Aggregation-Induced Emission (AIE) Probes in Fluorescent Sensing: Progress and Applications for Pesticide Detection
Abstract
1. Introduction
2. The Luminescence Mechanism of AIEgens and Detection Mechanisms Based on AIEgens Fluorescence Sensing
2.1. The Luminescence Mechanism of AIEgens
2.2. Detection Mechanism Based on AIEgens Fluorescence Sensing
3. Application of AIEgens in the Detection of Pesticide Residues in Fluorescence Sensors
3.1. Detection of Pesticides Using Organic Fluorescent Small-Molecule Sensors Based on AIEgens
3.2. Detection of Pesticides by Nanocomposite Sensors Based on AIEgens
3.3. Detection of Pesticides by Metal-Organic Framework Sensors Based on AIEgens
3.4. Detection of Pesticides by Supramolecular Assemblies Based on AIEgens
3.5. Detection of Pesticides by Porous Organic Polymer Sensors Based on AIEgens
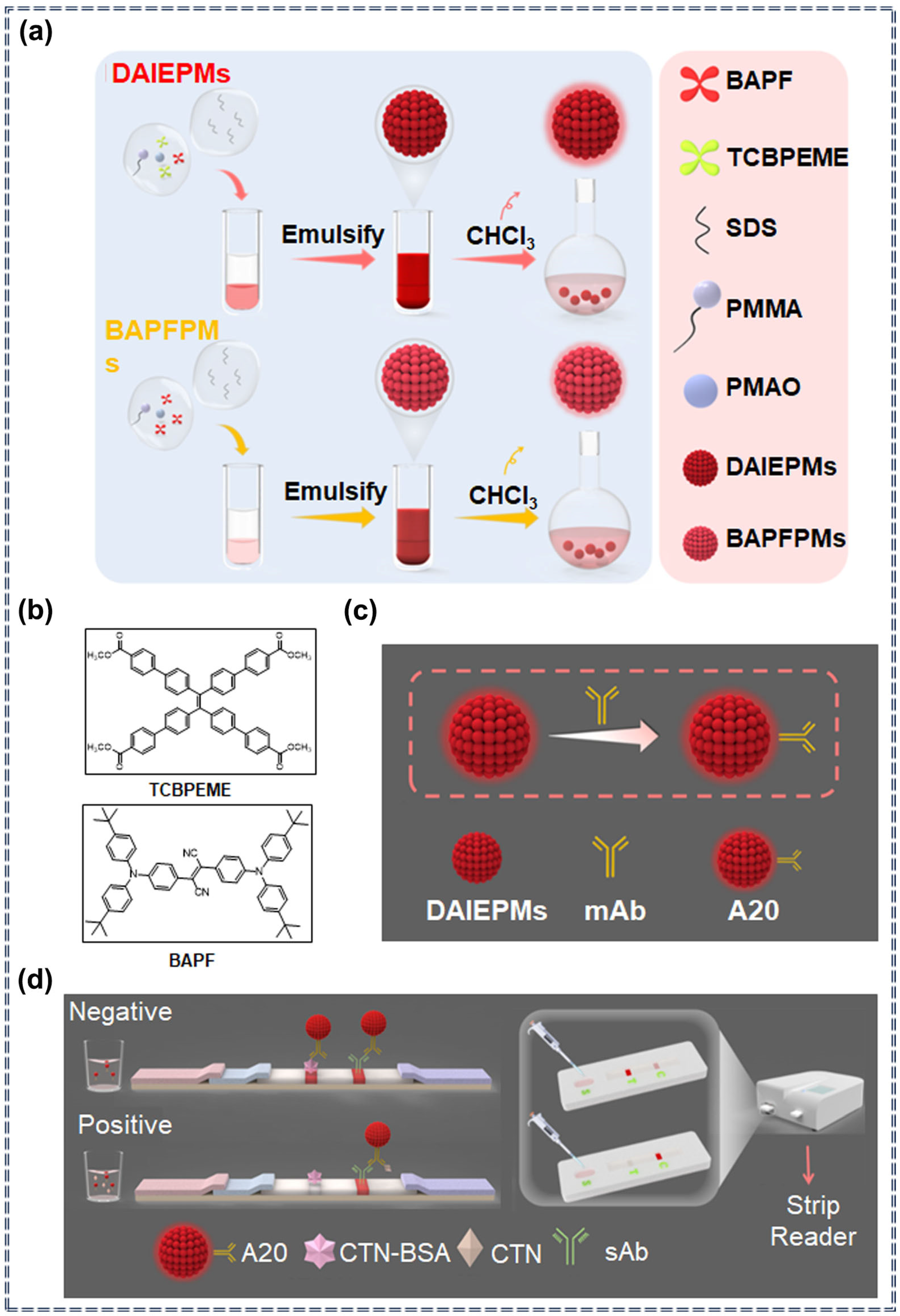
3.6. Detection of Pesticides by Lateral Flow Immunoassay Sensors Based on AIEgens
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase Inhibition-Based Biosensors for Pesticide Determination: A Review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Tian, Y.; Du, Y.; Ma, Y.; Zeng, S.; Gu, C.; Jiang, T.; Zhou, J. In Situ Recyclable Surface-Enhanced Raman Scattering-Based Detection of Multicomponent Pesticide Residues on Fruits and Vegetables by the Flower-like MoS2@Ag Hybrid Substrate. ACS Appl. Mater. Interfaces 2020, 12, 14386–14399. [Google Scholar] [CrossRef] [PubMed]
- Songa, E.A.; Okonkwo, J.O. Recent Approaches to Improving Selectivity and Sensitivity of Enzyme-Based Biosensors for Organophosphorus Pesticides: A Review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Fluorescent Peptide Probes for Organophosphorus Pesticides Detection. J. Hazard. Mater. 2020, 389, 122074. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Singh, R. Progress and Challenges in the Detection of Residual Pesticides Using Nanotechnology Based Colorimetric Techniques. Trends Environ. Anal. Chem. 2020, 26, e00086. [Google Scholar] [CrossRef]
- Haraux, E.; Tourneux, P.; Kouakam, C.; Stephan-Blanchard, E.; Boudailliez, B.; Leke, A.; Klein, C.; Chardon, K. Isolated Hypospadias: The Impact of Prenatal Exposure to Pesticides, as Determined by Meconium Analysis. Environ. Int. 2018, 119, 20–25. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, M.; Wang, C.; Wei, W.; Liu, Y. Enhancing Hydrogel-Based Long-Lasting Chemiluminescence by a Platinum-Metal Organic Framework and its Application in Array Detection of Pesticides and D-Amino Acids. Nanoscale 2020, 12, 4959–4967. [Google Scholar] [CrossRef]
- Zhou, P.; Han, K. ESIPT-Based AIE Luminogens: Design Strategies, Applications, and Mechanisms. Aggregate 2022, 3, e160. [Google Scholar] [CrossRef]
- Zhou, B.; Li, X. The Monitoring of Chemical Pesticides Pollution on Ecological Environment by GIS. Environ. Technol. Innov. 2021, 23, 101506. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of Pesticide Pollution at the Global Scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Liu, M.; Wei, J.; Wang, Y.; Ouyang, H.; Fu, Z. Dopamine-Functionalized Upconversion Nanoparticles as Fluorescent Sensors T for Organophosphorus Pesticide Analysis. Talanta 2019, 195, 706–712. [Google Scholar] [CrossRef]
