Abstract
The dynamics of tissue wound healing associated with dental implants placed in fresh extraction sockets or in healed alveolar ridges are not fully understood. Therefore, the aim of this preclinical in vivo investigation was to evaluate soft tissue healing and osseointegration after immediate implant placement, compared with delayed implant placement, using histomorphometric and immunohistochemical analyses. Methods: In 8 dogs, immediate postextraction implants and delayed implants were evaluated. After 2 and 8 weeks of healing, dissected tissue blocks were processed for calcified and decalcified histological analysis. Histometric measurements for soft tissue height and width were performed. Immunohistochemical analysis for the evaluation of bone metabolism, immune response and angiogenesis was performed. Results: At 2 weeks, histometric analysis showed that peri-implant soft tissue height was significantly greater in the immediate implant group (4.47 mm ± 0.78) compared to the delayed implant group (2.92 mm ± 0.51, p = 0.028), primarily due to the connective tissue height. Immunohistochemical analysis indicated significantly higher alkaline phosphatase (ALP) (6.85 ± 5.17 vs. 3.56 ± 2.31, p < 0.05) and matrix metalloproteinase (MMP2) activity (1.59 ± 1.64 vs. 0.58 ± 0.48, p < 0.05) in immediate implants, suggesting increased osteogenic and matrix metalloproteinase activity. At 8 weeks, the peri-implant soft tissue height remained higher in the immediate implant group (3.43 mm ± 0.83) compared to the delayed implant group (2.4 mm ± 0.37, p = 0.088), although the difference was less pronounced. Immunohistochemical differences between groups diminished in late healing stages. Conclusions: Soft tissue dimensions around immediate implants differ from those established around staged implants placed on healed alveolar ridges. Moreover, osseointegration dynamics around immediate implants may occur at a different rate compared with staged implants.
1. Introduction
There is evidence from both preclinical and clinical studies that following tooth loss/extraction, the alveolar process will suffer significant resorption and loss of bone volume [1,2]. To counteract these changes, the placement of immediate implants in fresh extraction sockets has been advocated. However, this implant surgical protocol, not only fails to prevent these dimensional changes, but rather accelerates alveolar bone resorption during wound healing [3,4]. This resorptive process is not influenced by the immediacy of implant placement, but by other factors, such as the thickness of the buccal bone plate, the space between the implant surface and the inner bone plate (gap) and the implant position related to the bone plates [5]. However, the simultaneous healing processes of implant osseointegration and socket wound healing after are still not fully understood. Similarly, there is evidence that the resulting resorptive changes do not affect only to the hard tissues, but may also influence the volume and final position of the peri-implant soft tissues [6]; this may be the reason for the unpredictable aesthetic outcomes reported with this implant placement protocol [7]. While some investigations studying the morphogenesis and healing of the peri-implant mucosa after immediate implant placement have reported soft tissue height dimension that is similar when compared with implants placed in healed ridges [2,8], in others, immediate implant placement results in larger dimensions of the peri-implant mucosa biological width, either due to a larger epithelial component [9], higher supracrestal connective tissue dimension [10] or both.
Other investigations have used immunohistochemical and biochemical techniques to study the biological phases during wound healing after implant placement, such as angiogenesis [11], inflammatory and granulation tissue formation [12], osteogenesis and bone formation [13,14]. These early healing events, however, have not been adequately studied after immediate implant placement, which may influence not only how implants osseointegrate, but also the outcome of the peri-implant hard and soft tissues after this implant surgical protocol. It was, therefore, the objective of this preclinical in vivo investigation to evaluate these healing events after immediate implant placement using histomorphometric and immunohistochemical techniques and comparting these results with the standard protocol of delayed implant placement.
2. Materials and Methods
This experimental in vivo investigation was designed in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for preclinical research [15] and was conducted in compliance with the Spanish and European Union regulations (European Communities Council Directive 86/609/EEC) on experimental in vivo experimentation. The experimental part of the study was carried out at the ‘Veterinary Teaching Hospital of the University of Santiago in Lugo, Spain’ once the study protocol was approved by the Ethical Committee of the Rof Codina Foundation (Lugo, Spain) (code: AELUOO1/14/INVMED/OUTROS (04)/FMG/05). This manuscript reports the immunohistochemical changes during early bone healing and the histomorphometric outcomes during the healing of the peri-implant soft tissues when comparing two implant placement protocols. The results on osseointegration (de novo bone formation) and the histomorphometrical results on the alveolar ridge alterations have been published previously [16].
2.1. Experimental Animals, Housing and Husbandry
Eight female Beagle dogs (between 1.5 and 2 years old, weighting between 15 and 20 kg), were selected and identified through a subcutaneous RFID chip. The dogs were monitored daily during the experimental phase of the investigation by experienced veterinarians. The animals were fed on a soft pellet diet and maintained in purpose-designed kennels in a 12:12 light/dark cycle at 22–21 °C. Prior to the study, all animals were monitored for any signs or symptoms of systemic disease and inspected to ensure the absence of oral disease. During the surgical interventions, the animals were premedicated with acepromazine (0.05 mg/kg/i.m., Calmo Meosan, Pfizer, Madrid, Spain) and morphine (0.3 mg/kg/i.m., Morfina Braun 2%, B. Braun Medical, Barcelona, Spain).
2.2. Experimental Design and Experiments
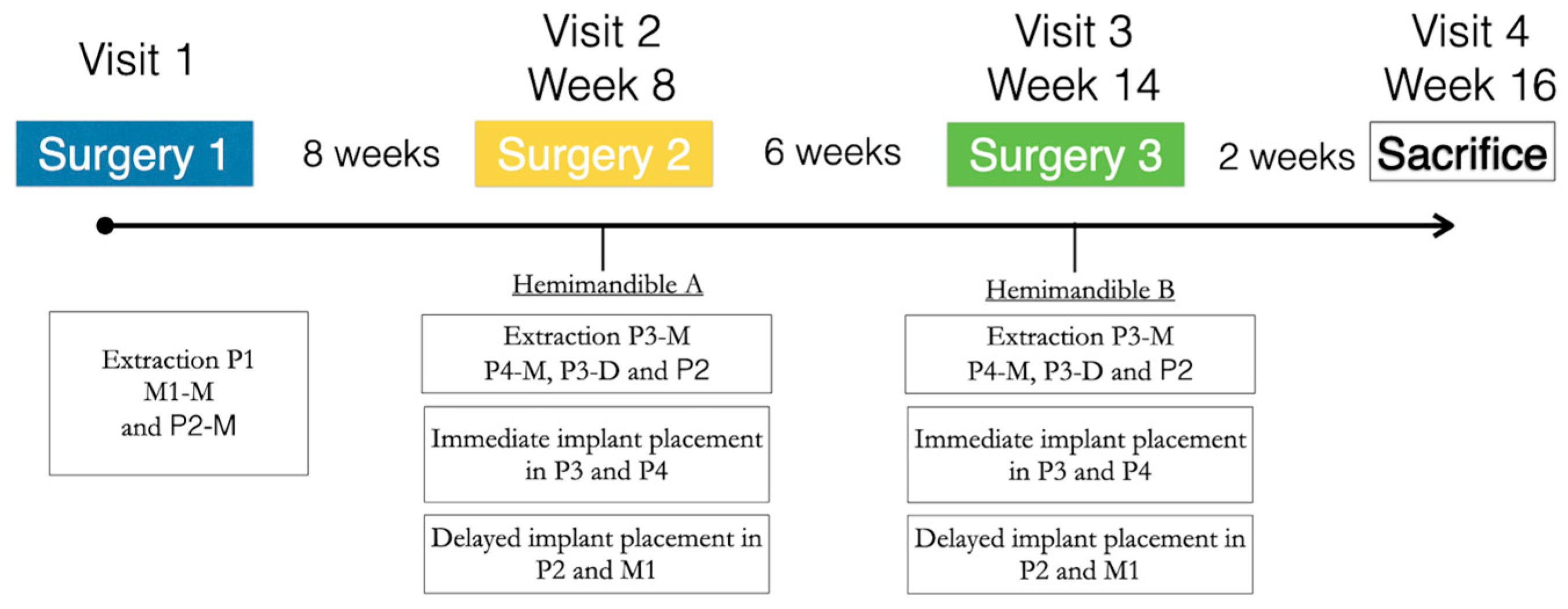
The study evaluated two surgical implant placement protocols, delayed and immediate (control and test groups, respectively) at two healing periods, 2 and 8 weeks (Figure 1). The detailed description of the experimental model has been previously reported [17]. In brief, the first intervention consisted of the extraction of first premolar (P1) and the mesial roots of the second premolar and first molar (P2 and M1) in one hemimandible. After a healing period of 2 months, implants were placed in these healed ridges (control sites; M1 and P1), while the remaining roots were extracted, and implants were placed immediately in these fresh extraction sockets (test sites—P3 and P4) (Figure 2a). The same surgical intervention was repeated on the other hemimandible 6 weeks after (Figure 2). After 2 weeks of healing following the last surgery, the animals were euthanized, providing therefore one hemimandible with a healing period of 2 weeks, while the contralateral hemimandibles provided 8 weeks healing samples. All subjects successfully completed the study protocol with no reported adverse events.
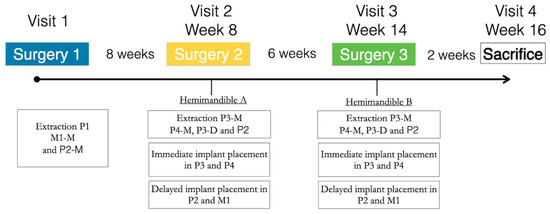
Figure 1.
Schematic representation of the study timeline.
