Preparation, Characterization, and Application of P(aluminum chloride-co-diallyldimethylammonium chloride) Hybrid Flocculant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Template Polymer
2.3. Characterization of the Polymer
2.4. Decolorization Test
3. Results and Discussion
3.1. Optimizing Synthesis Conditions
3.1.1. Effect of Modifier KH570 Concentration
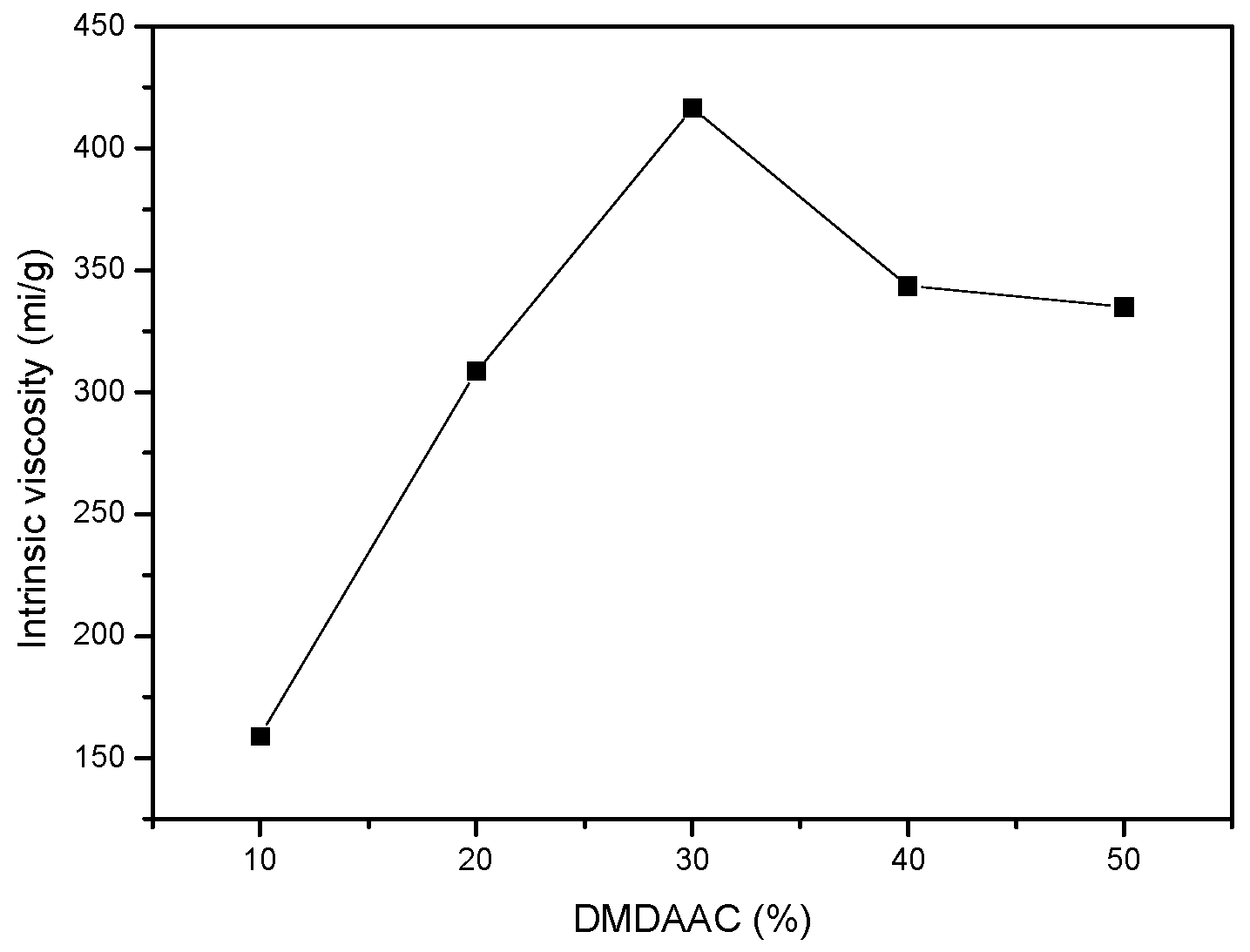
3.1.2. Effect of DMDAAC Concentration
3.1.3. Effect of Initiator Concentration
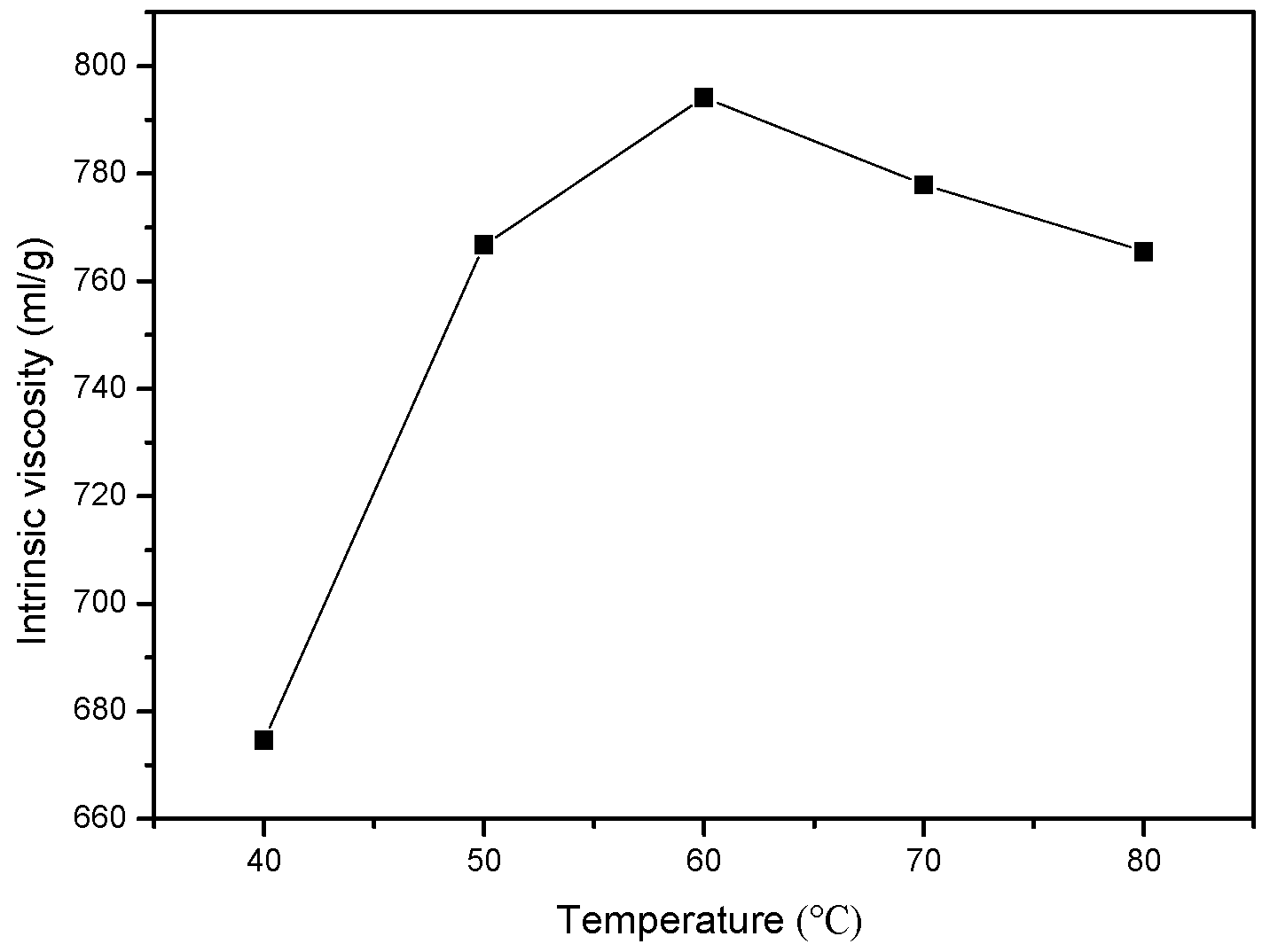
3.1.4. Effect of Reaction Temperature
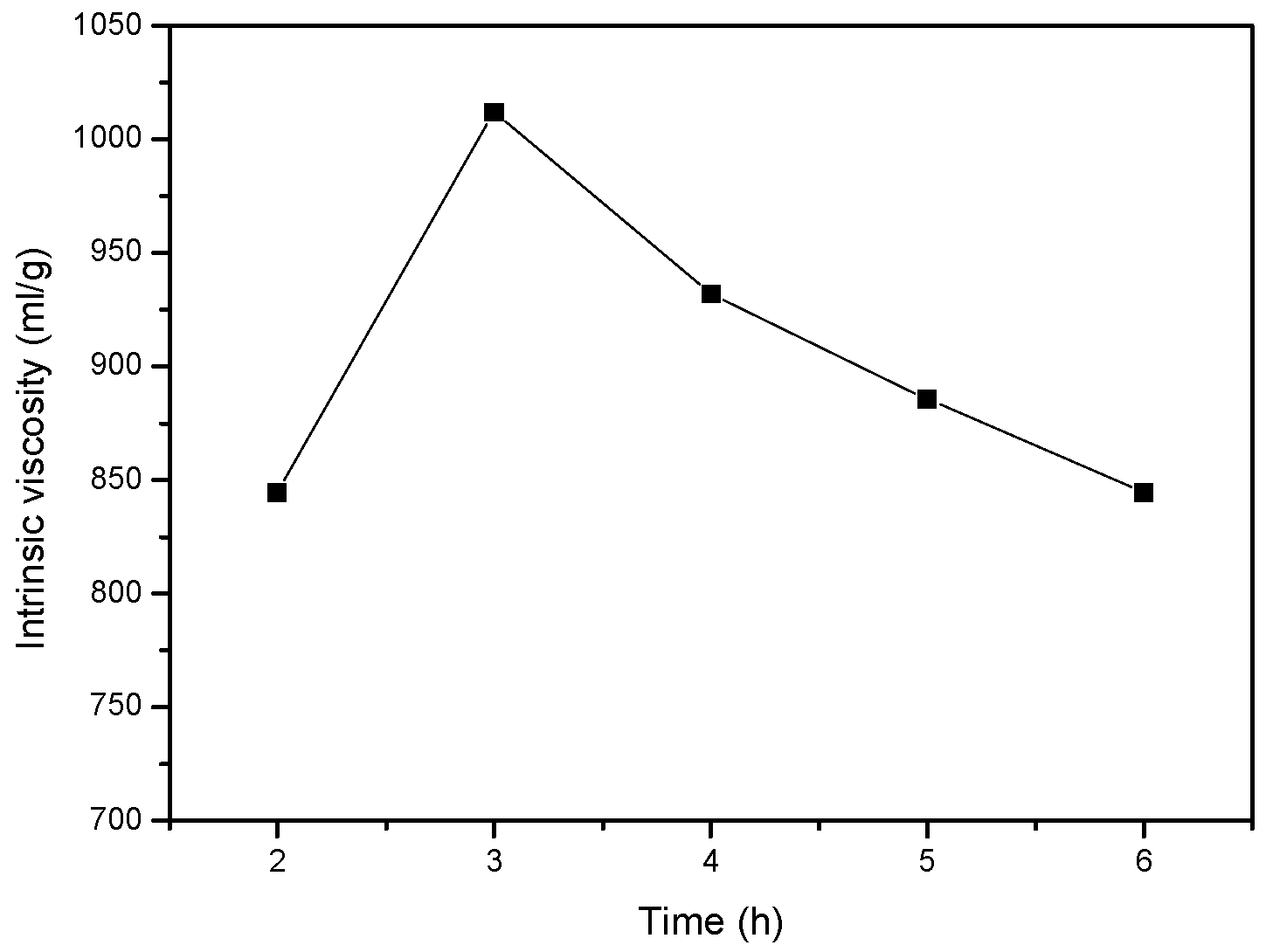
3.1.5. Effect of Reaction Time
3.2. Characterization
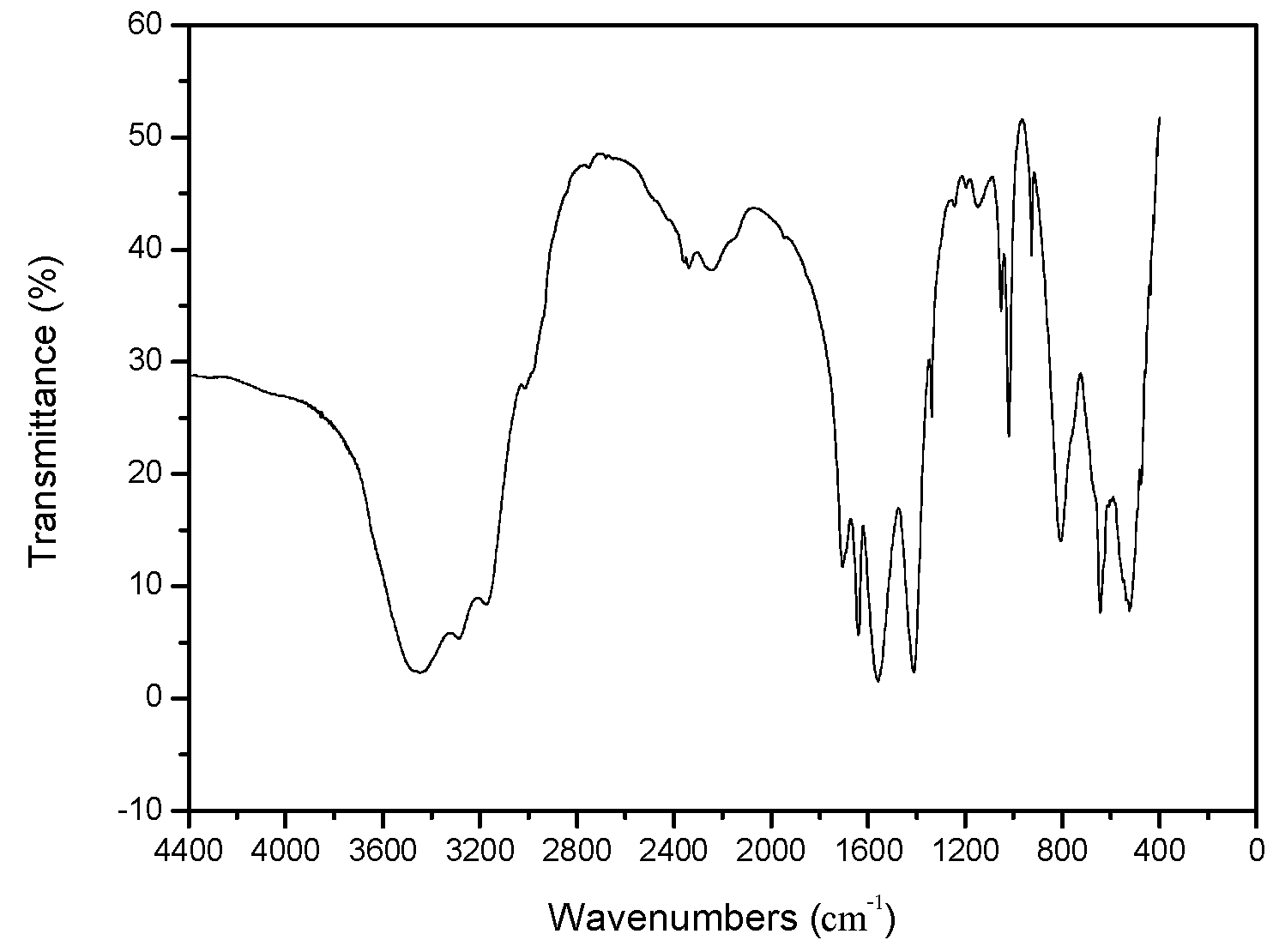