- Ishaq, Z.; Nawaz, M.A. Analysis of Contaminated Milk with Organochlorine Pesticide Residues Using Gas Chromatography. Int. J. Food Prop. 2018, 21, 879–891. [Google Scholar] [CrossRef]
- Tazarv, M.; Faraji, H.; Moghimi, A.; Azizinejad, F. Bursting-bubble Flow Microextraction Combined with Gas Chromatography to Analyze Organophosphorus Pesticides in Aqueous Samples. J. Sep. Sci. 2021, 44, 2965–2971. [Google Scholar] [CrossRef]
- Dane, A.J.; Havey, C.D.; Voorhees, K.J. The Detection of Nitro Pesticides in Mainstream and Sidestream Cigarette Smoke Using Electron Monochromator-Mass Spectrometry. Anal. Chem. 2006, 78, 3227–3233. [Google Scholar] [CrossRef]
- Tabibi, A.; Jafari, M.T. High Efficient Solid-Phase Microextraction Based on a Covalent Organic Framework for Determination of Trifluralin and Chlorpyrifos in Water and Food Samples by GC-CD-IMS. Food Chem. 2022, 373, 131527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Z.; Yang, G.; Wang, W.; Li, X.; Li, R.; Wu, Y. Microwave Pretreatment and Gas Chromatography–Mass Spectrometry Determination of Herbicide Residues in Onion. Food Chem. 2008, 108, 322–328. [Google Scholar] [CrossRef]
- Hassan, J.; Farahani, A.; Shamsipur, M.; Damerchili, F. Rapid and Simple Low Density Miniaturized Homogeneous Liquid–Liquid Extraction and Gas Chromatography/Mass Spectrometric Determination of Pesticide Residues in Sediment. J. Hazard. Mater. 2010, 184, 869–871. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Sarzanini, C.; Costantino, G.; Fungi, M. Determination of Herbicides by Solid Phase Extraction Gas Chromatography–Mass Spectrometry in Drinking Waters. Anal. Chim. Acta 2006, 578, 241–249. [Google Scholar] [CrossRef]
- Notardonato, I.; Salimei, E.; Russo, M.V.; Avino, P. Simultaneous Determination of Organophosphorus Pesticides and Phthalates in Baby Food Samples by Ultrasound–Vortex-Assisted Liquid–Liquid Microextraction and GC–IT/MS. Anal. Bioanal. Chem. 2018, 410, 3285–3296. [Google Scholar] [CrossRef]
- Wang, H.; Qu, B.; Liu, H.; Ding, J.; Ren, N. Analysis of Organochlorine Pesticides in Surface Water of the Songhua River Using Magnetoliposomes as Adsorbents Coupled with GC-MS/MS Detection. Sci. Total Environ. 2018, 618, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Harshit, D.; Charmy, K.; Nrupesh, P. Organophosphorus Pesticides Determination by Novel HPLC and Spectrophotometric Method. Food Chem. 2017, 230, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Quan, J.; Hu, Z. Detection of Organophosphorus Pesticides in Wheat by Ionic Liquid-Based Dispersive Liquid-Liquid Microextraction Combined with HPLC. J. Anal. Methods Chem. 2018, 2018, 8916393. [Google Scholar] [CrossRef]
- Simpson, A.J.; Simpson, M.J.; Soong, R. Environmental Nuclear Magnetic Resonance Spectroscopy: An Overview and a Primer. Anal. Chem. 2018, 90, 628–639. [Google Scholar] [CrossRef]
- Xu, N.; Ding, Y.; Ai, H.; Fei, J. Acetylene Black-Ionic Liquids Composite Electrode: A Novel Platform for Electrochemical Sensing. Microchim. Acta 2010, 170, 165–170. [Google Scholar] [CrossRef]
