Effects of Lactic Acid and Ascorbic Acid Mixture on Quality Properties of Wild Red Deer (Cervus elaphus) Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Preparation of Spray Solution
2.3. pH Determination
2.4. Instrumental Color Measurement
- When 0 < ∆E < 1, the observer does not notice the difference;
- When 1 < ∆E < 2, only an experienced observer may notice the difference;
- When 2 < ∆E < 3.5, an inexperienced observer also notices the difference;
- When 3.5 < ∆E < 5, a clear difference in color is noticed;
- When ∆E > 5, the observer notices two different colors.
2.5. Microbiological Evaluation
2.6. Instrumental Texture Measurement
2.7. Analysis of Sarcoplasmic and Myofibrillar Proteins by Sodium Dodecyl-Sulphate Polyacrylamide Gel Electrophoresis
2.7.1. Isolation of Sarcoplasmic and Myofibrillar Proteins
2.7.2. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis Conditions
2.8. Statistical Analysis
3. Results and Discussion
3.1. pH
3.2. Surface Color
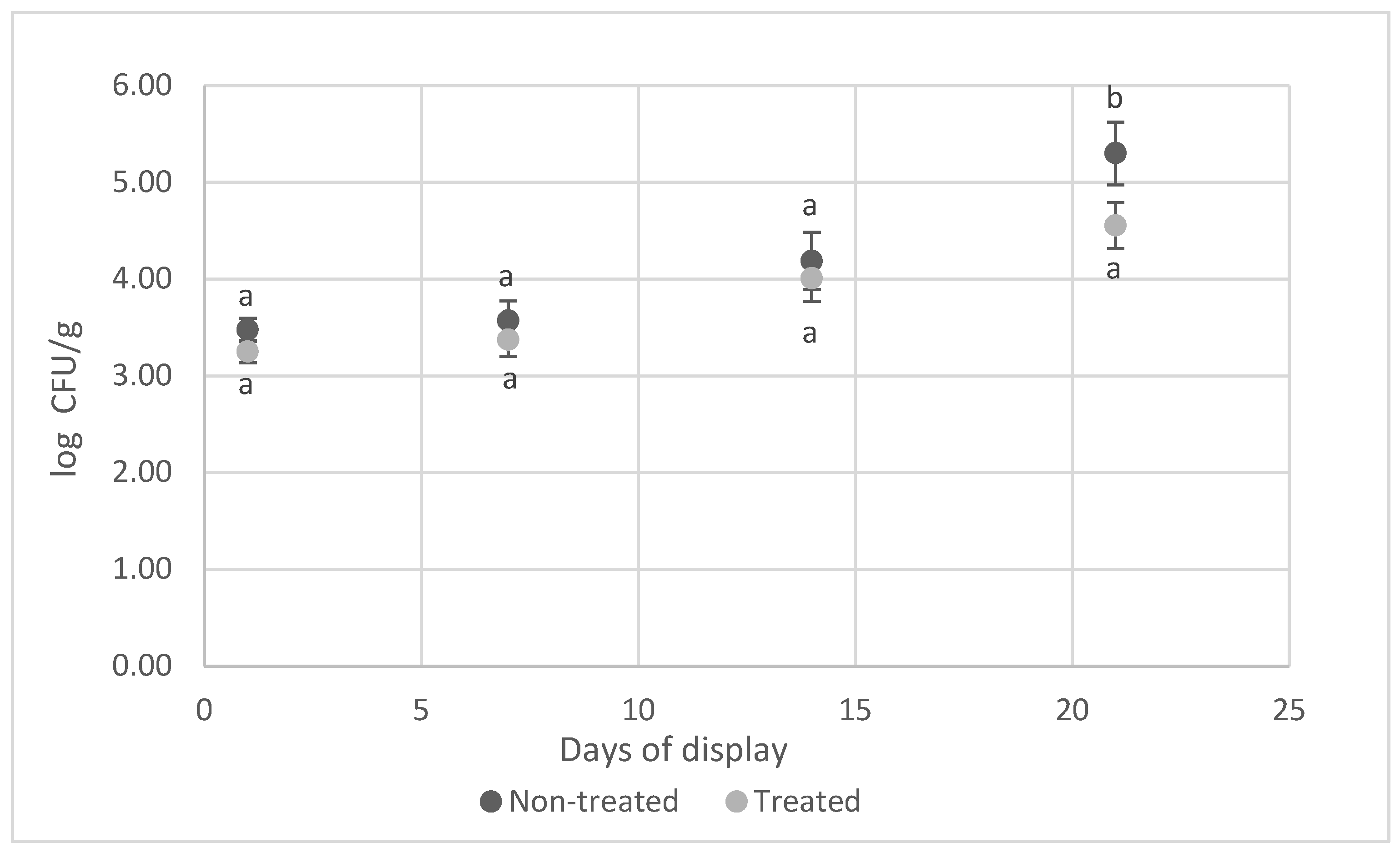
3.3. Microbiological Evaluation
3.4. Texture Analysis
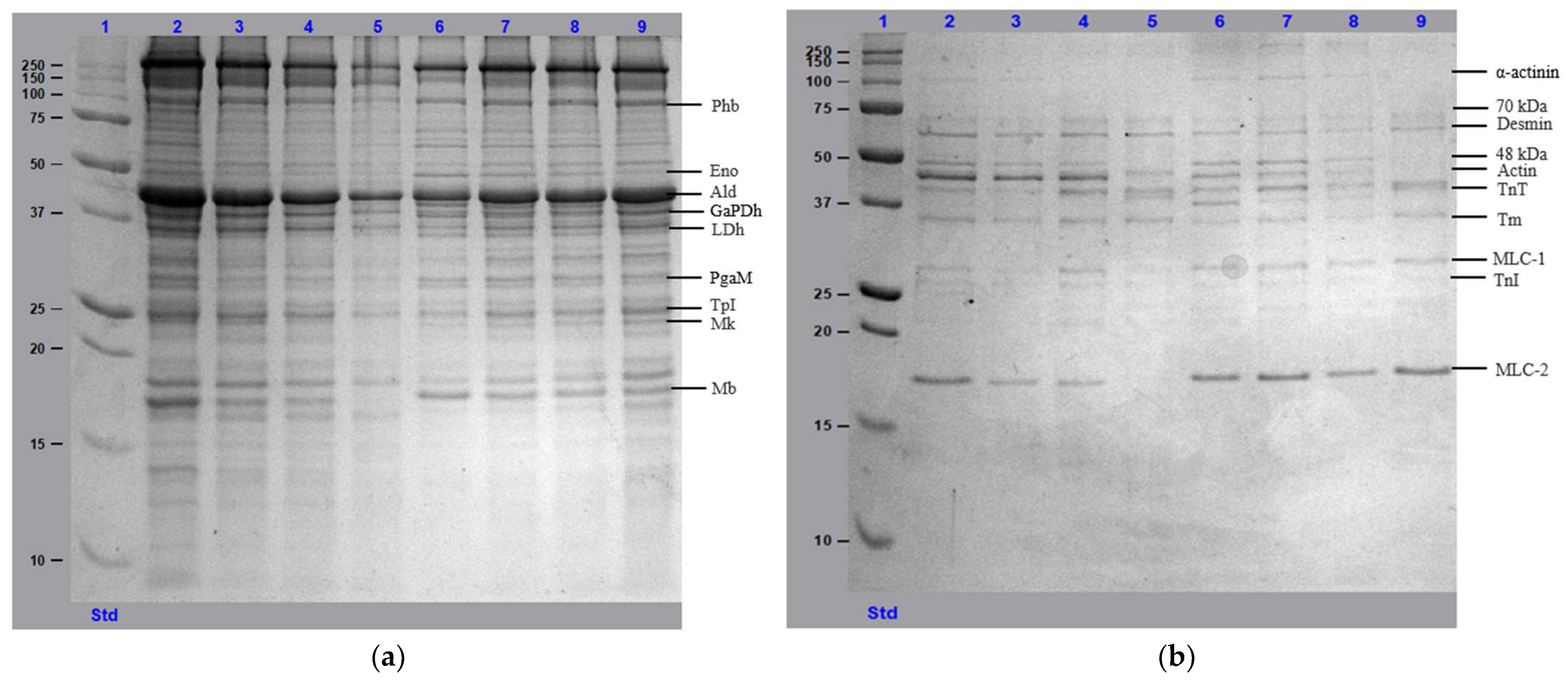
