Evaluation of Biodentine Tricalcium Silicate-Based Cement after Chlorhexidine Irrigation
Abstract
1. Introduction
2. Materials and Methods
3. Results
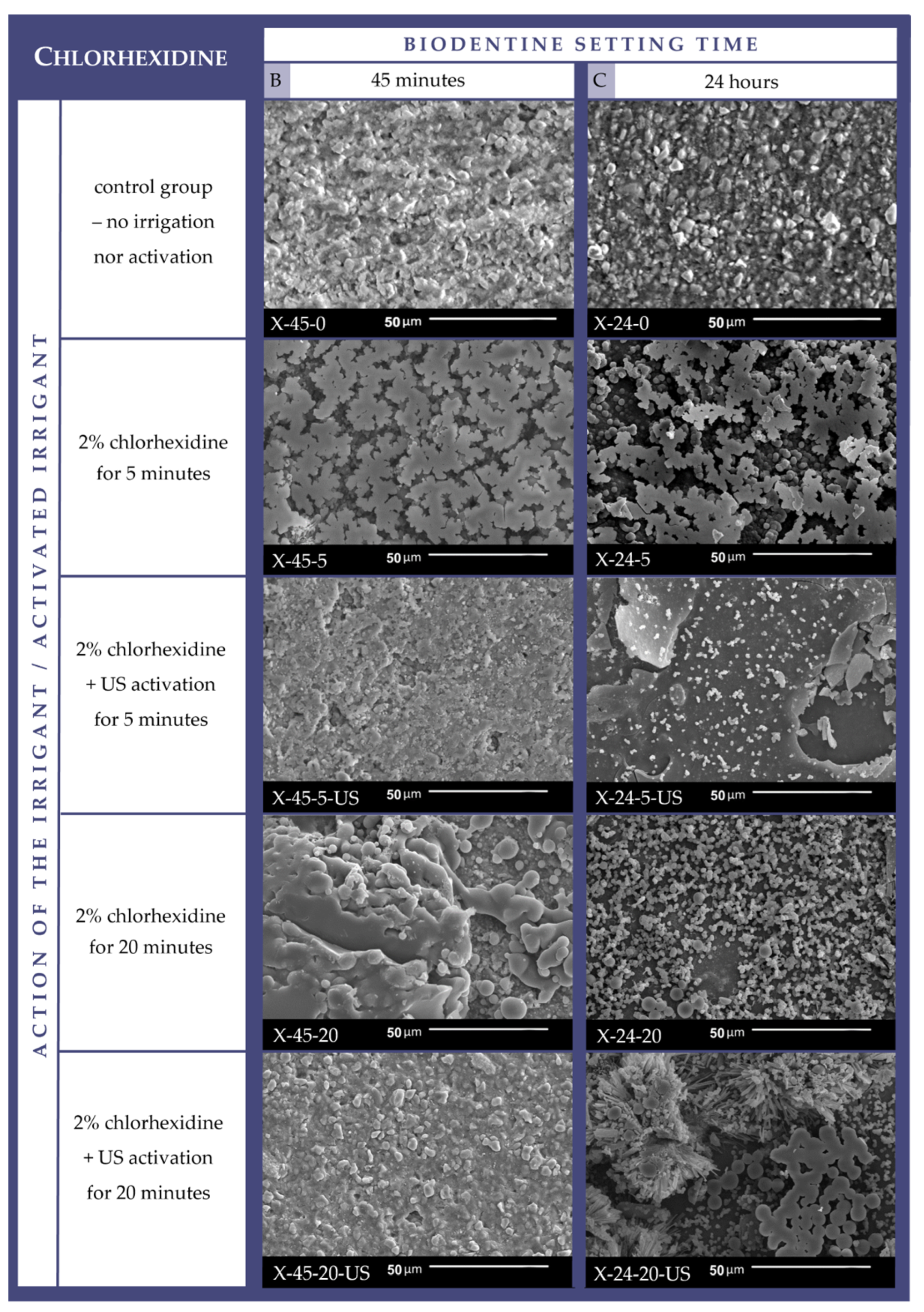
3.1. Cement Surface Microappearance
3.2. Cement Surface Chemical Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European Society of Endodontology Position Statement: Management of Deep Caries and the Exposed Pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G. Bioactive Dental Materials: The Current Status. Materials 2022, 15, 2016. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. The Use of Bioceramics in Endodontics—Literature Review. Clujul Med. 2016, 89, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Rodríguez-Lozano, F.J.; Llena, C.; Sauro, S.; Forner, L. Bioactivity of Bioceramic Materials Used in the Dentin-Pulp Complex Therapy: A Systematic Review. Materials 2019, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the Physical Properties of Tricalcium Silicate Cement-Based Root-End Filling Materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium Silicate-Based Cements: Composition, Properties, and Clinical Applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]
- Camilleri, J. Biodentine™: The Dentine in a Capsule or More? Septodont Clinical Insights. Available online: https://www.septodontcorp.com/wp-content/uploads/2018/02/Biodentine-Article-0118-LOW.pdf (accessed on 24 June 2024).
