Degradation of a Sauce-Glazed Ware from the Song Dynasty Salvaged Out of Water at the Dalian Island Wharf: Part II—The Effect of Surface-Attached Marine Organism Remains
Abstract
:1. Introduction
2. Methods and Samples
2.1. Methods
2.2. Samples
3. Experimental Results
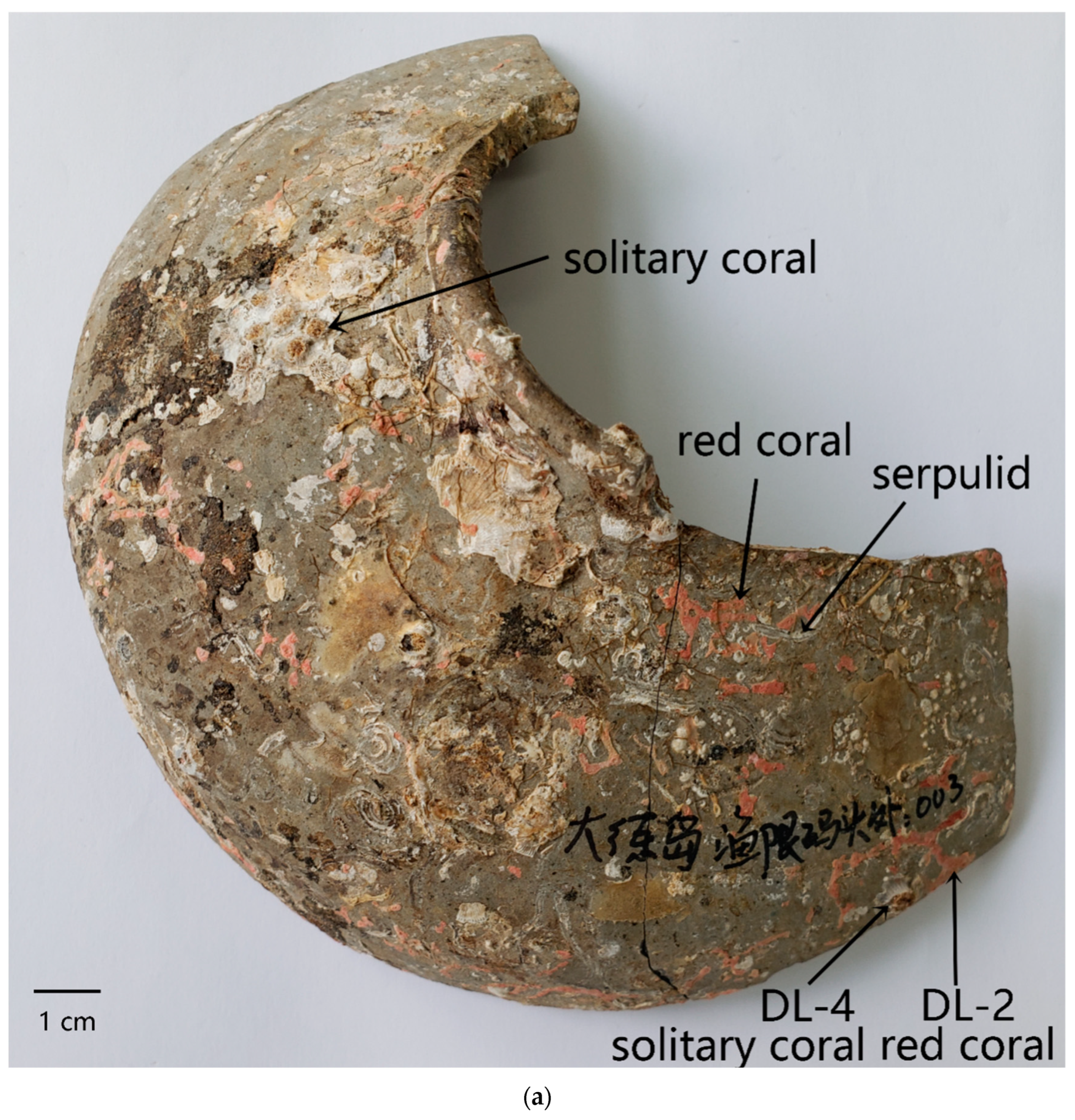
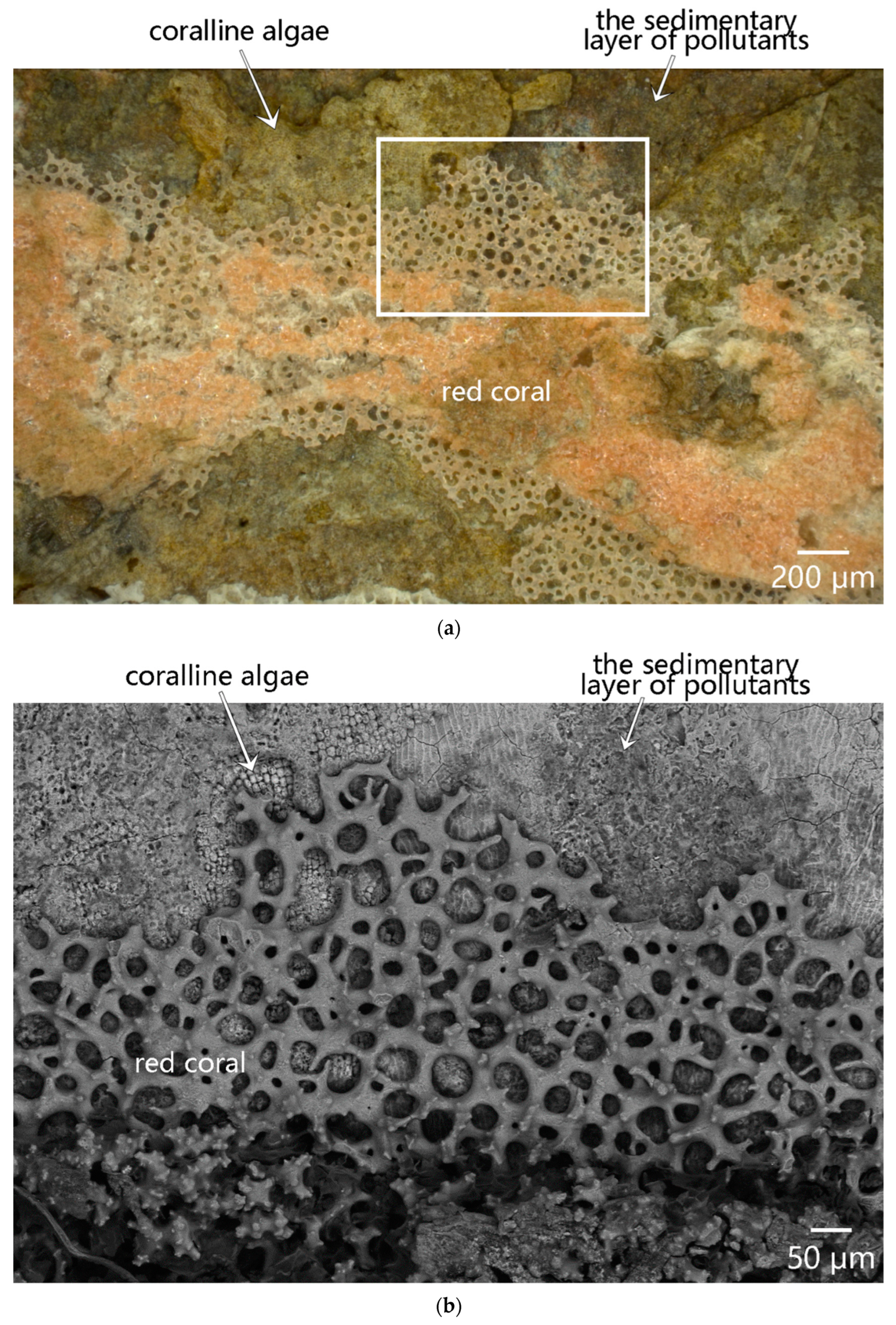
3.1. Morphology of the Attached Marine Organism Remains
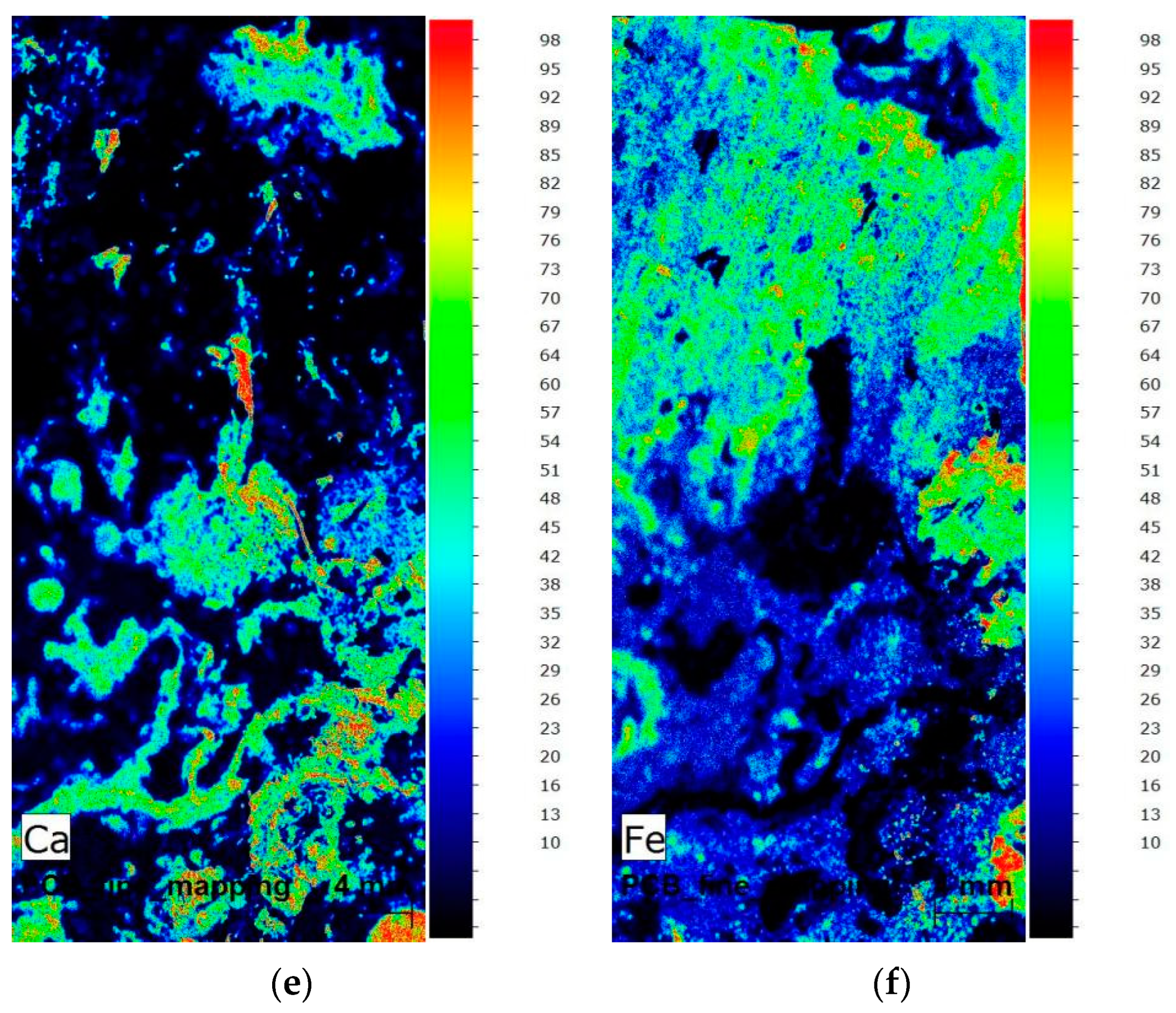
3.2. EDXRF Analysis Results of the Attached Marine Organism Remains
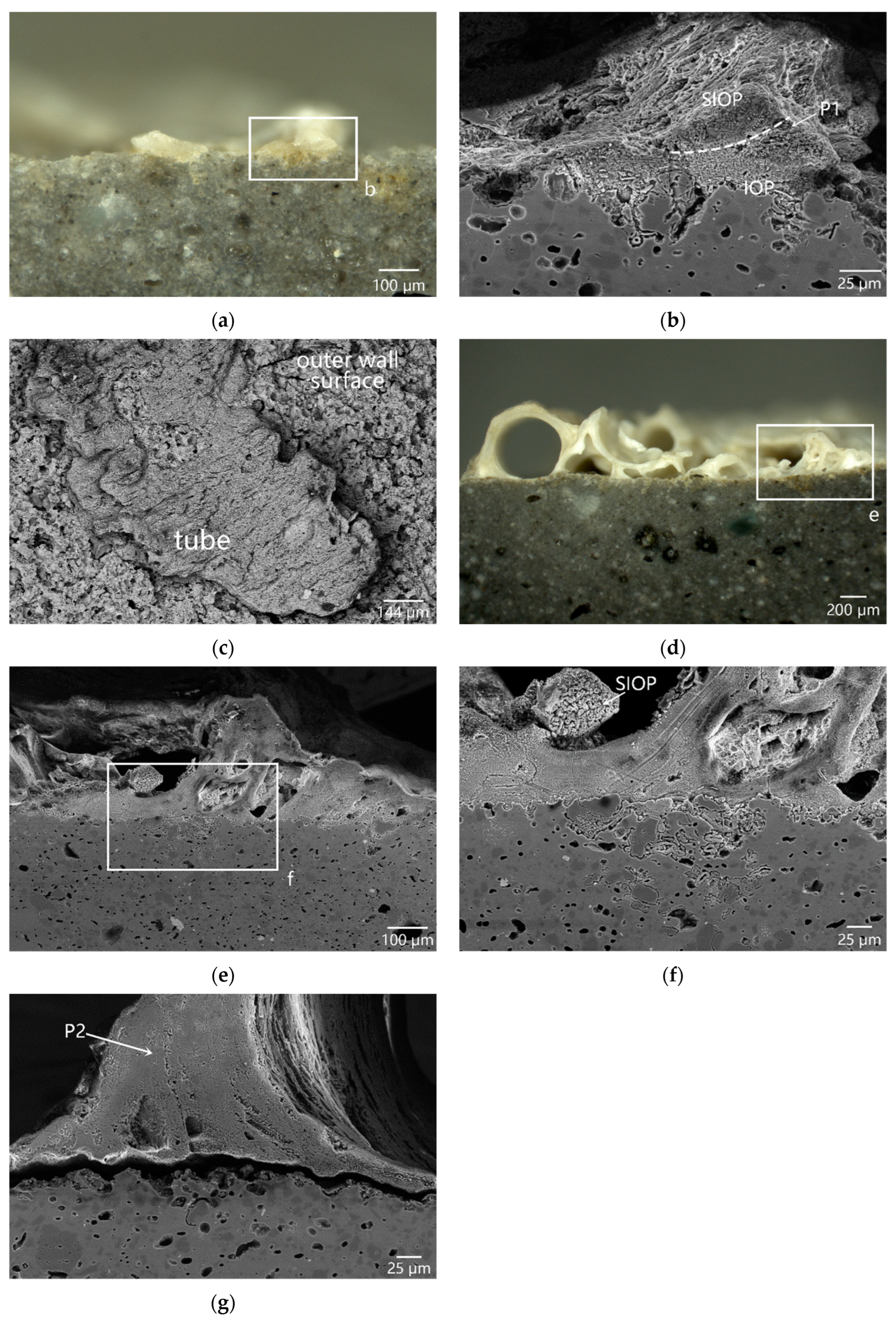
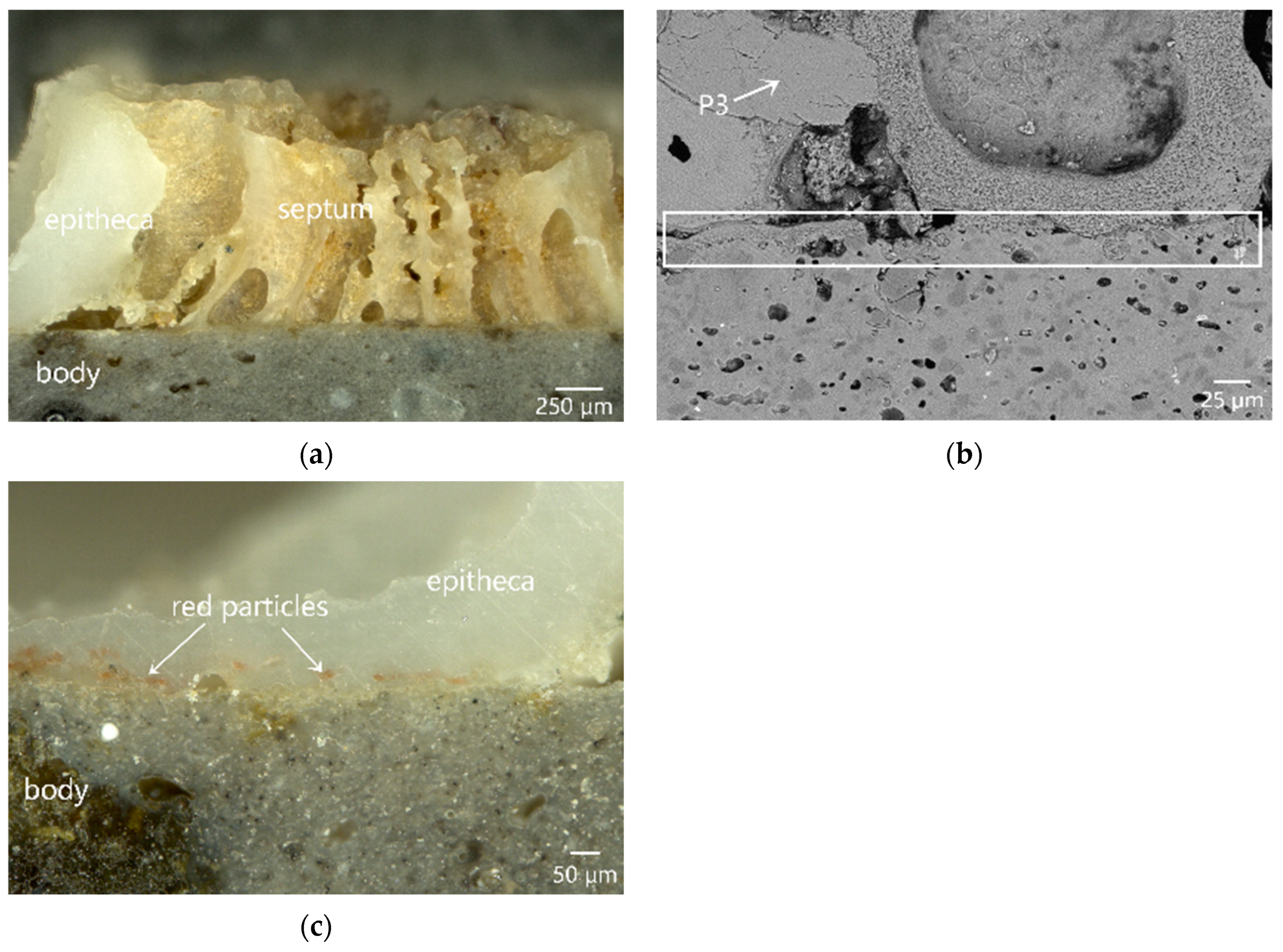
3.3. Microstructure and Micro-Area Composition of the Attached Marine Organism Remains
3.3.1. Unitary Organism: Serpulid and Solitary Coral
3.3.2. Modular Organisms: Bryozoans and Red Coral
3.3.3. Biological Overlapping Phenomenon between Coralline Algae and Red Coral
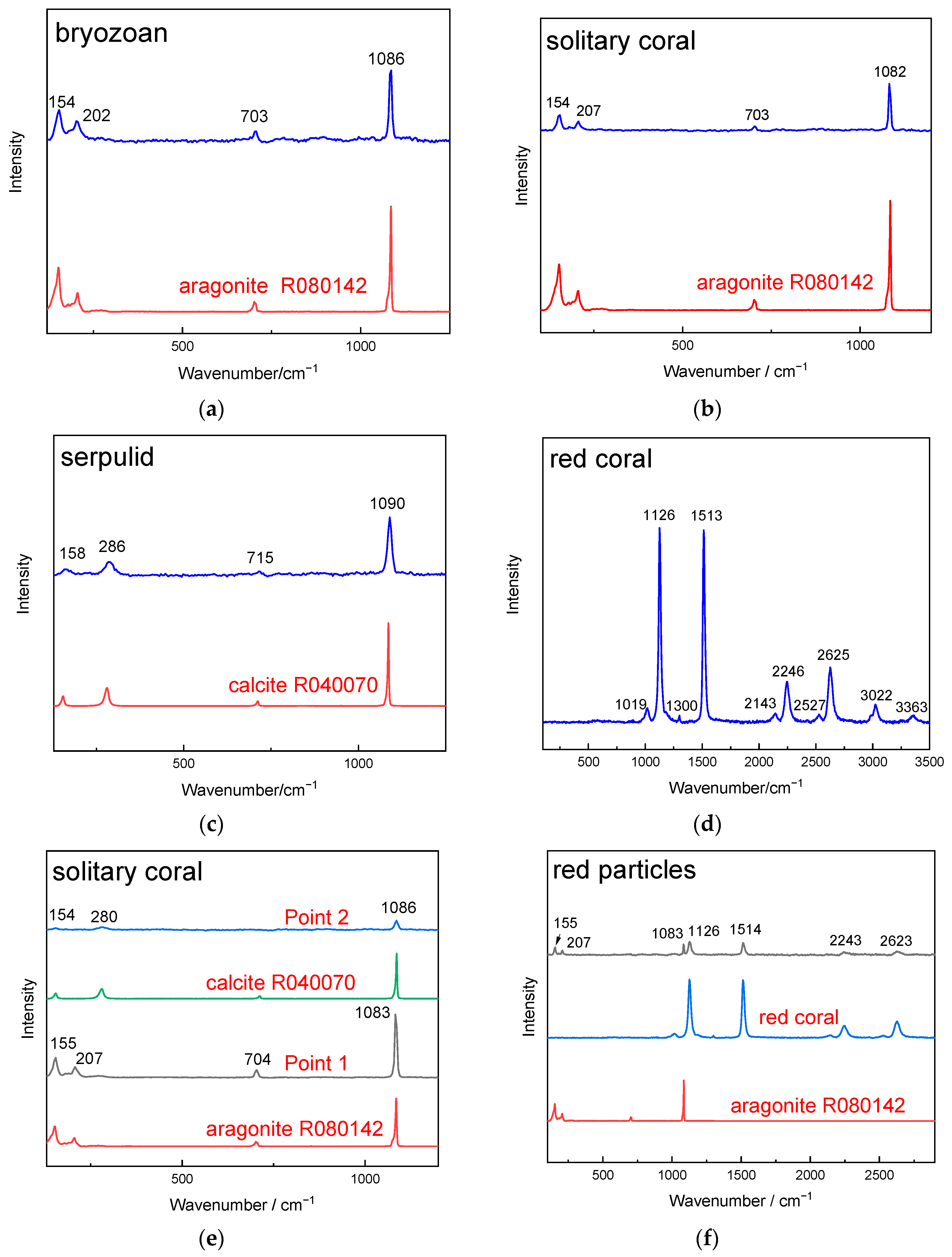
3.4. μ-Raman Analysis Results of Attached Marine Organism Remains
4. Discussion
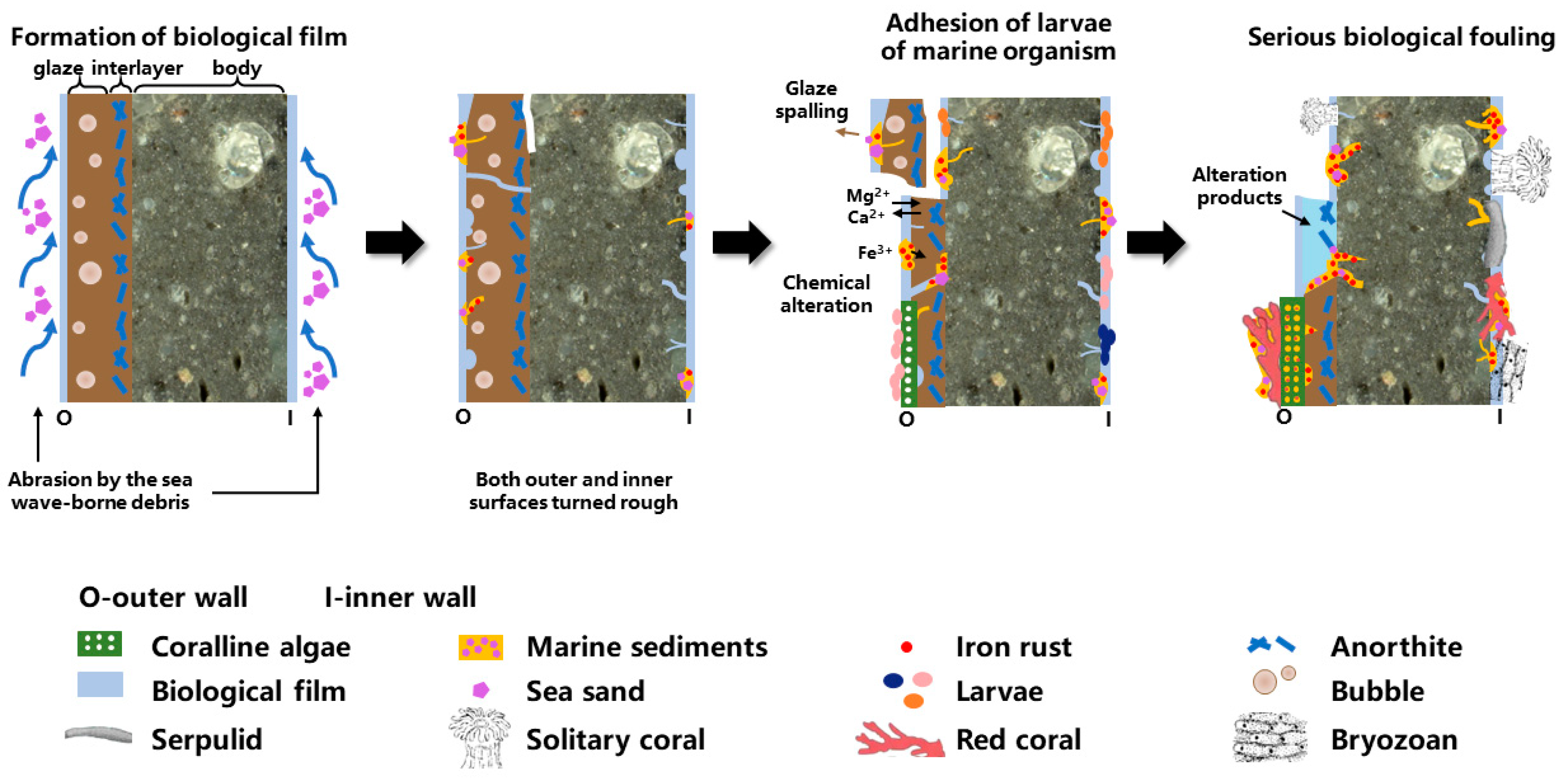
4.1. Effect of Surface Roughness on the Attachment of Marine Organisms
4.2. Effect of Bio-Attachment on the Degradation of Shard DL
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dromgoole, S. UNESCO Convention on the Protection of the Underwater Cultural Heritage. Int. J. Mar. Coast. Law 2001, 18, 59–108. [Google Scholar]
- Pearson, C. Conservation of Marine Archaeological Objects; Butterworth & Co. Ltd.: London, UK, 1987; pp. 16–22, 107–109, 261–264. [Google Scholar]
- Buys, S.; Oakley, V. Conservation and Restoration of Ceramics; Routledge: New York, NY, USA, 2014. [Google Scholar]
