Preservative Effect of a Gelatin-Based Film Including a Gelidium sp. Flour Extract on Refrigerated Atlantic Mackerel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initial Alga Flour Composition and Aqueous Extract Preparation
2.2. Preparation of the Film Systems
2.3. Fish Processing and Sampling
2.4. Microbiological Analyses of Fish Muscle
2.5. Chemical Analyses Related to Quality Loss
2.6. Statistical Analysis
3. Results
3.1. Alga Flour Composition
3.2. Evaluation of Microbial Growth in Atlantic Mackerel Muscle under Different Packaging Systems
3.3. Evolution of the pH and TMA Values
3.4. Assessment of Lipid Hydrolysis Development
3.5. Determination of Lipid Oxidation Evolution
4. Discussion
4.1. Antimicrobial Activity
4.2. Lipid Oxidation Development
4.3. Lipid Hydrolysis Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ofosu, F.K.; Daliri, E.B.M.; Lee, B.H.; Yu, X. Current trends and future perspectives on omega-3 fatty acids. Res. J. Biol. 2017, 5, 11–20. [Google Scholar]
- Tilami, S.K.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Sikorski, Z.E.; Kolakowski, E. Endogenous enzyme activity and seafood quality: Influence of chilling, freezing, and other environmental factors. In Seafood Enzymes. Utilization and Influence on Postharvest Seafood Quality; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 451–487. [Google Scholar]
- Özoğul, Y. Methods for freshness quality and deterioration. In Handbook of Seafood and Seafood Products Analysis; Nollet, L., Toldrá, F., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 189–214. [Google Scholar]
- Ghali, A.; Dave, D.; Budge, S.; Brooks, M. Fish spoilage mechanisms and preservation: Review. Amer. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, G.; Barbieri, F.; Montanari, C.; Gardini, F. Application of natural antimicrobial strategies in seafood preservation. In Innovative Technologies in Seafood Processing; Özogul, Y., Ed.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2020; pp. 243–262. [Google Scholar]
- Cai, L.; Li, X.; Wu, X.; Lv, Y.; Liu, X.; Li, J. Effect of chitosan coating enriched with ergothioneine on quality changes of Japanese sea bass (Lateolabrax japonicas). Food Bioprocess Technol. 2014, 7, 2281–2290. [Google Scholar] [CrossRef]
- Yerlikaya, P.; Aydan Yatmaz, H.; Kadir Topuz, O. Applications of edible films and coatings in aquatic foods. In Innovative technologies in seafood processing; Özoğul, Y., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 71–91. [Google Scholar]
