Abstract
Per- and polyfluoroalkyl substances (PFAS) are diverse synthetic chemicals manufactured over seven decades. It is an aliphatic molecule with a basic hydrophobic structure of carbon and fluorine linked to a hydrophilic end group. Due to their physicochemical properties associated with the unique structure, PFAS has been used in a wide variety of applications including aqueous film-forming foams (AFFF), paper, carpets, non-stick cookware, etc. as they make products resistant to water, heat, and stains. These molecules have drawn great attention recently for their unique properties, high stability and low degradability, and so-called “Forever Chemicals”. PFAS has the strongest carbon-fluorine bond which makes them persistent in the environment. Hence it contaminates natural resources and endangers public health. This review discusses the discovery, development, and evolution of PFAS from the wonder chemical era to a nightmare chemical era, exposure and its impacts on human health and the environment, current remediation techniques, and future trends of PFAS molecules and related products. The primary objective of this review is to identify knowledge gaps on PFAS contamination, remediation methods, and possible PFAS alternatives.
1. Introduction and Historical Development of PFAS
1.1. Historical Development of PFAS
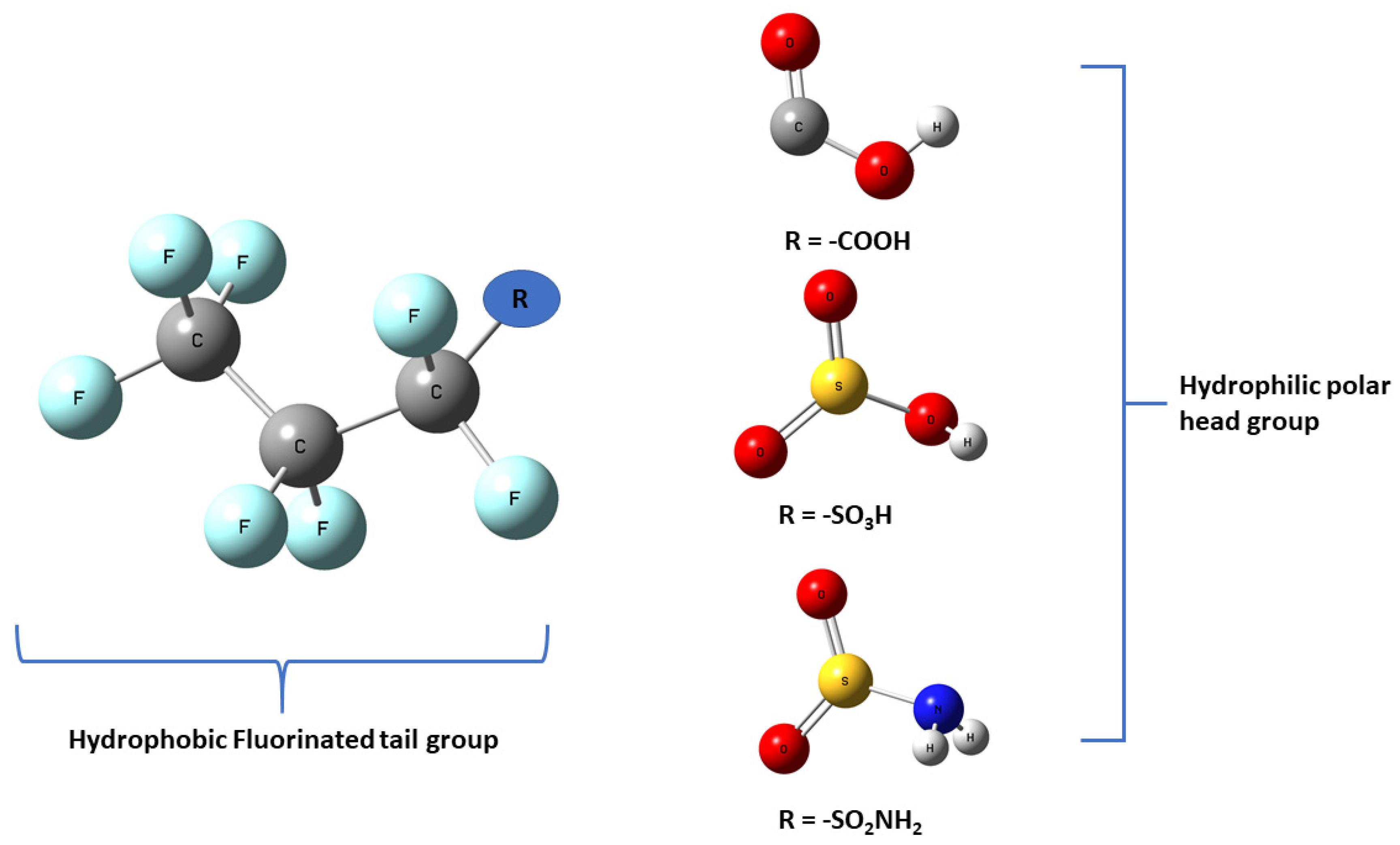
Life depends on the interactions between carbon and hydrogen, forming crucial bonds essential for many organic molecules. However, by the early 1930s scientists had been successful in creating the strongest single bond with carbon (C) replacing the hydrogen (H) with fluorine (F) atom in the backbone (Bond Energy: 536 kJ/mol) [1]. This leads to the development of the most resilient compounds in the synthetic chemical world today, so-called per- and poly-fluoroalkyl substances denoted by the acronym PFAS. PFAS is a group of over 5000 different synthetic aliphatic substances that contains the hydrophobic backbone (tail group) generally a C backbone with F atoms saturating most C atoms and at least one functional group (R) such as carboxylate (-COO−), sulfonate (-SO3−), amin (-NH2), sulphonamide (-SO2NH2), alcohol (-OH) and phosphonate (-PO32−) as a hydrophilic end group (head group) [2,3] as shown in Figure 1.
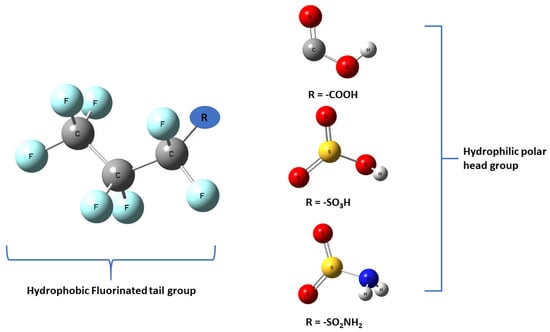
Figure 1.
Schematic representation of the structure of PFAS molecule. R stands for the functional head group.
Synthesizing and introducing this extensive synthetic family of compounds in the 1950’s, PFAS dubbed “forever chemicals” due to their high environmental persistence. PFAS became modern science’s “wonder chemical” because of its uniquely useful chemical and physical properties including innate chemical and thermal stability and lipid and water-repelling properties. These chemical and physical properties are due to the molecular structure of PFAS as the highly fluorinated tail part makes the molecule lipophobic and hydrophobic but the polar functional or the head group makes the molecule interact with polar molecules [4].
Polychlorotrifluoroethylene (PCTFE) was discovered in 1934 by Fritz Schloffer and Otto Scherer as the first form of the PFAS molecule and it was commercialized in the early 1950s. While working in DuPont laboratories in searching for an alternative to existing fluorocarbon-based refrigerants, Roy J. Plunkett and his assistant Jack Rebok accidentally found polytetrafluoroethylene (PTFE), a white waxy solid [5], which was later trademarked as Teflon and used in World War II, the Apollo space mission to the Teflon-coated kitchen pan in the modern world. During the Manhattan Project, DuPont used Teflon in warheads, liquid-fuel tanks and in pipes to hold toxic uranium hexafluoride at the uranium plant in Oak Ridge, Tennessee. Tetrafluoroethylene (C2F4) (TFE) was used as the monomer to synthesis polymerized TFE or Teflon using either suspension polymerization or emulsion polymerization. At the same time a multinational company, Minnesota Mining and Manufacturing Co. (3M) developed the PFAS by producing perfluorooctane sulfonic acid (PFOS) which can be used in fabric stains and water-repellant sprays including Scotchgard. Teflon was used in the Apollo space mission as seals on ventilators and in emerging medical technologies as a coating material for catheters [6,7].
Dupont started to produce Teflon on a commercial scale after the Second World War to facilitate it in other products such as cookware, stain repellents in fabrics and textiles, industrial coatings, and countless other products [7,8]. Meanwhile, Joseph A Simons worked on making more complex fluorocarbons under mild conditions to minimize the explosion during working with chemicals, referred as the “Simons Process”. This process involves electrochemical fluorination of hydrocarbons. When manufacturing PFOA, octanoyl chloride or octanoic acid was used as the starting material. In 1945 3M utilized the Simons Process to manufacture perfluorooctanoic acid (PFOA), which has properties that resist heat, oil, stains, grease, and water. Later in 1960s, 3M manufactured aqueous film-forming foam (AFFF) as “Legacy PFOS AFFF or Light Water”. This was first used by the U.S. Naval firefighting services as they showed advancements in fire suppression performance and increased firefighting safety [6,7,8,9].
1.2. Current Classification of PFAS
Buck et al. [10] defined PFAS as an aliphatic substance containing a –CnF2n+1 (where n is at least 1) moiety within its structure while the OECD/UNEP Global PFC Group proposed to include the chemical with at least one aliphatic perfluoro carbon moiety (–CnF2n–) as PFAS [11]. However, the exact definition is still under discussion. PFAS molecules can be also categorized as short-chain (with ≤7 carbon atoms in the chain) and long-chain with ≥8 carbon atoms in the chain) compounds (See the different color classifications in Table 1). Long-chain molecules have a higher bioaccumulation ability when compared with short-chain molecules. The unique chemical structure gives PFAS molecules a strong dielectric property which makes them excellent electrical insulators and able to prevent the electric current through materials.

