Technological Aspects of Sintering Low-Quality Wolframite Concentrate with Potassium Carbonate
Abstract
1. Introduction
2. Materials and Methods
3. The Results and Discussion
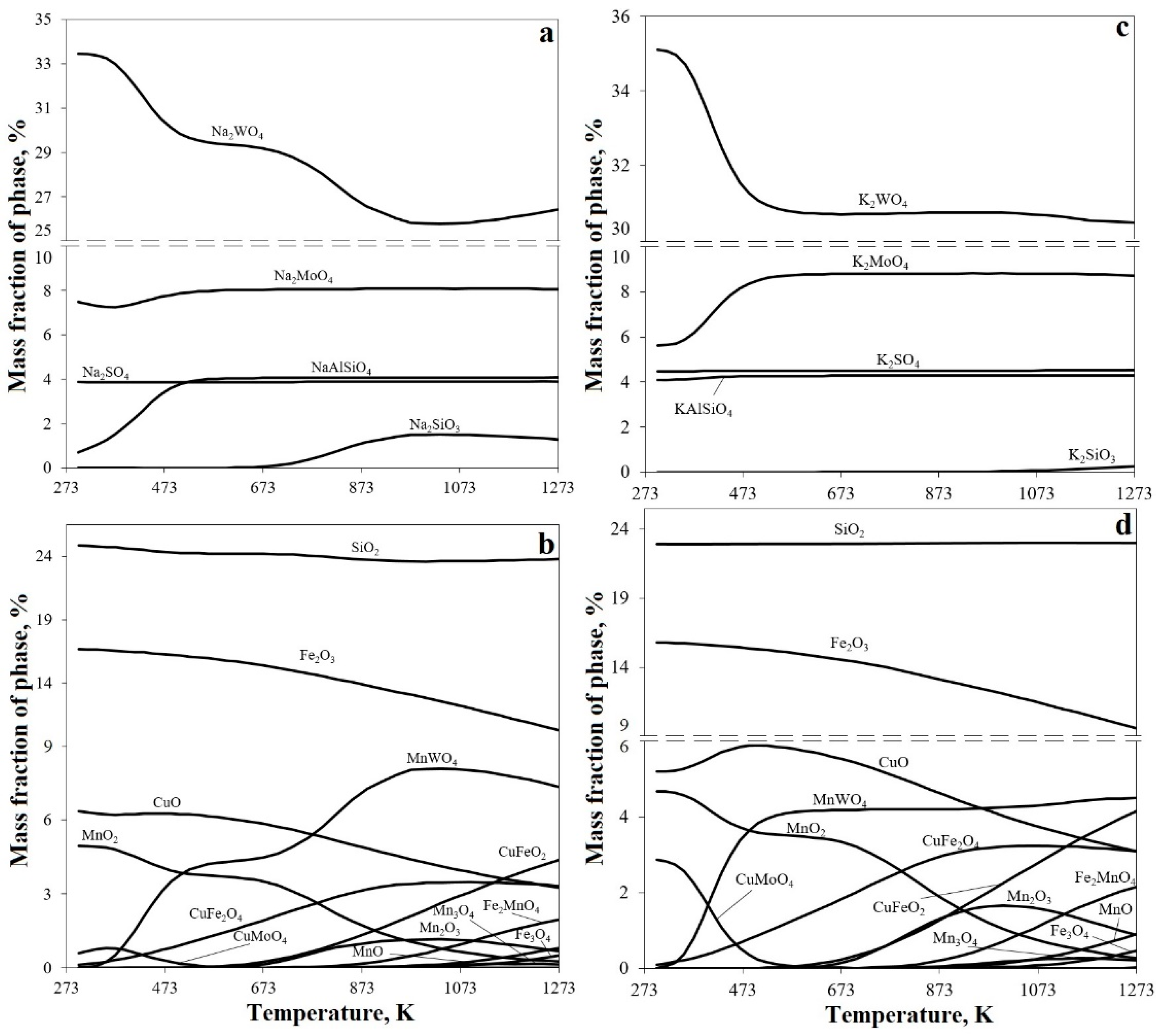
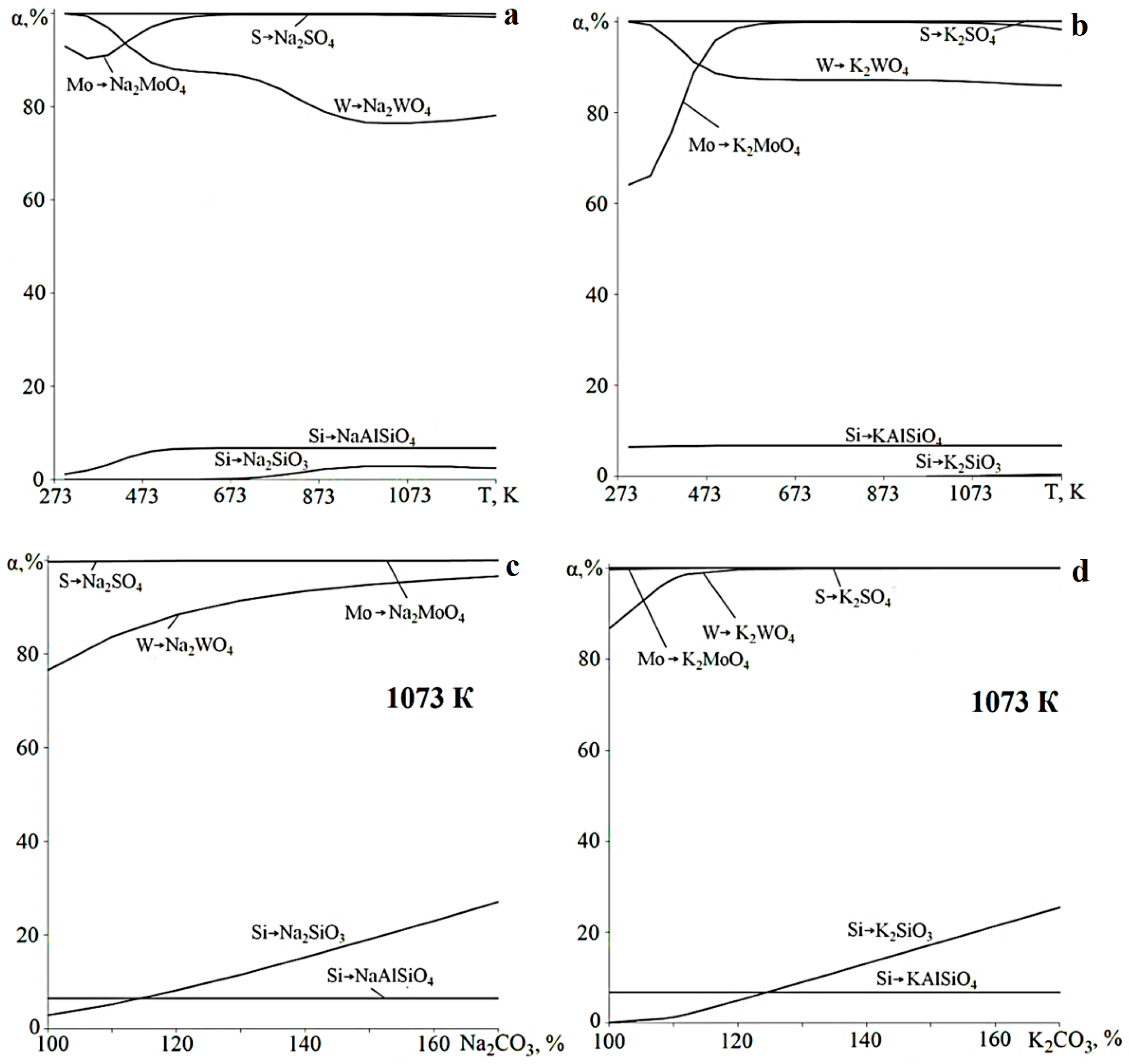
3.1. Thermodynamic Assessment of the Main Reactions
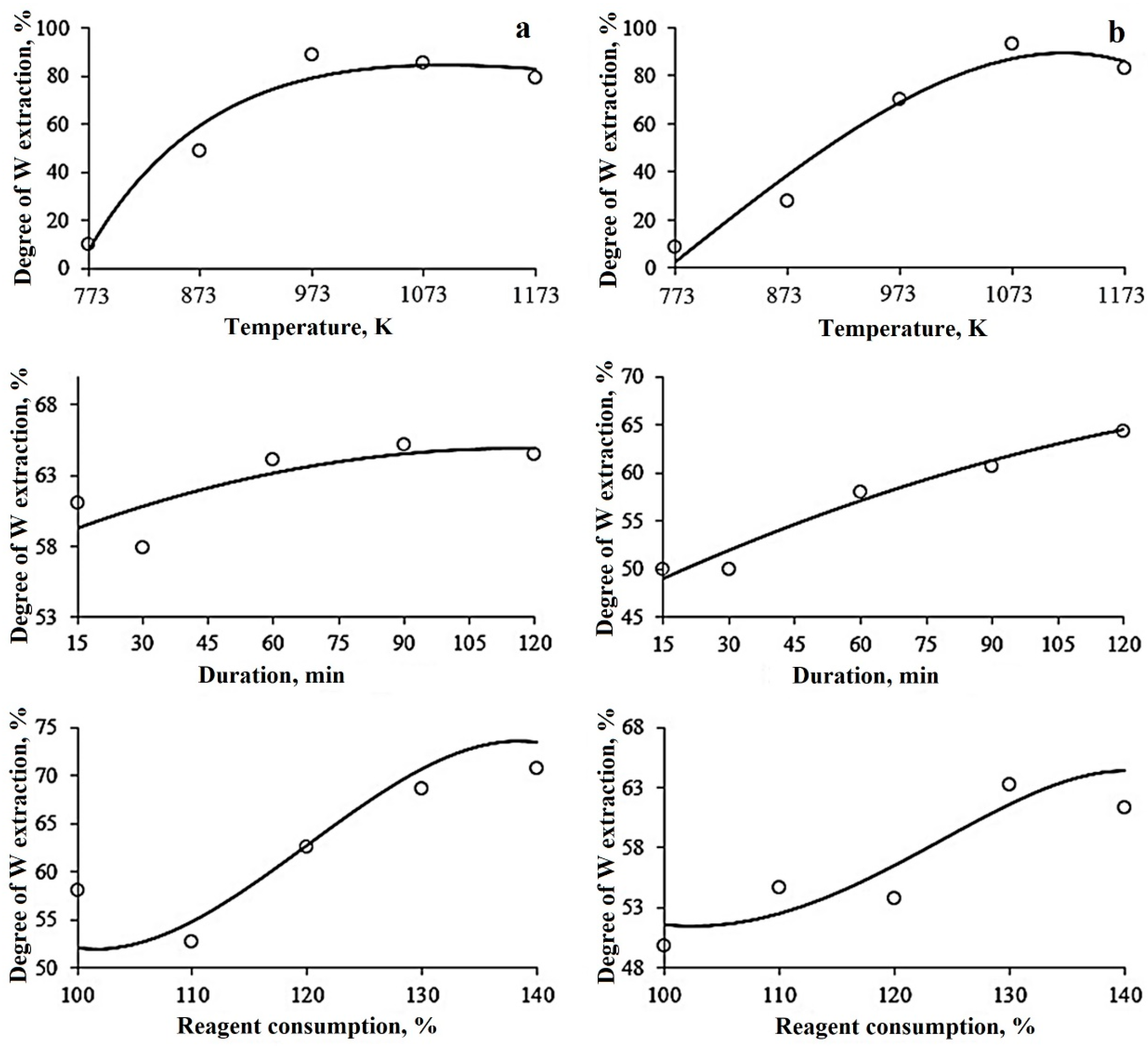
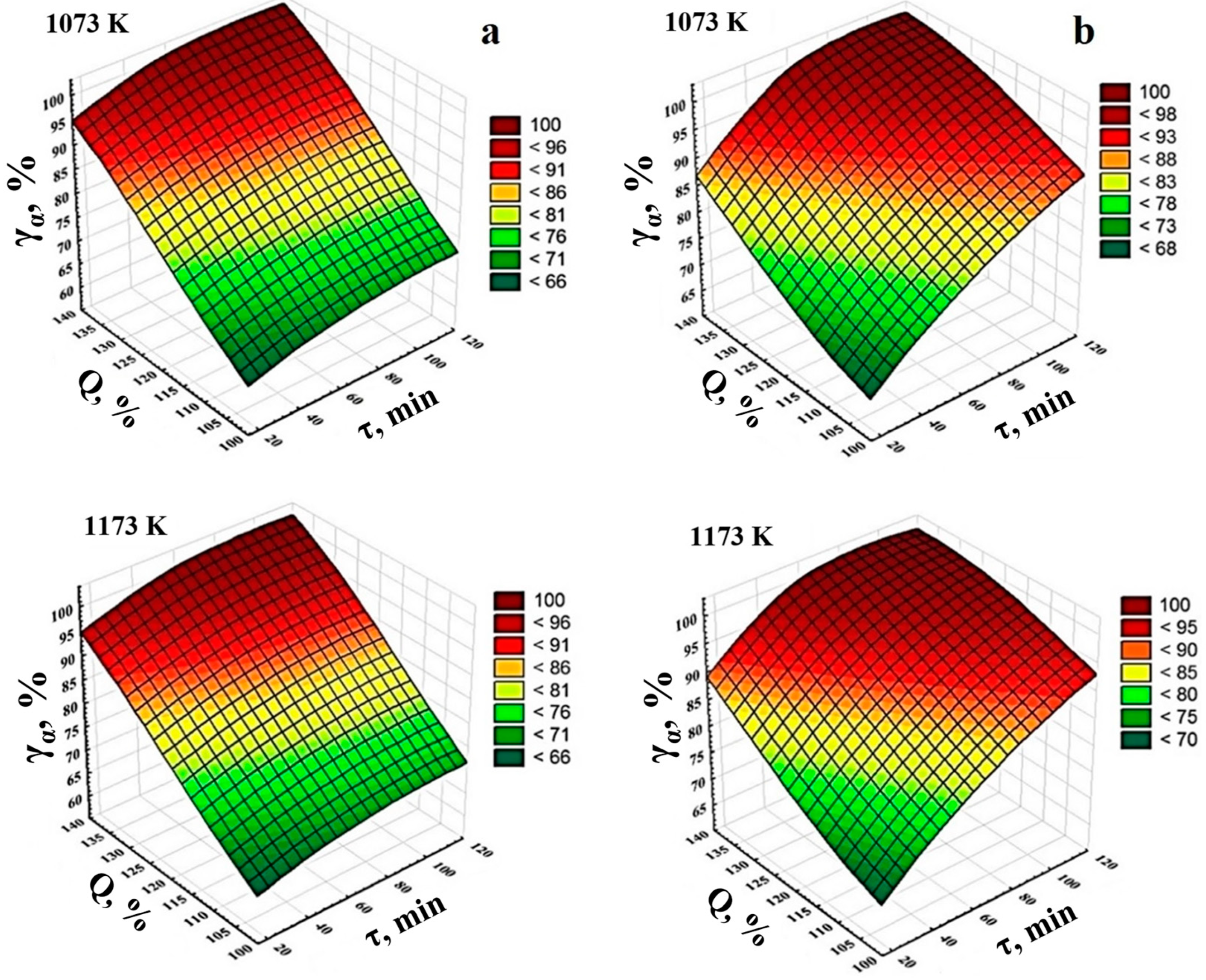
3.2. Optimization of Sintering Treatment Parameters Using the Method of Probabilistically Deterministic Planning of the Experiment
3.3. Industrial Testing of Laboratory Data on Sintering of Wolframite Concentrate with Sodium and Potassium Carbonates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zelikman, A.N.; Crane, O.E.; Samsonov, G.V. Rare Metal Metallurgy; Metallurgy: Moscow, Russia, 1978; pp. 1–560. [Google Scholar]
- Lassner, E.; Schubert, W.D. Tungsten: Properties, Chemistry, Technology of the Elements, Alloys and Chemical Compounds; Springer Science+Business Media: New York, NY, USA, 1999; pp. 179–214. [Google Scholar]
- Xu, L.; Zhao, B. Extraction of Sodium Tungstate from Tungsten Ore by Pyrometallurgical Smelting. Metals 2023, 13, 312. [Google Scholar] [CrossRef]