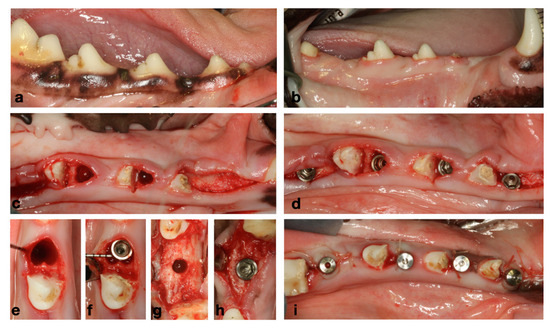
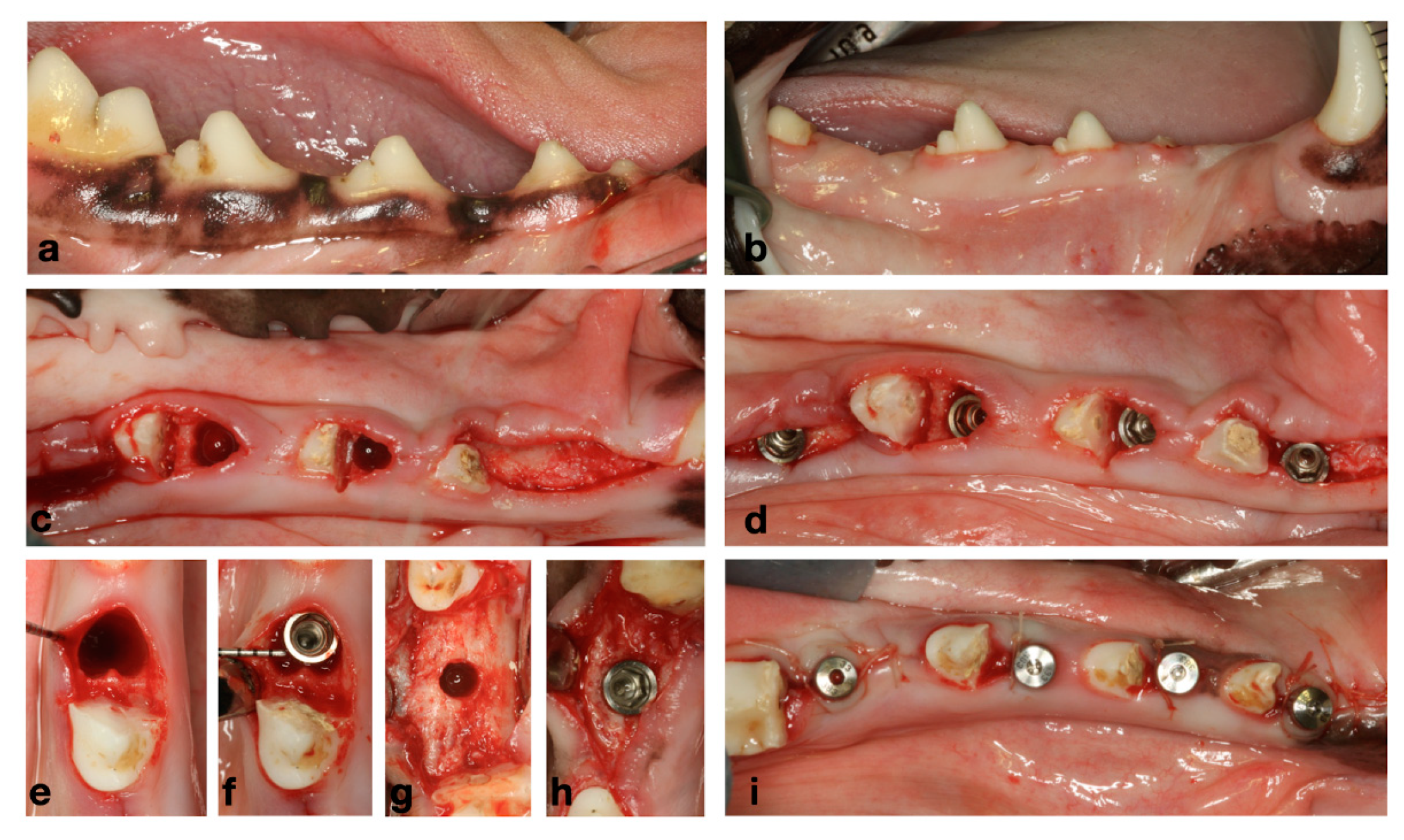
Figure 2.
(a) Baseline clinical situation. (b) Healed ridges after 2 months of healing from initial extractions. (c) Flapless extraction of messiah roots of P3 and P4. (d) Immediate and delayed implant placement. (e,f) Immediate implant placement. (g,h) Delayed implant placement. (i) Healing abutments and transmucosal healing.
2.3. Materials
Commercially available ZirTi® surface Premium® straight dental implants (Sweden & Martina, Padua, Italy) with a 0.8 mm coronal polished collar, diameter of 3.3 mm and lengths varying between 9.0 and 11.5 mm were used.
2.4. Veterinarian Care and Postoperative Protocol
In all the surgical interventions, the experimental animals were first sedated with propofol (2 mg/kg/i.v., Propovet, Abbott Laboratories, Kent, UK), then general anesthesia (2.5–4% of isoflurane) was applied under mechanically induced respiration (Isoba-vet, Schering-Plough, Madrid, Spain) and finally lidocaine 2% with epinephrine 1:100,000 (2% Xylocaine Dental, Dentsply, York, PA, USA) was infiltrated as local anesthetic.
Postoperative pain and inflammation were managed with morphine (0.3 mg/kg, i.m.) during the first 24 h, followed by meloxicam (0.1 mg/kg, s.i.d., p.o., Metacam, Boehringer Ingelheim España, Barcelona, Spain) for 3 days. Antibiotic therapy with amoxicillin (22 mg/kg/s.i.d./s.c., Amoxoil retard, Syva, León, Spain) was used for 7 days postoperatively.
Then the animals were fed with a soft diet and plaque control was attained by brushing the surgical area with a conventional manual toothbrush and applying an antiseptic solution containing 0.12% chlorhexidine (CHX) and 0.05% cetylpyridinium chloride (CPC) (PerioAid Tratamiento, Laboratorios Dentaid, Barcelona, Spain) on all mandibular tooth sites on a 2 days/week regime. The surgical areas and the implants were inspected once a week recording the health of the peri-implant mucosa and documenting any signs of inflammation or complications. Each experimental phase resulted in the placement of two immediate and two delayed implants per hemimandible, consistent across all specified healing intervals.
2.5. Euthanasia
Two weeks after the last surgery, the animals were euthanized with an overdose of sodium pentothal (40–60 mg/kg/i.v., Dolethal, Vetoquinol, Paris, France). The mandibles were freed from their attached tissues, sectioned into halves, and placed into sealable containers with formalin 4% solution and stored (at 5 °C) until histological processing.
2.6. Histological Preparation and Histomorphometry Analysis
Using a randomization protocol, 50% of the tissue blocks were processed for ground sectioning according to the methods described by Donath and Breuner (1982), while the remaining were decalcified following the modification of the “fracture technique” [18]. The histological outcomes of the undecalcified ground sections have been reported in a prior publication [16].
The present manuscript reports the histological and immunohistochemical results of the decalcified specimens processed through the modified “fracture technique”. These samples were first partially decalcified in ethylenediamine tetra acetic acid (EDTA, Laboratoriumdiscounter, Velsen-Noord, Netherlands) and then sectioned into 4 portions, respectively: mesio-buccal, mesio-lingual, disto-buccal and disto-lingual. These tissue portions were then dissected from the implant surface and independently processed via complete decalcification in EDTA, dehydration in serial steps of ethanol concentrations, secondary fixated in OsO4 and immersed in EPON (EPON Fluka chemie, Buchs, Switzerland) [19]. The resulting tissue blocks were sectioned with the microtome set at 7 µm and stained in Mallory’s trichrome or hematoxylin/eosin.
For each implant, the most representative decalcified buccal section was selected for the histological study and examined with a Nikon Eclipse Ti microscope (Nikon, Heidelberg, Germany) equipped with a dedicated image analysis software (Q-500MC; Nikon). All measurements were evaluated by a single experienced examiner (DP). Intraclass correlation coefficient tests were performed to estimate the intraexaminer reproducibility, resulting in an intraclass correlation coefficient of 0.997. Sixty-two section preparations were analyzed, resulting in 31 vestibular and 31 lingual sections.
In addition, measurements of the soft tissue dimensions were performed in the undecalcified ground sections.