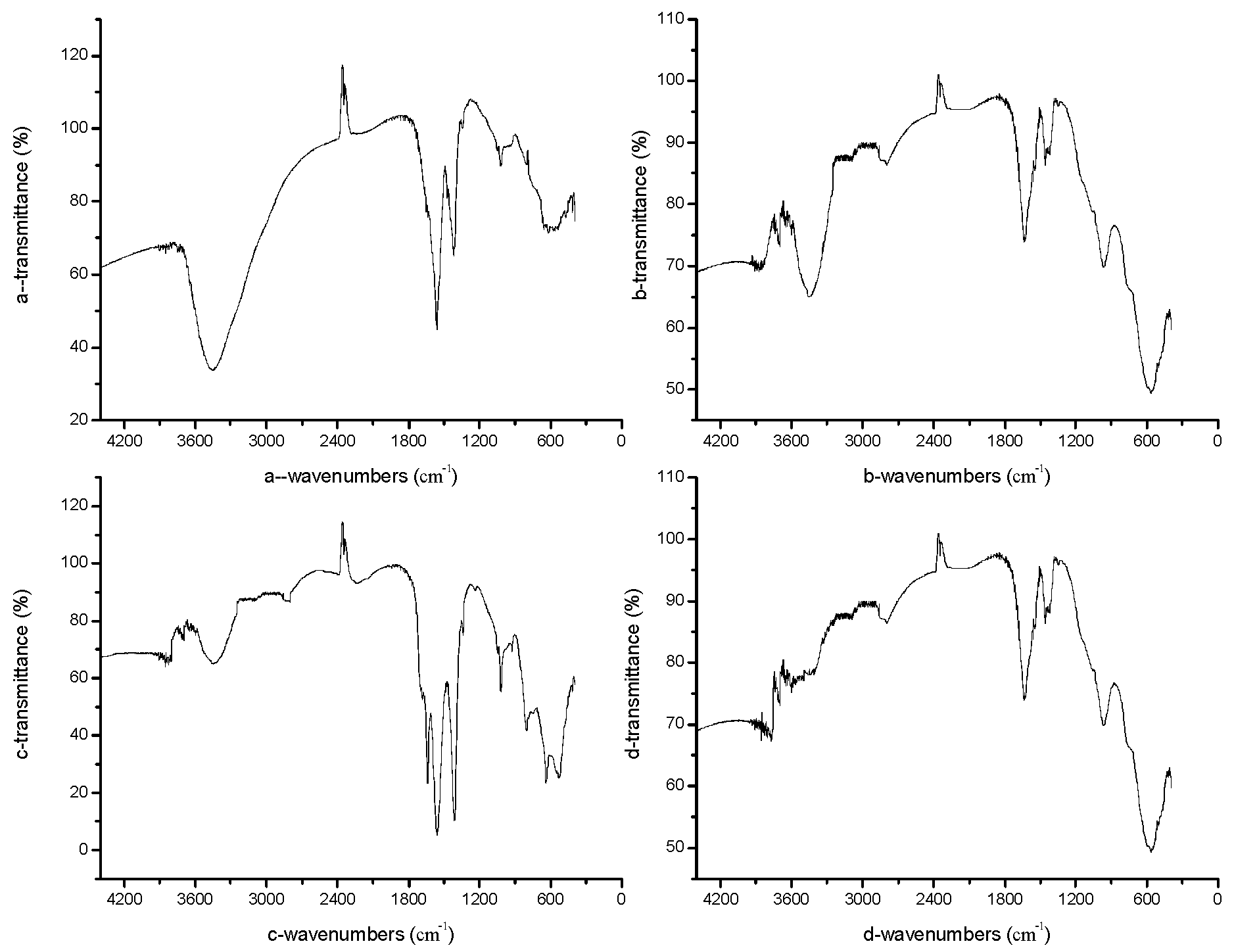
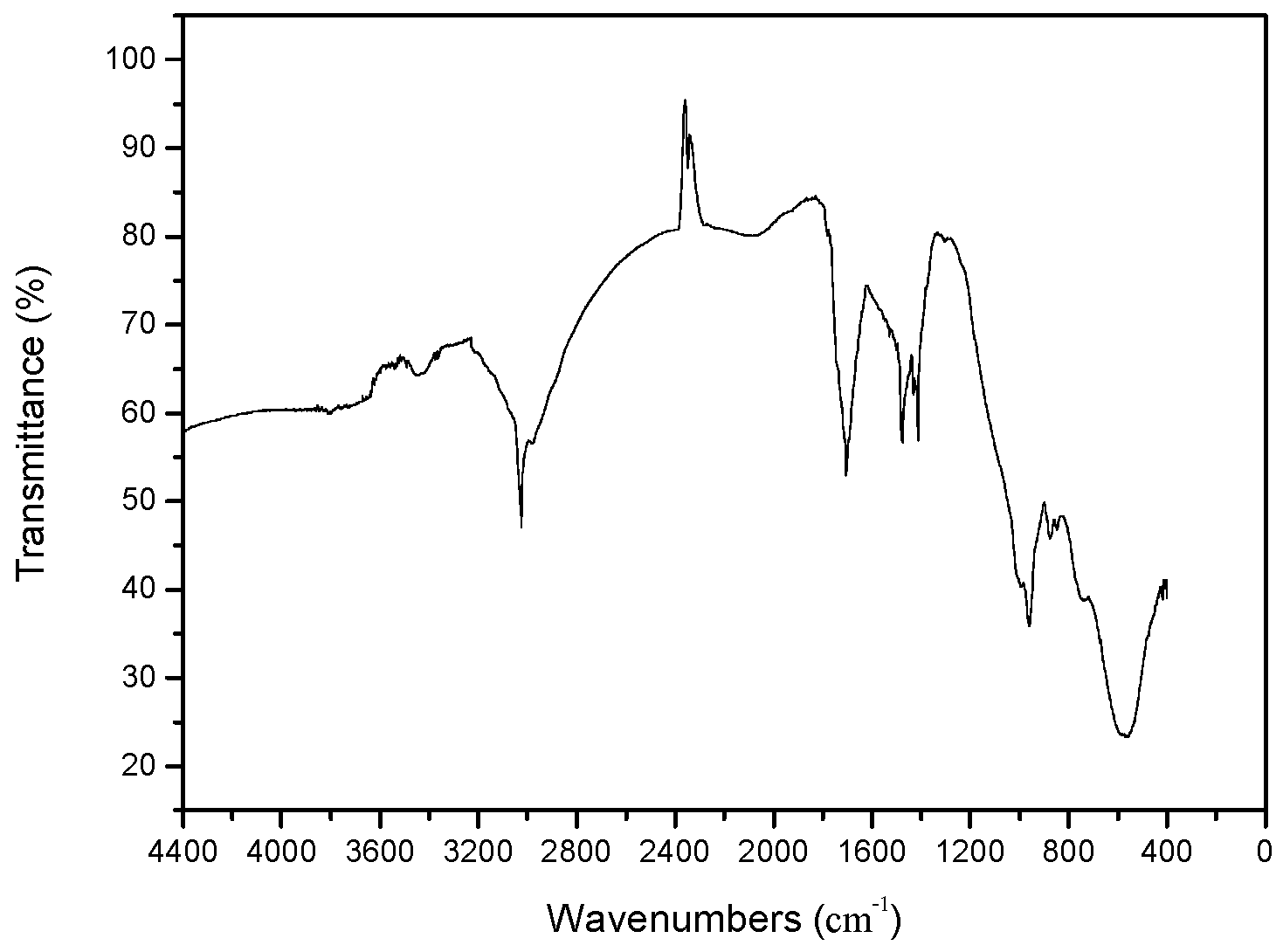
3.2.1. FTIR
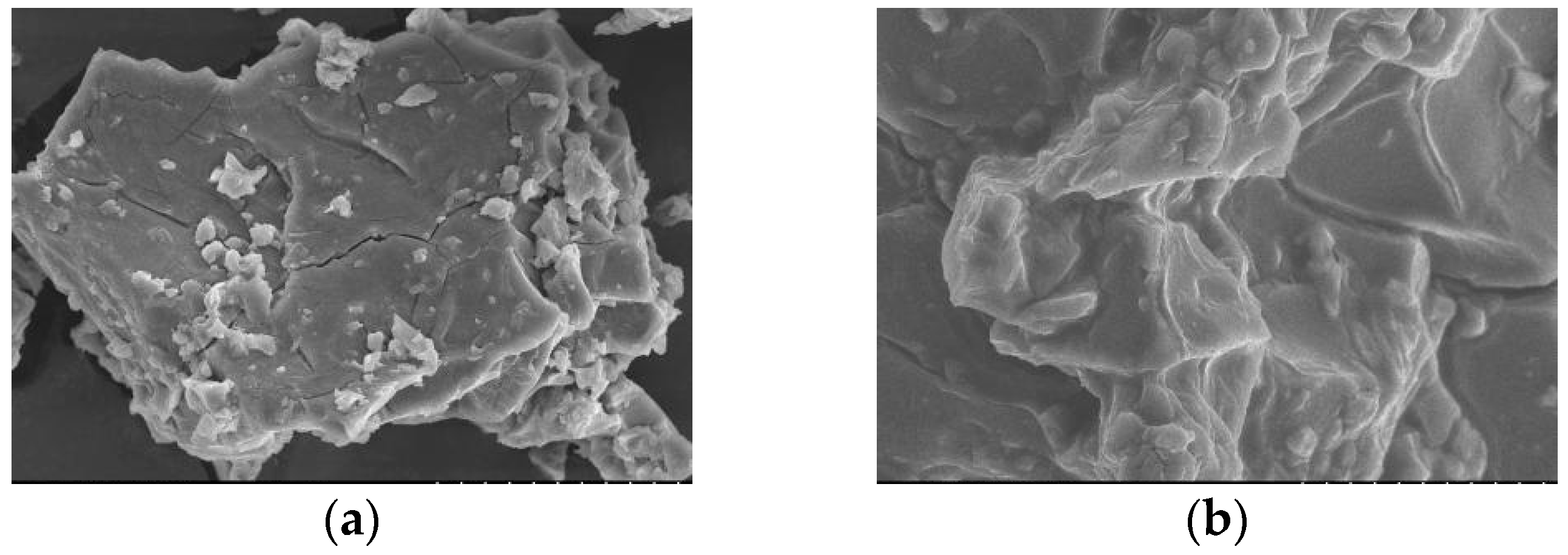
3.2.2. SEM
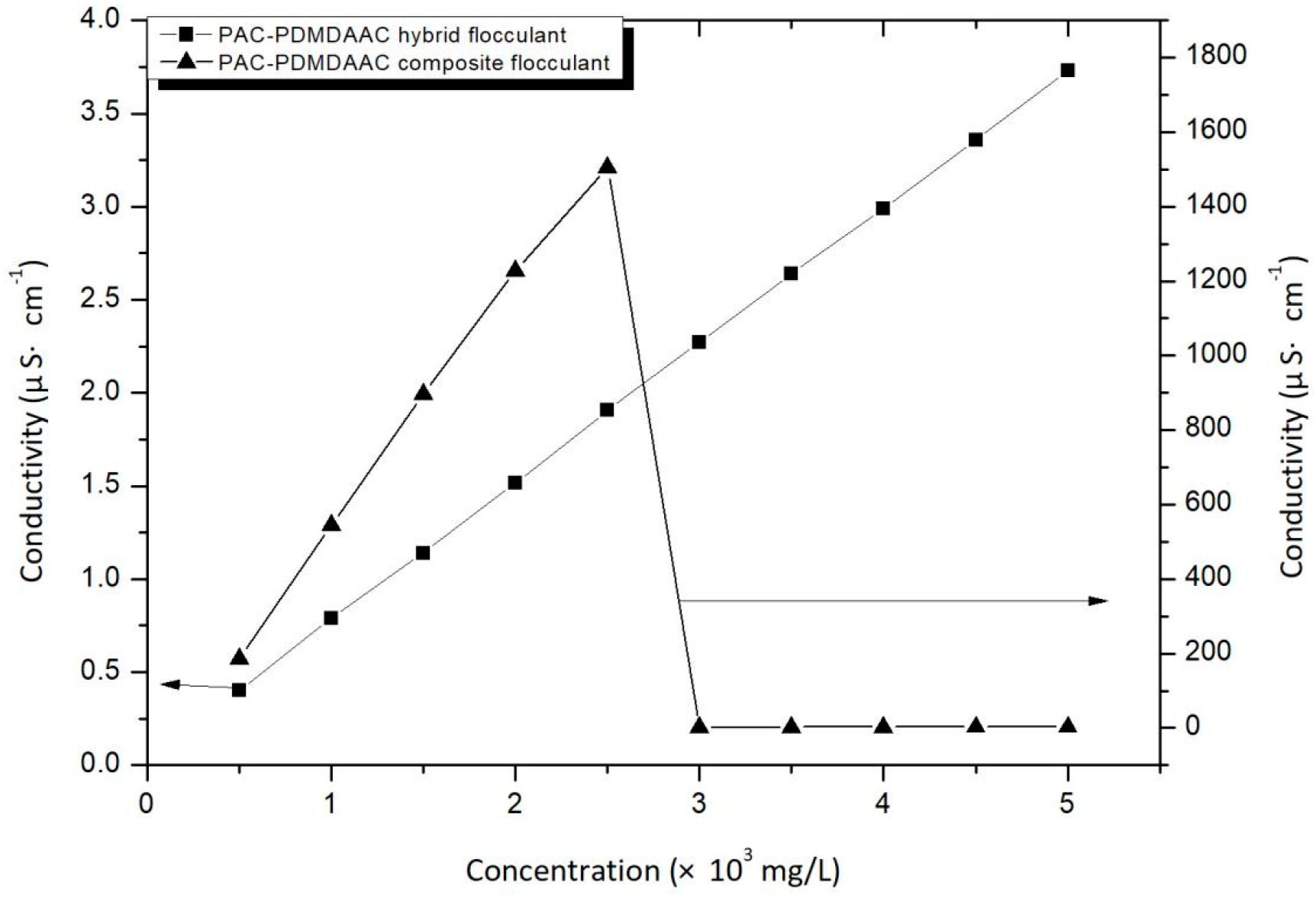
3.2.3. Electrical Conductivity Test
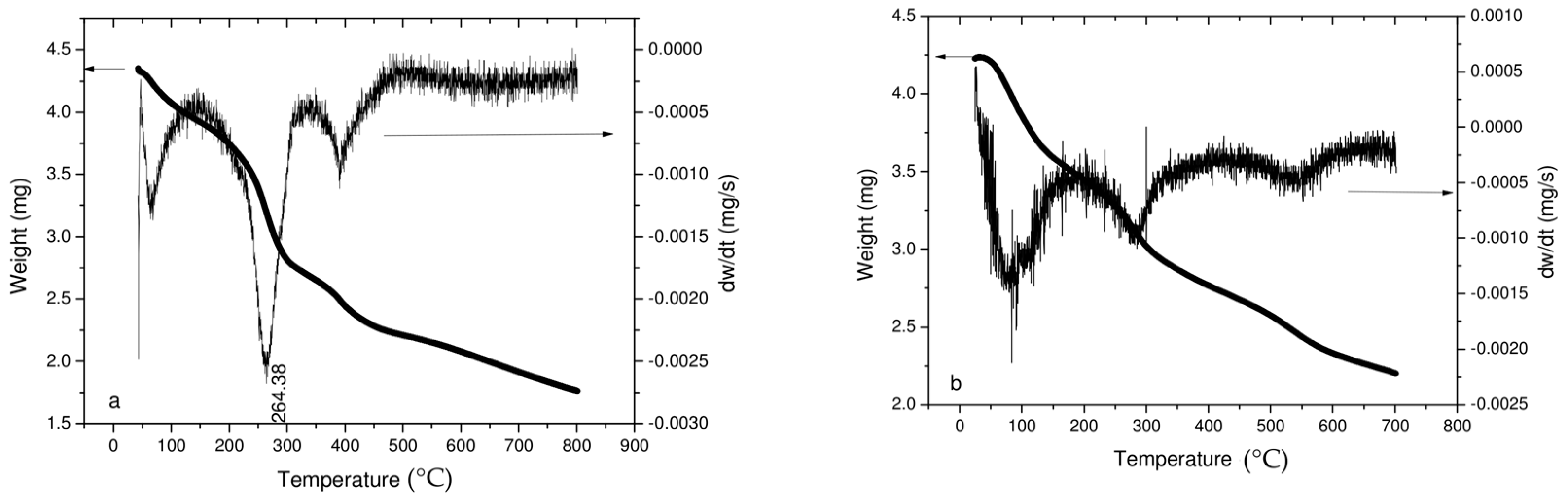
3.2.4. TGA
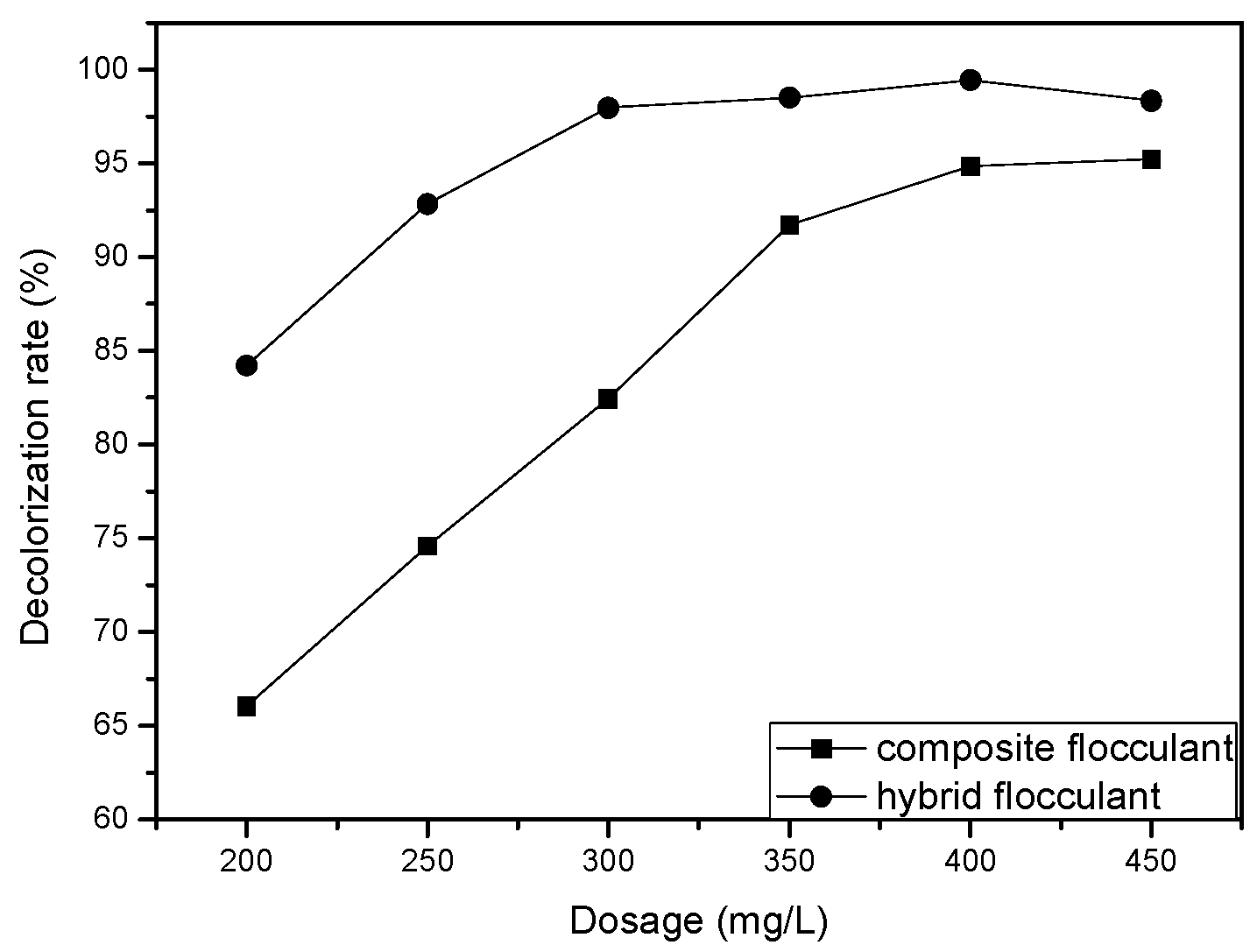
3.3. Flocculation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, Q.Q.; Zheng, H.L. Preparation, Characterization, and flocculation performance of P(acrylamide-co-diallyldimethylammonium chloride) by UV-initiated template polymerization. J. Appl. Polym. Sci. 2015, 132, 41747. [Google Scholar] [CrossRef]
