Abstract
This study explores the association of respiratory muscle strength with aerobic endurance kinetics among athletes, with a specific focus on maximal oxygen consumption (VO2max). Previous research has elucidated the complex interactions between respiratory and skeletal muscles during exercise, highlighting the critical role of efficient respiration in maximizing athletic performance. The interplay between active skeletal muscles and respiratory muscles, especially the influence of respiratory muscle fatigue on exercise capacity, is well-documented. High-intensity exercise has been shown to activate the respiratory muscle metaboreflex, which can restrict blood flow to working muscles, thereby impacting the energy required for respiration. A total of 41 athletes, drawn from the disciplines of biathlon, judo, and cross-country, participated in this study. Respiratory function tests (RFTs) were administered to assess various respiratory parameters, including changes in chest circumference. Additionally, maximal oxygen consumption (VO2max) and heart rate were measured during a treadmill test. To explore the associations between VO2max and ventilatory parameters—namely, ventilation (VE), oxygen consumption (VO2), carbon dioxide production (VCO2)—as well as respiratory metrics, linear regression analysis was employed. Based on the standardized regression coefficients (β), it was found that maximum expiratory pressure (MEP) (mean ± SD: 130.95 ± 42.82) and inspiratory diaphragmatic circumference values were significantly associated with VE, VO2, and VCO2. Conversely, the other predictor variables did not exhibit a significant effect on VE (mean ± SD: 134.80 ± 36.69), VO2 (mean ± SD: 3877.52 ± 868.47 mL), and VCO2 (mean ± SD: 4301.27 ± 1001.07 mL). Similarly, measurements of chest circumference (mean ± SD: 91.40 ± 10.72 cm), MEP, and diaphragmatic circumference during inspiration (mean ± SD: 95.20 ± 10.21 cm) were significantly associated with VO2max (mean ± SD: 58.52 ± 10.74 mL/kg/min), while the remaining predictor variables did not demonstrate a significant effect on VO2max. Additionally, a multiple linear regression analysis was conducted to examine the combined effects of respiratory muscle strength and ventilatory factors on VO2max. The model, which included interaction terms, explained 89.9% of the variance in VO2max (R2 = 0.899, adjusted R2 = 0.859). Significant interactions were found between MIP and VE (B = −0.084, p = 0.006), as well as MEP and VE (B = 0.072, p = 0.012). These findings suggest that respiratory muscle strength plays a more substantial role in determining VO2max in individuals with higher ventilatory efficiency, highlighting the importance of both respiratory strength and breathing efficiency in aerobic performance. Our findings underscore the importance of considering respiratory muscle strength in assessing and enhancing athletes’ aerobic performance. Integrating objective measurements such as maximal inspiratory and expiratory pressure assessments into routine performance evaluations allows coaches and sports scientists to monitor changes in respiratory function over time and adjust training protocols accordingly.
1. Introduction
The capacity for proper respiration, which includes supplying enough oxygen to the working muscles and removing carbon dioxide, depends on the efficiency of gas exchange during both external and internal respiration [1,2]. The gold standard for evaluating functional response through gas analysis is the cardiopulmonary exercise test (CPET), which helps identify functional and pathophysiological limitations [1]. This test is particularly important for scuba divers, pilots, soldiers, and professional athletes who experience high levels of physical stress. During physical activity, lung ventilation increases to meet the oxygen needs of the skeletal muscles and activates the accessory respiratory muscles alongside the diaphragm [3]. Respiratory muscle function depends on the strength and endurance of these muscles [4]. Some studies have shown that inspiratory muscles can become fatigued after short periods of intense exercise. This phenomenon underscores the significant physiological demands placed on the respiratory system during vigorous physical activity [5,6] and after long periods of moderate-intensity exercise [7]. Also, during these exercises, the respiratory muscles share the amount of oxygen consumed [8,9]. It has been determined that fatigue occurs in the diaphragm muscle with increased respiratory need during high-intensity exercises of 85% VO2max and above. Also, during these exercises, the respiratory muscles share the amount of oxygen consumed [10]. The findings from previous studies mentioned above underscore the shared demand for cardiac output and oxygen consumption between active skeletal muscles and respiratory muscles during exercise.
Optimizing respiration plays a pivotal role in enhancing the efficiency of working skeletal muscles by facilitating the accelerated delivery of blood to the relevant regions. This interplay highlights the interconnectedness of respiratory and skeletal muscle function in supporting overall exercise performance and underscores the importance of respiratory optimization strategies in maximizing athletic potential [11]. However, if the endurance of the respiratory muscles is not sufficient, it is predicted that exercise performance may decrease due to early fatigue of the diaphragm [11]. High-intensity exercise causes peripheral vasoconstriction [12]. In addition, it triggers the respiratory muscle metaboreflex, a high sympathetic nerve activity that limits blood flow to the working muscles and thus the energy output and consumption required for breathing [12,13].
The demanding work of the muscles used for inhalation has significant effects on the nervous system and the heart. In healthy people, voluntary resistance during inhalation that leads to muscle fatigue has been found to cause increases over time in muscle sympathetic nerve activity, heart rate, and mean arterial pressure. This is accompanied by a gradual decrease in arterial blood flow to inactive limbs [14]. Research also suggests that the reflex triggered by the fatigue of the inspiratory muscles is activated during full-body exercise. Specifically, blood flow to the legs is inversely related to the effort of breathing during high-intensity exercise, and changes in leg blood vessel resistance are directly related to the amount of noradrenaline (norepinephrine) released [15]. During prolonged intense full-body exercise, the response of the reflex associated with fatigue of the inspiratory muscles may limit exercise performance [16]. However, training the inspiratory muscles can delay the activation of the respiratory muscle reflex, potentially improving performance [12].
Our study represents a rare investigation that comprehensively evaluates both respiratory muscle parameters. The literature suggests that respiratory muscle fatigue typically does not manifest during exercise at intensities below approximately 80% of VO2max [17]. In another study, it was observed that during intense exercise (>85% of VO2max) in highly trained individuals, respiratory muscles require approximately 15–16% of VO2max and cardiac output, whereas, in untrained individuals, this proportion is ≤10% [18]. Considering these studies, our study aimed to investigate the impact of athletes’ respiratory muscle strength, as measured by maximal inspiratory and expiratory pressure, on aerobic endurance kinetics, specifically VO2max. Given the widely acknowledged importance of respiratory muscle strength in athletic performance, understanding the association between respiratory muscle strength and aerobic endurance kinetics can inform training strategies aimed at optimizing performance and minimizing fatigue, ultimately enhancing athletes’ competitive edge. It is worth mentioning that MIP/MEP are also quasi-isometric and static measures and it was suggested that dynamic assessments may be more suitable in an athletic environment [19]. Therefore, our study not only advances scientific knowledge but also has practical implications for enhancing athletic performance and improving training regimens in various sports contexts. In light of this information, we hypothesize that respiratory muscle strength will be associated with the kinetics of maximal oxygen consumption in athletes.
2. Materials and Methods
2.1. Study Design and Participant Selection
The participants, who were selected by the convenience sample method, included in the research consisted of 14 biathlon, 14 judo, and 13 cross-country skiing athletes (22 men and 19 women), who applied to the Ministry of Youth and Sports, Department of Athlete Health, Performance and Service Quality Standards in Ankara, Türkiye to become volunteer participants (Table 1). It is noteworthy that all participants in the study were of Caucasian descent. Determination of the requisite sample size for this study was conducted through the utilization of G-power Software 3.1.9.7, developed by the University of Dusseldorf, Germany, aiming for a power of 0.80 and an effect size of 0.30 [20]. This analysis indicated a minimum sample size of 38 participants for a regression analysis involving predictors. Inclusion criteria were being a non-smoker and not having a respiratory tract disease such as asthma, pulmonary tuberculosis, emphysema, or chronic bronchitis. Athletes on medication, especially those on cardiac glycoside or β-receptor antagonist-derived drugs, were not included in the study. Moreover, none of the participants had reported any history of lung disease in the past three months, nor had they undergone any prior respiratory muscle strength training. All athletes under the Ministry of Youth and Sports undergo regular health examinations to detect any illnesses. For our study, we selected athletes who had previously undergone these examinations. Importantly, all participants were athletes actively competing at the national level, demonstrating a high level of physical fitness and athletic proficiency within the study group. Additionally, no participants had recently recovered from injuries or were under significant medical treatment that could influence the study outcomes. Athletes who met the inclusion criteria were evaluated on the same day. The study was approved by the Gazi University Ethics Committee (No: 2022-941). The study was conducted according to the Declaration of Helsinki. All participants signed an informed consent form.