- Selivanov, E.N.; Pikulin, K.V.; Galkova, L.I.; Gulyaeva, R.I.; Petrova, S.A. Kinetics and mechanism of natural wolframite interactions with sodium carbonate. Int. J. Miner. Metall. Mater. 2019, 26, 1364–1371. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, B. A Fundamental Study on the Preparation of Sodium Tungstate from Wolframite via the Smelting Process. Metals 2024, 14, 299. [Google Scholar] [CrossRef]
- Baimbetov, B.; Moldabayeva, G.; Yeleuliyeva, A.; Jumankulova, S.; Taimassova, A.; Adilzhan, Z.; Baisultanov, R.; Yakob, E.; Serikbayev, V. Prospects of Processing Tungsten Ores from the Akchatau Deposit. Processes 2024, 12, 77. [Google Scholar] [CrossRef]
- Zhao, B.; Su, K.; Ma, X. Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System. Metals 2021, 11, 2014. [Google Scholar] [CrossRef]
- Yang, L.; Li, C.; Cao, C.; Xue, X.; Gong, D.; Wan, L. Kinetics of Low-Grade Scheelite Leaching with a Mixture of Sodium Phosphate and Sodium Fluoride. Metals 2022, 12, 1759. [Google Scholar] [CrossRef]
- Paulino, J.F.; Afonso, J.C.; Mantovano, J.L.; Vianna, C.A.; Silva Dias da Cunha, J.W. Recovery of tungsten by liquid-liquid extraction from a wolframite concentrate after fusion with sodium hydroxide. Hydrometallurgy 2012, 12–128, 121–124. [Google Scholar] [CrossRef]