- Luka, B.; Martina, C.; Anita, J.; Antonija, J.; Ivo, M.; Mladen, M.; Tanja, P.; Mladen, P. Conservation of Underwater Archaeological Finds Manual; Luka, B., Ed.; International Centre for Underwater Archaeology in Zadar: Zadar, Croatia, 2014. [Google Scholar]
- Li, N.S. Research on the Protection of Porcelain Excavated from the Ocean; Science Press: Beijing, China, 2016. [Google Scholar]
- Wang, Y.R.; Zhu, T.Q.; Yang, G.C.; Tan, X.; Ye, D.; Chen, H. The method to soften the concretions of ceramics in the “Nanhai I” Shipwreck of China Southern Song Dynasty (1127–1279AD). Herit. Sci. 2018, 6, 4. [Google Scholar] [CrossRef]
- Carmona, N.; García-Heras, M.; Gil, C.; Villegas, M.A. Chemical degradation of glasses under simulated marine medium. Mater. Chem. Phys. 2005, 94, 92–102. [Google Scholar] [CrossRef]
- Tournie, A.; Ricciardi, P.; Colomban, P. Glass corrosion mechanisms: A multiscale analysis. Solid State Ion. 2008, 179, 2142–2154. [Google Scholar] [CrossRef]
- Palomar, T.; Llorente, I. Decay processes of silicate glasses in river and marine aquatic environments. J. Non-Cryst. Solids 2016, 449, 20–28. [Google Scholar] [CrossRef]
- Palomar, T. Characterization of the alteration processes of historical glasses on the seabed. Mater. Chem. Phys. 2018, 214, 391–401. [Google Scholar] [CrossRef]
- Elkin, D.; Argüeso, A.; Grosso, M.; Murray, C.; Vainstub, D.; Bastida, R.; Dellino-Musgrave, V. Archaeological research on HMS Swift: A British Sloop-of-War lost off Patagonia, Southern Argentina, in 1770. Int. J. Naut. Archaeol. 2007, 36, 32–58. [Google Scholar] [CrossRef]
- Aloise, P.; Ricca, M.; Russa, M.F.; Ruffolo, S.A.; Belfiore, C.M.; Padeletti, G.; Crisci, G.M. Diagnostic analysis of stone materials from underwater excavations: The case study of the Roman archaeological site of Baia (Naples, Italy). Appl. Phys. A 2013, 114, 655–662. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.D.; Yang, Z.L.; Xu, C.; Lu, X. Degradation Mechanism of a Sauce-GlazedWare of the Song Dynasty Salvaged out of theWater at Dalian Island Wharf: Part I—The Effect of the Surface-Attached Composite Coagula. Materials 2023, 16, 1176. [Google Scholar] [CrossRef]
- Vuorisalo, T.; Tuomi, J. Unitary and Modular Organisms: Criteria for Ecological Division. Oikos 1986, 47, 382–385. [Google Scholar] [CrossRef]
- Pineda-Krch, M.; Poore, A.G. Spatial interactions within modular organisms: Genetic heterogeneity and organism fitness. Theor. Popul. Biol. 2004, 66, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bastida-Zavala, J.R. Serpulids (Annelida: Polychaeta) from the Eastern Pacific, including a brief mention of Hawaiian serpulids. Zootaxa 2008, 1722, 1–61. [Google Scholar] [CrossRef]
- Ten Hove, H.A.; Kupriyanova, E.K. Taxonomy of Serpulidae (Annelida, Polychaeta): The state of affairs. Zootaxa 2009, 2036, 1–126. [Google Scholar]
- Yang, Y.; Guo, Y.; Zhang, Y.; Zou, Y.; Wei, J.; Liang, L. Contribution of coral composition to color red in the uniform color space CIE 1976L*a*b*. Desalin. Water Treat. 2019, 168, 384–393. [Google Scholar] [CrossRef]
- Taylor, P.D.; Kudryavtsev, A.B.; Schopf, J.W. Calcite and aragonite distributions in the skeletons of bimineralic bryozoans as revealed by Raman spectroscopy. Invertebr. Biol. 2008, 127, 87–97. [Google Scholar] [CrossRef]
- Addadi, L.; Berman, A.; Oldak, J.M.; Weiner, S. Structural and stereochemical relations between acidic macromolecules of organic matrices and crystals. Connect. Tissue Res. 1989, 21, 127–135. [Google Scholar] [CrossRef]