- Tsoukalas, D.; Kendler, S.; Lerfall, J.; Nordeng Jakobsen, A. The effect of fishing season and storage conditions on the quality of European plaice (Pleuronectes platessa). LWT-Food Sci. Technol. 2022, 170, 114083. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.O.; Ravishankar, C.N.; Gopal, T.S. Active packaging of fishery products. Fish. Technol. 2010, 47, 1–18. [Google Scholar]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.S. Smart packaging systems for food applications. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Kuley, E.; Özoğul, F.; Polat, A. Advances in Packaging. In Innovative Technologies in Seafood Processing; Özoğul, Y., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 45–69. [Google Scholar]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Aider, M. Chitosan applications for active biobased films production and potential in the food industry: Review. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Andrade, P.; Barbosa, M.; Pedro Matos, R.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M.; Salejhabadi, A.; Nafchi, A.M.; Kumar, S.U.; Khalil, H.P.S. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their byproducts. Trend Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Cazón, P.; Velázquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging. Food Hydroc. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Development of active gelatin films by means of valorization of food processing waste. Food Hydroc. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Sandsdalen, E.; Haug, T.; Stensvag, K.; Styrvold, O. The antibacterial effect of a polyhydroxylated fucophlorethol from the marine brown alga, Fucus vesiculosus. World J. Microb. Biotechnol. 2003, 19, 777–782. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Arulkumar, A.; Rosemary, T.; Paramasivam, S.; Rajendran, R.B. Phytochemical composition, in vitro antioxidant, antibacterial potential and GC–MS analysis of red seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatal. Agricult. Biotechnol. 2018, 15, 63–71. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carb. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Zhai, H.; Qi, B.; Hao, S.; Huang, H.; Yang, X. Isolation, purification and monosaccharide composition analysis of polysaccharide from Gelidium amansii. Food Ferment. Indust. 2020, 7, 57–62. [Google Scholar]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macrom. 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhang, Q.; Wang, Y.; Yang, Q.; Yu, H.; Li, H.; Chen, J.; Fu, L. Sulfated polysaccharides from red seaweed Gelidium amansii: Structural characteristics, antioxidant and anti-glycation properties, and development of bioactive films. Food Hydroc. 2021, 119, 106820. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Barrio, L.P.; Zhao, M.; Tiwari, B.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Nilsson, A.E.; Krewer, C.; Östergren, K.; et al. Alternative protocols for the production of more sustainable agar-based extracts from Gelidium sesquipedale. Algal Res. 2021, 55, 102254. [Google Scholar] [CrossRef]