3.5. Protein Profile Analysis Using SDS-PAGE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soriano, A.; Cruz, B.; Gómez, L.; Mariscal, C.; García Ruiz, A. Proteolysis, physicochemical characteristics and free fatty acid composition of dry sausages made with deer (Cervus elaphus) or wild boar (Sus scrofa) meat: A preliminary study. Food Chem. 2006, 96, 173–184. [Google Scholar] [CrossRef]
- Vergara, H.; Gallego, L.; García, A.; Landete-Castillejos, T. Conservation of Cervus elaphus meat in modified atmospheres. Meat Sci. 2003, 65, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, E. Consumer acceptance—Meat quality aspects. In Consistency of Quality: Abstracts and Proceedings of the 11th International Meat Symposium, Centurion, South Africa, 29–30 January 2003; Agricultural Research Council (ARC): Hatfield, South Africa, 2003; pp. 146–159. [Google Scholar]
- Hoffman, L.C.; Wiklund, E. Game and venison—Meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef]
- Polak, T.; Rajar, A.; Gašperlin, L.; Žlender, B. Cholesterol concentration and fatty acid profile of red deer (Cervus elaphus) meat. Meat Sci. 2008, 80, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Bureš, D.; Bartoň, L.; Kotrba, R.; Hakl, J. Quality attributes and composition of meat from red deer (Cervus elaphus), fallow deer (Dama dama) and Aberdeen Angus and Holstein cattle (Bos taurus). J. Sci. Food Agric. 2015, 95, 2299–2306. [Google Scholar] [CrossRef]
- Milczarek, A.; Janocha, A.; Niedziałek, G.; Zowczak-Romanowicz, M.; Horoszewicz, E.; Piotrowski, S. Health-promoting properties of the wild-harvested meat of roe deer (Capreolus capreolus L.) and red deer (Cervus elaphus L.). Animals 2021, 11, 2108. [Google Scholar] [CrossRef]
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj (accessed on 29 September 2024).
- European Commission. Commission Regulation (EU) No 150/2011 of 18 February 2011 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards farmed and wild game and farmed and wild game meat. Off. J. Eur. Union 2011, 46, 14–16. [Google Scholar]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Available online: http://data.europa.eu/eli/reg/2002/178/oj (accessed on 29 September 2024).
- Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. Available online: http://data.europa.eu/eli/reg/2004/852/oj (accessed on 29 September 2024).