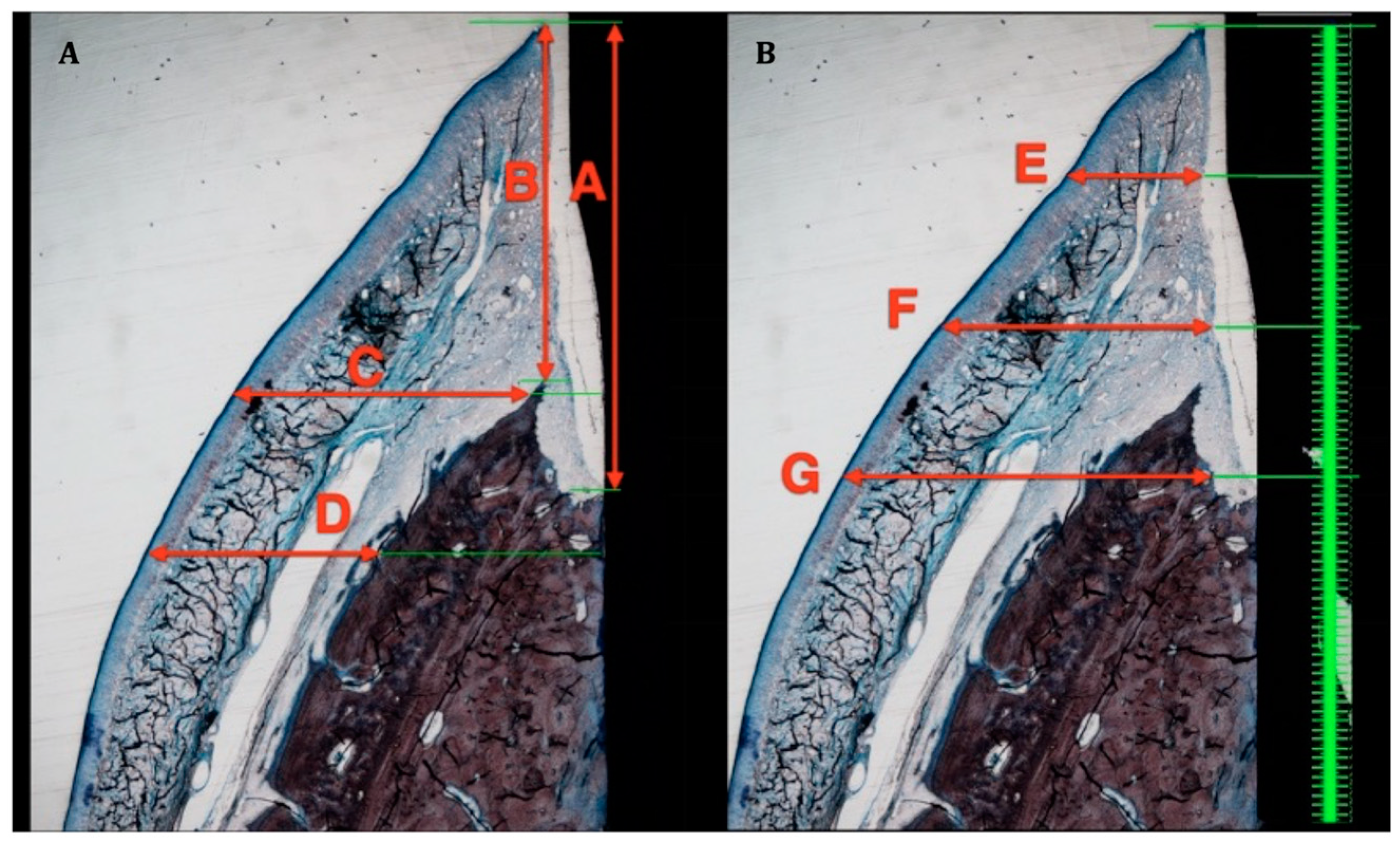
The following vertical and horizontal linear distances, expressed in mm, were measured on the buccal aspects of both decalcified and undecalcified sections (Figure 3 and Figure 4):
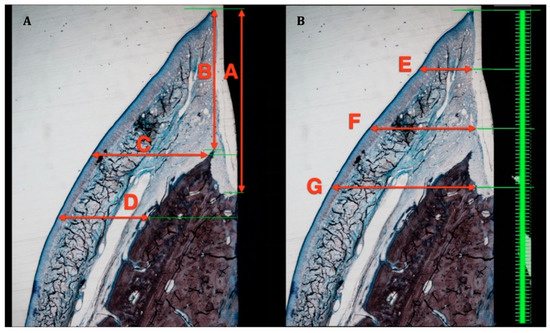
Figure 3.
Soft tissue measurement in ground sections. (A): A—Biologic width. B—Supracrestal soft tissue height. C—Soft tissue thickness at implant shoulder. D—Soft tissue thickness 1 mm apical to implant shoulder. (B): E—Soft tissue thickness 1 mm apical to free gingival margin. F—Soft tissue thickness 2 mm apical to free gingival margin. G—Soft tissue thickness 3 mm apical to free gingival margin.
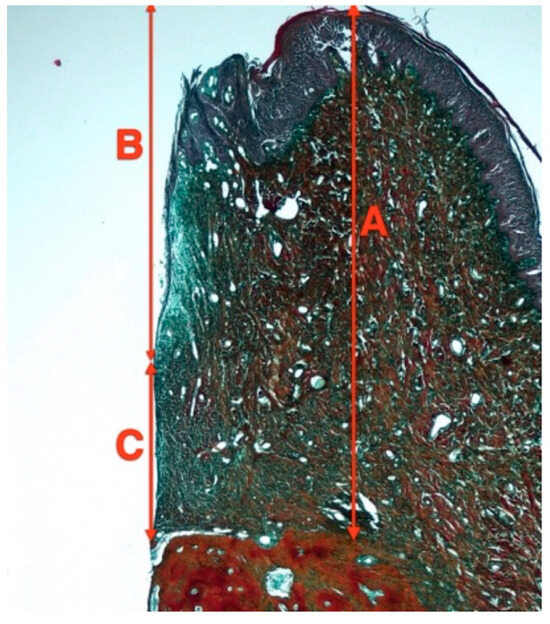
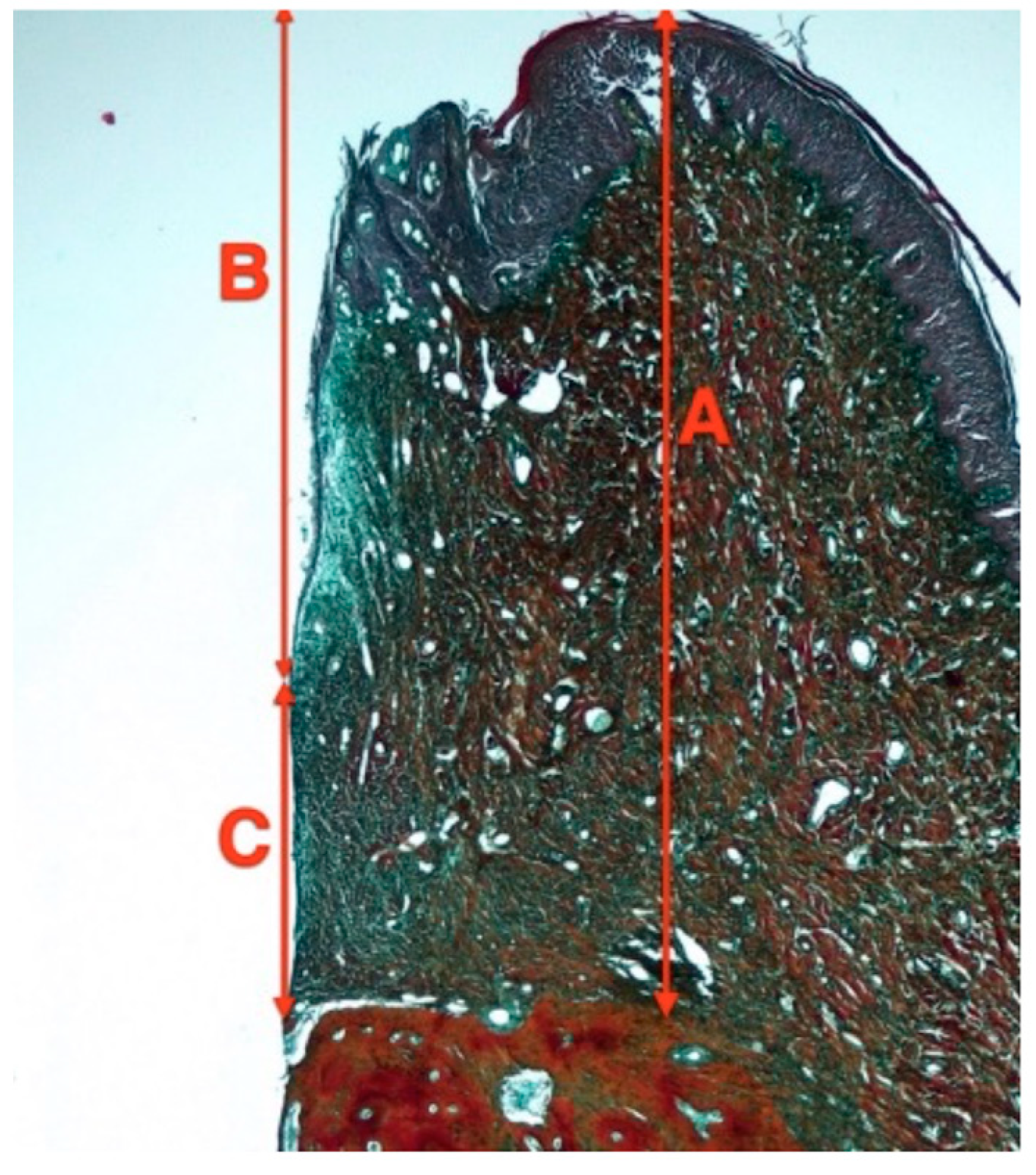
Figure 4.
Soft tissue measurements in decalcified sections. A—Biologic width, B—Junctional epithelium heigh. C—Connective tissue height.
- Height of the Peri-implant mucosa (PM-B)
- Height of the supra-crestal soft tissues (PM-Bc)
- Height of the barrier epithelium (PM-aJE)
- Height of the connective tissue (aJE-B)
These measurements used the following landmarks:
- Shoulder of the implant (I)
- Most coronal bone to implant contact (B)
- Most coronal bone crest (Bc)
- Margin of the peri-implant mucosa (PM)
- Apical border of the junctional epithelium (aJE)
From the ground sections, the thickness of the peri-implant mucosa was measured on a horizontal plane, perpendicular to the long axis of the implant, at five different reference levels: (i) at the level of the implant platform and 1 mm apically; and (ii) 1, 2, and 3 mm apical to the free gingival margin (Figure 3).
2.7. Immunohistochemical Analysis
Selected decalcified sections (27 sections) were processed for immunohistochemistry using the “Master Polymer Plus Detection System (Peroxidase)” kit (Master Diagnóstica; Granada, Spain), which contains a micropolymer-based secondary antibody, suitable for mouse and rabbit monoclonal and polyclonal primary antibodies, and DAB (3,3′-diaminobenzidine) as visualization solution.
The following primary antibodies were selected based on their role in bone metabolism, immune response and angiogenesis (Table 1):

Table 1.
Antibodies used for immunohistochemical analysis.