- Mulenshi, J.; Chelgani, S.C.; Rosenkranz, J. Mechanochemical Treatment of Historical Tungsten Tailings: Leaching While Grinding for Tungsten Extraction Using NaOH. Sustainability 2021, 13, 3258. [Google Scholar] [CrossRef]
- Gaur, R.P.S. Modern hydrometallurgical production methods for tungsten. JOM 2006, 58, 45–49. [Google Scholar] [CrossRef]
- Srinivas, K.; Sreenivas, T.; Natarajan, R.; Padmanabhan, N.P.H. Study on the recovery of tungsten from a composite wolframite-scheelite concentrate. Hydrometallurgy 2000, 58, 43–50. [Google Scholar] [CrossRef]
- Medvedev, A.S.; Aleksandrov, P.V.; Razykov, B.Z.; Rodionov, A.O.; Sannikova, O.V. Prospects for involvement of low-grade molybdenum and tungsten concentrates in metallurgical processing. Metallurgist 2013, 57, 261–267. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Z.; Li, H.; Li, Y.; Liu, M.; Chen, S.; Liang, Y. A new technology for production of high purity paratungstate ammonium from low grade tungsten concentrate. J. Cent. South Univ. Technol. 1996, 3, 171–176. [Google Scholar] [CrossRef]
- Misra, P.; Suri, A.K.; Gupta, C.K. Recovery of pure tungsten oxide from low grade wolframite concentrates by adsorption-desorption on activated charcoal. Miner. Eng. 1990, 3, 625–630. [Google Scholar] [CrossRef]
