Derivatives for Synergistic Photodynamic and Photothermal Therapy. Chem. Asian J. 2020, 15, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jiang, A.; Fang, H.; Chen, Y.; Guo, Z. Optical properties of natural small molecules and their applications in imaging and nanomedicine. Adv. Drug Deliv. Rev. 2021, 179, 113917. [Google Scholar] [CrossRef]
- Koh, E.; Chaturvedi, A.K.; Javitt, G.; Brandis, A.; Fluhr, R. Multiple paths of plant host toxicity are associated with the fungal toxin cercosporin. Plant Cell Environ. 2023, 46, 2542–2557. [Google Scholar] [CrossRef]
- Tang, Z.; Li, J.; Lin, F.; Bao, W.; Zhang, S.; Guo, B.; Huang, S.; Zhang, Y.; Rao, Y. Cercosporin-bioinspired photoreductive activation of aryl halides under mild conditions. J. Catal. 2019, 380, 1–8. [Google Scholar] [CrossRef]
- Newman, A.G.; Townsend, C.A. Molecular Characterization of the Cercosporin Biosynthetic Pathway in the Fungal Plant Pathogen Cercospora nicotianae. J. Am. Chem. Soc. 2016, 138, 4219–4228. [Google Scholar] [CrossRef]
- Mastrangelopoulou, M.; Grigalavicius, M.; Berg, K.; Ménard, M.; Theodossiou, T.A. Cytotoxic and Photocytotoxic Effects of Cercosporin on Human Tumor Cell Lines. Photochem. Photobiol. 2019, 95, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Grigalavicius, M.; Mastrangelopoulou, M.; Arous, D.; Juzeniene, A.; Ménard, M.; Skarpen, E.; Berg, K.; Theodossiou, T.A. Photodynamic Efficacy of Cercosporin in 3D Tumor Cell Cultures. Photochem. Photobiol. 2020, 96, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Brückner, C. Tolyporphin-An Unusual Green Chlorin-like Dioxobacteriochlorin. Photochem. Photobiol. 2017, 93, 1320–1325. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, S.P.; Rai, A.K.; Mohapatra, T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. 2011, 64, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Barnhart-Dailey, M.; Zhang, Y.; Zhang, R.; Anthony, S.M.; Aaron, J.S.; Miller, E.S.; Lindsey, J.S.; Timlin, J.A. Cellular localization of tolyporphins, unusual tetrapyrroles, in a microbial photosynthetic community determined using hyperspectral confocal fluorescence microscopy. Photosynth Res. 2019, 141, 259–271. [Google Scholar] [CrossRef]
- Morlière, P.; Mazière, J.C.; Santus, R.; Smith, C.D.; Prinsep, M.R.; Stobbe, C.C.; Fenning, M.C.; Golberg, J.L.; Chapman, J.D. Tolyporphin: A natural product from cyanobacteria with potent photosensitizing activity against tumor cells in vitro and in vivo. Cancer Res. 1998, 58, 3571–3578. [Google Scholar]
- Wyckmans, M.; Bervoets, A. A review on PUVA pricks-A debilitating adverse event. Photodermatol. Photoimmunol. Photomed. 2023, 39, 185–192. [Google Scholar] [CrossRef]
- Liu, X.P.; Zhang, W.S.; Wang, Y.N.; Ye, W.Q.; Xu, Z.R. In situ monitoring PUVA therapy by using a cell-array chip-based SERS platform. Anal. Chim. Acta 2022, 1189, 339224. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Dharmaratne, P.; Wang, B.; Lau, K.M.; Lee, C.C.; Cheung, D.W.S.; Chan, J.Y.W.; Yue, G.G.L.; Lau, C.B.S.; Wong, C.K.; et al. Hypericin and Pheophorbide a Mediated Photodynamic Therapy Fighting MRSA Wound Infections: A Translational Study from In Vitro to In Vivo. Pharmaceutics 2021, 13, 1399. [Google Scholar] [CrossRef]
- Li, T.; Hou, X.; Deng, H.; Zhao, J.; Huang, N.; Zeng, J.; Chen, H.; Gu, Y. Liposomal hypocrellin B as a potential photosensitizer for age-related macular degeneration: Pharmacokinetics, photodynamic efficacy, and skin phototoxicity in vivo. Photochem. Photobiol. Sci. 2015, 14, 972–981. [Google Scholar] [CrossRef]


| Inclusion Criteria |
| Papers describing photodynamic therapy |
| Original research papers |
| Papers describing clinical trials |
| Exclusion criteria |
| Works in a language other than English and Polish |
| Works that do not describe PDT using natural photosensitizers |