- Bone Metabolism: Alkaline phosphatase (ALP), Osteopontin (OPN), Matrix Metalloproteinase 2 (MMP2) and tartrate-resistant acid phosphatase (TRAP)
- Immune response: CD4 and Myeloperoxidase (MPO)
- Angiogenesis: Vascular Endothelial Growth Factor (VEGF)
Two independent evaluators (DP and FV) measured in duplicate the peri-implant soft tissue dimensions from selected decalcified sections were the junctional epithelium and underlying supracrestal connective tissue were preserved. In case of discrepancy, a third evaluator (MS) was consulted. A score range from 1 to 4 was established to evaluate the quality of the preparation and sections with a quality score of 4 (worst) were discarded from the analysis.
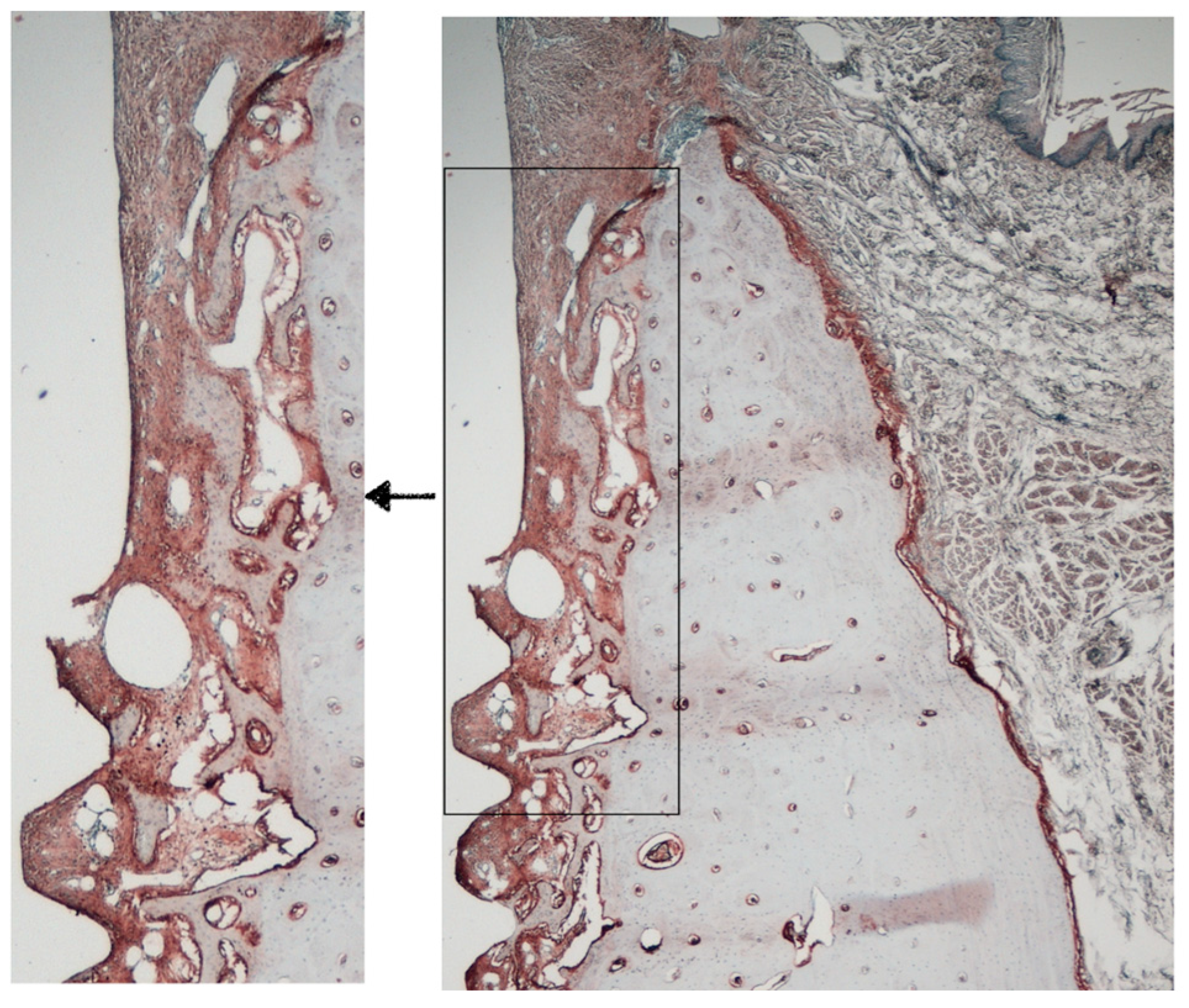
The resulting sections were observed under light microscope (Leica DMR, Leica Geosystems AG; St Gallen, Switzerland) under 2.5× magnification (Leica DFC, Leica Geosystems AG) and analyzed using ImageJ and the plugin IHC profiler (ImageJ 1.53f51 (Java 1.8.0_345 (64-bit)), National Institutes of Health, New York, NY, USA) [20]. In these slides, one region of interest (ROI) of 4 × 1 mm was chosen (412,830 pixels) using the implant shoulder as reference (Figure 5). The intensity of antibody staining was categorized according to the percentage of pixels being positively detected into: High positive, Positive, Low positive and Negative. To reduce false positives, only high positive and positive stained values were considered in the analysis. For TRAP analysis, a 20 × 20 equally sized grill was used and the percentage of chambers that contained TRAP-positive cells was calculated with Adobe Photoshop [20]. All images were evaluated via a blinded experienced examiner (RP).
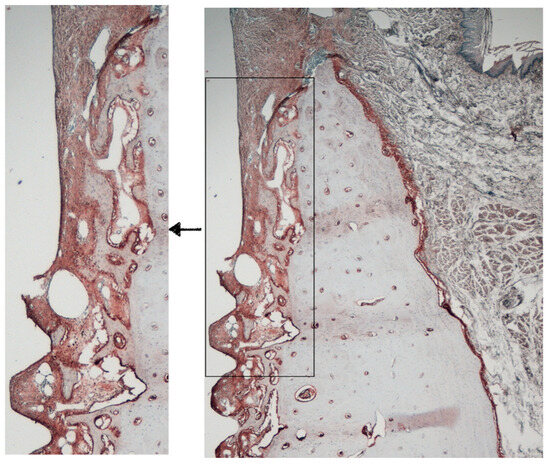
Figure 5.
Area of interest for immunohistochemical analysis.
2.8. Statistical Analysis
Data from continuous outcome measurements were expressed as means (±SD), with the dog considered as the statistical unit of analysis. Comparisons between groups were performed using parametric one-way analysis of variance (ANOVA) for normalized data sets with a general linear model. The Bonferroni correction was applied for multiple comparisons.
In cases where the normality test or equal variance test failed, the Kruskal–Wallis one-way ANOVA test on ranks was employed, followed by Dunn’s method for multiple comparisons. All statistical analyses were conducted using SPSS® (IBM SPSS Statistics, London, UK). Statistical significance was set at p < 0.05.
3. Results
3.1. Preclinical Observations
All subjects successfully completed the study protocol with no reported adverse events, and all implants successfully achieved osseointegration. Throughout the study, no animals exhibited behavioral changes or alterations in their eating and drinking habits.
3.2. Histology and Histomorphometry
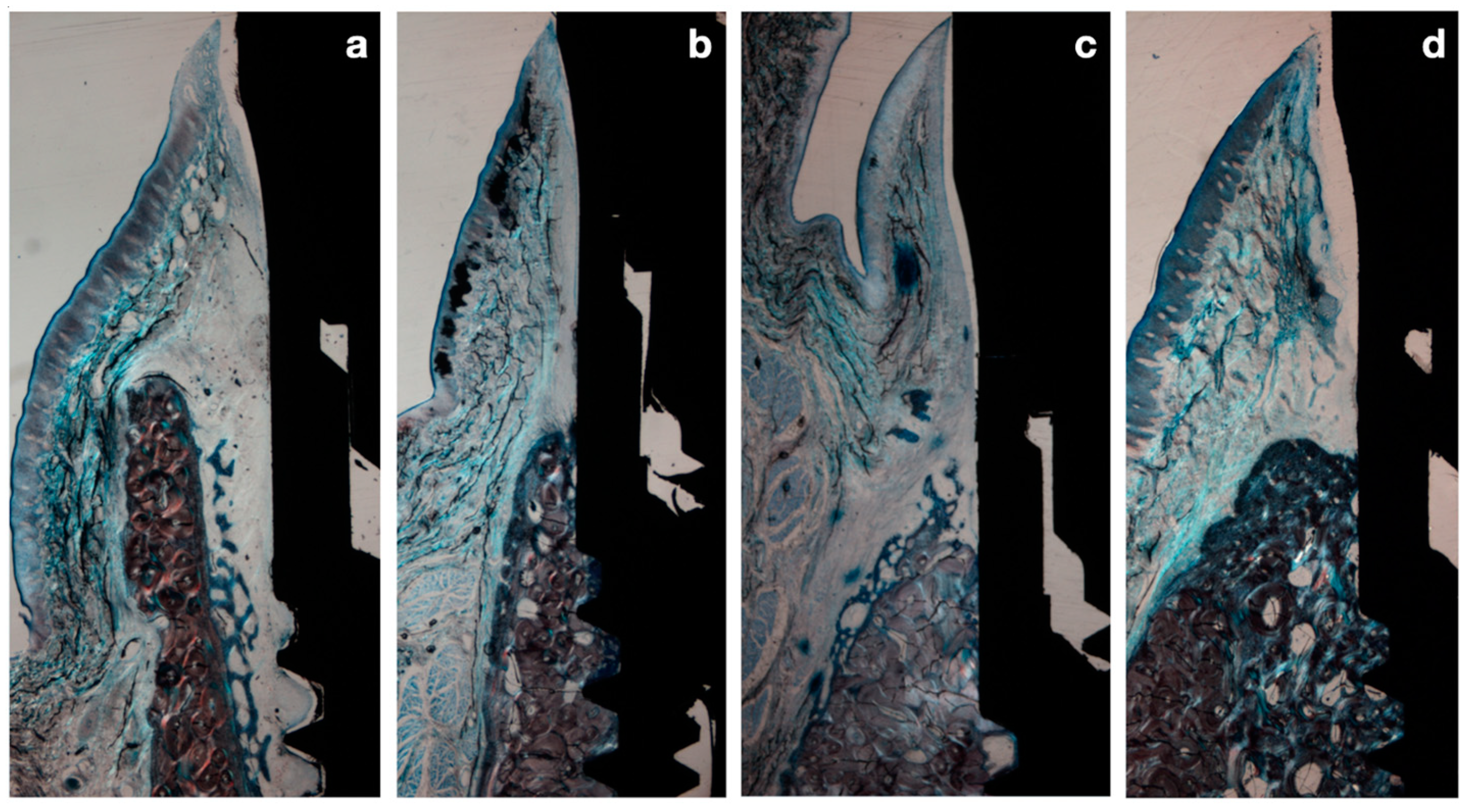
At 2 weeks posthealing, the immediate implants demonstrated a peri-implant mucosa covered by keratinized oral epithelium, which transitioned into sulcular, and junctional epithelium closely adhered to the abutment surface. Apical to the junctional epithelium, the connective tissue extended apically 2–5 mm until the first bone-to-implant contact. The buccal and lingual bone walls exhibited areas of bundle bone and woven bone in varying proportions.
