Plant-Origin Additives from Boswellia Species in Emulgel Formulation for Radiotherapy Skin Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characteristics of Extracts and Essential Oil from Boswellia Species
2.2.1. FTIR Spectrum
2.2.2. Antioxidant Activity by 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Assay
- —absorbance of blank sample;
- —average absorbance of samples.
2.2.3. Total Phenolic Content Assay
2.3. Composition and Obtaining of Topical Formulations
2.3.1. Emulsion
2.3.2. Emulgel
2.4. Physicochemical Analysis of Formulations
2.4.1. Visual Assessment and pH
2.4.2. Stability
2.4.3. Microscopic Structure
2.4.4. Rheological Properties
- —yield stress, Pa;
- τ—shear stress, Pa;
- k—consistency index, Pa·sn;
- —shear rate, s−1;
- —flow index, -.
2.5. Antioxidant Properties and Polyphenols Content
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Obtained Formulations
3.2. Rheological Properties
3.3. Antioxidant Properties and Polyphenols Content of Formulations
3.4. Sensory Analysis
4. Discussion
4.1. Composition and Characterization of Formulations
4.1.1. Visual Assessment and pH
4.1.2. Microscopic Structure
4.1.3. Rheological Properties
4.2. Antioxidant Properties and Polyphenols Content
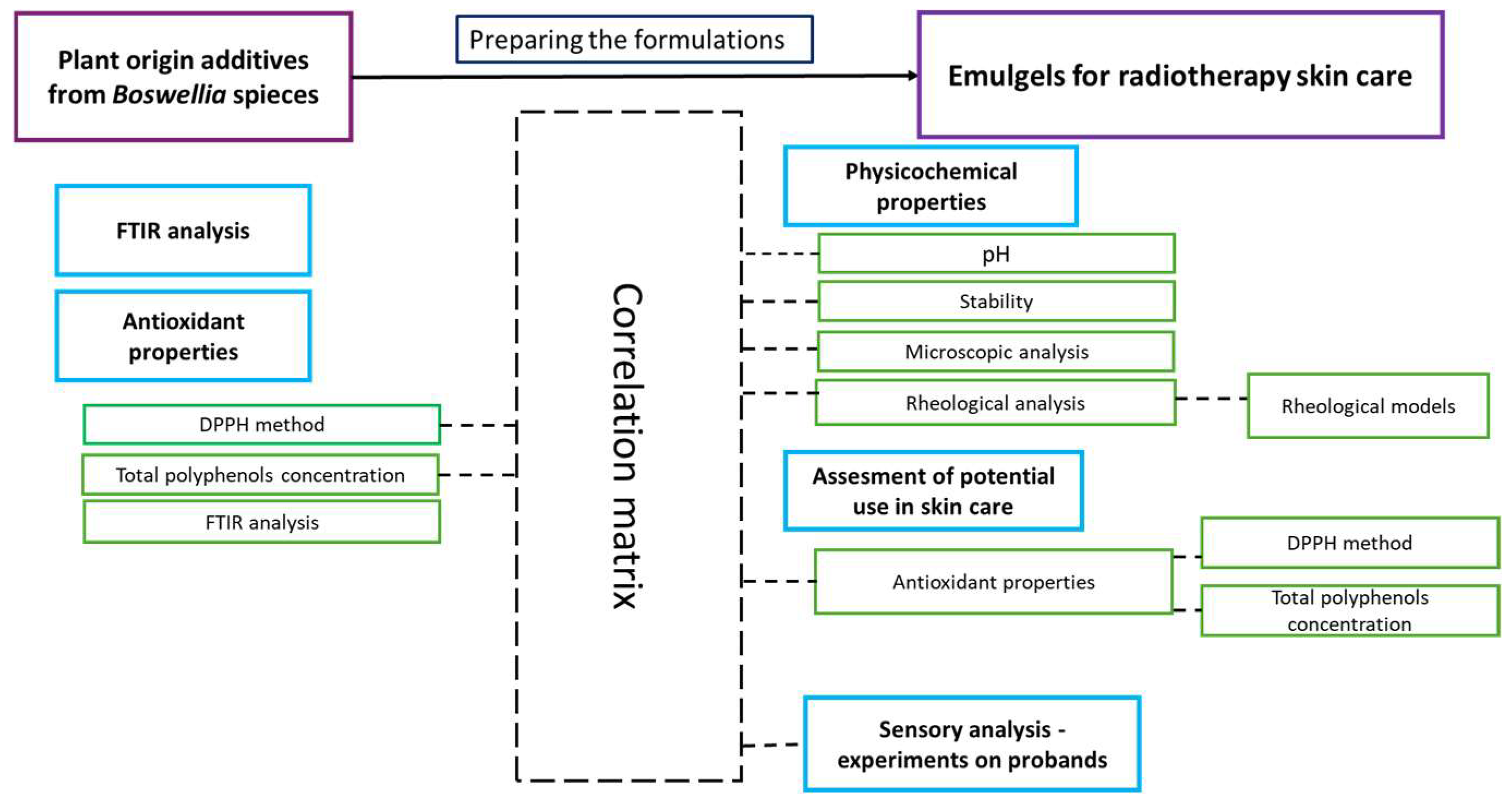
4.3. Correlation Matrix of Instrumental Analysis Results and Sensory Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeman, E.M.; Schreiber, E.C.; Tepper, J.E. 27–Basics of Radiation Therapy. In Abeloff’s Clinical Oncology, 6th ed.; Niederhuber, J.E., Armitage, J.O., Kastan, M.B., Doroshow, J.H., Tepper, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 431–460.e3. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Goździcka, W.J. Plant and Herbal Extracts as Ingredients of Topical Agents in the Prevention and Treatment Radiodermatitis: A Systematic Literature Review. Cosmetics 2022, 9, 63. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Gondhowiardjo, S.S.; Rosa, A.A.; Lievens, Y.; El-Haj, N.; Polo Rubio, J.A.; Prajogi, G.B.; Helgadottir, H.; Zubizarreta, E.; Meghzifene, A.; et al. Global Radiotherapy: Current Status and Future Directions-White Paper. JCO Glob. Oncol. 2021, 7, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Rucińska, M. Radiotherapy in the combined treatment. Nowotw. J. Oncol. 2022, 7, 366–372. [Google Scholar] [CrossRef]
- Bensadoun, R.J.; Humbert, P.; Krutman, J.; Luger, T.; Triller, R.; Rougier, A.; Seite, S.; Dreno, B. Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: Recommendations from a multinational expert panel. Cancer Manag. Res. 2013, 5, 401–408. [Google Scholar] [CrossRef]
- Radiation Therapy Side Effects. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/radiation/effects-on-different-parts-of-body.html (accessed on 29 April 2024).
- Zasadziński, K.; Spałek, M.J.; Rutkowski, P. Modern Dressings in Prevention and Therapy of Acute and Chronic Radiation Dermatitis—A Literature Review. Pharmaceutics 2022, 14, 1204. [Google Scholar] [CrossRef]