- Gajdár, J.; Barek, J.; Fischer, J. Antimony Film Electrodes for Voltammetric Determination of Pesticide Trifluralin. J. Electroanal. Chem. 2016, 778, 1–6. [Google Scholar] [CrossRef]
- Cai, Y.; Fang, J.; Wang, B.; Zhang, F.; Shao, G.; Liu, Y. A Signal-on Detection of Organophosphorus Pesticides by Fluorescent Probe Based on Aggregation-Induced Emission. Sens. Actuators B Chem. 2019, 292, 156–163. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, Q.; Li, J.; Yan, S.; Shen, J.; Liu, L.; Yin, M. An AIE Star Polymer with Enhanced Co-Delivery of Drug and Gene for Synergistic Pest Control. Chin. J. Chem. 2023, 41, 2671–2678. [Google Scholar] [CrossRef]
- Patel, D.A.; Anand, T.; Jali, B.R.; Sahoo, S.K. 4,4′-Sulfonyldianiline Derived AIE Luminogen for the Detection of Ofloxacin. ChemPlusChem 2024. [Google Scholar] [CrossRef]
- Xu, T.; Fu, Q.; Qingru, Z.; Wang, Z.; Liu, X.; Xiao, S.; Jiang, X.; Lu, Y.; Gong, Z.; Wu, Y.; et al. A Simple Fluorescence Pyrocatechol–Polyethyleneimine Detection Method for 3-MCPD. Anal. Methods 2024, 16, 276–283. [Google Scholar] [CrossRef]
- Xue, J.; Mao, K.; Cao, H.; Feng, R.; Chen, Z.; Du, W.; Zhang, H. Portable Sensors Equipped with Smartphones for Organophosphorus Pesticides Detection. Food Chem. 2024, 434, 137456. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.H.; Hui, B.Y.K.; Chin, K.L.O.; Zhu, Q.; Liu, X.; Xu, J. Recent Advances in Aggregation-Induced Emission (AIE)-Based Chemosensors for the Detection of Organic Small Molecules. Mater. Chem. Front. 2023, 7, 5561–5660. [Google Scholar] [CrossRef]
- Adarsh, N.; Krishnan, M.S.; Ramaiah, D. Sensitive Naked Eye Detection of Hydrogen Sulfide and Nitric Oxide by Aza-BODIPY Dyes in Aqueous Medium. Anal. Chem. 2014, 86, 9335–9342. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Niu, L.Y.; Shao, N.; Yang, Q.Z. BODIPY-Based Fluorescent Probe for Dual-Channel Detection of Nitric Oxide and Glutathione: Visualization of Cross-Talk in Living Cells. Anal. Chem. 2019, 91, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N. Metal-Coordinated Fluorescent and Luminescent Probes for Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). Coord. Chem. Rev. 2021, 427, 213581. [Google Scholar] [CrossRef]
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew. Chem. Int. Ed. 2011, 50, 9672–9675. [Google Scholar] [CrossRef]
- Kumar, N. Recent Developments of Fluorescent Probes for the Detection of Gasotransmitters (NO, CO and H2S). Coord. Chem. Rev. 2013, 257, 2335–2347. [Google Scholar] [CrossRef]
- Hammers, M.D.; Taormina, M.J.; Cerda, M.M.; Montoya, L.A.; Seidenkranz, D.T.; Parthasarathy, R.; Pluth, M.D. A Bright Fluorescent Probe for H2S Enables Analyte-Responsive, 3D Imaging in Live Zebrafish Using Light Sheet Fluorescence Microscopy. J. Am. Chem. Soc. 2015, 137, 10216–11022. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.W.; Zhang, B.; Tu, Q.; Wang, J.; Yuan, M.-S. Non-Biological Fluorescent Chemosensors for Pesticides Detection. Talanta 2022, 240, 123200. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, J.; Sun, D.W. Grouping Illuminants by Aggregation-Induced Emission (AIE) Mechanisms for Designing Sensing Platforms for Food Quality and Safety Inspection. Trends Food Sci. Technol. 2023, 134, 232–246. [Google Scholar] [CrossRef]
