Enhancing Greywater Treatment: High-Efficiency Constructed Wetlands with Seashell and Ceramic Brick Substrates
Abstract
:Featured Application
Abstract
1. Introduction
2. Material and Methods
2.1. CW System and Characteristics of the Support Bed
2.2. Operation of the Constructed Wetland System
2.3. Synthetic Greywater
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Synthetic Greywater
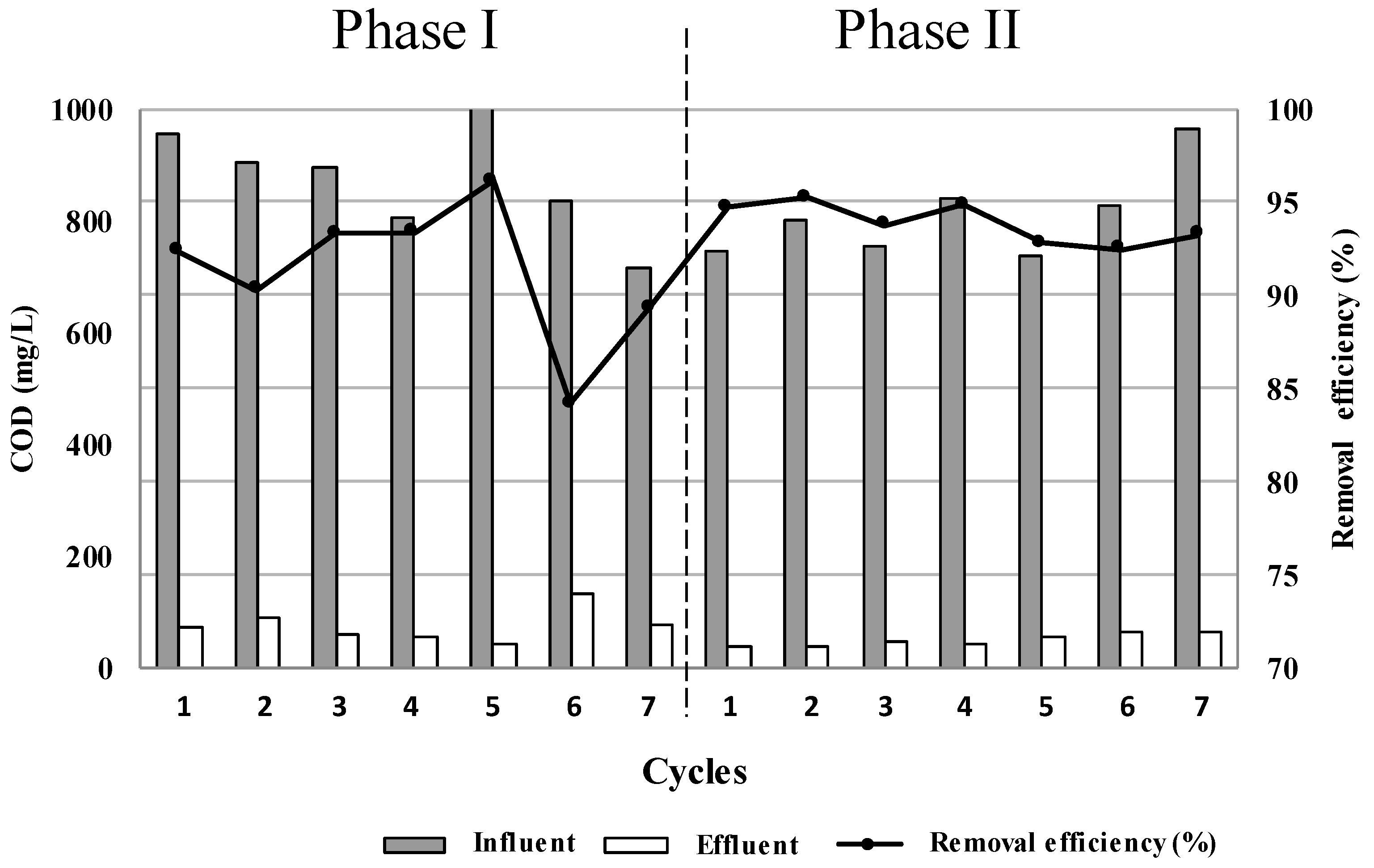
3.2. Operation and Performance of the CW with the Studied Substrates
3.3. Assessment of Echinodorus Subalatus Growth and Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Children’s Fund and World Health Organization. Progress on Household Drinking Water. Sanitation and Hygiene 2000–2017. Special Focus on Inequalities. New York, 2019; pp. 1–140. Available online: https://www.who.int/publications/i/item/9789241516235 (accessed on 24 August 2024).