- Tanur, A.E.; Gunari, N.; Sullan, R.M.; Kavanagh, C.J.; Walker, G.C. Insights into the composition, morphology, and formation of the calcareous shell of the serpulid Hydroides dianthus. J. Struct. Biol. 2010, 169, 145–160. [Google Scholar] [CrossRef]
- Rodgers, B.A. The Archaeologist’s Manual For Conservation; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004. [Google Scholar]
- Feng, S.; Li, F.; Li, S. Introduction to Marine Science; Higher Education Press: Beijing, China, 1999. [Google Scholar]
- Huggett, M.J.; Carpizo-Ituarte, E.J.; Nedved, B.T.; Hadfield, M.G. Formation and Function of the Primary Tube During Settlement and Metamorphosis of the Marine Polychaete Hydroides elegans (Haswell, 1883) (Serpulidae). Biol. Bull. 2021, 240, 82–94. [Google Scholar] [CrossRef]
- Vinn, O.; Zatoń, M.; Tovar-Hernández, M.A. Tube microstructure and formation in some feather duster worms (Polychaeta, Sabellidae). Mar. Biol. 2018, 165, 98. [Google Scholar] [CrossRef]
- Vinn, O.; Ten Hove, H.A.; Mutvei, H.; KirsimÄE, K. Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida). Zool. J. Linn. Soc. 2008, 154, 633–650. [Google Scholar] [CrossRef]
- Bromley, R.G.; Heinberg, C. Attachment strategies of organisms on hard substrates: A palaeontological view. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 429–453. [Google Scholar] [CrossRef]
- Taylor, P.D.; Lombardi, C.; Cocito, S. Biomineralization in bryozoans: Present, past and future. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1118–1150. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Yang, M.X.; Wang, F.Z. Observations of Surface Microstructures of Corallium Elatius by SEM. In Proceedings of the 2009 China Gems & Jewelry Academic Conference, Beijing, China, 12–16 May 2009; pp. 115–117. [Google Scholar]
