Abstract
The depletion of conventional light petroleum reserves has intensified the search for alternative sources, notably, low-quality heavy oils and byproducts from heavy crude processing, to meet the global demand for fuels, energy, and petrochemicals. Heavy crude oil (HO) and extra heavy crude oil (EHO) represent nearly 70% of the world’s reserves but require extensive upgrading to satisfy refining and petrochemical specifications. Their high asphaltene content results in elevated viscosity and reduced API gravity, posing significant challenges in extraction, transportation, and refining. Advanced catalytic approaches are crucial for efficient asphaltene removal and the conversion of heavy feedstocks into valuable light fractions. Kaolin, an aluminosilicate mineral, has emerged as a key precursor for zeolite synthesis and a promising catalyst in upgrading processes. This article provides a comprehensive exploration of kaolin’s geological origins, chemical properties, and structural characteristics, as well as the various modification techniques designed to improve its catalytic performance. Special focus is given to its application in the transformation of heavy crudes, particularly in facilitating asphaltene breakdown and enhancing light distillate yields. Finally, future research avenues and potential developments in kaolin-based catalysis are discussed, emphasizing its vital role in addressing the technological challenges linked to the growing reliance on heavier crude resources.
1. Introduction
Kaolin, which is an essential mineral found abundantly on the earth’s surface, has been extensively studied in both ancient and modern geological research. Named after a mountain region in southeast China, where it was first discovered and mined, kaolin translates to “gem” and is highly valued in various industrial applications [1,2]. Kaolin, a hydrated aluminosilicate mineral, predominantly comprises the crystalline mineral kaolinite [3], which is a triclinic, two-layer clay mineral that belongs to the class of phyllosilicates. The theoretical chemical composition of kaolinite is represented by the formula Al2Si2O5(OH)4, which translates to 46.54% SiO2, 39.5% Al2O3, and 13.96% H2O by weight. However, other minerals can be found in kaolin raw materials, in addition to kaolinite, including halloysite, dickite, nacrite, mica, and quartz, as well as metal oxides such as K2O, CaO, TiO2,Fe2O3,Na2O, MgO, MnO, and P2O5 as impurities. These minerals are typically found in hydrothermal deposits and are often combined with kaolinite. Halloysite, for instance, has relatively few pure deposits, with one of the few working commercial halloysite deposits located on the North Island of New Zealand.
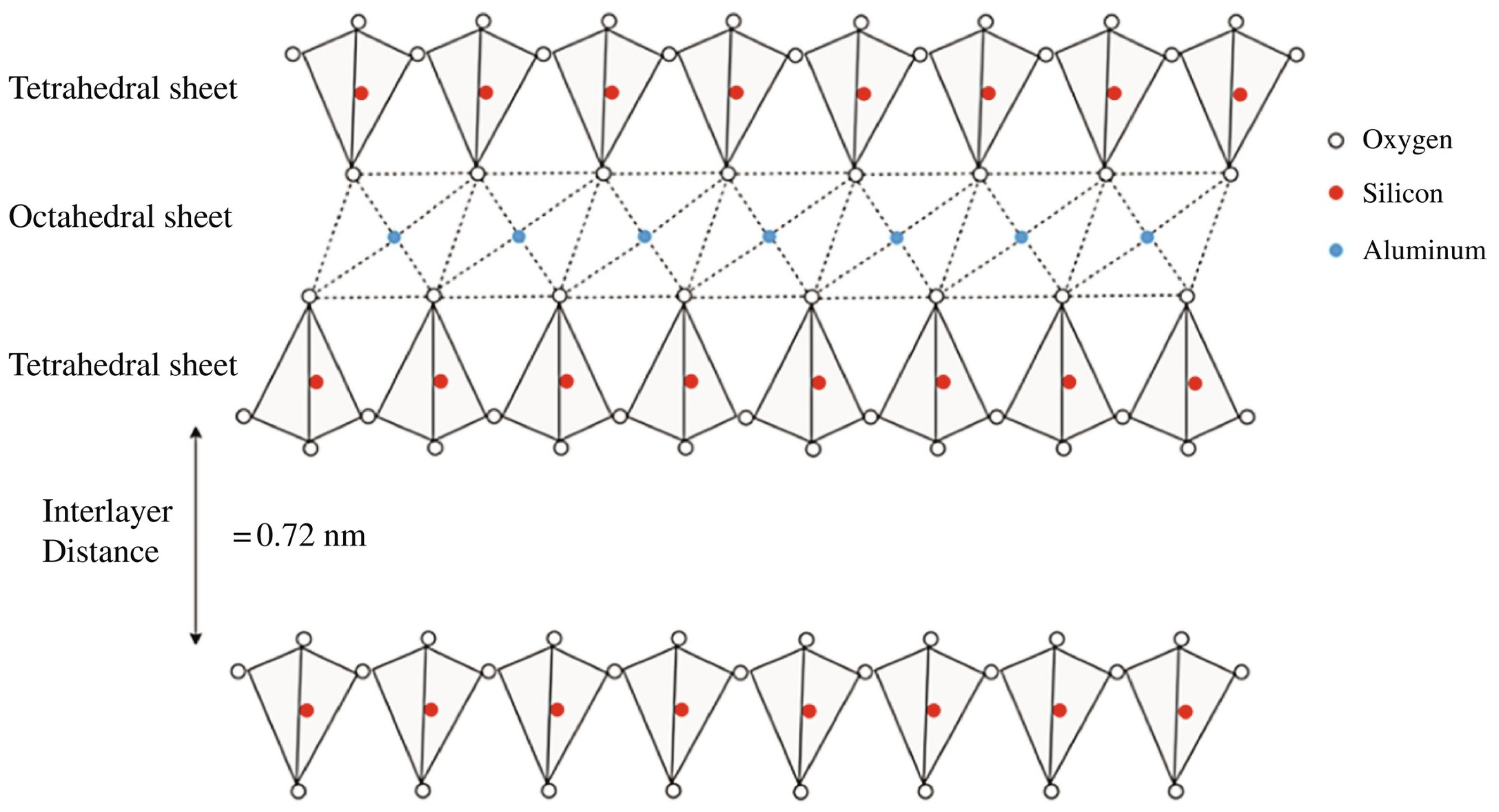
The structure of kaolinite is characterized by a layered arrangement consisting of alternating tetrahedral and octahedral sheets (Figure 1) [4]. The tetrahedral sheets are composed of silicon–oxygen (SiO4) tetrahedra, in which each silicon atom is surrounded by four oxygen atoms, forming a tetrahedral shape. These tetrahedra are linked together in a hexagonal pattern through shared oxygen atoms, creating a continuous two-dimensional sheet of SiO4. Directly bonded to the tetrahedral sheet is the octahedral sheet, which is composed of aluminum–oxygen–hydroxyl (AlO6(OH)4) octahedra. In this configuration, each aluminum atom is surrounded by six oxygen or hydroxyl groups, forming an octahedral shape. The octahedral sheets are connected to the tetrahedral sheets through shared oxygen atoms, which act as bridging atoms between the silicon and aluminum layers. This composite structure results in a two-layered arrangement in which one tetrahedral sheet is bonded to one octahedral sheet, forming a “1:1 layer”. The layers are held together by hydrogen bonds between the hydroxyl groups of the octahedral sheet and the oxygen atoms of the tetrahedral sheet in adjacent layers.
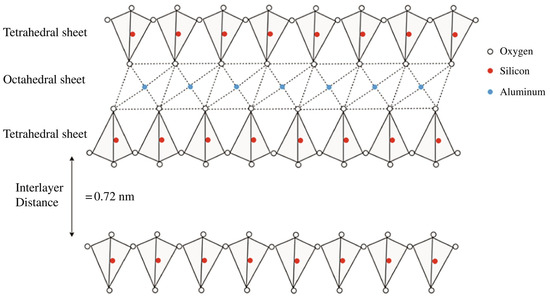
Figure 1.
Schematic structure of the kaolin minerals. Figure extracted from A. Hamza et al. (2023) [4]. Reproduced with the permission of Elsevier.
Based on its structure, kaolin exhibits unique properties, such as its non-swelling nature, small particle size, well-defined dimensions, specific chemical structure, elemental makeup, and low cation exchange capacity (CEC) [5]. These characteristics make it suitable for use in coatings and materials requiring dimensional stability, as well as for food-grade applications due to its minimal reactivity with other compounds [6]. Its small particle size and dimensional properties enhance the wettability, smoothness, and durability of materials, while its chemical composition contributes to its utility in environments requiring chemical and heat resistance. Therefore, kaolin’s versatility and abundance render it a valuable raw material for numerous applications across various industrial sectors. Advancements in kaolin-based materials have enabled their use in multiple forms, particularly in paper manufacturing as a filler and coating agent to enhance printing quality. Its low abrasiveness, combined with its softness and low thermal and electrical conductivity, further increases its commercial value. Although kaolin primarily serves applications in the paper industry, it is also extensively used in ceramics, rubber, plastics, paints, adhesives, and catalysis [7].
Recent advancements have facilitated the production of microporous zeolite frameworks and mesoporous aluminosilicates from kaolin [8,9,10,11]. However, its natural acidity and surface area are lower compared to smectites, palygorskite, and sepiolite, which is attributed to the limited substitution in the kaolin layer [12]. Consequently, enhancing the acidity of kaolin requires the incorporation of specific components into its structure [13]. Research indicates that acid-treated kaolin can improve catalytic efficacy, increase specific surface area, and introduce new acid sites on the surface of the material. This characteristic makes kaolin suitable for various chemical processes, such as enhancing the production of aromatic compounds and improving deoxidation activity [14,15].
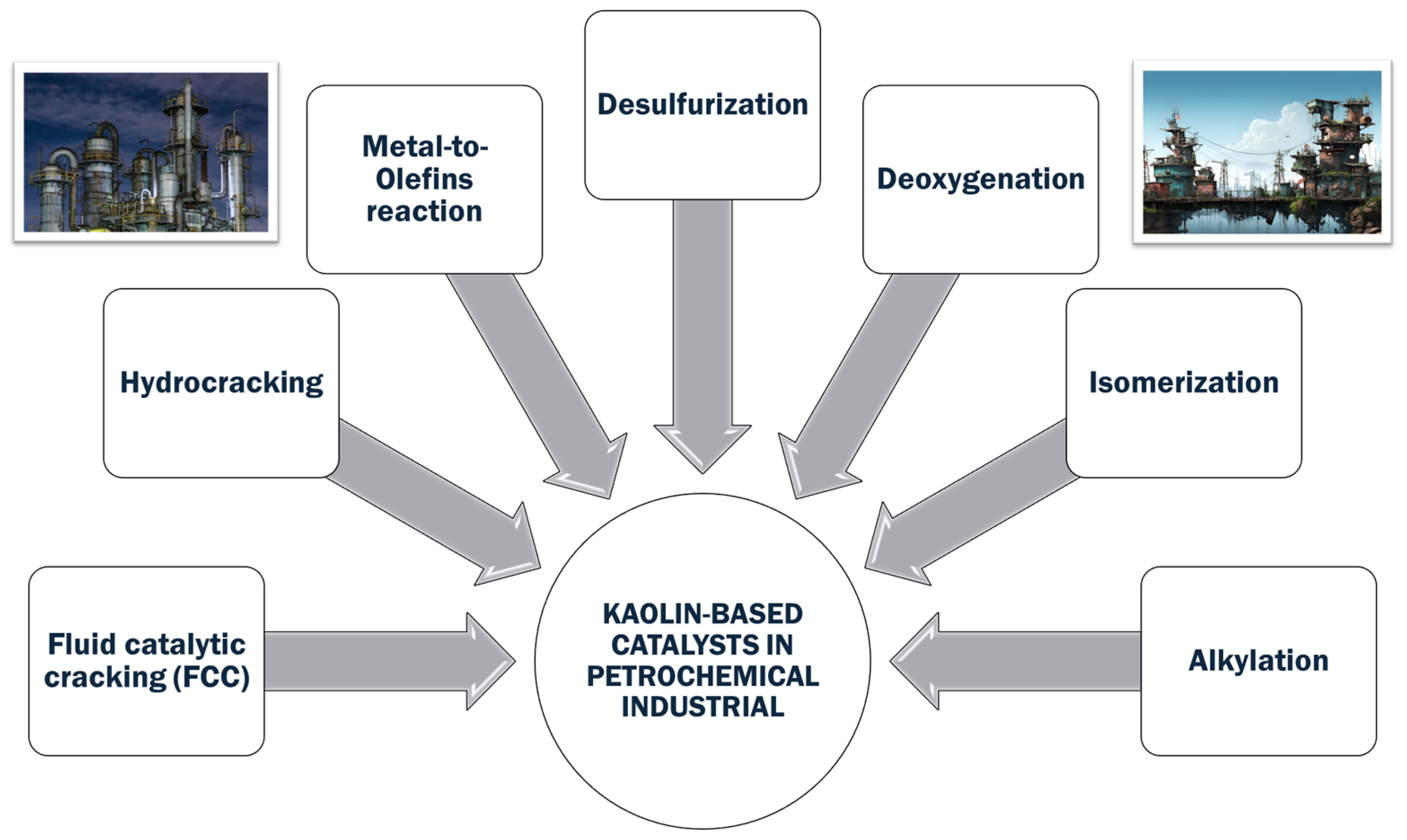
Moreover, kaolin has gained prominence in the petrochemical sector as a catalyst and precursor for many significant industrial processes [16]. As mentioned before, the modification of kaolin-introducing acid sites is a crucial methodology for its use in catalytic cracking reactions, in which kaolin-based catalysts are used to break down large hydrocarbon molecules into more valuable light olefins like ethylene and propylene. In the context of petrochemical applications, kaolin has been extensively studied for its role in producing zeolites and mesoporous materials. Zeolites synthesized from kaolin exhibit excellent thermal stability, acidity, and pore structure, making them effective catalysts for a wide range of chemical reactions. These properties are particularly beneficial in fluid catalytic cracking units, in which zeolite-based catalysts derived from kaolin help optimize the conversion of heavy crude oil into lighter, more valuable products [17]. Moreover, kaolin’s ability to improve the mechanical strength and thermal stability of catalysts further underscores its importance in the petrochemical industry. The incorporation of kaolin into catalyst formulations enhances their resistance to sintering and deactivation, thereby extending the catalyst’s operational life and efficiency. This durability is essential in high-temperature processes, such as hydrocracking and catalytic reforming, in which maintaining catalyst performance over prolonged periods is critical [18].
In addition to its catalytic applications, kaolin also plays a significant role in the production of proppants used in hydraulic fracturing [19,20]. Proppants are materials injected into shale formations to keep the fractures open, allowing for the extraction of oil and gas. Kaolin-based proppants offer high strength and conductivity, ensuring efficient resource recovery and reducing the environmental impact of drilling operations. Recent research has also explored the potential of kaolin in environmental applications related to the petrochemical industry. Modified kaolin has been investigated for its capacity to adsorb and remove pollutants from wastewater, including heavy metals and organic contaminants [21,22]. This application not only helps mitigate the environmental footprint of petrochemical processes but also contributes to sustainable industrial practices.
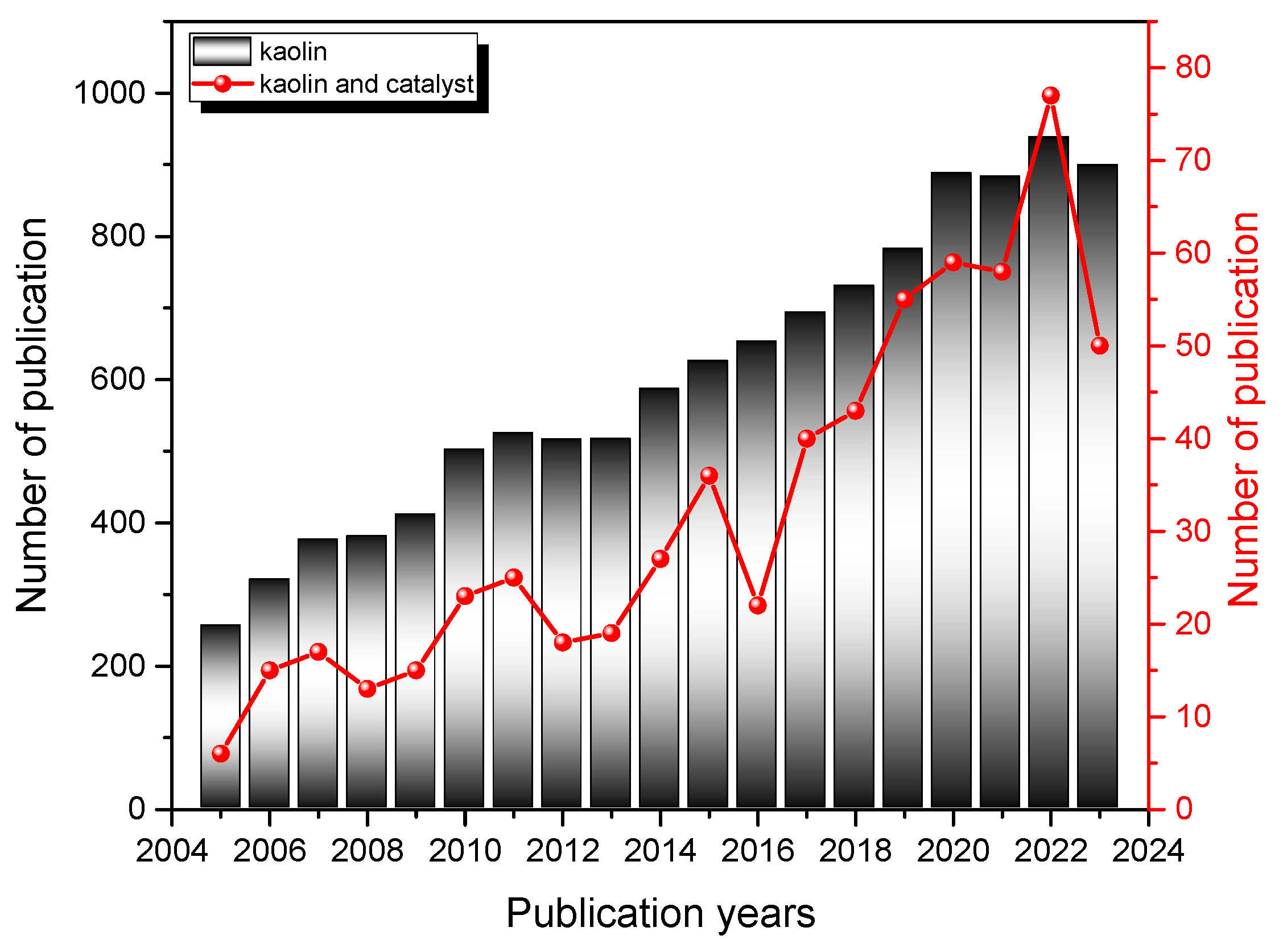
As research and technology continue to advance, the significance of kaolin in the petrochemical sector is expected to grow, driving innovation and efficiency in this critical industry. Figure 2 shows a bibliometric analysis that highlights how interest in kaolin-based materials in general (columns), and kaolin applied in the catalysis field in particular (line), as a research topic has grown in recent years.