- Castillo, A.; Lucia, L.M.; Goodson, K.J.; Savell, J.W.; Acuff, G.R. Comparison of Water Wash, Trimming, and Combined Hot Water and Lactic Acid Treatments for Reducing Bacteria of Fecal Origin on Beef Carcasses. J. Food Prot. 1998, 61, 823–828. [Google Scholar] [CrossRef]
- Aymerich, T.; Picouet, P.A.; Monfort, J.M. Decontamination technologies for meat products. Meat Sci. 2008, 78, 114–129. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Smith, J.V.; Broadbent, J.R. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 88, 256–260. [Google Scholar] [CrossRef]
- Naveena, B.M.; Muthukumar, M.; Sen, A.R.; Babji, Y.; Murthy, T.R.K. Improvement of shelf-life of buffalo meat using lactic acid, clove oil, and vitamin C during retail display. Meat Sci. 2006, 74, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Canto, A.C.V.C.S.; Costa-Lima, B.R.C.; Suman, S.P.; Monteiro, M.L.G.; Viana, F.M.; Salim, A.P.A.A.; Nair, M.N.; Silva, T.J.P.; Freitas, M.Q. Color attributes and oxidative stability of longissimus lumborum and psoas major muscles from Nellore bulls. Meat Sci. 2016, 121, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Quilo, S.A.; Pohlman, F.W.; Dias-Morse, P.N.; Brown, A.H., Jr.; Crandall, P.G.; Story, R.P. Microbial, instrumental color and sensory characteristics of inoculated ground beef produced using potassium lactate, sodium metasilicate or peroxyacetic acid as multiple antimicrobial interventions. Meat Sci. 2010, 84, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Sánchez-García, C. Nutritional composition of game meat from wild species harvested in Europe. Meat Nutr. 2021, 77–100. [Google Scholar] [CrossRef]
- Fooks, L.J.; Gibson, G.R. In vitro investigations of the effect of probiotics and prebiotics on selected human intestinal pathogens. FEMS Microbiol. Ecol. 2002, 39, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Badoni, M. Effects of peroxyacetic acid, acidified sodium chlorite or lactic acid solutions on the microflora of chilled beef carcasses. Int. J. Food Microbiol. 2004, 91, 43–50. [Google Scholar] [CrossRef]
- Makras, L.; De Vuyst, L. The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int. Dairy J. 2006, 16, 1049–1057. [Google Scholar] [CrossRef]
- Manzoor, A.; Jaspal, M.H.; Yaqub, T.; Haq, A.U.; Nasir, J.; Avais, M.; Asghar, B.; Badar, I.H.; Ahmad, S.; Yar, M.K. Effect of lactic acid spray on microbial and quality parameters of buffalo meat. Meat Sci. 2020, 159, 107923. [Google Scholar] [CrossRef]
- Mols, M.; Van Kranenburg, R.; Van Melis, C.; Moezelaar, R.; Abee, T. Analysis of acid-stressed Bacillus cereus reveals a major oxidative response and inactivation-associated radical formation. Environ. Microbiol. 2010, 12, 873–885. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Lactic acid concentrations that reduce microbial load yet minimally impact colour and sensory characteristics of beef. Meat Sci. 2017, 129, 169–175. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Gaby, S.K.; Singh, V.N.; Vitamin, C. Vitamin Intake and Health: A Scientific Review; Gaby, S.K., Bendich, A., Eds.; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Friedrich, L.; Siró, I.; Dalmadi, I.; Horváth, K.; Ágoston, R.; Balla, C. Influence of various preservatives on the quality of minced beef under modified atmosphere at chilled storage. Meat Sci. 2008, 79, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, S.; Liu, X.; Lei, Y.; Bai, Y.; Yang, Y.; Li, H.; Liu, Y.; Xiao, Y.; Xie, P.; et al. Effects of lactic acid and ascorbic acid electrostatic spraying on the physicochemical attributes and microbial diversity of beef aged at mild temperature (10° C). Meat Sci. 2024, 214, 109532. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.S.; Tatol, M. Colour difference∆ EA survey. Mach. Graph. Vis. 2012, 20, 383–411. [Google Scholar]