- Deng, X.; Xu, Z.; Zhang, Z.; Zhang, W.; Li, J.; Zheng, L.; Chen, X.; Pan, Y.; Qiu, P.; Wang, D.; et al. In Vivo 3-Photon Fluorescence Imaging of Mouse Subcortical Vasculature Labeled by AIEgen Before and After Craniotomy. Adv. Funct. Mater. 2022, 32, 2205151. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Wang, J.; Li, F.; Jiang, H.; Liu, L. Recent Progress on the Construction and Application of Dithienylethene-Based Photochromic AIEgens. Luminescence 2023, 39, e4546. [Google Scholar] [CrossRef]
- Barman, D.; Narang, K.; Parui, R.; Zehra, N.; Khatun, M.N.; Adil, L.R.; Iyer, P.K. Review on Recent Trends and Prospects in π-Conjugated Luminescent Aggregates for Biomedical Applications. Aggregate 2022, 3, e172. [Google Scholar] [CrossRef]
- Chen, P.; Lv, P.; Guo, C.-S.; Wang, R.P.; Su, X.; Feng, H.T.; Tang, B.Z. Enantioselective Recognition Based on Aggregation-Induced Emission. Chin. Chem. Lett. 2023, 34, 108041. [Google Scholar] [CrossRef]
- Ye, F.Y.; Hu, M.; Zheng, Y.S. Advances and Challenges of Metal Ions Sensors Based on AIE Effect. Coord. Chem. Rev. 2023, 493, 215328. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, S.; Zeng, Z.; Tang, B.Z. Functional Scaffolds from AIE Building Blocks. Matter 2020, 3, 1862–1892. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Li, Z.; Tang, B.Z.; Wang, D. Bioinspired AIE Nanomedicine: A Burgeoning Technology for Fluorescence Bioimaging and Phototheranostics. Adv. Mater. 2024, 36, 2406047. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Wang, Q.; Wu, H.; Zhao, J.W.; Cao, X. Research Progress on AIE Cyanostilbene-Based Self-Assembly Gels: Design, Regulation and Applications. Coord. Chem. Rev. 2022, 471, 214753. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Alam, P.; Bai, H.; Bhalla, V.; Bryce, M.R.; Cao, M.; Chen, C.; Chen, S.; Chen, X.; et al. Aggregation-Induced Emission (AIE), Life and Health. ACS Nano 2023, 17, 14347–14405. [Google Scholar] [CrossRef]
- Lee, K.W.; Chen, H.; Wan, Y.; Zhang, Z.; Huang, Z.; Li, S.; Lee, C.-S. Innovative Probes with Aggregation-Induced Emission Characteristics for Sensing Gaseous Signaling Molecules. Biomaterials 2022, 289, 121753. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Hong, Y.; Dong, Y.; Häußler, M.; Lam, J.W.Y.; Li, Z.; Guo, Z.; Guo, Z.; Tang, B.Z. Fluorescent “Light-up” Bioprobes Based on Tetraphenylethylene Derivatives with Aggregation-Induced Emission Characteristics. Chem. Commun. 2006, 3705–3707. [Google Scholar] [CrossRef]
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Synthesis, Light Emission, Nanoaggregation, and Restricted Intramolecular Rotation of 1,1-Substituted 2,3,4,5-Tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Luminogenic Polymers with Aggregation-Induced Emission Characteristics. Prog. Polym. Sci. 2012, 37, 182–209. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, M.; Xiao, H.; Wang, L.; Chen, Z.; Liu, S.; Li, J.; Li, S.; James, T.D. “Irregular” Aggregation-Induced Emission Luminogens. Coord. Chem. Rev. 2020, 418, 213358. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, Z.; Su, H.; Zhang, P.; Liu, J.; Niu, G.; Li, S.; Wang, Z.; Kwok, R.T.K.; Ni, X.-L.; et al. Exploration of Biocompatible AIEgens from Natural Resources. Chem. Sci. 2018, 9, 6497–6502. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Hong, Y.; Tang, B.Z. Fabrication of Fluorescent Nanoparticles Based on AIE Luminogens (AIE Dots) and Their Applications in Bioimaging. Mater. Horiz. 2016, 3, 283–293. [Google Scholar] [CrossRef]
- Hu, R.; Kang, Y.; Tang, B.Z. Recent Advances in AIE Polymers. Polym. J. 2016, 48, 359–370. [Google Scholar] [CrossRef]
- He, T.; Wang, H.; Chen, Z.; Liu, S.; Li, J.; Li, S. Natural Quercetin AIEgen Composite Film with Antibacterial and Antioxidant Properties for In Situ Sensing of Al3+ Residues in Food, Detecting Food Spoilage, and Extending Food Storage Times. ACS Appl. Bio Mater. 2018, 1, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Daga, P.; Dey, N. Exploring Various Photochemical Processes in Optical Sensing of Pesticides by Luminescent Nanomaterials: A Concise Discussion on Challenges and Recent Advancements. ACS Omega 2023, 8, 44395–44423. [Google Scholar] [CrossRef]
- Mirres, A.C.d.M.; Silva, B.E.P.d.M.d.; Tessaro, L.; Galvan, D.; Andrade, J.C.d.; Aquino, A.; Joshi, N.; Conte-Junior, C.A. Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors 2022, 12, 572. [Google Scholar] [CrossRef]
- Naghibi, S.; Chen, T.; Jamshidi Ghahfarokhi, A.; Tang, Y. AIEgen-Enhanced Protein Imaging: Probe Design and Sensing Mechanisms. Aggregate 2021, 2, e41. [Google Scholar] [CrossRef]
- Niu, H.; Ye, T.; Yao, L.; Lin, Y.; Chen, K.; Zeng, Y.; Li, L.; Guo, L.; Wang, J. A Novel Red-to-near-Infrared AIE Fluorescent Probe for Detection of Hg2+ with Large Stokes Shift in Plant and Living Cells. J. Hazard. Mater. 2024, 475, 134914. [Google Scholar] [CrossRef]
- Asad, M.; Anwar, M.I.; Abbas, A.; Younas, A.; Hussain, S.; Gao, R.; Li, L.K.; Shahid, M.; Khan, S. AIE Based Luminescent Porous Materials as Cutting-Edge Tool for Environmental Monitoring: State of the Art Advances and Perspectives. Coord. Chem. Rev. 2022, 463, 214539. [Google Scholar] [CrossRef]
- Lee, M.M.S.; Yu, E.Y.; Yan, D.; Chau, J.H.C.; Wu, Q.; Lam, J.W.Y.; Ding, D.; Kwok, R.T.K.; Wang, D.; Tang, B.Z. The Role of Structural Hydrophobicity on Cationic Amphiphilic Aggregation-Induced Emission Photosensitizer-Bacterial Interaction and Photodynamic Efficiency. ACS Nano 2023, 17, 17004–17020. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, Y.; Hou, Y.; Zhong, G.; Gao, R.; Wu, J.; Shen, B.; Zhang, X. Detection of Carboxylesterase 1 and Carbamates with a Novel Fluorescent Protein Chromophore Based Probe. Dyes Pigm. 2021, 192, 109444. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, P.; Xia, Y.; Li, F.; Zhang, M. A Simple AIE Probe to Pesticide Trifluralin Residues in Aqueous Phase: Ultra-Fast Response, High Sensitivity, and Quantitative Detection Utilizing a Portable Platform. Talanta 2023, 269, 125352. [Google Scholar] [CrossRef]
- Zhou, N.; Cai, M.; Zheng, S.; Guo, H.; Yang, F. First Organic Fluorescent Sensor for Pesticide Paclobutrazol Based on Tetraphenylimidazole Schiff Base. Sens. Actuators B Chem. 2024, 417, 136051. [Google Scholar] [CrossRef]