| Photosensitizer | Therapeutic Regimen | Findings | Article |
|---|---|---|---|
| Curcumin | Six sessions of PDT of dentures and oral cavity, three times a week for half a month using a CUR solution of 5 μg/mL and light with a wavelength ranging from 440 to 460 nm | Reduction in mean number of CFU/mL of Candida from denture surface and palatal mucosa after 6- and 12-week follow-up after CUR-PDT for the treatment of stomatitis in patients who are heavy smokers | Labban et al. [35] |
| Use of mouthwash with 20 mL of 30 mg/L CUR solution for 5 min, its removal, and exposure to blue light with a wavelength of 455 +/− 30 nm for 5 min | CFU reduction at 1 and 2 h after CUR-PDT | Leite et al. [36] | |
| Rinsing the mouth three times with 15 mL of 25 mg/L or 100 mg/L curcumin solution for 1 min, followed by irradiation of the mouth for 6 min with 450 nm light | Reduction in microbial count 24 h after CUR-PDT | Ricci Donato et al. [37] | |
| Rinsing the mouth with a 1 mg/L curcumin solution for 2 min, followed by irradiation of the buccal and lingual surfaces of the teeth sequentially for 3 min with 450 +/− 10 nm light | Reduction in S. mutans in orthodontic patients after CUR-PDT; this effect was enhanced when 0.1% SDS solution was added to curcumin solution | Panhoca et al. [38] | |
| Rinsing the mouth with 20 mL of 1.5 g/L curcumin solution for 5 min, followed by irradiation of the oral surface for 5 min with 468 nm light and application of the low-intensity laser PBM-T protocol | Cure of 12 of 14 cancer patients from oral mucositis after CUR-PDT | Pinheiro et al. [39] | |
| Infusion of 2.5 mg/mL curcumin solution into canal 180 s before irradiation, subsequent cleansing with MTAD | Achieving the highest push-back bond strength (PBS) at two levels: cervical and midline after CUR-PDT | Saini et al. [40] | |
| Application of a mask containing 1% curcumin for 20 min, followed by exposure to 445 nm light; sessions at 3-day intervals, for a total of two treatments per week, administered continuously for 2 weeks | Lesion removal 54.7 ± 21.5% in the treatment of mild to moderate acne with CUR-PDT | Zhang et al. [41] | |
| Hypericin | Application of 0.25% hypericin ointment to each index lesion, covering it with an opaque dressing or clothing for 18 to 24 h, followed by exposure to visible light in the range of 500 to 650 nm; Light treatment started at 5 J/cm2 and increased by 1 J/cm2 with each treatment, until a mild skin reaction was observed in the treated lesions or until a maximum light dose of 12 J/cm2 was reached. Lesions were treated twice a week for each 6-week cycle | Achieving 49% success rate in treating cutaneous T-cell lymphoma with early-stage mycosis fungoides using HYP-PDT | Kim et al. [54] |
| Application of hypericin ointment with a concentration of 2 mg/mL to the lesion and 10 mm of surrounding skin in a layer 1 mm thick under an occlusive dressing, removing it after 2 h, and irradiating the area with a PDT lamp emitting incoherent red light with a wavelength of 580 to 680 nm. The total light dose was 75 J cm2 for 15–20 min. The aforementioned lesions were treated with PDT at weekly intervals; the average treatment period was 6 weeks. The number of PDT treatments depended on the clinical features of the treated areas and persistent fluorescence during photodynamic diagnosis. | A 50% complete clinical response was achieved in cases of cutaneous keratosis, 28% in patients with superficial basal cell carcinoma, and 40% in patients with Bowen’s disease by PDT with H. perforatum extract | Kacerovska et al. [55] | |