- Tundisi, J.G.; Matsumara-Tundisi, T.A. Água (Water); Scienza: São Carlos, SP, Brazil, 2020; pp. 1–130. [Google Scholar] [CrossRef]
- UNEP. A Snapshot of the World’s Water Quality: Towards a Global Assessment; United Nations Environment Programme: Nairobi, Kenya, 2016; pp. 1–162. [Google Scholar]
- Stamatelatou, K.; Tsagarakis, K.P. Sewage Treatment Plants: Economic Evaluation of Innovative Technologies for Energy Efficiency; IWA Publishing: London, UK, 2015; pp. 1–337, Number 104536. Available online: https://library.oapen.org/handle/20.500.12657/43789 (accessed on 24 August 2024).
- Santos, J.; Rodrigues, S.; Magalhães, M.; Rodrigues, K.; Pereira, L.; Marinho, G. A state-of-the-art review (2019–2023) on constructed wetlands for greywater treatment and reuse. Environ. Chall. 2024, 16, 100973. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Mir, T.U.G.; Rahayu, F.; Suhara, C.; Anjli, A.; Chopra, C.; Singh, R.; Prakash, A.; El Messaoudi, N.; et al. Eco-friendly and safe alternatives for the valorization of shrimp farming waste. Environ. Sci. Pollut. Res. 2024, 31, 38960–38989. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, V.M.S. Wetland System Built with Native Macrophyte Echinodorus Subalatus for Gray Water Treatment. Ph.D. Thesis, Centro de Ciências, Universidade Federal do Ceará, Fortaleza, Brazil, 2022. Available online: http://repositorio.ufc.br/handle/riufc/69601 (accessed on 24 August 2024).
- Abed, S.N.; Scholz, M. Chemical simulation of greywater. Environ. Technol. 2016, 37, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2005; pp. 1–9750. ISBN 978-087553-287. [Google Scholar]
- Rodier, J. Análisis de las Aguas (Spanish Edition); Omega: Barcelona, Spain, 1990. [Google Scholar]
- Bani-Melhem, K.; Al-Kilani, M.R.A. Comparison between iron and mild steel electrodes for the treatment highly loaded greywater using an electrocoagulation technique. Arab. J. Chem. 2023, 16, 105119. [Google Scholar] [CrossRef]
- Uthirakrishnan, U.; Manthapuri, V.; Harafan, A.; Chellam, P.V.; Karuppiah, T. The regime of constructed wetlands in greywater treatment. Water Sci. Technol. 2022, 85, 3169–3183. [Google Scholar] [CrossRef]
- Baracuhy, V.S. Monitoring Gray Water Quality and Socio-Environmental Assessment of Users in an Agricultural Production Unit in Cabaceiras-PB. Master’s Thesis, Federal University of Campina Grande, Paraíba, Brazil, 2014. Available online: http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/17022 (accessed on 24 August 2024).
- Paulo, P.L.; Azevedo, C.; Begosso, L.; Galbiati, A.F.; Boncz, M.A. Natural systems treating greywater and blackwater on-site: Integrating treatment. reuse and landscaping. Ecol. Eng. 2013, 50, 95–100. [Google Scholar] [CrossRef]
- Liao, Y.; Wam, Z.; Cao, X.; Jiang, L.; Feng, L.; Zheng, H.; Ji, F. The importance of rest phase and pollutant removal mechanism of tidal flow constructed wetlands (TFCW) in rural grey water treatment. Chemosphere 2023, 311, 137010. [Google Scholar] [CrossRef]