- Bergamonti, L.; Bersani, D.; Csermely, D.; Lottici, P.P. The Nature of the Pigments in Corals and Pearls: A Contribution from Raman Spectroscopy. Spectrosc. Lett. 2011, 44, 453–458. [Google Scholar] [CrossRef]
- Wray, J.L. Calcareous Algae; Elsevier Scientific Pub.: Rio de Janeiro, Brazil, 1998. [Google Scholar]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Abarzua, S.; Jakubowski, S. Biotechnological investigation for the prevention of biofouling. I. Biological and biochemical principles for the prevention of biofouling. Mar. Ecol. Prog. Ser. 1995, 123, 301–312. [Google Scholar] [CrossRef]
- Raman, S.; Kumar, R. Interfacial morphology and nanomechanics of cement of the barnacle, Amphibalanus reticulatus on metallic and non-metallic substrata. Biofouling 2011, 27, 569–577. [Google Scholar] [CrossRef]
- Conradt, R. Chemical Durability of Oxide Glasses in Aqueous Solutions: A Review. J. Am. Ceram. Soc. 2008, 91, 728–735. [Google Scholar] [CrossRef]
- Lombardo, T.; Gentaz, L.; Verney-Carron, A.; Chabas, A.; Loisel, C.; Neff, D.; Leroy, E. Characterisation of complex alteration layers in medieval glasses. Corros. Sci. 2013, 72, 10–19. [Google Scholar] [CrossRef]
- Gowers, K.R.; Millard, S.G. On-site linear polarization resistance mapping of reinforced concrete structures. Corros. Sci. 1993, 35, 1593–1600. [Google Scholar] [CrossRef]
- Rodríguez, P.; Ramírez, E.; González, J.A. Methods for studying corrosion in reinforced concrete. Mag. Concr. Res. 1994, 46, 81–90. [Google Scholar] [CrossRef]
- Li, Q.; Ye, X. Surface deterioration analysis for probabilistic durability design of RC structures in marine environment. Struct. Saf. 2018, 75, 13–23. [Google Scholar] [CrossRef]
