Upgrading Biomass Wastes to Graphene Quantum Dots with White-Light-Emitting Features in the Solid State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis and Purification of GQDs
2.3. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Selva Sharma, A.; Lee, N.Y. Comprehensive review on fluorescent carbon dots and their applications in nucleic acid detection, nucleolus targeted imaging and gene delivery. Analyst 2024, 149, 4095–4115. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.; Tian, Z.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D. Electrochemical Tuning of Luminescent Carbon Nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Gozali Balkanloo, P.; Mohammad Sharifi, K.; Poursattar Marjani, A. Graphene quantum dots: Synthesis, characterization, and application in wastewater treatment: A review. Mater. Adv. 2023, 4, 4272–4293. [Google Scholar] [CrossRef]
- Lai, S.; Jin, Y.; Shi, L.; Zhou, R.; Zhou, Y.; An, D. Mechanisms behind excitation- and concentration-dependent multicolor photoluminescence in graphene quantum dots. Nanoscale 2020, 12, 591–601. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Li, Q. The toxicity of graphene quantum dots. RSC Adv. 2016, 6, 89867–89878. [Google Scholar] [CrossRef]
- Dorontić, S.; Jovanović, S.; Bonasera, A. Shedding Light on Graphene Quantum Dots: Key Synthetic Strategies, Characterization Tools, and Cutting-Edge Applications. Materials 2021, 14, 6153. [Google Scholar] [CrossRef]