- Cai, Y.; Qiu, Z.; Lin, X.; Zeng, W.; Cao, Y.; Liu, W.; Liu, Y. Self-Assembled Nanomaterials Based on Aggregation-Induced Emission of AuNCs: Fluorescence and Colorimetric Dual-Mode Biosensing of Organophosphorus Pesticides. Sens. Actuators B Chem. 2020, 321, 128481. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Huang, Q.; Li, W.; Yu, Q.; Zhu, L.; Zhu, T.; Liu, S.; Chi, Z. Amphiphilic Polymer-Mediated Aggregation-Induced Emission Nanoparticles for Highly Sensitive Organophosphorus Pesticide Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 32689–32696. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Luo, X.; Luo, P.; Han, Z.; Peng, Q.; Li, K.; Hou, H.; Zang, S.Q. AIE Ligand-Based Silver Clusters Used for Ethion Detection. Mater. Chem. Front. 2021, 5, 7982–7986. [Google Scholar] [CrossRef]
- Wu, X.; Wang, P.; Hou, S.; Wu, P.; Xue, J. Fluorescence Sensor for Facile and Visual Detection of Organophosphorus Pesticides Using AIE Fluorogens-SiO2-MnO2 Sandwich Nanocomposites. Talanta 2019, 198, 8–14. [Google Scholar] [CrossRef]
- Xu, Y.; Pu, Y.; Jiang, H.; Huang, Y.; Shen, C.; Cao, J.; Jiang, W. Highly Sensitive Fluorescent Sensing Platform for Imidacloprid and Thiamethoxam by Aggregation-Induced Emission of the Zr(IV) Metal-Organic Framework. Food Chem. 2021, 375, 131879. [Google Scholar] [CrossRef]
- Zhang, K.; Elder, T.; Cheng, Z.; Zhan, K.; Peng, Y.; Li, M. Cellulose Nanofiber-Templated Metal-Organic Frameworks for Fluorescent Detection of Methyl Parathion Pesticides. J. Environ. Chem. Eng. 2024, 12, 112670. [Google Scholar] [CrossRef]
- Wei, D.; Li, M.; Wang, Y.; Zhu, N.; Hu, X.; Zhao, B.; Zhang, Z.; Yin, D. Encapsulating Gold Nanoclusters into Metal–Organic Frameworks to Boost Luminescence for Sensitive Detection of Copper Ions and Organophosphorus Pesticides. J. Hazard. Mater. 2022, 441, 129890. [Google Scholar] [CrossRef]
- Fan, L.; Tong, C.; Cao, Y.; Long, R.; Wei, Q.; Wang, F.; Tong, X.; Shi, S.; Guo, Y. Highly Specific Esterase Activated AIE plus ESIPT Probe for Sensitive Ratiometric Detection of Carbaryl. Talanta 2022, 246, 123517. [Google Scholar] [CrossRef]
- Ge, J.; Wang, L.J.; Pan, X.; Zhang, C.; Wu, M.Y.; Feng, S. Colorimetric and Ratiometric Supramolecular AIE Fluorescent Probe for the On-Site Monitoring of Fipronil. Analyst 2023, 148, 5395–5401. [Google Scholar] [CrossRef]
- Kang, Z.; Yang, J.; Jiang, J.; Zhao, L.; Zhang, Y.; Tu, Q.; Wang, J.; Yuan, M.-S. Pillar[5]Arenes Modified Tetraphenylethylene as Fluorescent Chemosensor for Paraquat Detection. Sens. Actuators B Chem. 2022, 370, 132436. [Google Scholar] [CrossRef]
- Kaur, J.; Malegaonkar, J.N.; Bhosale, S.V.; Singh, P.K. An Anionic Tetraphenyl Ethylene Based Simple and Rapid Fluorescent Probe for Detection of Trypsin and Paraoxon Methyl. J. Mol. Liq. 2021, 333, 115980. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z. Creation of Carbazole-Based Fluorescent Porous Polymers for Recognition and Detection of Various Pesticides in Water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Wang, Z. Micro-/Mesoporous Fluorescent Polymers and Devices for Visual Pesticide Detection with Portability, High Sensitivity, and Ultrafast Response. ACS Appl. Mater. Interfaces 2022, 14, 5815–5824. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, F.; Wang, Z. AIE-Active Fluorescent Porous Polymers for Recognizable Detection of Imidacloprid and Structure–Property Relationship. Chem. Mater. 2022, 34, 10701–10710. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Xiao, X.; Shu, X.; Fei, D.; Guang, Y.; Zhou, Y.; Lai, W. High-Performance Fluorescent Microspheres Based on Fluorescence Resonance Energy Transfer Mode for Lateral Flow Immunoassays. Anal. Chem. 2023, 95, 17860–17867. [Google Scholar] [CrossRef]
- Awiaz, G.; Lin, J.; Wu, A. Recent Advances of Au@Ag Core–Shell SERS-based Biosensors. Exploration 2023, 3, 20220072. [Google Scholar] [CrossRef]
- Xia, X.; Shi, B.; Wang, L.; Liu, Y.; Zou, Y.; Zhou, Y.; Chen, Y.; Zheng, M.; Zhu, Y.; Duan, J.; et al. From Mouse to Mouse-ear Cress: Nanomaterials as Vehicles in Plant Biotechnology. Exploration 2021, 1, 9–20. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Yu, Y.; Wang, J.; Liu, J.; Ihara, H.; Qiu, H. Composite Materials Based on Covalent Organic Frameworks for Multiple Advanced Applications. Exploration 2023, 3, 20220144. [Google Scholar] [CrossRef]
- Timofeev, K.L.; Kulinich, S.A.; Kharlamova, T.S. NH2-Modified UiO-66: Structural Characteristics and Functional Properties. Molecules 2023, 28, 3916. [Google Scholar] [CrossRef]
- Lammert, M.; Reinsch, H.; Murray, C.A.; Wharmby, M.T.; Terraschke, H.; Stock, N. Synthesis and Structure of Zr (IV)- and Ce (IV)-Based CAU-24 with 1,2,4,5-Tetrakis (4-Carboxyphenyl) Benzene. Dalton Trans. 2016, 45, 18822–18826. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yang, Y.-W. Macrocycle-Based Fluorochromic Systems. Cell Rep. Phys. Sci. 2024, 5, 101873. [Google Scholar] [CrossRef]
- Cui, R. A Tb-MOF Anion, Porous Coordination Framework Constructed with Oxalate Ligand: Crystal Structure, Adsorption Properties, and Luminescence Sensing. Dyes Pigm. 2021, 195, 109669. [Google Scholar] [CrossRef]


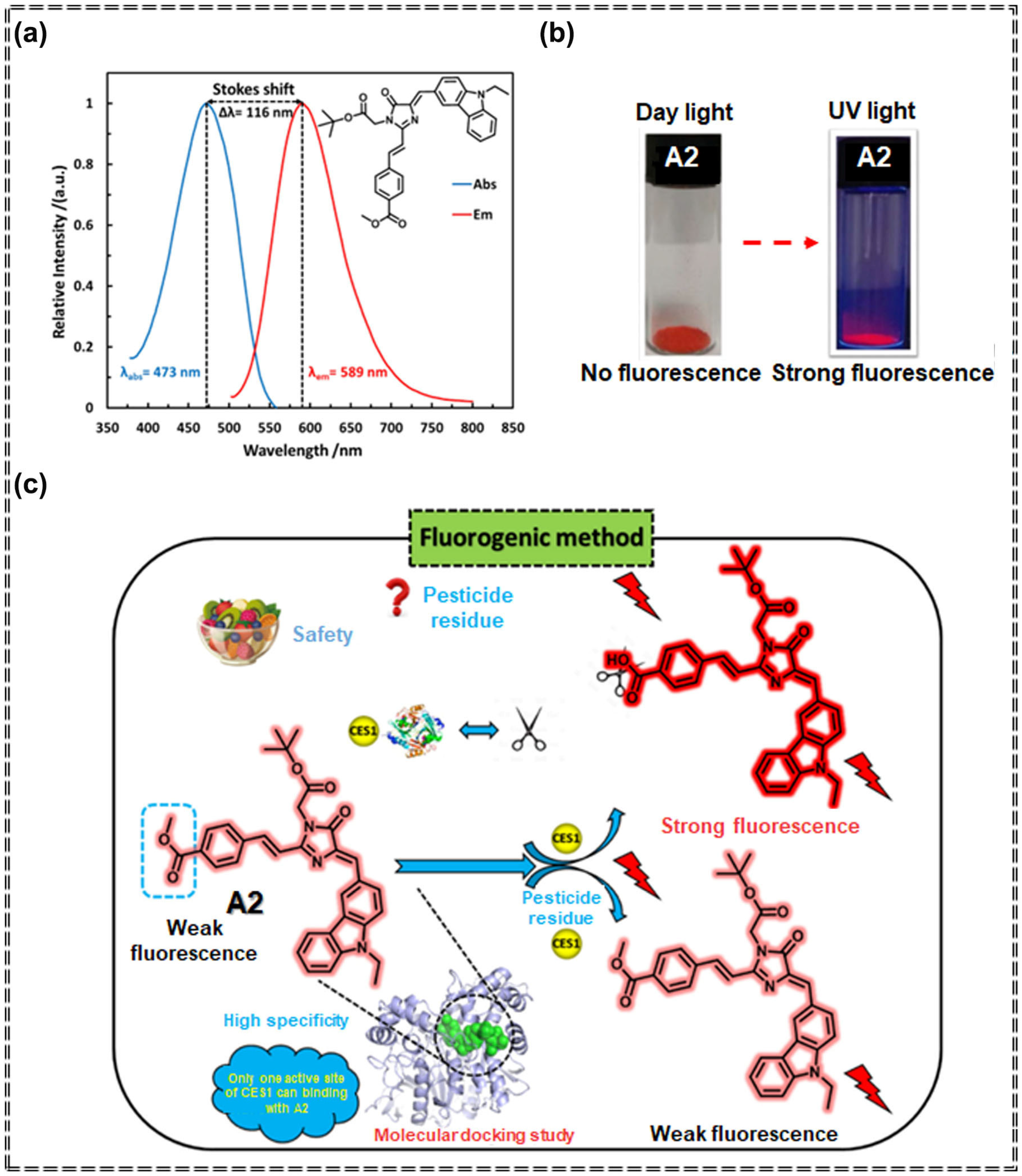


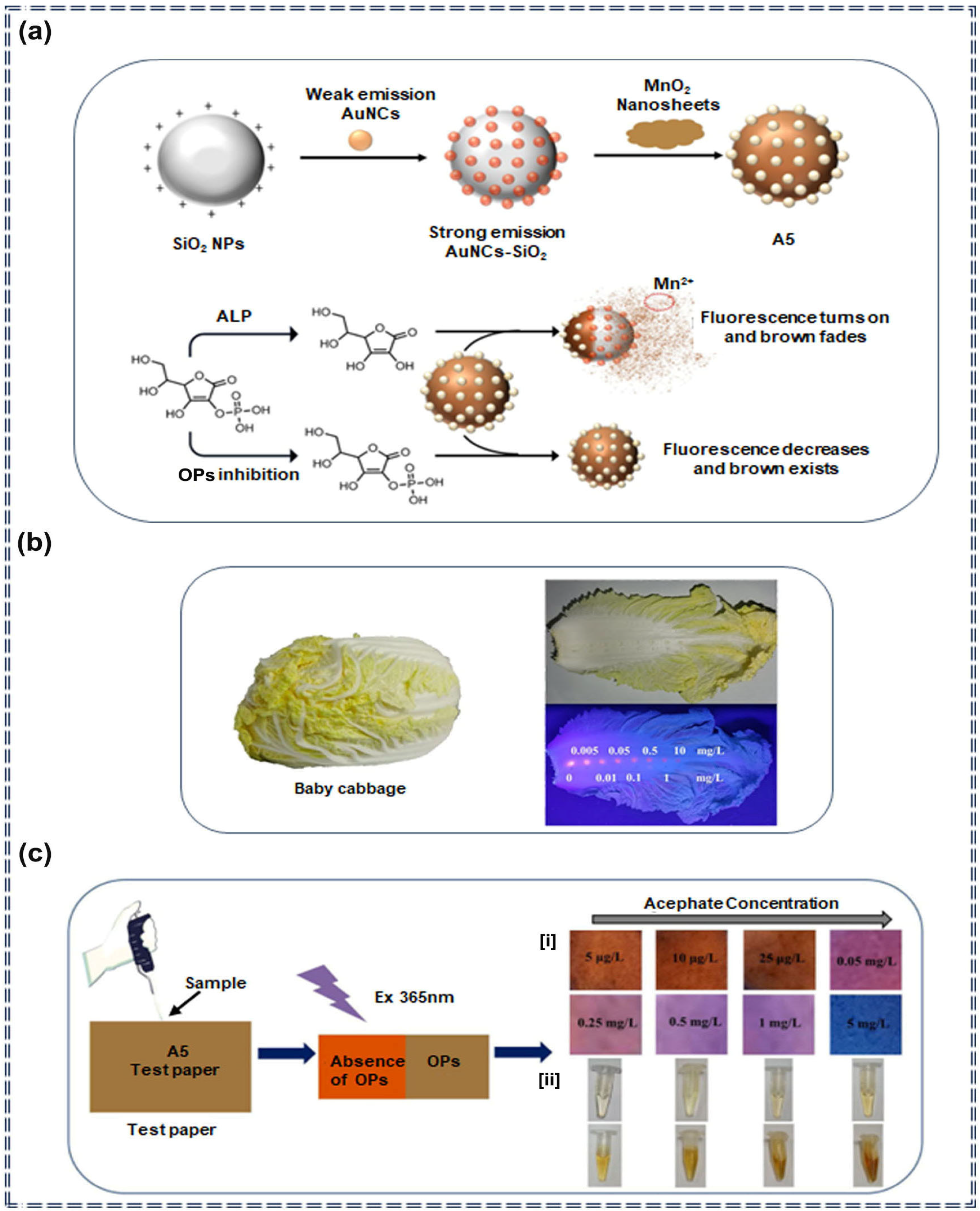
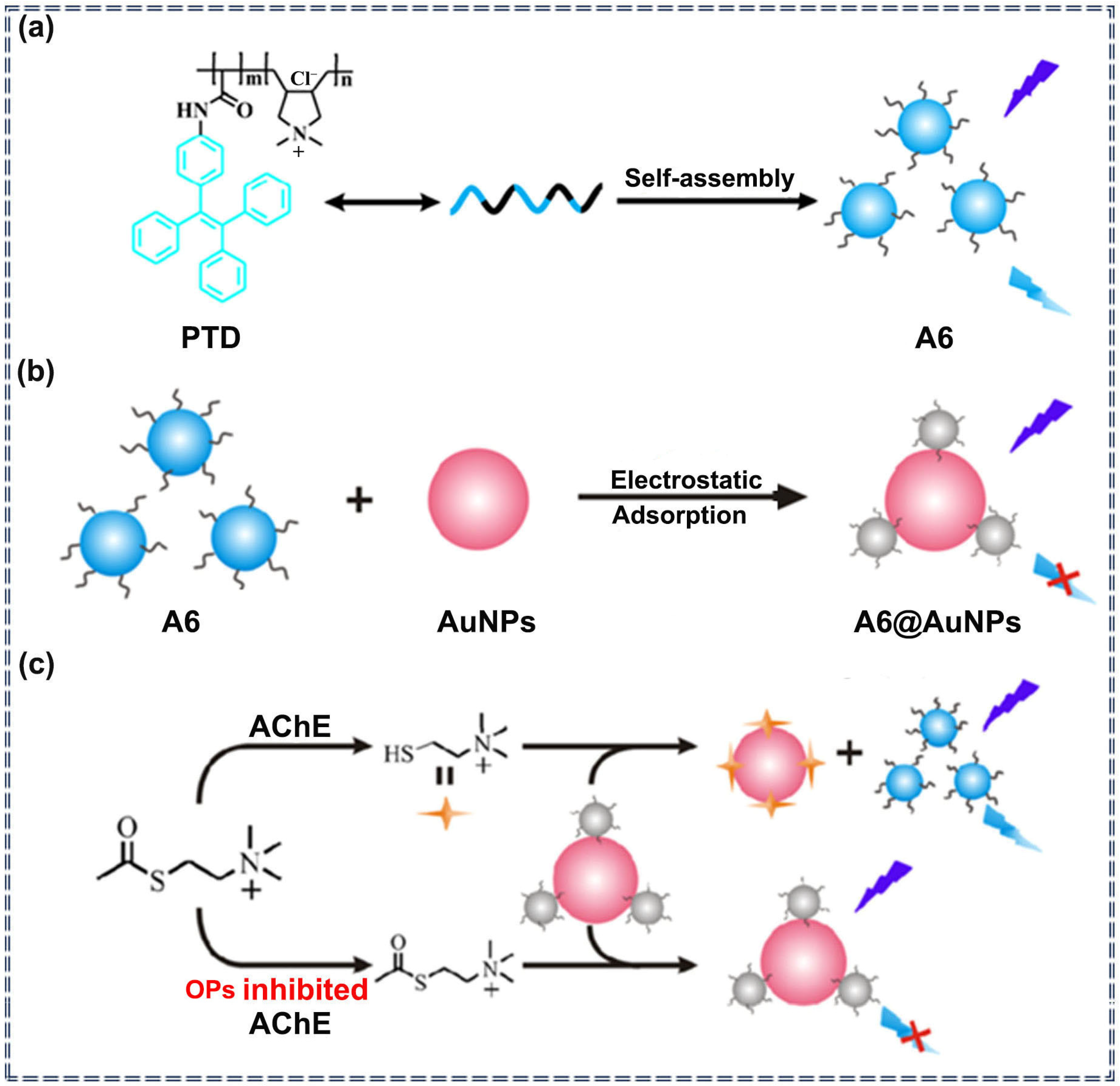
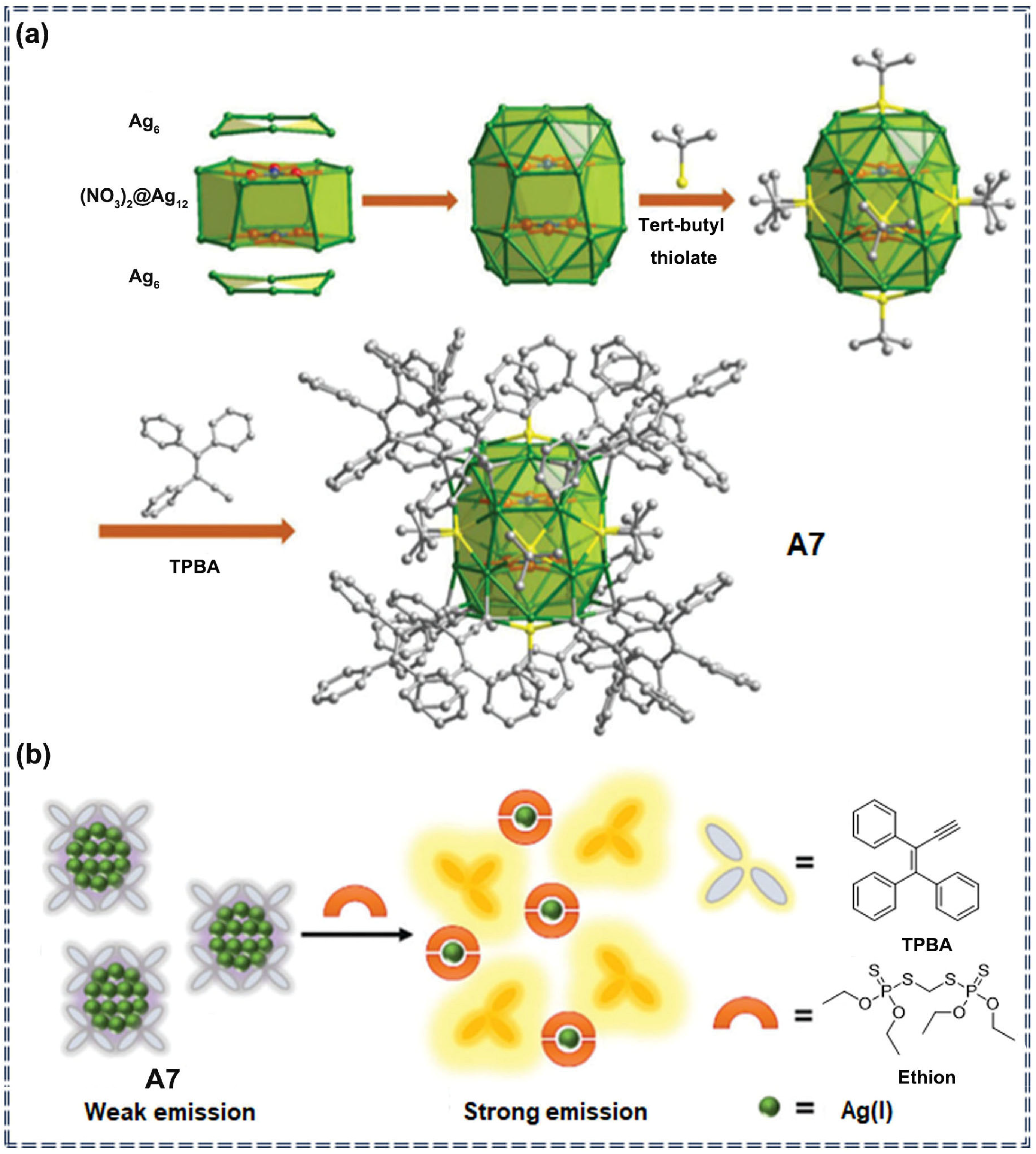

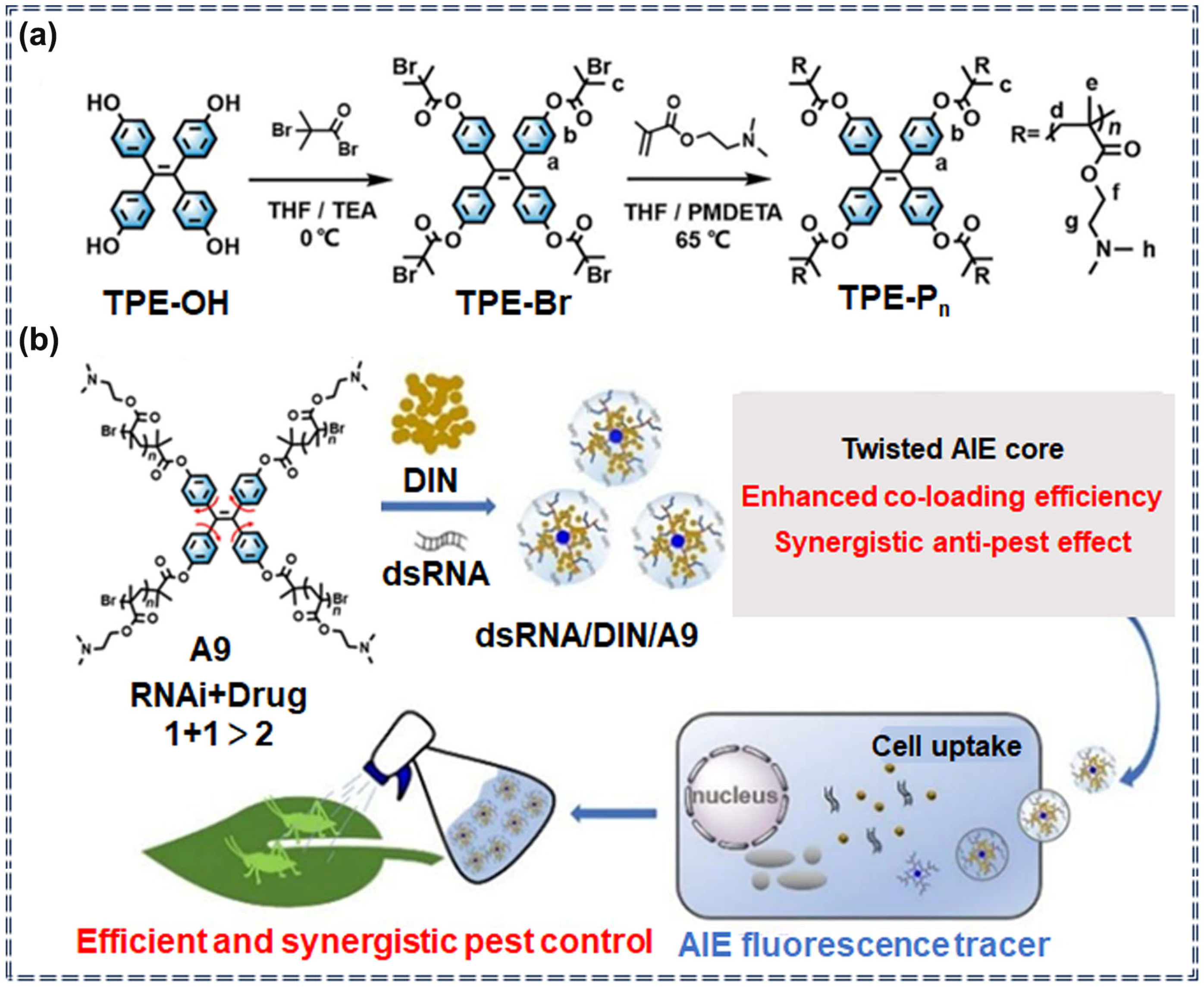
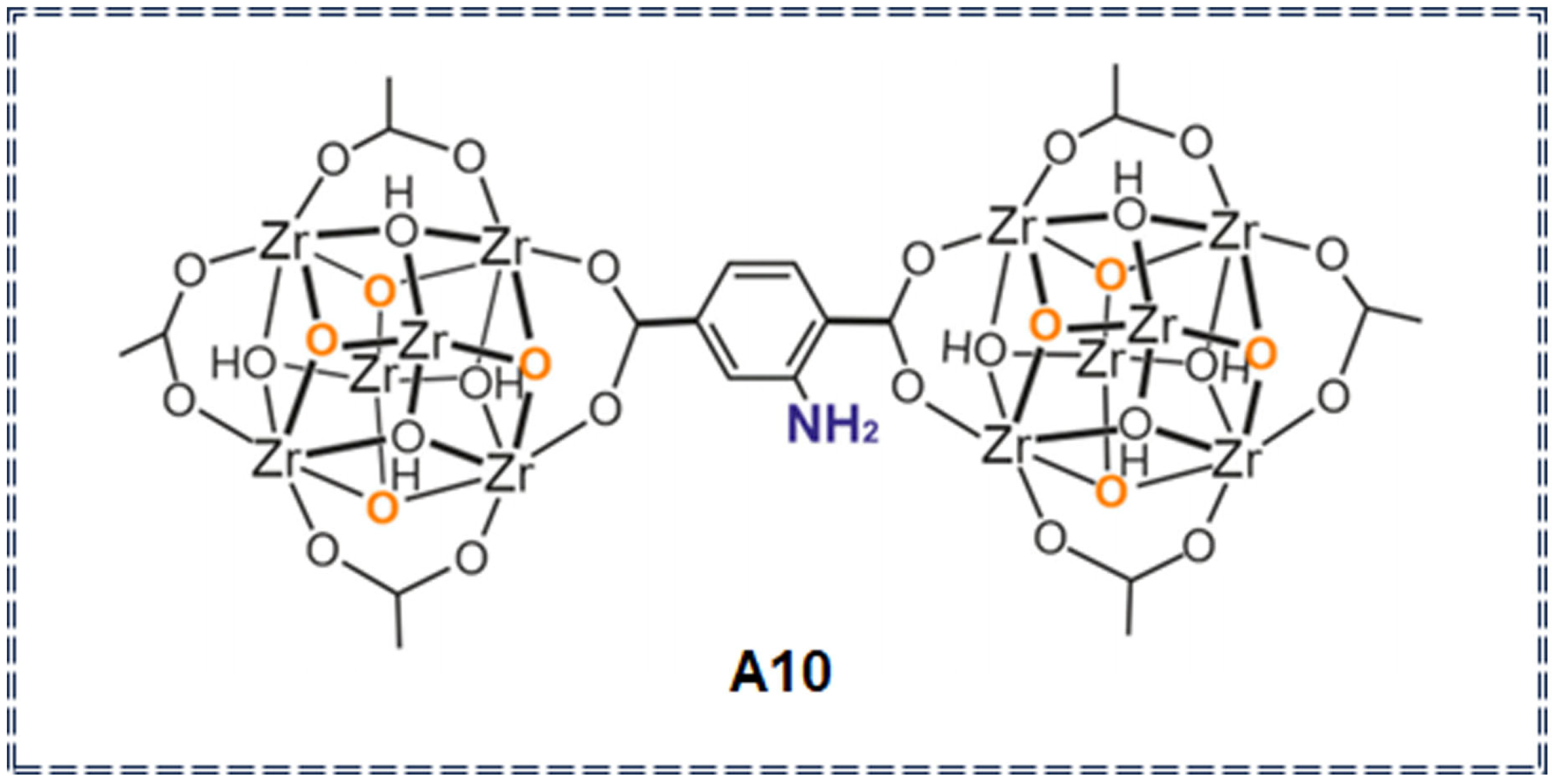
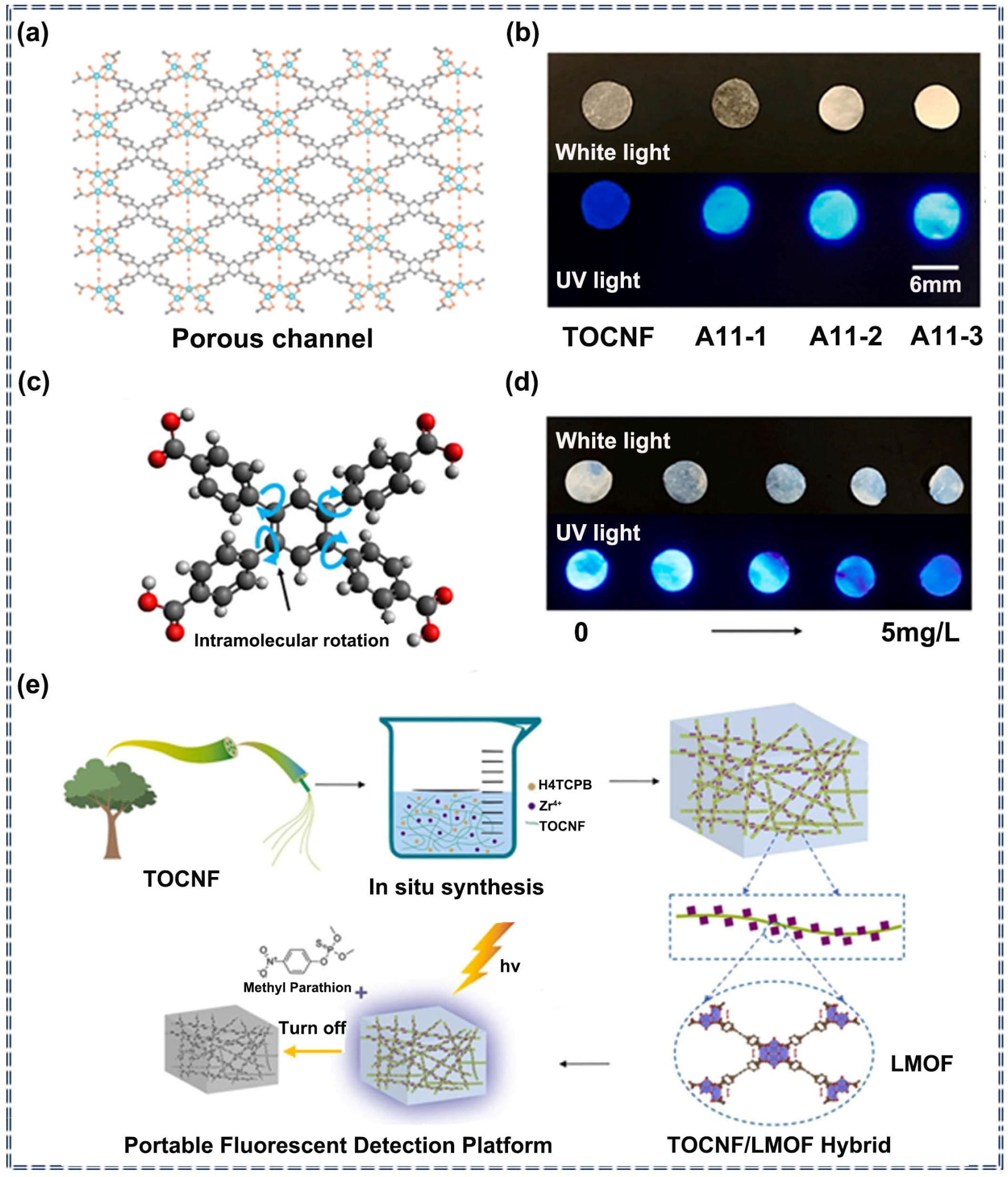
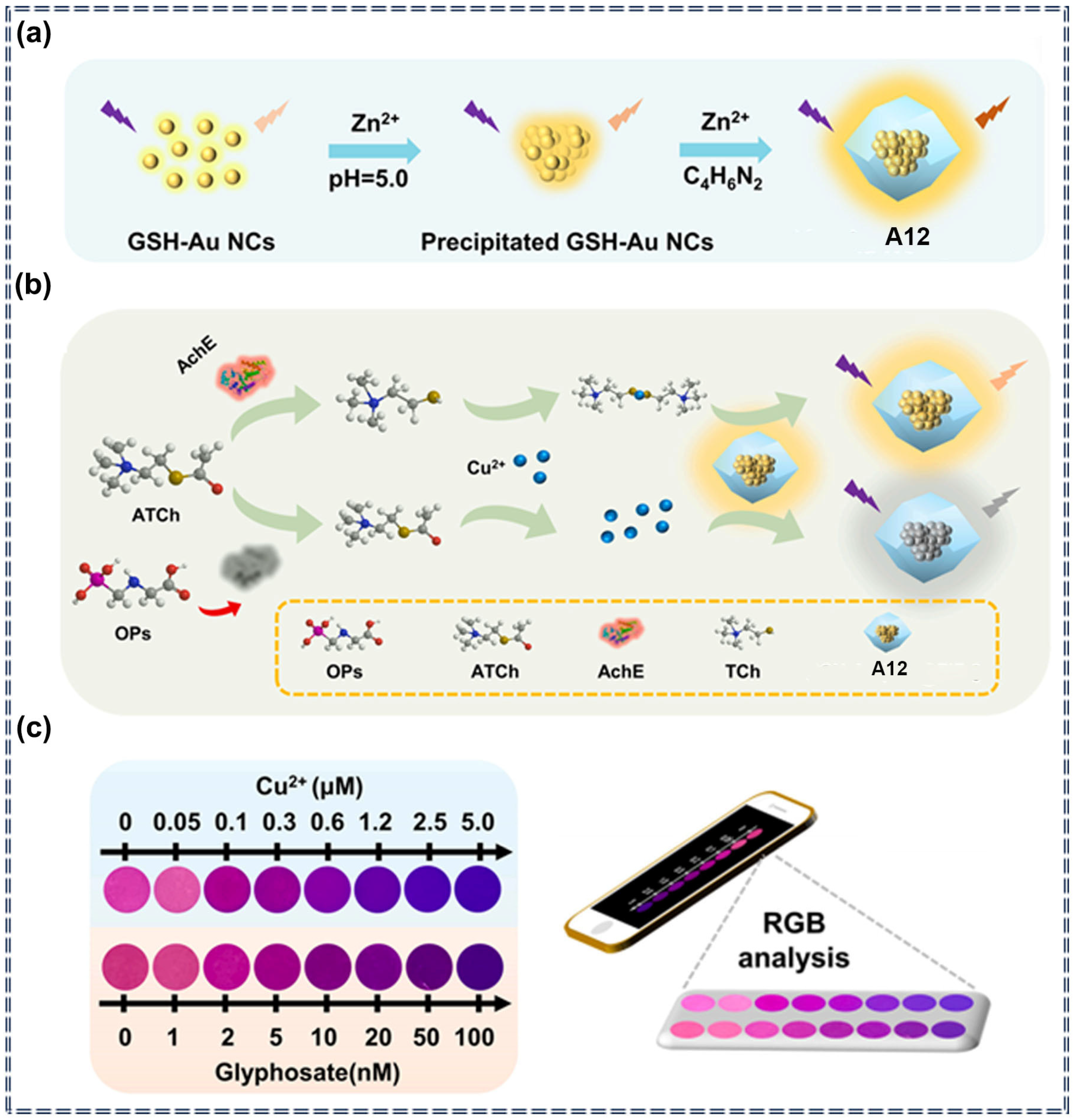



| Sensor Type | Sensor | Analyte | LOD | Detection Range | Sensing Type | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Small molecules | A1 | Organophosphorus pesticides | 0.008 mg/L | 0.009–22.5 mg/L | Turn-on | Specific recognition and chemical interaction | [27] |
| A2 | Carbamate pesticides | 27.8 ng/mL | 0 μg/mL–50 g/mL | Turn-off | Specific recognition and chemical interaction | [69] | |
| A3 | Trifluralin | 6.28 μg/L | 20–90 μg/L | Turn-off | Photophysical quenching process | [70] | |
| A4 | Paclobutrazol | 9.3 × 10−8 M | 2 × 10−7–9 × 10−6 M | Turn-on | Photophysical change process and solubility change | [71] | |
| Nanocomposites | A5 | Organophosphorus pesticides | 0.4 μg/L (Fluorescence) 0.09 μg/L (colorimetric) | 1–50 mg/L (Fluorescence) 0.1–50 mg/L (colorimetric) | Turn-off | Specific recognition and molecular self-assembly | [72] |