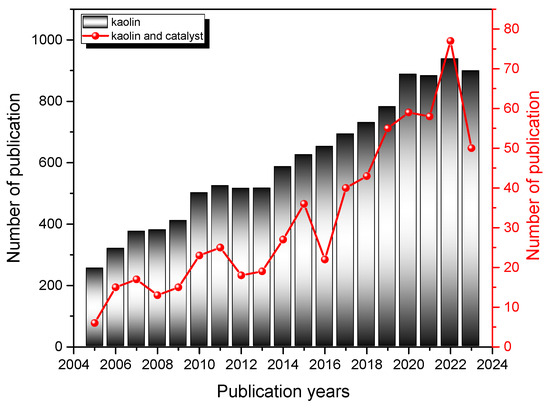
Figure 2.
Number of publications containing the words “kaolin” and “kaolin and catalysis” per year in the period (2005–2024) in the Web of Science (WOS) database. Columns and lines correspond to searches with words in the title, abstract, or keywords, as reported in the inset.
Herein, a comprehensive overview of kaolin is provided, exploring the physicochemical properties of kaolin that make it suitable for catalysis in the petrochemical industry. The review comprises six sections, as follows: it begins with an introduction (Section 1), followed by a geological perspective on the classification of kaolin based on its origin in Section 2. Section 3 discusses worldwide kaolin clay production, demand patterns, and market trends. Section 4 delves into key chemical, structural, and functional properties of kaolin. Section 5 covers modification processes to enhance its industrial applicability, while Section 6 unlocks the potential advanced applications of kaolin in various industries. Section 7 specifically focuses on kaolin in petrochemical catalysis, from fundamental principles to recent innovations. The discussion concludes with Section 8, highlighting promising solutions and future outlooks to ensure the continued prominence of kaolin in modern industrial catalysis for petrochemical processes, including recent advancements in the development of new materials.
2. Classification of Kaolin Based on the Origin: A Geological Perspective
Kaolins can be classified based on their origin into primary categories of pure aluminum silicate minerals with hydroxyl groups, such as kaolinite, dickite, halloysite, and nacrite. These minerals share the chemical formula [Al2(OH)4Si2O5], are predominantly found as fine particles, small sheet-like crystals, or clusters of white earth (<2 μm), and are seldom observed as macroscopic crystals [23]. A summary of the historical milestones in the classification of kaolin minerals is provided below [24,25,26]. The term “kaolinite” was first applied by Johanson and Blake in 1867 based on samples from Kau Ling (High Ridge), a hill near Jauchau Fu, China [27,28]. “Dickite” was named by Allan Brugh Dick in 1908 from specimens found on the Island of Anglesey, Wales, UK [27,28]. The mineral “nacrite” was identified by Brongniart in 1807 at the Einigkeit mine in Brand-Erbisdorf, Freiberg, Saxony, Germany [27,28]. Lastly, “halloysite” was first described by Berthier in 1826 from samples collected in Liege, Belgium [29,30].
Kaolin minerals, which are crucial for various industrial applications, are distributed globally, but only a few deposits meet high quality standards. The grade classification of raw kaolin typically influences its price and suitability for different industrial uses. The main constituents of kaolin are SiO2, which makes up about 46%, and Al2O3, which makes up around 38% [27]. The quality of natural kaolin fillers is regulated by ISO 3262, Part 8, which specifies that high-quality kaolin must contain at least 90% kaolinite (a hydrated aluminum silicate). Commercial kaolin typically comprises kaolinite as the primary component, along with other minor minerals that vary depending on the deposit’s characteristics. This material, which is commonly referred to as kaolin or China clay [28], is extensively used in various industries. Notably, dickite and nacrite are not subject to large-scale exploitation, and this overview does not cover other industrial minerals that also contain significant amounts of kaolinite.
The mineral kaolinite, which imparts the characteristic white hue to kaolin, is prevalent in sedimentary formations akin to kaolin deposits found worldwide. Kaolin is found as sedimentary rock, and its deposits are broadly classified into primary (residual) and secondary (redeposited) types [7]. Primary kaolin forms in situ from the weathering of feldspar-rich crystalline rocks, such as granite (feldspar, muscovite, and quartz) [29]. This process, known as “kaolinization”, disintegrates the rocks into smaller fragments, which are subsequently transported by wind or water. Then, these primary deposits remain at their original location of formation. The modification may involve hydrothermal transformation or weathering under humid, acidic climatic conditions (involving CO2-containing waters). Primary deposits often contain 15% to 30% kaolin, with the remainder consisting of unaltered granite components like feldspar, muscovite, and quartz. In contrast, secondary kaolin deposits result from the erosion of primary deposits, with the eroded materials being transported and subsequently deposited in sedimentary environments like streams, lakes, and basins. Smaller and lighter kaolin particles can travel longer distances, eventually settling in lakes, estuaries, and lagoons to form secondary deposits. In other words, secondary kaolin deposits are generated when existing kaolin deposits undergo reworking through alteration or other geological processes, leading to the accumulation of new kaolinite layers over older ones. Primary deposits include China clay found in Cornwall, England [30]. Secondary deposits, exemplified by those in Georgia and South Carolina (USA), are also a result of this process [31,32]. Impurities in secondary kaolin deposits may include graphite, anatase, pyrite, quartz, muscovite, and smectite. The color of kaolinite can vary from white to grayish-white, depending on the presence of these impurities. There are notable differences between primary and secondary kaolin deposits. Primary clays tend to have larger particle sizes and lower concentrations of anatase and iron oxide compared to secondary clays. Then, the formation of kaolin involves complex geological processes that differentiate primary and secondary deposits, each with distinct characteristics and compositions. These distinctions are critical for various industrial applications, including their role in catalysis for petrochemical processes.
3. Worldwide Kaolin Clay Production, Demand Patterns, and Market Trends
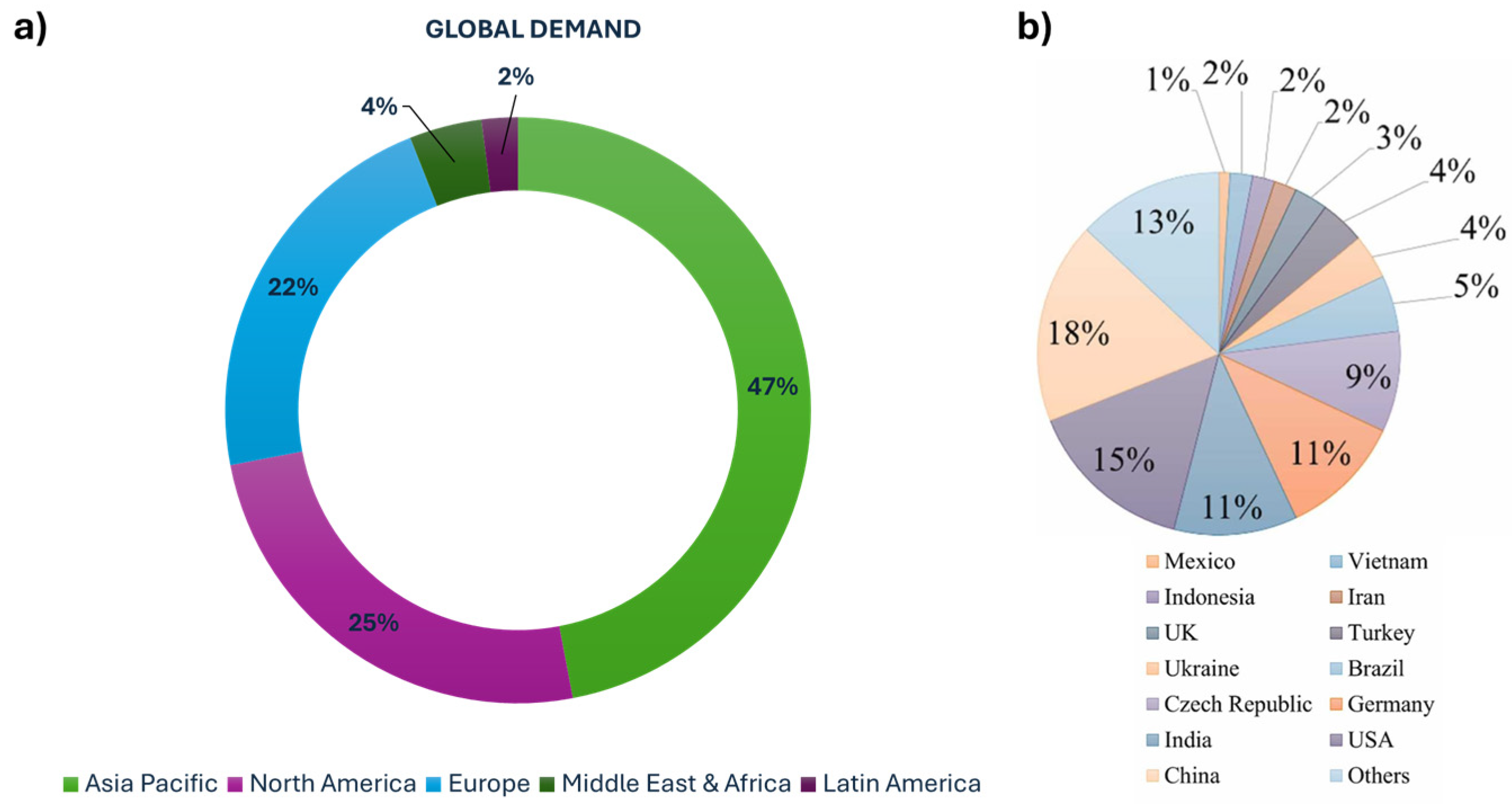
Due to the versatile applications of kaolin, global demand is expected to continue growing. According to “Precedence Research Market Company” (Figure 3a) [33], the regional demand for kaolin in 2022 showed that the Asia–Pacific region had the highest demand at 47% of 24.8 million metric tons (MMts), according to the stats released by the United States Geological Survey (USGS) in 2022, while the Middle East, Africa, and Latin America had the lowest demand at 6%. Other regions, including Europe and North America, demonstrated moderate demand for the mineral. This high demand correlates with the global production pattern of kaolin.
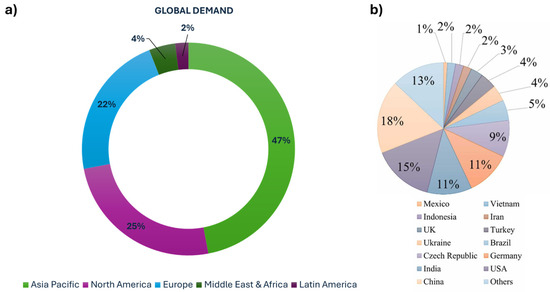
Figure 3.
(a) Regional demand [33] and (b) production by country of kaolin clay [34].
Over the past 15 years, kaolin production worldwide has shown an upward trend, increasing by 22% from 31.5 million tonnes (Mt) in 2010 to 38.5 Mt in 2018. In 2018, the United States led in kaolin production, with 7.3 Mt, followed by Germany (4.3 Mt), India (4.1 Mt), the Czech Republic (3.5 Mt), China (3.2 Mt), Brazil (2.0 Mt), Turkey (1.9 Mt), and Ukraine (1.8 Mt), according to data provided by the study on the EU’s list of critical raw materials in 2020 [34]. Between 2009 and 2016, kaolin production increased by an average of 500 kilotons (kT) per year, with a significant surge of +5 Mt in 2017, mainly due to contributions from the United States, India, Germany, and the Czech Republic (Figure 3b). Developing countries like India and China have steadily increased their kaolin processing capabilities and expanded their industrial production capacity, leading to greater participation in the global kaolin trade. However, these countries primarily produce rough-processed kaolin, with most of their kaolin sales directed to geo-developing nations.
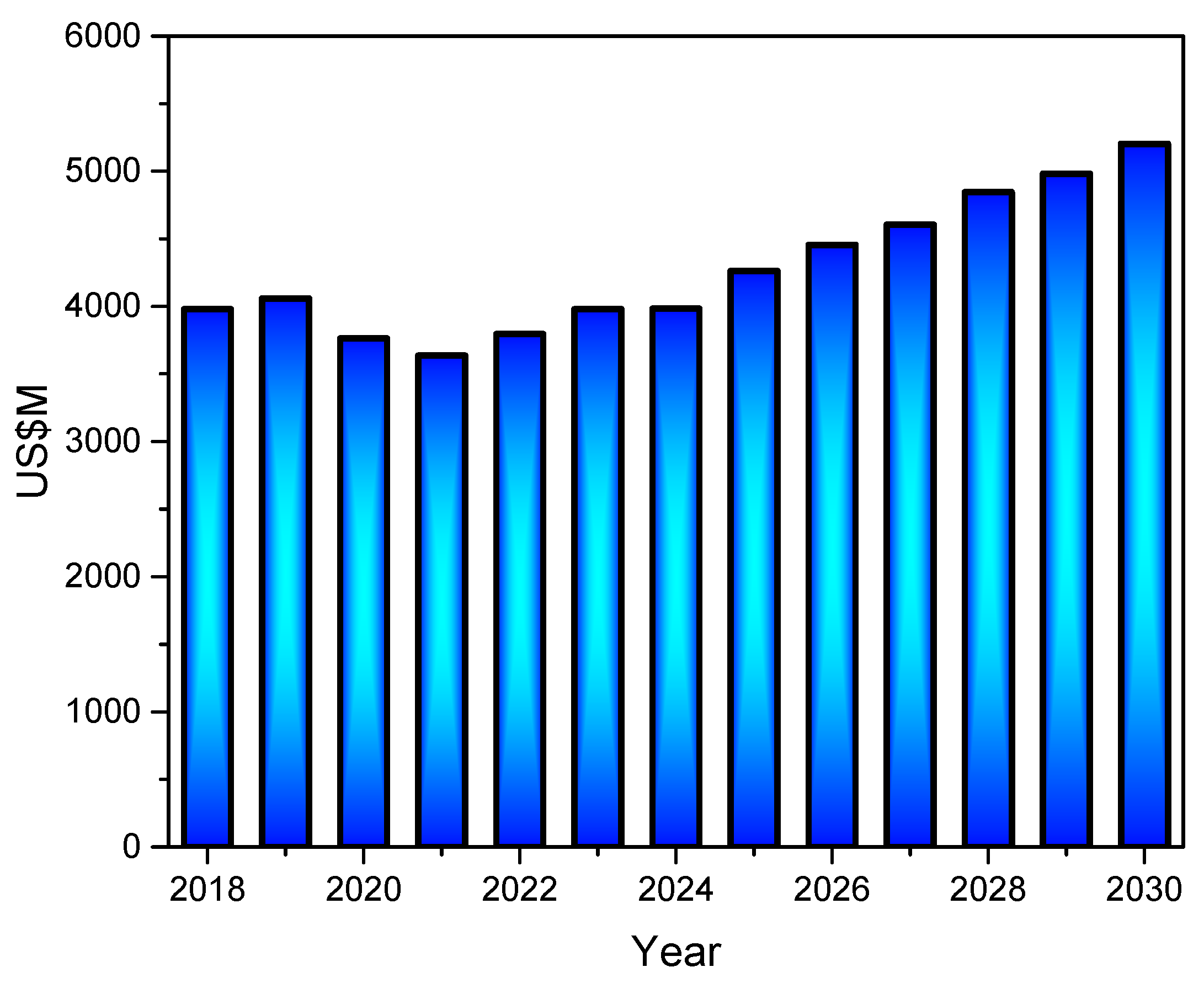
According to market research, the kaolin market was valued at USD 3.98 billion in 2023 and is projected to increase at a compound annual growth rate (CAGR) of 3.9% from 2024 to 2030, with a revenue forecast in 2030 of USD 5.20 billion (Figure 4) [35]. The growing investment in the construction and infrastructure sectors is augmenting the demand for paints and coatings, ceramic-based products, and cement, thereby driving market growth.
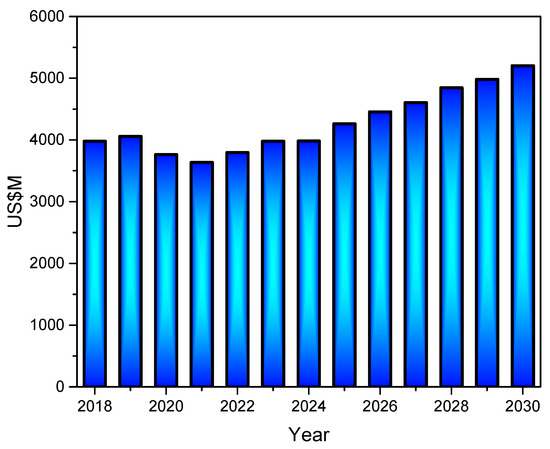
Figure 4.
Global kaolin market from 2018 to 2030, following Grand View Research database [35].