Table 1.
Demographic information of the research group.
2.2. Procedures
To ensure accurate results, we implemented various measures to minimize the impact of factors that could affect respiratory function and VO2max. We controlled respiratory diseases and medication use and instructed participants to maintain their normal training routines. Any deviations from their usual training schedules were reported and documented. To account for differences in dietary habits, participants were required to follow a standardized pre-test meal protocol at least 2 h before each testing session. This was aimed at reducing the influence of recent food intake on exercise performance and respiratory function. In addition, all tests in the laboratory environment were conducted while maintaining strict limits: the temperature ranged from 18 to 23 °C, and the relative humidity was kept below 70% to ensure consistency across trials. Furthermore, participants were asked to refrain from consuming caffeine or alcohol for 24 h before testing and to avoid strenuous exercise for 48 h before the testing sessions to minimize fatigue-related effects. These measures were taken to ensure that the respiratory and aerobic performance measurements accurately reflected the participants’ baseline capabilities under standardized conditions. Before starting the test period, demographic information of the athletes (age, year of sport, smoking habits, medication) was recorded first, and then Respiratory Function Tests (RFT) were performed to evaluate respiration and respiratory muscle strength. The circumference measurements of the athletes were recorded by measuring the axilla and subcostal circumferences. Then, the athletes’ VO2max values were measured on the treadmill with a CPET K5 device, and the data were recorded. Finally, respiratory function tests for athletes in each sport were conducted from 10:00 to noon, and cardiopulmonary performance tests were conducted from 16:00 to 18:00. These measurements were carried out by expert personnel, including a specialist physician and a training science specialist, and took place over three days.
Upon scrutinizing the gender distributions within the various disciplines, it was discerned that male cross-country skiing athletes exhibited divergent training ages compared to their female counterparts. Furthermore, statistical analyses of heights and body weights revealed notable differences favoring male athletes across all three disciplines (p < 0.05). Conversely, similar values were observed between male and female athletes in other assessed parameters. These findings underscore potential gender-specific disparities in training backgrounds and anthropometric characteristics within the studied athlete cohorts, highlighting the importance of considering gender-related factors in athletic performance analyses.
2.3. Data Collection Tools
2.3.1. Measurement of Chest Circumference
To assess the chest circumference accurately, two distinct methods were employed:
Axillary Circumference Measurement: This technique entailed the assessment of chest circumference at two pivotal junctures—during maximal expiration and maximal inspiration. The circumference was gauged from the level of the chest apex, encircling the axilla (or armpit), employing a rigid tape measure to ensure precision [19].
Subcostal Circumference Measurement: This approach aimed to capture variations in diaphragmatic circumference. Measurements were conducted during specific respiratory phases—mid-inspiration, maximal expiration, and maximal inspiration. The circumference was evaluated just below the ribcage, utilizing the same rigid tape measure for consistency across assessments [21].
2.3.2. Assessment of Respiratory Muscle Strength
Athletes were briefed on the procedures before the tests. Respiratory function and respiratory muscle strength were assessed utilizing a digital spirometer (Pony FX Cosmed, Italy). The evaluations were conducted with athletes seated comfortably in an upright position. Throughout the tests, participants utilized a mouthpiece and wore a nose clip. They were instructed to seal their lips tightly around the mouthpiece to prevent any air leakage from the spirometer. To familiarize athletes with the device’s operation, a few trial tests were conducted before the formal assessments. Each test was repeated thrice with a maximum rest period of three minutes between trials. The highest measurement score obtained was utilized for statistical analysis. During the maximal voluntary ventilation (MVV) test, athletes were directed to breathe deeply, rapidly, and forcefully for 12 s. Following the completion of the test, athletes were asked to briefly hold their breath to prevent respiratory alkalosis, and the MVV value was recorded. MVV: The greatest amount of air that can be inhaled and exhaled within a given period. MIP (Maximal Inspiratory Pressure): The maximum pressure generated during inhalation. MEP (Maximal Expiratory Pressure): The maximum pressure generated during exhalation. To evaluate respiratory muscle strength, MIP and MEP tests were administered. For the MIP test, athletes were instructed to fully exhale before taking a deep, rapid, and forceful inhalation. Conversely, for the MEP test, athletes were prompted to inhale fully before exhaling swiftly and forcefully. Each test was repeated thrice with rest intervals between measurement cycles. The most favorable results obtained from all tests were selected for inclusion in the subsequent analysis [22].
Additionally, the MIP and MEP tests, which provide information about respiratory muscle strength using the intraoral pressure method, are tests accepted by the European Respiratory Society (ERS) and American Thoracic Society (ATS). These tests are recognized as non-invasive methods for measuring respiratory muscle strength. During exercise, respiratory muscle activation can be assessed by intramuscular EMG or surface electrodes. However, invasive procedures should be performed under medical supervision, while action potential measurements taken using surface electrodes can be challenging to control during high-intensity exercise due to factors like electrode displacement or excessive interference. In previous studies that examined the relationship between respiratory muscle strength and exercise, the MIP/MEP method (which measures intraoral pressure) has been used [23,24].
2.3.3. Assessment of Aerobic Capacity (VO2max)
In this study, maximal oxygen uptake (VO2max) was evaluated using a portable cardiopulmonary exercise test system (Cosmed K5, Italy, Serial No: 2019030706), renowned for its precision in automatically analyzing expiratory gases. The system was calibrated with a known gas mixture (5.0% CO2 and 16.0% O2) to ensure accuracy in measurements. The VO2max test protocol started with a 2-min warm-up phase at a constant speed of 5.0 km/h on the treadmill. After this initial phase, the treadmill speed increased incrementally by 0.016 km/h every second, providing a gradual rise in intensity. This specific speed increment was selected to ensure a smooth transition between workloads while pushing the participants toward their maximal aerobic capacity. Athletes were monitored throughout the test, with adjustments continuing until they met at least three of the following termination criteria: a rating of perceived exertion (RPE) of 17 or higher on the Borg scale, verbal indication of exhaustion, no further increase in oxygen consumption despite rising workload, a respiratory quotient (RQ) of 1.15 or above, achieving 85% or more of their maximal heart rate, or a plateau in heart rate despite increasing effort. The average values recorded during the final 30 s of the test were used to calculate the participants’ maximal oxygen consumption, normalized to their body weight [25]. The measurements included minute ventilation (VE), oxygen uptake (VO2), and carbon dioxide production (VCO2), all captured in real time by the gas analysis system. To avoid bias, different researchers conducted the test administration and data analysis, ensuring that the study’s blinding technique was effectively implemented.