- Kiprian, D.; Szykut-Badaczewska, A.; Gradzińska, A.; Czuwara, J.; Rudnicka, L. How to manage radiation-induced dermatitis? Nowotw. J. Oncol. 2022, 72, 86–95. [Google Scholar] [CrossRef]
- Michalewska, J. Odczyny popromienne w radioterapii oraz popromienne zapalenie skóry. Lett. Oncol. Sci. 2017, 14, 104–109. [Google Scholar] [CrossRef]
- Amber, K.T.; Shiman, M.I.; Badiavas, E.V. The use of antioxidants in radiotherapy-induced skin toxicity. Integr. Cancer Ther. 2014, 13, 38–45. [Google Scholar] [CrossRef]
- Kim, J.H.; Kolozsvary, A.J.; Jenrow, K.A.; Brown, S.L. Mechanism of radiation-induced skin injury and implications for future clinical trials. Int. J. Radiat. Biol. 2013, 89, 311–318. [Google Scholar] [CrossRef]
- Fowble, B.; Yom, S.S.; Yuen, F.; Arron, S. Skin Care in Radiation Oncology. A Practical Guide; Springer: Cham, Switzerland, 2016; pp. 31–45. [Google Scholar]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef] [PubMed]
- McQuestion, M. Evidence-based skin care management in radiation therapy. Semin. Oncol. Nurs. 2006, 23, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kodiyan, J.; Amber, K.T. Topical antixodidants in radiodermatitis: A clinical review. J. Palliat. Nurs. 2015, 21, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Wszołek, K.; Piotrowska, A. Analysis of the composition of selected cosmetics for oncological patients. Kosmetol. Estet. 2019, 8, 575–580. [Google Scholar]
- Msomi, N.Z.; Simelane, M.B.C. 11. Herbal Medicine. In Herbal Medicine; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Kleinschek, K.S.; Smrke, D.M.; Kreft, S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015, 54, 740–751. [Google Scholar] [CrossRef]
- Skalska-Kamińska, A.; Woźniak, A.; Paduch, R.; Kocjan, R.; Rejdak, R. Herbal preparation extract for skin after radiotherapy treatment. Part One—Preclinical tests. Acta Pol. Pharm. Drug Res. 2014, 71, 781–788. [Google Scholar]
- Griñan-Lison, C.; Blaya-Cánovas, J.L.; López-Tejada, A.; Ávalos-Moreno, M.; Navarro-Ocón, A.; Cara, F.E.; González-González, A.; Lorente, J.A.; Marchal, J.A.; Granados-Principal, S. Antioxidants for the Treatment of Breast Cancer: Are We There Yet? Antioxidants 2021, 10, 205. [Google Scholar] [CrossRef]
- Thornfeldt, C.R. Chapter 12: Rośliny jako kosmeceutyki. In Kosmeceutyki, 2nd ed.; Draelos, Z.D., Ed.; Elsevier Edra Urban & Partner: Wrocław, Poland, 2009; pp. 87–98. [Google Scholar]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, J. Effective tool for assessment of the quality of barrier creams–relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharmacol. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Kalinowska, M.; Płońska, A.; Trusiak, M.; Gołębiewska, E.; Gorlewska-Pietluszenko, A. Comparing the extraction methods, chemical composition, phenolic contents and antioxidant activity of edible oils from Cannabis sativa and Silybum marianu seeds. Sci. Rep. 2022, 12, 20609. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, B.; Lee, Y.S.; Kim, T.Y. Role of superoxide dismutase 3 in skin inflammation. J. Dermatol. Sci. 2012, 67, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, S.H. Biophysical properties and finger print in Boswellia sp. Burseraceae. Saudi J. Biol. Sci. 2019, 26, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Srujana, T.S.; Babu, K.R.; Rao, B.S.S. Phytochemical Investigation and Biological Activity of Leaves Extract of Plant Boswellia serrata. Pharma. Innov. 2012, 1, 22–46. [Google Scholar]
- Mishra, S.; Bishnoi, R.S.; Maurya, R.; Jain, D. Boswellia serrata roxb.–A bioactive herbs with various pharmacological activities. Asian J. Pharm. Clin. Res. 2020, 13, 33–39. [Google Scholar] [CrossRef]
- Alraddadi, B.G.; Shin, H.-J. Biochemical Properties and Cosmetic Uses of Commiphora myrrha and Boswellia serrata. Cosmetics 2022, 9, 119. [Google Scholar] [CrossRef]
- Sharma, V.; Nayak, S.K.; Paul, S.R.; Choudhary, B.; Ray, S.S.; Pal, K. 9–Emulgels. In Woodhead Publishing Series in Biomaterials, Polymeric Gels: Characterization, Properties and Biomedical Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 251–264. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef]
- Chandak, S.M.; Trivedi, S.S.; Wadher, K.J.; Umekar, M. Desing, Development and Ex vivo Characterization of Boswellia serrata Loaded Emulgel. Int. Pharm. Phytochem. Res. 2020, 12, 78–84. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kulawik-Pióro, A.; Szczurek, M. Nanoemulsion Gel Formulation Optimization for Burn Wounds: Analysis of Rheological and Sensory Properties. Processes 2020, 8, 1416. [Google Scholar] [CrossRef]
- Auerswald, S.; Schreml, S.; Meier, R.; Blancke Soares, A.; Niyazi, M.; Marschner, S.; Belka, C.; Canis, M.; Haubner, F. Wound monitoring of pH and oxygen in patients after radiation therapy. Radiat. Oncol. 2019, 14, 199. [Google Scholar] [CrossRef]
- Hu, S.C.; Hou, M.F.; Luo, K.H.; Chuang, H.Y.; Wei, S.Y.; Chen, G.S.; Chiang, W.; Huang, C.J. Changes in biophysical properties of the skin following radiotherapy for breast cancer. J. Dermatol. 2014, 41, 1087–1094. [Google Scholar] [CrossRef]
- Dong, L.; Liu, C.; Cun, D.; Fang, L. The effect of rheological behavior and microstructure of the emulgels on the release and permeation profiles of Terpinen-4-ol. Eur. J. Pharm. Sci. 2015, 12, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kulawik-Pióro, A.; Osak, E.; Mendrycka, M.; Trześniewska-Ofiara, Z. Bigels as novel systems for the delivery active compounds from Centella asiatica. Soft Mater. 2023, 21, 316–338. [Google Scholar] [CrossRef]
- Gallegos-Infante, J.A.; Galindo-Galindo, M.d.P.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; González-Laredo, R.F. Effect of Aqueous Extracts of Quercus resinosa on the Mechanical Behavior of Bigels. Sci. Pharm. 2022, 90, 73. [Google Scholar] [CrossRef]