- Bohlouli, A.; Reza Afshar, M.; Aboutalebi, M.R.; Seyedein, S.H. Optimization of tungsten leaching from low manganese wolframite concentrate using Response Surface Methodology (RSM). Int. J. Refract. Hard Met. 2016, 61, 107–114. [Google Scholar] [CrossRef]
- Gelsing, R.J.H.; Stein, H.N.; Stevels, J.M. The phase diagram K2WO4–WO3. Recl. Trav. Chim. Pays-Bas. 1965, 84, 1452–1458. [Google Scholar] [CrossRef]
- Selivanov, E.N.; Pikulin, K.V.; Gulyaeva, R.I.; Galkova, L.I. Kinetics of the Natural Wolframite Interaction with Sodium and Potassium Carbonates. Mater. Sci. Forum. 2020, 989, 440–447. [Google Scholar] [CrossRef]
- Malyshev, V.P. Probabilistic-Deterministic Planning of the Experiment; Alma-Ata Sci.: Alma-Ata, Kazakhstan, 1981; Volume 116. [Google Scholar]
- Malyshev, V.P. Mathematical Planning of Metallurgical and Chemical Experiment; Nauka: Alma-Ata, Kazakhstan, 1977. [Google Scholar]
- Ibishev, K.S.; Malyshev, V.P.; Kim, S.V.; Sarsembaev, B.S.; Egorov, N.B. Preparation of nanosized nickel powder by direct-current electrolysis combined with high-voltage spark discharge. Plasma Chem. 2017, 51, 219–223. [Google Scholar] [CrossRef]