2.4. Statistical Procedures
Statistical analysis was performed using IBM SPSS Version 21 (IBM Corp., released 2012, Armonk, NY, USA). The normal distribution of the data was assessed visually and using the Shapiro-Wilk test. Both linear regression and multiple linear regression with interaction terms were applied to examine the relationships between VO2max and the respiratory parameters (MIP, MEP, MAC, ACI, ACE, MSC, SCI, SCE, and MVV), as well as ventilatory efficiency (VE), oxygen consumption (VO2), and carbon dioxide production (VCO2). Linear regression was used to assess the individual contributions of each respiratory parameter to VO2max. Additionally, multiple linear regression was performed with interaction terms to evaluate how respiratory muscle strength (MIP, MEP) interacts with VE, VO2, and VCO2 to predict VO2max and the model fit was assessed using R-squared (R2), adjusted R-squared, and the F-statistic. Moreover, key statistical values considered in the analysis included the β (beta) coefficient, which indicates the change in the dependent variable (VO2max) for each unit change in the independent variable, and the t-statistic, which tests whether a coefficient is significantly different from zero. A p-value of less than 0.05 was considered statistically significant. Additionally, Zero-order correlations were evaluated to determine the direct relationship between two variables without adjusting for other influences, and Partial correlations were used to assess the strength of the relationship between two variables while controlling for other variables. The significance of the coefficients was tested using t-values and p-values, with statistical significance set at p < 0.05.
3. Results
The statistical analyses of the VO2max, VE, VO2, and VCO2 parameters are presented in tables below.
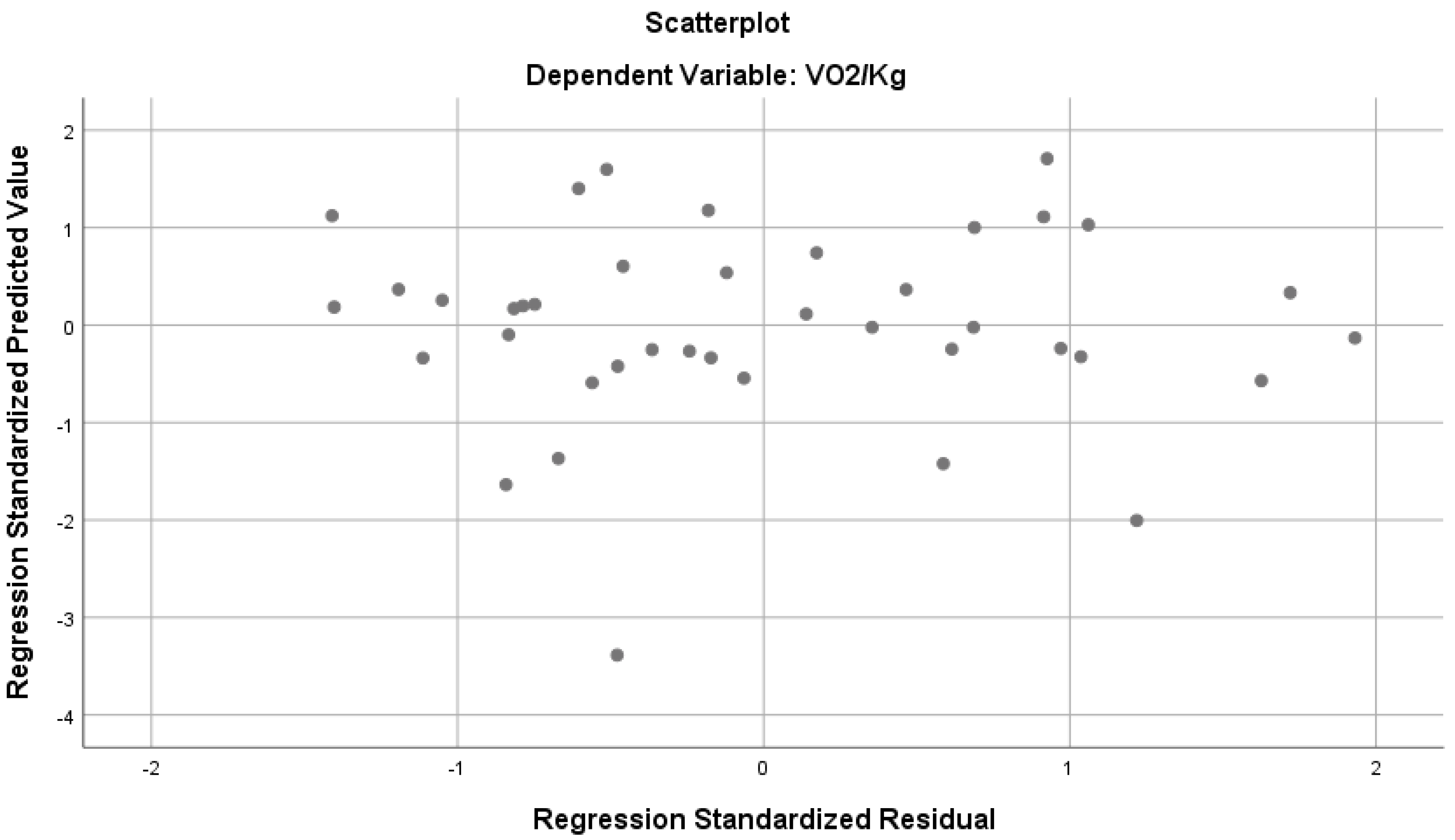
According to Table 2, the parameters indicate a strong and significant relationship with VO2max/kg (R = 0, 715, R2 = 0.511, p < 0.05). The above-mentioned variables together explain 51% of the total variance in VO2max/kg (Figure 1). According to the standardized regression coefficient (β), mid-axilla circumference, MEP and inspiratory subcostal circumference values have a significant effect on VO2max, while the other predictor variables do not seem to have a significant effect on VO2max. When examining the bilateral and partial correlations between the predictor variables and VO2max, we found a negative correlation between the MIP, mid-axilla, and axilla circumference measurements during inspiration and expiration, and the mid-subcostal and subcostal circumference measurements during inspiration and expiration. Also, a positive relationship was determined between MEP and MVV values (Figure 1)

Table 2.
Regression analysis results regarding VO2max/kg (Mean ± S.D. = 58.52 ± 10.74 mL/kg/min) prediction of the parameters.
Figure 1.
Regression analysis results predicting VO2max/kg from various parameters.