In the delayed implant group, the structure and organization of the soft tissues were similar, although the height of the connective tissue was smaller, ranging from 1 to 2 mm (Figure 6).
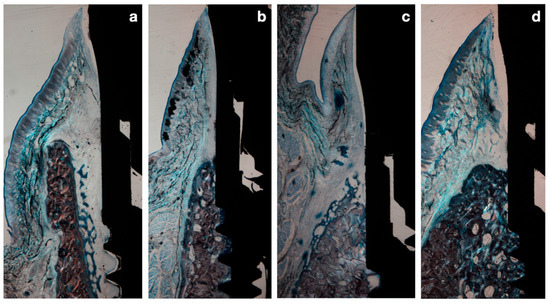
Figure 6.
(a) Immediate implant after 2 weeks healing. (b) Immediate implant after 8 weeks healing. (c) Delayed implant after 2 weeks healing. (d) Delayed implant after 8 weeks healing.
After 8 weeks of healing, in the immediate implant group, the gap between the implant surface and the inner buccal bone plate was filled with lamellar bone in direct contact with the implant surface. In both groups, the structural organization and peri-implant mucosal dimensions were similar; however, the apico-coronal dimensions of the supracrestal tissues in the delayed implants were smaller (Figure 6).
3.3. Histometric Results
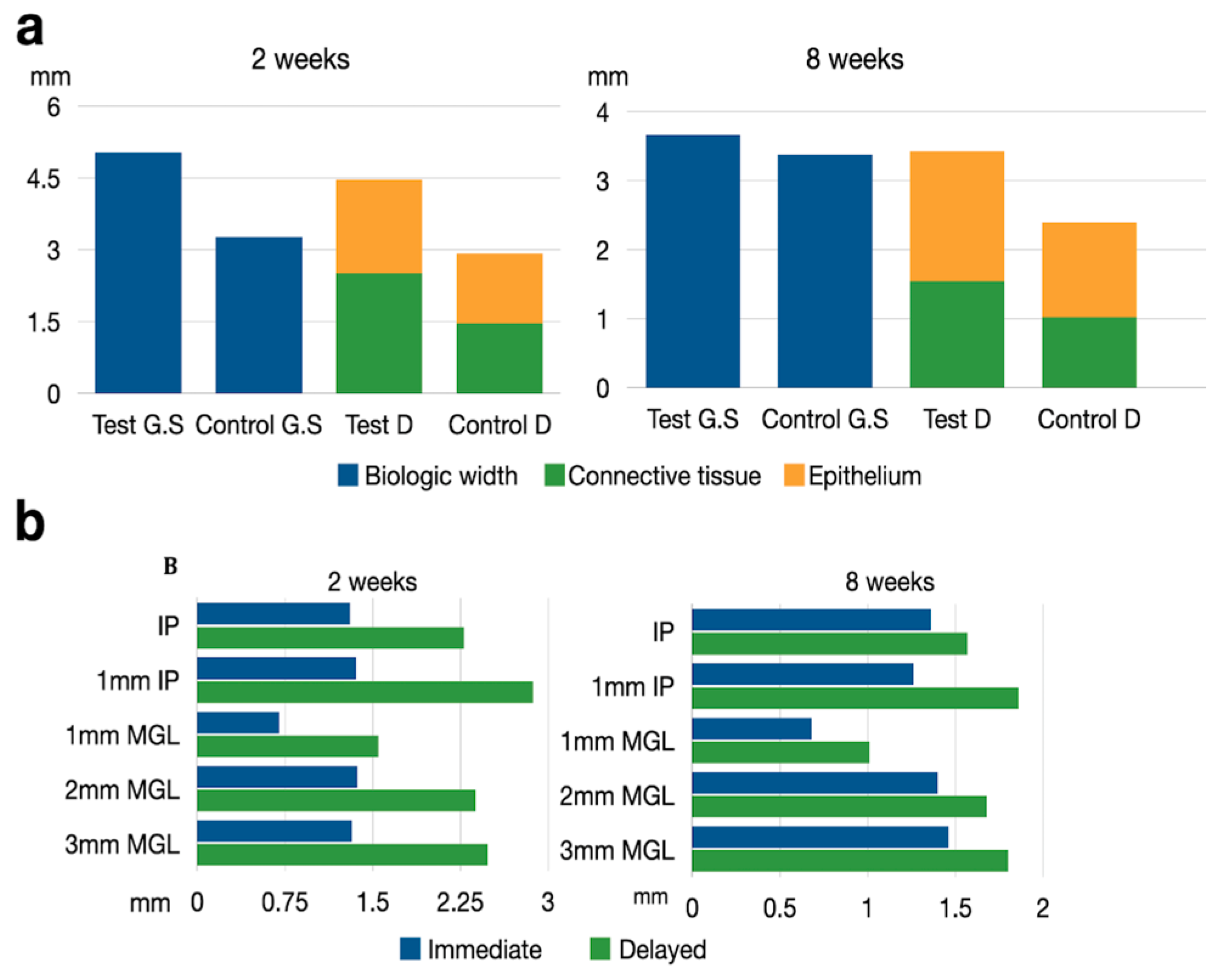
Histometric results are presented in Table 2. At 2 weeks, the mean peri-implant soft tissue height (PM-BC) of the immediate implants (test group) was 4.47 mm (±0.78) while for the delayed implants (control group), it was 2.92 mm (±0.51), making these differences statistically significant (p = 0.028). These differences were mainly due to the connective tissue height (aJE-B), being 2.52 mm (±0.65) in the test group and 1.46 mm (±0.325) in the control group. At 8 weeks, these statistically significant differences remained, albeit of a smaller dimension. At this healing timepoint, the mean peri-implant soft tissue height (PM-BC) of the immediate implants (test group) was 3.43 mm (±0.83) while in the delayed implants (control group), it was 2.4 mm (±0.37); (p = 0.088) (Table 2).

Table 2.
Biologic width dimensions (mean ± SD values).
The height and thickness of the peri-implant mucosa was measured in both undecalcified and decalcified sections (Figure 7a). Although the mean soft tissue height was consistently higher in the undecalcified sections, with a mean difference ranging between 0.2 to 0.98 mm, these differences between methods were not statistically significant (Table 2).
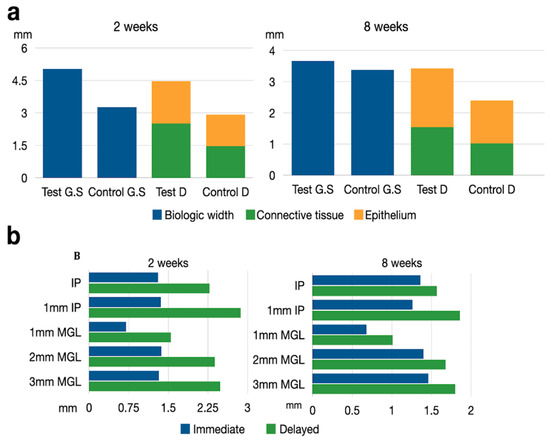
Figure 7.
(a) Schematic drawing representing the biologic width measurements. Test GS = Immediate implant in ground section. Control GS = Delayed implant in ground section. Test D = Immediate implant in decalcified sections. Control D = Delayed implant in decalcified sections. (b) Schematic drawing representing the soft tissue thickness at different levels after 2 and 8 weeks healing at test and implant sites. IP = Implant platform. MGL = Mucogingival line.
Table 3 depicts the thickness of the peri-implant soft tissues. At two weeks, a consistently thicker tissue was observed in the delayed implant group with respect to the immediate implant group, at all the defined reference points. The mean differences varied between 0.69 mm (immediate 1.37 mm ± 0.34 vs. delayed 2.38 mm ± 0.41) 2 mm apically to the free gingival margin and 1.51 mm (immediate 1.36 mm ± 0.3 vs. delayed 2.8 mm ± 0.31) 1 mm apically from the implant shoulder. In most cases, these differences were statistically significant (Table 3).

Table 3.
Soft tissue thickness (mean ± SD values).
At eight weeks, the control group still presented thicker soft tissues, although differences were smaller and not statistically significant (Figure 7b).