- Arshad, W.; Khan, H.M.S.; Akhtar, N.; Mohammad, I.S. Polymeric emulgel carrying Cinnamomum tamala extract: Promising delivery system for potential topical applications. Braz. J. Pharm. Sci. 2020, 56, e18318. [Google Scholar] [CrossRef]
- de Lafuente, Y.; Ochoa-Andrade, A.; Parente, M.E.; Palena, M.C.; Jimenez-Kairuz, A.F. Preparation and evaluation of caffeine bioadhesive emulgels for cosmetic applications based on formulation design using QbD tools. Int. J. Cosmet. Sci. 2020, 42, 548–556. [Google Scholar] [CrossRef]
- Ergin, A.D.; Inal, O.; Barkat, A. In vitro and ex vivo assessments of surfactant-free topical curcumin emulgel. J. Res. Pharm. 2023, 27, 544–556. [Google Scholar] [CrossRef]
- Di Mambro, V.M.; Fonseca, M.J. Assays of physical stability and antioxidant activity of a topical formulation added with different plant extracts. J. Pharm. Biomed. Anal. 2005, 37, 287–295. [Google Scholar] [CrossRef]
- Al-Yasiry, A.; Kiczorowska, B. Frankincense—Therapeutic properties. Adv. Hyg. Exp. Med. 2016, 70, 380–391. [Google Scholar] [CrossRef]
- Singh, H.P.; Yadav Kumar, I.; Chandra, D.; Jain, D.A. In Vitro Antioxidant and Free Radical Scavenging Activity of Different Extracts of Boerhavia diffusa and Boswellia serrata. Int. J. Pharma Sci. Res. 2012, 3, 503–511. [Google Scholar]
- Siddiqui, M.Z. Boswellia serrata, a potential anti-inflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef]
- Mehta, M.; Dureja, H.; Garg, M. Development and optimization of boswellic acid-loaded proniosomal gel. Drug Deliv. 2016, 23, 3072–3081. [Google Scholar] [CrossRef]
- Kora, A.J.; Sashidhar, R.B.; Arunachalam, J. Aqueous extract of gum olibanum (Boswellia serrata): A reductant and stabilizer for the biosynthesis of antibacterial silver nanoparticles. Process Biochem. 2012, 47, 1516–1520. [Google Scholar] [CrossRef]
- Geetha, V.; Chakravarthula, S.N. Chemical composition and anti-inflammatory activity of Boswellia ovalifoliolata essential oils from leaf and bark. J. For. Res. 2017, 29, 373–381. [Google Scholar] [CrossRef]
- Fatimah, A.; Albasher, G.; Alharbi, R.I.; Asaggabi, N.S. Antifungal Potential of Aqueous Extract of Boswellia carteri. J. Pure Appl. Microbiol. 2019, 13, 2375–2381. [Google Scholar] [CrossRef]
- Almeida, I.F.; Maleckova, J.; Saffi, R.; Monteiro, H.; Góios, F.; Amaral, M.H.; Costa, P.C.; Garrido, J.; Silva, P.; Pestana, N.; et al. Characterization of an antioxidant surfactant-free topical formulation containing Castanea sativa leaf extract. Drug Dev. Ind. Pharm. 2015, 41, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudlou, Y.; Asghari Ghajari, M.; Tavasoli, S. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef]