- Gorb, S.N. Biological attachment devices: Exploring nature’s diversity for biomimetics. Philos. Trans. R. Soc. A 2008, 366, 1557–1574. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.F. A fracture mechanical analysis of fouling release from nontoxic antifouling coatings. Prog. Org. Coat. 2001, 43, 188–192. [Google Scholar] [CrossRef]
- Morse, D.E.; Morse, A.N.C. Enzymatic Characterization of the Morphogen Recognized by Agaricia humilis (Scleractinian Coral) Larvae. Biol. Bull. 1991, 181, 104–122. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ricca, M.; Belfiore, C.M.; Ruffolo, S.A.; De Buergo Ballester, M.A.; Crisci, G.M. The Contribution of Earth Sciences to the Preservation of Underwater Archaeological Stone Materials: An Analytical Approach. Int. J. Conserv. Sci. 2015, 6, 335–348. [Google Scholar]
- Carpizo-Ituarte, E.; Hadfield, M.G. Stimulation of Metamorphosis in the Polychaete Hydroides elegans Haswell (Serpulidae). Biol. Bull. 1998, 194, 14–24. [Google Scholar] [CrossRef]
- Hadfield, M.G. The D P Wilson Lecture. Research on settlement and metamorphosis of marine invertebrate larvae: Past, present and future. Biofouling 1998, 12, 9–29. [Google Scholar] [CrossRef]
- Allemand, D.; Ferrier-Pagès, C.; Furla, P.; Houlbrèque, F.; Puverel, S.; Reynaud, S.; Tambutté, É.; Tambutté, S.; Zoccola, D. Biomineralisation in reef-building corals: From molecular mechanisms to environmental control. Comptes Rendus Palevol 2004, 3, 453–467. [Google Scholar] [CrossRef]
- Gowell, M.R.; Coombes, M.A.; Viles, H.A. Rock-protecting seaweed? Experimental evidence of bioprotection in the intertidal zone. Earth Surf. Process. Landf. 2015, 40, 1364–1370. [Google Scholar] [CrossRef]