Table 1.
PFAS classification according to the Carbon chain length.
PFAS substances are also grouped into two broad categories as polymeric and non-polymeric molecules. PFOA and PFOS are two well-known non-polymeric PFAS substances and these non-polymeric substances can be further divided as perfluoroalkyl and polyfluoroalkyl substances. Perfluoroalkyl substances have totally fluorinated carbon chain where polyfluoroalkyl substances have at least one (but not all) fluorinated carbon atom in the chain.
Polymeric PFAS substances are sub grouped as fluoropolymers (ex: Teflon/PTFE), side-chain fluorinated polymers, and perfluoropolyethers [2,10,11]. Physical characteristics like molar mass, molar volume, surface area, and melting point of PFAS are increasing with the number of carbon atoms in the chain. PFAS is also well-known for its non-stick, low-friction properties, and water-repellent properties. But due to having a very strong C–F bond PFAS molecules are also chemically inert and hence they remain stable and unreactive in the environment for a long time or forever and referred asForever Chemicals”. Thus, they became biologically and environmentally persistent and show bioaccumulation potential. PFAS molecules are water-soluble and dissolve in water and infiltrate groundwater using natural solubility pathways. This can lead to ecological impacts and drinking water supplies [12,13,14,15].
1.3. Health Impacts of PFAS Exposure
The persistence of PFAS in the environment and their accumulation in human tissues have raised significant health concerns. Numerous studies have documented the adverse effects of PFAS exposure on various organ systems, highlighting the need for increased awareness and regulatory actions.
PFAS exposure has been shown to induce metabolic dysregulation and phenotypic changes in hepatocytes, leading to hepatotoxicity, oxidative stress, and inflammatory responses. These changes underscore the potential health risks associated with PFAS exposure, particularly regarding liver health [16,17,18,19].
In children, PFAS exposure has been linked to a slight decrease in antibody responses and potential associations with childhood infections, particularly with specific compounds such as PFOS, PFOA, PFHxS, and PFNA. This suggests that even low levels of PFAS exposure during childhood could lead to compromised immune function and increased susceptibility to infections [20].
PFAS also impacts female reproductive health, with evidence linking exposure to conditions like polycystic ovary syndrome (PCOS), endometriosis, premature ovarian insufficiency (POI), and diminished ovarian reserve (DOR). These findings emphasize the need for further research to understand the mechanisms by which PFAS affect ovarian function and reproductive health [21].
Furthermore, PFAS accumulates in the liver and kidneys, where they can interfere with liver transporters and contribute to adverse health outcomes, such as increased serum cholesterol levels. Understanding the interaction between PFAS and liver transporters is crucial for assessing the risks associated with PFAS exposure [22].
PFAS have been found to induce immune-toxic effects at low levels, potentially leading to reduced immune responses and increased susceptibility to infections and diseases. Computational models, such as agent-based modelling (ABM) and physiologically based kinetic (PBK) models, are being used to understand PFAS mechanisms better and inform risk assessments and regulatory decisions [23]. PFAS exposure is also associated with a range of health issues, including thyroid disorders, kidney dysfunction, liver damage, and an increased risk of certain cancers [24].
Additionally, PFAS exposure has been linked to DNA methylation changes, which may disrupt normal gene expression and contribute to the development of cancer and other health disorders. The potential effects of PFAS on pregnancy outcomes, including complications and impacts on fetal development, further underscore the need for comprehensive research on PFAS exposure, particularly in vulnerable populations like pregnant women and children [21,25].
The binding of PFAS to serum proteins varies based on carbon chain length, affecting their toxicity and elimination of half-lives. Understanding this variability is essential for predicting PFAS behavior in human serum and assessing associated health risks [26].
Epidemiological studies have demonstrated that PFAS exposure is associated with increased risks of certain cancers, reduced immune function, and developmental delays in infants. Moreover, PFAS may disrupt endocrine functions, leading to metabolic and thyroid disorders, particularly in vulnerable populations such as postmenopausal women [27]. Evidence from studies in the Czech Republic suggests that PFAS may have immunosuppressive effects, increasing the risk of immune-mediated diseases like eczema and allergies [28].
Finally, PFAS pose significant health risks due to their ability to cause oxidative stress, immune system disruption, metabolic impairment, and alterations in gut microbiota. These molecular mechanisms contribute to enhanced toxicity, particularly in susceptible populations, underscoring the urgent need for research to develop effective mitigation strategies [29]. Additionally, short-chain PFAS can modulate human cytochrome P450 enzymes, impacting xenobiotic metabolism and potentially affecting overall human health [30,31].
The above growing body of evidence on PFAS highlights their significant health risks, particularly in vulnerable populations. The wide-ranging effects of PFAS on metabolic processes, immune function, reproductive health, and cancer risk necessitate continued research and regulatory efforts to mitigate exposure and protect public health.
Over the past several years the term PFAS has raised public, scientific, and regulatory concerns because of their persistence, and some early evidence by the Environmental Protection Agency (EPA), suggested the detention of traces of PFOA detected in blood in almost all U.S. citizens [2,12,13,14,15]. A survey by the National Health and Nutrition Examination (NHANES) in 2011–2012 suggested that PFAS was detected in the blood of 97% of U.S. citizens tested. These statistics alarmed the public and the responsible parties as PFAS exposure will lead to major health impacts such as cancer, thyroid disorders, developmental issues, and immune system problems and made PFAS, a “nightmare chemical” in the world. In this manuscript, a comprehensive review of the background of PFAS was performed, how it developed and evolved from a wonder chemical to a nightmare chemical, current applications, human exposure, and related health impacts and remediation, and the future of PFAS is described to fill in gaps in the literature. The specific objectives of this review are:
- How PFAS were discovered and their initial applications.
- Why was PFAS considered a “wonder chemical” and later a “nightmare chemical”?
- How widespread is PFAS contamination globally and what is its environmental stability?
- What are the known environmental and health impacts of PFAS?
- What are the Effective PFAS remediation methods and future use of PFAS?
In addition to the comprehensive coverage of the above five objectives, this review takes an interdisciplinary approach by integrating insights from chemistry, environmental sciences, public health, and policy analysis to provide an inclusive understanding of PFAS-related issues. Additionally, a detailed discussion on the historical context and narrative of the evolution of PFAS was provided, and extended discussion on initial to modern applications making the manuscript more engaging and accessible to a wider audience. A global perspective was also provided by comparing regional differences in contamination levels and regulatory responses, and various remediation methods and highlight the potential regulations, ongoing research, and emerging alternatives. This review emphasizes the importance of involving diverse stakeholders in addressing PFAS contamination, ensuring that the proposed solutions are practical, sustainable, and socially acceptable.
2. Applications and Environmental Ubiquity of PFAS
2.1. PFAS Applications from World War II to the AI Era
Having a unique molecular structure, PFAS accounts for various properties including high thermal stability and amphiphilic properties as it can simultaneously show both hydrophobic and hydrophilic properties hence used in different applications including consumer products, industrial uses, medical and healthcare and construction, and automotive applications. This section overviews the previous and current applications of different PFASs and their ubiquitous presence after decades of manufacturing and consumption. Table 2 summarizes the applications and related properties and functions of PFAS employed. It also overviews the maximum and minimum PFAS concentrations used in each product category.

Table 2.
Applications and properties of the PFAS employed.
The production cost of PFAS is 100–1000 times more expensive than conventional hydrocarbon surfactant per unit volume. Hence, used only when a smaller amount of PFAS can replace a larger quantity of non-fluorinated chemicals with the same performance or when there is no other substance that can deliver the required performance. PFAS has both hydrophobic and oleophobic perfluorocarbon moieties, so it can produce effective surfactants and surface protectors. PFAS-based fluor surfactants can lower the surface tension of water from 72 mNm−1 to 16 mNm−1, making them useful in a wide range of surfactants and repellants [32].
PFAS such as PTFE or Teflon have been used in cooking pans since the mid-20th century. The first Teflon-coated non-stick frying pan was introduced to the market in the early 1960s and gained popularity ever since due to its convenience and ease of cleaning. Production expanded globally by producing non-stick frying and baking pans, and other kitchen utensils. Due to having resistance to heat, water, grease, and stain and extremely low friction coefficient, PFAS are used in various other industries such as food wrapping, pesticides, paints, drink can-lining materials, military, leather, aerospace, textile, surgical instruments, lubricant & oil, firefighting foams, cosmetics, plastics, etc. PFAS are used in solvent-water adhesives to have complete contact between joining surfaces and retard foaming, PFAS can reduce cement shrinkage and use to stabilize the aqueous foam to produce flowable concrete mixtures, PFAS are used in supercritical carbon dioxide fluid to synthesize ceramics including titanium dioxide and silver chloride, PFAS also use to lower the surface tension and enhance the wetting, penetration, antifogging characteristics in household cleaning products and they can be used on glass, metals, or plastics as an anti-mist film to prevent surface fogging in humid environments such as bathrooms and automobile windshields [32].
PFAS are also used in energy-related materials such as semiconductors, solar collectors, photovoltaic cells, and lithium-ion batteries (LiB) which can power electric vehicles (EVs). The fluoropolymers PTFE and PVDF (polyvinylidene difluoride) are also used as binders in lithium (Li) batteries (both rechargeable and non-rechargeable) to provide a stronger connection between the current collector and the electrode. Binder materials with these PFASs are essential during the manufacturing process. Due to its thermal, chemical, and electrochemical stability, PFAS is used in Li batteries to enhance performance, durability, and safety. Moreover, PFAS is also used as electrolytes in LiB, as LiB electrolytes must be conductive, electrochemically stable, low volatile, and flammable. A group of researchers analyzed samples of soil, sediments, and surface water collected in neighboring LiB manufacturing plants in Minnesota, Kentucky, Belgium and France and found the presence of bis-perfluoroalkyl sulfonimides (bis-FASIs), a subgroup of PFAS in parts per billion concentrations [39,40].