3.4. Immunohistochemical Results
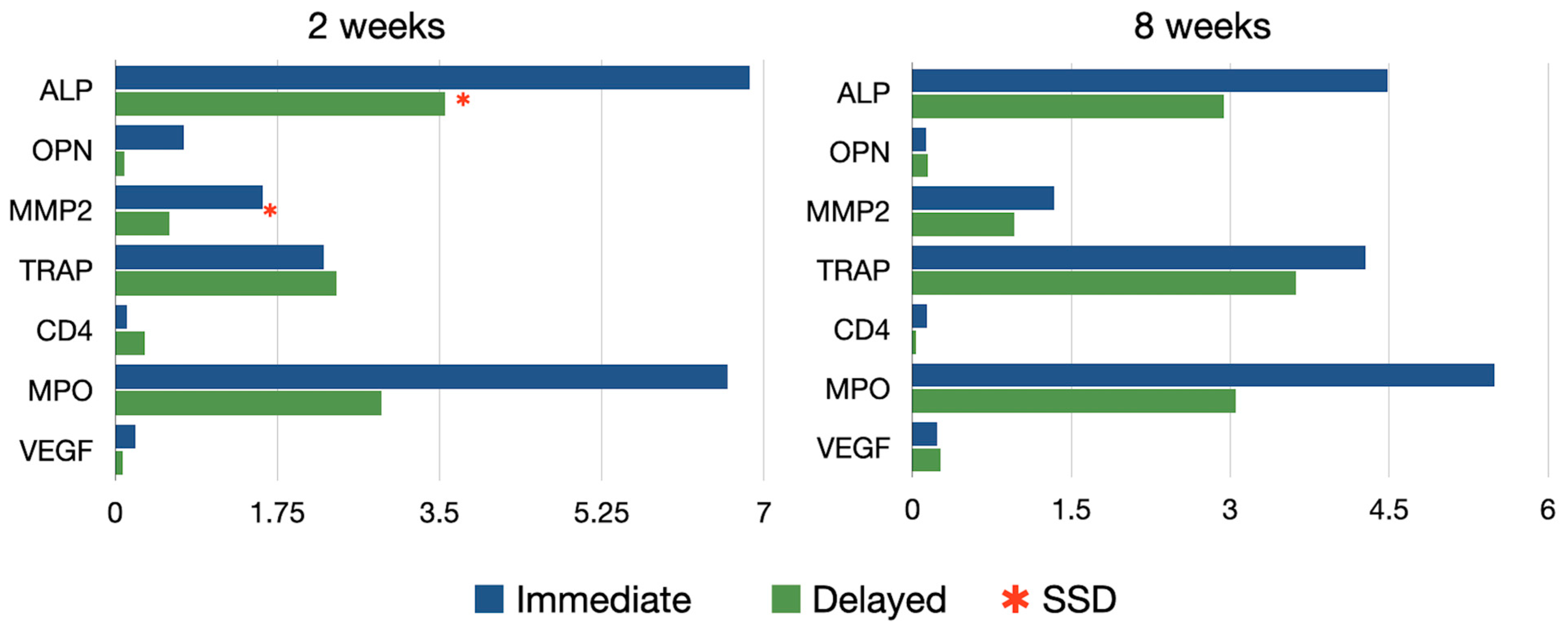
This analysis resulted from the measurement of 115 decalcified sections (64 test and 51 controls) in 30 implant sites (Figure 8).
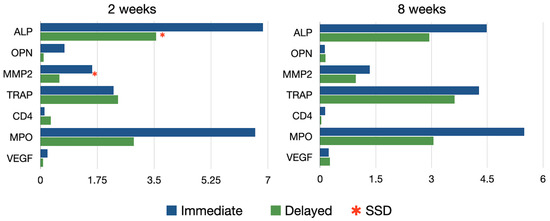
Figure 8.
Immunohistochemical expression of all the analyzed antibodies after 2 and 8 weeks healing.
3.5. Bone Metabolism
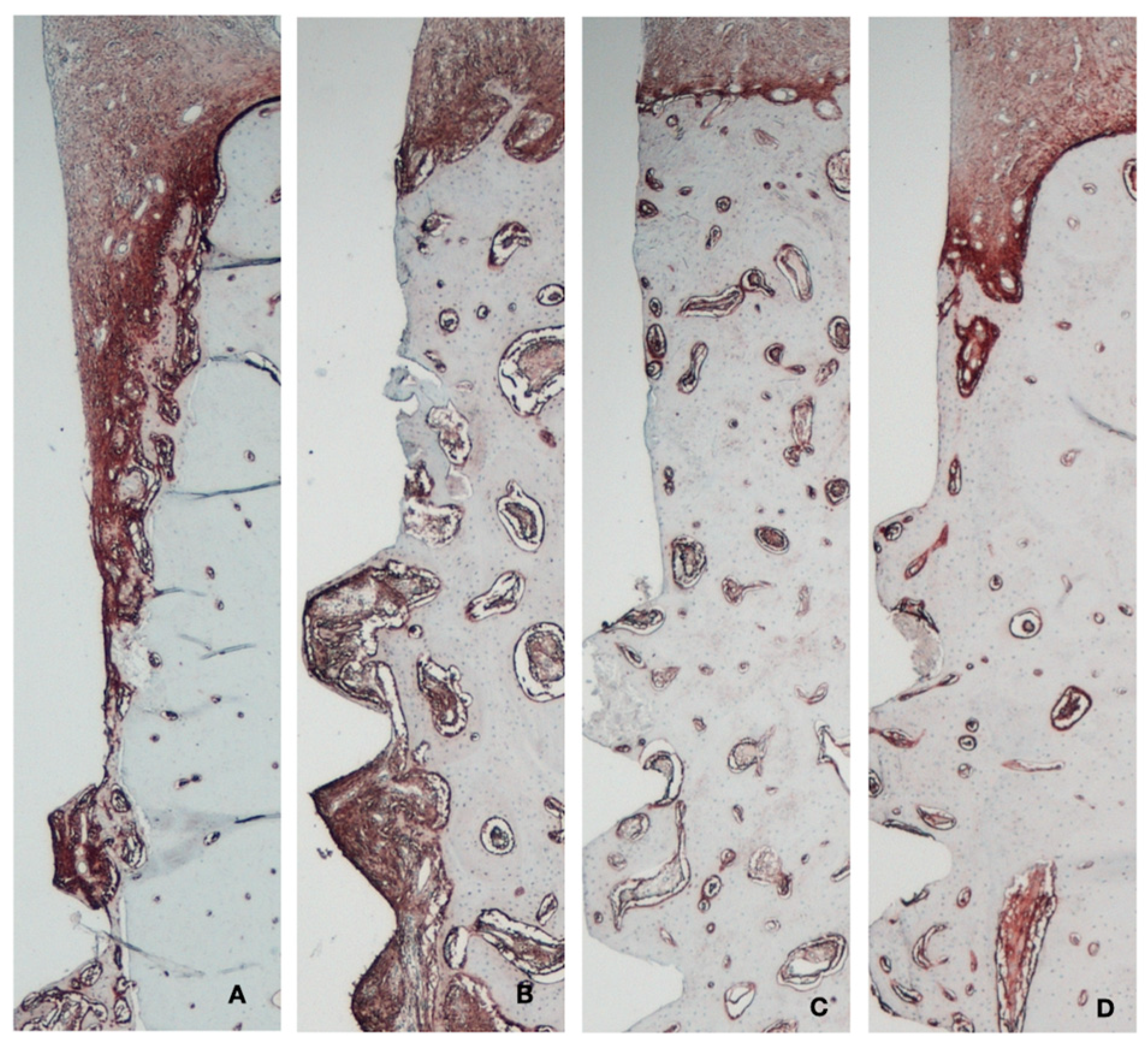
Alkaline phosphatase (ALP), expressing osteogenic potential and identified via a dark red staining, was predominantly observed at 2 weeks of healing in the immediate implants (Figure 9), particularly in the bone areas adjacent to the implant threads and at the coronal area of the socket facing the gap. At the same healing time, in the delayed implants, ALP activity was detected only in the trabecular bone near the thread area. After 8 weeks of healing, ALP expression was localized at the marginal bone crest level in both groups, with no significant differences between them.
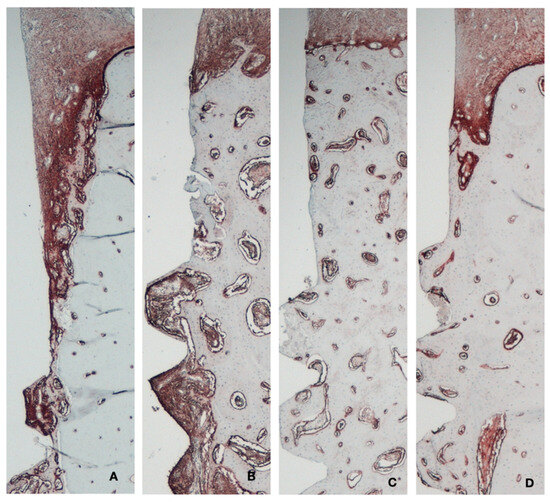
Figure 9.
Immunohistochemical analysis of alkaline phosphatase. (A) Immediate implant 2 weeks. (B) Delayed implant 2 weeks. (C) Immediate implant 8 weeks. (D) Delayed implant 8 weeks.
In the quantitative analysis of ALP intensity, the immediate implant group exhibited significantly higher ALP activity compared to the control group at 2 weeks (6.85 ± 5.17 vs. 3.56 ± 2.31, respectively; p < 0.05). This difference between the groups diminished at 8 weeks (4.48 ± 3.31 vs. 2.93 ± 1.38, respectively) (Table A1).
At two weeks of healing, MMP2 was detected as a dark red staining and was highly expressed within the gap area in the immediate implants. In contrast, in the delayed implants group, activity was only detected at the level of the marginal bone crest. By 8 weeks, a similar staining pattern was observed in both the test and control groups, with higher expression localized at the marginal bone crest level only. In the quantitative analysis of MMP2 intensity, the test group exhibited significantly higher activity compared to the control group at 2 weeks (1.59 ± 1.64 vs. 0.58 ± 0.48, respectively; p < 0.05). This difference between groups diminished at 8 weeks, although a tendency for higher expression in the immediate implant group was still noted, albeit without statistical significance.
TRAP staining, indicating osteoclastic activity, was primarily observed at two weeks in the bone area in contact with the implants in the immediate implant group. After 8 weeks of healing, TRAP staining was more prevalent around the vestibular cortical plate in the test group, whereas in the control group, it was observed in the peri-implant bone area. No significant differences between groups were observed at any healing time.
3.6. Inflammatory Reaction
No evidence of inflammatory reaction was observed in any of the groups. Likewise, no statistical differences were found in the intensity of MPO and CD4 among the groups at 2 and 8 weeks.