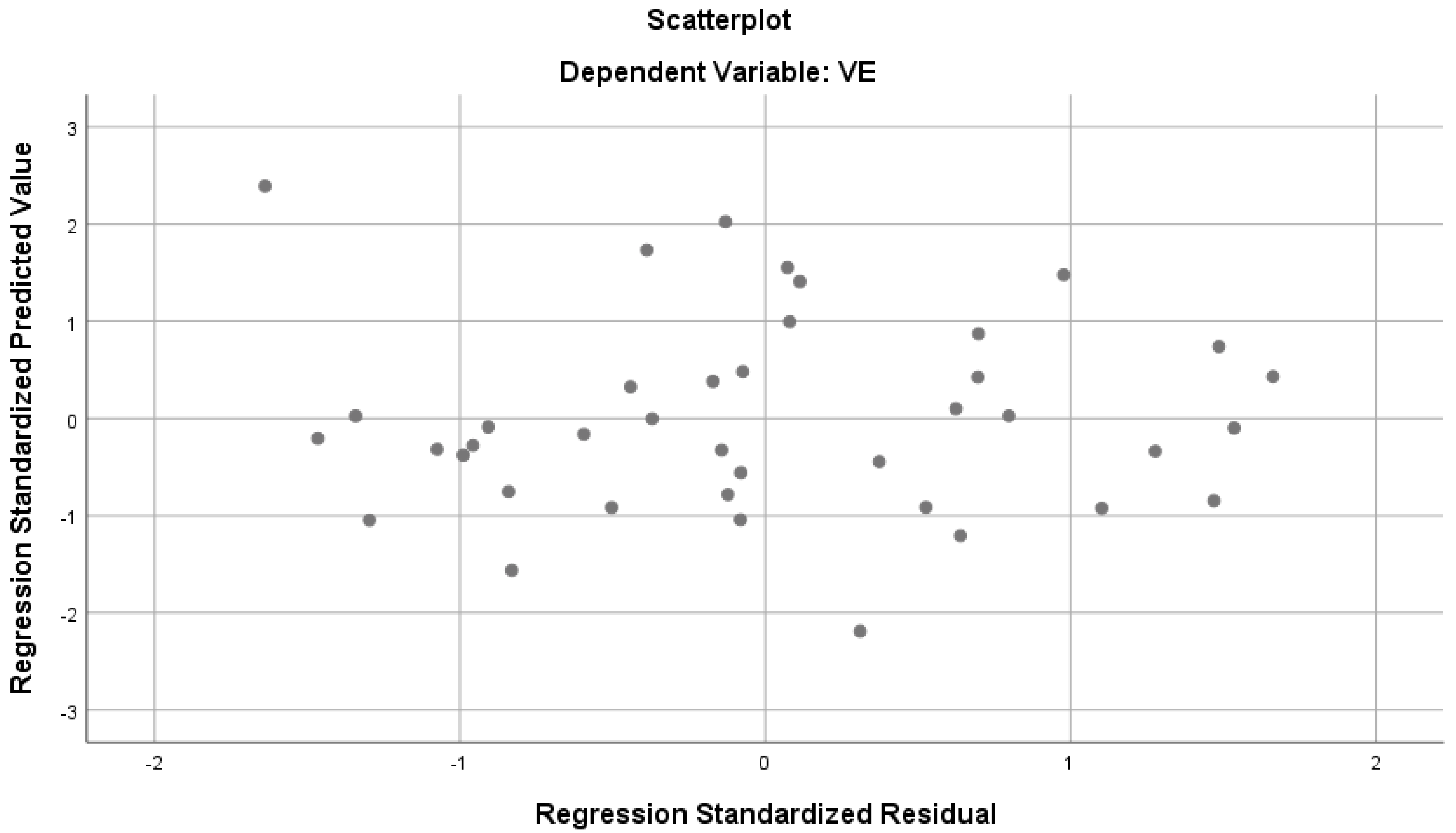
According to Table 3, the parameters show a strong and significant relationship with VE (R = 0.760, R2 = 0.577, p < 0.05). The mentioned variables together explain 57% of the total variance in VE (Figure 2). According to the standardized regression coefficient (β), the values of MEP and inspiratory subcostal circumference measurement have a significant effect on VE, while the other predictor variables do not seem to have a significant effect on VE. When the bilateral and partial correlations between the predictor variables and VE were examined, low-level correlations were found between MIP and VE, and moderate and positive correlations with other parameters.

Table 3.
Regression analysis results regarding VE (Mean ± S.D. = 134.80 ± 26.69 mL) prediction of the parameters.
Figure 2.
Regression analysis results predicting VE from various parameters.
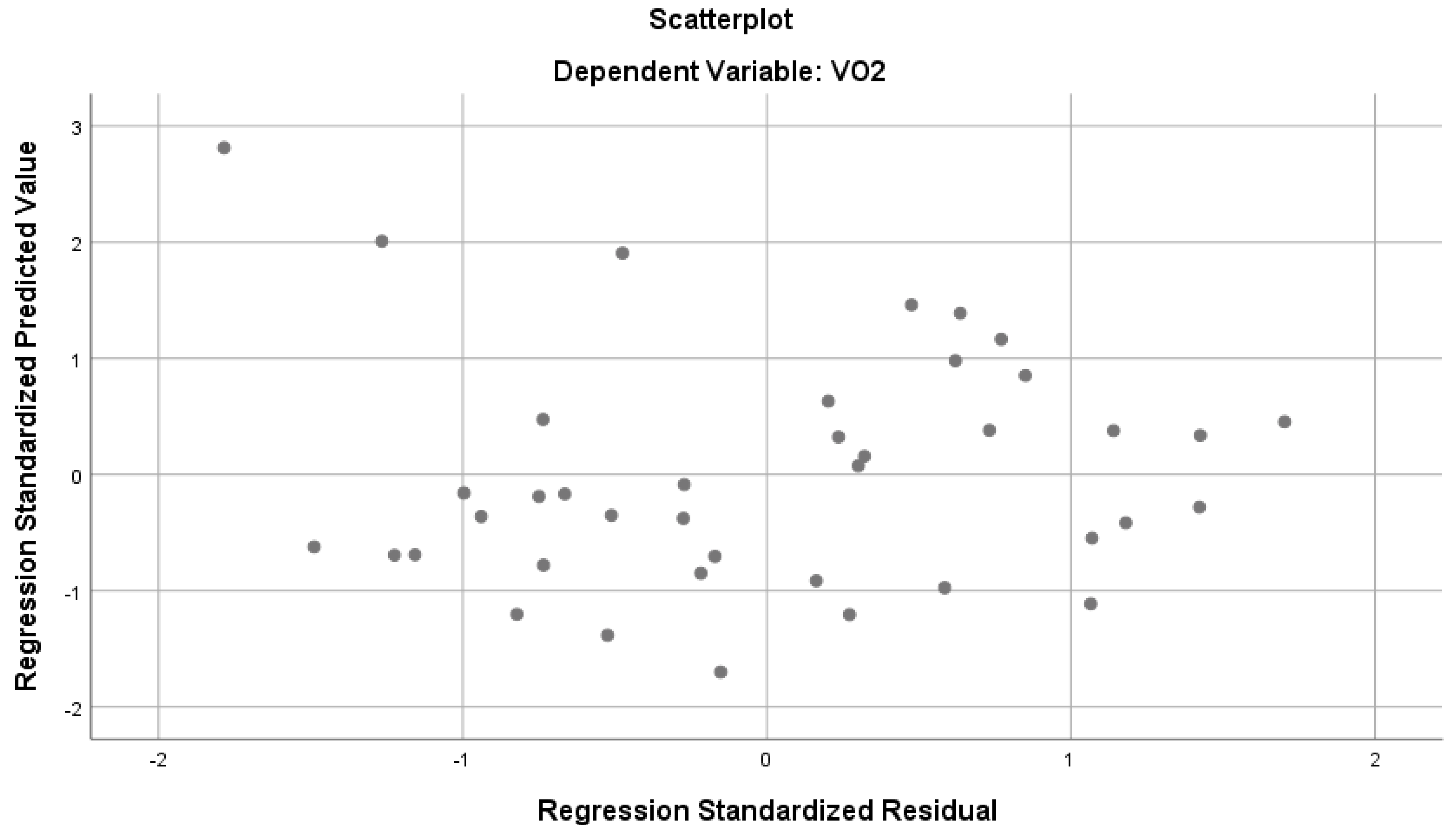
Table 4 demonstrates that the parameters have a strong and significant relationship with VO2 (R = 0.814, R2 = 0.663, p < 0.05). The mentioned variables together explain 66% of the total variance in VO2 (Figure 3).According to the standardized regression coefficient (β), the values of MEP and subcostal circumference on inspiration have a significant effect on VO2, while the other predictor variables do not seem to have a significant effect on VO2. When the bilateral and partial correlations between predictor variables and VO2 were examined, low-level correlations were found between MIP and VO2, and moderate and positive correlations with other parameters.

Table 4.
Regression analysis results regarding VO2 (Mean ± S.D. = 3877.52 ± 868.47 mL) prediction of the parameters.
Figure 3.
Regression analysis results predicting VO2 from various parameters.