- Malkondu, Ö.; Karapinar Kazandağ, M.; Kazazoğlu, E. A Review on Biodentine, a Contemporary Dentine Replacement and Repair Material. Biomed Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Septodont. Biodentine™: Active Biosilicate Technology™; Scientific File; Septodont: Saint-Maur-des-Fossés, France, 2010. [Google Scholar]
- Aksoy, M.K.; Oz, F.T.; Orhan, K. Evaluation of Calcium (Ca2+) and Hydroxide (OH−) Ion Diffusion Rates of Indirect Pulp Capping Materials. Int. J. Artif. Organs 2017, 40, 641–646. [Google Scholar] [CrossRef]
- Koubi, G.; Colon, P.; Franquin, J.C.; Hartmann, A.; Richard, G.; Faure, M.O.; Lambert, G. Clinical Evaluation of the Performance and Safety of a New Dentine Substitute, Biodentine, in the Restoration of Posterior Teeth—A Prospective Study. Clin. Oral Investig. 2013, 17, 243–249. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of Human Dental Pulp Capped with Biodentine and Mineral Trioxide Aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Martens, L.C.; Vandenbulcke, J.; Jacquet, W.; Bottenberg, P.; Cauwels, R.G.E.C. Efficacy of Three Different Pulpotomy Agents in Primary Molars: A Randomized Control Trial. Int. Endod. J. 2017, 50, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Lu, J.X.; Zeng, Q.; Zhao, W.; Li, W.Q.; Ling, J.Q. Comparison of Mineral Trioxide Aggregate and Calcium Hydroxide for Apexification of Immature Permanent Teeth: A Systematic Review and Meta-Analysis. J. Formos. Med. Assoc. 2016, 115, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Abdulkhader, S.Z. Full Pulpotomy with Biodentine in Symptomatic Young Permanent Teeth with Carious Exposure. J. Endod. 2018, 44, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.B.; Pieroni, K.A.M.G.; Nelson-Filho, P.; Silva, R.A.B.; Hernandéz-Gatón, P.; Lucisano, M.P.; Paula-Silva, F.W.G.; de Queiroz, A.M. Furcation Perforation: Periradicular Tissue Response to Biodentine as a Repair Material by Histopathologic and Indirect Immunofluorescence Analyses. J. Endod. 2017, 43, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Kokate, S.; Shah, R. Management of a Large Periapical Lesion Using BiodentineTM as Retrograde Restoration with Eighteen Months Evident Follow Up. J. Conserv. Dent. 2013, 16, 573–575. [Google Scholar] [CrossRef]
- Estrela, C.; Decurcio, D.d.A.; Rossi-Fedele, G.; Silva, J.A.; Guedes, O.A.; Borges, Á.H. Root Perforations: A Review of Diagnosis, Prognosis and Materials. Braz. Oral Res. 2018, 32, 133–146. [Google Scholar] [CrossRef]
- Palatyńska-Ulatowska, A.; Buła, K.; Klimek, L. Influence of Sodium Hypochlorite and Ultrasounds on Surface Features and Chemical Composition of Biodentine Tricalcium Silicate-Based Material. Dent. Mater. J. 2020, 39, 587–592. [Google Scholar] [CrossRef]
- American Association of Endodontists (AAE). Glossary of Endodontic Terms. Available online: https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/ (accessed on 28 June 2024).