3.7. Angiogenesis
The expression of VGEF was more intense in the immediate implant group at two weeks, especially in the more coronal areas, although these differences were not statistically significant.
4. Discussion
With the aim of understanding the differences that occur during the healing process of immediate and delayed implants, we conducted this investigation. The results from the present in vivo experimental investigation demonstrated that during the early healing of immediate implants in fresh extraction sockets, there was a significantly higher vertical dimension of the supracrestal soft tissues compared to implants placed in healed ridges, although these differences tended to diminish over time. Conversely, soft tissues were thicker in the delayed implant group, especially in the early healing period. The immunohistochemical analysis revealed a significantly higher expression of the proteins ALP and MMP2 at immediate implant sites, predominantly in the early healing period. Additionally, a higher expression of TRAP was observed at immediate implant sites after 8 weeks healing, although differences between groups were not statistically significant.
The results from this study regarding the soft tissue dimensions, agree with previous publications studying this implant surgical protocol. During the early healing stage, the coronal aspect of the immediate implants is first filled with a connective tissue matrix [21], that gradually converts into bone [1], which results in a higher soft tissue dimension at this early healing stage (two weeks) when compared with the stage implant placement. However, with time, these differences were gradually reduced. While the biological width dimensions in the group of immediate implants at the late healing time (8 weeks) were shorter than what has been stated in previous reports [2,8,10,17], the soft tissue dimensions reported in the delayed implant sites were coherent with those reported in similar experimental studies at similar healing times [22,23]. These differences among experimental studies could be due to variations in the experimental protocols, as some studies performed immediate implants without raising a flap, while others raised a flap, which may influence the bone resorptive process during healing [24].
The present results when comparing immediate versus delayed implant placement also differ with those from previous publications from our research group [17] that reported no significant differences in biological width, nor in epithelial or connective tissue dimensions. These discrepant results could be explained by the specific triangular implant morphology used in this investigation [17]. Another influencing factor could be the implant diameter, since the alveolar crests in the Beagle dog experimental model are rather narrow and the use of very congruent implant diameters, such as 4.1 mm, could influence the resorption of the socket bone walls [25,26,27]. Also, the apico-coronal implant position, the proximity of the implant to the bony walls and the different implant-abutment interphase configurations, are factors that may influence the bone resorptive process during the healing of dental implants [28,29].
The present investigation also evaluated the possible influence of the implant placement protocol, (immediate versus delayed) on the resulting peri-implant mucosa. While in the immediate implants the soft tissue thickness remained virtually unchanged between 2 and 8 weeks, it significantly decreased in the delayed implants during this period. This finding may be explained by the fact that the significant reduction in the hard tissues that occur after immediate implant placement are partially compensated by soft tissue enlargement [30]. These results are congruent with the previous report from this in vivo experimental investigation that reported a significant reduction in the thickness of the bony walls in the delayed implant placement group, compared with the immediate implants [16].
The reported immunohistochemical differences between immediate and delayed may indicate that the bone healing after immediate implant placement differs from the healing around implants placed in healed ridges. Specifically, alkaline phosphatase (ALP), a marker of osteogenesis and osteoblast phenotyping [31], has been observed in previous reports around dental implants, 2 weeks after their placement, in the transition from connective tissue to mineralized osteoid [32]. Other studies have reported that the peak of ALP expression occurs between weeks 1 and 3, thus representing primary mineralization around dental implants [33]. These findings correlate with those from the present investigation, demonstrating a significantly higher ALP activity during the early healing of immediate implants, which require a higher bone apposition to fill the gap between the implant surface and the socket bone walls.
MMP2 is a biomarker of increased bone metabolism, being deposited in areas of intense bone modeling with a relevant role in bone resorption [34]. It is usually expressed during the early stages of bone formation [35]. In this investigation, MMP2 was expressed significantly higher at immediate implants after 2 weeks of healing, which translates to an increased MMP2 expression during the proliferative stage of socket healing [36].
Another key biomarker in bone metabolism is TRAP, a glycoprotein expressed during bone resorption, which is secreted by mature osteoclasts, activated macrophages and dendritic cells [31,37]. Our results did not find differences between groups, although there was a trend towards higher TRAP expression in delayed healing implants during the late healing phases. These results are in accordance with the histological outcomes reported in the previous report of this investigation, where the buccal bone resorption was more pronounced in the delayed implant group at 8 weeks [16].
Overall, these immunohistochemical results highlight a higher expression of bone metabolism biomarkers during the early healing phases, especially on immediate implants. Similarly, Yi et al. [38] observed that after 8 weeks healing, immediate implants achieved higher bone to implant contact when compared with delayed implants. Sanz Martin et al. [17] also observed higher bone-to-implant contact in immediate implants than in delayed implants after 4 weeks of healing. Moreover, studies in rodents have shown an increased bone osseointegration in dental implants facing periodontal ligament remnants in postextraction sockets, compared to implants around pristine bone [39,40].
The results from this experimental in vivo investigation should be interpreted with caution considering the potential limitations of the experimental design, which may not allow for direct human translation of the outcomes. Furthermore, the use of different surgical approaches when placing the implants (flapped in the delayed implants versus flapless in the immediate implants) may account for different bone resorption patterns [24]. However, we chose these different surgical protocols since these are the ones regularly used in clinical practice. Another limitation is related to the inherent limitations of the histological methods utilized; on one hand the use of decalcified sections for the soft tissue histomorphometry, although providing thinner sections and allowing for more precise location of the limits of the epithelium and the bone to implant contact, are more difficult to orientate and several samples had to be excluded of from the analysis due to the presence of alterations that limited the precise measurement of the tissue dimensions. When comparing measurements from decalcified versus undecalcified sections, however, differences were not statistically significant between methods. Regarding the immunohistochemical analysis, one limitation could be the semiquantitative nature of the results, with the possibility of including false positives. However, to avoid this problem, only positive and highly positive results were included [20]. Also, there was no agreement whether the immunohistochemical analysis should be performed from decalcified or from ground sections, as proposed by some authors [41].
5. Conclusions
In conclusion and within the limitations of this in vivo experimental investigation, the soft tissue height was greater both in its epithelial and connective tissue components around immediate implant placement, compared with delayed implants, especially at the early healing phases. Conversely, the soft tissue thickness was greater around the delayed implants, especially in these early stages. The immunohistochemical results revealed that implants placed into fresh extraction sockets demonstrated an increased bone metabolism expressed by a higher osteoblastic and osteoclastic activity, especially in the early healing stages.
Author Contributions
R.P.: Data retrieval, data analysis, performed histological measurements and writing the manuscript. J.S.-E.: Data analysis, participated in the surgical procedures, protocol design and manuscript editing. D.P.: performed the histomorphometry evaluation. F.V.: Participated in the surgical procedures, protocol design, manuscript editing. F.L.: Participated in the surgical procedures. M.S.: Participated in the protocol design and manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by a research grant from Sweden/Martina dental implants (Padova, Italy).
Institutional Review Board Statement
This experimental in vivo investigation was designed in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for preclinical research and was conducted in compliance with the Spanish and Euro-pean Union regulations (European Communities Council Directive 86/609/EEC) on experimental in vivo experimenta-tion. The experimental part of the study was carried out at the ‘Veterinary Teaching Hospital of the University of Santi-ago in Lugo, Spain’ once the study protocol was approved by the Ethical Committee of the Rof Codina Foundation (Lugo, Spain) (code: AELUOO1/14/INVMED/OUTROS (04)/FMG/05).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare to have no conflicts of interest.
Appendix A

Table A1.
Antigen Reactivity of Immunohistochemical Analysis (Mean ± SD Values).
Table A1.
Antigen Reactivity of Immunohistochemical Analysis (Mean ± SD Values).
| Time | Group | Alkaline Phosphatase | Matrix Metalloproteinase 2 | Osteopontin | Tartrate Resistant Acid Phosphatase | Vascular Endothelial Growth Factor | Lymphocites CD4 | Myeloperoxidase |
|---|---|---|---|---|---|---|---|---|
| 2 wk | Test | 6.85 ± 5.17 * | 1.59 ± 1.64 * | 0.11 ± 0.18 | 2.25 ± 1.99 | 0.22 ± 0.46 | 0.13 ± 0.18 | 6.61 ± 7.31 |
| 2 wk | Control | 3.56 ± 2.31 | 0.58 ± 0.48 | 0.09 ± 0.14 | 2.39 ± 2.05 | 0.08 ± 0.14 | 0.31 ± 0.64 | 2.87 ± 1.24 |
| 8 wk | Test | 4.48 ± 3.31 | 1.34 ± 1.85 | 0.13 ± 0.33 | 4.28 ± 1.55 | 0.23 ± 0.42 | 0.13 ± 0.27 | 5.49 ± 3.9 |
| 8 wk | Control | 2.93 ± 1.38 | 0.95 ± 1.48 | 0.14 ± 0.33 | 3.62 ± 2.21 | 0.26 ± 0.29 | 0.03 ± 0.04 | 3.05 ± 2.33 |
* Significantly different from control group 2 weeks (p < 0.05).