- Skopov, G.V.; Bulatov, K.V.; Skopin, D.Y. The Rate of Lead Sulfide Distillation from Polymetal Concentrate and Polymetal Matte Prepared from it. Metallurgist 2015, 59, 168–176. [Google Scholar] [CrossRef]
- Shoshay, Z.; Sapinov, R.V.; Sadenova, M.A.; Beisekenov, N.A.; Varbanov, P.S.; Suyundikov, M. Application of Probabilistic-Deterministic Method for Experiment Planning of Hydrometallurgical Processing of Various Wastes for Gold Extraction. Chem. Eng. Trans. 2022, 94, 1135–1140. [Google Scholar]
- Borshynbayev, A.; Omarov, K.; Mustafin, Y.; Havlíček, D.; Absat, Z.; Muratbekova, A.; Kaikenov, D.; Pudov, A.; Shuyev, N. A Study of copper leaching from the tailings of the Karagaily (Republic of Kazakhstan) concentrating factory using an electric hydropulse discharge. J. Serb. Chem. Soc. 2022, 87, 925–937. [Google Scholar] [CrossRef]
- Barshabayeva, A.; Balpanova, N.; Aitbekova, D.; Baikenov, M.; Aubakirov, Y.; Khalikova, Z.S.; Tusipkhan, A.; Tulebaeva, B.; Gulzhan, T. The Influence of Various Factors on Nanocatalyst Activity during Benzothiophene Hydrogenation. Appl. Sci. 2022, 12, 12792. [Google Scholar] [CrossRef]