Another widespread global use of PFAS is artificial turf fields. More than 13,000 artificial turfs are utilized across the United States and many of the regular users of these fields are children, adolescents, and young adults. Regular exposure can cause health risks. Artificial turfs include fibers, infill, and backing. Crumb rubber granules, created by shredded recycled end-of-life tires are used as infill substances. This contains hundreds of chemical agents including fluorotelomer alcohols (FTOHs), a class of PFAS. A recent study on crumb rubber infill samples concluded that they exhibit carcinogenic characteristics and has possible human and environmental health risks when exposed [41,42,43].
2.2. Environmental Exposure and Ubiquity
The strong C-F bond in PFAS, due to the small size and high electronegativity of fluorine, makes PFAS chemically inert, with low molecular polarity, amphiphilicity and high stability against thermal, chemical, oxidative and reductive degradation [44,45]. Depending on the carbon chain length, volatility, water-solubility, and the headgroup functionality make a significant impact on their distribution in the environment. The short-chain PFAS molecules are more volatile and have a high mobility. Hence, they can travel via the atmosphere and can enter water cycles even located far from the areas of direct exposure. Meanwhile, long-chain PFAS molecules are less volatile and more hydrophobic. Hence, they are more easily bound to solid matrices like soil. Table 3 shows how the unique properties of PFAS contributed to specific industrial applications.

Table 3.
Unique Properties of PFAS and Resulting Industrial Applications.
The two sources of release of PFAS into the environment are point sources and non-point sources. Point sources are discrete and stationary meanwhile non-point sources are diffuse sources of unknown origin or location. Industrial/manufacturing facilities, firefighting training sites, wastewater treatment plants (WWTPs), and landfills are typically categorized as point sources and atmospheric transport of volatile PFAS, consumer products breakdown, surface runoff, and precipitation can be categorized as non-point sources [51,52]. One primary point source of PFAS contamination of surface and ground water is the AFFF used in firefighting training exercises. Since the US military has been the largest consumer of AFFFs for firefighting training exercises, PFAS contamination in water due to AFFF has been widely documented. The US Air Force has identified nearly 200 Department of Defense (DoD) installations where AFFF has been used [53] in firefighting foam and the foam can be washed away to the nearby water resources and soil. Per-fluoroethylcyclohexanesulfonate (PFECHS) is used in aircraft hydraulic fluid as an erosion inhibitor and in 2011, it was identified in a predatory fish in the Great Lakes and the surface water of the lakes [54,55,56,57,58].
Surface water and groundwater play a vital role in societal demands such as drinking water, water transport, and irrigation [59]. However, both surface water and ground water are severely polluted with organic waste and suspended solids [60,61]. Moreover, it has been identified that unlike other organic pollutants, perfluoroalkyl acids (PFAAs) are water soluble and have been detected in both surface water including lakes, rivers, and tributaries, and in ground water in similar concentrations in the range of 1-1000 ng/L [62]. PFAS enters to the WWTPs from various pathways like domestic, industrial and landfill and making these facilities one of the primary sources of PFAS contamination in surface water. Some studies have shown that perfluorobutanesulfonic acid (PFBS), a short-chain PFAS molecule used in food packaging, floor wax and carpets was detected in wastewater, drinking water, and surface water compared to perfluorooctane sulfonic acid (PFOS), a long-chain PFAS molecule [38].
Table 4 summarizes the PFAS concentration in the global aquatic environment. A study by Skaggs and Logue analyzed the residential tap water in Brookings, South Dakota (USA) and found PFHxA and PFOA in 268 ng/L and 213 ng/L concentrations respectively [63] where a different study found PFHxA, PFOS, PFOA, and PFBS PFAS molecules in residence and public drinking water facilities in Gustavus and Alaska (USA). Groundwater samples in North Carolina (USA) show 20–4773 ng/L of total PFAS concentration [64] and in Hurlburt Field AFB, Florida detected PFOA, PFOS, and PFBS substances in 109 ng/L, 830 ng/L, and 9250 ng/L concentrations respectively [65].
Many PFAS-related products with different fields of applications are routinely released to the aquatic systems. As most PFAS compounds transport readily in water due to their high-water solubility and comparatively low molecular weight, they are more likely to rapidly spread in aquatic systems. Perfluorobutanoic acid (PFBA) and PFBS are the most common short-chain PFAS compounds found in drinking water, sewage sludge and sediments in a large quantity. As PFAS is distributed from the primary manufacturer to the commercial user to final disposal, it can be released to the environment in both controlled and unrolled pathways and since the degradation methods have not been well characterized, PFAS are ubiquitous environmental contaminant and incredibly resilient in typical environmental conditions [66,67]. PFAS has been detected in groundwater globally with different concentration levels. Among different groundwater contamination pathways, the release AFFFs from firefight training facilities has been recognized as the major cause [68,69,70].

Table 4.
PFAS occurrence in water bodies in different locations.
Table 4.
PFAS occurrence in water bodies in different locations.
| Water Source | PFAS | Concentration (ng/L) | Location/Region |
|---|---|---|---|
| Sea Water | PFBS | 0.02875 | Northwestern Atlantic Margin [71] |
| PFOS | 0.22675 | ||
| PFOA | 0.2155 | ||
| br-PFOS | 0.14275 | ||
| PFHxS | 0.065 | ||
| Groundwater | PFOA | 58 | A US Department of Defense site near a fire training area where AFFF was used [72] |
| PFBA | 25 | ||
| PFOS | 25 | ||
| PFHxS | 160 | ||
| PFBS | 45 | ||
| PFOA | 90.8 | Rural areas in eastern China [73] | |
| PFBA | 33.1 | ||
| PFOS | 19.2 | ||
| PFHxS | 0.9 | ||
| PFBS | 4.8 | ||
| PFNA | 4.7 | ||
| Drinking Water | PFOS | 0.4 | USA |
| PFOA | 0.3 | ||
| GenX | 0.5 | ||
| PFBA | 2.0 | ||
| PFBS | 0.3 | ||
| PFPeA | 2.0 | ||
| PFDA | 0.9 | ||
| River | GenX | 14.8 | North Carolina, USA [64] |
| PFOS | 12.5 | ||
| PFPeA | 12.2 | ||
| Wastewater | PFBA | 1740 | Milan, Italy (Municipalities: Assago, Buccinasco, Corsico, Cesano and Boscone) [74] |
| PFPeA | 5320 | ||
| PFHxA | 2290 | ||
| PFHpA | 1570 | ||
| PFOA | 1820 | ||
| PFOS | 3770 | ||
| landfill leachate (2:1 solid-liquid ratio) | PFBA | 6400 | USA, China, Australia, Norway, Spain [75] |
| PFPeA | 530 | ||
| PFHxA | 310 | ||
| PFHpA | 36 | ||
| PFBS | 43 | ||
| PFHxS | 56 | ||
| PFOS | 13 | ||
| Lakes and surface water | PFBA | 2.6 | Nevada, USA [76] |
| PFPeA | 52.3 | ||
| PFHxA | 80.7 | ||
| PFHpA | 11.6 | ||
| PFOA | 27.3 | ||
| PFUA | 0.5 | ||
| PFBS | 17.7 | ||
| PFPeS | 2.3 | ||
| PFHxS | 11.2 | ||
| PFOS | 12.9 | ||
| PFDS | 4.3 |
Agricultural uses of contaminated irrigation water and landfills are among other reasons for groundwater contamination. Groundwater contamination is also directly impacted for the PFAS contamination of drinking water. Drinking water is an important source for humans, and PFAS in drinking water leads to a significant burden in human blood serum over a lifetime [69,70].
2.3. Freshwater Systems
Include ecosystems such as lakes, rivers, wetlands streams, and groundwater and are essential for humans and wildlife in the water supply. Unfortunately, several studies have found that PFAS substances have been detected in various freshwater bodies. The PFAS concentration in freshwater is influenced by the seasonal variation and the presence of multiple non-point and point sources [77]. Not only freshwater but also marine water (sea waters and oceans), which covers 70% of the Earth, has also been contaminated by PFAS-related substances. An interesting study done recently found PFOS, PFNA, and PFBS with a total PFAS concentration of 0.06-1.73 ng/gdw in surface sediments in the Bearing Sea in the western Arctic Ocean. The wide variety of properties made PFAS produce unlimited products in different fields of applications and developed as an environmental contaminant globally without control and detection until the early 2000s. As a result, it has become a threat to human and environmental health and is being recognized as a global issue [78].