- Pierrat, P.; Gaumet, J.-J. Graphene quantum dots: Emerging organic materials with remarkable and tunable luminescence features. Tetrahedron Lett. 2020, 61, 152554. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, B.; Guo, G.; Li, Y.; Li, T.; Zhu, Y.; Wang, X.; Chen, D. Bright Tunable Multicolor Graphene Quantum Dots for Light-Emitting Devices and Anticounterfeiting Applications. ACS Appl. Nano Mater. 2023, 6, 3245–3253. [Google Scholar] [CrossRef]
- Nie, Q.; Jia, L.; Luan, J.; Cui, Y.; Liu, J.; Tan, Z.; Yu, H. Graphene quantum dots/BiOCl visible-light active photocatalyst for degradation of NO. Chem. Eng. Sci. 2024, 285, 119614. [Google Scholar] [CrossRef]
- Selvakumar, T.; Rajaram, M.; Natarajan, A.; Harikrishnan, L.; Alwar, K.; Rajaram, A. Highly Efficient Sulfur and Nitrogen Codoped Graphene Quantum Dots as a Metal-Free Green Photocatalyst for Photocatalysis and Fluorescent Ink Applications. ACS Omega 2022, 7, 12825–12834. [Google Scholar] [CrossRef]
- Vijaya Kumar Saroja, A.P.; Garapati, M.S.; ShyiamalaDevi, R.; Kamaraj, M.; Ramaprabhu, S. Facile synthesis of heteroatom doped and undoped graphene quantum dots as active materials for reversible lithium and sodium ions storage. Appl. Surf. Sci. 2020, 504, 144430. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, J.; Li, Y.; Wang, L.; Dong, Y.; Jiang, Z.; Fan, C.; Cao, Y.; Sheng, R.; Liu, A.; et al. Graphene Quantum Dot Reinforced Electrospun Carbon Nanofiber Fabrics with High Surface Area for Ultrahigh Rate Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 11669–11678. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Xia, R.; Li, Y.; Zhao, J.; Lin, L.; Cao, J.; Wang, Q.; Liu, Y.; Guo, H. Graphene-quantum-dots-induced MnO2 with needle-like nanostructure grown on carbonized wood as advanced electrode for supercapacitors. Carbon 2020, 162, 114–123. [Google Scholar] [CrossRef]
- Zahir, N.; Magri, P.; Luo, W.; Gaumet, J.J.; Pierrat, P. Recent Advances on Graphene Quantum Dots for Electrochemical Energy Storage Devices. Energy Environ. Mater. 2022, 5, 201–214. [Google Scholar] [CrossRef]
- Mahalingam, S.; Manap, A.; Omar, A.; Low, F.W.; Afandi, N.F.; Chia, C.H.; Rahim, N.A. Functionalized graphene quantum dots for dye-sensitized solar cell: Key challenges, recent developments and future prospects. Renew. Sustain. Energy Rev. 2021, 144, 110999. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L.; et al. Efficiency Enhancement of Perovskite Solar Cells through Fast Electron Extraction: The Role of Graphene Quantum Dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef]
- Li, M.; Ni, W.; Kan, B.; Wan, X.; Zhang, L.; Zhang, Q.; Long, G.; Zuo, Y.; Chen, Y. Graphene quantum dots as the hole transport layer material for high-performance organic solar cells. Phys. Chem. Chem. Phys. 2013, 15, 18973. [Google Scholar] [CrossRef]
- Lyu, B.; Li, H.-J.; Xue, F.; Sai, L.; Gui, B.; Qian, D.; Wang, X.; Yang, J. Facile, gram-scale and eco-friendly synthesis of multi-color graphene quantum dots by thermal-driven advanced oxidation process. Chem. Eng. J. 2020, 388, 124285. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, J.; Li, W.; Zhang, J.; Wang, L. Yellow fluorescent graphene quantum dots as a phosphor for white tunable light-emitting diodes. RSC Adv. 2019, 9, 9301–9307. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ga, L.; Ai, J. Rapid Synthesis of Highly Fluorescent Nitrogen-Doped Graphene Quantum Dots for Effective Detection of Ferric Ions and as Fluorescent Ink. ACS Omega 2019, 4, 15842–15848. [Google Scholar] [CrossRef]
- Deka, M.J.; Chowdhury, D. CVD Assisted Hydrophobic Graphene Quantum Dots: Fluorescence Sensor for Aromatic Amino Acids. ChemistrySelect 2017, 2, 1999–2005. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Ankitha, M.; Pillai, V.K.; Alwarappan, S. Graphene quantum dots for biosensing and bioimaging. RSC Adv. 2024, 14, 16001–16023. [Google Scholar] [CrossRef]
- Mohammed-Ahmed, H.K.; Nakipoglu, M.; Tezcaner, A.; Keskin, D.; Evis, Z. Functionalization of graphene oxide quantum dots for anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2023, 80, 104199. [Google Scholar] [CrossRef]
- Bak, S.; Kim, D.; Lee, H. Graphene quantum dots and their possible energy applications: A review. Curr. Appl. Phys. 2016, 16, 1192–1201. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Tian, R.; Zhong, S.; Wu, J.; Jiang, W.; Shen, Y.; Jiang, W.; Wang, T. Solvothermal method to prepare graphene quantum dots by hydrogen peroxide. Opt. Mater. 2016, 60, 204–208. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Wang, S.; Chen, G. Microwave-Assisted Rapid Exfoliation of Graphite into Graphene by Using Ammonium Bicarbonate as the Intercalation Agent. Ind. Eng. Chem. Res. 2017, 56, 9341–9346. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Electrochemical Method To Prepare Graphene Quantum Dots and Graphene Oxide Quantum Dots. ACS Omega 2017, 2, 8343–8353. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Park, J.; Hyun, D.; Yang, J.; Lee, H. Generation of graphene quantum dots by the oxidative cleavage of graphene oxide using the oxone oxidant. New J. Chem. 2015, 39, 2425–2428. [Google Scholar] [CrossRef]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-Scale and Controllable Synthesis of Graphene Quantum Dots from Rice Husk Biomass: A Comprehensive Utilization Strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, P.; Magri, P.; Gaumet, J.-J. Solid-State Fluorescent Graphene-Based Quantum Dots. WO2023194683, 12 October 2023. [Google Scholar]
- Pierrat, P.; Magri, P.; Gaumet, J.-J. Solid-State Fluorescent Graphene-Based Quantum Dots. FR3134385, 13 October 2023. [Google Scholar]
- Shao, T.; Wang, G.; An, X.; Zhuo, S.; Xia, Y.; Zhu, C. A reformative oxidation strategy using high concentration nitric acid for enhancing the emission performance of graphene quantum dots. RSC Adv. 2014, 4, 47977–47981. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, Y.-H.; Lee, C.-L.; Lee, M.; Choi, H.C.; Lee, T.-W.; Rhee, S.-W. Electroluminescence from Graphene Quantum Dots Prepared by Amidative Cutting of Tattered Graphite. Nano Lett. 2014, 14, 1306–1311. [Google Scholar] [CrossRef]