- Jiang, S.J.; Feng, X.R. Flocculation Performance and Shearing Capacity Study on Al30 Morphology in Polyhydroxy Aluminium. Asian J. Chem. 2013, 25, 9875–9878. [Google Scholar] [CrossRef]
- Ji, X.M.; Li, Z.H. Response Surface Methodology Approach to Optimize Parameters for Coagulation Process Using Polyaluminum Chloride (PAC). Water 2024, 16, 1470. [Google Scholar] [CrossRef]
- Roach, J.D.; Lane, R.F. Comparative study of the uses of poly(4-vinylpyridine) and poly(diallyldimethylammonium) chloride for the removal of perchlorate from aqueous solution by polyelectrolyte-enhanced ultrafiltration. Water Res. 2011, 45, 1387–1393. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wu, Q. Preparation of composite coagulant for the removal of microplastics in water. Water Environ. Res. 2023, 95, 10969. [Google Scholar] [CrossRef]
- Wu, L.M.; Deng, J.J. Understanding synergistic mechanisms of silicate decorated polyaluminium chloride and organic polymer flocculation for enhancing polymer-flooding wastewater treatment. Process Saf. Environ. Prot. 2023, 170, 1–10. [Google Scholar] [CrossRef]
- Jiang, S.J.; Li, X.E.; Feng, X.R. Synthesis and Characterization of PAC-PDMDAAC Organic-Inorganic Hybrid Polymer Flocculants. Acta. Polym. Sin. 2014, 4, 466–472. [Google Scholar]
- Shen, X.; Gao, B.Y. Application of composite flocculants for removing organic matter and mitigating ultrafiltration