3. Human Exposure and Health Impacts
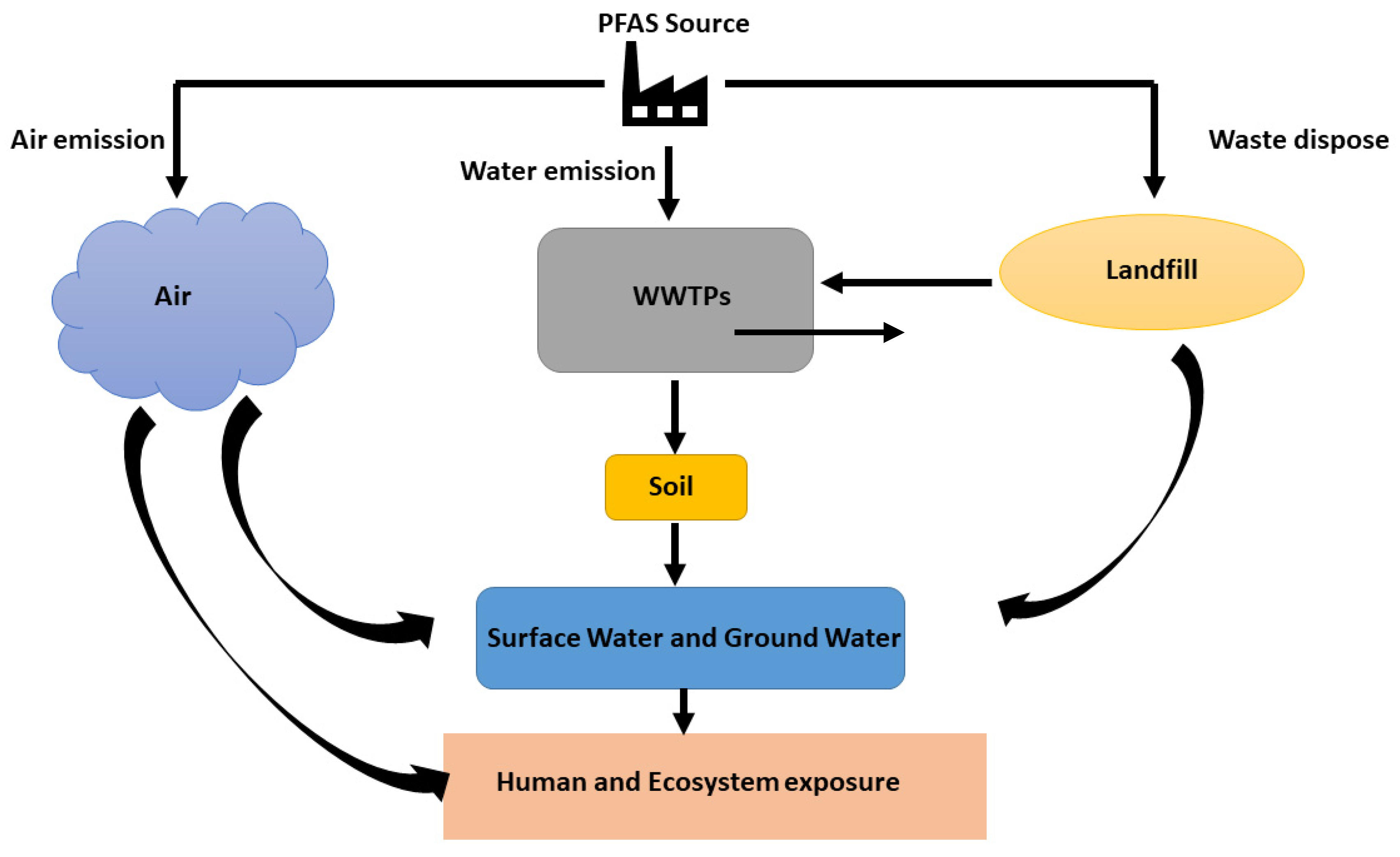
As discussed, earlier PFAS can be present in our drinking water (both public and private drinking water wells), soil, landfills, waste disposal sites and hazardous waste sites, food or food packages like pizza boxes and candy wraps, household products like fabric, cook-ware and cleaning products and firefighting foams [56]. The major source of PFAS waste is from industrial and municipal WWTPs where this can be directly contributed to the PFAS in the atmosphere and freshwater systems and indirectly spread into the sewage, sludge, and biosolids for agricultural use [6,79]. Figure 2 illustrates the environmental distribution of PFAS and possible human and environmental exposure pathways. Untreated PFAS has become a major issue due to the widespread environmental presence, bioaccumulation, persistence, and adverse health effects as they can end up in plants and food chain [80]. A study showed that several PFAAs were detected with the ng/L concentration range and perfluorooctanoic acid (PFOA) with a median concentration of 67 ng/L to 697 ng/L, in an effluent in the WWTPs in New York. This high persistence of PFAA in the environment and the widespread detection creates concerns about possible human and animal PFAS exposure. Due to the irreplaceability of some applications in some PFAs and fluorinated PFAS alternatives, detecting and evaluating the environmental impacts of PFAS-related substances has become a primary concern. It has become a major risk to humans and other living organisms as these chemicals can enter the human body through digestion and respiratory systems and accumulate in the body [81,82].
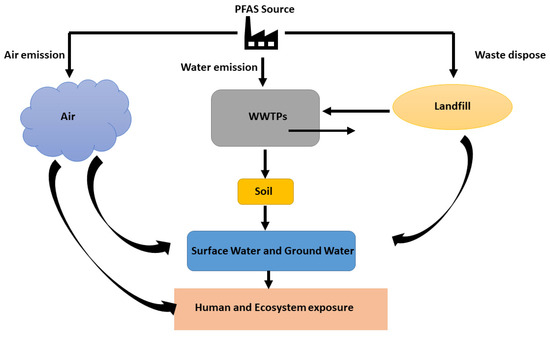
Figure 2.
Schematic representation of PFAS emission sources and human and ecosystem exposure.
Drinking water exposure is identified as one of the most concerning pathways [68,83]. Recent studies using human blood samples have found PFOS, PFOA, perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS) in human serum and plasma proteins. Some of these chemicals were detected in animal blood samples collected in remote regions of the world and due to the widespread occurrence and health effects, major U.S. companies have terminated PFOS and PFHxS production in 2002. Due to the unique structure, most PFAS substances remain untreated at the WWTPs, and the effluent from WWTPs or untreated sewage discharges to the environment or landfill. Therefore, both WWTPs and landfill leachate are two major sources of releasing PFAS into the aquatic environment. For many decades, landfills have been identified as a long-term disposal of solid waste from residential, commercial, and industrial sources and PFAS have been detected in both historic and active landfills [1,54,75]. Mansoner et al. reported that landfill leachate and effluent of WWTPs have PFAS concentrations of 93,100 ng/L and 6950 ng/L. Before 2002, US EPA set the drinking water health advisory limits for PFOS and PFOA to 150 µg/L and 1 µg/L and it was reduced to 0.2 µg/L and 0.4 µg/L in 2009 and further reduced to a combined value of 70 ng/L in 2016 [84]. PFOA and PFOS are still present in significant concentrations and some short-chain PFAS substances like HFPO-DA(GenX), PFHxA, PFHxS, and PFBA have been detected in drinking water sources and tap water worldwide. This can lead to chronic exposure and bioaccumulation and the human population consuming a larger amount of PFAS-contaminated drinking water for a longer period can cause major health problems, such as alternations in lipid metabolism, kidney, and testicular cancer, impaired liver functions, chronic kidney damage, cardiovascular disease, pregnancy-induced hypertension or pre-eclampsia, reduced fetal growth, preterm birth, low birth weight, childhood obesity, and increased miscarriage risk [79,85,86].
When considering the environmental persistency and health impacts, particle size plays an important role as the composition. Size fraction information involves analyzing PFAS particles or aggregates based on their size distribution. This can influence the transport and mobility in the environment and the bioavailability and potential uptake by organisms. Particles with 0.1–2 µm size range are not as efficiently removed by the Brownian diffusion as other size ranges. Studies has investigated that the majority of PFOA and other perfluoroalkyl carboxylates tends to accumulate in the finest particles <0.14 µm where perfluoroalkyl sulfonate substances are in coarser particles as 1.38–3.81 µm [87,88].