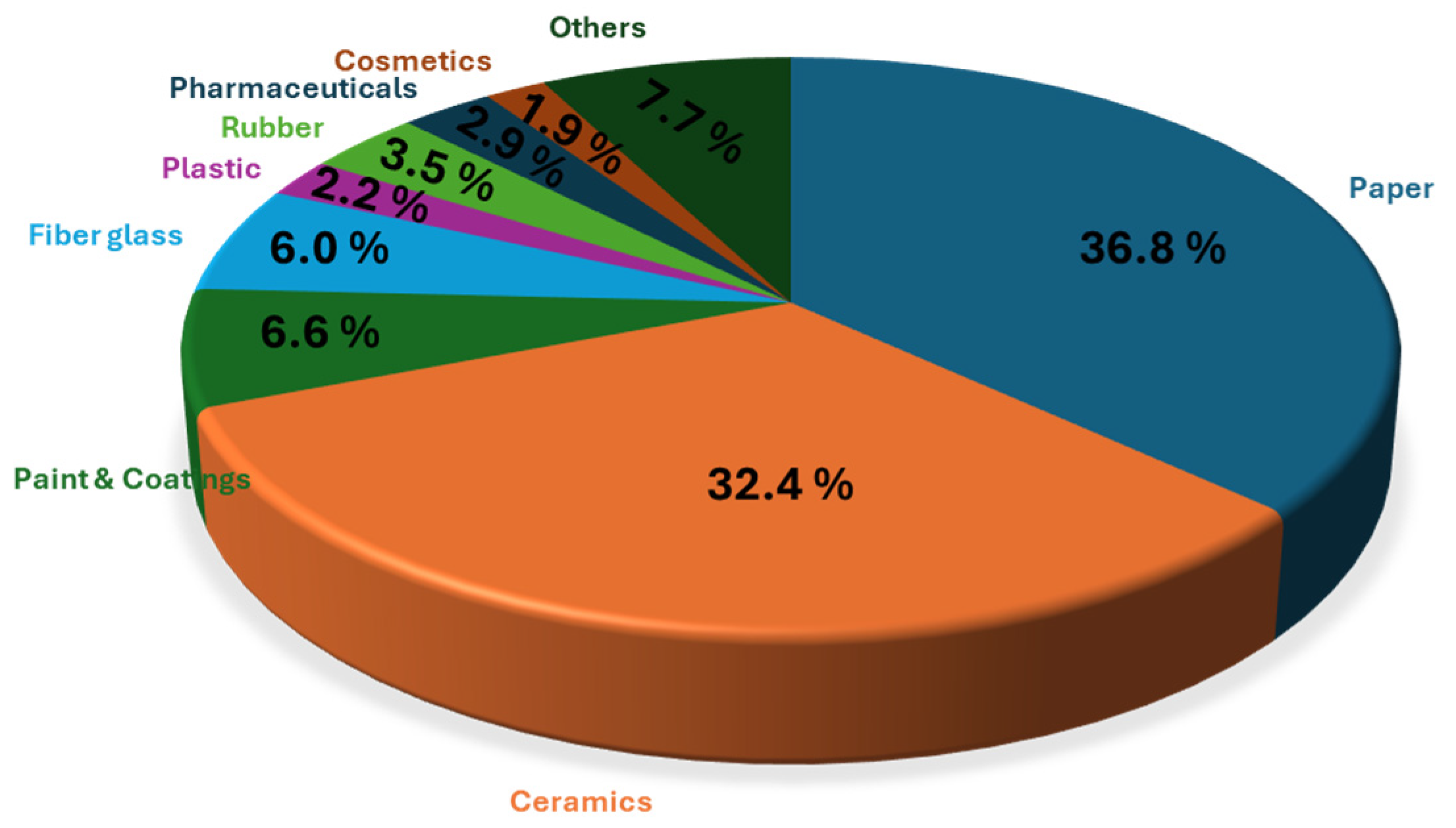
Regarding trends of the global kaolin market, the paper industry led the kaolin market, holding nearly 37.0% of the revenue share in 2023 (Figure 5) [36]. Kaolin improves paper’s brightness, opacity, smoothness, and printability, thereby enhancing ink absorption and pigment holdout. The ceramics segment was the second largest, with an expected revenue CAGR of 5.4% due to kaolin’s fine particle size, chemical inertness, and high fusion temperature, making it ideal for porcelain and bone china. Paints and coatings also showed significant growth potential, driven by construction demand and kaolin’s properties like better suspension and water resistance. Conversely, the rubber segment is projected to decline due to the rise of substitutes and increased use of calcined products for heavy-duty insulation rubber. The sector encompassing other applications, which represents approximately 8%, includes areas such as catalysis, environmental remediation, medicine, agriculture, etc.
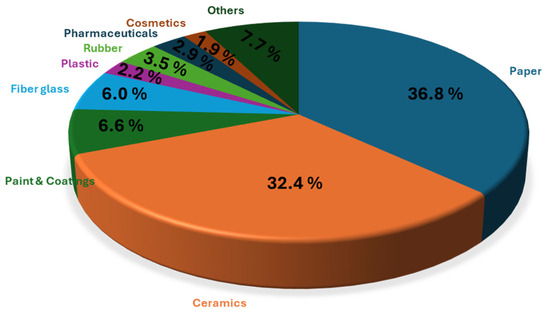
Figure 5.
Application insights of the global kaolin market for 2024, according to the Grand View Research database [36].
4. Key Properties of Kaolin: Chemical, Structural, and Functional Insights
Kaolin’s unique properties, including its chemical inertness, adsorbent capabilities, non-swelling nature, and soothing and detoxifying effects, make it a valuable material for diverse applications. This clay appears bright white upon extraction, but it can develop yellow or rust-colored stains due to contact with iron oxide. Naturally, kaolin exists as a finely textured powder with the following characteristics [37]:
- Chemistry: Unprocessed kaolin has the chemical formula Al2Si2O5(OH)4. Kaolinite, an aluminum silicate mineral, is formed through the chemical weathering of aluminum-rich feldspars found in pegmatites and granites.
- Structure: Kaolin consists of hexagonal crystals ranging from 0.1 to 10 μm in size. The layered structure of these crystals enhances the desirable properties of kaolin.
- Chemically Inert: Kaolin’s lack of reactivity makes it ideal for use in pharmaceuticals, cosmetics, and various industrial applications. Its neutral pH ensures it does not alter the chemical composition of products or promote bacterial growth.
- Adsorbent: Kaolin’s natural absorbency allows it to remove unwanted pollutants, pathogens, and other components from mixtures, thereby enhancing its effectiveness in various applications.
- Non-swelling: The hydrogen bonds between kaolinite crystals prevent water molecules from infiltrating the layers, resulting in kaolin’s non-swelling properties. This makes it a stable additive that does not alter the physical properties of mixtures or products.
- Molecular Stability: Kaolinite exhibits non-expanding behavior and minimal isomorphous replacement due to its high molecular stability. While kaolinite is the least reactive clay, its sensitivity to pH can influence metal adsorption. Metal adsorption occurs through the release of hydrogen (H+) ions at the mineral’s edge sites and on the exposed planar surfaces of the silica and alumina sheets.
- Soothing Properties: Kaolin’s calming properties make it useful for soothing red, irritated skin, benefiting those with sensitive or sunburned skin by reducing redness and irritation.
- Detoxification: Kaolin’s cleansing properties help remove dirt, oil, and impurities from the skin, revitalizing and freshening the complexion by eliminating dead skin cells and debris.
- Gentle Exfoliation: The slightly abrasive texture of kaolin clay enables it to exfoliate the skin gently, promoting a smoother, more luminous complexion by clearing away dead skin cells and unclogging pores.
- Healing Qualities: Kaolin clay is believed to have healing properties and can help treat skin issues such as psoriasis, eczema, and acne. While more research is needed to confirm its efficacy for specific conditions, kaolin clay is widely used in the cosmetic and skincare industries due to its versatility, gentleness, and beneficial properties for various skin issues.
5. Modification Processes of Kaolin: Enhancing Industrial Applicability
Kaolin is a widely available and cost-effective layered silicate mineral. Prior to any modifications, the raw material is collected, crushed, and sieved to achieve the desired particle size. However, unprocessed kaolin often exhibits limitations such as low purity, large particle size, hardness, and suboptimal performance, rendering it inadequate for modern industrial applications. To overcome these limitations and increase kaolin’s value and economic effectiveness across various industries, various methods have been developed to enhance its specific surface area, catalytic activity, and adsorption efficiency. Its high specific surface area, ion exchange capacity, and unique layered structure provide excellent reactive activity and adsorption capabilities.
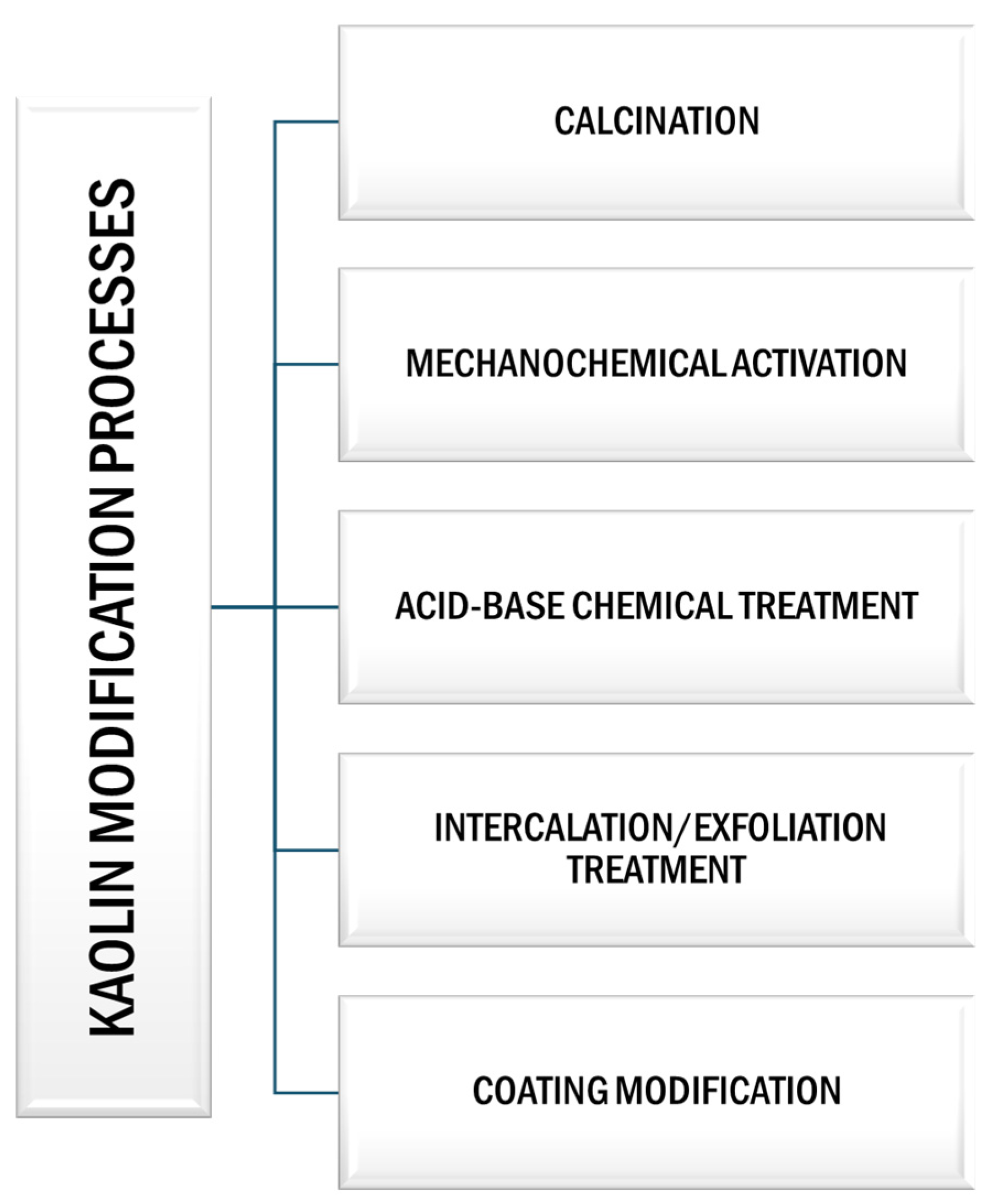
These modification techniques include heat treatment by calcination, mechanochemical activation, chemical (acid–base) modification, intercalation/exfoliation treatment, and coating modification, among others (Figure 6).
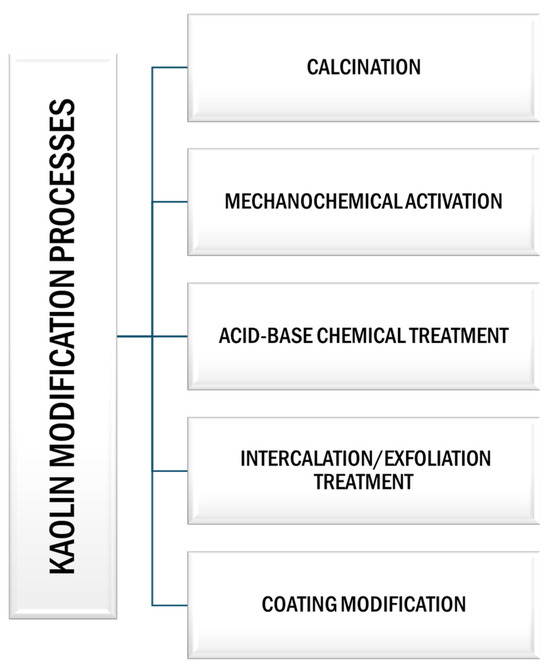
Figure 6.
Schematic representation of different modification processes of kaolin.
5.1. Calcination Process
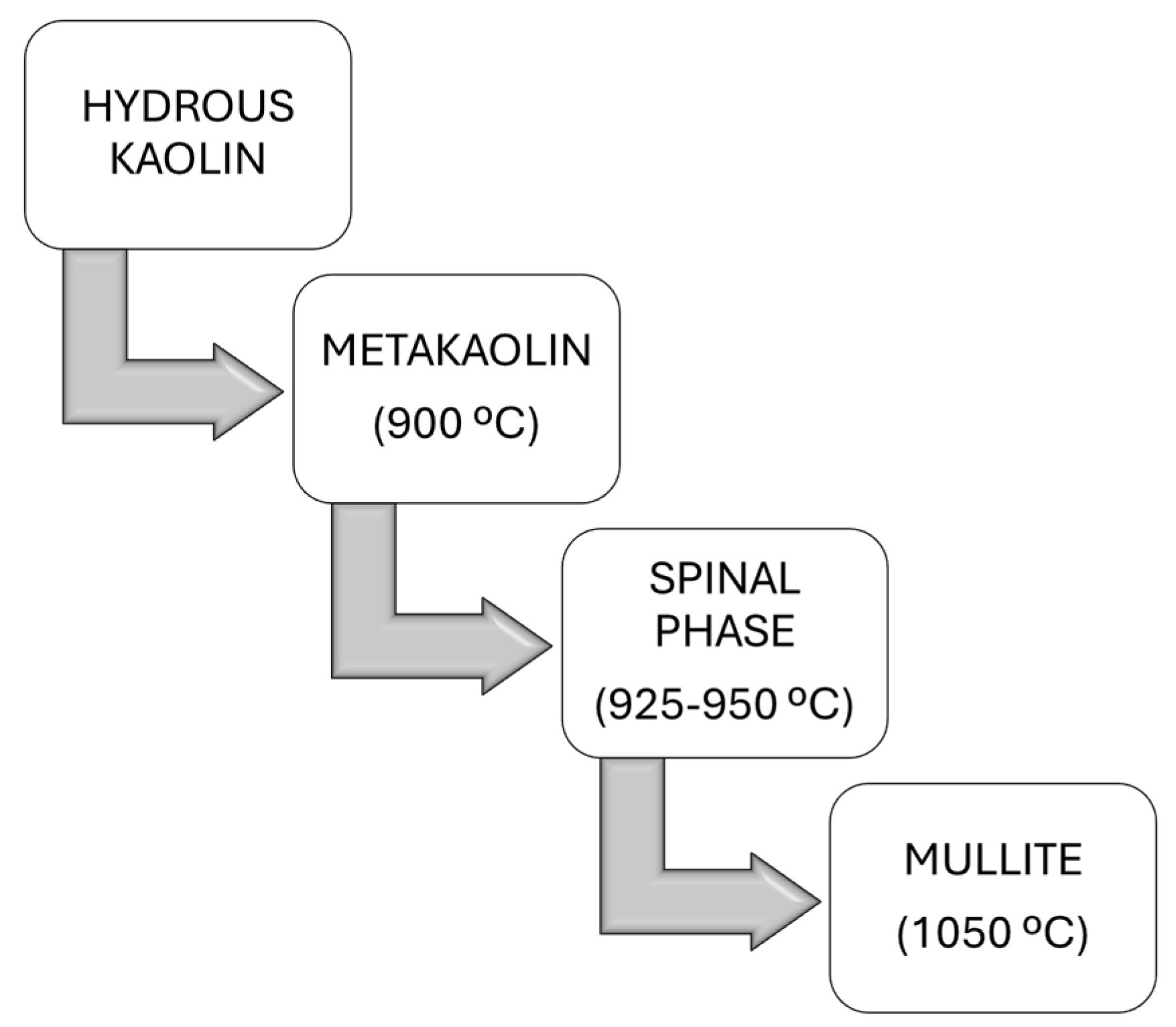
Kaolin containing an attached water molecule is classified as hydrous. The chemical formula of hydrous kaolin is ((Al2O3)(2SiO2)(2H2O)), and this form of kaolin, also referred to as washed kaolin, is extracted from raw kaolin in the earth’s crust through a process commonly known as levigation. The water molecule can be removed from hydrous clay through calcination, a process involving heating the clay to high temperatures, which enhances its color. This process produces calcined clay. By varying the calcination temperature, the following distinct phases can be achieved (Figure 7): metakaolin (550–900 °C), spinel (925–1050 °C), or mullite (>1050 °C) [38].
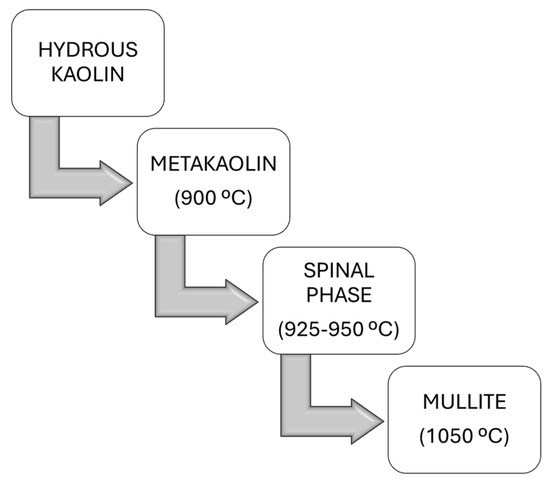
Figure 7.
Phase transition of hydrous kaolin to mullite via calcination temperature adjustment.
Endothermic dehydroxylation, or dehydration, is the process by which disordered metakaolin (Al2Si2O7) is formed through the removal of structural hydroxyl groups and the emission of water vapor [39,40]. The dehydroxylation of hydroxyl groups in kaolinite begins at 800 °C, leading to the disintegration of its crystal lattice and resulting in an amorphous structure of metakaolin enriched with active alumina and silica. This dehydroxylation process is activated when kaolin undergoes thermal treatment at 800 °C and can persist up to 900 °C, as detailed in Equation (1).