- Ferreira, M.; Ramos-Oliveira, C.; Magalhães, R.; Martins, N.; Ozório, R.O.A.; Salgado, J.M.; Belo, I.; Oliva-Teles, A.; Peres, H. Fermented agar byproduct and sunflower cake mixture as feedstuff for European seabass (Dicentrarchus labrax). Anim. Feed Sci. Technol 2024, 315, 116048. [Google Scholar] [CrossRef]
- Mouga, T.; Fernandes, I.B. The red seaweed giant Gelidium (Gelidium corneum) for new biobased materials in a circular economy framework. Earth 2022, 3, 788–813. [Google Scholar] [CrossRef]
- Agarwal, P.; Kayala, P.; Chandrasekaran, N.; Mukherjee, A.; Shah, S.; Thomas, J. Antioxidant and antibacterial activity of Gelidium pusillum (Stackhouse) against Aeromonas caviae and its applications in aquaculture. Aquac. Int. 2021, 29, 845–858. [Google Scholar] [CrossRef]
- Lim, G.O.; Hong, Y.H.; Song, K.B. Incorporating grapefruit seed extract into Gelidium corneum-whey protein isolate blend packaging film increases the shelf life of fish paste. J. Food Sci. Nutr. 2008, 13, 370–374. [Google Scholar] [CrossRef]
- Cui, M.; Zhou, R.; Wang, Y.; Zhang, M.; Liu, K.; Ma, C. Beneficial effects of sulfated polysaccharides from the red seaweed Gelidium pacificum Okamura on mice with antibiotic-associated diarrhea. Food Funct. 2020, 11, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Viedma, J.; Aguilera, J.M.; Flores, M.; Lemus-Mondaca, R.; Larrazabal, M.J.; Miranda, J.M.; Aubourg, S.P. Protective effect of red algae (Rhodophyta) extracts on essential dietary components of heat-treated salmon. Antioxidants 2021, 10, 1108. [Google Scholar] [CrossRef]
- Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Antimicrobial activity of red alga flour (Gelidium sp.) and its effect on quality retention of Scomber scombrus during refrigerated storage. Foods 2022, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.G.; Trigo, M.; Zhang, B.; Aubourg, S.P. Effect of alga flour extract on lipid damage evolution in heated fish muscle system. Antioxidants 2022, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods for Analysis of the Association of Analytical Chemistry, 15th ed.; Association of Official Chemists. Inc.: Arlington, VA, USA, 1990; pp. 931–937. [Google Scholar]
- Trigo, M.; Nozal, P.; Miranda, J.M.; Aubourg, S.P.; Barros-Velázquez, J. Antimicrobial and antioxidant effect of lyophilized Fucus spiralis addition on gelatin film during refrigerated storage of mackerel. Food Cont. 2022, 131, 108416. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Lesieur, S.; Labarre, D.; Jayakrishnan, A. Periodate oxidation of sodium alginate in water and in ethanol–water mixture: A comparative study. Carb. Polym. 2005, 340, 1425–1429. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Vieites Baptista de Sousa, J.; Villa, T.; Barros-Velázquez, J. Histamine and cadaverine production by bacteria isolated from fresh and frozen albacore (Thunnus alalunga). J. Food Prot. 1999, 62, 933–939. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Vieites Baptista de Sousa, J.; Villa, T.; Barros-Velázquez, J. Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. J. Food Prot. 1998, 61, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gigirey, B.; Vieites Baptista de Sousa, J.; Villa, T.; Barros-Velázquez, J. Characterization of biogenic amine-producing Stenotrophomonas maltophilia strains isolated from white muscle of fresh and frozen albacore tuna. Int. J. Food Microb. 2000, 57, 19–31. [Google Scholar] [CrossRef]
- Tozawa, H.; Erokibara, K.; Amano, K. Proposed modification of Dyer’s method for trimethylamine determination in codfish. In Fish Inspection and Quality Control; Kreuzer, R., Ed.; Fishing News Books Ltd.: London, UK, 1971; pp. 187–190. [Google Scholar]
- Bligh, E.; Dyer, W. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Herbes, S.E.; Allen, C.P. Lipid quantification of freshwater invertebrates: Method modification for microquantitation. Can. J. Fish. Aquat. Sci. 1983, 40, 1315–1317. [Google Scholar] [CrossRef]
- Lowry, R.; Tinsley, I. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef]
- Chapman, R.; McKay, J. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J. Am. Oil Chem. Soc. 1949, 26, 360–363. [Google Scholar] [CrossRef]
- Vyncke, W. Direct determination of the thiobarbituric acid value in trichloroacetic acid extracts of fish as a measure of oxidative rancidity. Fette. Seifen. Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R. A comparison between conventional and fluorescence detection methods of cooking-induced damage to tuna fish lipids. Z. Lebensm. Unters. Forsch. 1995, 200, 252–255. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R. Polyunsaturated fatty acids in tuna phospholipids: Distribution in the sn-2 location and changes during cooking. J. Agric. Food Chem. 1996, 44, 585–589. [Google Scholar] [CrossRef]
- Rustad, T. Lipid oxidation. In Handbook of Seafood and Seafood Products Analysis; Nollet, L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 87–95. [Google Scholar]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Recent highlights in sustainable biobased edible films and coatings for fruit and vegetable applications. Foods 2024, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Said, N.S.; Sarbon, N.M. Physical and mechanical characteristics of gelatin-based films as a potential food packaging material: A review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E. Polysaccharide-based biodegradable films: An alternative in food packaging. Polysaccharides 2022, 3, 761–775. [Google Scholar] [CrossRef]
- Kuda, T.; Ikemori, T. Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem. 2009, 112, 575–581. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.; Critchley, A.; Van de Velde, F.; Ribeiro-Claro, P. Identification of selected seaweed polysaccharides (phycocolloides) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydroc. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- El-Baroty, G.S.; El-Baz, F.K.; Abd-Elmoein, A.; El-Baky, H.H.A.; Ali, M.M.; Ibrahim, A.E. Evaluation of glycolipids of some Egyptian marine algae as a source of bioactive substances. Electr. J. Environm. Agric. Food Chem. 2011, 10, 2114–2128. [Google Scholar]