References
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Sukekava, F.; Wennstrom, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Vina-Almunia, J.; Candel-Marti, M.E.; Cervera-Ballester, J.; Garcia-Mira, B.; Calvo-Guirado, J.L.; Penarrocha-Oltra, D.; Penarrocha-Diago, M. Buccal bone crest dynamics after immediate implant placement and ridge preservation techniques: Review of morphometric studies in animals. Implant. Dent. 2013, 22, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.; Salvi, G.E.; Iorio Siciliano, V.; Cafiero, C.; Blasi, A.; Lang, N.P. Dimensional ridge alterations following immediate implant placement in molar extraction sites: A six-month prospective cohort study with surgical re-entry. Clin. Oral. Implant. Res. 2009, 20, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Sanz, M. Immediate implants at fresh extraction sockets: From myth to reality. Periodontol. 2000 2014, 66, 132–152. [Google Scholar] [CrossRef]
- De Rouck, T.; Collys, K.; Cosyn, J. Immediate single-tooth implants in the anterior maxilla: A 1-year case cohort study on hard and soft tissue response. J. Clin. Periodontol. 2008, 35, 649–657. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Cortellini, P.; Graziani, F.; Cairo, F.; Lang, N.P.; Abundo, R.; Conforti, G.P.; Marquardt, S.; Rasperini, G.; Silvestri, M.; et al. Immediate versus delayed implant placement after anterior single tooth extraction: The timing randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 215–224. [Google Scholar] [CrossRef]
- Blanco, J.; Alves, C.C.; Nunez, V.; Aracil, L.; Munoz, F.; Ramos, I. Biological width following immediate implant placement in the dog: Flap vs. flapless surgery. Clin. Oral. Implant. Res. 2010, 21, 624–631. [Google Scholar] [CrossRef]
- Vignoletti, F.; de Sanctis, M.; Berglundh, T.; Abrahamsson, I.; Sanz, M. Early healing of implants placed into fresh extraction sockets: An experimental study in the beagle dog. III: Soft tissue findings. J. Clin. Periodontol. 2009, 36, 1059–1066. [Google Scholar] [CrossRef]
- Rimondini, L.; Bruschi, G.B.; Scipioni, A.; Carrassi, A.; Nicoli-Aldini, N.; Giavaresi, G.; Fini, M.; Mortellaro, C.; Giardino, R. Tissue healing in implants immediately placed into postextraction sockets: A pilot study in a mini-pig model. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2005, 100, e43–e50. [Google Scholar] [CrossRef]
- Mair, B.; Fuerst, G.; Kubitzky, P.; Tangl, S.; Bergmeister, H.; Losert, U.; Watzek, G.; Gruber, R. The anti-angiogenic substance TNP-470 impairs peri-implant bone formation: A pilot study in the rabbit metaphysis model. Clin. Oral. Implant. Res. 2007, 18, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Vigon, A.; Martinez-Villa, S.; Suarez, I.; Vignoletti, F.; Sanz, M. Histomorphometric and immunohistochemical evaluation of collagen containing xenogeneic bone blocks used for lateral bone augmentation in staged implant placement. Int. J. Implant. Dent. 2017, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef] [PubMed]
- Thieu, M.K.L.; Stoetzel, S.; Rahmati, M.; El Khassawna, T.; Verket, A.; Sanz-Esporrin, J.; Sanz, M.; Ellingsen, J.E.; Haugen, H.J. Immunohistochemical comparison of lateral bone augmentation using a synthetic TiO2 block or a xenogeneic graft in chronic alveolar defects. Clin. Implant. Dent. Relat. Res. 2023, 25, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Abrahamsson, I. Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. J. Clin. Periodontol. 2012, 39 (Suppl. S12), 6–27. [Google Scholar] [CrossRef]
- Vignoletti, F.; Sanz-Esporrin, J.; Sanz-Martin, I.; Nunez, J.; Luengo, F.; Sanz, M. Ridge alterations after implant placement in fresh extraction sockets or in healed crests: An experimental in vivo investigation. Clin. Oral. Implant. Res. 2019, 30, 353–363. [Google Scholar] [CrossRef]
- Sanz-Martin, I.; Vignoletti, F.; Nunez, J.; Permuy, M.; Munoz, F.; Sanz-Esporrin, J.; Fierravanti, L.; Shapira, L.; Sanz, M. Hard and soft tissue integration of immediate and delayed implants with a modified coronal macrodesign: Histological, micro-CT and volumetric soft tissue changes from a pre-clinical in vivo study. J. Clin. Periodontol. 2017, 44, 842–853. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J. Clin. Periodontol. 1994, 21, 189–193. [Google Scholar] [CrossRef]
- Schroeder, H.E. Ultrastructure of the junctional epithelium of the human gingiva. Helv. Odontol. Acta 1969, 13, 65–83. [Google Scholar]
- Cha, J.K.; Pla, R.; Vignoletti, F.; Jung, U.W.; Sanz-Esporrin, J.; Sanz, M. Immunohistochemical characteristics of lateral bone augmentation using different biomaterials around chronic peri-implant dehiscence defects: An experimental in vivo study. Clin. Oral. Implant. Res. 2021, 32, 569–580. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Araujo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Abrahamsson, I.; Welander, M.; Lang, N.P.; Lindhe, J. Morphogenesis of the peri-implant mucosa: An experimental study in dogs. Clin. Oral. Implant. Res. 2007, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Cochran, D.L. Crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged and submerged implants in the canine mandible. J. Periodontol. 2000, 71, 1412–1424. [Google Scholar] [CrossRef]
- Blanco, J.; Nunez, V.; Aracil, L.; Munoz, F.; Ramos, I. Ridge alterations following immediate implant placement in the dog: Flap versus flapless surgery. J. Clin. Periodontol. 2008, 35, 640–648. [Google Scholar] [CrossRef]
- Monje, A.; Chappuis, V.; Monje, F.; Munoz, F.; Wang, H.L.; Urban, I.A.; Buser, D. The Critical Peri-implant Buccal Bone Wall Thickness Revisited: An Experimental Study in the Beagle Dog. Int. J. Oral. Maxillofac. Implant. 2019, 34, 1328–1336. [Google Scholar] [CrossRef]
- Ellis, R.; Chen, S.; Davies, H.; Fitzgerald, W.; Xu, J.; Darby, I. Primary stability and healing outcomes of apically tapered and straight implants placed into fresh extraction sockets. A pre-clinical in vivo study. Clin. Oral. Implant. Res. 2020, 31, 705–714. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Qiao, S.; Zhang, X.; Lai, H.; Gu, Y. Peri-implant tissue alteration around tissue-level and bone-level implants in fresh extraction sockets: A histomorphometric study in dogs. Ann. Transl. Med. 2021, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Ferrari, D.; Herten, M.; Kirsch, A.; Schaer, A.; Schwarz, F. Influence of platform switching on crestal bone changes at non-submerged titanium implants: A histomorphometrical study in dogs. J. Clin. Periodontol. 2007, 34, 1089–1096. [Google Scholar] [CrossRef]
- Caneva, M.; Salata, L.A.; de Souza, S.S.; Baffone, G.; Lang, N.P.; Botticelli, D. Influence of implant positioning in extraction sockets on osseointegration: Histomorphometric analyses in dogs. Clin. Oral. Implant. Res. 2010, 21, 43–49. [Google Scholar] [CrossRef]
- Chappuis, V.; Engel, O.; Shahim, K.; Reyes, M.; Katsaros, C.; Buser, D. Soft Tissue Alterations in Esthetic Postextraction Sites: A 3-Dimensional Analysis. J. Dent. Res. 2015, 94, 187S–193S. [Google Scholar] [CrossRef]
- Halling Linder, C.; Ek-Rylander, B.; Krumpel, M.; Norgard, M.; Narisawa, S.; Millan, J.L.; Andersson, G.; Magnusson, P. Bone Alkaline Phosphatase and Tartrate-Resistant Acid Phosphatase: Potential Co-regulators of Bone Mineralization. Calcif. Tissue Int. 2017, 101, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Stucki, U.; Schmid, J.; Hammerle, C.F.; Lang, N.P. Temporal and local appearance of alkaline phosphatase activity in early stages of guided bone regeneration. A descriptive histochemical study in humans. Clin. Oral. Implant. Res. 2001, 12, 121–127. [Google Scholar] [CrossRef]
- Sela, J.; Gross, U.M.; Kohavi, D.; Shani, J.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Primary mineralization at the surfaces of implants. Crit. Rev. Oral. Biol. Med. 2000, 11, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, L.; Tan, Y. Different roles of matrix metalloproteinase 2 in osteolysis of skeletal dysplasia and bone metastasis (Review). Mol. Med. Rep. 2020, 23, 70. [Google Scholar] [CrossRef]
- Dew, G.; Murphy, G.; Stanton, H.; Vallon, R.; Angel, P.; Reynolds, J.J.; Hembry, R.M. Localisation of matrix metalloproteinases and TIMP-2 in resorbing mouse bone. Cell Tissue Res. 2000, 299, 385–394. [Google Scholar] [CrossRef]
- Accorsi-Mendonca, T.; Paiva, K.B.; Zambuzzi, W.F.; Cestari, T.M.; Lara, V.S.; Sogayar, M.C.; Taga, R.; Granjeiro, J.M. Expression of matrix metalloproteinases-2 and -9 and RECK during alveolar bone regeneration in rat. J. Mol. Histol. 2008, 39, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Alexandersen, P.; Karsdal, M.A.; Qvist, P.; Schaller, S.; Tankó, L.B. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur. J. Clin. Pharmacol. 2006, 62, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.Y.; Park, Y.S.; Pippenger, B.E.; Lee, B.; Miron, R.J.; Dard, M. Dimensional Changes Following Immediate and Delayed Implant Placement: A Histomorphometric Study in the Canine. Int. J. Oral. Maxillofac. Implant. 2017, 32, 541–546. [Google Scholar] [CrossRef]
- Pei, X.; Wang, L.; Chen, C.; Yuan, X.; Wan, Q.; Helms, J.A. Contribution of the PDL to Osteotomy Repair and Implant Osseointegration. J. Dent. Res. 2017, 96, 909–916. [Google Scholar] [CrossRef]
- Yuan, X.; Pei, X.; Zhao, Y.; Li, Z.; Chen, C.H.; Tulu, U.S.; Liu, B.; Van Brunt, L.A.; Brunski, J.B.; Helms, J.A. Biomechanics of Immediate Postextraction Implant Osseointegration. J. Dent. Res. 2018, 97, 987–994. [Google Scholar] [CrossRef]
- Schwarz, F.; Rothamel, D.; Herten, M.; Ferrari, D.; Sager, M.; Becker, J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: An immunohistochemical study in dogs. Clin. Oral. Implant. Res. 2008, 19, 642–652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).