2Al2Si2O5(OH)4 → 2Al2Si2O7 + 4H2O
This induces the breakdown of the crystal lattice structure, resulting in the formation of an amorphous metakaolin structure, which is rich in both active silica and alumina. Research indicates that metakaolin is not merely a mixture of amorphous alumina (Al2O3) and silica (SiO2) but rather has a complex amorphous structure with hexagonal layers and some degree of long-range order that is not fully crystalline [41]. Further heating to temperatures between 925 and 950 °C transforms metakaolin into a phase known as aluminum–silicon spinel (Si3Al4O12), also referred to as a gamma-alumina-type structure. This transformation is represented by the following reaction (Equation (2)):
2Al2Si2O7 → Si3Al4O12 + SiO2
The calcination temperature significantly affects the environment of the aluminum atoms within the metakaolin, leading to variations in the material’s structure and properties, including its mechanical strength and thermal conductivity [42]. During calcination at 1050 °C, the spinel phase (Si3Al4O12) nucleates and subsequently transforms into mullite (3Al2O3·2SiO2) and highly crystalline cristobalite (SiO2), as illustrated by the following reaction (Equation (3)):
3Si3Al4O12 → 2Si2Al6O13 + 5SiO2
5.2. Mechanochemical Activation
Mechanochemical activation is a modification technique that involves the reduction of particle size, leading to the increased pore volume and surface area of kaolinite materials [43]. This process enhances the surface reactivity of kaolinite and improves its ion exchange capacity. Mechanochemical activation can be achieved through specific micronization processes (dry pulverization of kaolin raw materials, vibratory mills, jet mills, cyclone autogenous mills, high-speed mechanical impact, ultrafine pulverizes, etc.) or industrial grinding techniques (wet grinding, extrusion, chemical immersion, and dry superfine grinding) [44]. During mechanochemical activation, kaolinite particles undergo delamination, resulting in the formation of large spheroidal agglomerates from the delaminated particles. The delaminated product is a wet xerogel with a randomly structured network, in which hydroxyl units interact to form coordinated water molecules. This interaction is a result of proton migration between hydroxyl units, a process known as prototropy (Equations (4) and (5)).
OH− ⇔ H+ + O2−
H+ + OH− ⇔ H2O
The grinding process leads to a decrease in the structural order of kaolinite, which coexists with disordered materials. The degree of structural order in the delaminated precursor significantly influences the physicochemical properties of the mechanochemically activated kaolinite. Specifically, kaolinite with high structural order experiences a more pronounced effect from mechanochemical activation compared to kaolinite with lower structural order [45]. Excessive grinding, however, can negatively impact the kaolinite crystal structure, transforming it into an amorphous material with reduced surface area and pore volume. This alteration affects the overall performance and application potential of the kaolinite material in various industrial processes.
5.3. Acid–Base Chemical Treatment
Chemical activation is a widely utilized method for modifying kaolin clay, with numerous studies documenting the improvement of surface properties through this procedure. The chemically modified kaolin acquires improved physical and chemical properties, making it suitable for more specialized applications, particularly in catalysis, to improve the activity and product selectivity of chemical reactions of interest [16]. This technique has been extensively researched not only for kaolin but also for enhancing the surface and catalytic properties of fibrous clays, such as sepiolite and palygorskite, and smectites clays, including saponite and montmorillonite [37].
5.3.1. Acid Activation
Acid-activated kaolinite is widely utilized in adsorption and ion exchange due to its enhanced properties post-activation, but also for increasing the catalytic properties and adsorption capacity due to the acid activation process improves the acidity, surface area, pore size, and volume of kaolinite [46,47]. This treatment leads to dealumination, the removal of mineral impurities, the disaggregation of kaolinite particles, and the dissolution of the external layers, altering both the structure and chemical composition of the material [48]. As a result, acid-activated kaolinite is an excellent precursor for synthesizing solid acid catalysts used for catalytic cracking in petrochemical processes [49]. The activation process not only increases the porosity of kaolinite but also enhances its acid centers and surface area, making it suitable as an inorganic host for intercalation and exfoliation. Acid activation involves the protonation of aluminol (AlOH) groups by hydrogen ions from the acidic medium, which leads to dealumination and an increase in the Si/Al ratio of the synthesized materials. This process facilitates absorption by increasing the cation exchange capacity (CEC), pore volume, and surface area [50,51], thereby enhancing the material’s ability to physically absorb water. However, high concentrations of acid can reduce the structural and coordinated water in kaolinite, which is reflected in increased endothermic peaks during recrystallization and dehydroxylation. Regarding solubility, several factors influence this parameter, such as the type of acid used, kaolinite-to-acid ratio, operating temperature, leaching period, and particle size. Generally, kaolinite solubility increases with higher acid concentrations and longer leaching periods, although excessive leaching can decrease the surface area [52].
Inorganic acids are more effective than organic acids in generating new surface acid sites, although they may cause the kaolinite structure to collapse due to excessive leaching of the octahedral layer. Conversely, organic acids preserve the structure of kaolinite better but are less effective at generating new acid sites. Specific surface area and pore volume increase with higher molarity of the activator and longer activation times. Under the same conditions, kaolinite solubility in HCl is less than in H2SO4, with the order of solubility and subsequent increase in surface area as follows: CH3COOH < H3PO4 < HCl < H2SO4 < HClO4 < HNO3 [48,52,53]. Recent interest has focused on synthesizing solid acid zeolites from kaolinite for the petrochemical industry, as the process is cost-effective and produces zeolites with enhanced pore size and structure compared to conventional zeolites [17,54,55]. Both organic and inorganic acids can chemically activate kaolinite, highlighting its potential as a precursor for zeolite synthesis in converting heavy molecules in petrochemical processes [56]. The process requires higher calcination temperatures (550 to 950 °C) to effectively activate kaolinite and obtain metakaolin due to the strong hydrogen bonds between its layers, which make the material resistant to chemical attack. The reactions involved in the dealumination process are as follows (Equations (6) and (7)):
3H2SO4 + Al2Si2O5(OH)4 → Al2(SO4)3 + 2H4SiO4 + H2O
3SO3 + Al2O3 → Al2(SO4)3
Research has shown that kaolin’s strong passivity makes it less susceptible to the effects of acid treatment alone. However, metakaolin, which is produced by heating kaolin, shows increased reactivity when subjected to acid treatment. Calcined kaolin clay, when converted to metakaolin and subsequently treated with hydrochloric acid (HCl) under reflux conditions, can achieve high surface areas, even with minimal alumina removal. This process results in metakaolin with both stable surface areas and significant Brønsted acidity. The properties of the treated metakaolin, including its surface area, stability, and pore size, are influenced by the calcination temperature of the clay and the amount and temperature of HCl treatment. Deviations from the optimal calcination temperature range can significantly reduce alumina solubility and surface area. Additionally, while excessive HCl treatment can enhance the surface area, it may also lead to the over-removal of aluminum, compromising the catalytic activity of the treated metakaolin [57]. Belver Carolina et al. [58] investigated the acid activation of metakaolin by treating it with 6 M hydrochloric acid at room temperature and at 90 °C under reflux for 6 and 24 h. They found that at room temperature, the structure and properties of metakaolin remained unchanged. However, a six-hour reflux treatment removed most octahedral Al3+ cations, forming an amorphous silica phase with a surface area of up to 219 m2/g. Conversely, a more potent acid treatment (reflux for 24 h) resulted in further amorphization of the silica, reducing the surface area to 23 m2/g. These acid-treated solids have potential as substrates for catalysts and adsorbents.
5.3.2. Alkaline Activation
Alkali activation significantly modifies the acidity, surface area, pore size, and volume, as well as the adsorption strength of kaolinite, making it an ideal precursor for solid basic catalysts [59]. This process involves the deprotonation of aluminol and silanol groups, resulting in the simultaneous dealumination and desilication of the kaolinite material [60,61]. Alkali activation is also effective in developing various basic zeolites with low Si/Al ratios, including K–F zeolite, 13X zeolite, A, P, and X zeolites, zeolite N, Na–Y zeolite, MCM-41, and zeolite NaA [62,63,64,65,66,67]. The alkali activation of kaolinite and other clays has received significantly less research attention than acid treatment. Given the solubility of silica and aluminates in alkaline solutions, the gradual leaching of silicic and aluminic layers can be expected during alkali activation. Recent studies, however, have reported the selective removal of silicon sheets to create γ-alumina with intriguing properties [68]. During these alkaline treatments, concentrated solutions were used both at ambient temperature and under reflux conditions. As a result, metakaolin was broken down and transformed into highly crystalline K-F zeolite. Treatments were performed using 1 M and 5 M potassium hydroxide (KOH) solutions, with the strength of the treatment influencing the formation of zeolite crystals and the breakdown of metakaolinite layers. This strong crystallinity led to extremely low surface areas in the treated samples [58].
Interestingly, aluminum is more readily dissolved in acidic treatments than silicon, whereas the opposite is observed in alkali treatments. X-ray diffraction (XRD) patterns reveal significant alterations in the crystal structure after acid treatments, while XRD peaks generally become more distinct following alkali treatments. This suggests that alkali treatments have a smaller impact on mineral structures, although they often create new mineral phases. The dissolution of amorphous mineral particles during alkali treatment enhances basal reflections, effectively “cleaning” the minerals [69]. Considering the high concentrations of silicon dissolved in alkaline solutions, the exterior silica sheets of the minerals may also be removed. In kaolin, the presence of SiO2 undergoes a conversion process resulting in the formation of free SiO2, which readily reacts with alkaline substances [70]. Sodium hydroxide modification facilitates the leaching of silicon from calcined kaolin, increasing the small pore structure [71]. As the duration of alkali treatment increases, the pore size distribution of calcined coal-series kaolin becomes wider, its specific surface area decreases, its pore volume increases, and its cracking activity and selectivity improve.
5.4. Intercalation/Exfoliation Treatment
Intercalation involves the effective insertion of a neutral polar organic substance into the interlamellar spaces of an inorganic host lattice, resulting in nanosized composite materials with a regularly spaced stack. Wide interlamellar spaces are crucial as they prevent interactions between randomly dispersed layers within a consistent polymer matrix, thereby facilitating the exfoliation of the synthesized material. The unique, notably non-centrosymmetric structure of kaolinite renders it an ideal host for intercalation [72], although the efficiency of intercalation is influenced by factors such as the crystallinity and the particle size of the host material. Higher crystallinity typically results in an increased rate of intercalation, while smaller particle sizes generally lead to a reduced intercalation rate [73]. This is due to the fact that a decrease in particle size is associated with a reduction in structural order, which affects the intercalation process. The primary challenge impeding the use of kaolinite as a host material is its rigid interlamellar spacing and absence of charge. Consequently, only a limited range of interlamellar compounds—such as methanol, acetamide, octadecyl amine, dimethylformamide, potassium acetate, N-dimethylformamide, deuterated dimethylsulfoxide, and dimethylsulfoxide (DMSO)—are capable of intercalating directly into the kaolinite layers. Nonetheless, after introducing initial guest compounds into kaolinite, it is possible for further intercalation to occur with additional organic guests that cannot penetrate the kaolinite layers directly [74]. This can be achieved through the covalent grafting of small polar molecules, such as DMSO or methanol, which form Al–O–C bonds with the aluminol basal plane of the kaolinite, thereby expanding the interlamellar spacing [75]. This expansion creates an environment in which subsequent guest compounds can replace the initially intercalated ones. Another approach to facilitating intercalation involves mechanochemical methods, in which the organic guest is co-ground with the inorganic host to achieve the desired insertion [76].
5.5. Coating Modification
Coating modification is an effective technique for enhancing the properties of kaolinite by preventing powder agglomeration and improving fluidity and dispersion characteristics [6]. This process involves applying a coating layer to kaolinite particles, which significantly influences their performance and structural integrity [32,77,78].
One of the primary advantages of coating modification is its ability to inhibit powder agglomeration [79]. Uncoated kaolinite particles tend to clump together due to van der Waals forces and other intermolecular interactions, which can lead to reduced processability and performance. By applying a coating, these interactions are minimized as the coating creates a protective layer around the particles. This protective layer effectively prevents clumping, allowing the particles to remain dispersed and resulting in a more homogeneous powder. Consequently, the coated kaolinite demonstrates improved flow properties and is better suited for various applications.
In addition to addressing agglomeration, the application of a coating layer enhances the fluidity of kaolinite powders [80]. Improved fluidity is crucial for several processes, including mixing, handling, and application in industries in which uniform powder distribution is essential. The coating modification improves the dispersion properties of kaolinite, ensuring that the particles are distributed more evenly when used in composite materials or as fillers. This uniform distribution contributes to the consistency and quality of the final product, making it more effective in diverse formulations.
Beyond improving fluidity and dispersion, coating modification plays a vital role in reinforcing the structural integrity of kaolinite [81]. The coating layer enhances the mechanical strength of the powder by enveloping the kaolinite particles, which helps to maintain their structural cohesion under mechanical stress or during processing. This reinforcement improves the stability and performance of the kaolinite in various applications, ensuring that the powder remains effective even under challenging conditions.
The success of coating modification depends on several factors, with reaction conditions and the specific surface area of the kaolinite particles being particularly critical. Reaction conditions, such as temperature, pH, and coating material concentration, significantly impact the quality and uniformity of the coating layer. Additionally, the specific surface area of the kaolinite particles influences how well the coating adheres and covers the particles. Generally, larger surface areas allow for better coating coverage and more effective modification.
6. Unlocking the Potential: Advanced Applications of Kaolin in Industry
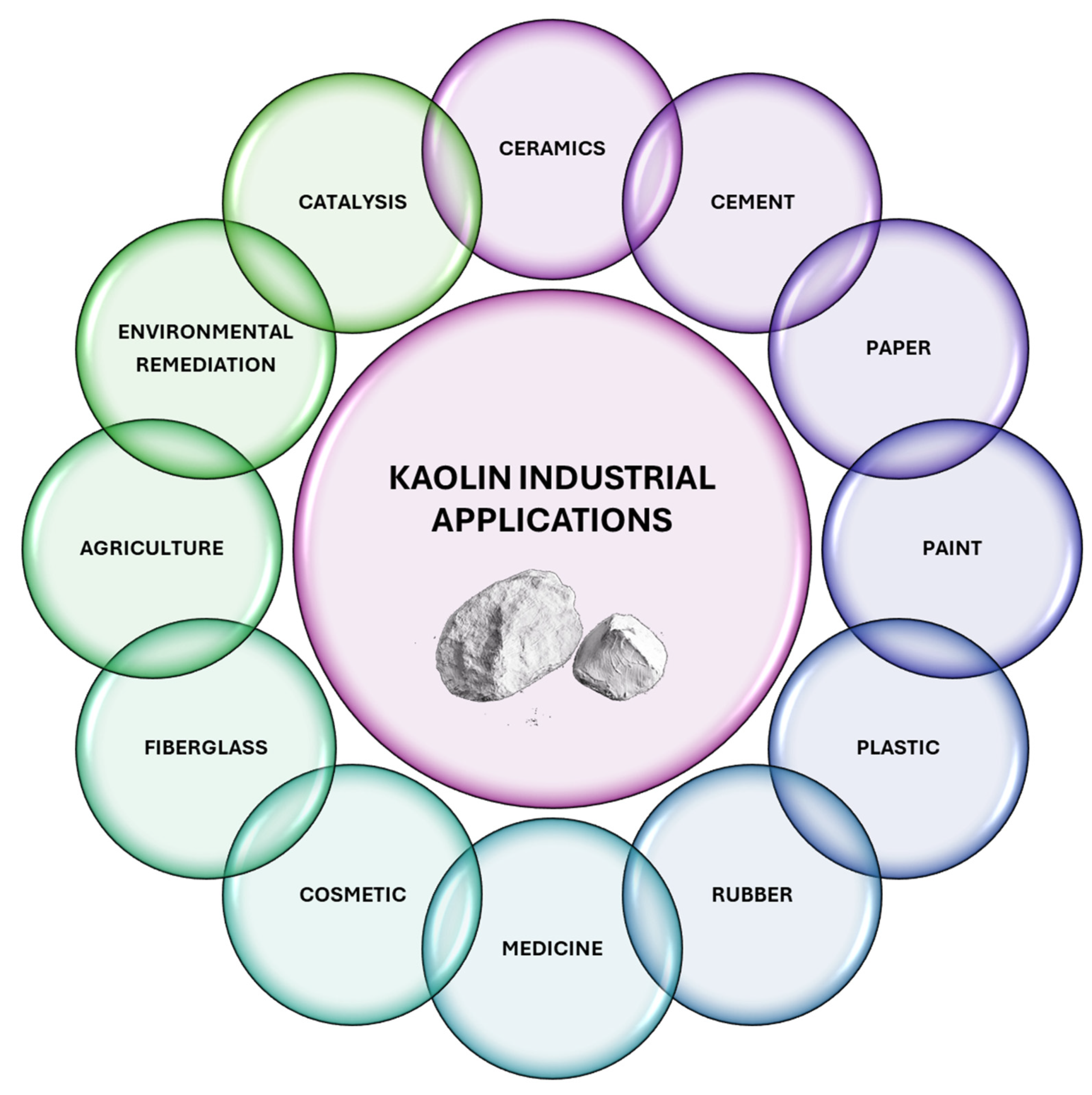
Kaolin, which is primarily composed of the clay mineral kaolinite, has garnered significant interest due to its wide-ranging applications across numerous industries. Its unique physical and chemical properties, such as high brightness, fine particle size, and chemical inertness, make it indispensable in various domains, such as ceramics, cement, paper, paint, plastics, rubber, pharmaceutical, cosmetic, fiberglass, environmental decontamination, and catalysis. This section delves into the multifaceted applications of kaolin, underscoring its pivotal role in advancing technological innovations and its economic significance in industrial processes (Figure 8).