- Zeid, A.H.A.; Aboutabl, E.A.; Sleem, A.A.; El-Rafie, H.M. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carb. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef]
- Jo, W.; Song, N.; Lee, J.; Song, K. Physical properties and antimicrobial activities of a persimmon peel/red algae composite film containing grapefruit seed extract. Food Sci. Biotechnol. 2014, 23, 1169–1172. [Google Scholar] [CrossRef]
- García-Soto, B.; Miranda, J.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Rodríguez-Martínez, A.; Barros-Velázquez, J.; Aubourg, S.P. Effect of biodegradable film (lyophilized alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Dovale, G.; Rodríguez, A.; Aubourg, S.P. Antioxidant and antimicrobial behavior of alga Gracilaria gracilis extracts during hake (Merluccius merluccius) chilled storage. Bulg. Chem. Comm. 2018, 50, 118–124. [Google Scholar]
- Arulkumar, A.; Satheeshkumar, K.; Paramasivam, S.; Rameshthangam, P.; Miranda, J.M. Chemical biopreservative effects of red seaweed on the shelf life of black tiger shrimp (Penaeus monodon). Foods 2020, 9, 634. [Google Scholar] [CrossRef]
- Pokorný, J. Browning from lipid-protein interactions. Prog. Food Nutr. Sci. 1981, 5, 421–428. [Google Scholar]
- Farvin, K.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Mohabati, M.; Eslami, S.; Sohrabipour, J.; Miri, R. Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iran. J. Pharm. Res. 2013, 12, 339–348. [Google Scholar]
- Widowati, I.; Lubac, D.; Puspita, M.; Bourgougnon, N. Antibacterial and antioxidant properties of the red alga Gracilaria verrucosa from the North coast of Java, Semarang, Indonesia. Int. J. Latest Res. Sci. Technol. 2014, 3, 179–185. [Google Scholar]
- Reboleira, J.; Ganhão, R.; Mendes, S.; Adão, P.; Andrade, M.; Vilarinho, F.; Sanches-Silva, A.; Sousa, D.; Mateus, A.; Bernardino, S. Optimization of extraction conditions for Gracilaria gracilis extracts and their antioxidative stability as part of microfiber food coating additives. Molecules 2020, 25, 4060. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kong, L.M.; Xie, Y.Y.; Wang, C.; Wang, X.L.; Wang, Y.B.; Fu, L.L.; Zhou, T. Purification, structural characterization, and biological activities of degraded polysaccharides from Porphyra yezoensis. J. Food Biochem. 2021, 45, e13661. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Cholinesterase inhibitory activity, antioxidant properties, and phytochemical composition of Chlorococcum sp. extracts. J. Food Biochem. 2021, 45, e13395. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Back, S.; Song, K. Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Pack. Shelf Life 2018, 18, 157–163. [Google Scholar] [CrossRef]