- Naguman, P.N.; Karimova, L.M.; Tokaeva, Z.M.; Shinbaev, U.B. A multifactor simulation of the process of leaching oxidized molybdenum products in sulfuric acid solutions. Russ. J. Non-Ferr. Met. 2009, 50, 330–334. [Google Scholar] [CrossRef]
- Makhabetov, Y.; Myngzhassar, Y.; Abdirashit, A. Modeling the Ferrosilicomanganese Smelting Process using Manganese-rich Slag. Acta Metall. Slovaca 2024, 30, 29. [Google Scholar] [CrossRef]
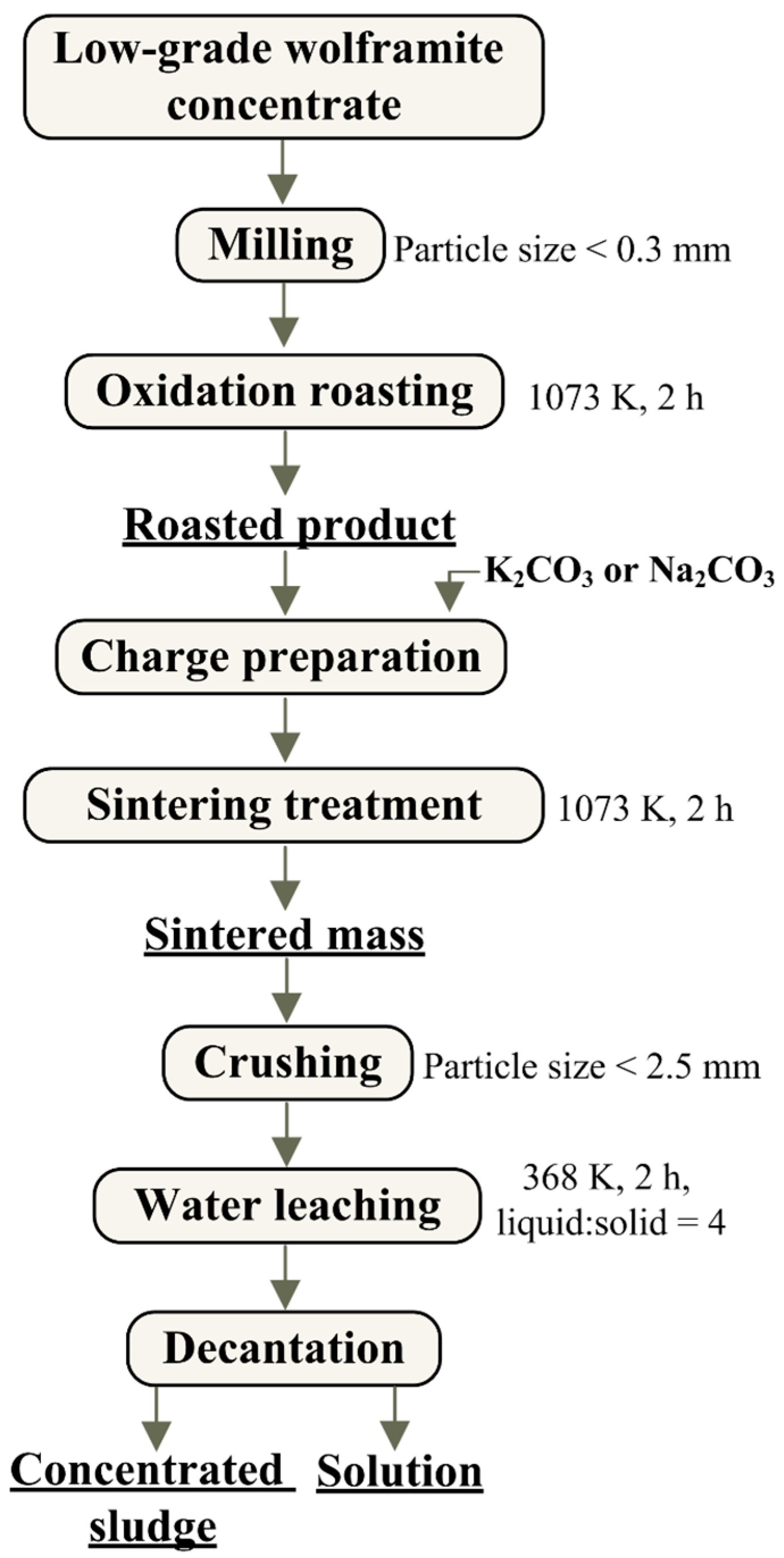
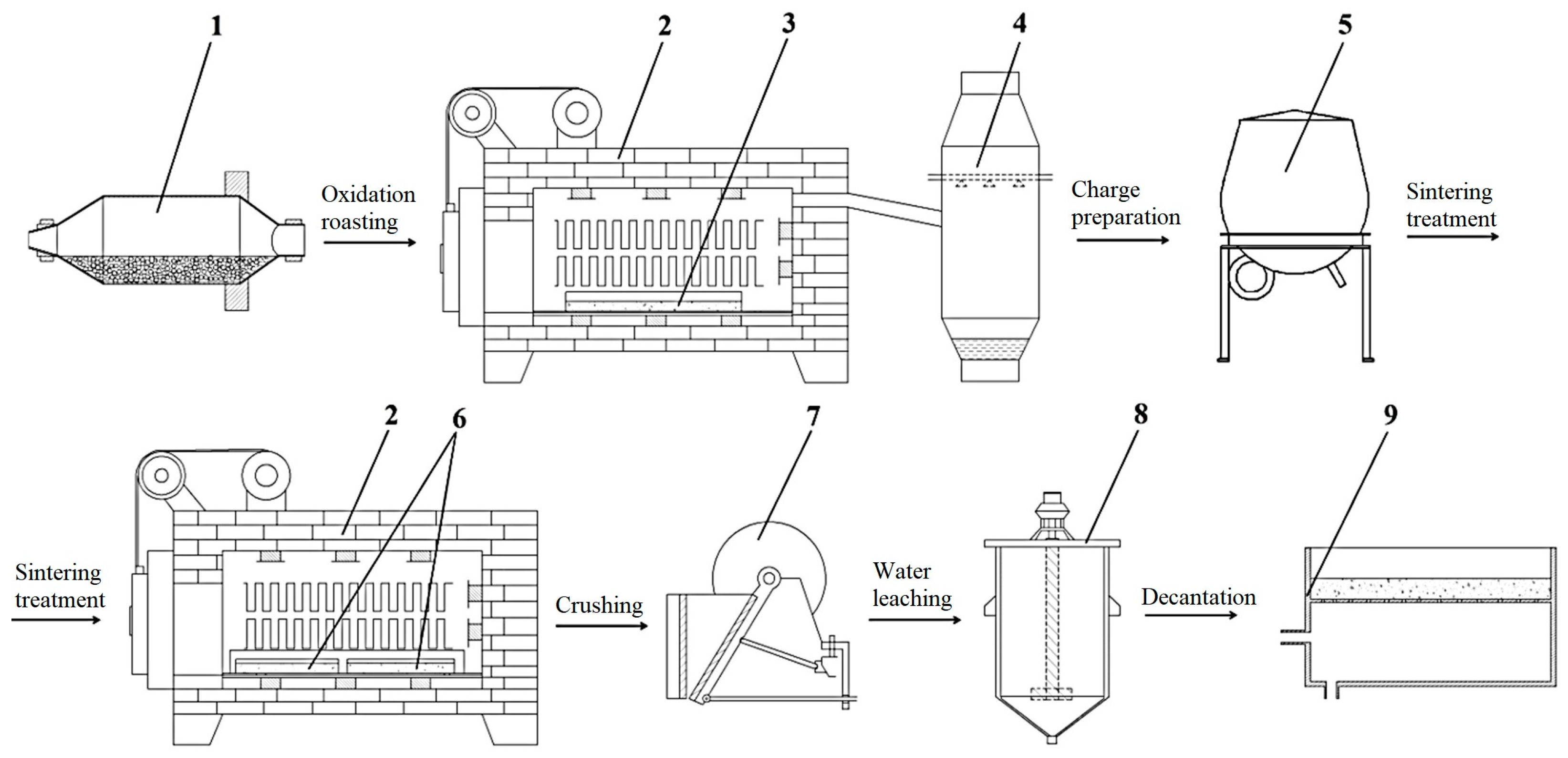
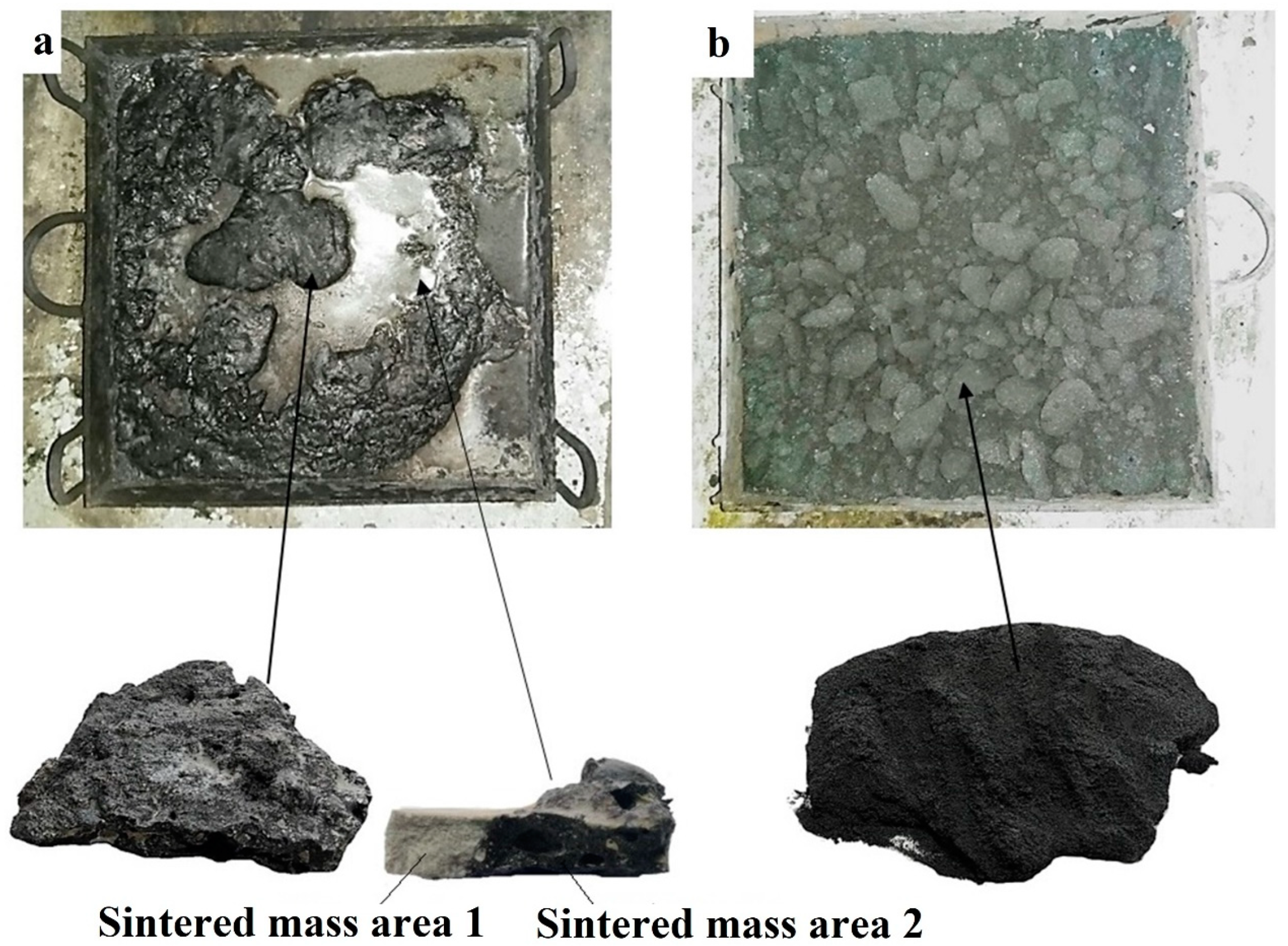
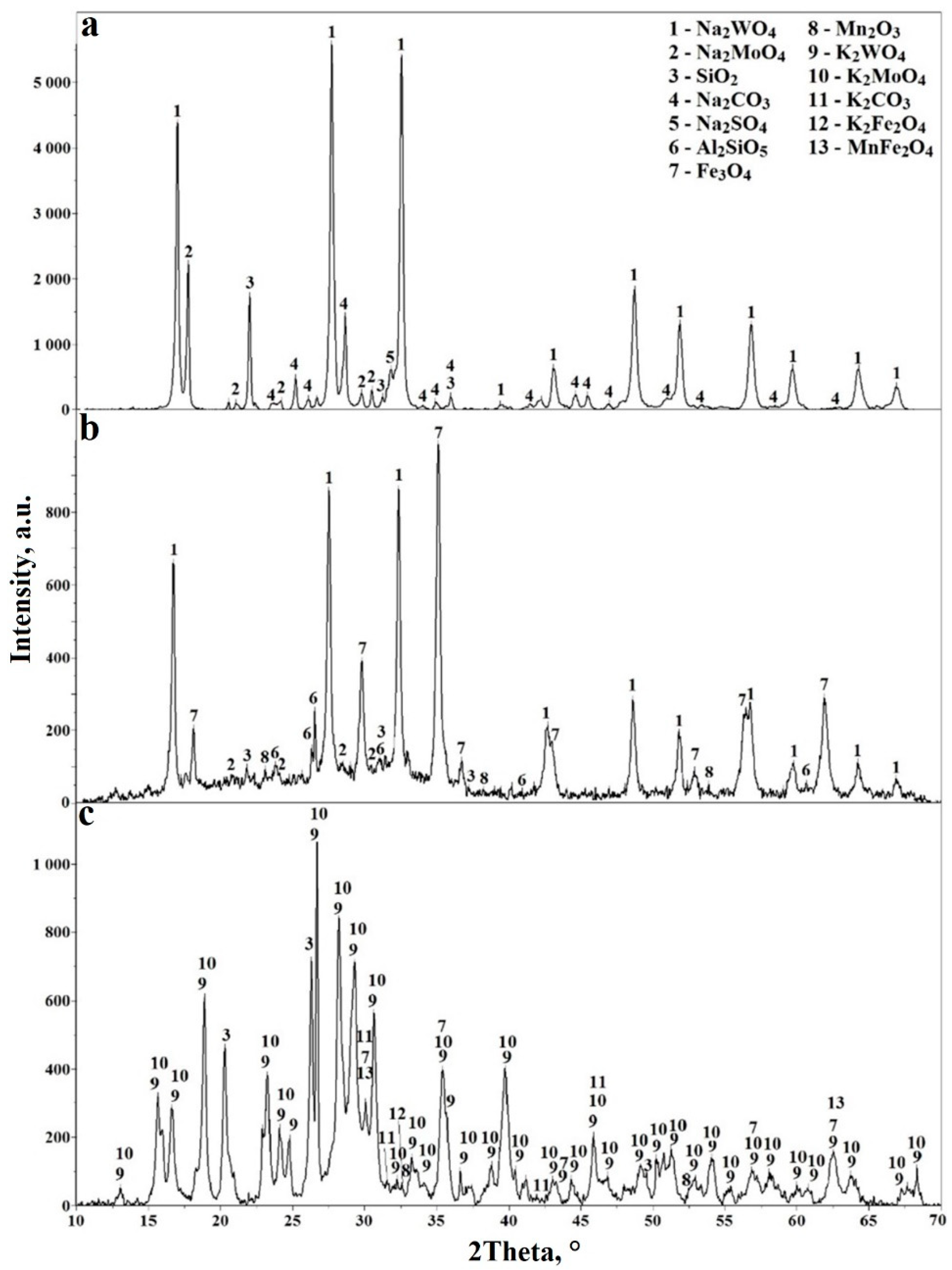
| Concentrate | Content, Mass. % | |||||||
|---|---|---|---|---|---|---|---|---|
| W | Si | S | Mo | Mn | Fe | Cu | Al | |
| Initial | 22.9 | 11.6 | 10.7 | 4.3 | 3.6 | 12.2 | 6.2 | 1.4 |
| Post-Treatment | 24.7 | 13.5 | 1.01 | 4.41 | 3.69 | 13.4 | 6.35 | 1.44 |
| Coefficient | Level | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| T, K | 773 | 873 | 973 | 1073 | 1173 |
| τ, min | 15 | 30 | 60 | 90 | 120 |
| Q, % | 100 | 110 | 120 | 130 | 140 |
| Experience Number | T, K | τ, min | Q, % | γα for Various Systems | |
|---|---|---|---|---|---|
| Na2CO3 System | K2CO3 System | ||||
| 1 | 773 | 15 | 100 | 4.6 | 5.3 |
| 2 | 773 | 60 | 120 | 11.5 | 9.9 |