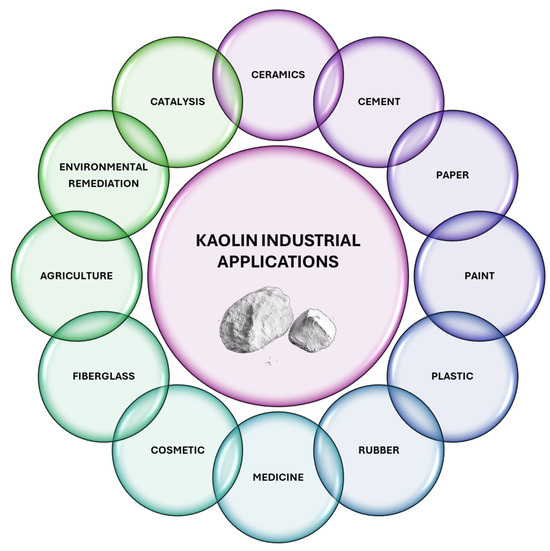
Figure 8.
Schematic representation of industrial applications of kaolin-based materials.
6.1. Ceramic Industry
The industry of ceramics encompasses a wide range of products, including dinnerware, sanitaryware, tile, electrical porcelain, pottery, and refractories, all of which utilize kaolin [82]. Both kaolin and ball clay (kaolinitic clay) are primary ingredients in many ceramic products. The specific ceramic product and the conditions of the manufacturing plant determine the parameters of the kaolin clay used. Key variables include the proportions of kaolin, other clays, silica, and flux in the ceramic body. The consistent chemical composition of kaolin is essential to ensure stable firing and vitrifying properties [83].
Ceramics, which are made by applying high temperatures to earthen materials, date back to prehistoric times when early humans used clay for cooking and found that heat could solidify shapes made from plastic clays [84]. Over time, ceramics evolved into an engineering profession. The properties of ceramic materials vary based on their clay mineral composition, particle size, organic content, and non-clay minerals, with kaolinite being the most crucial due to its beneficial physical and chemical properties.
Key properties provided by kaolin and ball clay include plasticity, green strength, dry strength, fired strength and color, refractoriness, thermal conductivity, water absorption, and controlled shrinkage [7,32,85,86]. Plasticity refers to a material’s ability to deform under stress without breaking and to retain its shape once the stress is removed. Measuring plasticity can be performed by assessing water content, penetration resistance, or deformation under stress. Green strength, which is measured by the breaking strength of a clay bar, must be sufficient for handling without damage. Drying shrinkage occurs as clay dries, and firing shrinkage happens during firing; both are influenced by water content and particle size. Dry strength, which is higher than green strength, relates to particle size and is essential for the final product’s durability [87]. During firing, kaolinite transforms, losing its structure and forming new phases at high temperatures, such as mullite [88,89,90]. The fired color of kaolinite is white, while ball clays are light cream. The modulus of rupture (MOR) of fired pieces, which indicates strength, is significantly higher than that of dried pieces.
In refractories, kaolin and ball clay have been used since the early 1800s for products like firebricks and insulating bricks [6]. Refractory clays must withstand high temperatures and are measured using pyrometric cones that indicate heat resistance. Flint clays, while refractory, lack plasticity and are mixed with plastic clays for shaping. Companies like Villeroy & Boch and Royal Doulton rely on kaolin to achieve the desired aesthetic and functional properties of their ceramic products.
6.2. Cement-Based Material Industry
Concrete is the most commonly used building material worldwide, with ordinary Portland cement (OPC) traditionally serving as the binding agent. Cement is made by combining materials rich in lime, silica, alumina, and iron oxide. This blend is then sintered and ground, with gypsum added as a setting retardant. As reducing carbon dioxide emissions becomes crucial in combating global warming, it is essential to seek alternative low-emission binding agents for concrete. Kaolin, which is a good source of alumina and silica, also contributes to a whiter cement. Although kaolin’s use in cement is currently minimal, the potential of metakaolin as a pozzolan is considerable [91]. The reactive alumina and silica in metakaolin react with excess calcium to form calcium aluminum silicate, enhancing the concrete’s strength, workability, and durability while preventing autogenous shrinkage. Recently, metakaolin, a partially calcined form of kaolin, has been introduced as a pozzolanic additive in high-strength cement. Moreover, studies have shown that incorporating metakaolin can boost the strength of oil well cements by up to 40%. Therefore, Kaolin-based cement materials offer excellent versatility, making their development prospects highly promising. The LafargeHolcim company utilizes kaolin in their products to enhance performance.
6.3. Paper Industry
Kaolin is essential in the paper industry and is primarily used for coating and filling [77,92]. As a filler, kaolin mixes with cellulose fibers in wood pulp, enhancing the paper’s internal structure. For coating, it combines with water, adhesives, and additives, improving smoothness, brightness, gloss, opacity, and printability. Uncoated paper does not meet high-quality printing standards, but the fine particle size and platy shape of kaolinite provide a smooth, dense, and uniformly porous surface for better ink absorption.
Kaolin’s hydrophilic nature makes it easily dispersible in aqueous systems, which is ideal for coating formulations applied to paper surfaces at high speeds. Proper rheology, which should be Newtonian or thixotropic, ensures even coating distribution [93]. Kaolin grades used for coatings typically have particles, of which 80% are less than 2 μm. Delaminated kaolins, which are used in lightweight coatings (LWC), offer a shingle-like structure that enhances ink holdout and smoothness, reducing the paper’s weight and lowering postal costs for publications. Rheology is crucial for kaolin in paper coatings, with viscosity affected by particle size, shape, surface area, and impurities [80,94]. Engineered kaolins are designed to improve specific properties like opacity, gloss, and brightness. Kaolin fillers, typically coarser and less bright, mix with paper pulp to enhance brightness, opacity, smoothness, and printability, offering a cost-effective alternative to pulp [95]. Despite the rise of calcium carbonate fillers in neutral/alkaline papermaking, kaolin remains significant in certain applications.
Additionally, kaolin serves as a fiber extender in automotive gaskets, replacing asbestos due to health concerns, and as a cost-effective extender for titanium dioxide in paper filling and coating, maintaining brightness and opacity while reducing costs [6]. Calcined kaolins, with their high brightness and opacity, enhance light scattering and overall paper quality. Major companies, like Georgia-Pacific and Imerys, utilize kaolin in their paper manufacturing processes to improve surface properties and printability.
6.4. Paint Industry
Kaolin is a critical component in the paint industry, albeit with a smaller market compared to its use in paper coating and filling. Annually, around 600,000 tons of kaolin are incorporated into paints worldwide as extender pigments [96]. It has extensive applications in water-based interior latex paints and is also utilized in oil-based exterior industrial primers. Calcined and delaminated kaolins are particularly favored in interior water-based paints, which are characterized by pigment volume concentrations (PVC) between 50% and 70%. In contrast, semi-gloss and high-gloss water-based systems employ fine particle size kaolins at PVCs below 50% [92].
Kaolin’s fine particle size, with 98% of particles being less than 2 μm, significantly enhances paint properties such as suspension, viscosity, and leveling. As an economical extender for titanium dioxide (TiO2), the dominant pigment in paints, kaolin effectively reduces production costs [97,98]. The high aspect ratio and thin plate-like structure of delaminated kaolins contribute to a smoother paint film surface and improved sheen. The incorporation of calcined kaolins in paint formulations improves the scrubbability and mechanical strength of the paint films. These kaolins also enhance washability, facilitate stain removal, and improve enamel holdout by preventing enamel penetration into the paint structure. In flat paints, calcined kaolin increases hiding power, film toughness, and scrubbability, although it may compromise stain resistance [99]. Paint formulations can be optimized for specific performance criteria by blending various extenders and pigments.
In essence, kaolin’s role in the paint industry is multifaceted, providing cost-efficient improvements to multiple paint properties. Its contributions to suspension, viscosity, and surface smoothness and its function as a TiO2 extender underscore its value in both water-based and oil-based paint formulations. The Krebs & Hiebert and BASF companies utilize kaolin to enhance the performance characteristics of their coatings, such as opacity and durability.
6.5. Plastic Industry
Kaolin-based materials are widely utilized as fillers in the plastics industry due to their various beneficial properties. Kaolin enhances the surface finish of plastics, mitigates cracking and shrinkage during curing, and conceals the fiber pattern in fiberglass-reinforced composites [100]. Additionally, kaolin contributes to improved thermal stability, high impact strength, and resistance to both chemical action and weathering [101,102]. It also aids in regulating the flow properties of plastic materials. The concentration of kaolin fillers in plastic formulations can range from 15% to as high as 60%.
A primary application of kaolin is in polyvinyl chloride (PVC) coatings for wire and cable insulation [103]. Calcined kaolin, along with silane-treated kaolin, is employed to enhance electrical resistance and reduce costs. Calcination, which is typically performed at approximately 1000 °C, lowers the surface energy of kaolin, imparting some degree of hydrophobicity and making it a preferred filler for PVC. Surface modification with silanes further increases this hydrophobicity, optimizing the performance of kaolin in electrical insulation. The effectiveness of kaolin as a filler is highly influenced by particle size, with finer particles generally offering better reinforcement of physical properties across various polymers. Enhanced strength and impact resistance in plastics, such as polypropylene and PVC, can be achieved through the use of fine kaolin particles combined with coupling agents that facilitate chemical bonding between the filler and the polymer matrix [104]. Silane treatments improve the dispersion of kaolin and its interaction with the polymer [92]. Furthermore, the thin plate-like shape of kaolinite benefits certain polymers by improving the flexural modulus, dimensional stability, surface smoothness, and barrier properties [105].
Recent developments continue to emphasize the advantages of kaolin in plastics, with ongoing research focusing on optimizing its properties for enhanced performance in diverse plastic applications. The versatile role of kaolin in improving the physical characteristics and cost efficiency of plastics underscores its value as a critical filler material in the industry. For example, the DuPont company incorporates kaolin in the manufacturing of certain plastic products to enhance stiffness and thermal stability.
6.6. Rubber Industry
In the rubber manufacturing sector, kaolin is a valued material due to its ability to reinforce and stiffen rubber compounds while being more cost-effective than alternative pigments [106]. Although carbon black dominates as the pigment for black rubber products, kaolin is widely utilized for its advantages in non-black rubber [107].
Kaolin’s role is categorized based on particle size, distinguishing between hard and soft clays. Hard clays, which are characterized by their fine particles, are essential in products demanding high durability and wear resistance, such as in the production of shoe heels, tires, conveyor belts, and bicycle tires. These clays provide the necessary stiffness to uncured rubber, thereby preventing deformation during manufacturing. They are also crucial in mitigating mechanical molding challenges in hard rubber goods, including household items, toys, and various novelties [108]. Moreover, they find application in gloves, adhesives, butyl inner tubes, reclaimed rubber, and neoprene compounds.
On the other hand, soft clays are preferred in rubber formulations in which cost efficiency is a primary concern and wear resistance is of lesser importance [109]. These clays are incorporated into a range of products, including tire bead insulation, household items, blown sponges, and rubber toys. They allow for higher filler loadings and enable more rapid extrusion than hard clays. High-aspect ratio, delaminated kaolins are specifically used in white sidewall tires to effectively mitigate air leakage. Additionally, kaolins that have been surface-modified are employed to improve dispersion and reinforcement within rubber compounds [110].
6.7. Medicine Industry
Related to medicine, kaolin is valued in the pharmaceutical industry for its multifunctional roles due to its inherent properties of high purity and stability [111]. Primarily, kaolin serves as an excipient in various formulations, in which it functions as a binding agent and a filler [112,113]. Its use in tablet formulations is particularly notable, as it contributes to the structural integrity of the tablets, ensuring that they remain intact during storage and handling. The adsorption properties of kaolin are also exploited in the preparation of suspensions and gels, in which it helps to stabilize the formulation by preventing the settling of active ingredients.
Moreover, kaolin’s high surface area and particle size distribution make it effective in adsorbing impurities and controlling the release of active pharmaceutical ingredients (APIs) [114]. Its inert nature ensures minimal interaction with other components of the pharmaceutical formulation, making it suitable for sensitive applications. Recent developments have also explored the use of kaolin in controlled-release drug delivery systems, in which its role in modifying the release kinetics of drugs is under investigation [115]. The annual usage of kaolin in pharmaceutical applications underscores its importance as a reliable and versatile component in the production of various medicinal products. Companies like Merck utilize kaolin in the formulation of specific medications, benefiting from its inertness and safety.
6.8. Cosmetic Industry
Kaolin is extensively utilized for its desirable properties in a range of formulations related to the cosmetic industry [93,116]. Its primary function is as an absorbent agent, effectively removing excess oils and impurities from the skin, which makes it a valuable component in facial masks and powders [117]. The fine, platy structure of kaolin contributes to a smooth texture in cosmetic products, enhancing their application and feel on the skin.
Kaolin’s mildness and inert nature render it suitable for use in products designed for sensitive skin. It helps in stabilizing emulsions and preventing the separation of components in creams and lotions. Additionally, its high absorption capacity is advantageous in controlling the oiliness of cosmetic formulations, which improves their performance and longevity [118]. Recent advancements have also explored kaolin’s role in the formulation of mineral-based cosmetics, in which its inclusion contributes to the opacity and coverage of products. The widespread use of kaolin in cosmetics highlights its versatility and efficacy in enhancing both the functional and aesthetic qualities of personal care products. Major cosmetic brands, such as L’Oréal and Estée Lauder, incorporate kaolin in their products for its beneficial effects in skin care.
6.9. Fiberglass Industry
Kaolin plays a vital role in the fiberglass industry, in which it is a key raw material in the production of various fiberglass products [37]. Fiberglass is widely used for applications such as thermal and acoustic insulation, reinforcement in plastics, textile yarns, electronic circuit boards, and even paper, cloth, and roofing shingles. The production of fiberglass requires a blend of several components, with kaolin being a significant part of this mix. The primary materials for fiberglass include silica, kaolin, and limestone, which are complemented by smaller amounts of boric acid, soda ash, and sodium sulfate. Kaolin’s contribution is crucial due to its specific properties, which help in achieving the desired characteristics of fiberglass. For instance, kaolin must meet the following stringent chemical specifications: it should contain approximately 38.5 ± 0.6% alumina (Al2O3), 45.0 ± 0.5% silica (SiO2), no more than 1.5 ± 0.3% titanium dioxide (TiO2), and a maximum of 0.6% iron oxide (Fe2O3) [119]. These specifications ensure that the kaolin integrates well with other materials and contributes to the strength, durability, and overall quality of the final product. The extensive use of kaolin in fiberglass production is reflected in the substantial quantities required annually. The kaolin used is typically processed to a dry form to meet the specific demands of fiberglass production. This large-scale use underscores kaolin’s importance in the fiberglass sector, in which its properties enhance the performance and reliability of various fiberglass applications. Its role in providing stability, improving mechanical strength, and contributing to the structural integrity of fiberglass products makes it an indispensable component in this industry.
6.10. Agriculture Industry
One of the primary uses of kaolin in agriculture is as a pesticide and growth enhancer. Kaolin-based sprays are applied to crops to form a protective film that helps to deter insect pests, reduce heat stress, and prevent sunburn on plants [120]. This physical barrier not only protects the plants but also reduces the need for chemical pesticides, promoting a more sustainable agricultural practice. Additionally, kaolin improves soil health and fertility [121]. When used as a soil amendment, kaolin enhances water retention and nutrient availability, contributing to better root development and overall plant growth. Its fine particle size and high surface area allow it to retain moisture and release it slowly to the plants, which is particularly beneficial in drought-prone areas. Recent studies have also highlighted the role of kaolin in improving the quality of harvested produce [122]. The reflective properties of kaolin sprays help to manage the light environment around the plants, leading to improved photosynthesis and potentially higher yields. Moreover, kaolin treatments can reduce the incidence of certain fungal diseases by creating a hostile environment for the growth of pathogens.
In terms of environmental impact, kaolin is a naturally occurring mineral that is non-toxic and environmentally friendly, making it an ideal choice for sustainable agriculture. Its use can lead to reduced chemical inputs, lower production costs, and healthier ecosystems. The versatility and efficacy of kaolin in various agricultural applications underscore its importance as a tool for enhancing crop production and promoting sustainable farming practices.
6.11. Environmental Decontamination
Kaolin is crucial in environmental remediation, owing to its superior adsorption capabilities and chemical resilience. A key application of kaolin in this domain is the extraction of heavy metals and organic pollutants from polluted water and soil. Its fine particles and extensive surface area offer abundant active sites for contaminant adsorption, making kaolin a highly efficient material for water purification and soil enhancement. In water treatment processes, kaolin is utilized to adsorb and remove toxic metals such as lead, cadmium, and mercury [50,123,124]. Its high cation exchange capacity allows kaolin to bind with these metal ions, reducing their mobility and bioavailability in aquatic systems. This helps in preventing the entry of these harmful substances into the food chain and protecting aquatic life and human health.
Kaolin is also employed in the treatment of industrial effluents. It can be used to coagulate and flocculate suspended particles in wastewater, facilitating their removal through sedimentation or filtration [125,126]. This application is particularly valuable in industries such as mining, textiles, and paper manufacturing, in which large volumes of wastewater are generated. Moreover, kaolin’s role in soil remediation involves the immobilization of pollutants, thus preventing their leaching into groundwater [127]. By incorporating kaolin into contaminated soils, the mobility of contaminants can be significantly reduced, making the environment safer for plant growth and reducing the risk of groundwater contamination. Recent research has focused on enhancing the remediation capabilities of kaolin by modifying its surface with various functional groups or combining it with other materials like activated carbon or biochar [128,129]. These modifications aim to increase kaolin’s adsorption capacity and selectivity for specific pollutants, thus broadening its applicability in environmental cleanup efforts.
Overall, kaolin’s natural abundance, non-toxic nature, and effectiveness in pollutant adsorption make it a valuable resource in the field of environmental remediation. Its use contributes to cleaner water and soil, thereby supporting ecological health and sustainability.
6.12. Catalysis
Kaolin is widely recognized for its catalytic applications beyond the petrochemical industry due to its high surface area, porosity, and chemical inertness. This mineral can be utilized both as catalyst support and as a stand-alone catalyst for the synthesis of microspherical zeolitic molecular sieves and silica aluminophosphate (SAPO) sieves [16]. In environmental catalysis, kaolin-supported catalysts are effective in breaking down organic pollutants in water and soil, such as pesticides and dyes, and are also used in the reduction of nitrogen oxides (NOx) in automotive and industrial exhaust treatment [130]. In fine chemical synthesis, kaolin acts as a catalyst in the production of pharmaceutical intermediates, enhancing reaction selectivity and yields, and is utilized in hydrogenation reactions crucial for manufacturing various fine chemicals and pharmaceuticals [131,132]. Kaolin-based catalysts also play a significant role in renewable energy processes, aiding in the conversion of biomass into biofuels and in water-splitting reactions to produce hydrogen [133,134]. In the polymer industry, kaolin serves as a catalyst support in polymerization reactions [135], facilitating the production of polymers with desired properties, and oxidation reactions essential for manufacturing a range of industrial products. The green chemistry applications of kaolin include the development of catalytic converters that enhance reaction efficiency and minimize waste and photocatalysis for environmental cleanup processes under light irradiation [136,137].