| A6 | Paraoxon | 0.38 ng/mL | 0.8–60 ng/mL | Turn-off | Specific recognition and molecular self-assembly | [73] | |
| A7 | Ethion | 0.96 mM | 0–50.0 mM | Turn-on | chemical interaction | [74] | |
| A8 | Organophosphorus pesticides | 1 μg/L | 1–100 μg/L | Turn-off | Specific recognition | [75] | |
| A9 | / | / | / | / | Specific recognition | [28] | |
| Organic metal frameworks | A10 | Imidacloprid (IM), Thiamethoxam (TH) pesticides | 5.57 μg/L (IM) 0.98 μg/L (TH) | 1–20,000 μg/L | / | solubility change | [76] |
| A11 | Methyl parathion | 1.3 × 10−3 mg/L | 0.1–5 mg/L | Turn-off | Noncovalent interaction | [77] | |
| A12 | Glyphosate | 0.28 nM | 0–100 nM | Turn-off | Specific recognition and chemical interaction | [78] | |
| Supramolecular assemblies | A13 | Carbaryl | 0.007 μg/L | 0.02–2.00 μg/L | Ratiometric | Specific recognition and photophysical change process | [79] |
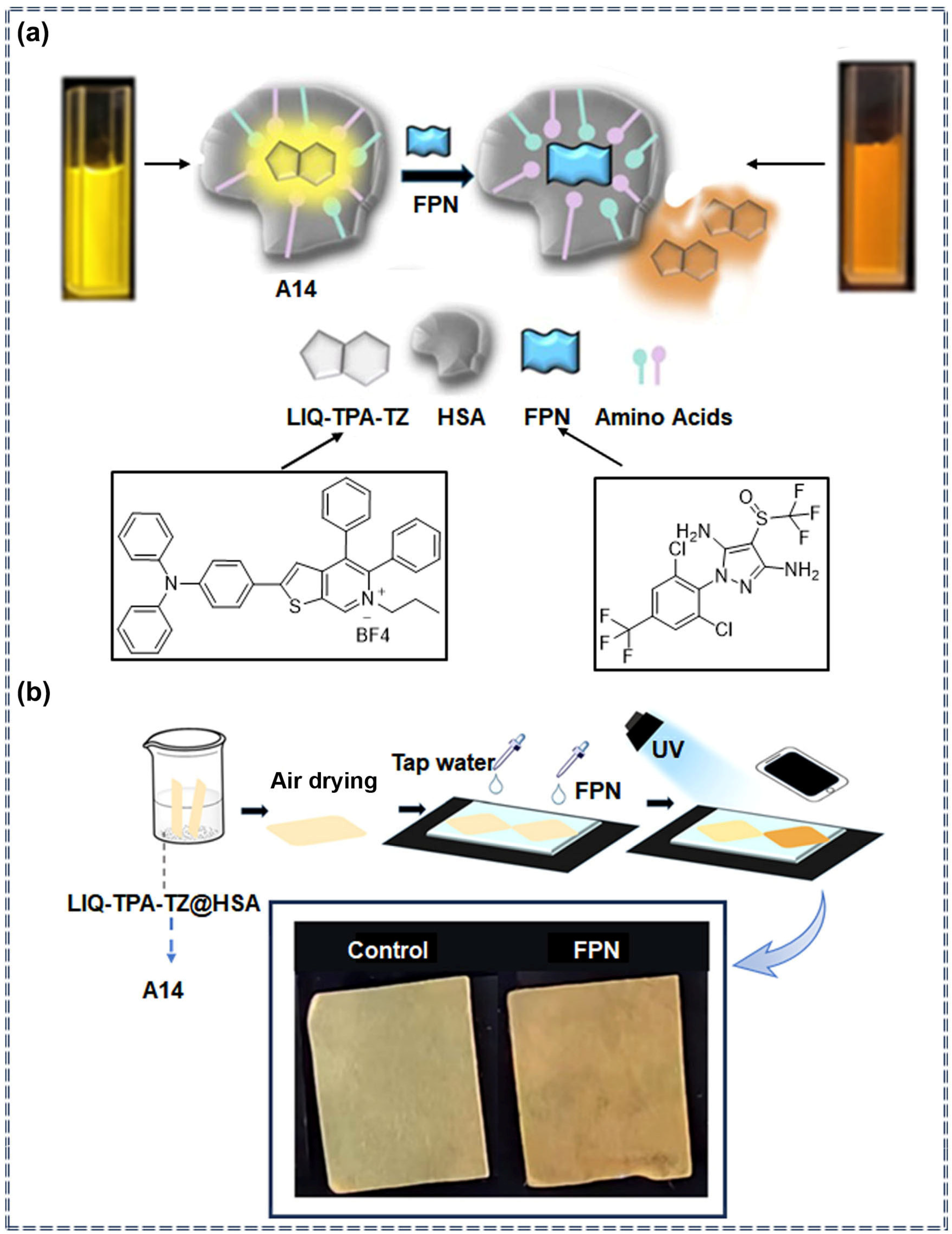
| A14 | Fipronil | 0.05 μM | 0–1 μM | Ratiometric | Specific recognition and photophysical change process | [80] | |
| A15 | Paraquat | 154.1 nM | 0–120 μM | Turn-on | Noncovalent interaction | [81] | |
| A16 | Paraoxon methyl | 501 μM | 0–45 mM | Turn-on | Specific recognition and molecular self-assembly | [82] | |
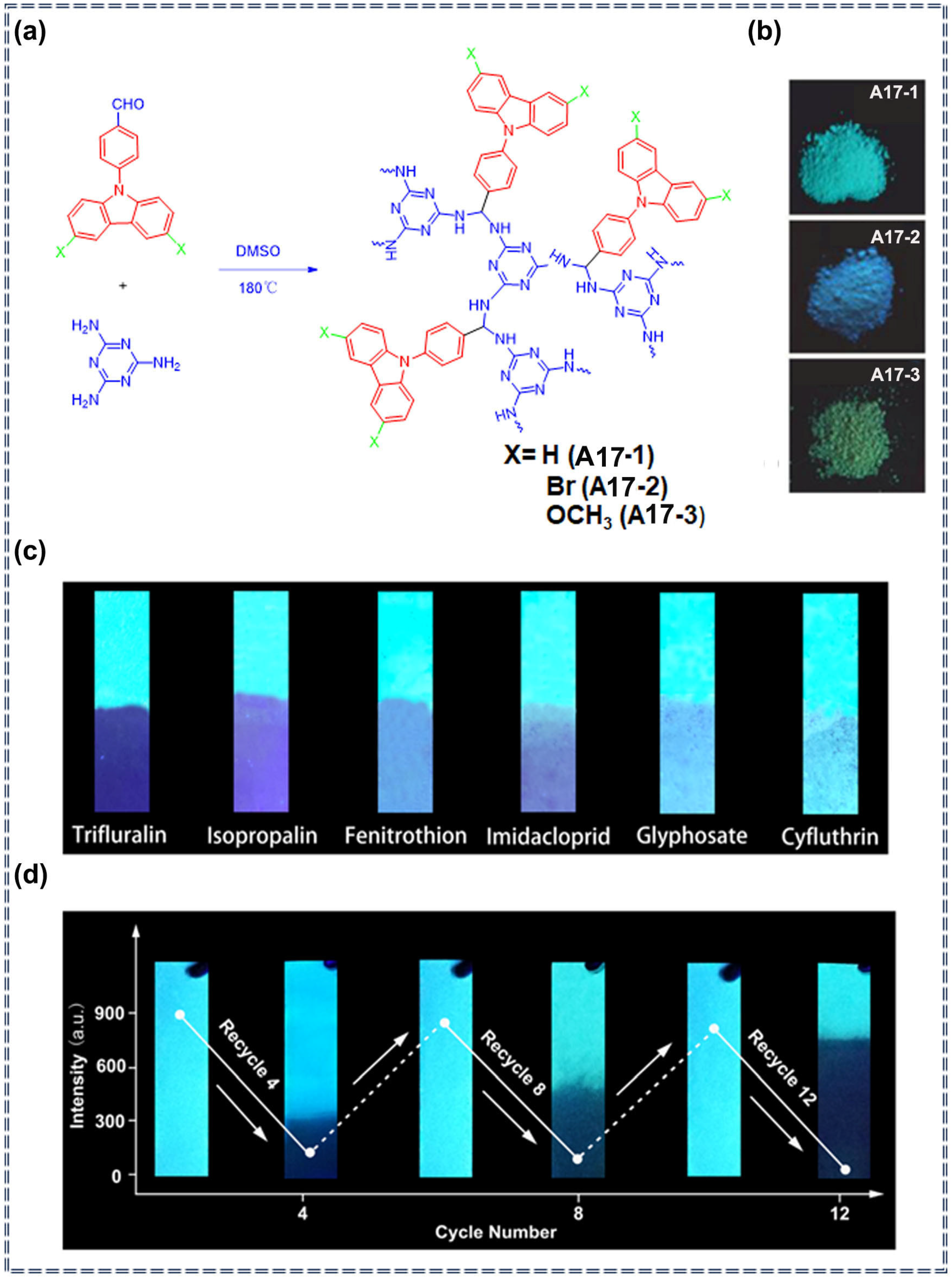
| Porous organic polymers | A17 | Trifluralin, Isopropalin, Glyphosate, Fenitrothion, Imidacloprid, Cyfluothrin | / | / | Turn-off | Photophysical quenching process | [83] |
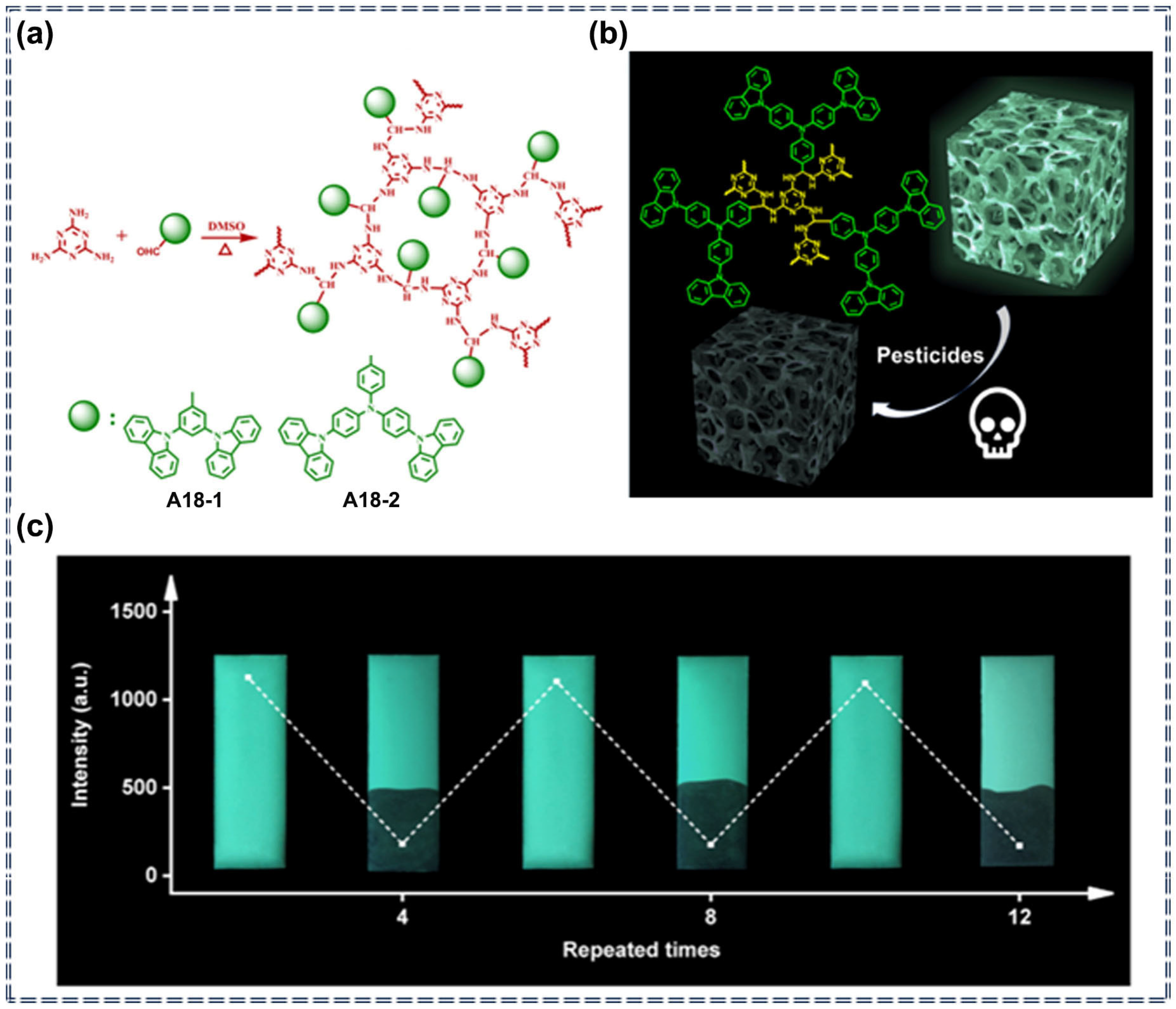
| A18 | Imidacloprid, Cyfluothrin, Triflumizole, Lambda-cyhalothrin | 30–1100 ppb (A18-1) 83–4207 ppb (A18-2) | / | Turn-off | Photophysical quenching process | [84] | |
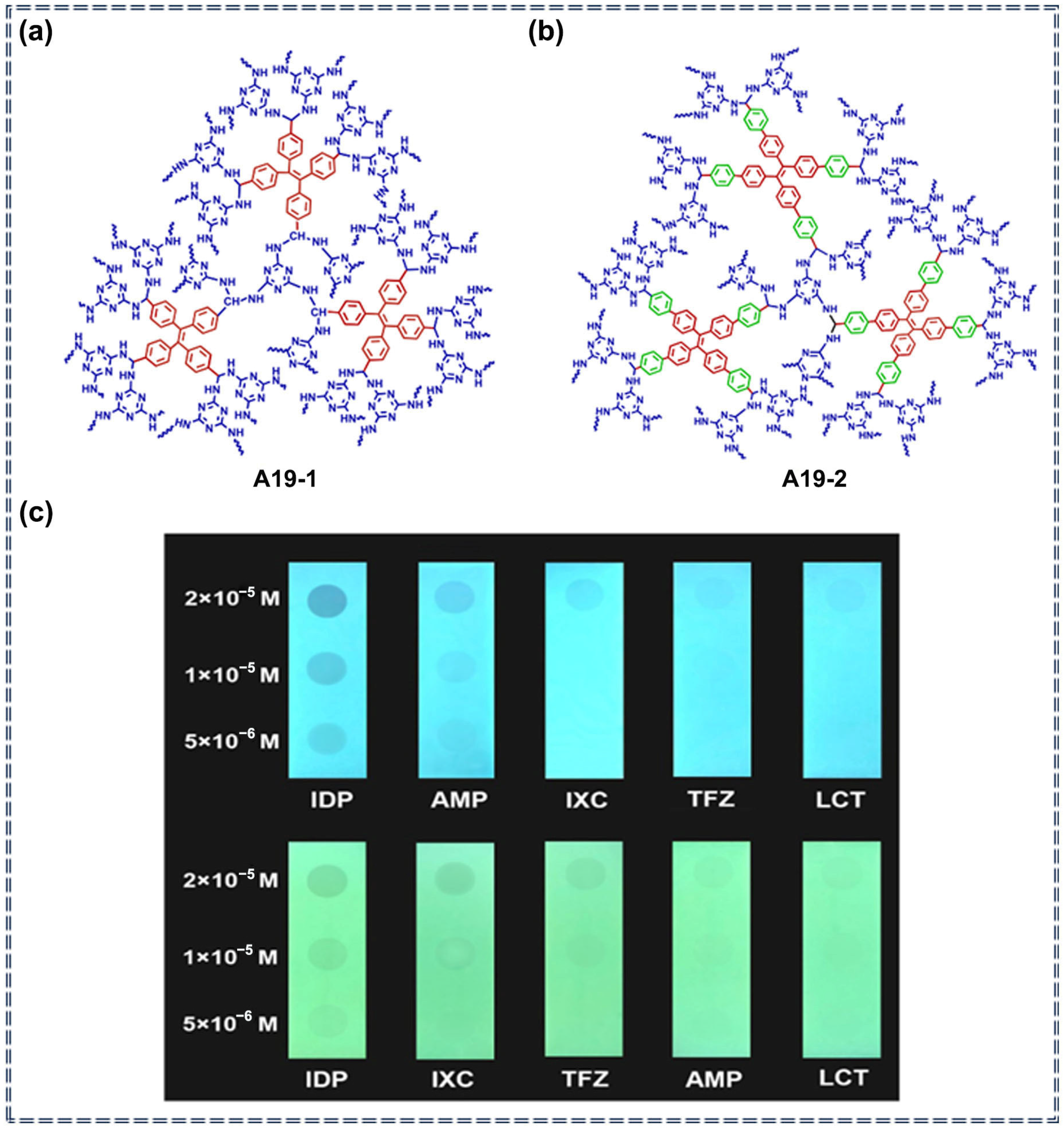
| A19 | Imidacloprid, Triflumizole, Lambda-Cyhalothrin, Acetamiprid and Indoxacarb | 28–2570 ppb (A19-1) 0.13–2.54 ppm (A19-2) | / | Turn-off | Photophysical quenching process | [85] | |
| Lateral flow immunoassays | A20 | Chlorothalonil | 1.2 pg/mL | 0–500 ng/mL | Turn-off | Specific recognition | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, B.; Li, H.; Ren, S.; Wu, J.-R.; Wang, H. Aggregation-Induced Emission (AIE) Probes in Fluorescent Sensing: Progress and Applications for Pesticide Detection. Appl. Sci. 2024, 14, 8947. https://doi.org/10.3390/app14198947
Zha B, Li H, Ren S, Wu J-R, Wang H. Aggregation-Induced Emission (AIE) Probes in Fluorescent Sensing: Progress and Applications for Pesticide Detection. Applied Sciences. 2024; 14(19):8947. https://doi.org/10.3390/app14198947
Chicago/Turabian StyleZha, Bowen, Hui Li, Susu Ren, Jia-Rui Wu, and Haitao Wang. 2024. "Aggregation-Induced Emission (AIE) Probes in Fluorescent Sensing: Progress and Applications for Pesticide Detection" Applied Sciences 14, no. 19: 8947. https://doi.org/10.3390/app14198947
APA StyleZha, B., Li, H., Ren, S., Wu, J.-R., & Wang, H. (2024). Aggregation-Induced Emission (AIE) Probes in Fluorescent Sensing: Progress and Applications for Pesticide Detection. Applied Sciences, 14(19), 8947. https://doi.org/10.3390/app14198947