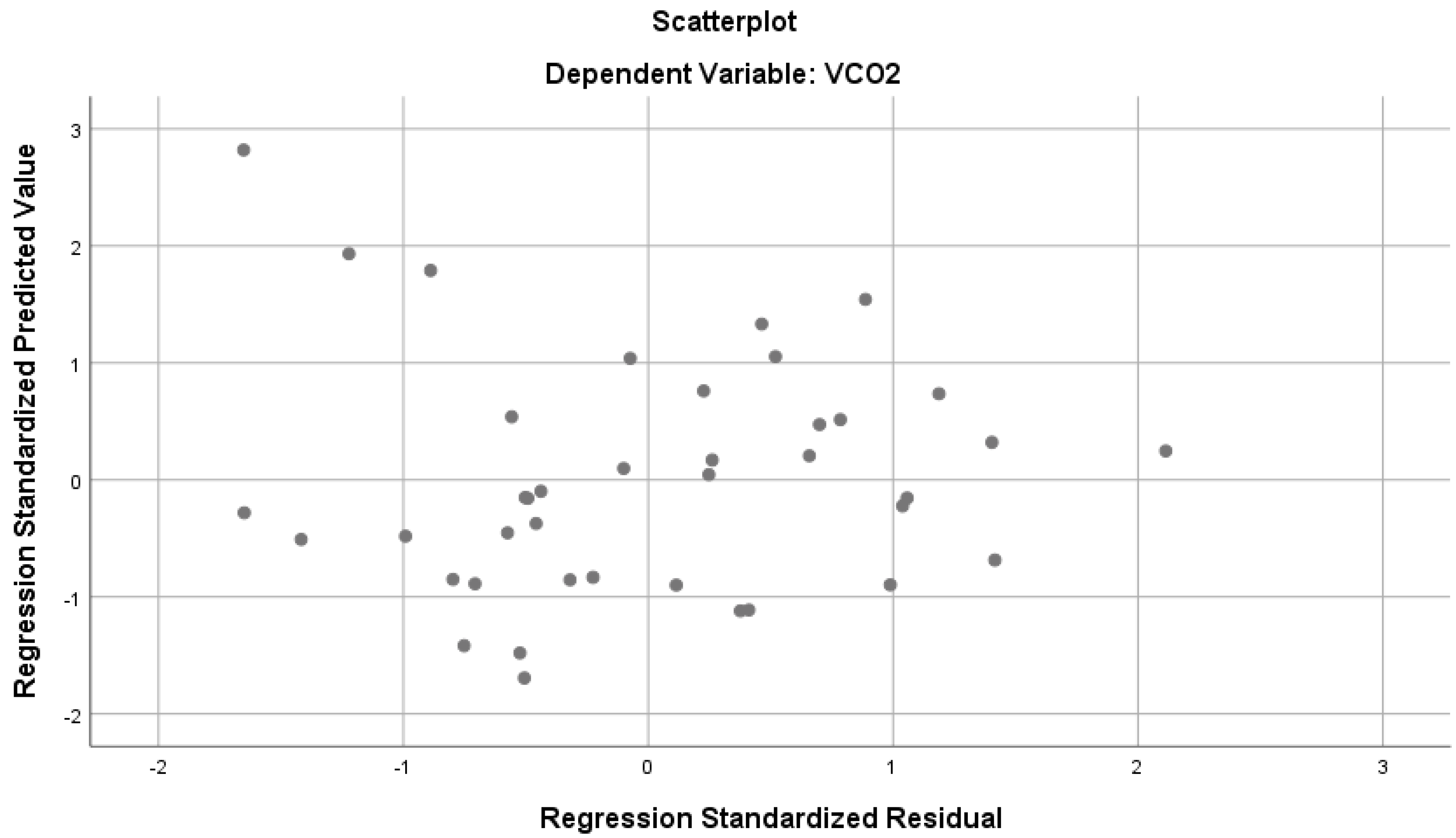
In Table 5, there is a strong and significant relationship with VCO2 (R = 0.802, R2 = 0.643, p < 0.05). These variables combined account for 64% of the total variance in VCO2 (Figure 4). According to the standardized regression coefficient (β), the values of MEP and inspiratory subcostal circumference measurement have a significant effect on VCO2, while other predictor variables do not seem to have a significant effect on VCO2. When the bilateral and partial correlations between the predictor variables and VCO2 were examined, low-level correlations were found between MEP and VCO2, and moderate and positive correlations with other parameters.

Table 5.
Regression analysis results regarding VCO2 (Mean ± S.D. = 4301.27 ± 0.1001 mL) prediction of the parameters.
Figure 4.
Regression analysis results predicting VCO2 from various parameters.
In Table 6, a multiple linear regression analysis with interaction terms was conducted to explore the relationship between VO2max (VO2/kg) and respiratory muscle strength (MIP, MEP), along with physiological factors (VE, VO2, and VCO2). The model was statistically significant and explained approximately 89.9% of the variance in VO2max (R2 = 0.899, adjusted R2 = 0.859, F(11, 28) = 22.60, p < 0.001). The interaction between MIP and VE was found to be significant (β = −0.084, p = 0.006), indicating that the effect of inspiratory muscle strength on VO2max is moderated by ventilatory efficiency. Specifically, as VE increases, the impact of MIP on VO2max becomes more pronounced. Additionally, the interaction between MEP and VE was also significant (B = 0.072, p = 0.012), suggesting that expiratory muscle strength interacts positively with VE to influence VO2max. However, other interaction terms, such as MIP with VO2 (B = 0.002, p = 0.315) and MIP with VCO2 (B = 0.001, p = 0.608), were not statistically significant. Similarly, no significant interactions were found between MEP with VO2 or VCO2. This suggests that while respiratory muscle strength interacts with ventilatory efficiency, it does not interact significantly with oxygen consumption or carbon dioxide production to affect VO2max.

Table 6.
Multiple Linear Regression analysis results (with interaction terms) for VO2max prediction.
4. Discussion
This study aimed to investigate the relationships between athletes’ respiratory muscle strength, assessed via MIP and maximal expiratory pressure MEP, and aerobic endurance, as characterized by maximal oxygen consumption kinetics. It is noteworthy that approximately 16% of oxygen intake during exercise is utilized by the respiratory muscles. During maximal exercise (85% and above of maximum heart rate), approximately 14–16% of cardiac output is directed to the respiratory muscles [15]. Considering this aspect, the impact of respiratory muscles on performance is significant and cannot be ignored [26]. Our study found a negative correlation between VO2max and measurements of the circumference at the mid-axilla and axilla during both inspiration and expiration, as well as at the mid-subcostal and subcostal regions during inspiration and expiration.
In our study, we observed positive correlations between MEP and MVV, suggesting an augmented ventilatory response during exercise, where greater MEP and MVV capacity result in increased respiratory frequency. There are studies indicating an increase in maximum minute ventilation due to CO2 elevation during exercise [27,28]. This, in turn, facilitates the swift elimination of carbon dioxide and enhanced oxygen uptake during physical exertion.
Furthermore, Dempsey et al. (2008) found that the respiratory muscle load during maximal exercise has a significant impact on tidal volume and CO in healthy trained individuals [29]. Another study conducted by Johnson et al. (2007) reported improvements in cycling performance following specific inspiratory muscle training, accompanied by an increase in anaerobic work capacity [30]. Additionally, Vasiccova et al. (2017) reported that improving inspiratory muscle strength through respiratory muscle training led to an enhancement in swimmers’ maximum underwater swimming distance [31]. Lastly, differences in respiratory muscles between sports disciplines may result from variations in anaerobic or aerobic demands. The diverse nature of the group was crucial for enhancing the study’s quality and diversity. In the literature, Klusiewicz (2014) could not find a correlation between the MIP value and absolute or relative VO2max values in male athletes but did find a correlation between the MIP value and absolute or relative VO2max values in female athletes. In our study, no significant relationship could be found between relative VO2max and MIP measurement results, which suggests that the athletes in our study reached their VO2max value before reaching their maximum inspiratory muscle strength potential [32].