Kaolin’s role in catalysis is notably significant, but its most critical application is within the petrochemical industry. Here, kaolin is pivotal in fluid catalytic cracking (FCC), which is a crucial process that transforms heavy hydrocarbons into valuable lighter products, such as gasoline and petrochemical feedstocks. The upcoming section will offer an in-depth exploration of kaolin’s fundamental contributions to petrochemical catalysis, which stands as the most relevant point of this review study.
7. Kaolin in Petrochemical Catalysis: From Fundamentals to Innovations
The petrochemical industry underpins much of modern life and the global economy, driving advancements in everything from everyday consumer products to high-tech innovations. The utilization of kaolin in catalytic processes holds substantial relevance for the petrochemical industry, offering promising advancements in reaction efficiency and selectivity.
This section delves into the significant role of kaolin in catalytic operations relevant to this critical industry. Initially, an overview of the current state of the global petrochemical industry will be provided. Subsequently, the petrochemical processes designed to transform heavy feedstocks into light distillates will be described. Finally, we will detail the specific catalytic reactions—such as cracking, isomerization, and alkylation—in which kaolin is utilized. By analyzing recent studies and industrial implementations, we aim to elucidate the practical impacts and enhancements that kaolin contributes to these crucial petrochemical processes.
7.1. The Current Global Oil Landscape: Production Giants, Geopolitical Tensions, and Market Volatility
The global oil industry remains one of the most powerful sectors in the world economy. The data and information shown below have been taken from the bibliographic source Statista, which is a statistics portal for market data, research, and studies (80,000 topics from 22,500 sources in 170 industries) [138].
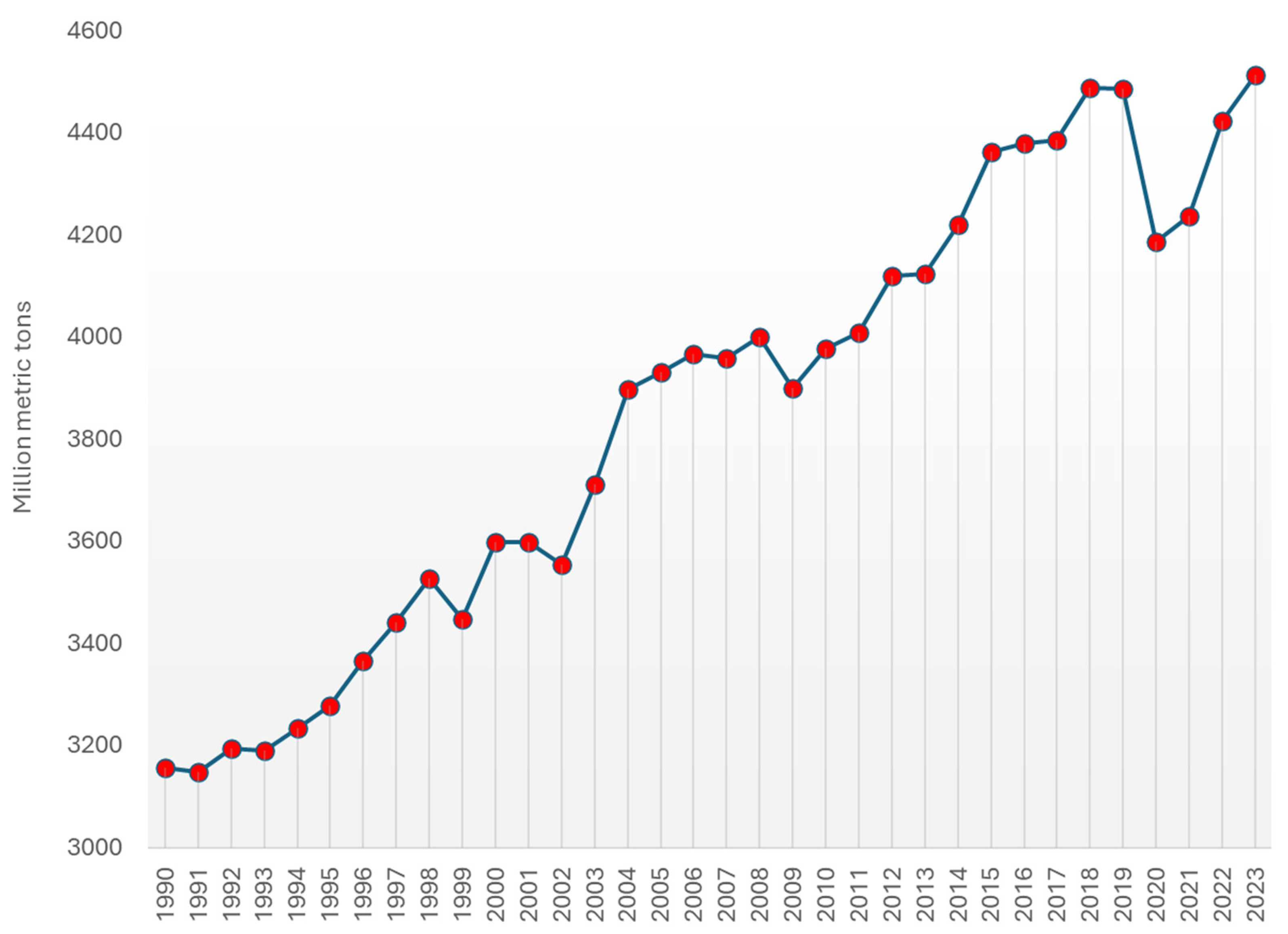
Annually, more than 4 billion tons of crude oil are produced (Figure 9) [139], with a significant portion—approximately one-third—originating from the United States, Saudi Arabia, and Russia. Although Middle Eastern countries continue to hold over half of the world’s proven oil reserves (Table 1) [140], the United States has surpassed these nations in production volumes, largely thanks to advancements in shale oil and tar sand extraction. In 2023, the U.S. alone accounted for more than one-fifth of the daily global oil production.
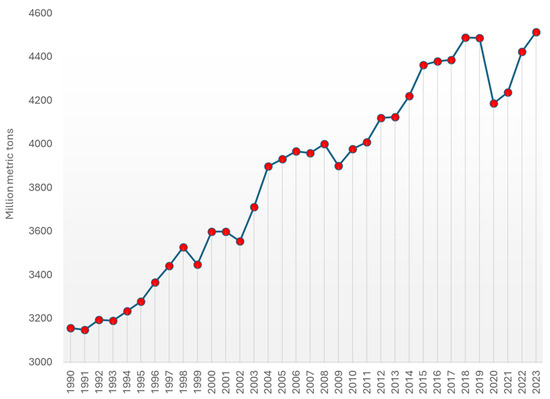
Figure 9.
World oil production from 1990 to 2023 (in million metric tons) [139].

Table 1.
Percentage distribution of global oil reserves from 1992 to 2020, by region [140].
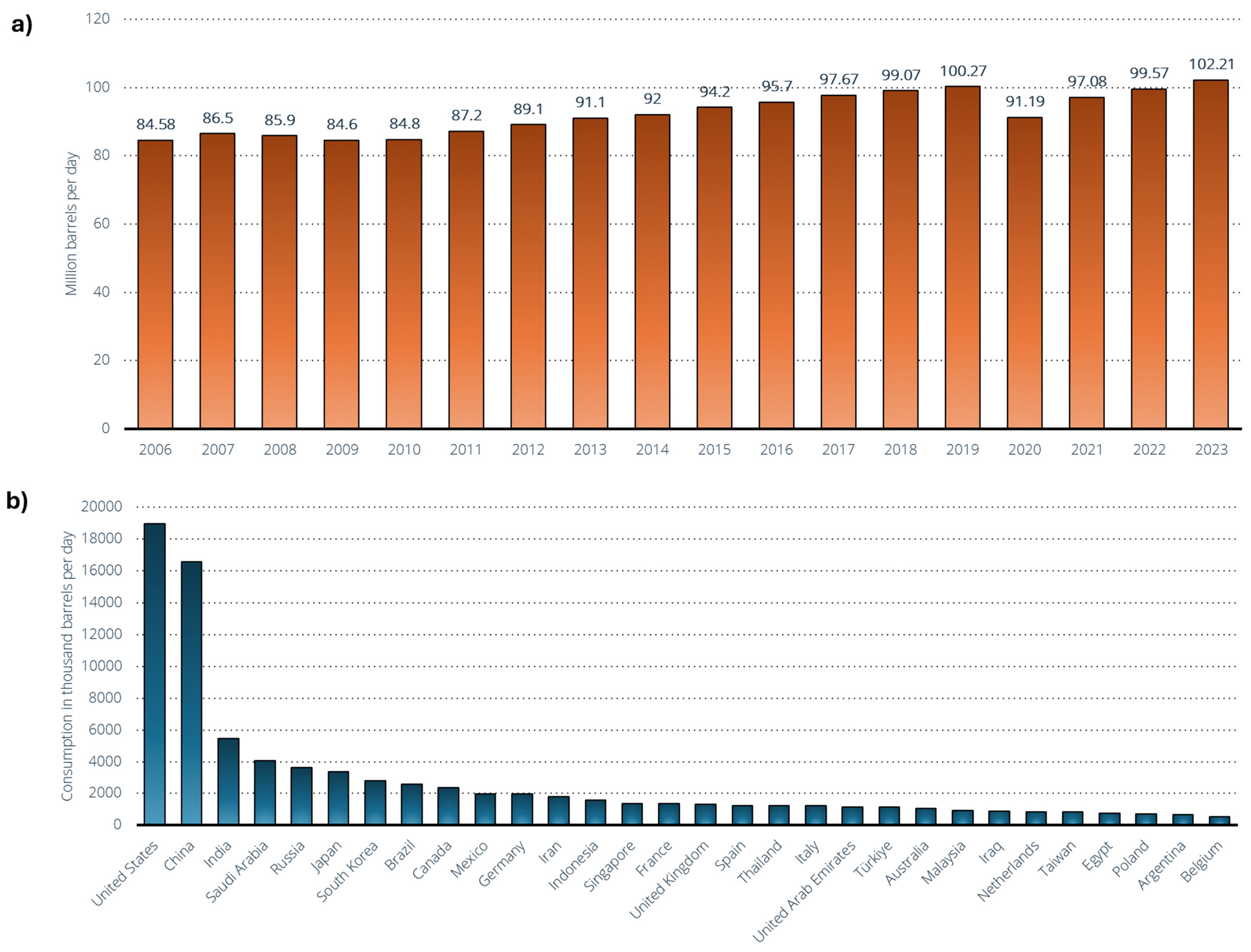
Figure 10 represents the demand for crude oil worldwide from 2005 to 2023 (Figure 10a) and the leading oil-consuming countries worldwide in 2023 (Figure 10b). Global oil demand hovers around 102 million barrels per day in 2023 [141], but the source anticipates that economic activity and corresponding oil demand will rise by the end of the year, with projections indicating that it could exceed 104 million barrels per day. The road transport sector is the largest oil consumer worldwide, comprising nearly half of the global oil demand, primarily due to the heavy reliance on petroleum-based motor fuels. The Organization of the Petroleum Exporting Countries (OPEC) projects that global demand for oil products will reach 110 million barrels per day by 2045 [141], with transportation fuels like gasoline and diesel expected to remain the most consumed products. Diesel and gasoil demand are projected to increase to 30.1 million barrels per day by 2045, up from 27.6 million barrels in 2021. Gasoline demand is forecasted to reach 27.6 million barrels per day by 2045.
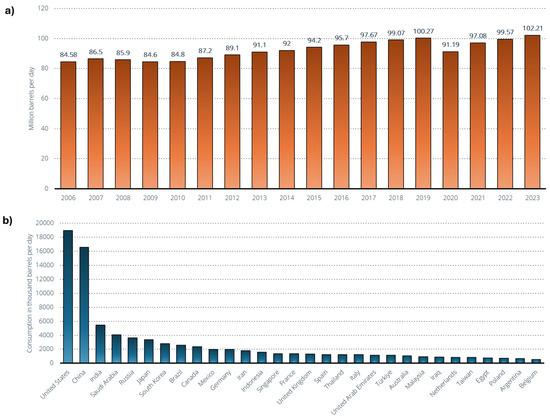
Figure 10.
(a) Demand for crude oil worldwide from 2005 to 2023 [141]. (b) Leading oil-consuming countries worldwide in 2023 (in 1000 barrels per day) [142].
The United States and China rank as the leading oil consumers worldwide, each using 19 million and 16.6 million barrels per day, respectively [142]. The United States has seen a rapid increase in its oil usage, making it one of the leading importers despite possessing significant domestic reserves. Over the past decade, the proportion of global oil consumption from Europe and North America has begun to decline, while consumption in the Asia–Pacific region and other areas has increased. As alternative energy sources become more cost-effective and new transportation technologies gain prominence, global oil consumption is anticipated to reach its peak in the near future.
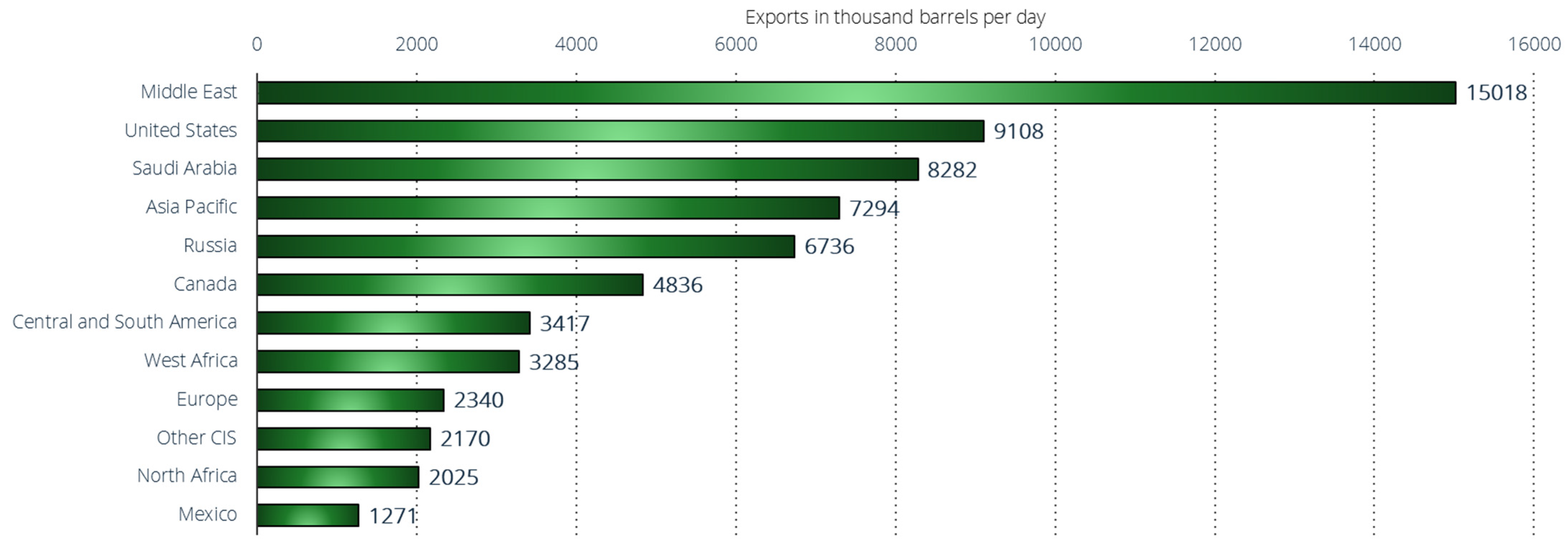
The Middle East, as a region, is the world’s largest oil exporter, with approximately 15 million barrels per day exported in 2022 (Figure 11) [143]. Approximately one-third of global oil production originates from the Middle East. Many countries in this region are members of OPEC, which is an organization that wields considerable influence by collectively setting oil prices. OPEC was founded in Iraq in 1960 and currently comprises 13 member nations. Beyond the Middle East, the United States, Saudi Arabia, Asia–Pacific region, and Russia are the principal oil-exporting nations, each exporting in a range of 7 to 9 million barrels per day in 2022. Russia has maintained a significant and stable role in global oil exports over the past decade. Nowadays, the reliance on Russian oil exports continues to pose a significant challenge to the global economy. Russia, although not the top exporter, remains a crucial geopolitical supplier, pumping over 11 million barrels per day. The lack of readily available substitutes for Russian oil further complicates the situation, emphasizing the global market’s vulnerability. In contrast, the United States has emerged as a major player more recently. Since 2010, U.S. petroleum exports have nearly quadrupled, surpassing Saudi Arabia’s export volumes in 2021.
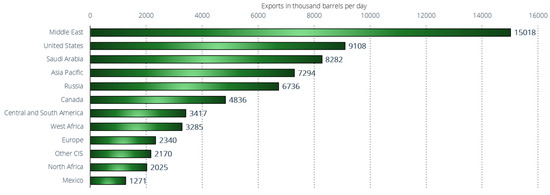
Figure 11.
Leading crude oil and oil products exporters worldwide in 2022, by region or country (in 1000 barrels per day) [143].
In response to fluctuating market conditions, OPEC and its allies have extended their production cuts until 31 December 2025. These reductions, amounting to nearly 6% of global demand, set the collective production ceiling at 39.7 million barrels per day. The primary motivations for these cuts are to support oil prices and to stabilize the crude oil market amidst anticipated demand declines. However, this measure does not fully address the existing bottleneck in refining capacity. Recent studies by Wood Mackenzie indicate that over 20% of refining capacity may be at risk of closure due to diminishing gasoline margins and increasing pressure to reduce carbon dioxide emissions [144]. China has significantly expanded its refining capabilities, with the China Petroleum and Chemical Corporation (Sinopec) now operating one of the largest distillation capacities. Despite these advancements, the United States remains the leading oil refiner, processing more than 18 million barrels per day as of 2023 [139].