- Stejskal, N.; Miranda, J.M.; Martucci, J.F.; Ruseckaite, R.A.; Barros-Velázquez, J.; Aubourg, S.P. Quality enhancement of refrigerated hake muscle by active packaging with a protein concentrate from Spirulina platensis. Food Bioprocess Technol. 2020, 13, 1110–1118. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Quitral, V.; Larraín, M.A.; Rodríguez, A.; Gómez, J.; Maier, L.; Vinagre, J. Autolytic degradation and microbiological activity in farmed Coho salmon (Oncorhynchus kisutch) during chilled storage. Food Chem. 2007, 104, 369–375. [Google Scholar] [CrossRef]
- Labuza, T. Kinetics of lipid oxidation in foods. CRC Crit. Rev. Food Technol. 1971, 2, 355–405. [Google Scholar] [CrossRef]
- Miyashita, K.; Takagi, T. Study on the oxidative rate and prooxidant activity of free fatty acids. J. Am. Oil Chem. Soc. 1986, 63, 1380–1384. [Google Scholar] [CrossRef]
- Babakhani, A.; Farvin, K.; Jacobsen, C. Antioxidative effect of seaweed extracts in chilled storage of minced Atlantic mackerel (Scomber scombrus): Effect on lipid and protein oxidation. Food Bioprocess Technol. 2016, 9, 352–364. [Google Scholar] [CrossRef]
- Barros-Velázquez, J.; Miranda, J.M.; Ezquerra-Brauer, J.M.; Aubourg, S.P. Impact of icing systems with aqueous, ethanolic and ethanolic-aqueous extracts of alga Fucus spiralis on microbial and biochemical quality of chilled hake (Merluccius merluccius). Int. J. Food Sci. Technol. 2016, 51, 2081–2089. [Google Scholar] [CrossRef]
- Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Effect of an icing medium containing the alga Fucus spiralis on the microbiological activity and lipid oxidation in chilled megrim (Lepidorhombus whiffiagonis). Food Cont. 2016, 59, 290–297. [Google Scholar] [CrossRef]
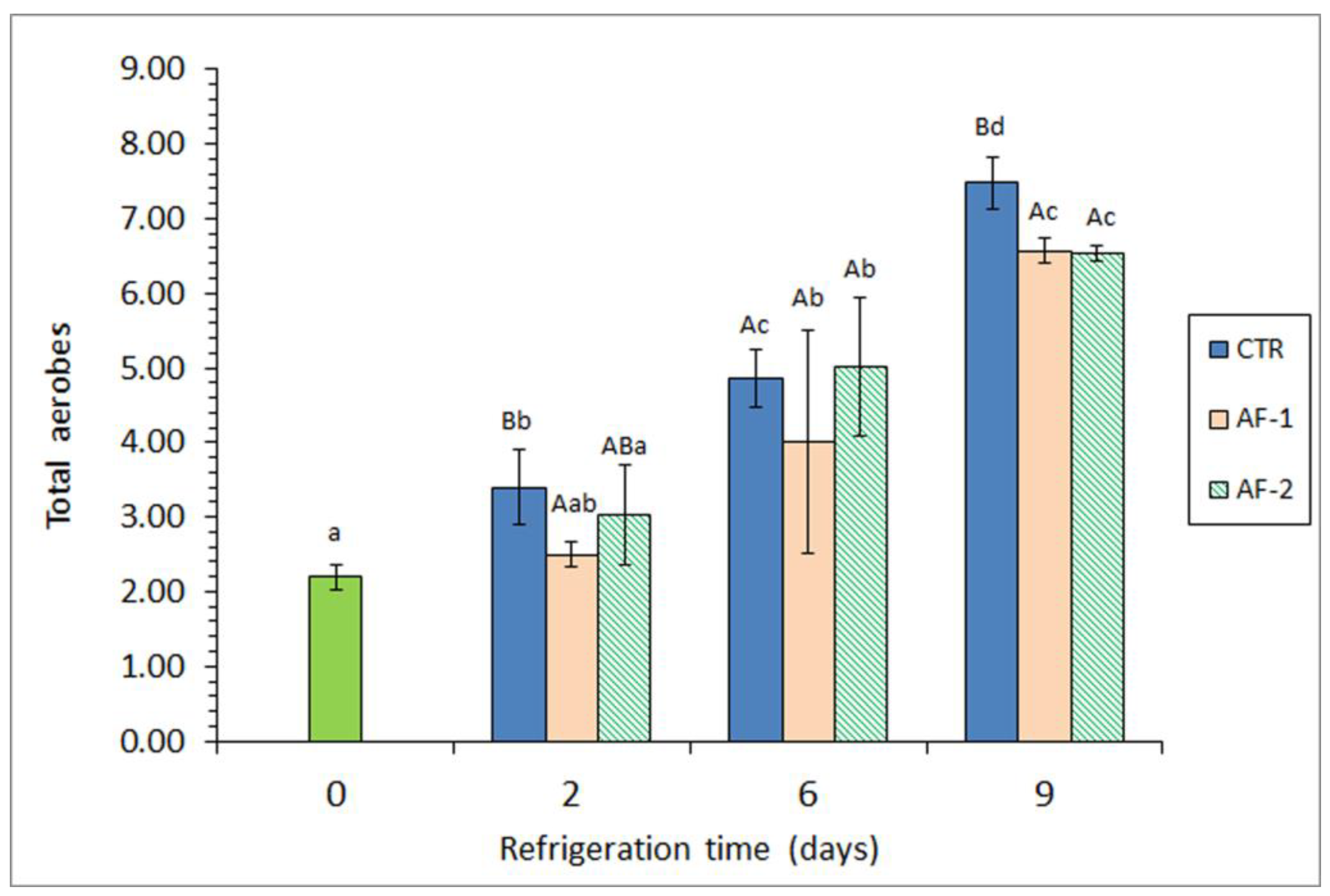

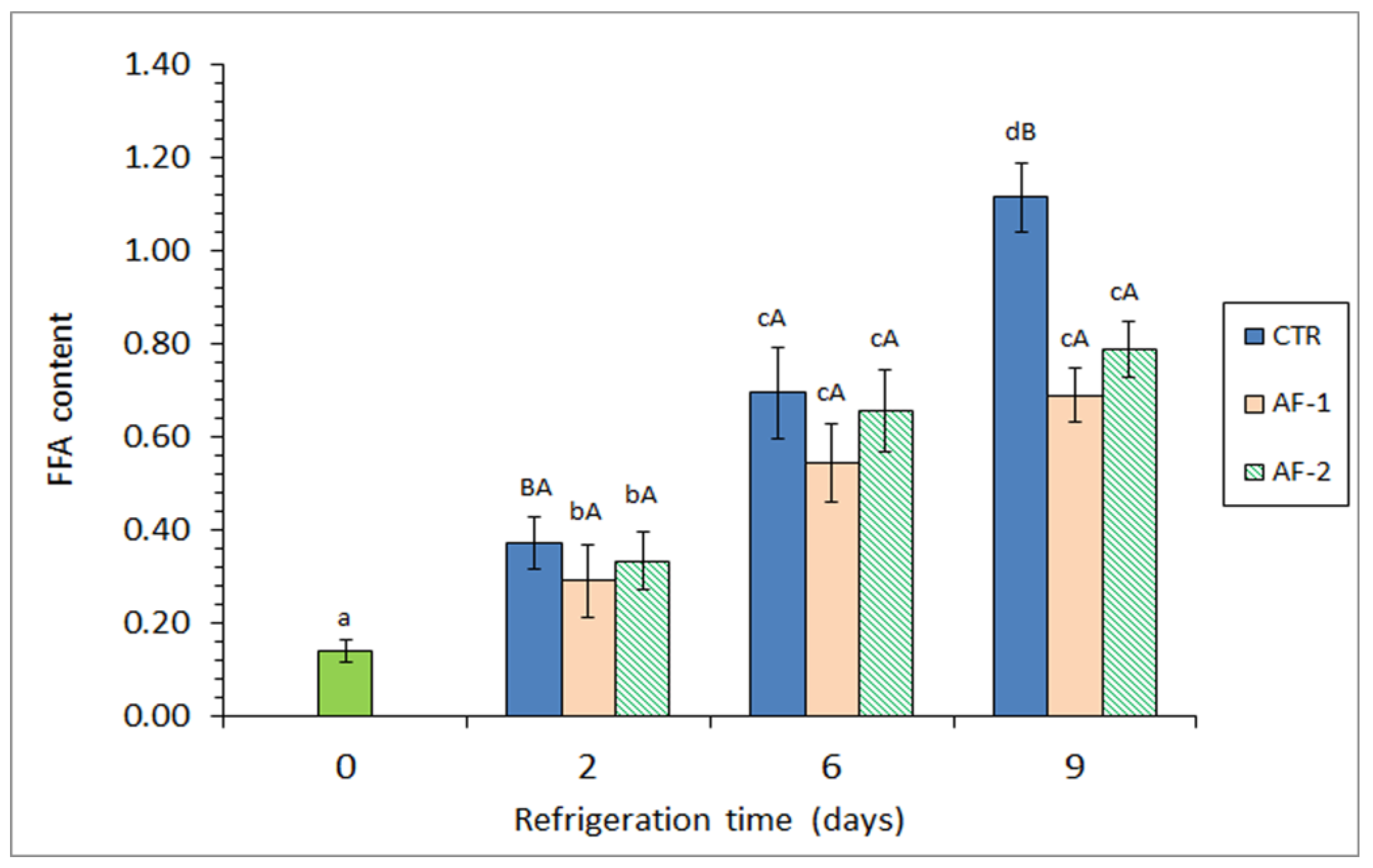
| Microbial Group | Packaging Condition | Refrigeration Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 2 | 6 | 9 | ||
| Enterobacteriaceae | CTR | 1.00 ± 0.00 a | 1.86 ± 0.52 Ab | 2.33 ± 0.35 Ab | 4.21 ± 0.65 Ac |
| AF-1 | 1.00 ± 0.00 a | 1.53 ± 0.20 Ab | 1.73 ± 0.88 Ab | 3.23 ± 0.35 Ac | |
| AF-2 | 1.00 ± 0.00 a | 1.75 ± 0.64 Ab | 2.28 ± 0.87 Ab | 3.73 ± 0.41 Ac | |
| Psychrotrophs | CTR | 2.82 ± 0.24 a | 4.68 ± 0.79 Bb | 6.32 ± 0.32 Bc | 8.78 ± 0.15 Bd |
| AF-1 | 2.82 ± 0.24 a | 2.93 ± 0.35 Aa | 5.16 ± 0.32 Ab | 7.83 ± 0.30 Ac | |
| AF-2 | 2.82 ± 0.24 a | 4.68 ± 0.39 Bb | 6.44 ± 0.15 Bc | 7.54 ± 0.55 Ad | |
| Lipolytic bacteria | CTR | 2.00 ± 0.00 a | 2.26 ± 0.24 Aa | 3.13 ± 0.41 Ab | 4.95 ± 1.09 Ac |
| AF-1 | 2.00 ± 0.00 a | 2.66 ± 0.58 Aab | 2.43 ± 0.51 Aab | 3.64 ± 1.05 Ab | |
| AF-2 | 2.00 ± 0.00 a | 2.16 ± 0.28 Aab | 3.00 ± 0.89 Abc | 3.63 ± 0.46 Ac | |