Previous studies in the literature have mainly focused on explaining the impact of respiratory muscle strengthening training on VO2max. For instance, Lomax et al. (2011) found that a 4-week program of inspiratory muscle training enhanced the performance of the Yo-Yo test for two groups of 12 male football players competing in the national league. The training involved one set of 30 breaths twice daily at 50% of maximal inspiratory mouth pressure (PImax) and a pre-training inspiratory muscle warm-up of two sets of 30 breaths at 40% of PImax. This improvement was reported to be significant when compared to a control group [33]. In another study, Volianitis et al. (2001) reported a study they conducted on 14 female competitive rowers at the commencement of rowing which found that the athletes’ VO2max value after respiratory muscle warm-up exercise performed with branch-specific general warm-up was higher than that of the experimental group [34]. In contrast, Amonette and Dupler (2002) reported in a study conducted on 12 male competitive triathletes and marathon runners (N = 8 research, N = 4 control group) that breathing exercises (MIP 15%, performed twice a day for a maximum of 30 breaths for 4 weeks) did not cause an improvement in VO2max capacity [35]. In another study conducted on young football players, it was reported that there was no significant change in VO2max values after a four-week respiratory muscle training program [36]. Likewise, Romer et al. (2002) conducted a study on 16 trained male cyclists (age = 29 ± 3.3 research group, age = 30 ± 2.6 control group), and they showed that inspiratory muscle training (6 weeks) caused no significant change in the VO2max values of the subjects after respiratory muscle training. However, there is evidence that maximal increasing exercise attenuates the perceptual response to exercise, and there is evidence of improved performance in competitive cyclists after inspiratory muscle training [9].
During high-intensity endurance exercise, there is increased production of metabolic CO2. This excess CO2 needs to be removed by increasing ventilation. If the respiratory muscles are not strong or enduring enough to meet the increased demand for ventilation, the levels of CO2 in the tissues and blood will rise, leading to metabolic acidosis. This can cause both skeletal and respiratory muscles to fail. Therefore, expiratory muscle strength is important in this context [36]. We found a moderate positive correlation between MEP and VCO2 in our study, demonstrating the significance of expiratory muscle strength. Our study also revealed that subcostal circumference measurements in MEP during inspiration significantly affected VE. In line with these findings, it is predicted that increased development of respiratory muscles will result in higher air intake into the lungs per minute, leading to improved sports performance [37].
Efficient utilization of the respiratory muscles plays a critical role in sustaining prolonged aerobic activities. Enhanced respiratory muscle efficiency is commonly associated with improved exercise performance and overall endurance capacity [38]. Numerous studies within the literature have explored the efficacy of respiratory muscle training (RMT) as a means to augment exercise performance and optimize athletic outcomes, indicating its potential value in both elite and recreational athletes. For instance, Illi et al. (2012) conducted a comprehensive systematic review and meta-analysis, demonstrating the significant benefits of RMT in improving exercise performance among healthy individuals [39]. Building upon this foundation, Kowalski et al. (2024) explored the practical applications of RMT specifically within the context of endurance sports, shedding light on its potential implications for athletes [40]. Additionally, Haj Ghanbari et al. (2013) conducted meta-analyses, providing compelling evidence for the efficacy of RMT in enhancing athletic performance across various disciplines [41]. Another study conducted by Shei (2018) contributed to the body of knowledge by reviewing the ergogenic effects of RMT in healthy individuals, further substantiating its potential benefits in exercise settings [42]. Likewise, Lazovic et al. (2015) investigated respiratory adaptations in athletes across different sports disciplines, highlighting the versatility and applicability of RMT strategies [43]. Lastly, Hackett (2020) conducted a study focusing on specific respiratory muscle adaptations in males engaged in both endurance and strength training, providing valuable insights into the physiological mechanisms underlying RMT benefits [44]. The studies mentioned above emphasize that RMT is an effective strategy for improving exercise performance and athletic outcomes in diverse populations and settings. Endurance training is thought to delay the onset of respiratory muscle fatigue and improve the efficiency of oxygen utilization. As respiratory muscle development progresses, it is expected to increase air intake volume, leading to a positive impact on sports performance [3]. Additionally, enhancing the strength of respiratory muscles not only enhances performance but also helps in preventing injuries. For instance, previous studies demonstrate significant improvements in respiratory muscle strength and functional outcomes, indicating potential benefits for injury prevention and recovery [45]. Increased respiratory muscle strength can assist athletes in maintaining proper breathing mechanics during intense exercise, reducing the risk of fatigue-related injuries and respiratory distress [46].
Furthermore, research has found inconsistencies in the effects of inspiratory muscle training on athletic performance. Some studies show significant benefits, while others do not demonstrate meaningful improvements. These discrepancies may be due to the use of standard inspiratory muscle training protocols without considering individual differences [47]. Additionally, RMT is associated with extra stress and training load. Therefore, such training methods should not be unconditionally recommended without considering the broader context [48].
Lastly, our study found a negative correlation between VO2max and chest circumference measurements at rest, during inspiration, and expiration, as well as diaphragm circumference measurements at rest, during inspiration, and expiration. In contrast, positive correlations were observed between MEP and MVV values. In a resting state, the diaphragm or external intercostal muscles alone are sufficient for adequate respiration. However, during intense exercise, the effectiveness of the diaphragm decreases, and accessory muscles such as the scalene muscles, pectoralis minor, and sternocleidomastoid muscles gradually come into play to assist with inspiration [49]. During calm breathing, expiration is passive because the chest wall and lungs tend to return to their original state due to their elastic properties. During exercise, the internal intercostal muscles become active, responsible for forceful expiration. The contraction of these expiratory muscles, which reduces intrathoracic volume, increases lung compression [50]. Hence, this suggests that when exercising, the respiratory system is more active. This may be due to stronger expiratory muscles and a higher maximum voluntary ventilation, leading to a faster breathing rate. This helps in quicker removal of carbon dioxide and faster absorption of oxygen.
It is well-documented that there are two distinct phases of respiratory increase when physical activity begins. Initially, there is a sudden and noticeable rise in respiration, followed by a more gradual increase in both the depth and frequency of breathing. During the onset of exercise, even before chemical signals are released, the cerebral motor cortex becomes more active, sending neural signals to the respiratory center. This neural activation is responsible for the initial spike in respiration. The second phase of respiratory increase is driven by physiological changes in the arterial blood, such as temperature elevation and alterations in chemical composition. As exercise duration progresses, factors such as increased temperature, CO2, and hydrogen ion (H+) concentrations stimulate greater oxygen consumption in the muscles and an expansion of the arteriovenous oxygen difference (a-v O2 difference). This, in turn, activates chemoreceptors and the respiratory center, leading to enhanced respiratory frequency and depth [51].
During intense exercise, tidal volume can increase by up to 50% of vital capacity. However, as maximal workload is approached, tidal volume begins to decline, and the increase in minute VE becomes dependent on an elevated breathing frequency. Individuals naturally adjust both the depth and frequency of respiration to maintain optimal ventilatory efficiency. Well-trained athletes can utilize up to 95% of their MVV during exercise, whereas untrained individuals typically reach only 60–70% of their MVV. These interconnected physiological responses underscore the critical role of respiratory muscles during maximal exertion. Following prolonged exercise, fatigue may develop in the diaphragm and other respiratory muscles, further emphasizing their importance in sustaining performance [52].
Despite the valuable insights gained from this study, several limitations should be acknowledged. First, the uneven distribution of participants across the sports groups in the study may limit the generalizability of the findings. However, the impact of this on the analysis results has been carefully considered, and the findings have been interpreted with these limitations in mind. Future research with a more homogeneous sample within each sport can support these findings and enhance generalizability. Second, all participants in this study were of Caucasian descent. While this was a homogeneous sample that allowed for controlled comparisons within this group, it limits the external validity of the findings. Research has shown that physiological factors, including respiratory function and oxygen consumption, can vary across ethnicities. As a result, caution should be exercised when applying these findings to athletes of different ethnic backgrounds, and future studies should aim to include more ethnically diverse populations to determine whether similar associations hold across various groups. Third, although the measurement techniques used—such as respiratory function tests, maximal oxygen consumption (VO2max), and diaphragmatic circumference assessments—are widely accepted and validated in general athletic populations, the specific validation of these methods for athletes in sports such as biathlon, judo, and cross-country skiing was not conducted. It is important to ensure that the methods used are reliable and valid for the specific populations being studied, as variations in sport-specific respiratory and aerobic demands could impact the accuracy of the measurements. Moreover, considering the potential interactions observed in our study, key outcomes such as MIP, MEP, and various physiological measurements—including VO2max, VE, and VCO2 are interrelated. These associations are influenced by factors such as training level, age, gender, and exercise intensity. A more detailed examination of these complex interactions may yield a deeper understanding of the mechanisms underlying respiratory and metabolic performance. The way these variables interact has significant implications for the development of personalized training programs aimed at optimizing athletic performance.