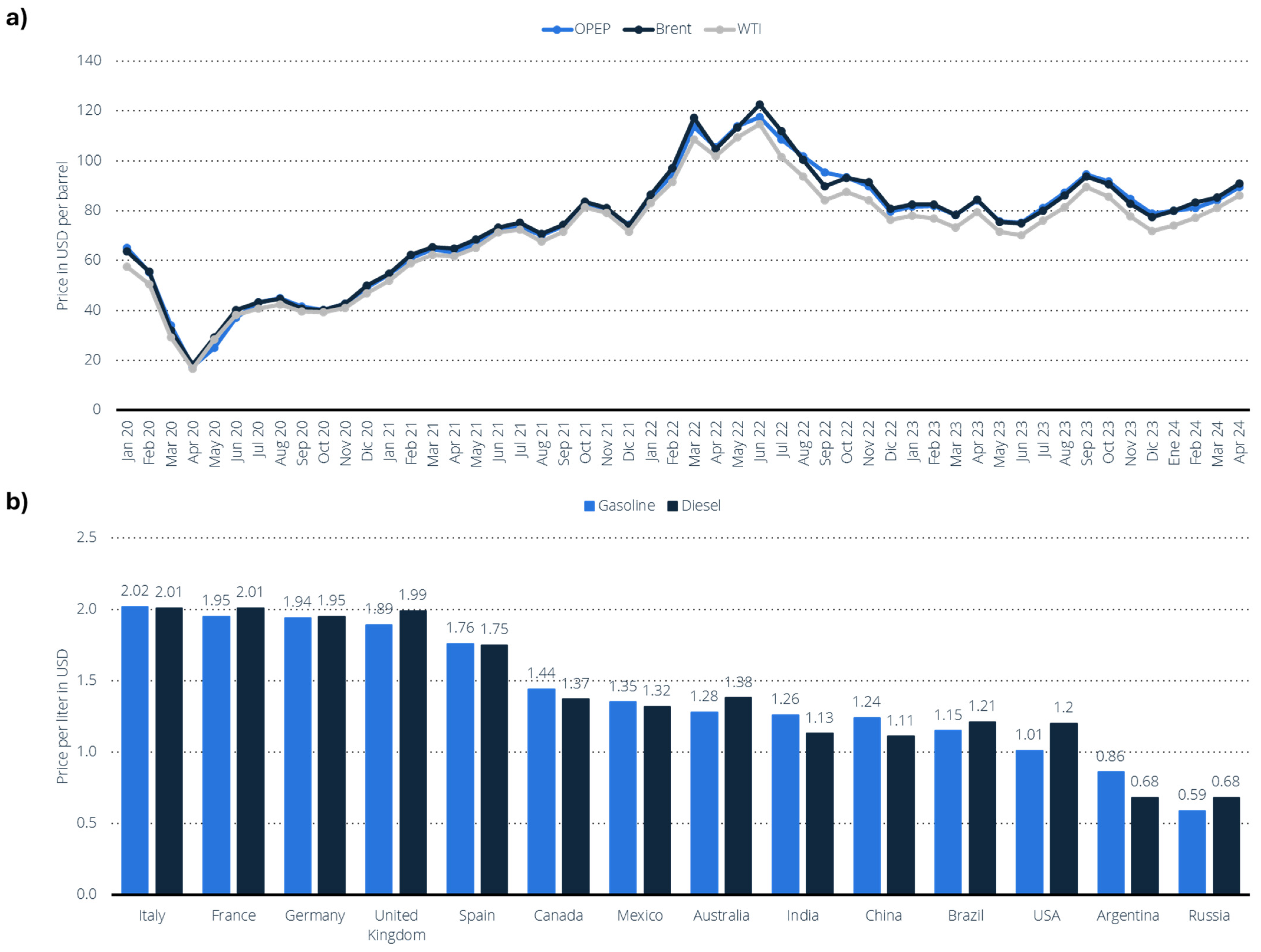
Regarding price volatility, the oil market has experienced significant price fluctuations (Figure 12a) due to a combination of factors, including the COVID-19 pandemic, sanctions on Russia, and geopolitical tensions in the Middle East, particularly the ongoing conflict between Gaza and Israel [145]. Indeed, the prices of derived oil products, such as gasoline and diesel, have also been impacted, varying according to the country (Figure 12b) [146]. In January 2022, the spot prices for key crude oils (OPEC, Brent, and WTI) were around USD 85 per barrel. However, prices surged to as high as USD 140 per barrel within the next six months. More recently, in January 2024, the prices of these key benchmarks had fallen below USD 90 per barrel, reflecting a complex and shifting market landscape. Nonetheless, ongoing regional conflicts suggest that prices may rise again by the end of the year.
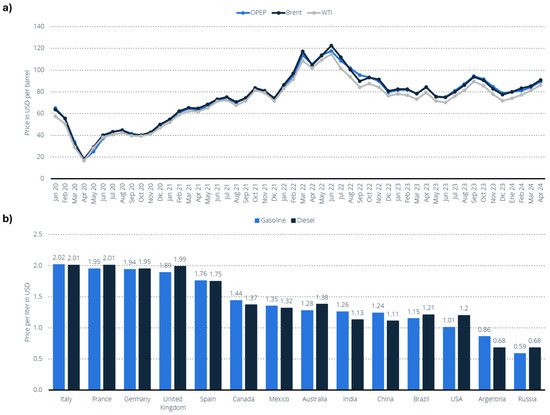
Figure 12.
(a) Average price of Brent, OPEC, and West Texas Intermediate (WTI) crude oil from January 2020 to April 2024 (in dollars per barrel) [145]. (b) Average price per liter of gasoline and diesel (gasoil) in certain countries as of October 2023 (in dollars per liter USD) [146].
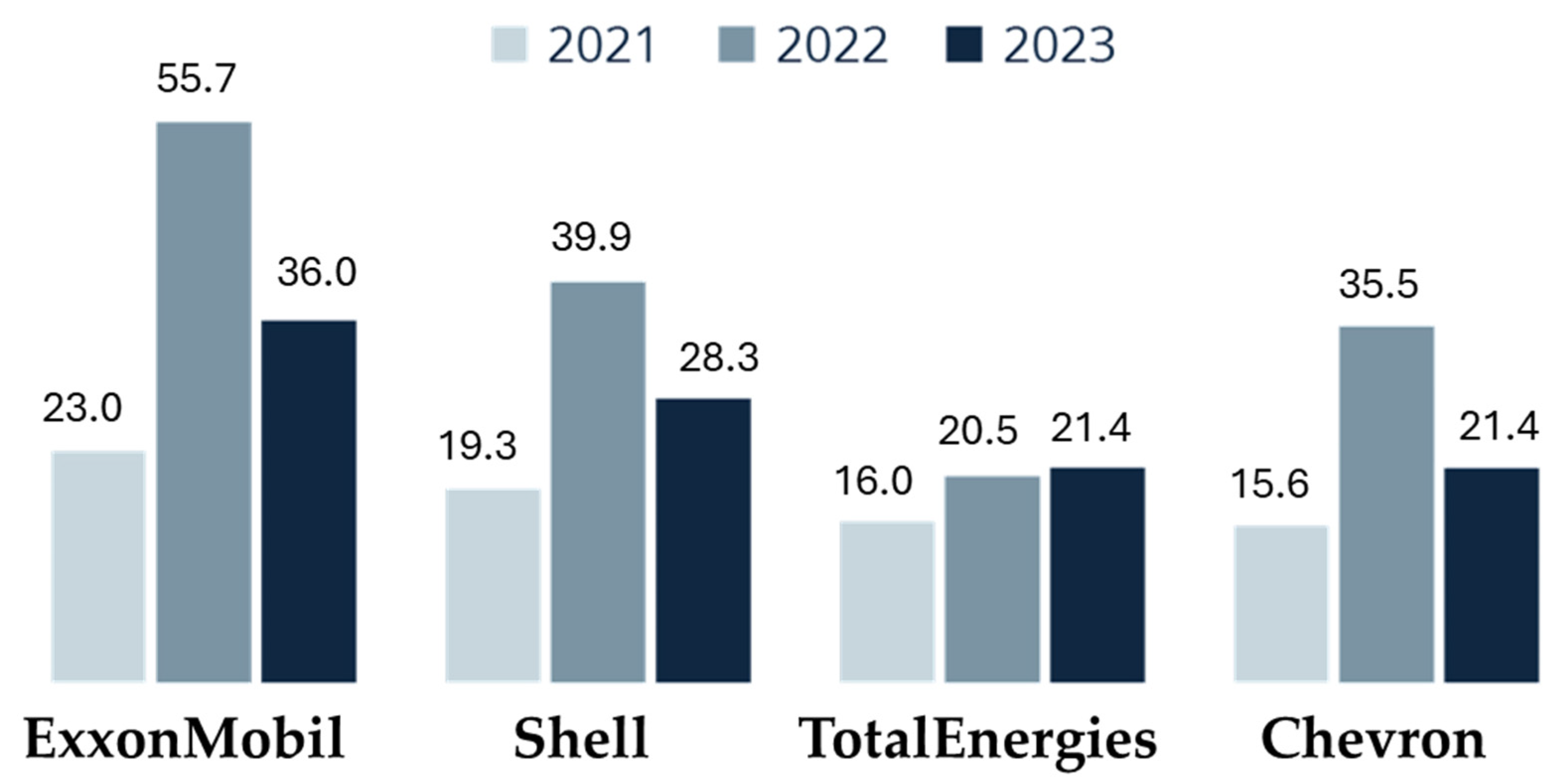
In turbulent times, oil companies often emerge as significant beneficiaries (Figure 13) [147]. For instance, ExxonMobil reported a net profit of USD 11.4 billion between January and March 2023, more than double its earnings from the same period in 2022. This pattern of increased profitability, although less pronounced, is mirrored by other major industry players such as Shell, Chevron, and TotalEnergies.
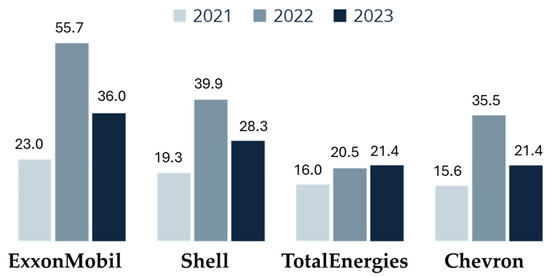
Figure 13.
Net earnings of oil and gas companies in the first quarters of 2021, 2022, and 2023 (in billions of USD) [147].
In summary, the global oil industry is characterized by high production levels, complex geopolitical dynamics, and significant economic impacts. The interplay between supply and demand, refining capacity constraints, and geopolitical tensions continues to shape the industry’s trajectory and profitability.
7.2. Petrochemical Processes for Transforming Heavy Feedstocks into Light Distillates
The petrochemical industry employs a range of sophisticated processes to convert heavy feedstocks into lighter, more valuable distillates [148,149]. Heavy feedstocks, such as crude oils with high molecular weights, often contain undesirable components and have limited applications in their raw form. By converting these heavy feedstocks into lighter distillates, the industry can produce more valuable and versatile products, such as gasoline, jet fuel, and diesel, which are essential for meeting global energy demands and compliance with environmental regulations. Light distillates offer improved performance characteristics and are in higher demand due to their utility in various applications, from transportation to industrial processes. This transformation not only enhances the economic value of the raw materials but also optimizes the overall efficiency and sustainability of the petrochemical production processes. The principal petrochemical processes for converting heavy feedstocks into light distillates are outlined as follows:
- Cracking: Cracking is a fundamental process in the petrochemical industry, breaking down large hydrocarbon molecules into smaller, more useful fragments. Thermal cracking utilizes high temperatures to achieve this breakdown, while catalytic cracking employs catalysts to enhance the reaction efficiency and selectivity [150]. Catalytic cracking is particularly important for producing high-octane gasoline and other valuable light hydrocarbons [151].
- Reforming: Reforming enhances the quality of lighter hydrocarbons by restructuring their molecular configuration [152]. This process is crucial for producing high-octane gasoline components and aromatic hydrocarbons such as benzene, toluene, and xylenes. Catalytic reforming typically involves the use of a platinum-based catalyst to facilitate the rearrangement of hydrocarbons, improving their performance in various applications.
- Hydrocracking: Hydrocracking combines high pressure, hydrogen, and a catalyst to convert heavy hydrocarbons into lighter, more saturated products [153]. This process effectively produces a wide range of lighter hydrocarbons, including high-quality diesel and jet fuels. Hydrocracking is valued for its ability to produce a high yield of desired products while reducing the sulfur and nitrogen content of the end products.
- Distillation: Distillation is a key separation technique that utilizes differences in boiling points to divide crude oil into various fractions [154]. Fractional distillation is commonly employed to separate the crude oil into light and heavy fractions, which are further processed into specific products. This method is crucial for isolating components such as gasoline, kerosene, and diesel.
- Steam cracking: Steam cracking is a high-temperature process that uses steam to break down hydrocarbons into lighter molecules, including ethylene and propylene [155]. This process is pivotal for producing key building blocks for the petrochemical industry, such as ethylene, which is used to manufacture a wide range of products including plastics and synthetic rubber.
- Extraction and refining: Extraction and refining processes involve the separation of valuable components from heavy feedstocks using physical or chemical methods [156]. Techniques such as solvent extraction and hydroprocessing are employed to purify and enhance the quality of the extracted components, making them suitable for further processing or direct use.
Each of these processes plays a crucial role in the petrochemical industry, enabling the transformation of heavy feedstocks into a diverse array of lighter, more valuable products. The selection and optimization of these processes are vital for meeting the demands of the industry and advancing the development of new and improved petrochemical products.
7.3. Complementary Processes in the Petrochemical Industry
While the primary focus of conversion processes in the petrochemical industry often revolves around transforming heavy feedstocks into lighter distillates, there are a number of additional processes that play a crucial role in optimizing and enhancing the quality of the final products. These processes, including alkylation, polymerization, and oxidation, do not directly involve the conversion of heavy raw materials but are nonetheless essential for improving the properties and value of petrochemical products.
1. Alkylation: Alkylation is a critical refining process in which light hydrocarbons, such as olefins, react with isoparaffins in the presence of an acid catalyst to produce high-octane gasoline components [157]. This process significantly enhances the performance characteristics of gasoline, particularly in terms of increasing the octane rating, which is key to preventing engine knocking and improving fuel efficiency. While alkylation does not contribute to the direct conversion of heavy feedstocks, it plays a pivotal role in refining lighter hydrocarbons into more valuable, high-performance products. The production of alkylate through this process is vital in meeting the growing demand for cleaner-burning, high-octane fuels.
2. Polymerization: Polymerization is another important process that does not involve breaking down heavy feedstocks but rather transforming small monomer molecules, such as propylene and butylene, into larger polymer chains. These polymers are used extensively in the production of plastics, fibers, and resins, which are foundational materials in both consumer and industrial applications. Polymerization can be achieved through various techniques, such as addition and condensation polymerization, depending on the desired characteristics of the final product. By converting simple hydrocarbons into complex, high-value materials, polymerization enables the petrochemical industry to diversify its product portfolio and contribute to the manufacturing of goods that are indispensable in modern life, from packaging to automotive parts [158].
3. Oxidation and other chemical reactions: Oxidation processes introduce oxygen into hydrocarbon molecules to create a range of oxygenated compounds, including alcohols, aldehydes, and carboxylic acids. These products are essential building blocks in numerous chemical industries, from pharmaceuticals to plastics, and are integral to the production of lubricants, solvents, and other functional materials [159]. While oxidation itself does not reduce heavy feedstocks, it provides critical intermediate products that enhance the functionality of hydrocarbon derivatives. In addition to oxidation, other chemical reactions—such as isomerization, hydrogenation, and dehydrogenation—are employed to further refine and modify hydrocarbons, optimizing their properties for specific industrial applications [160,161]. For instance, isomerization improves the octane rating of light naphtha, while hydrogenation processes are crucial for removing sulfur and saturating unsaturated hydrocarbons. These reactions contribute significantly to the overall versatility and efficiency of the petrochemical industry, allowing for the fine-tuning of products to meet stringent market demands and environmental regulations. Although alkylation, polymerization, and oxidation do not directly transform heavy feedstocks into lighter distillates, they are indispensable to the petrochemical value chain. These processes enhance the performance, quality, and variety of the products derived from hydrocarbons, enabling the industry to meet diverse market needs. They underscore the intricate nature of petrochemical refining, in which both conversion and complementary processes work together to maximize the value extracted from crude oil and natural gas, ensuring a broad spectrum of high-quality end products.
7.4. Kaolin-Driven Catalysis in Petrochemical Processes
Kaolin’s journey as a catalyst in the petrochemical industry began with its discovery as an effective support material in the late 20th century [162]. Initially, its superior thermal stability and resistance to coke formation made it an ideal candidate for enhancing catalyst performance in FCC. The material’s layered aluminosilicate structure offered a robust framework that improved reaction efficiency and extended catalyst life. A significant advantage of kaolinite is its low cost, making it an economical alternative to other catalyst supports like mullite (3Al2O3·2SiO2), alumina (Al2O3), and cordierite (2MgO·2Al2O3·5SiO2). In fact, the cost of kaolinite is approximately 100 times lower than that of alumina [163]. Additionally, kaolinite requires a lower sintering temperature (1250 °C) than alumina (1600 °C). Beyond these economic benefits, kaolinite exhibits greater flexural strength and lower density than alumina. Kaolinite can also be combined with other materials, such as dolomite, natural hydroxyapatite (Ca10(PO4)6(OH)2), and calcite (CaCO3), further enhancing its utility as a versatile and cost-effective catalyst support.
As research progressed, kaolin’s applications expanded beyond support roles; it also emerged as a stand-alone catalyst in the synthesis of zeolites and silicoaluminophosphate molecular sieves (SAPOs) [16]. This versatility has positioned kaolin as a key player in various catalytic processes in addition to FCC, including hydrocracking, isomerization, and alkylation, among others (Figure 14). This section explores the evolution of kaolin’s role from a support material to an independent catalyst, detailing its significant contributions to optimizing catalytic processes and advancing the production of high-value petrochemical products. A summary is presented in Table 2, which outlines the catalytic processes utilizing kaolin-based catalysts. The table provides a description of each process, highlights the specific role of kaolin in these reactions, and details the advantages it imparts in each case.
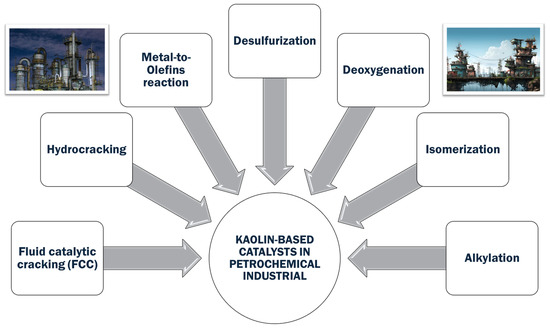
Figure 14.