| 3 | 773 | 30 | 110 | 2.1 | 5.6 |
| 4 | 773 | 120 | 140 | 17.0 | 11.9 |
| 5 | 773 | 90 | 130 | 15.3 | 10.2 |
| 6 | 973 | 15 | 120 | 88.7 | 41.8 |
| 7 | 973 | 60 | 110 | 88.1 | 80.1 |
| 8 | 973 | 30 | 140 | 95.0 | 63.2 |
| 9 | 973 | 120 | 130 | 96.6 | 100.0 |
| 10 | 973 | 90 | 100 | 76.7 | 65.7 |
| 11 | 873 | 15 | 110 | 12.1 | 7.3 |
| 12 | 873 | 60 | 140 | 56.0 | 31.4 |
| 13 | 873 | 30 | 130 | 38.0 | 13.5 |
| 14 | 873 | 120 | 100 | 70.1 | 40.1 |
| 15 | 873 | 90 | 120 | 68.5 | 47.3 |
| 16 | 1173 | 15 | 140 | 100.0 | 100.0 |
| 17 | 1173 | 60 | 130 | 93.6 | 97.3 |
| 18 | 1173 | 30 | 100 | 67.2 | 67.1 |
| 19 | 1173 | 120 | 120 | 57.0 | 69.6 |
| 20 | 1173 | 90 | 110 | 79.5 | 80.1 |
| 21 | 1073 | 15 | 130 | 99.8 | 95.1 |
| 22 | 1073 | 60 | 100 | 71.3 | 70.8 |
| 23 | 1073 | 30 | 120 | 87.4 | 100.0 |
| 24 | 1073 | 120 | 110 | 81.9 | 100.0 |
| 25 | 1073 | 90 | 140 | 85.8 | 100.0 |
| Average | 62.55 | 56.54 | |||
| Reagent Used | Content, % | |||||||
|---|---|---|---|---|---|---|---|---|
| W | Mo | Si | Fe | Mn | Al | P | Cu | |
| K2CO3 | 1.21 | 0.12 | 18.3 | 18.2 | 8.01 | 1.69 | 0.28 | 9.39 |
| Na2CO3 | 1.89 | 0.26 | 13.2 | 23.9 | 6.56 | 1.39 | 0.21 | 7.69 |
| Function | R | tR |
|---|---|---|
| Experiment with Na2CO3 | ||