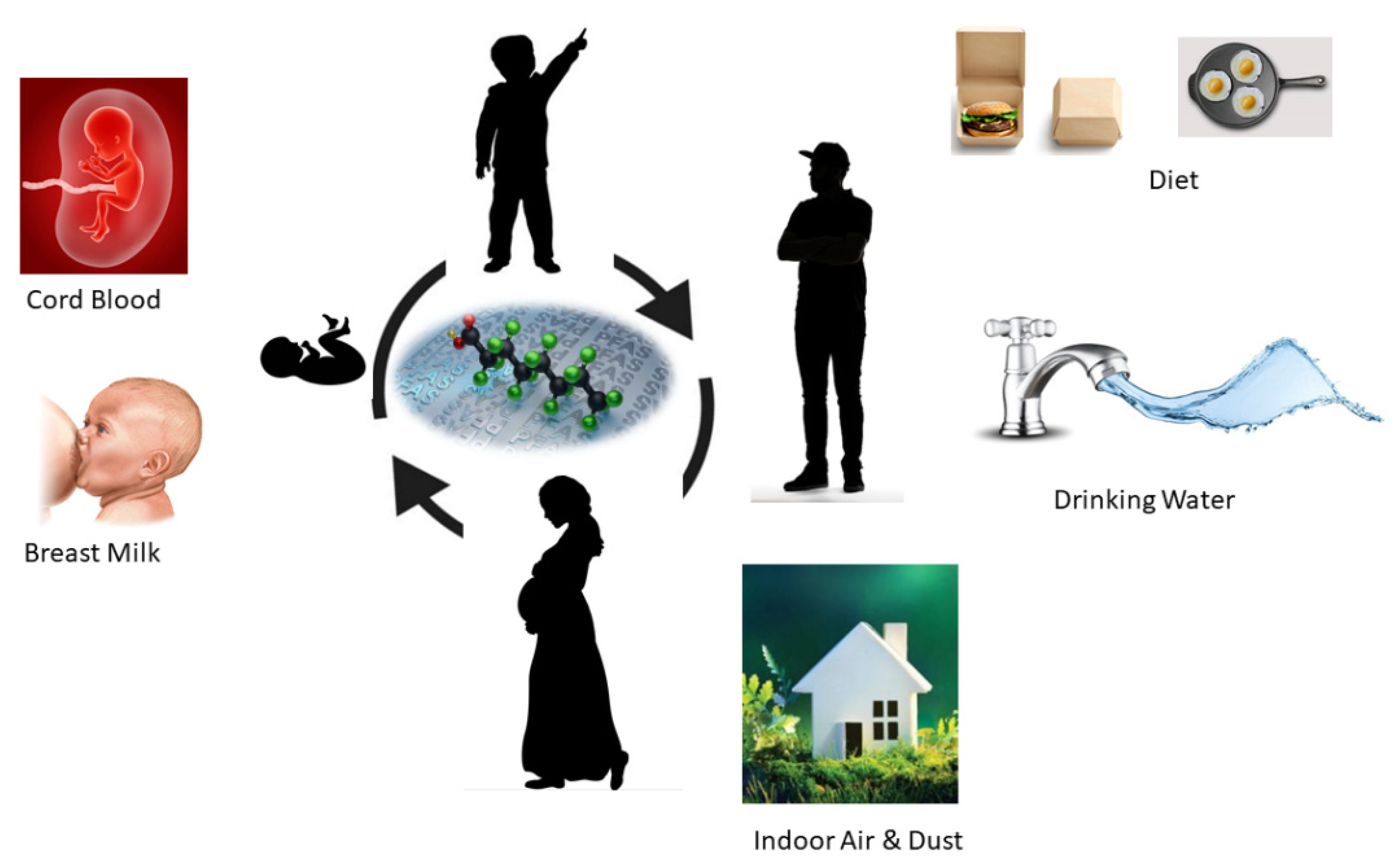
Humans can be exposed to PFAS through various pathways as shown in Figure 3. PFAS is used in many consumer products and has been detected in commonly used households like jackets, carpets, building materials, cleansers, food contact materials, and paints. PFAS also can migrate from fluorochemical-treated food contact materials into food simulants such as butter, water, and vinegar. Even though drinking water and food are considered as the primary exposure pathways for PFAS, humans and especially children can be exposed to household dust through ingestion. Inhalation and dermal contact. Other PFAS-related household products such as textiles, food packaging, building materials, carpets, and rugs can also be exposed to the human body through air or physical absorption. According to the European Food Safety Authority (EFSA), fish and other seafood accounted for 86% of dietary PFAS exposure in adults. A recent study showed PFAS contamination in breast milk in the USA. This study used breast milk collected from 50 US mothers and detected 39 PFAS samples including 9 short-chain and 30 long-chain PFAS molecules with a total PFAS concentration of 52–1850 pg/L. Some studies have found the possible detection of PFAS in blood samples of the umbilical cord, the lifeline between a mother and baby proving that we are exposed to PFAS even before we come to the world.
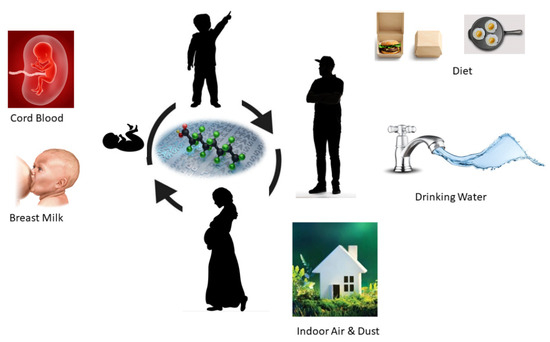
Figure 3.
Direct PFAS exposure pathways in humans in different stages of human lifetime.
4. Remediation and the Future Use of PFAS
4.1. Remediation Methods
As the toxicological effects increase on wildlife and humans, due to the bioaccumulation and extreme persistence of the environment, remediation of PFAS has grown concerns rapidly. Effective 8th of July 2024, the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA or Superfund), the Environmental Protection Agency (EPA) is designating PFOS and PFOA, two of the oldest and most used PFAS molecules are hazardous including their salts and structural isomers. But over the last decades, there are many PFAS that have been produced including PFOA, PFOS, and their precursors in large quantities and require more regulatory attention [89].
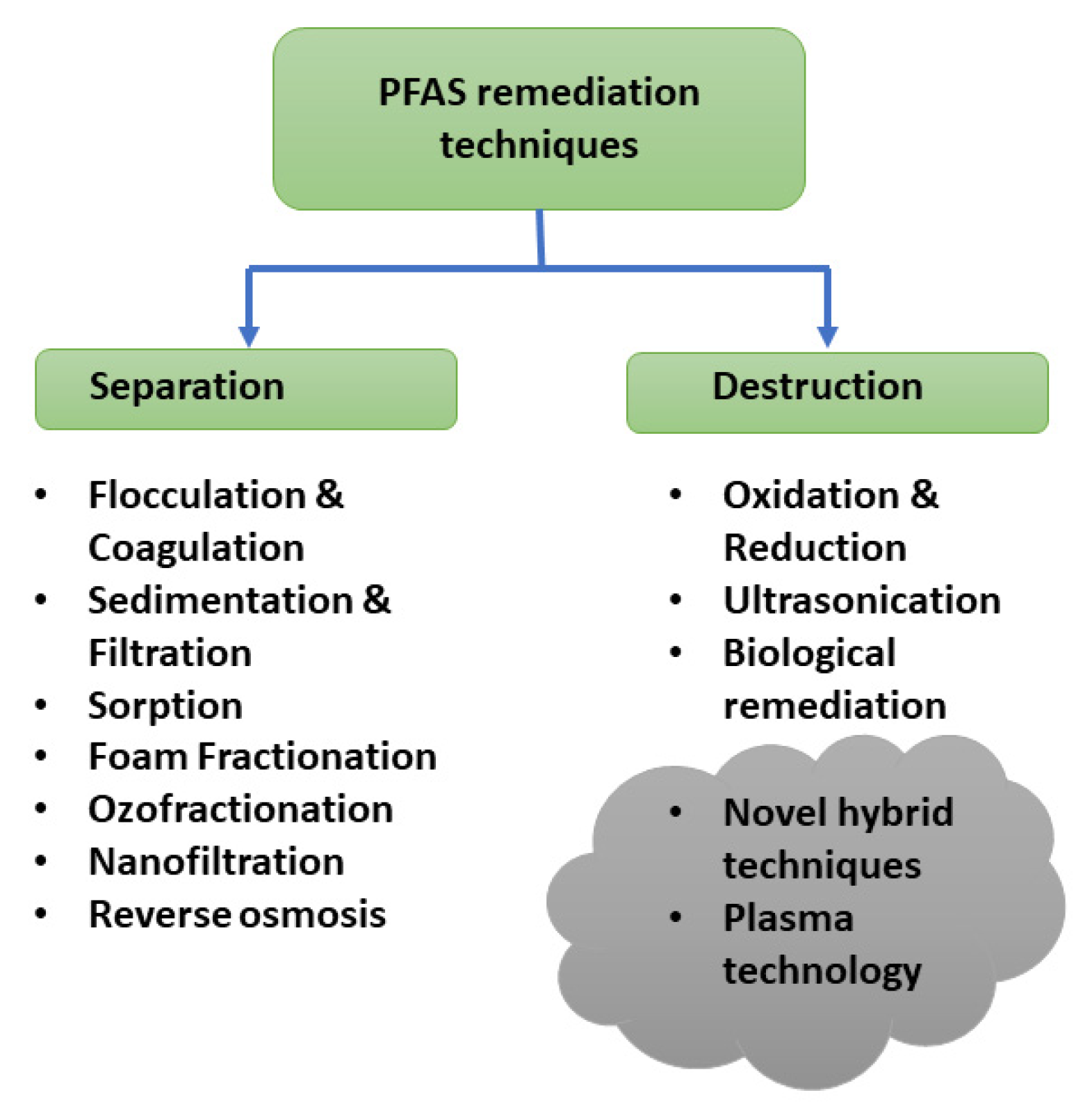
The current PFAS remediation techniques for water treatment can be categorized as separation and degradation. Figure 4 illustrates the different techniques in each category adopted from the existing remediation techniques for the other contaminants. Adsorption is an effective, eco-friendly, and economical separation method designed to be used to treat PFAS contaminants. It is important to select a suitable absorbent with an appropriate adsorption capacity and efficiency based on the sorbent properties such as pore structure. Solution pH is also an important factor in adsorption as pH can change the surface charge. The widely used adsorption technique in PFAS removal is using activated carbon, ion exchange resins, or polymers. But this concentrates PFAS from the liquid phase to the solid phase and it requires suitable treatment approaches. This has been considered as a disadvantage of this process. Other commonly used separation methods are filtration technologies including nanofiltration and reverse osmosis [90]. These filtration processes are effective for removing all PFAS chains but are more expensive than adsorption methods. Foam fractionation is also one of the emerging technologies, that introduces gas bubbles into the liquid to concentrate surface-active PFAS molecules at the gas-liquid bubble interface and separate for further treatment or disposal. Ozofractionation combines foam fractionation with ozone, which is widely used in the aquaculture industry. Even though it can be used to remove a wide range of contaminants with different concentrations of PFAS, it will not be a suitable technique to achieve low regulatory limits in ng/L and need other treatment processes to enhance the destruction effect.
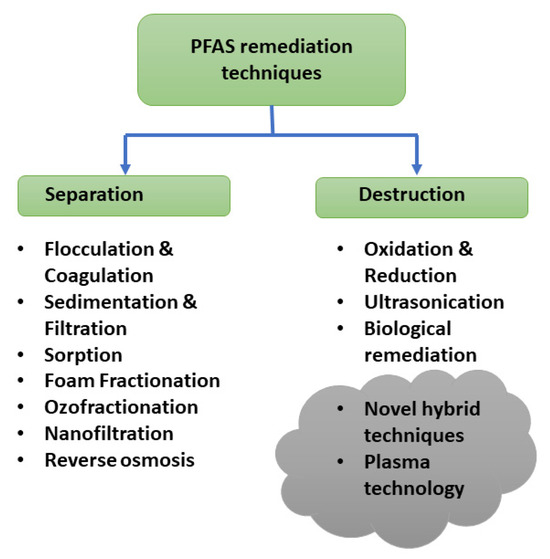
Figure 4.