- International Association of Microbiological Societies; International Commission on Microbiological Specifications for Foods; Roberts, T.A. Microorganisms in Foods: Sampling for Microbiological Analysis, Principles and Specific Applications; Blackie Acad. & Professional: London, UK, 1986. [Google Scholar]
- De Huidobro, F.R.; Miguel, E.; Blázquez, B.; Onega, E. A comparison between two methods (Warner–Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef]
- Jonas, G.; Mertens, L.; Verheyen, D.; Crauwels, P.; Michiels, C.; Van Impe, J. Combined effects of high hydrostatic pressure and sodium nitrite on color, water holding capacity and texture of frankfurter. J. Phys. Conf. Ser. 2017, 950, 042006. [Google Scholar] [CrossRef]
- Csehi, B.; Szerdahelyi, E.; Pásztor-Huszár, K.; Salamon, B.; Tóth, A.; Zeke, I.; Jónás, G.; Friedrich, L. Changes of protein profiles in pork and beef meat caused by high hydrostatic pressure treatment. Acta Aliment. 2016, 45, 565–571. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Aktaş, N.; Aksu, M.I.; Kaya, M. The effect of organic acid marination on tenderness, cooking loss and bound water content of beef. J. Muscle Foods 2003, 14, 181–194. [Google Scholar] [CrossRef]
- Oreskovich, D.C.; Bechtel, P.J.; McKeith, F.K.; Novakofski, J.; Basgall, E.J. Marinade pH affects textural properties of beef. J. Food Sci. 1992, 57, 305–311. [Google Scholar] [CrossRef]
- Seuss, I.; Martin, M. The influence of marinating with food acids on the composition and sensory properties of beef. Fleischwirtschaft 1993, 73, 292–295. [Google Scholar]
- Goddard, B.L.; Mikel, W.B.; Conner, D.E.; Jones, W.R. Use of organic acids to improve the chemical, physical, and microbial attributes of beef strip loins stored at −1° C for 112 days. J. Food Prot. 1996, 59, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O. Meat spoilage and evaluation of the potential storage life of fresh meat. J. Food Prot. 1983, 46, 444–452. [Google Scholar] [CrossRef]
- Jose, M.T.; Iyer, P.; Prabhakaran, P. Influence of pH on the keeping quality of beef. Kerala J. Vet. Sci. 1984, 15, 135–139. [Google Scholar]
- Kotula, K.L.; Thelappurate, R. Microbiological and sensory attributes of retail cuts of beef treated with acetic and lactic acid solutions. J. Food Prot. 1994, 57, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Stivarius, M.R.; Pohlman, F.W.; McElyea, K.S.; Waldroup, A.L. Effects of hot water and lactic acid treatment of beef trimmings prior to grinding on microbial, instrumental color and sensory properties of ground beef during display. Meat Sci. 2002, 60, 327–334. [Google Scholar] [CrossRef]
- Pierson, M.D.; Collins-Thompson, D.L.; Ordal, Z.J. Microbiological, sensory, and pigment changes of aerobically and anaerobically packaged beef. Food Technol. 1970, 24, 129–133. [Google Scholar]
- Renerre, M.; Labas, R. Biochemical factors influencing metmyoglobin formation in beef muscles. Meat Sci. 1987, 19, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.; Anjaneyulu, A.S.R. Quality improvement of ground buffalo meat by preblending and vacuum packaging. Fleischwirtsch. Int. 1997, 5, 15–19. [Google Scholar]
- Capita, R.; Alonso-Calleja, C.; Rodríguez-Pérez, R.; Moreno, B.; García-Fernández, M.D.C. Influence of poultry carcass skin sample site on the effectiveness of trisodium phosphate against Listeria monocytogenes. J. Food Prot. 2002, 65, 853–856. [Google Scholar] [CrossRef]