- Gholami, M.; O’Sullivan, A.D.; Mackey, H.R. Nutrient treatment of greywater in green wall systems: A critical review of removal mechanisms, performance efficiencies and system design parameters. J. Environ. Manag. 2023, 345, 118917. [Google Scholar] [CrossRef]
- Oteng-Peprah, M.; Acheampong, M.A.; Devries, N.K. Greywater characteristics. treatment systems. reuse strategies and user perception-a review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef]
- Júnior, R.M.; Passoni, C.M.; Santos, M.F.; Bernardes, F.S.; Magalhães Filho, F.J.C.; Paulo, P.L. Assessment of surfactant removal capacity and microbial community diversity in a greywater-treating constructed wetland. Resources 2023, 12, 38. [Google Scholar] [CrossRef]
- Hampel, M.; Mauffrett, A.; Pazdro, K.; Blasco, J. Anionic surfactant linear alkylbenzene sulfonates (LAS) in sediments from the Gulf of Gdask (southern Baltic Sea. Poland) and its environmental implications. Environ. Monit. Assess. 2012, 184, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Sezerino, P.H.; Santos, M.O.; Pelissari, C.; Celis, G.S.; Philipp, L.S. Horizontal constructed wetlands applied on decentralized wastewater treatment. Rev. Eng. Const. Civil 2015, 2, 1–10. [Google Scholar]
- Sotiropoulou, M.; Stefanatou, A.; Schiza, S.; Petousi, I.; Stasinakis, A.S.; Fountoulakis, M.S. Removal of microfiber in vertical flow constructed wetlands treating greywater. Sci. Total. Environ. 2023, 858, 159723. [Google Scholar] [CrossRef] [PubMed]
- Kotsia, D.; Deligianni, A.; Fyllas, N.M.; Stasinakis, A.S.; Fountolakis, M.S. Converting treatment wetlands into “treatment gardens”: Use of ornamental plants for greywater treatment. Sci. Total. Environ. 2020, 744, 140889. [Google Scholar] [CrossRef] [PubMed]
- Nema, A.; Prasad, R.; Sharma, D.; Yadav, K.; Chistian, R.A.; Hibrahim, H. Performance evaluation of different macrophytes in small-scale vertical flow constructed wetlands for greywater treatment using principal component analysis. Can. J. Chem. Eng. 2023, 101, 1321–1334. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Taha, D.S.; Hassan, W.H.; Lakhera, S.K.; Ansar, S.; Pradhan, S. Subsurface flow constructed wetlands for treating of simulated cadmium ions-wastewater with presence of Canna indica and Typha domingensis. Chemosphere 2023, 338, 139469. [Google Scholar] [CrossRef]
- Pinheiro, V.S. Application of Dissolved Air Flotation for Treating Hard Water in the Seridó/RN Region. M.Sc. Thesis, Federal University of Pernambuco, Recife, Brazil, 2011. [Google Scholar]
- Khalifa, M.E.; El-Reash, Y.G.A.; Ahmed, M.I.; Rizk, F.W. Effect of media variation on the removal efficiency of pollutants from domestic wastewater in constructed wetland systems. Ecol. Eng. 2020, 143, 105668. [Google Scholar] [CrossRef]
- Zak, D.; Hupter, M.; Cabezas, A.; Juransiski, G.; Audet, J.; Kleeberg, A.; Goldhammer, T. Sulphate in freshwater ecosystems: A review of sources, biogeochemical cycles, ecotoxicological effects and bioremediation. Earth Sci. Rev. 2021, 212, 103446. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Stability of calcium carbonate and magnesium carbonate in rainwater and nitric acid solutions. Energy Convers. Manag. 2006, 47, 3059–3068. [Google Scholar] [CrossRef]