PFAS remediation techniques.
4.2. Ultrasonication or Sonolysis
Uses sound waves at frequencies between 20 kHz to 1100 kHz generated by a transducer with a crystal that converts electrical current to sound waves (mechanical energy) [91,92,93]. This process creates microbubbles in water and with the bubble collation in the compression cycle, the temperature within the bubble can be increased up to 5000 K and release significant energy. Due to the high surface activity and hydrophobicity, PFAS molecules like PFOA and PFOS are absorbed onto the bubble-water interface and create perfluorocarbon intermediates by cleaving the C-C and C-S bonds. Microbial degradation is a natural remediation technique which relies on the inherent ability of living organisms such as bacteria and fungi and their enzymes to degrade chemical bonds in pollutants such as PFAS. This is eco-friendly, sustainable and cost-effective but rare in nature [94]. Hybrid technology is defined as a combination of two or more individual treatment processes. Hybrid technologies are more effective than individual treatment processes, allowing research and building innovative methods for cost-effective and large-scale PFAS remediation [89].
4.3. Possible PFAS Alternatives and Manage PFAS in Sewage
Considering the number of PFAS-related products and benefits, eliminating PFAS from the world or making a PFAS-free world is unrealistic. Instead of an immediate ban on PFAS, researchers are suggesting phasing out, where people can limit unnecessary use [95]. They recommend differentiating thousands of PFAS-related products into three major categories:
- (a)
- Non-essential—Products that are not essential for the functioning of society, health, and safety (ex: ski waxes)
- (b)
- Substitutable—Products that perform important functions but alternatives with an equivalent property have been developed (ex: some water-resistant textiles, Fluorine-free foam, non-fluorinated water repellents)
- (c)
- Essential—Products that are essential for the functioning of society, health, and safety, and the alternatives have not developed yet (ex: some medical devices)
A few potential alternatives are the use of fluorine-free foam such as F3 instead of AFFF, choosing untreated fabrics or fabrics with an alternative stain-resistant coating that does not contain PFAS, and using cookware made from stainless steel, cast iron, ceramics, or glass instead of Teflon coated products. It is also important to encourage and support PFAS-free alternatives in their manufacturing processes. Estimating and treating the PFAS in sewage from households and businesses is an important process to protect public health and the environment [96]. The initial and most important step of this is to control the source by phasing out the PFAS products, generating regulations, and educating the public about the PFAS-free alternatives. It is necessary to improve wastewater treatment by implementing advanced filtration technologies and using effective technologies to destroy PFAS in sludge. Sludge management is important as sewage sludge with high PFAS concentrations should be disposed of in a way that minimizes environmental contamination. It is also important to do regular monitoring of PFAS levels in the wastewater and treated effluent and invest in research and development of cost-effective and efficient PFAS removal technologies.
5. Summary and Conclusions
Per- and poly-fluoroalkyl (PFAS) substances are a group of manmade chemicals with hydrophobic carbon chains and hydrophilic polar functional groups. This makes PFAS a unique chemical molecule with various properties such as high chemical and heat resistance, hydrophobicity, low friction coefficient, and lipophobicity. Hence it has been used in various applications since it was first discovered during World War II. Due to the high thermal and chemical stability and having the strongest carbon-fluorine bond in the backbone chain, these molecules have been extremely persistent and stable in the environment. PFAS substances are identified as major environmental contaminants and detected in various freshwater, groundwater, marine water resources and soil and landfill leachate. This has become a major health concern since PFAS has been found in human and animal tissues.
This review outlines the discovery and development of PFAS and discusses the various applications to date. The human exposure and health effects of PFAS substances and current PFAS remediation techniques were evaluated. Long-chain PFAS degradation can result in novel PFAS substances that can be released into the environment without a complete analysis and hence establishing a strong literature background on these techniques is an important factor. The following are some of the key findings from the current remediation techniques we found through this extensive review:
- It is important to develop techniques that can remove and destroy PFAS simultaneously without generating toxic waste.
- Develop or improve ion exchange methods, foam fractionation, and ozone fractionation in an effective way to treat short-chain PFAS molecules.
- Develop and investigate the destruction mechanisms.
- Investigate the efficiency of current treatment methods.
However, it is important to develop current remediation techniques and investigate new remediation techniques to destroy PFAS contamination at a successful rate is important. Since PFAS has been used in most consumer products and various applications it will be a challenge to replace PFAS from the composition, but it is important to experiment with PFAS alternatives to a better future.
Author Contributions
Conceptualization: J.N.M., Writing-original draft preparation: D.C.P., Writing-Review and Editing: J.N.M., Visualization: D.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge Udaysinh Patil graduate student of NJIT and Sriram Kaluri from Monroe Township High School, NJ for assisting us with tables.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, M.; Zhao, X.; Zhao, D.; Soong, T.-Y.; Tian, S. Poly- and Perfluoroalkyl Substances (PFAS) in Landfills: Occurrence, Transformation and Treatment. Waste Manag. 2023, 155, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Zhang, J.; Thiessen, P.A.; Chirsir, P.; Kondic, T.; Bolton, E.E. Per- and Polyfluoroalkyl Substances (PFAS) in PubChem: 7 Million and Growing. Environ. Sci. Technol. 2023, 57, 16918–16928. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.-T.; Li, Q. Physicochemical properties and interactions of perfluoroalkyl substances (PFAS)—Challenges and opportunities in sensing and remediation. Sci. Total Environ. 2023, 905, 166764. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, R.J. The history of polytetrafluoroethylene: Discovery and development. In High Performance Polymers: Their Origin and Development; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Gaines, L.G.T. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef]
- Wang, Z.; Dewitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- Gaber, N.; Bero, L.; Woodruff, T.J. The Devil they Knew: Chemical Documents Analysis of Industry Influence on PFAS Science. Ann. Glob. Health 2023, 89, 4013. [Google Scholar] [CrossRef]
- Annunziato, K.M.; Doherty, J.; Lee, J.; Clark, J.M.; Liang, W.; Clark, C.W.; Nguyen, M.; Roy, M.A.; Timme-Laragy, A.R. Chemical characterization of a legacy aqueous film-forming foam sample and developmental toxicity in zebrafish (Danio rerio). Environ. Health Perspect. 2020, 128, ehp6470. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; Van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; DeWitt, J.C.; Knappe, D.R.U.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. [Google Scholar] [CrossRef]
- Sadia, M.; Kunz, M.; ter Laak, T.; De Jonge, M.; Schriks, M.; van Wezel, A.P. Forever legacies? Profiling historical PFAS contamination and current influence on groundwater used for drinking water. Sci. Total Environ. 2023, 890, 164420. [Google Scholar] [CrossRef] [PubMed]
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the regulation of per- and polyfluoroalkyl substances (PFAS): A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef]
- Kotthoff, M.; Bücking, M. Four chemical trends will shape the next decade’s directions in perfluoroalkyl and polyfluoroalkyl substances research. Front. Chem. 2018, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Sznajder-Katarzyńska, K.; Surma, M.; Cieślik, I. A Review of Perfluoroalkyl Acids (PFAAs) in terms of Sources, Applications, Human Exposure, Dietary Intake, Toxicity, Legal Regulation, and Methods of Determination. J. Chem. 2019, 2019, 2717528. [Google Scholar] [CrossRef]
- Alijagic, A.; Sinisalu, L.; Duberg, D.; Kotlyar, O.; Scherbak, N.; Engwall, M.; Orešič, M.; Hyötyläinen, T. Metabolic and phenotypic changes induced by PFAS exposure in two human hepatocyte cell models. Environ. Int. 2024, 190, 108820. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ling, X.; He, S.; Cui, H.; Yang, Z.; An, H.; Wang, L.; Zou, P.; Chen, Q.; Liu, J.; et al. PPARα/ACOX1 as a novel target for hepatic lipid metabolism disorders induced by per- and polyfluoroalkyl substances: An integrated approach. Environ. Int. 2023, 178, 108138. [Google Scholar] [CrossRef] [PubMed]
- Louisse, J.; Fragki, S.; Rijkers, D.; Janssen, A.; van Dijk, B.; Leenders, L.; Staats, M.; Bokkers, B.; Zeilmaker, M.; Piersma, A.; et al. Determination of in vitro hepatotoxic potencies of a series of perfluoroalkyl substances (PFASs) based on gene expression changes in HepaRG liver cells. Arch. Toxicol. 2023, 97, 1113–1131. [Google Scholar] [CrossRef]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ. Int. 2020, 134, 105220. [Google Scholar] [CrossRef]
- Antoniou, E.E.; Dekant, W. Childhood PFAS exposure and immunotoxicity: A systematic review and meta-analysis of human studies. Syst. Rev. 2024, 13, 176. [Google Scholar] [CrossRef]
- Safta, D. Per- and Polyfluorinated Substances (PFAS); a Literature Review. Undergrad. J. Public Health 2024, 8, 6064. [Google Scholar] [CrossRef]
- Vujic, E.; Ferguson, S.S.; Brouwer, K.L.R. Effects of PFAS on human liver transporters: Implications for health outcomes. Toxicol. Sci. 2024, 200, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Iulini, M.; Russo, G.; Crispino, E.; Paini, A.; Fragki, S.; Corsini, E.; Pappalardo, F. Advancing PFAS risk assessment: Integrative approaches using agent-based modelling and physiologically-based kinetic for environmental and health safety. Comput. Struct. Biotechnol. J. 2024, 23, 2763–2778. [Google Scholar] [CrossRef] [PubMed]
- Habib, Z.; Song, M.; Ikram, S.; Zahra, Z. Overview of Per- and Polyfluoroalkyl Substances (PFAS), Their Applications, Sources, and Potential Impacts on Human Health. Pollutants 2024, 4, 136–152. [Google Scholar] [CrossRef]
- Ogbuewu, I.; Nnaji, J. Human Health Impacts of Perfluoroalkyl Substances, Micro- and Nanoplastics Contamination of Drinking Water. Arch. Ecotoxicol. 2023, 5, 75–82. [Google Scholar] [CrossRef]
- Fischer, F.C.; Ludtke, S.; Thackray, C.; Pickard, H.M.; Haque, F.; Dassuncao, C.; Endo, S.; Schaider, L.; Sunderland, E.M. Binding of Per- and Polyfluoroalkyl Substances (PFAS) to Serum Proteins: Implications for Toxicokinetics in Humans. Environ. Sci. Technol. 2024, 58, 1055–1063. [Google Scholar] [CrossRef]
- Forever Chemicals: The Persistent Effects of Perfluoroalkyl and Polyfluoroalkyl Substances on Human Health. 2023. [Online]. Available online: www.thelancet.com (accessed on 21 September 2024).