- Acuff, G.R.; Vanderzant, C.; Savell, J.W.; Jones, D.K.; Griffin, D.B.; Ehlers, J.G. Effect of acid decontamination of beef subprimal cuts on the microbiological and sensory characteristics of steaks. Meat Sci. 1987, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bosilevac, J.M.; Nou, X.; Barkocy-Gallagher, G.A.; Arthur, T.M.; Koohmaraie, M. Treatments using hot water instead of lactic acid reduce levels of aerobic bacteria and Enterobacteriaceae and reduce the prevalence of Escherichia coli O157 on preevisceration beef carcasses. J. Food Prot. 2006, 69, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Shivas, S.D.; Kropf, D.H.; Hunt, M.C.; Kastner, C.L.; Kendall, J.L.A.; Dayton, A.D. Effects of ascorbic acid on display life of ground beef. J. Food Prot. 1984, 47, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, J.; Cierach, M.; Żmijewski, T. Effects of aging treatment and freezing/thawing methods on the quality attributes of beef from Limousin × Holstein-Friesian and Hereford × Holstein-Friesian crossbreeds. Meat Sci. 2018, 137, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Albenzio, M.; della Malva, A.; Santillo, A.; Loizzo, P.; Sevi, A. Proteolytic pattern of myofibrillar protein and meat tenderness as affected by breed and aging time. Meat Sci. 2013, 95, 281–287. [Google Scholar] [CrossRef]
- Silva, L.H.P.; Rodrigues, R.T.S.; Assis, D.E.F.; Benedeti, P.D.B.; Duarte, M.S.; Chizzotti, M.L. The relationship between water-M.L. Explaining meat quality of bulls and steers by differential proteome and phosphoproteome analysis of skeletal muscle. J. Proteom. 2019, 199, 51–66. [Google Scholar] [CrossRef]
- Buts, B.; Claeys, E.; Demyer, D. Relation between concentration of troponin-T, 30,000-Dalton, and titin on SDS–PAGE and tenderness of bull longissimus dorsi. In Proceedings of the 32nd European Meeting of Meat Research Workers, Ghent, Belgium, 24–29 August 1986; pp. 24–29. [Google Scholar]
- Gault, N.F.S. The relationship between water-holding capacity and cooked meat tenderness in some beef muscles as influenced by acidic conditions below the ultimate pH. Meat Sci. 1985, 15, 15–30. [Google Scholar] [CrossRef]
- Wenham, L.M.; Locker, R.H. The effect of marinading on beef. J. Sci. Food Agric. 1976, 27, 1079–1084. [Google Scholar] [CrossRef]
- Stanton, C.; Light, N. The effects of conditioning on meat collagen: Part 4—The use of pre-rigor lactic acid injection to accelerate conditioning in bovine meat. Meat Sci. 1990, 27, 141–159. [Google Scholar] [CrossRef]
- Eilers, J.D.; Vellve, L.T.; Ma, M.; Baldwin, R.R.; Harris, J.S.; Oltjen, J.W. Evaluation of calcium chloride and lactic acid injection on chemical, microbiological and descriptive attributes of mature cow beef. Meat Sci. 1994, 38, 443–451. [Google Scholar] [CrossRef]
- Laville, E.; Savell, J.W.; Reed, R.B.; Meullenet, J.F.; Schilling, D.R.; McKeith, F.K. Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J. Agric. Food Chem. 2009, 57, 10755–10764. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Kerry, J.P.; Mullen, A.M. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010, 85, 115–120. [Google Scholar] [CrossRef]
- Joo, S.T.; Kauffman, R.G.; Kim, B.C.; Park, G.B. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef]
- Marino, R.; Albenzio, M.; della Malva, A.; Caroprese, M.; Santillo, A.; Sevi, A. Changes in meat quality traits and sarcoplasmic proteins during aging in three different cattle breeds. Meat Sci. 2014, 98, 178–186. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Kitamura, S.; Tanabe, S.; Nishimura, T.; Nakajima, I.; Chikuni, K. N-terminal amino acid sequences of troponin T fragments, including 30 kDa one, produced during postmortem aging of bovine longissimus muscle. Meat Sci. 2004, 67, 19–24. [Google Scholar] [CrossRef]