- Touré, B.; Faye, B.; Kane, A.W.; Lo, C.M.; Niang, B.; Boucher, Y. Analysis of Reasons for Extraction of Endodontically Treated Teeth: A Prospective Study. J. Endod. 2011, 37, 1512–1515. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Palatyńska-Ulatowska, A.; Klimek, L. The Effect of Irrigation with Citric Acid on Biodentine Tricalcium Silicate-Based Cement: SEM-EDS In Vitro Study. Materials 2022, 15, 3467. [Google Scholar] [CrossRef]
- Zehnder, M. Root Canal Irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Zamany, A.; Safavi, K.; Spångberg, L.S.W. The Effect of Chlorhexidine as an Endodontic Disinfectant. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Wilkoński, W.; Jamróz-Wilkońska, L. Protokół Płukania Kanałów Korzeniowych Według Zaleceń Działu Badawczego Polskiego Towarzystwa Endodontycznego. Mag. Stomatol. 2013, 246, 92–94. [Google Scholar]
- Boutsioukis, C.; Arias-Moliz, M.T. Present Status and Future Directions—Irrigants and Irrigation Methods. Int. Endod. J. 2022, 55, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.N.; Pinto, E.B.; Galo, R.; Falci, S.G.M.; Mesquita, A.T. Passive Ultrasonic Irrigation in Root Canal: Systematic Review and Meta-Analysis. Acta Odontol. Scand. 2019, 77, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic Irrigants: Different Methods to Improve Efficacy and Related Problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef]
- Aggarwal, V.; Singla, M.; Miglani, S.; Kohli, S. Comparative Evaluation of Push-out Bond Strength of ProRoot MTA, Biodentine, and MTA Plus in Furcation Perforation Repair. J. Conserv. Dent. 2013, 16, 462–465. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Palatyńska-Ulatowska, A.; Klimek, L. How Does Ethylenediaminetetraacetic Acid Irrigation Affect Biodentine? A Multimethod Ex Vivo Study. Materials 2024, 17, 1230. [Google Scholar] [CrossRef]
- Villat, C.; Grosgogeat, B.; Seux, D.; Farge, P. Conservative Approach of a Symptomatic Carious Immature Permanent Tooth Using a Tricalcium Silicate Cement (Biodentine): A Case Report. Restor. Dent. Endod. 2013, 38, 258–264. [Google Scholar] [CrossRef]