- Miloslav, S.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; Fang, Y.; Xu, K.; He, S.; Zhao, M. Autotrophic denitrification in constructed wetlands: Achievements and challenges. Bioresour. Technol. 2020, 318, 123778. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério do Meio Ambiente (MMA). Resolução nº 430. de 17 de Março de 2011; Conselho Nacional do Meio Ambiente (CONAMA): Brasília, Brazil, 2011; Available online: https://www.legisweb.com.br/legislacao/?id=114770 (accessed on 24 August 2024).
- Punyapwar, S.; Mutnuri, S. Diversity and functional annotation of microorganisms in French vertical flow constructed wetland treating greywater. World J. Microbiol. Biotechnol. 2020, 36, 148. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total. Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, L.; Eddy, H.P. Wastewater Engineering: Treatment and Resource, 5th ed.; McGraw-Hill: New York, NY, USA, 2016; p. 2018. [Google Scholar]
- Cheng, G.; Li, Q.; Su, Z.; Sheng, S.; Fu, J. Preparation, optimization, and application of sustainable ceramsite substrate from coal fly ash/waterworks sludge/oyster shell for phosphorus immobilization in constructed wetlands. J. Clean. Prod. 2018, 175, 572–581. [Google Scholar] [CrossRef]
- Deng, S.; Chen, Y. A study by response surface methodology (RSM) on optimization of phosphorus adsorption with nano spherical calcium carbonate derived from waste. Water Sci Technol. 2019, 79, 188–197. [Google Scholar] [CrossRef]
- Žibien, G.; Dapkien, M.; Kazakeviien, J.; Radzeviius, A. Phosphorus removal in a vertical flow constructed wetland using dolomite powder and chippings as filter media. J. Water Secur. 2015, 1, 46–52. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of various emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Nguyen, T.H.H.; Soda, S.; Vu, N.D.; Pham, T.T. White hard clam (Meretrix lyrata) shells media to improve phosphorus removal in lab-scale horizontal sub-surface flow constructed wetlands: Performance. removal pathways. and lifespan. Bioresour. Technol. 2020, 312, 123602. [Google Scholar] [CrossRef]
- Almuktar, A.A.A.N.; Suhail, N.A.; Scholz, M. Wetlands for wastewater treatment and subsequent recycling of treated effluent: A review. Environ. Sci. Pollut. Res. 2018, 25, 23595–23623. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef]
- Pérez-Lopez, M.E.; Arreaola-Ortiz, A.E.; Zamora, P.M. Evaluation of detergent removal in artificial wetlands (biofilters). Ecol. Eng. 2018, 122, 135–142. [Google Scholar] [CrossRef]
- Thomas, R.; Gough, R.; Freeman, C. Linear alkylbenzene sulfonate (LAS) removal in constructed wetlands: The role of plants in the treatment of plants the treatment of a typical pharmaceutical and personal care product. Ecol. Eng. 2017, 106, 415–422. [Google Scholar] [CrossRef]