- Ghafoor, K.; Mohamed, I.A.; Doğu, S.; Uslu, N.; Fadimu, G.J.; Al Juhaimi, F.; Babiker, E.; Özcan, M.M. The Effect of Heating Temperature on Total Phenolic Content, Antioxidant Activity, and Phenolic Compounds of Plum and Mahaleb Fruits. Int. J. Food Eng. 2019, 15, 20170302. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Ayala-Zavala, J.F.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; de Miranda, M.R.A.; Silva-Espinoza, B.A. Using Sensory Evaluation to Determine the Highest Acceptable Concentration of Mango Seed Extract as Antibacterial and Antioxidant Agent in Fresh-Cut Mango. Foods 2018, 7, 120. [Google Scholar] [CrossRef]
- McBride, N.; Hogan, S.A.; Kerry, J.P. Comparative addition of rosemary extract and additives on sensory and antioxidant properties of retail packaged beef. Int. J. Food Sci. Technol. 2007, 42, 1201–1207. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanuskiene, K.; Janulis, V. Formulation of gels and Emulgels with Malus domestica Borkh: Apple extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 372. [Google Scholar] [CrossRef]
- Tasneem, R.; Khan, H.; Zaka, H.S.; Khan, P. Development and cosmeceutical evaluation of topical emulgel containing Albizia lebbeck bark extract. J. Cosmet. Dermatol. 2021, 21, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Togni, S.; Maramaldi, G.; Di Pierro, F.; Biondi, M. A cosmeceutical formulation based on boswellic acids for the treatment of erythematous eczema and psoriasis. Clin. Cosmet. Investig. Dermatol. 2014, 7, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Iram, F.; Khan, S.A.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523. [Google Scholar] [CrossRef]
- Mun, S.; Kim, Y.L.; Kang, C.G.; Park, K.H.; Shim, J.Y.; Yong, P.K. Development of Reduced Fat Mayonnaise using 4αGT a semodified Rice Starch and Xanthan Gum. Int. J. Biol. Macromol. 2009, 44, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.; Khan, H.M.S.; Rasool, F.; Khan, K.U.R.; Usman, F.; Ghalloo, B.A.; Umair, M.; Babalghith, A.O.; Kamran, M.; Aadil, R.M.; et al. Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging. Molecules 2022, 27, 5990. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Tavakolifar, B.; Huseini, H.F.; Mosavat, S.H.; Heydari, M. The Effects of Boswellia serrata Gum Resin on the Blood Glucose and Lipid Profile of Diabetic Patients: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Evid.-Based Integr. Med. 2018, 23, 2515690X18772728. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Cel, K.; Sroka, Z. The mechanistic insights into the role of pH and solvent on antiradical and prooxidant properties of polyphenols—Nine compounds case study. Food Chem. 2023, 407, 134677. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, C.; Romero, A.; Aguilar, J.M.; Cordobés, F.; Guerrero, A. Temperature and pH as factors influencing droplet size distribution and linear viscoelasticity of O/W emulsions stabilized by soy and gluten proteins. Food Hydrocoll. 2010, 24, 783–791. [Google Scholar] [CrossRef]