- Borkar, S.A.; Ataide, I. Biodentine Pulpotomy Several Days after Pulp Exposure: Four Case Reports. J. Conserv. Dent. 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Hashem, D.F.; Foxton, R.; Manoharan, A.; Watson, T.F.; Banerjee, A. The Physical Characteristics of Resin Composite-Calcium Silicate Interface as Part of a Layered/Laminate Adhesive Restoration. Dent. Mater. 2014, 30, 343–349. [Google Scholar] [CrossRef]
- Attur, K.; Joy, M.; Karim, R.; Anil Kumar, V.; Deepika, C.; Ahmed, H. Comparative Analysis of Endodontic Smear Layer Removal Efficacy of 17% Ethylenediaminetetraacetic Acid, 7% Maleic Acid, and 2% Chlorhexidine Using Scanning Electron Microscope: An in Vitro Study. J. Int. Soc. Prev. Community Dent. 2016, 6, S160–S165. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshida, K.; Suzuki, R.; Nakamura, H. Root Canal Irrigation with Citric Acid Solution. J. Endod. 1996, 22, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Buła, K.; Palatyńska-Ulatowska, A.; Klimek, L. Biodentine Management and Setting Time with Vicat and Vickers Evaluation; a Survey-Based Study on Clinicians’ Experience. Arch. Mater. Sci. Eng. 2020, 103, 75–85. [Google Scholar] [CrossRef]
- Lapinska, B.; Klimek, L.; Sokolowski, J.; Lukomska-Szymanska, M. Dentine Surface Morphology after Chlorhexidine Application-SEM Study. Polymers 2018, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Ghabraei, S.; Afkhami, F.; Shamshiri, A.R.; Mohammadi, Z. Comparison of Cytotoxicity between Mineral Trioxide Aggregate Mixed with Chlorhexidine and Common Endodontic Regeneration Medicaments on Periodontal Ligament Stem Cells: An in Vitro Study. Front. Dent. 2023, 20, 1. [Google Scholar] [CrossRef]
- Hernandez, E.P.; Botero, T.M.; Mantellini, M.G.; McDonald, N.J.; Nör, J.E. Effect of ProRoot® MTA Mixed with Chlorhexidine on Apoptosis and Cell Cycle of Fibroblasts and Macrophages in Vitro. Int. Endod. J. 2005, 38, 137–143. [Google Scholar] [CrossRef]