- Matias, L.Q.; Soares, G. Morphology and micromorphology of the seed coats of species of Echinodorus (Alismataceae) from Brazilian Northeastern. Bol. Mus. Para. Emílio Goeldi Ciênc. Nat. 2009, 4, 165–173. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total. Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef]
- Tan, S.; Sha, Y.; Sun, L.; Li, Z. Abiotic Stress-Induced Leaf Senescence: Regulatory Mechanisms and Application. Int. J. Mol. Sci. 2023, 24, 11996. [Google Scholar] [CrossRef]
- Marcelino, G.C.; Morais, A.A. Analysis of a prototype of constructed wetlands in the treatment of industrial dairy effluents. Res. Soc. Dev. 2022, 11, 12811830520. [Google Scholar] [CrossRef]
- Forgiarini, F.R.; Rizzi, E.S. Efficiency of different macrophytes in the removal of biodegradable organic matter in constructed wetland with vertical flow in subtropical climate. Sci. Eng. J. 2016, 25, 79–86. [Google Scholar] [CrossRef]
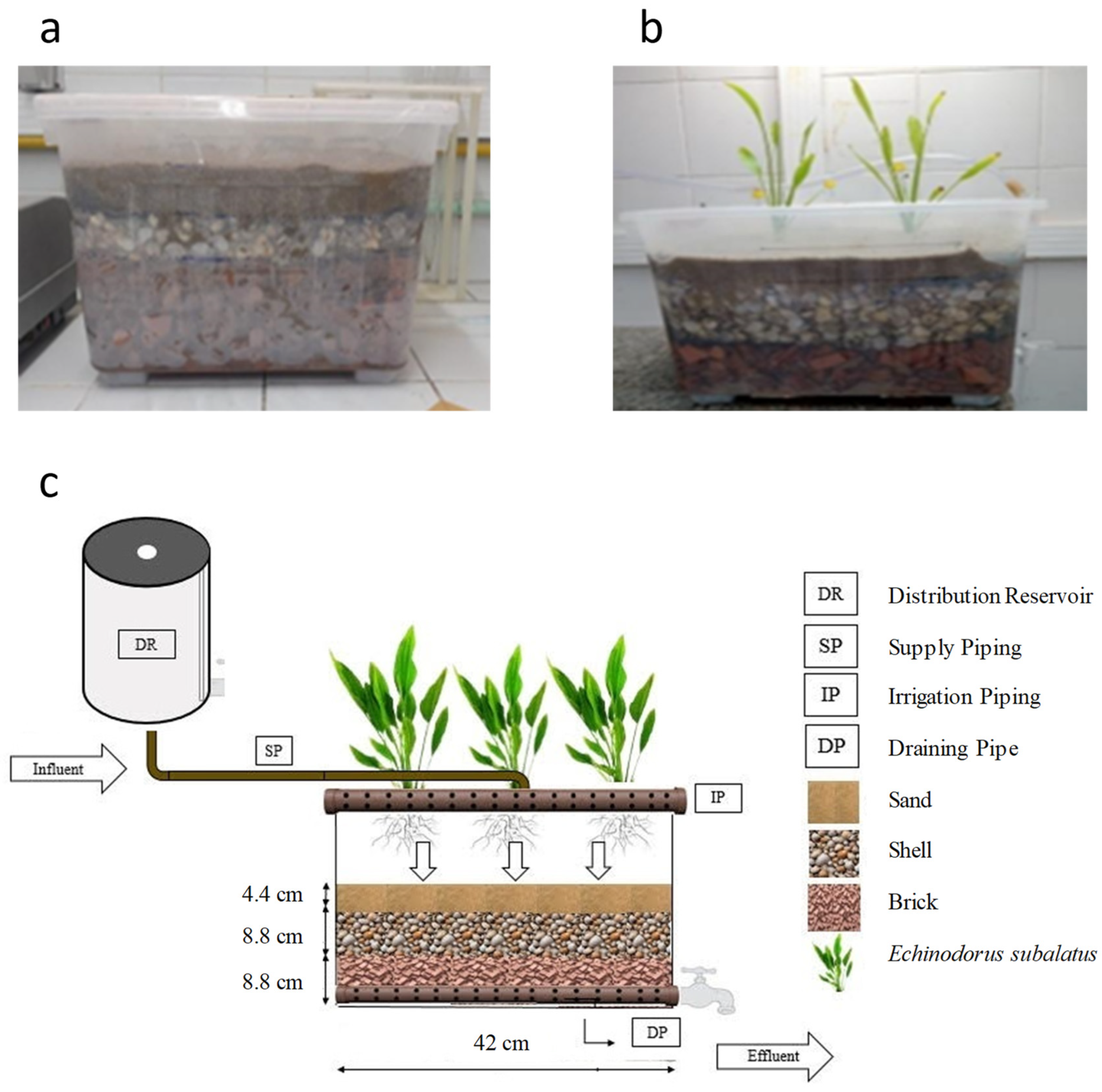

| Parameter | Minimum | Maximum | Average |
|---|---|---|---|
| pH | 6.61 | 7.11 | 6.83 ± 0.20 |
| EC (μS/cm) | 1256.0 | 1452.0 | 1430.0 ± 60.4 |
| Hardness (mgCaCO3/L) | 100.0 | 175.0 | 135.7 ± 23.5 |
| COD (mg/L) | 713.7 | 1030.0 | 843.7 ± 95.0 |
| Phosphorus (mg/L) | 29.6 | 39.8 | 35.3 ± 3.4 |
| Ammonium (mg/L) | 9.7 | 33.8 | 20.5 ± 6.4 |
| Nitrate (mg/L) | 2.8 | 4.1 | 3.4 ± 0.5 |
| Nitrite (mg/L) | 51.5 | 60.3 | 57.7 ± 2.6 |
| LAS (mg/L) | 3.4 | 6.1 | 4.3 ± 0.9 |
| DO (mg/L) | 1.2 | 3.1 | 1.9 ± 0.5 |
| TS (mg/L) | 1360 | 1614 | 1498 ± 82 |
| TDS (mg/L) | 628 | 736 | 700 ± 32 |
| TSS (mg/L) | 640 | 897 | 751 ± 80 |
| Reference | Colonizing Species | Influent COD (mg/L) | Cycle Time (Days) | Efficiency (%) |
|---|---|---|---|---|
| This study | Echinodorus subalatus | 843.7 | 7 | 94.0 |
| Bermudez [7] | Echinodorus subalatus | 1851.7 | 7 | 88.4 |
| Sotiropoulou et al. [21] | Zantedeschia aethiopica | 500–2500 | 1 | 92.0–97.0 |
| Kotsia et al. [22] | Pittosporum tobira Polygala myrtifolia Hedera helix (ivy) | 350 | 7 | 96.0 |
| Nema et al. [23] | Hymenocallis littoralis Phragmites australis Canna indica Colocasia esculenta | 143.3 | 0.50 | 55.4 46.7 52.5 50.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feitosa, A.P.; Rodrigues, K.; Martins, W.E.; Rodrigues, S.M.P.R.; Pereira, L.; Silva, G.M.M. Enhancing Greywater Treatment: High-Efficiency Constructed Wetlands with Seashell and Ceramic Brick Substrates. Appl. Sci. 2024, 14, 9011. https://doi.org/10.3390/app14199011
Feitosa AP, Rodrigues K, Martins WE, Rodrigues SMPR, Pereira L, Silva GMM. Enhancing Greywater Treatment: High-Efficiency Constructed Wetlands with Seashell and Ceramic Brick Substrates. Applied Sciences. 2024; 14(19):9011. https://doi.org/10.3390/app14199011
Chicago/Turabian StyleFeitosa, Adriano P., Kelly Rodrigues, Waleska E. Martins, Sara M. P. R. Rodrigues, Luciana Pereira, and Glória M. M. Silva. 2024. "Enhancing Greywater Treatment: High-Efficiency Constructed Wetlands with Seashell and Ceramic Brick Substrates" Applied Sciences 14, no. 19: 9011. https://doi.org/10.3390/app14199011