- Kawabata, Y.; Kato, E.; Iwanami, M. Enhanced Long-Term Resistance of Concrete with Marine Sessile Organisms to Chloride Ion Penetration. J. Adv. Concr. Technol. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Lv, J.; Mao, J.; Ba, H. Influence of Crassostrea gigas on the permeability and microstructure of the surface layer of concrete exposed to the tidal zone of the Yellow Sea. Biofouling 2015, 31, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.M.; Pitman, A.J.; Henderson, S.M. Developments in the understanding of the biology of marine wood boring crustaceans and in methods of controlling them. Int. Biodeterior. Biodegrad. 1999, 43, 197–205. [Google Scholar] [CrossRef]
- Sangeetha, R.; Kumar, R.; Doble, M.; Venkatesan, R. Barnacle cement: An etchant for stainless steel 316L? Colloids Surf. B Biointerfaces 2010, 79, 524–530. [Google Scholar] [CrossRef] [PubMed]







| Organism Remains | Na | Mg | Al | Si | S | Cl | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Serpulid | 3.43 | 3.46 | 6.06 | 0.54 | 0.26 | 0.70 | 79.99 | 3.90 | |
| Solitary coral | 1.07 | 0.38 | 0.96 | 1.51 | 1.79 | 2.47 | 0.12 | 87.02 | 0.48 |
| Red coral | 4.65 | 0.56 | 1.31 | 0.66 | 0.23 | 0.36 | 88.04 | 3.07 | |
| Bryozoan | 0.15 | 1.51 | 7.57 | 13.26 | 0.42 | 0.45 | 1.16 | 70.64 | 3.47 |
| Na | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Mn | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 0.59 | 1.16 | 24.47 | 49.93 | 0.28 | 1.39 | 3.50 | 1.50 | 1.67 | 0.22 | 15.03 | |
| P2 | 1.65 | 5.07 | 0.67 | 1.75 | 0.24 | 0.52 | 1.01 | 0.07 | 87.03 | 0.07 | 0.49 | 0.49 |
| P3 | 2.27 | 2.66 | 13.19 | 31.14 | 0.04 | 2.08 | 3.22 | 4.00 | 3.42 | 1.17 | 1.64 | 34.97 |
| P4 | 7.54 | 4.97 | 20.82 | 0.12 | 0.35 | 2.63 | 1.04 | 11.78 | 0.08 | 3.10 | 47.22 | |
| P5 | 1.80 | 3.29 | 1.16 | 1.82 | 3.66 | 6.7 | 13.52 | 0.72 | 63.03 | 0.12 | 0.65 | 2.59 |
| P6 | 3.00 | 3.22 | 0.76 | 2.68 | 17.24 | 4.49 | 11.17 | 2.38 | 32.02 | 0.94 | 1.25 | 20.28 |