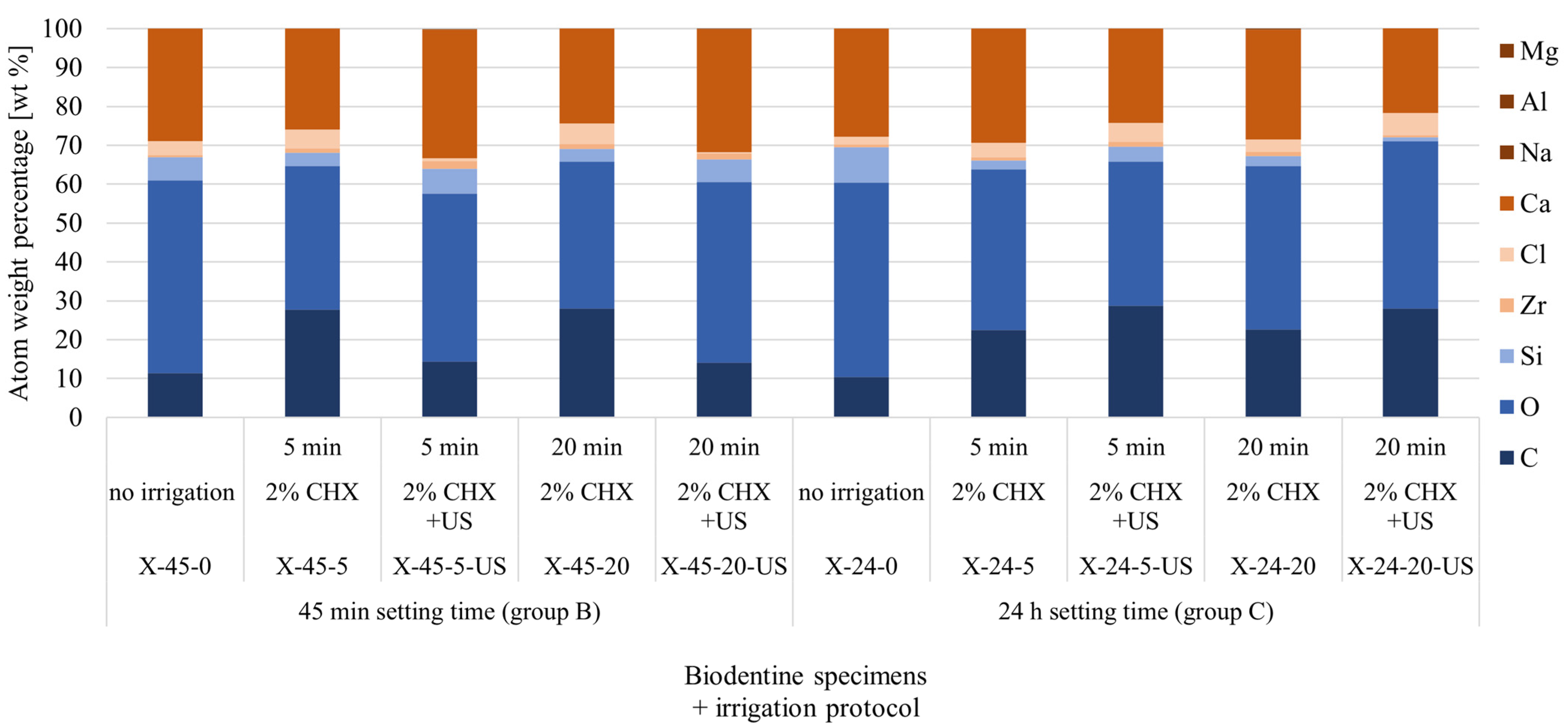
| Element | 45 min Setting Time (Group B) | 24 h Setting Time (Group C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X-45-0 | X-45-5 | X-45-5-US | X-45-20 | X-45-20-US | X-24-0 | X-24-5 | X-24-5-US | X-24-20 | X-24-20-US | |
| No Irrigation | 2% CHX; 5 min | 2% CHX + US; 5 min | 2% CHX; 20 min | 2% CHX + US; 20 min | No Irrigation | 2% CHX; 5 min | 2% CHX + US; 5 min | 2% CHX; 20 min | 2% CHX + US; 20 min | |
| C | 11.35 | 27.8 | 14.4 | 28.0 | 14.1 | 10.41 | 22.5 | 28.7 | 22.6 | 28.0 |
| O | 49.61 | 36.9 | 43.2 | 37.8 | 46.5 | 49.93 | 41.3 | 37.1 | 42.0 | 43.1 |
| Si | 5.96 | 3.4 | 6.3 | 3.3 | 5.8 | 9.09 | 2.3 | 3.9 | 2.6 | 1.0 |
| Zr | 0.56 | 1.1 | 2.0 | 1.2 | 1.6 | 0.74 | 0.8 | 1.2 | 1.1 | 0.5 |
| Cl | 3.64 | 4.9 | 0.7 | 5.3 | 0.2 | 2.09 | 3.7 | 4.8 | 3.2 | 5.7 |
| Ca | 28.87 | 25.9 | 33.2 | 24.4 | 31.7 | 27.74 | 29.4 | 24.3 | 28.2 | 21.8 |
| Na | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 |
| Al | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mg | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, K.; Palatyńska-Ulatowska, A.; Klimek, L. Evaluation of Biodentine Tricalcium Silicate-Based Cement after Chlorhexidine Irrigation. Appl. Sci. 2024, 14, 8702. https://doi.org/10.3390/app14198702
Dąbrowska K, Palatyńska-Ulatowska A, Klimek L. Evaluation of Biodentine Tricalcium Silicate-Based Cement after Chlorhexidine Irrigation. Applied Sciences. 2024; 14(19):8702. https://doi.org/10.3390/app14198702
Chicago/Turabian StyleDąbrowska, Katarzyna, Aleksandra Palatyńska-Ulatowska, and Leszek Klimek. 2024. "Evaluation of Biodentine Tricalcium Silicate-Based Cement after Chlorhexidine Irrigation" Applied Sciences 14, no. 19: 8702. https://doi.org/10.3390/app14198702
APA StyleDąbrowska, K., Palatyńska-Ulatowska, A., & Klimek, L. (2024). Evaluation of Biodentine Tricalcium Silicate-Based Cement after Chlorhexidine Irrigation. Applied Sciences, 14(19), 8702. https://doi.org/10.3390/app14198702