| Application of 1.5 mL of 0.5% H. perforatum extract containing 0.1% hypericin to half of the face for 10 min under occlusion. The face was then illuminated simultaneously with 630 nm red light and 520 nm green light from a 35 mW⁄cm2 LED light-emitting device for 10 min (total light dose was 21 J/cm2). Participants received a total of four treatments at 1-week intervals and were observed 1 and 4 weeks after the last treatment. | One week after the last PDT treatment with H. perforatum extract, a 56.5% reduction in acne lesions was observed. | Kim et al. [56] | |
| Riboflavin | Conditioning the surface of the posts with RF at a concentration of 25 mol/L, followed by irradiation with a green laser at 540 nm for 60 s | RF-PDT can be an effective surface conditioner for PEEK inserts | Al Deeb et al. [63] |
| RF pretreatment of PEEK posts and exposure to light at 632 nm, 150 mW power, and power density of 23.43 J/cm2 continuously for 1 min | RF-PDT is an alternative to PEEK post surface conditioning | Alkhudhairy et al. [64] | |
| Brushing the canal with a 150 g/mL riboflavin solution activated with 660 nm LED light at 150 mW for about 60 s | RF-PDT showed significantly lower bond integrity values than the other methods tested in the force test of pressing the fibrous insert into the root dentin | AlGhamdi et al. [65] | |
| Phycocyanin | Applying a phycocyanin solution of 1000 μg/mL to the surface of healthy dentin, leaving it for 5 min, followed by irradiation with a 220 mW diode laser at a wavelength of 635 nm and an energy density of 61.2 J/cm2 for 3 min under continuous water rinsing and air drying | RF-PDT can be recommended as an antimicrobial method and does not adversely affect bonding to healthy dentin using a universal adhesive in a self-etching protocol | Hashemikamangar et al. [74] |
| Emodin | Application of n-Emo gel with emodin to the wound surface for five minutes, followed by irradiation with a light-emitting diode with a wavelength of 450 ± 10 nm, output intensity of 1000 ± 1400 mW/cm2, and energy density of 60–80 J/cm2 for one minute | n-Emo-gel-PDT may act as an adjunct to conventional wound care in the treatment of postoperative complications at the donor site after free gingival graft surgery | Yaghobee et al. [82] |
| Photosensitizer | Characteristic Information Described in the Article about the Mechanisms of Action of Each Photosensitizer | Article |
|---|---|---|
| Curcumine | Inhibits the NF-κB pathway. Can induce autophagy, inhibit EMT, and invasion and migration of tumor cells. | [30] |
| Hypericin | It can induce stress in the ER, leading to the release of calcium ions into the cytoplasm. Increasing their levels can activate various enzymes and lead to mitochondrial damage. | [46] |
| Riboflavin | It can activate MAPK (mitogen-activated protein kinase) pathways such as p38 and JNK, leading to further activation of apoptotic mechanisms. | [59] |
| Phycocyanin | Depending on the dose of PS and light, it can activate either apoptosis or necrosis. | [67] |
| Anthraquinones | Some anthraquinones may additionally inhibit the defense mechanisms of cancer cells, leading to the accumulation of ROS and increasing the effectiveness of PDT. | [75] |
| Furanocoumarins | Cycloaddition occurs, and a covalent bond is formed between the psoralen and the pyrimidine bases. During the subsequent phases of UV irradiation, additional interactions can occur between the psoralen monoadduct and the pyrimidine base, resulting in the formation of cross-links between DNA strands. | [84] |
| Pheophorbide a | Significantly increases the levels of pro-apoptotic proteins while decreasing the levels of anti-apoptotic proteins. Inhibits proliferation, migration, and invasion of cancer cells. It works by inhibiting the EMT process and reducing the expression of matrix metalloproteinases, which are key in the process of tumor invasion. | [93] |
| Alkaloids | It readily binds to DNA, increasing the efficiency of singlet oxygen generation. Upon photoexcitation, berberine causes guanine-specific oxidation in DNA, leading to DNA damage that can induce apoptosis or cell death by necrosis. | [105] |