To address the lack of specific recommendations for coaches and athletes, the study’s findings suggest several practical applications. Coaches could focus on improving the efficiency of respiratory mechanics, rather than solely aiming for increased chest expansion, by incorporating breathing exercises that target both inspiratory and expiratory muscles, such as diaphragmatic breathing and controlled exhalation. Athletes should consider integrating RMT into their routine to enhance MEP, which was positively correlated with VO2max and VE. Additionally, individualized assessments of respiratory patterns and chest mobility could help tailor training programs to optimize oxygen uptake and endurance, especially in athletes engaged in high-intensity or endurance sports. Finally, environmental factors such as altitude and temperature should be considered during training, as they might influence the relationship between respiratory parameters and performance.
Coaches can enhance athletes’ respiratory muscle strength by integrating targeted RMT into their programs. For endurance sports, focus on exercises that improve both inspiratory and expiratory muscle strength, while strength and power sports may benefit from shorter, intense breathing exercises aimed at boosting expiratory strength. Tailor RMT to the athlete’s competition level, using moderate resistance for beginners and advanced resistance for elite athletes. Individual assessments of lung capacity and respiratory patterns should guide adjustments to training programs. Additionally, adapt training to environmental factors like altitude and temperature to optimize respiratory performance.
5. Conclusions
Our investigation elucidates the substantial association with respiratory muscle strength, notably was measured through maximal inspiratory pressure and MEP, on various physiological parameters related to aerobic endurance among athletes. Our results show significant associations between MEP and inspiratory diaphragmatic circumference values with VE, VO2, and VCO2. Moreover, the measured values of diaphragmatic circumference during inspiration and MEP were significantly associated with VO2max. These findings underscore the pivotal role of respiratory muscle strength in shaping athletes’ aerobic fitness levels. Incorporating specific respiratory muscle training into athletes’ conditioning regimens can improve their respiratory muscle strength, thereby enhancing their overall aerobic capacity and endurance. Such insights provide valuable implications for tailored training strategies aimed at optimizing athletes’ respiratory function and overall endurance capacity.
Author Contributions
G.D. and B.K. conceived this study. V.O.Ç., H.İ.C., D.I.A. and M.R.-I. wrote the methodology. G.D., H.İ.C., V.S. and M.R.-I. undertook the statistical analysis. G.D., B.K., V.O.Ç., H.İ.C., M.R.-I., D.I.A. and V.S. developed the manuscript. G.D., B.K., V.O.Ç., H.İ.C., M.R.-I., D.I.A. and V.S. critically revised the manuscript and approved the final manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Gazi University Ethics Committee (Date: 21 June 2022; No: 2022-941). Informed consent was obtained from all participants.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns and restrictions on sensitive information.
Acknowledgments
The authors are grateful to all the participants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Iterson, E.H.; Cho, L.; Tonelli, A.; Finet, J.E.; Laffin, L.J. All-cause mortality predicted by peak oxygen uptake differs depending on spirometry pattern in patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2021, 8, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- KKasiak, P.; Kowalski, T.; Rębiś, K.; Klusiewicz, A.; Ładyga, M.; Sadowska, D.; Wilk, A.; Wiecha, S.; Barylski, M.; Poliwczak, A.R.; et al. Is the Ventilatory Efficiency in Endurance Athletes Different?—Findings from the NOODLE Study. J. Clin. Med. 2024, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, P.; Road, J.; Altalag, A.; Altalag, A.; Road, J.; Wilcox, P. Exercise Testing. In Pulmonary Function Tests in Clinical Practice; Springer: London, UK, 2009; pp. 157–216. [Google Scholar]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef] [PubMed]
- Degens, H.; Stasiulis, A.; Skurvydas, A.; Statkeviciene, B.; Venckunas, T. Physiological comparison between non-athletes, endurance, power and team athletes. Eur. J. Appl. Physiol. 2019, 119, 1377–1386. [Google Scholar] [CrossRef]
- Volianitis, S.; Mcconnell, A.K.; Koutedakis, Y.; Mcnaughton, L.; Backx, K.; Jones, D.A. Inspiratory muscle training improves rowing performance. Med. Sci. Sports Exerc. 2001, 33, 803–809. [Google Scholar] [CrossRef]
- Boussana, A.; Galy, O.; Le Gallais, D.; Hue, O. The effect of an Olympic distance triathlon on the respiratory muscle strength and endurance in triathletes. J. Exerc. Rehabil. 2020, 16, 356–362. [Google Scholar] [CrossRef]
- Mador, M.J.; Acevedo, F.A. Effect of respiratory muscle fatigue on subsequent exercise performance. J. Appl. Physiol. 1991, 70, 2059–2065. [Google Scholar] [CrossRef]
- Romer, L.M.; McConnell, A.K.; Jones, D.A. Effects of inspiratory muscle training on timetrial perfor-mance in trained cyclists. J. Sports Sci. 2002, 20, 547–562. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Amann, M.; Romer, L.M.; Mıller, J.D. Respiratory System Determinants Of Peripheral Fa-tigue And Endurance Performance. Med. Sci. İn Sports Exerc. 2008, 40, 457–461. [Google Scholar] [CrossRef]
- Babcock, M.A.; Pegelow, D.F.; Harms, C.A.; Dempsey, J.A. Effects of Respiratory Muscle Unloading on Exercise-induced Diaphragm Fatigue. Cardiopulm. Phys. Ther. J. 2002, 13, 32. [Google Scholar] [CrossRef]
- Harms, C.A. Effect of skeletal muscle demand on cardiovascular function. Med. Sci. Sports Exerc. 2000, 32, 94. [Google Scholar] [CrossRef]
- Romer, L.M.; Lovering, A.T.; Haverkamp, H.C.; Pegelow, D.F.; Dempsey, J.A. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J. Physiol. 2006, 571, 425–439. [Google Scholar] [CrossRef]
- Sheel, A.W.; Derchak, P.A.; Morgan, B.J.; Pegelow, D.F.; Jacques, A.J.; Dempsey, J.A. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J. Physiol. 2001, 537, 277–289. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Archiza, B.; Ramsook, A.H.; Mitchell, R.A.; Peters, C.M.; Molgat-Seon, Y.; Henderson, W.R.; Koehle, M.S.; Boushel, R.; Sheel, A.W. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp. Physiol. 2017, 102, 1535–1547. [Google Scholar] [CrossRef]
- Welch, J.F.; Archiza, B.; Guenette, J.A.; West, C.R.; Sheel, A.W. Effect of diaphragm fatigue on subsequent exercise tolerance in healthy men and women. J. Appl. Physiol. 2018, 125, 1987–1996. [Google Scholar] [CrossRef]
- Wetter, T.J.; Harms, C.A.; Nelson, W.B.; Pegelow, D.F.; Dempsey, J.A. Influence of respiratory muscle work on VO2 and leg blood flow during submaximal exercise. J. Appl. Physiol. 1999, 87, 643–651. [Google Scholar] [CrossRef]