membrane fouling in surface water treatment: The role of composite ratio. Environ. Sci. Water Res. Technol. 2019, 5, 2242–2250. [Google Scholar] [CrossRef]
- KhaiErn, L.; Norhashimah, M. Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater: A review. Chem. Eng. J. 2012, 203, 370–386. [Google Scholar]
- Ariffin, A.; Razali, M.A.A. PolyDADMAC and polyacrylamide as a hybrid flocculation system in the treatment of pulp and paper mills waste water. Chem. Eng. J. 2012, 179, 107–111. [Google Scholar] [CrossRef]
- Tripathy, J.; Mishra, A. Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review. Water 2024, 16, 1481. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Gao, B.Y. Comparison of coagulation behavior and floc characteristics of titanium tetrachloride(TiCl4) and polyaluminum chloride(PACl) with surface water treatment. Chem. Eng. J. 2011, 166, 544–550. [Google Scholar] [CrossRef]
- Chao, L.; Wang, H.W. Design of SiO2-TiO2-P(AM-DMC) cationic composite flocculant and optimization of the flocculation process using response surface methodology. J. Disper. Sci. Technol. 2021, 42, 1132–1145. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, B. Evaluation of the flocculation performance of carboxymethyl chitosan-graft-polyacrylamide, a novel amphoteric chemically bonded composite flocculant. Water Res. 2012, 46, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhao, D.Y. Preparation and performance evaluation of hybrid polymer flocculants (PAC-PAM), and comparison experiments with other flocculants. Environ. Prog. Sustain. 2022, 41, 13829. [Google Scholar] [CrossRef]
- Chen, M.J.; Zou, C.J. Characterization and flocculation evaluation of a new organic-inorganic hybrid polymer flocculant (PAC-AM-DMC). J. Appl. Polym. Sci. 2021, 138, 51388. [Google Scholar] [CrossRef]
- Bharti, S.; Mishra, S. Synthesis, Characterization and Application of Polymethyl Methacrylate Grafted oatmeal: A potential Flocculant for Wastewater Treatment. Int. J. Environ. Res. 2016, 10, 169–178. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Liu, B. Preparation, Characterization, and Application of P(aluminum chloride-co-diallyldimethylammonium chloride) Hybrid Flocculant. Appl. Sci. 2024, 14, 8708. https://doi.org/10.3390/app14198708
Feng X, Liu B. Preparation, Characterization, and Application of P(aluminum chloride-co-diallyldimethylammonium chloride) Hybrid Flocculant. Applied Sciences. 2024; 14(19):8708. https://doi.org/10.3390/app14198708
Chicago/Turabian StyleFeng, Xinrui, and Bei Liu. 2024. "Preparation, Characterization, and Application of P(aluminum chloride-co-diallyldimethylammonium chloride) Hybrid Flocculant" Applied Sciences 14, no. 19: 8708. https://doi.org/10.3390/app14198708