- Eve, A.A.; Tunc, E.; Mehta, D.; Yoo, J.Y.; Yilmaz, H.E.; Emren, S.V.; Akçay, F.A.; Erdogan, Z.M. PFAS and their association with the increased risk of cardiovascular disease in postmenopausal women. Toxicol. Sci. 2024, 200, 312–323. [Google Scholar] [CrossRef]
- Rudzanova, B.; Vlaanderen, J.; Kalina, J.; Piler, P.; Zvonar, M.; Klanova, J.; Blaha, L.; Adamovsky, O. Impact of PFAS exposure on prevalence of immune-mediated diseases in adults in the Czech Republic. Environ. Res. 2023, 229, 115969. [Google Scholar] [CrossRef]
- Mao, X.; Liu, Y.; Wei, Y.; Li, X.; Liu, Y.; Su, G.; Wang, X.; Jia, J.; Yan, B. Threats of per- and poly-fluoroalkyl pollutants to susceptible populations. Sci. Total Environ. 2024, 921, 171188. [Google Scholar] [CrossRef] [PubMed]
- Solan, M.E.; Lavado, R. Effects of short-chain per-and polyuoroalkyl substances (PFAS) on human cytochrome P450 (CYP450) enzymes and human hepatocytes: An in vitro study. Preprint 2023. [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Minnesota Pollution Control Agency—Maya Gilchrist and L. McLain. PFAS in the Textile and Leather Industries. 2023. [Online]. Available online: www.pca.state.mn.us (accessed on 21 September 2024).
- Guidance on PFAS Exposure, Testing, and Clinical Follow-Up; The National Academies Press: Washington, DC, USA, 2022. [CrossRef]
- Minnesota Department of Agriculture. PFAS in Pesticides: Interim Report for the Legislature; Minnesota Department of Agriculture: Saint Paul, MN, USA, 2024. [Google Scholar]
- Zabaleta, I.; Blanco-Zubiaguirre, L.; Baharli, E.N.; Olivares, M.; Prieto, A.; Zuloaga, O.; Elizalde, M.P. Occurrence of per- and polyfluorinated compounds in paper and board packaging materials and migration to food simulants and foodstuffs. Food Chem. 2020, 321, 126746. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, H.D.; Venier, M.; Wu, Y.; Eastman, E.; Urbanik, S.; Diamond, M.L.; Shalin, A.; Schwartz-Narbonne, H.; Bruton, T.A.; Blum, A.; et al. Fluorinated Compounds in North American Cosmetics. Environ. Sci. Technol. Lett. 2021, 8, 538–544. [Google Scholar] [CrossRef]
- Dewapriya, P.; Chadwick, L.; Gorji, S.G.; Schulze, B.; Valsecchi, S.; Samanipour, S.; Thomas, K.V.; Kaserzon, S.L. Per- and polyfluoroalkyl substances (PFAS) in consumer products: Current knowledge and research gaps. J. Hazard. Mater. Lett. 2023, 4, 100086. [Google Scholar] [CrossRef]
- Guelfo, J.L.; Ferguson, P.L.; Beck, J.; Chernick, M.; Doria-Manzur, A.; Faught, P.W.; Flug, T.; Gray, E.P.; Jayasundara, N.; Knappe, D.R.U.; et al. Lithium-ion battery components are at the nexus of sustainable energy and environmental release of per- and polyfluoroalkyl substances. Nat. Commun. 2024, 15, 5548. [Google Scholar] [CrossRef] [PubMed]
- Rensmo, A.; Savvidou, E.K.; Cousins, I.T.; Hu, X.; Schellenberger, S.; Benskin, J.P. Lithium-ion battery recycling: A source of per- and polyfluoroalkyl substances (PFAS) to the environment? Environ. Sci. Process. Impacts 2023, 25, 1015–1030. [Google Scholar] [CrossRef]
- Murphy, M.; Warner, G.R. Health impacts of artificial turf: Toxicity studies, challenges, and future directions. Environ. Pollut. 2022, 310, 119841. [Google Scholar] [CrossRef]
- Zuccaro, P.; Licato, J.; Davidson, E.A.; Thompson, D.C.; Vasiliou, V. Assessing extraction-analysis methodology to detect fluorotelomer alcohols (FTOH), a class of perfluoroalkyl and polyfluoroalkyl substances (PFAS), in artificial turf fibers and crumb rubber infill. Case Stud. Chem. Environ. Eng. 2023, 7, 100280. [Google Scholar] [CrossRef]
- Zuccaro, P.; Thompson, D.C.; de Boer, J.; Watterson, A.; Wang, Q.; Tang, S.; Shi, X.; Llompart, M.; Ratola, N.; Vasiliou, V. Artificial turf and crumb rubber infill: An international policy review concerning the current state of regulations. Environ. Challenges 2022, 9, 100620. [Google Scholar] [CrossRef]
- Podder, A.; Sadmani, A.A.; Reinhart, D.; Chang, N.-B.; Goel, R. Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Eze, C.G.; Okeke, E.S.; Nwankwo, C.E.; Nyaruaba, R.; Anand, U.; Okoro, O.J.; Bontempi, E. Emerging contaminants in food matrices: An overview of the occurrence, pathways, impacts and detection techniques of per- and polyfluoroalkyl substances. Toxicol. Rep. 2024, 12, 436–447. [Google Scholar] [CrossRef]
- Carnero, A.R.; Lestido-Cardama, A.; Loureiro, P.V.; Barbosa-Pereira, L.; de Quirós, A.R.B.; Sendón, R. Presence of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in food contact materials (FCM) and its migration to food. Foods 2021, 10, 1443. [Google Scholar] [CrossRef]
- Verma, S.; Lee, T.; Sahle-Demessie, E.; Ateia, M.; Nadagouda, M.N. Recent advances on PFAS degradation via thermal and nonthermal methods. Chem. Eng. J. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.K.; Käfer, F.; Deng, J. Review of essential use of fluorochemicals in lithographic patterning and semiconductor processing. J. Micro/Nanopatterning, Mater. Metrol. 2022, 21, 010901. [Google Scholar] [CrossRef]
- Banzhaf, S.; Filipovic, M.; Lewis, J.; Sparrenbom, C.J.; Barthel, R. A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs). AMBIO 2016, 46, 335–346. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, S.; Zhang, Y.; Wu, R.; Pang, J.; Li, W. Vitreous surgery for macular hole-related retinal detachment after phacoemulsification cataract extraction: 10-year retrospective review. Eye 2012, 26, 1058–1064. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Ruyle, B.J.; Pickard, H.M.; LeBlanc, D.R.; Tokranov, A.K.; Thackray, C.P.; Hu, X.C.; Vecitis, C.D.; Sunderland, E.M. Isolating the AFFF signature in coastal watersheds using oxidizable PFAS precursors and unexplained organofluorine. Environ. Sci. Technol. 2021, 55, 3686–3695. [Google Scholar] [CrossRef]
- Moody, C.A.; Field, J.A. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 2000, 34, 3864–3870. [Google Scholar] [CrossRef]
- Propp, V.R.; De Silva, A.O.; Spencer, C.; Brown, S.J.; Catingan, S.D.; Smith, J.E.; Roy, J.W. Organic contaminants of emerging concern in leachate of historic municipal landfills. Environ. Pollut. 2021, 276, 116474. [Google Scholar] [CrossRef]
- Solo-Gabriele, H.M.; Jones, A.S.; Lindstrom, A.B.; Lang, J.R. Waste type, incineration, and aeration are associated with per- and polyfluoroalkyl levels in landfill leachates. Waste Manag. 2020, 107, 191–200. [Google Scholar] [CrossRef]
- Travar, I.; Uwayezu, J.N.; Kumpiene, J.; Yeung, L.W. Challenges in the PFAS Remediation of Soil and Landfill Leachate: A Review. Adv. Environ. Eng. Res. 2020, 2, 1. [Google Scholar] [CrossRef]
- Munoz, G.; Michaud, A.M.; Liu, M.; Duy, S.V.; Montenach, D.; Resseguier, C.; Watteau, F.; Sappin-Didier, V.; Feder, F.; Morvan, T.; et al. Target and Nontarget Screening of PFAS in Biosolids, Composts, and Other Organic Waste Products for Land Application in France. Environ. Sci. Technol. 2021, 56, 6056–6068. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, Y.; Chen, H.; Chang, S.; Tian, Y.; Sun, H. Per- and polyfluoroalkyl substances and the contribution of unknown precursors and short-chain (C2–C3) perfluoroalkyl carboxylic acids at solid waste disposal facilities. Sci. Total Environ. 2020, 705, 135832. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ozaki, N.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Occurrence of per- and polyfluoroalkyl substances in aquatic environments and their removal by advanced oxidation processes. Chemosphere 2023, 330, 138666. [Google Scholar] [CrossRef]
- Wang, T.; Le, T.; Hu, J.; Ravindra, A.V.; Xv, H.; Zhang, L.; Wang, S.; Yin, S. Ultrasonic-assisted ozone degradation of organic pollutants in industrial sulfuric acid. Ultrason. Sonochem. 2022, 86, 106043. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Y.; Ma, L.; Lin, X.; Li, Q.; Li, Y.; Wang, X. Perfluoroalkyl substances in water, sediment, and fish from a subtropical river of China: Environmental behaviors and potential risk. Chemosphere 2022, 288, 132513. [Google Scholar] [CrossRef]