- Masuda, T.; Chinen, H.; Fukada, K. Effects of Protein Concentration and pH on Oil Droplet Size in O/W Emulsions Stabilized by Bovine Serum Albumin. Chem. Lett. 2014, 43, 598–600. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]




| Ingredient | E_P | Em_P | E_SCO2 | Em_SCO2 | E_SO | Em_SO | Em_hSCO2 | Em_hSO |
|---|---|---|---|---|---|---|---|---|
| % Mass. | ||||||||
| Distilled water | 59.60 | 29.80 | 56.10 | 28.05 | 55.10 | 27.55 | 28.05 | 27.55 |
| Hydrogel | - | 50.00 | - | 50.00 | - | 50.00 | 50.00 | 50.00 |
| Sunflower oil | 10.00 | 5.00 | 10.00 | 5.00 | 10.00 | 5.00 | 5.00 | 5.00 |
| Milk thistle oil | 10.00 | 5.00 | 10.00 | 5.00 | 10.00 | 5.00 | 5.00 | 5.00 |
| Glyceryl monostearate | 8.00 | 4.00 | 8.00 | 4.00 | 8.00 | 4.00 | 4.00 | 4.00 |
| Glycerin | 7.00 | 3.50 | 7.00 | 3.50 | 7.00 | 3.50 | 3.50 | 3.50 |
| Cetyl alcohol | 3.00 | 1.50 | 3.00 | 1.50 | 3.00 | 1.50 | 1.50 | 1.50 |
| Soxhlet extract from B. serrata | - | - | 2.50 | 1.25 | 2.50 | 1.25 | 1.25 | 1.25 |
| Select CO2 extract from B. serrata | - | - | 1.00 | 0.50 | - | - | 0.50 | - |
| Essential oil from B. carterii | - | - | - | - | 2.00 | 1.00 | - | 1.00 |
| D-panthenol | 2.00 | 1.0 | 2.00 | 1.00 | 2.00 | 1.00 | 1.00 | 1.00 |
| Mixture of phenoxyethanol and ethylhexylglycerin | 0.40 | 0.20 | 0.40 | 0.20 | 0.40 | 0.20 | 0.20 | 0.20 |
| Sample | Odor | Color | Consistency | Stability | pH | Mean Droplet Size of Dispersed Phase μm |
|---|---|---|---|---|---|---|
| E_P | none | white | creamy | stable | 6.69 ± 0.01 a | 19.0 ± 1.5 |
| Em_P | none | white | cream–gel | stable | 5.88 ± 0.03 b | 10.9 ± 0.5 |
| E_SCO2 | intense herbal scent | white | creamy | stable | 5.64 ± 0.01 bc | 8.1 ± 0.6 |
| Em_SCO2 | intense herbal scent | white | creamy | stable | 6.12 ± 0.01 ab | 15.1 ± 0.9 |
| E_SO | intense herbal scent | white | creamy | stable | 4.92 ± 0.06 cd | 6.6 ± 0.7 |
| Em_SO | intense herbal scent | white | creamy | stable | 4.57 ± 0.03 cd | 8.6 ± 0.3 |
| Em_hSCO2 | intense herbal scent | white | creamy | stable | 5.51 ± 0.01 cd | 20.9 ± 0.8 |
| Em_hSO | intense herbal scent | white | creamy | stable | 4.77 ± 0.03 d | 7.0 ± 0.6 |
| Sample | , Pa | k, Pa·sn | n, - | R2 | ƞ, Pas at = 50 s−1 |
|---|---|---|---|---|---|
| E_P | 49.27 | 1.372 | 0.686 | 0.999 | 1.35 ± 0.04 |
| Em_P | 36.03 | 4.629 | 0.378 | 0.976 | 1.20 ± 0.08 |
| E_SO | 16.96 | 66.53 | 0.136 | 0.925 | 3.21 ± 0.20 |
| Em_SO | 61.44 | 5.291 | 0.528 | 0.999 | 1.95 ± 0.12 |
| E_SCO2 | 24.07 | 154.3 | 0.161 | 0.929 | 6.50 ± 0.58 |
| Em_SCO2 | 122.6 | 57.19 | 0.247 | 0.990 | 5.62 ± 0.23 |
| Em_hSO | 116.8 | 80.47 | 0.214 | 0.982 | 6.05 ± 0.43 |
| Em_hSCO2 | 84.90 | 70.22 | 0.234 | 0.986 | 5.03 ± 0.30 |