- Yang, G.; Hu, H.; Zhou, Y.; Hu, Y.; Huang, H.; Nie, F.; Shi, W. Synthesis of one-molecule-thick single-crystalline nanosheets of energetic material for high-sensitive force sensor. Sci. Rep. 2012, 2, 698. [Google Scholar] [CrossRef]
- Upare, P.P.; Yoon, J.-W.; Kim, M.Y.; Kang, H.-Y.; Hwang, D.W.; Hwang, Y.K.; Kung, H.H.; Chang, J.-S. Chemical conversion of biomass-derived hexose sugars to levulinic acid over sulfonic acid-functionalized graphene oxide catalysts. Green Chem. 2013, 15, 2935. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-Doped Graphene Quantum Dots as a Novel Fluorescent Probe for Highly Selective and Sensitive Detection of Fe 3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef]
- Shi, S.-C.; Liu, H.-H.; Chen, T.-H.; Chen, C.-K.; Ko, B.-T. Preparation of aldehyde-graphene quantum dots from glucose for controlled release of anticancer drug. Front. Mater. 2023, 10, 1180745. [Google Scholar] [CrossRef]
- Zhu, S.; Bai, X.; Wang, T.; Shi, Q.; Zhu, J.; Wang, B. One-step synthesis of fluorescent graphene quantum dots as an effective fluorescence probe for vanillin detection. RSC Adv. 2021, 11, 9121–9129. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Patil, S.; Guin, D.; Ogale, S. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on–off–on probe for Ag+ ions. Nanoscale 2014, 6, 11664–11670. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Ojha, A.K. Charcoal derived graphene quantum dots for flexible supercapacitor oriented applications. New J. Chem. 2020, 44, 11085–11091. [Google Scholar] [CrossRef]