- Loi, J.X.; Chua, A.S.M.; Rabuni, M.F.; Tan, C.K.; Lai, S.H.; Takemura, Y.; Syutsubo, K. Water quality assessment and pollution threat to safe water supply for three river basins in Malaysia. Sci. Total Environ. 2022, 832, 155067. [Google Scholar] [CrossRef]
- Skaggs, C.S.; Logue, B.A. Ultratrace analysis of per- and polyfluoroalkyl substances in drinking water using ice concentration linked with extractive stirrer and high performance liquid chromatography—Tandem mass spectrometry. J. Chromatogr. A 2021, 1659, 462493. [Google Scholar] [CrossRef] [PubMed]
- Pétré, M.-A.; Genereux, D.P.; Koropeckyj-Cox, L.; Knappe, D.R.; Duboscq, S.; Gilmore, T.E.; Hopkins, Z.R. Per- and Polyfluoroalkyl Substance (PFAS) Transport from Groundwater to Streams near a PFAS Manufacturing Facility in North Carolina, USA. Environ. Sci. Technol. 2021, 55, 5848–5856. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Li, X.; Quinete, N. Occurrence, fate, sources and toxicity of PFAS: What we know so far in Florida and major gaps. TrAC Trends Anal. Chem. 2020, 130, 115976. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, 512. [Google Scholar] [CrossRef] [PubMed]
- Winchell, L.J.; Wells, M.J.; Ross, J.J.; Fonoll, X.; Norton, J.W.; Kuplicki, S.; Khan, M.; Bell, K.Y. Analyses of per- and polyfluoroalkyl substances (PFAS) through the urban water cycle: Toward achieving an integrated analytical workflow across aqueous, solid, and gaseous matrices in water and wastewater treatment. Sci. Total Environ. 2021, 774, 145257. [Google Scholar] [CrossRef]
- Grunfeld, D.A.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Kibbey, T.C.G.; O’carroll, D.M. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Babayev, M.; Capozzi, S.L.; Miller, P.; McLaughlin, K.R.; Medina, S.S.; Byrne, S.; Zheng, G.; Salamova, A. PFAS in drinking water and serum of the people of a southeast Alaska community: A pilot study. Environ. Pollut. 2022, 305, 119246. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.; Phelps, W.; Masarik, K.; Burke, K.; Zhang, C.; Schwartz, A.; Wang, M.; Nitka, A.L.; Schutz, J.; Trainor, T.; et al. Prevalence and Source Tracing of PFAS in Shallow Groundwater Used for Drinking Water in Wisconsin, USA. Environ. Sci. Technol. 2023, 57, 17415–17426. [Google Scholar] [CrossRef] [PubMed]
- Pétré, M.-A.; Salk, K.; Stapleton, H.; Ferguson, P.; Tait, G.; Obenour, D.; Knappe, D.; Genereux, D. Per- and polyfluoroalkyl substances (PFAS) in river discharge: Modeling loads upstream and downstream of a PFAS manufacturing plant in the Cape Fear watershed, North Carolina. Sci. Total Environ. 2022, 831, 154763. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Choyke, S.; Ferguson, P.L.; Andaya, C.; Burant, A.; Maizel, A.C.; Strathmann, T.J.; Higgins, C.P. Electrochemical Transformations of Perfluoroalkyl Acid (PFAA) Precursors and PFAAs in Groundwater Impacted with Aqueous Film Forming Foams. Environ. Sci. Technol. 2018, 52, 10689–10697. [Google Scholar] [CrossRef]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Moneta, B.G.; Feo, M.L.; Torre, M.; Tratzi, P.; Aita, S.E.; Montone, C.M.; Taglioni, E.; Mosca, S.; Balducci, C.; Cerasa, M.; et al. Occurrence of per- and polyfluorinated alkyl substances in wastewater treatment plants in Northern Italy. Sci. Total Environ. 2023, 894, 165089. [Google Scholar] [CrossRef]
- Coffin, E.S.; Reeves, D.M.; Cassidy, D.P. PFAS in municipal solid waste landfills: Sources, leachate composition, chemical transformations, and future challenges. Curr. Opin. Environ. Sci. Health 2023, 31, 100418. [Google Scholar] [CrossRef]
- Bai, X.; Son, Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total Environ. 2020, 751, 141622. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Yun, X.; Spooner, D.E.; Kurz, M.J.; McKenzie, E.R.; Sales, C.M. Exposure pathways and bioaccumulation of per- and polyfluoroalkyl substances in freshwater aquatic ecosystems: Key considerations. Sci. Total Environ. 2022, 822, 153561. [Google Scholar] [CrossRef]
- Garg, S.; Wang, J.; Kumar, P.; Mishra, V.; Arafat, H.; Sharma, R.S.; Dumée, L.F. Remediation of water from per-/poly-fluoroalkyl substances (PFAS)—Challenges and perspectives. J. Environ. Chem. Eng. 2021, 9, 105784. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, S.; Swank, A.; Sobus, J.R.; Howey, X.M.; Schmid, J.; Catron, T.; McCord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ. Health Perspect. 2020, 128, 47005. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Gugliandolo, E.; Cuzzocrea, S.; Crupi, R.; Britti, D. Current Review of Increasing Animal Health Threat of Per- and Polyfluoroalkyl Substances (PFAS): Harms, Limitations, and Alternatives to Manage Their Toxicity. Int. J. Mol. Sci. 2023, 24, 11707. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Wong, L.-Y.; Jia, L.T.; Kuklenyik, Z.; Calafat, A.M. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999−2008. Environ. Sci. Technol. 2011, 45, 8037–8045. [Google Scholar] [CrossRef]
- Post, G.B. Recent US State and Federal Drinking Water Guidelines for Per- and Polyfluoroalkyl Substances. Environ. Toxicol. Chem. 2021, 40, 550–563. [Google Scholar] [CrossRef]
- Göckener, B.; Weber, T.; Rüdel, H.; Bücking, M.; Kolossa-Gehring, M. Human biomonitoring of per- and polyfluoroalkyl substances in German blood plasma samples from 1982 to 2019. Environ. Int. 2020, 145, 106123. [Google Scholar] [CrossRef]
- Ojo, A.F.; Peng, C.; Ng, J.C. Combined effects and toxicological interactions of perfluoroalkyl and polyfluoroalkyl substances mixtures in human liver cells (HepG2). Environ. Pollut. 2020, 263, 114182. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Taniyasu, S.; Yamazaki, E.; Wei, S.; Wang, X.; Gai, N.; Kim, J.H.; Eun, H.; Lam, P.K.S.; Yamashita, N. Per- and Polyfluoroalkyl Substances in the Air Particles of Asia: Levels, Seasonality, and Size-Dependent Distribution. Environ. Sci. Technol. 2020, 54, 14182–14191. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lao, J.-Y.; Wang, Q.; Ruan, Y.; He, Y.; Lee, P.K.; Leung, K.M.; Lam, P.K. Per- and polyfluoroalkyl substances in the atmosphere of waste management infrastructures: Uncovering secondary fluorotelomer alcohols, particle size distribution, and human inhalation exposure. Environ. Int. 2022, 167, 107434. [Google Scholar] [CrossRef] [PubMed]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A review of the applications, environmental release, and remediation technologies of per- and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 8117. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Kazwini, T.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700. [Google Scholar] [CrossRef]
- Meegoda, J.N.; de Souza, B.B.; Casarini, M.M.; Kewalramani, J.A. A Review of PFAS Destruction Technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. [Google Scholar] [CrossRef]
- Kewalramani, J.A.; Wang, B.; Marsh, R.W.; Meegoda, J.N.; Freire, L.R. Coupled high and low-frequency ultrasound remediation of PFAS-contaminated soils. Ultrason. Sonochem. 2022, 88, 106063. [Google Scholar] [CrossRef]
- Berhanu, A.; Mutanda, I.; Taolin, J.; Qaria, M.A.; Yang, B.; Zhu, D. A review of microbial degradation of per- and polyfluoroalkyl substances (PFAS): Biotransformation routes and enzymes. Sci. Total Environ. 2023, 859, 160010. [Google Scholar] [CrossRef]
- Cousins, I.T.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; Scheringer, M.; Trier, X.; Vierke, L.; et al. The concept of essential use for determining when uses of PFASs can be phased out. Environ. Sci. Process. Impacts 2019, 21, 1803–1815. [Google Scholar] [CrossRef]
- Nguyen, H.T.; McLachlan, M.S.; Tscharke, B.; Thai, P.; Braeunig, J.; Kaserzon, S.; O’Brien, J.W.; Mueller, J.F. Background release and potential point sources of per- and polyfluoroalkyl substances to municipal wastewater treatment plants across Australia. Chemosphere 2022, 293, 133657. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).