- Bowker, B.C.; Fahrenholz, T.M.; Paroczay, E.W.; Eastridge, J.S.; Solomon, M.B. Effect of hydrodynamic pressure processing and aging on the tenderness and myofibrillar proteins of beef strip loins. J. Muscle Foods 2008, 19, 74–97. [Google Scholar] [CrossRef]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Influence of early postmortem protein oxidation on beef quality. J. Anim. Sci. 2004, 82, 785–793. [Google Scholar] [CrossRef]
- Lonergan, E.H.; Zhang, W.; Lonergan, S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010, 86, 184–195. [Google Scholar] [CrossRef]
| Characteristics | Day 1 | Day 7 | Day 14 | Day 21 | SE | |
|---|---|---|---|---|---|---|
| pH | Treated | 5.60bx | 5.57bx | 5.33ax | 5.38ax | 0.06745 |
| Non-treated | 5.68cy | 5.56bcx | 5.38ax | 5.31ax | 0.0843 | |
| L* | Treated | 30.2bx | 29.1abx | 27.5ax | 28.6abx | 0.56051 |
| Non-treated | 30.3ax | 29.4ax | 30.0ay | 29.5ax | 0.21213 | |
| a* | Treated | 9.4ax | 9.6ax | 11.1bx | 11bx | 0.44977 |
| Non-treated | 9.2ax | 10ax | 11.3bx | 11.8bx | 0.59494 | |
| b* | Treated | 2.0ax | 2.4bx | 2.1ay | 2.4bx | 0.10308 |
| Non-treated | 2.2bx | 2.2bx | 1.8ax | 2.4bx | 0.12583 | |
| Hue angle (°) | Treated | 0.21ax | 0.24bx | 0.18ax | 0.22bx | 0.0125 |
| Non-treated | 0.23bx | 0.21bx | 0.16ax | 0.20abx | 0.01472 | |
| Chroma | Treated | 9.6ax | 9.9ax | 11.3bx | 11.1bx | 0.43842 |
| Non-treated | 9.4ax | 10.3ax | 11.4bx | 12.0by | 0.58768 | |
| Characteristics | Day 1 | Day 7 | Day 14 | Day 21 | SE | |
|---|---|---|---|---|---|---|
| Hardness (N) | Treated | 22.45bx | 20.43bx | 10.22ax | 10.64ax | 3.206094 |
| Non-treated | 23.76bx | 22.61bx | 16.17ay | 12.52ax | 2.668772 | |
| Cohesiveness (-) | Treated | 0.37bx | 0.32ax | 0.29ax | 0.29ax | 0.018875 |
| Non-treated | 0.39bx | 0.34abx | 0.31ay | 0.29ax | 0.021747 | |
| Springiness (mm) | Treated | 0.80ax | 0.81ax | 0.80ax | 0.81ax | 0.002887 |
| Non-treated | 0.89by | 0.83ax | 0.89by | 0.88by | 0.014361 | |
| Chewiness (mJ) | Treated | 6.69bx | 5.23bx | 2.42ax | 2.56ax | 0.889616 |
| Non-treated | 8.36cx | 6.36bx | 4.46aby | 3.25ax | 1.356622 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enkhbold, M.; Lőrincz, A.; Elayan, M.; Friedrich, L.; Barkó, A.; Csurka, T.; Boros, A.; Hitka, G.; Varga-Tóth, A. Effects of Lactic Acid and Ascorbic Acid Mixture on Quality Properties of Wild Red Deer (Cervus elaphus) Meat. Appl. Sci. 2024, 14, 8915. https://doi.org/10.3390/app14198915
Enkhbold M, Lőrincz A, Elayan M, Friedrich L, Barkó A, Csurka T, Boros A, Hitka G, Varga-Tóth A. Effects of Lactic Acid and Ascorbic Acid Mixture on Quality Properties of Wild Red Deer (Cervus elaphus) Meat. Applied Sciences. 2024; 14(19):8915. https://doi.org/10.3390/app14198915
Chicago/Turabian StyleEnkhbold, Munkhnasan, Attila Lőrincz, Majd Elayan, László Friedrich, Annamária Barkó, Tamás Csurka, Anikó Boros, Géza Hitka, and Adrienn Varga-Tóth. 2024. "Effects of Lactic Acid and Ascorbic Acid Mixture on Quality Properties of Wild Red Deer (Cervus elaphus) Meat" Applied Sciences 14, no. 19: 8915. https://doi.org/10.3390/app14198915
APA StyleEnkhbold, M., Lőrincz, A., Elayan, M., Friedrich, L., Barkó, A., Csurka, T., Boros, A., Hitka, G., & Varga-Tóth, A. (2024). Effects of Lactic Acid and Ascorbic Acid Mixture on Quality Properties of Wild Red Deer (Cervus elaphus) Meat. Applied Sciences, 14(19), 8915. https://doi.org/10.3390/app14198915