| Chlorophyllin | Decreases the expression of the anti-apoptotic protein BCL-2 and increases the level of the pro-apoptotic protein Bax, which alters the BCL-2/Bax ratio, further promoting apoptosis of cancer cells. | [115] |
| Hypocrellin | It can affect mitochondrial oxidative stress-related signaling pathways regulated by proteins such as JunB and BAG-4. | [118] |
| Cercosporin | Causes cell death mainly by necrosis mechanism through damage to mitochondria and ER cell membranes. | [133] |
| Toliporphins | Nuclear membrane damage by transferring photo-oxidative damage from photoactivated TPs bound to the rough ER through inactivation of acyl-CoA:cholesterol-0-acyltransferase, a sensitive marker of ER membrane integrity. | [137] |
| Photosensitizer | Toxicity | Side Effects | Article |
|---|---|---|---|
| Curcumine | No serious adverse effects in clinical trials No adverse effects on healthy tissues in clinical trials | Mild sensation of warmth, mild erythema on both sides (resolving up to 2 h), mild pigment deposition on both cheeks after two treatments in both experimental and control groups in two patients (this pigmentation resolved spontaneously after 1-month follow-up) | [35,36,37,38,39,40,41] |
| Hypericin | No serious side effects in clinical trials | Mild local skin lesions and injection site reactions, burning and pain during irradiation | [54,55] |
| Riboflavin | No described side effects in clinical trials | No described side effects in clinical trials | [63,64,65] |
| Phycocyanin | No described side effects in clinical trials | No described side effects in clinical trials | [74] |
| Antrachinones | No described side effects in clinical trials | No described side effects in clinical trials | [82] |
| Furanocoumarins | No data available | No data available | [138,139] |
| Pheophorbide a | Conflicting data in in vitro studies: (1) low sensitivity to Pa-PDT of WRL-68 liver cells; (2) cytotoxic effects of Pa-PDT in four primary cultures of normal mammary epithelial cells; (3) cytotoxic effect of Pa-PDT of neutrophils | No serious side effects in in vivo mouse studies | [96,97,98,140] |
| Alkaloids | Less than 10% mortality of healthy HaCaT keratinocytes after bereberin administration without irradiation and about 53% mortality of these cells after in vitro irradiation | No data available | [103] |
| Chlorophyllin | No in vitro cytotoxicity against RAW 264.7 macrophages both in the dark and after illumination | No data available | [115] |
| Hypocrellin | Minor retinal and retinal pigments, epithelial damage, and low dermal phototoxicity under simulated sunlight 24 h after in vivo administration of liposomal form of hypocrellin B in a rat model of choroidal neovascularization | No data available | [141] |
| Cercosporin | No data available | No data available | |
| Toliporphins | Increased risk of damage to healthy tissues due to even distribution in muscle, liver, and tumor tissues | No in vivo side effects described in mice | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aebisher, D.; Przygórzewska, A.; Bartusik-Aebisher, D. Natural Photosensitizers in Clinical Trials. Appl. Sci. 2024, 14, 8436. https://doi.org/10.3390/app14188436
Aebisher D, Przygórzewska A, Bartusik-Aebisher D. Natural Photosensitizers in Clinical Trials. Applied Sciences. 2024; 14(18):8436. https://doi.org/10.3390/app14188436
Chicago/Turabian StyleAebisher, David, Agnieszka Przygórzewska, and Dorota Bartusik-Aebisher. 2024. "Natural Photosensitizers in Clinical Trials" Applied Sciences 14, no. 18: 8436. https://doi.org/10.3390/app14188436
APA StyleAebisher, D., Przygórzewska, A., & Bartusik-Aebisher, D. (2024). Natural Photosensitizers in Clinical Trials. Applied Sciences, 14(18), 8436. https://doi.org/10.3390/app14188436