- Mahesh, S.; Lekshmi, C.L.; Renuka, K.D.; Joseph, K. Simple and Cost-Effective Synthesis of Fluorescent Graphene Quantum Dots from Honey: Application as Stable Security Ink and White-Light Emission. Part. Part. Syst. Charact. 2016, 33, 70–74. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 300, 75–82. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794–7807. [Google Scholar] [CrossRef]
- Yu, T.; Wang, H.; Guo, C.; Zhai, Y.; Yang, J.; Yuan, J. A rapid microwave synthesis of green-emissive carbon dots with solid-state fluorescence and pH-sensitive properties. R. Soc. Open Sci. 2018, 5, 180245. [Google Scholar] [CrossRef]
- Ahmad, K.; Pal, A.; Pan, U.N.; Chattopadhyay, A.; Paul, A. Synthesis of single-particle level white-light-emitting carbon dots via a one-step microwave method. J. Mater. Chem. C 2018, 6, 6691–6697. [Google Scholar] [CrossRef]
- Zhou, D.; Li, D.; Jing, P.; Zhai, Y.; Shen, D.; Qu, S.; Rogach, A.L. Conquering Aggregation-Induced Solid-State Luminescence Quenching of Carbon Dots through a Carbon Dots-Triggered Silica Gelation Process. Chem. Mater. 2017, 29, 1779–1787. [Google Scholar] [CrossRef]
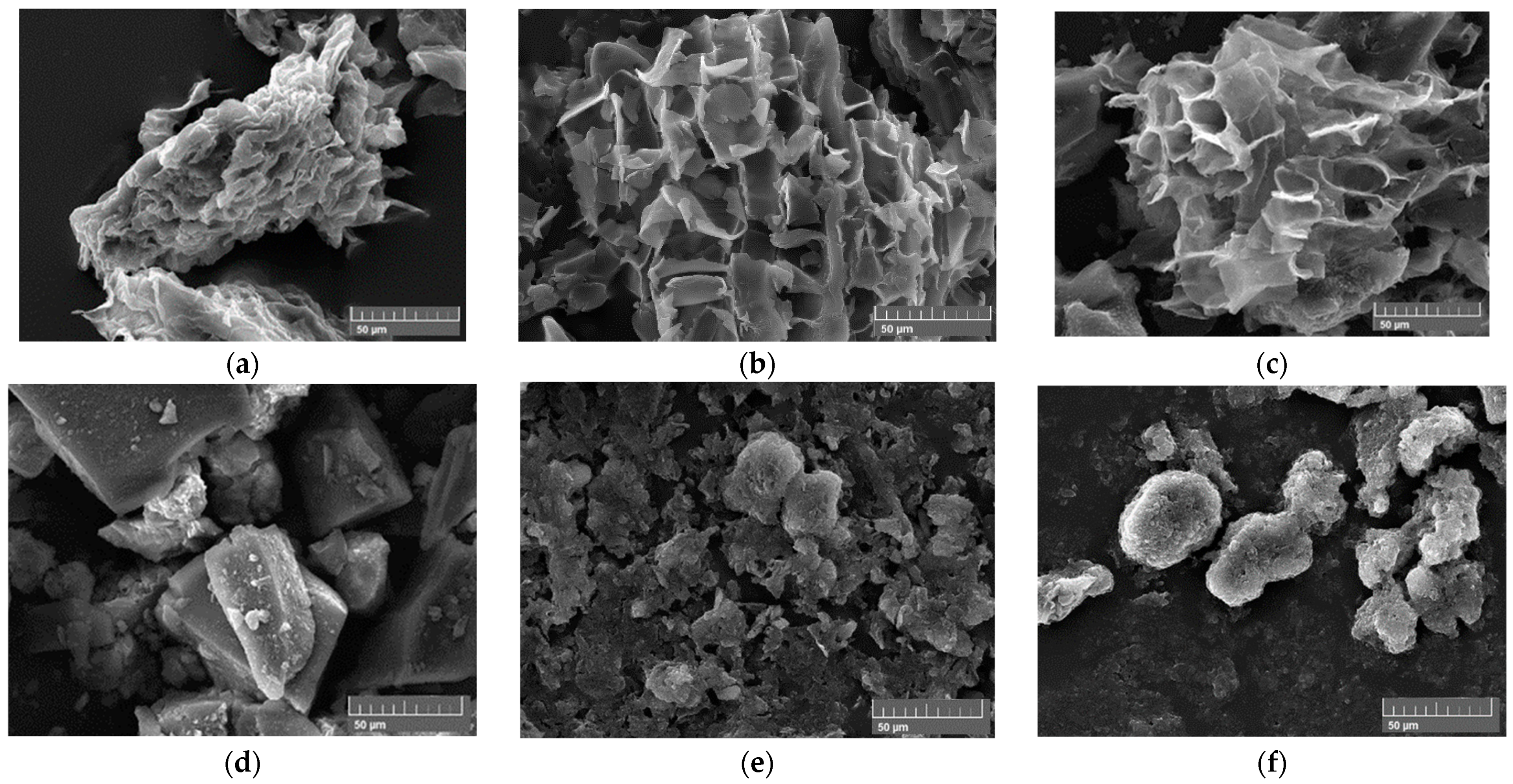
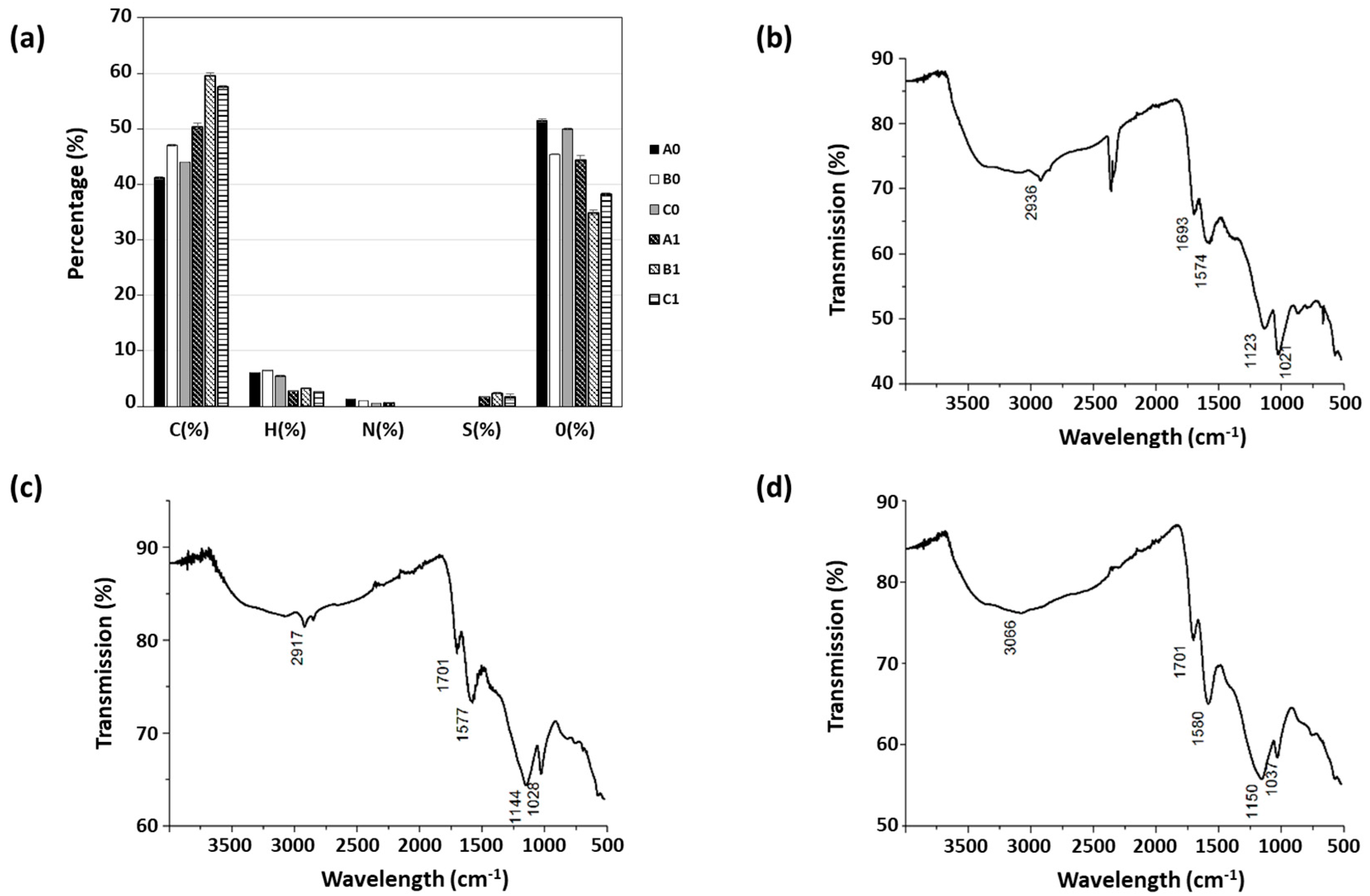
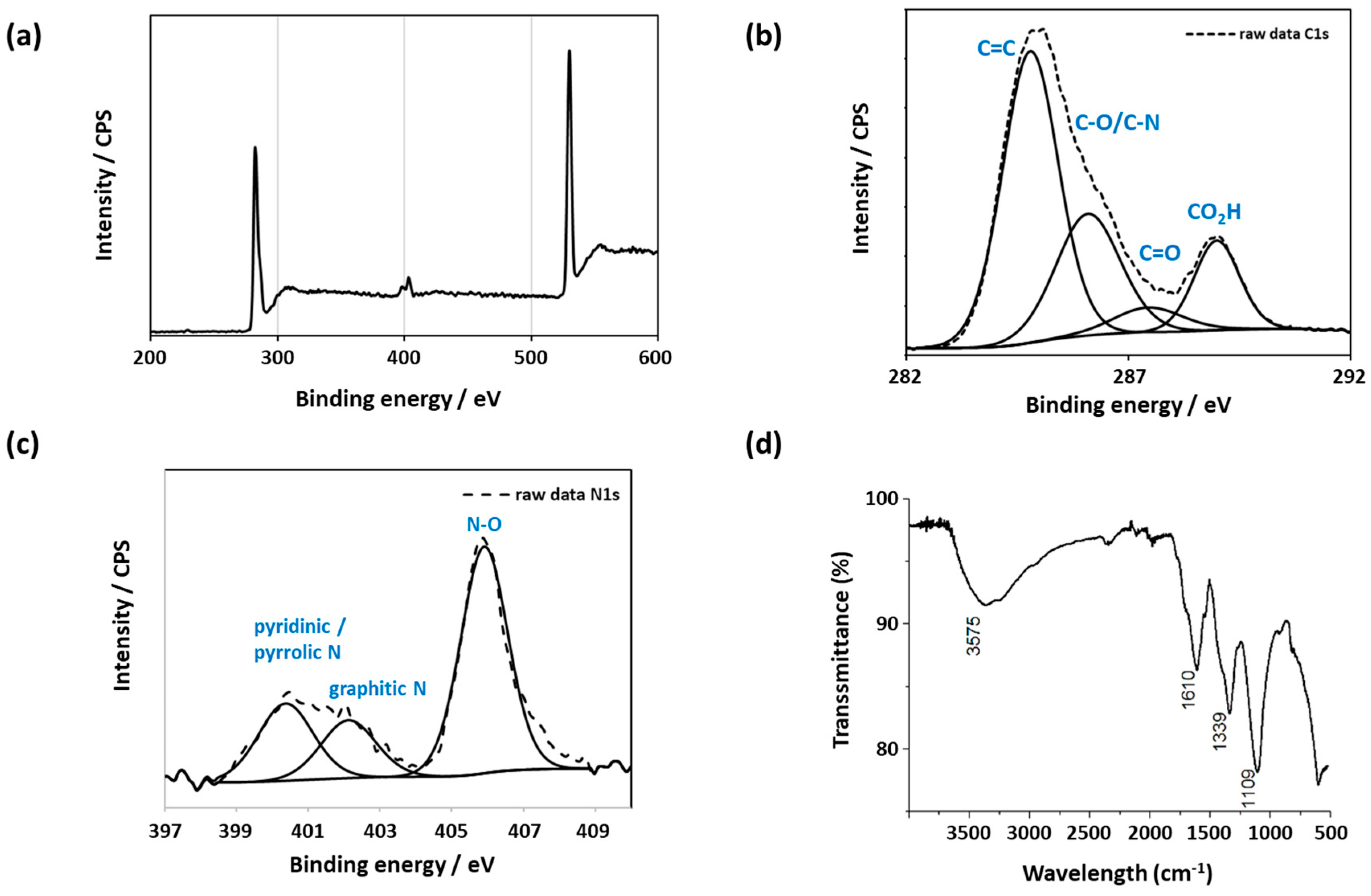
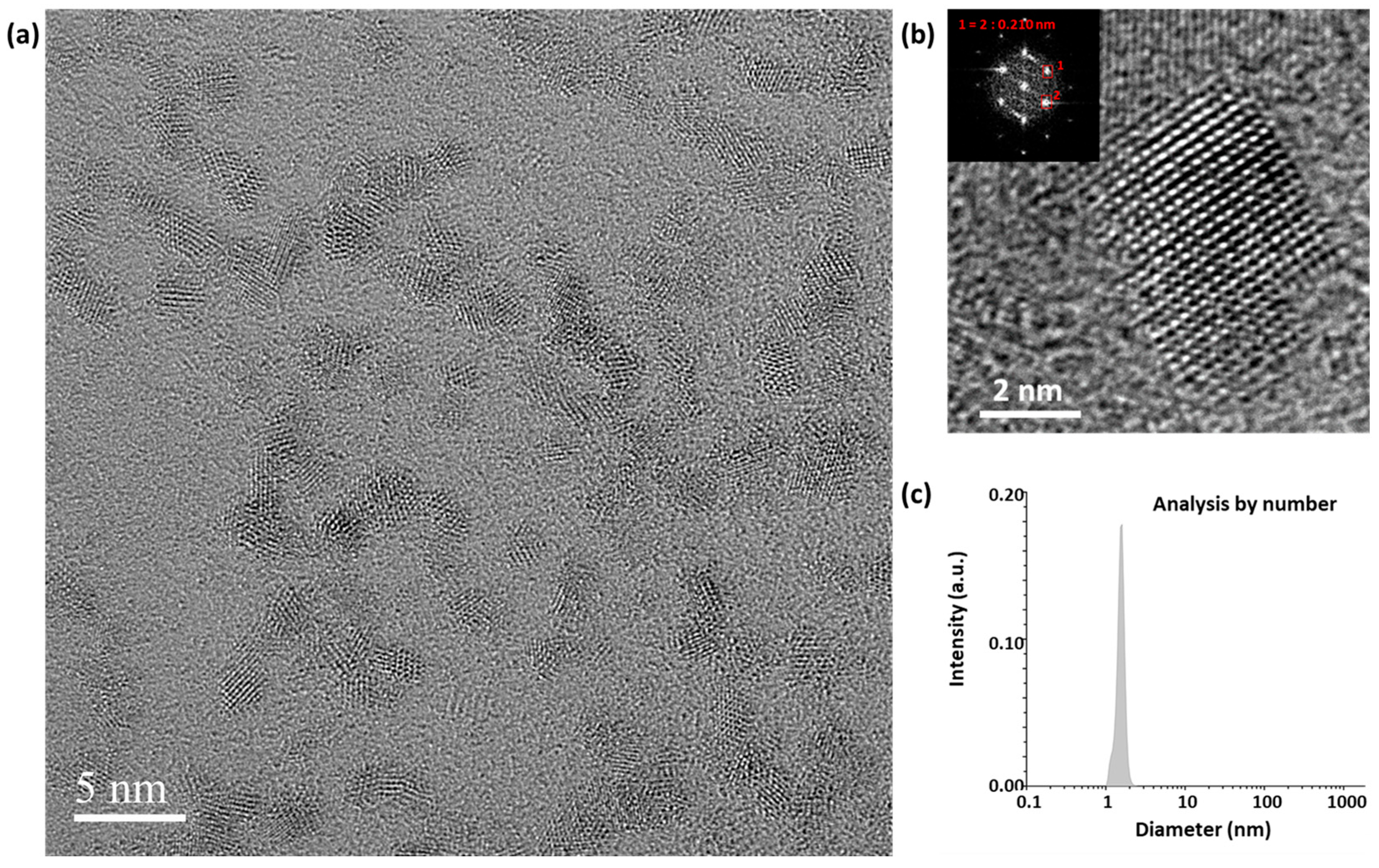
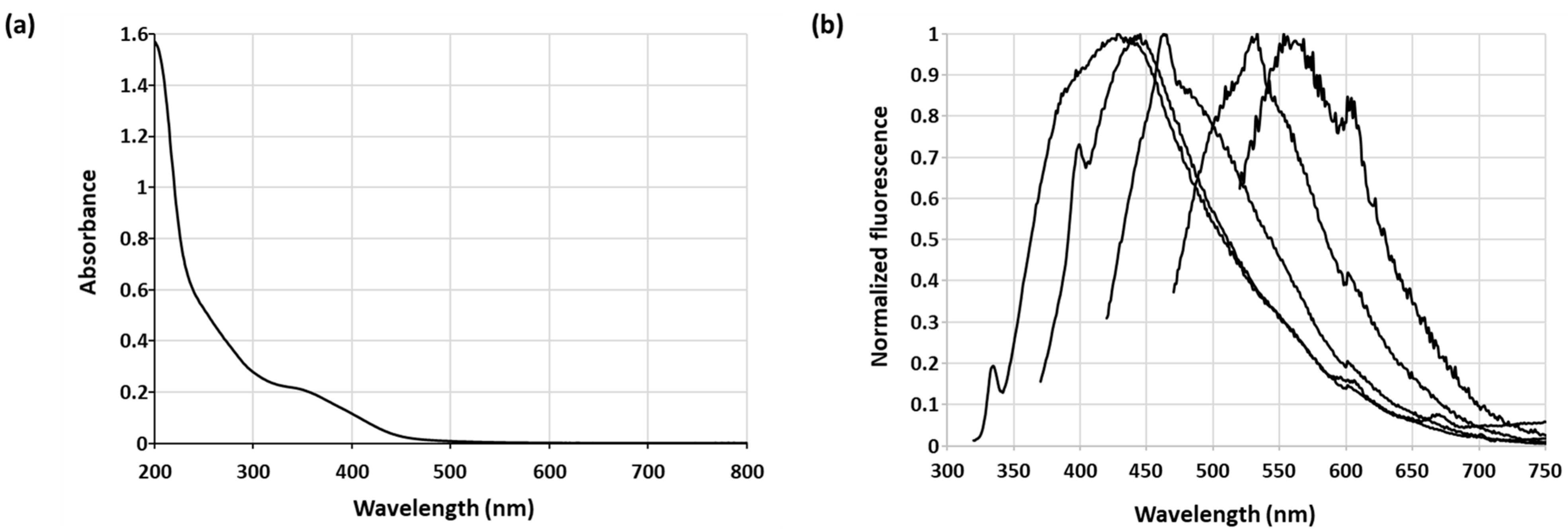
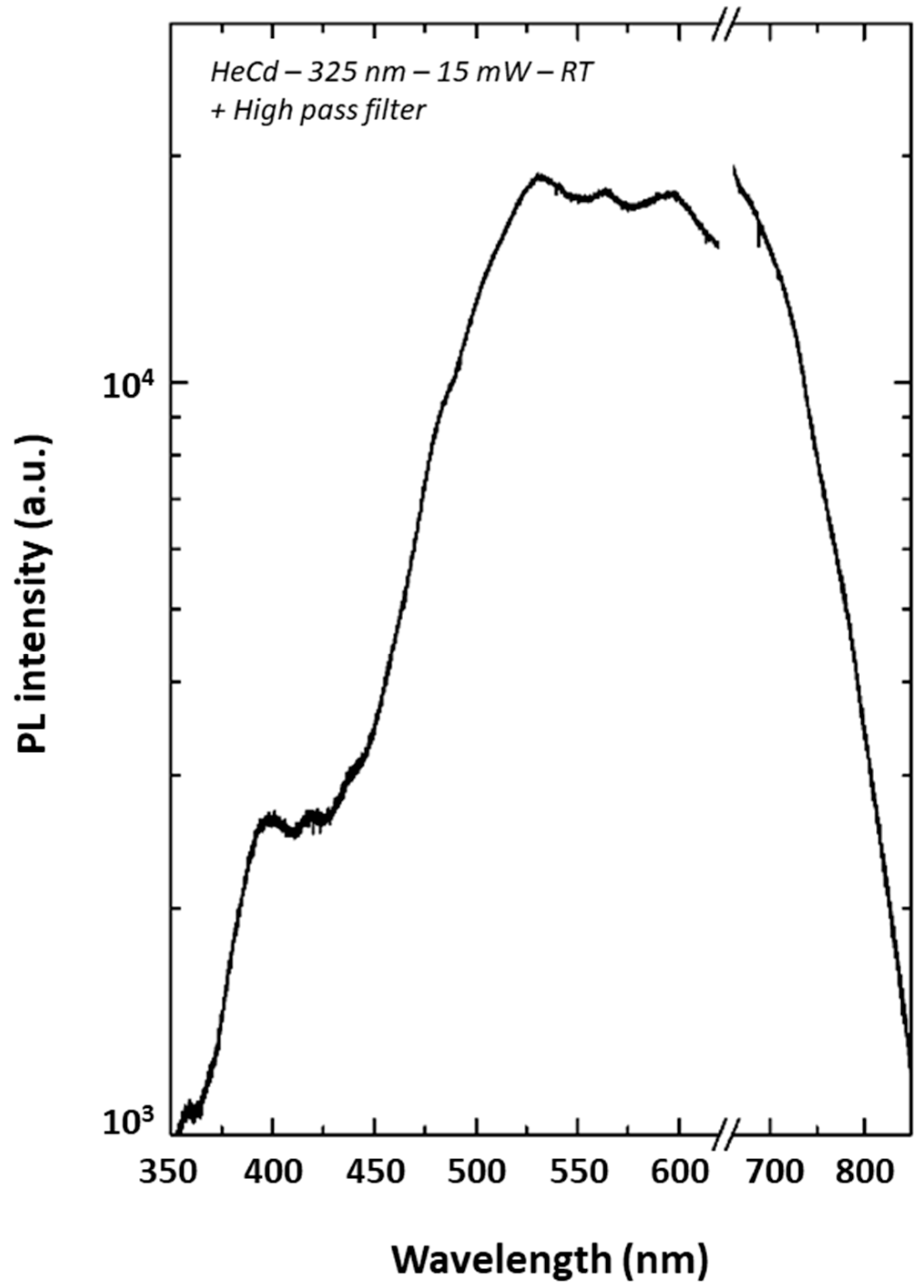
| Sample A2 | Sample B2 | Sample C2 | |
|---|---|---|---|
| C content (%) | 58.1 ± 2.1 | 64.7 ± 2.2 | 65.2 ± 2.4 |
| N content (%) | 5.4 ± 0.2 | 4.0 ± 0.1 | 4.9 ± 0.2 |