- Harms, C.A.; Wetter, T.J.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Hanson, P.; Dempsey, J.A. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J. Appl. Physiol. 1998, 85, 609–618. [Google Scholar] [CrossRef]
- Kowalski, T.; Klusiewicz, A. Powerbreathe S-Index test-guidelines and recommendations for practioners. Biomed. Hum. Kinet. 2023, 15, 225–228. [Google Scholar] [CrossRef]
- Smith, K.; Cook, D.; Guyatt, G.H.; Madhavan, J.; Oxman, A.D. Respiratory Muscle Training in Chronic Airflow Limitation: A Meta-Analysis1-3. Am. Rev. Respir. Dis. 1992, 145, 533–539. [Google Scholar] [CrossRef]
- Mondal, P.B.; Mridha, S. A study on selected anthropometric characteristics of heightweight matched female athletes and non-athletes. J. Sports Phys. Educ. 2015, 2, 41–45. [Google Scholar]
- Steier, J.; Kaul, S.; Seymour, J.; Jolley, C.; Rafferty, G.; Man, W.; Moxham, J. The value of multiple tests of respiratory muscle strength. Thorax 2007, 62, 975–980. [Google Scholar] [CrossRef]
- Martinez-Navarro, I.; Collado, E.; Hernando, B.; Hernando, C. Pulmonary and inspiratory muscle function response to a mountain ultramarathon. J. Sports Sci. Med. 2021, 20, 706. [Google Scholar] [CrossRef]
- Sinderby, C.A.; Beck, J.C.; Lindström, L.H.; Grassino, A.E. Enhancement of signal quality in esophageal recordings of diaphragm EMG. J. Appl. Physiol. 1997, 82, 1370–1377. [Google Scholar] [CrossRef]
- Onal, E.; Lopata, M.; Ginzburg, A.S.; O’Connor, T.D. Diaphragmatic EMG and transdiaphragmatic pressure measurements with a single catheter. Am. Rev. Respir. Dis. 1981, 124, 563–565. [Google Scholar]
- Powers, S.K.; Howley, E.T. Exercise Physiology: Theory and Application to Fitness and Performances. Med. Sci. Sports Exerc. 1995, 27, 466. [Google Scholar] [CrossRef]
- Janssens, L.; Brumagne, S.; McConnell, A.K.; Raymaekers, J.; Goossens, N.; Gayan-Ramirez, G.; Hermans, G.; Troosters, T. The assessment of inspiratory muscle fatigue in healthy individuals: A systematic review. Respir. Med. 2013, 107, 331–346. [Google Scholar] [CrossRef]
- Dinardi, R.R.; de Andrade, C.R.; da Cunha Ibiapina, C. Evaluation of the effectiveness of the external nasal dilator strip in adolescent athletes: A randomized trial. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1500–1505. [Google Scholar] [CrossRef]
- Dempsey, J.A.; McKenzie, D.C.; Haverkamp, H.C.; Eldridge, M.W. Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest 2008, 134, 613–622. [Google Scholar] [CrossRef]
- Johnson, M.A.; Sharpe, G.R.; Brown, P.I. Inspiratory muscle training improves cycling time-trial performance and anaerobic work capacity but not critical power. Eur. J. Appl. Physiol. 2007, 101, 761–770. [Google Scholar] [CrossRef]
- Vašíčková, J.; Neumannová, K.; Svozil, Z. The Effect of Respiratory Muscle Training on Fin-Swimmers’ Performance. J. Sports Sci. Med. 2017, 16, 521–526. [Google Scholar]
- Klusiewicz, A.; Długołęcka, B.; Charmas, M. Characteristics of the respiratory muscle strength of women and men at different training levels. Pol. J. Sport Tour. 2014, 21, 82–86. [Google Scholar] [CrossRef]
- Lomax, M.; Grant, I.; Corbett, J. Inspiratory muscle warm-up and inspiratory muscle training: Separate and combined effects on intermittent running to exhaustion. J. Sports Sci. 2011, 29, 563–569. [Google Scholar] [CrossRef]
- Volianitis, S.; McConnell, A.K.; Koutedakis, Y. Specific respiratory warm-up improves rowing performance and exertional dyspnea. Med. Sci. Sports Exerc. 2001, 33, 1189–1193. [Google Scholar] [CrossRef]
- Amonette, W.; Dupler, T. The effects of respiratory muscle training on VO2max, the ventilatory threshold and pulmonary function. J. Exerc. Physiol. 2002, 5, 29. [Google Scholar]
- Jurić, I.; Labor, S.; Plavec, D.; Labor, M. Inspiratory muscle strength affects anaerobic endurance in professional athletes. Arh. Hig. Rada Toksikol. 2019, 70, 42–48. [Google Scholar] [CrossRef]
- Migliaccio, G.M.; Russo, L.; Maric, M.; Padulo, J. Sports performance and breathing rate: What is the connection? A narrative review on breathing strategies. Sports 2023, 11, 103. [Google Scholar] [CrossRef]
- Romer, L.M.; Miller, J.D.; Haverkamp, H.C.; Pegelow, D.F.; Dempsey, J.A. Inspiratory muscles do not limit maximal incremental exercise performance in healthy subjects. Respir. Physiol. Neurobiol. 2007, 156, 353–361. [Google Scholar] [CrossRef]
- Illi, S.K.; Held, U.; Frank, I.; Spengler, C.M. Effect of Respiratory Muscle Training on Exercise Performance in Healthy Individuals. Sports Med. 2012, 42, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.; Granda, D.; Klusiewicz, A. Practical Application of Respiratory Muscle Training in Endurance Sports. Strength. Cond. J. 2024, 10-1519. [Google Scholar] [CrossRef]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; Sheel, A.W.; et al. Effects of respiratory muscle training on performance in athletes: A systematic review with meta-analyses. J. Strength. Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef] [PubMed]
- Shei, R.J. Recent Advancements in Our Understanding of the Ergogenic Effect of Respiratory Muscle Training in Healthy Humans: A Systematic Review. J. Strength. Cond. Res. 2018, 32, 2665–2676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lazovic, B.; Djelic, M.; Suzic-Lazic, J.; Takic, M.; Lazic, J.S. Respiratory adaptations in different types of sport. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2269–2274. [Google Scholar]
- Hackett, D.A. Lung function and respiratory muscle adaptations of endurance-and strength-trained males. Sports 2020, 8, 160. [Google Scholar] [CrossRef]
- Sapienza, C.M.; Wheeler, K. Respiratory muscle strength training: Functional outcomes versus plasticity. Semin. Speech Lang. 2006, 27, 236–244. [Google Scholar] [CrossRef]
- Aliverti, A. The respiratory muscle during exercise. Breathe 2016, 12, 165–168. [Google Scholar] [CrossRef]
- Shei, R.-J.; Paris, H.L.; Sogard, A.S.; Mickleborough, T.D. Mickleborough. Time to Move Beyond a ‘One-Size Fits All’ Approach to Inspiratory Muscle Training. Front. Physiol. 2021, 12, 766346. [Google Scholar] [CrossRef]
- McConnell, A.K.; Romer, L.M. Endurance training of respiratory muscles improves cycling performance in fit young cyclists. BMC Physiol. 2024, 4, 9. [Google Scholar]
- Yi, S.J.; Kim, J.S. The effects of respiratory muscle strengthening exercise using a sling on the amount of respiration. J. Phys. Ther. Sci. 2015, 27, 2121–2124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Patel, N.; Chong, K.; Baydur, A. Methods and applications in respiratory physiology: Respiratory mechanics, drive and muscle function in neuromuscular and chest wall disorders. Front. Physiol. 2022, 13, 838414. [Google Scholar] [CrossRef]
- Peters, C.M.; Sheel, A.W. Pulmonary Physiology and Response to Exercise. In Exercise and Sports Pulmonology: Pathophysiological Adaptations and Rehabilitation; Springer: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar]
- Saklica, D. Respiratory System And Its Adaptations To Exercise. In Functional Exercise Anatomy And Physiology For Physiotherapists; Springer: Cham, Switzerland, 2023; pp. 423–445. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).