| P7 | 0.48 | 0.77 | 0.37 | 0.21 | 95.36 | 0.01 | 0.05 | 0.29 |
| Na | Mg | Al | Si | Ca | Fe | P | S | |
|---|---|---|---|---|---|---|---|---|
| Tube of Serpulid on the surface of outer wall (P1) | 3.03 | 24.23 | 1.13 | 1.70 | 66.92 | 1.43 | 1.56 | |
| Tube of Serpulid on the surface of inner wall (P2) | 23.56 | 4.35 | 12.66 | 58.35 | 1.08 | |||
| Epitheca of solitary coral (P3) | 1.55 | 0.59 | 97.35 | 0.52 | ||||
| Bryozoans skeletons on the surface of outer wall (P4) | 3.95 | 5.65 | 86.41 | 3.99 | ||||
| Bryozoans skeletons on the surface of inner wall (P5) | 4.89 | 1.03 | 92.94 | 1.14 | ||||
| Red coral skeletons (P6) | 2.07 | 22.83 | 73.35 | 1.75 | ||||
| Foreign pollutants (P7) | 5.90 | 5.11 | 14.70 | 1.58 | 70.62 | 2.09 | ||
| Quartz in body (P8) | 1.66 | 94.29 | 4.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, R.; Li, W.; Yang, Z.; Xu, C.; Lu, X. Degradation of a Sauce-Glazed Ware from the Song Dynasty Salvaged Out of Water at the Dalian Island Wharf: Part II—The Effect of Surface-Attached Marine Organism Remains. Appl. Sci. 2024, 14, 8596. https://doi.org/10.3390/app14198596
Ding R, Li W, Yang Z, Xu C, Lu X. Degradation of a Sauce-Glazed Ware from the Song Dynasty Salvaged Out of Water at the Dalian Island Wharf: Part II—The Effect of Surface-Attached Marine Organism Remains. Applied Sciences. 2024; 14(19):8596. https://doi.org/10.3390/app14198596
Chicago/Turabian StyleDing, Rao, Weidong Li, Zelin Yang, Changsong Xu, and Xiaoke Lu. 2024. "Degradation of a Sauce-Glazed Ware from the Song Dynasty Salvaged Out of Water at the Dalian Island Wharf: Part II—The Effect of Surface-Attached Marine Organism Remains" Applied Sciences 14, no. 19: 8596. https://doi.org/10.3390/app14198596