| Quality Index | Packaging Condition | Refrigeration Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 2 | 6 | 9 | ||
| pH | CTR | 6.25 ± 0.02 a | 6.51 ± 0.02 Bb | 6.58 ± 0.15 Ab | 6.59 ± 0.02 Bb |
| AF-1 | 6.25 ± 0.02 a | 6.39 ± 0.10 ABab | 6.40 ± 0.16 Aab | 6.42 ± 0.08 Ab | |
| AF-2 | 6.25 ± 0.02 a | 6.29 ± 0.08 Aa | 6.44 ± 0.11 Aab | 6.50 ± 0.03 ABb | |
| TMA | CTR | 1.60 ± 0.42 a | 1.87 ± 0.47 Aa | 12.80 ± 1.7 Ab | 61.50 ± 15.7 Ac |
| AF-1 | 1.60 ± 0.42 a | 2.60 ± 0.78 Aa | 11.57 ± 2.3 Ab | 52.67 ± 23.6 Ac | |
| AF-2 | 1.60 ± 0.42 a | 2.80 ± 0.79 Aa | 11.00 ± 4.6 Ab | 50.20 ± 13.1 Ac | |
| Quality Index *** | Packaging Condition | Refrigeration Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 2 | 6 | 9 | ||
| PV | CTR | 0.98 ± 0.47 a | 1.24 ± 0.43 Aa | 3.05 ± 1.70 Aab | 5.03 ± 1.61 Cb |
| AF-1 | 0.98 ± 0.47 a | 0.72 ± 0.35 Aa | 2.11 ± 1.66 Aab | 2.27 ± 0.15 Bb | |
| AF-2 | 0.98 ± 0.47 ab | 1.57 ± 0.44 Aab | 0.68 ± 0.46 Aa | 1.60 ± 0.33 Ab | |
| TBA-i | CTR | 0.42 ± 0.12 a | 0.91 ± 0.17 Ab | 2.86 ± 1.07 Ac | 4.77 ± 0.76 Bd |
| AF-1 | 0.42 ± 0.12 a | 1.13 ± 0.45 Ab | 3.25 ± 1.39 Ac | 3.10 ± 1.77 ABc | |
| AF-2 | 0.42 ± 0.12 a | 1.18 ± 0.42 Ab | 1.84 ± 0.42 Ab | 1.77 ± 0.27Ab | |
| FR | CTR | 3.69 ± 1.56 a | 4.85 ± 0.77 Aa | 6.39 ± 0.65 Ab | 7.22 ± 0.29 Cc |
| AF-1 | 3.69 ± 1.56 a | 4.89 ± 0.17 Aa | 5.40 ± 0.61 Aab | 6.16 ± 0.58 Bb | |
| AF-2 | 3.69 ± 1.56 a | 4.44 ± 0.94 Aa | 5.84 ± 1.32 Aa | 4.86 ± 0.16 Aa | |
| PI | CTR | 2.27 ± 0.16 a | 2.04 ± 0.23 Aa | 1.97 ± 0.21 Aa | 1.88 ± 0.47 Aa |
| AF-1 | 2.27 ± 0.16 a | 2.20 ± 0.16 ABa | 1.98 ± 0.10 Aa | 1.94 ± 0.53 Aa | |
| AF-2 | 2.27 ± 0.16 a | 2.40 ± 0.10 Ba | 2.25 ± 0.02 Ba | 2.14 ± 0.12 Aa | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, L.; Gómez, A.; Trigo, M.; Miranda, J.M.; Barros-Velázquez, J.; Aubourg, S.P. Preservative Effect of a Gelatin-Based Film Including a Gelidium sp. Flour Extract on Refrigerated Atlantic Mackerel. Appl. Sci. 2024, 14, 8817. https://doi.org/10.3390/app14198817
López L, Gómez A, Trigo M, Miranda JM, Barros-Velázquez J, Aubourg SP. Preservative Effect of a Gelatin-Based Film Including a Gelidium sp. Flour Extract on Refrigerated Atlantic Mackerel. Applied Sciences. 2024; 14(19):8817. https://doi.org/10.3390/app14198817
Chicago/Turabian StyleLópez, Lucía, Antonio Gómez, Marcos Trigo, José M. Miranda, Jorge Barros-Velázquez, and Santiago P. Aubourg. 2024. "Preservative Effect of a Gelatin-Based Film Including a Gelidium sp. Flour Extract on Refrigerated Atlantic Mackerel" Applied Sciences 14, no. 19: 8817. https://doi.org/10.3390/app14198817
APA StyleLópez, L., Gómez, A., Trigo, M., Miranda, J. M., Barros-Velázquez, J., & Aubourg, S. P. (2024). Preservative Effect of a Gelatin-Based Film Including a Gelidium sp. Flour Extract on Refrigerated Atlantic Mackerel. Applied Sciences, 14(19), 8817. https://doi.org/10.3390/app14198817