| O content (%) | 34.7 ± 1.2 | 30.5 ± 1.0 | 29.6 ± 1.1 |
| S content (%) | 1.8 ± 0.1 | 0.8 ± 0.1 | 0.3 ± 0.1 |
| Precursors | Synthetic Methods | Purification Procedure | Size Range (nm) | Yield (wt%) | Ref. |
|---|---|---|---|---|---|
| Rice husks |
| Filtration with 0.22 µm | 3–6 | 15 | [34] |
| Plant leaves |
| NaOH and filtration with 0.22 µm | 5–6 | /* | [45] |
| Charcoal |
| Simple filtration and drying | 2–15 | /* | [46] |
| Honey |
| Centrifugation | 1–3 | /* | [47] |
| Coffee |
| Filtration with 0.22 µm, then dialysis for 2 days | 2–5 | 33 | [48] |
| Lignin |
| Dialysis for 2 days | 2–6 | 21 | [49] |
| Orange peel |
| Evaporation | 1–2 | 20 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magri, P.; Franchetti, P.; Gaumet, J.-J.; Maxit, B.; Diliberto, S.; Pierrat, P. Upgrading Biomass Wastes to Graphene Quantum Dots with White-Light-Emitting Features in the Solid State. Appl. Sci. 2024, 14, 8807. https://doi.org/10.3390/app14198807
Magri P, Franchetti P, Gaumet J-J, Maxit B, Diliberto S, Pierrat P. Upgrading Biomass Wastes to Graphene Quantum Dots with White-Light-Emitting Features in the Solid State. Applied Sciences. 2024; 14(19):8807. https://doi.org/10.3390/app14198807
Chicago/Turabian StyleMagri, Pierre, Pascal Franchetti, Jean-Jacques Gaumet, Benoit Maxit, Sébastien Diliberto, and Philippe Pierrat. 2024. "Upgrading Biomass Wastes to Graphene Quantum Dots with White-Light-Emitting Features in the Solid State" Applied Sciences 14, no. 19: 8807. https://doi.org/10.3390/app14198807