| Sample | Type of Vibrations and Corresponding Wavenumber cm−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ν-OH Alcohols, Phenols 3000– 3500 cm−1 Wide Band at 3500–3550 cm−1 for Carboxylic Acids | ν C-H Aromatic 3030 cm−1 | ν C-H Aliphatic Compound 2850–3000 cm−1 | ν C=O Aldehydes, Ketones, Acids, Esters 1600–1870 cm−1 | ν C=C in Alkenes 1600–1680 cm−1 | ν C=C Aromatic 1500–1610 cm−1 | δ OH Phenols, Alcohols 1330–1420 cm−1 | ν C-O 1050–1430 cm−1 | δ OH Phenols Alcohols 650–770 cm−1 or ν C-Cl 600–800 cm−1 | |
| Soxhlet extract | 3357.19 | - | 2969.41 | 1712.12 | - | - | 1453.57 1377.26 | 1046.90 | 879.38 |
| Select CO2 extract | 3578.17 | 3042.48 | 2957.32 | 1642.94 | 1642.94 | 1511.20 | 1381.69 | 1242.79 | 778.44 |
| Essential oil from B. carterii | 3446.06 | 3043.90 | 2957.74 | 1738.03 | 1643.38 | 1511.52 | 1447.08 1381.46 | 1242.57 | 778.30 |
| Correlations | Odor | Consistency | Cushion Effect | Spreadability | Oiliness | Absorption | Hydration | % Inhibition | Polyphenol Content | pH | Mean Droplet Size | Viscosity | Yield Point | k | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odor | 1.00 | ||||||||||||||
| Consistency | −0.07 | 1.00 | |||||||||||||
| Cushion effect | −0.39 | 0.11 | 1.00 | ||||||||||||
| Spreadability | 0.760 *** | 0.22 | −0.62 | 1.00 | |||||||||||
| Oiliness | −0.15 | −0.14 | 0.920 ** | −0.53 | 1.00 | ||||||||||
| Absorption | 0.28 | −0.09 | −0.919 ** | 0.62 | −0.808 *** | 1.00 | |||||||||
| Hydration | 0.625 **** | −0.03 | −0.08 | 0.60 | 0.07 | 0.04 | 1.00 | ||||||||
| % inhibition | 0.15 | −0.45 | 0.45 | −0.04 | 0.624 **** | −0.38 | 0.662 **** | 1.00 | |||||||
| Polyphenol content | 0.44 | 0.01 | 0.26 | 0.39 | 0.23 | −0.37 | 0.58 | 0.50 | 1.00 | ||||||
| pH | −0.38 | 0.36 | 0.36 | −0.62 | 0.30 | −0.33 | −0.51 | −0.35 | −0.644 **** | 1.00 | |||||
| Mean droplet size | 0.18 | −0.16 | −0.09 | −0.27 | 0.12 | 0.08 | −0.34 | −0.30 | −0.628 **** | 0.677 **** | 1.00 | ||||
| Viscosity | 0.43 | 0.31 | 0.11 | 0.48 | 0.20 | 0.07 | 0.29 | 0.21 | 0.36 | −0.12 | −0.09 | 1.00 | |||
| Yield point | 0.36 | 0.24 | −0.36 | 0.49 | −0.27 | 0.54 | −0.21 | −0.50 | −0.20 | −0.04 | 0.30 | 0.39 | 1.00 | ||
| k | 0.45 | 0.39 | 0.22 | 0.35 | 0.22 | −0.21 | 0.27 | 0.16 | 0.53 | −0.03 | −0.11 | 0.874 ** | 0.10 | 1.00 | |
| n | −0.30 | −0.14 | 0.15 | −0.57 | 0.07 | −0.33 | −0.57 | −0.43 | −0.29 | 0.38 | 0.39 | −0.764 *** | −0.05 | −0.57 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulawik-Pióro, A.; Goździcka, W.; Kruk, J.; Piotrowska, A. Plant-Origin Additives from Boswellia Species in Emulgel Formulation for Radiotherapy Skin Care. Appl. Sci. 2024, 14, 8648. https://doi.org/10.3390/app14198648
Kulawik-Pióro A, Goździcka W, Kruk J, Piotrowska A. Plant-Origin Additives from Boswellia Species in Emulgel Formulation for Radiotherapy Skin Care. Applied Sciences. 2024; 14(19):8648. https://doi.org/10.3390/app14198648
Chicago/Turabian StyleKulawik-Pióro, Agnieszka, Weronika Goździcka, Joanna Kruk, and Anna Piotrowska. 2024. "Plant-Origin Additives from Boswellia Species in Emulgel Formulation for Radiotherapy Skin Care" Applied Sciences 14, no. 19: 8648. https://doi.org/10.3390/app14198648