| γT | 0.97 | 24.5 |
| γτ | 0.73 | 2.69 |
| γQ | 0.83 | 4.63 |
| γα1 | 0.89 | 19.6 |
| Experiment with K2CO3 | ||
| γT | 0.97 | 33.8 |
| γτ | 0.98 | 34.8 |
| γQ | 0.84 | 4.92 |
| γα2 | 0.92 | 26.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikulin, K.V.; Galkova, L.I.; Vitkina, G.Y.; Karlina, A.I. Technological Aspects of Sintering Low-Quality Wolframite Concentrate with Potassium Carbonate. Appl. Sci. 2024, 14, 9000. https://doi.org/10.3390/app14199000
Pikulin KV, Galkova LI, Vitkina GY, Karlina AI. Technological Aspects of Sintering Low-Quality Wolframite Concentrate with Potassium Carbonate. Applied Sciences. 2024; 14(19):9000. https://doi.org/10.3390/app14199000
Chicago/Turabian StylePikulin, Kirill V., Lyudmila I. Galkova, Galina Y. Vitkina, and Antonina I. Karlina. 2024. "Technological Aspects of Sintering Low-Quality Wolframite Concentrate with Potassium Carbonate" Applied Sciences 14, no. 19: 9000. https://doi.org/10.3390/app14199000
APA StylePikulin, K. V., Galkova, L. I., Vitkina, G. Y., & Karlina, A. I. (2024). Technological Aspects of Sintering Low-Quality Wolframite Concentrate with Potassium Carbonate. Applied Sciences, 14(19), 9000. https://doi.org/10.3390/app14199000