Applications of kaolin-based catalysts in reactions of interest to the petrochemical industry.

Table 2.
Role, characteristics, and benefits of kaolin-based catalysts in petrochemical processes of industrial interest.
7.4.1. Fluid Catalytic Cracking (FCC)
FCC reactions are considered essential processes in the petrochemical industry and are designed to convert heavy petroleum fractions, such as vacuum residue, into lighter, more valuable products like gasoline and diesel (Figure 15) [164,165]. This process employs a solid catalyst in a fluidized bed to break down large hydrocarbon molecules into smaller, lighter ones through catalytic cracking [166]. Operating at high temperatures (500–550 °C) and utilizing an acidic catalyst, FCC significantly enhances the yield of desirable light hydrocarbons, thereby optimizing the overall efficiency and profitability of the refining process.
Figure 15.
Schematic representation of the FCC fractionation system process flow. Figure extracted from X. Qin et al. (2023) [165]. Reproduced with the permission of Elsevier.
Zeolite cracking catalysts are the most used in FCC processes and were introduced commercially in the early 1960s, initially incorporating type X or type Y zeolite within various matrix compositions that evolved from the amorphous catalysts of the 1940s and 1950s [167]. These early matrices moderated the zeolite’s high activity to prevent rapid deactivation due to coke formation. From the 1960s to the mid-1970s, FCC catalysts were modified to enhance conversion and liquid yield and reduce coke production, leading to improved gasoline octane, activity, and metal tolerance. The evolving demands for increased yields of light olefins and branched isomers in reformulated gasoline necessitate varied catalyst compositions, with over 150 catalyst grades available today. The 1970s and 1980s saw the advent of co-catalyst systems for emission control and the introduction of small pore zeolites. Zeolite Y is still the major active ingredient in FCC catalysts, but it has undergone many modifications since its introduction to meet the changing needs of the refining industry to produce high-quality FCC catalysts for today’s market. Milton, who initially patented the synthesis of zeolite Y, used precipitated silica, sodium aluminate, and caustic soda as starting materials [168]. This process required a 24 h aging step at lower temperatures before crystallization could begin. Later, researchers at the Davison Division of W. R. Grace and Co. refined the synthesis by incorporating sodium silicate and seed crystals, eliminating the need for the aging step [169].
Several studies have demonstrated the viability of kaolinite as a starting material for synthesizing microspherical zeolitic materials due to its resistance to attrition, thermal and hydrothermal stability, affordability, and availability [163,170,171]. A standard method involves mixing metakaolin (derived from kaolinite), raw kaolinite, sodium silicate, and water, which is followed by spray-drying the mixture before calcination to form the desired fluid microspheres. These microspheres are then mixed with an aqueous NaOH solution to form a slurry, which is stirred continuously during hydrothermal treatment to crystallize into faujasite zeolite. Proper monitoring of the crystallization process minimizes the formation of unwanted B zeolite. The resulting composite material may contain more than 15% faujasite zeolite with a Si/Al ratio greater than four. For instance, Haden et al. [172] and Brown et al. [170] have synthesized and patented catalytic cracking catalysts, including crystalline faujasite, X, and Y zeolites. In the approach proposed by Haden, the complete transformation of the material to zeolite Y is neither achieved nor intended. Instead, the clay undergoes calcination above the high-temperature kaolin exotherm to form an incipient mullite phase, rendering only a portion of the alumina active for zeolite synthesis. Initially, synthesis was performed on extrudates containing both high-temperature-calcined kaolin and metakaolin, typically in the presence of caustic in a mineral oil medium, yielding catalysts suitable for moving bed reactors. Subsequent patents reported by Haden et al. [173] expanded this technology to include spray-dried microspheres and aqueous slurries. In these methods, kaolin is spray-dried into microspheres appropriate for FCC, which are then calcined and treated with caustic to form about 25 to 30 wt% zeolite Y throughout the microsphere. Aging the caustic microsphere slurry at approximately 100°F for 6 to 12 h precedes crystallization at 180°F. The resulting slurry filtration produces a disilicate mother liquor. NaY zeolite with a Si/Al ratio of 4.53 was synthesized at 22.6% using this procedure. To enhance the yield of faujasite zeolite, they proposed using a deflocculating agent in slurries of hydrated kaolin with higher solids content to aid spray drying. The resulting microspheres were separated into two fractions (10/90 by weight) before calcination and alkali treatment, with calcination temperatures for the 10% and 90% fractions being below and above the clay exotherms, respectively. Mixing the two fractions before treating them with a caustic soda solution resulted in Y zeolite with improved attrition resistance and a higher yield of 52% within a shorter crystallization time. In addition to that, Brown et al. demonstrated that omitting the washing step after filtration allows some mother liquor to remain within the microsphere [170]. The material is then flash-dried and undergoes further treatment to reduce matrix surface area and decrease gas and coke production. A patent reported by Barry K. Speronello [174] employing similar technology necessitates the use of both metakaolin and high-temperature-calcined kaolin, along with seeds, to achieve crystallinities approaching 60% within the microsphere. By adjusting the crystallization time and the clay-type ratio, the extent of crystal formation can be modulated. Regardless of the specific conditions, the unconverted portion of the microsphere serves as a stable matrix that binds the zeolite and aids in cracking molecules that are too large to enter the zeolite supercage.
Zeolite Y synthesis can also begin with the acid treatment of metakaolin to remove alumina [175]. After removing enough alumina, the kaolin is combined with a caustic solution, left to age, and then crystallized to form pure zeolite Y crystals. The aluminum removal is crucial to achieving the appropriate soda–silica–alumina ratio necessary for zeolite Y formation. The calcination of kaolin to metakaolin is essential to activate the alumina within the clay. The calcination temperature and the quality and consistency of the clay source are critical factors. However, this method is not currently used in commercial practice. In 2007, Atta et al. [171] dealuminated metakaolin with an inorganic acid to synthesize X zeolite, increasing the Si/Al ratio to the desired value without using sodium silicate, as reported by Haden et al. They achieved 57% faujasite zeolite, consisting of 34% X zeolite and 23% Y zeolite. This result is attributed to the similar synthesis conditions for both zeolites, such as the Si/Al ratio, the pH value, aging, the reaction temperature, and time. However, longer aging periods have been shown to increase the purity and crystallinity of Y zeolite. Chandrasekhar et al. [176] explained that a higher percentage of Y zeolite could be achieved with extended aging periods, suggesting that Haden et al. could have generated a higher yield if they had increased the aging time. Recently, Ajayi et al. [177] synthesized NaY zeolite from potassium-rich Nigerian kaolinite clay, focusing on the effects of the metakaolinization temperature and aging on potassium’s role in zeolitization. Optimal conditions included calcination at 900 °C for 6 h, followed by aging for 8 days and synthesis at 95 °C for 72 h. Potassium was found to hinder NaY formation, affecting nucleation, crystal morphology, and stability. The NaY zeolite produced had a cubic shape, an ion exchange capacity of 4.72–4.94 meq/g, and a specific surface area of 672 m2/g, maintaining stability up to 600 °C. This indicates that potassium’s presence significantly impacts the synthesis and quality of NaY zeolite.
Based on that, the most important mineral currently used in the manufacture of carriers for FCC catalysts is kaolin. Kaolin’s largest use is in catalyst substrates for the catalytic cracking of petroleum. Because many catalysts are used at high temperatures and pressures, the refractory character of kaolin is appropriate for these applications. The purity of kaolin is critical in petroleum cracking operations, thus a processed kaolin with low levels of iron, titanium, alkali, and alkaline earth compounds is preferred [6]. Kaolin’s conversion to a zeolite in the preparation of cracking catalyst supports increases the surface area of the catalyst exposed in the reaction, enhancing its effectiveness. The low cost, high purity, and platelet shape of kaolin promote the formation of good pore structures, ease of acid leaching, and ease of conversion to zeolite [162]. The transformation of kaolin to zeolite not only augments the catalytic activity but also improves the mechanical strength and thermal stability of the catalysts, which are essential properties for enduring the rigorous conditions of FCC units.
Initial attempts to use pure zeolite for catalytic cracking of gas oil were unsuccessful because the high surface area and site density of the zeolite, combined with extended catalyst-oil contact times in early FCC units, led to a product stream with high levels of unwanted secondary reaction products, such as coke and light gases. This overcracking rapidly deactivated the pure zeolite catalyst due to coke formation. Indeed, Plank and Rosinski [178] discovered that diluting the zeolite in a matrix reduced overcracking and coke poisoning, resulting in a zeolite-containing FCC catalyst that significantly outperformed the previously used amorphous silica–alumina or acidified clay catalysts. Before the development of zeolite-containing FCC catalysts, amorphous FCC catalysts had been the dominant materials for twenty years.
Early formulations of zeolite FCC catalysts included rare earth exchanged zeolites (either type X or Y), kaolin clay, and a binder, which was often synthetic silica–alumina or peptized pseudoboehmite [179,180]. The in situ technology developed by Haden [173] was distinctive because it was binderless; particle hardness was achieved through the thermal treatment of spray-dried clay microspheres before zeolitization. No specific component was added to the spray dryer feed to bind the clay particles. While all three binders exhibited catalytic activity, their selectivity in cracking oil molecules differed. Reactions over these matrices were less selective compared to zeolite, producing more coke and gas and less gasoline. During the 1970s, when gasoline selectivity was crucial and FCC feedstocks were more paraffinic, the matrix cracking activity was seen as a drawback [167]. Adjustments in unit design to capitalize on zeolitic cracking and the increasing zeolite content in catalysts highlighted the need for improved attrition resistance, leading to the mid-1970s introduction of silica sol-based binders. These low-surface area binders were essentially inactive in gas oil cracking but produced much harder catalysts. The use of heavier feeds and the need for increased metal tolerance necessitated the reintroduction of active matrix components into FCC catalysts. Modern FCC catalysts must be extremely hard yet sufficiently porous to crack heavy, large oil molecules into smaller fractions suitable for the fuel oil range or small enough to be processed by the zeolite component. Additionally, matrices must mitigate the effects of contaminant metals, like vanadium and nickel, on gas and coke production and act as soda sinks. They should also synergize with zeolite to maximize transportation fuel yields and minimize coke and light gas formation.
In FCC catalyst formulations, clay is predominantly added as an inert densifier to enhance the apparent bulk density (ABD) without affecting the catalyst’s activity or selectivity. Kaolin is the preferred clay due to its ability to form high-solids pumpable slurries, its low fresh surface area, and its efficient packing properties attributed to its platelet structure. Typically, manufacturers acquire kaolin as a 60 to 65 wt-% slurry, dispersed with agents such as sodium silicate or tetrasodium pyrophosphate, and use it directly without additional processing [167]. Several key parameters of kaolin are critical for FCC catalyst production. The particle size of the kaolin must be small to ensure that the resultant catalyst has good ABD and attrition resistance. Typically, kaolin particles for FCC use have average sizes (measured by sedigraph) of 0.3 to 0.4 microns, with 90% of particles being approximately 1 micron. The content of iron and titania in the kaolin is also crucial. Elevated levels of these elements can cause undesirable secondary reactions, such as increased gas and coke formation and heightened CO combustion in the regenerator. For FCC catalysts, acceptable levels are typically below 3.0 wt-% for titania and between 0.4 and 0.8 wt-% for iron. Additionally, due to environmental regulations, the crystalline silica content in kaolin has become an important consideration, despite its typically low concentration in the clay. Minimizing crystalline silica presence is now more critical than ever due to recent regulatory changes. Advancements in this field were addressed, since the enhancement of FCC catalysts with modified kaolinite introduces porosity into the catalyst structure, which can significantly improve cracking activity, leading to the production of high-octane gasoline and increased yields of lower olefins such as butylene and propylene [181]. Barry et al. developed a novel porous mullite through the calcination of kaolinite at 1200 °C [174]. Their findings indicated that the synthesized mullite exhibited resistance to attrition due to the formation of strong inter-crystalline bonds. The mullite was impregnated with chloroplatinic acid using the incipient wetness method, and the resulting catalyst was employed in FCC units for CO oxidation.
Regarding binder based on kaolin materials, it is worth mentioning that the in situ type of FCC catalyst preparation, as introduced by Engelhard [182], eliminates the need for an added binder. The process begins by forming kaolin microspheres. Initially, these clay particles exhibit weak binding after spray drying, leading to breakdown if slurried in water. However, high-temperature calcination significantly enhances their attrition resistance and maintains their microspherical shape. This is crucial for the subsequent in situ crystallization step in a caustic solution, which further hardens the microspheres and facilitates the formation of intrastructural NaY zeolite. The high-temperature environment promotes the reaction of surface hydroxyls on the clay platelets, eliminating water and creating oxygen-bridged bonds between particles. During crystallization, substantial silica removal from the particles results in macropores that are subsequently filled by growing intrastructural zeolite, yielding significant porosity in the 30 to 100 Å range. The remaining matrix, now richer in alumina due to silica removal, exhibits notable acidity and high surface area, making it an effective matrix for bottom cracking. However, the large surface area and exposure to contaminant metals within the clay can lead to increased coke and gas production. Retaining some silica, as suggested by Brown [170], mitigates this undesired activity by reducing the overall surface area. Recent advancements involving metakaolin and seed technology have further decreased porosity and surface area, thereby enhancing the catalyst’s performance [183]. Subsequent studies introduced by Absil et al. showed the preparation of phosphorus-modified kaolinite blended with ZSM-5 zeolites [184]. This catalyst blend, containing 40 wt% ZSM-5 and approximately 2.3–2.5 wt% phosphorus, demonstrated good attrition resistance and was utilized in the cracking of Joliet sour heavy gas oil in a fixed-fluidized bed unit. The blend showed a marked improvement in the yields of butylene and propylene compared to the base catalyst, corroborating findings from earlier studies [185].
In the last two decades, FCC catalysts synthesized from kaolin-derived zeolites have been extensively studied due to their potential to enhance catalytic activity, selectivity, and resistance to deactivation [186]. Below, a summary will present key findings from various studies conducted between 2001 and 2023, highlighting the advancements in FCC catalyst performance attributed to different synthesis and modification techniques of kaolin-based zeolites. Initially, L. Patrylak et al. developed the synthesis and characterization of zeolite-containing microspheres and FCC catalysts based on Ukrainian kaolin, revealing that variations in synthesis conditions notably affect the micro- and mesoporosity of the samples [187]. This work established that the sodium cations in the zeolite act as weak Lewis acid sites, impacting ammonia retention and thus influencing catalytic activity. Notably, the transformation of zeolite phases during catalyst preparation led to increased mesoporosity and stable catalytic performance, which are crucial for modern FCC applications. Building on these foundational studies, research into Y zeolite catalysts highlighted that the unit cell size of Y zeolite plays a critical role in determining the product distribution during catalytic cracking [188]. A model predicting coke formation based on unit cell size was developed, showing that catalysts with larger unit cell sizes produce higher coke, LPG, and dry gas yields, whereas those with smaller sizes favor gasoline production.
The exploration of different kaolins, including those from Suzhou, has shown that the chemical and mineralogical properties of kaolin significantly affect catalyst performance [189]. Catalysts derived from Suzhou kaolin, which incorporate approximately 30% NaY zeolite, demonstrated superior activity, attrition resistance, and resistance to metal passivation, outperforming standard commercial catalysts in gasoline and coke selectivity. Further innovations in FCC catalysts involved novel approaches, such as the in situ synthesis of catalysts with high zeolite content, tailored for cracking resid feedstocks [190]. These catalysts showed enhanced pore structures, making large resid molecules more accessible to active sites, thus improving activity and selectivity for resid-containing feedstocks. Subsequent studies introduced advanced composites, such as NaY/kaolin microspheres combined with ZSM-5, to maximize propylene yield [191]. This catalyst exhibited superior meso- and macropores and acid sites, leading to a notable increase in propylene yield compared to traditional catalysts. The development of ZSM-5 from expanded perlite and kaolin [192], as well as β-zeolite synthesized from kaolin [9], showcased improved catalytic performance in FCC naphtha aromatization. These zeolites, which are characterized by unique pore systems and tunable crystallinity, offered higher catalytic activity and better product distribution. Evaluations of various components, including Y zeolite, kaolin, and alumina, in the cracking of heavy petroleum residues revealed that Y zeolite significantly increased coke formation, while kaolin and alumina contributed to higher coke yields compared to pure heavy residue [193]. This study highlighted the sensitivity of the TG technique in assessing coke formation during FCC. In another advancement, hydrodesulfurization (HDS) catalysts supported in γ-Al2O3-MB composites with H-type β-zeolite synthesized from kaolin demonstrated enhanced sulfur reduction capabilities [194]. Additionally, the modification of kaolin-based FCC catalysts with vanadium demonstrated that increased weak Lewis acidity improved sulfur reduction rates [195]. Catalysts with optimal vanadium content achieved substantial reductions in sulfur content, highlighting the effectiveness of this modification. The incorporation of β-zeolite improved the acidity and hydrogenation activity of the catalysts, achieving higher HDS efficiency and deeper sulfur removal compared to traditional supports. Recent innovations have focused on optimizing the synthesis conditions for β-zeolite from kaolin, enhancing the catalyst’s performance in the HDS of diesel [196]. By systematically adjusting pretreatment and crystallization conditions, catalysts with β-zeolite content showed improved HDS activities and compliance with ultra-clean diesel specifications. Further advancements included the synthesis of ZSM-5 zeolites on kaolin microspheres using an amine-free system [197]. This method resulted in small-sized ZSM-5 crystallites with high hydrothermal stability and acidity, promoting the production of light olefins during FCC. The evaluation of FCC catalysts derived from kaolin in conjunction with commercial catalysts in cracking polypropylene loads demonstrated that highly active commercial FCC catalysts were more efficient in producing gaseous products, although they also led to increased aromatic formation [198]. This underscored the importance of catalyst activity in controlling product distribution. Recent developments in synthesizing cocrystallized USY/ZSM-5 zeolites from kaolin revealed that combining these zeolites in the same material enhances the effectiveness of ZSM-5 as a propylene booster [199]. This improvement is attributed to the enhanced ability of ZSM-5 to crack larger olefins and suppress hydrogen transfer when synthesized with USY. One year later, a cost-effective kaolin-based catalyst for upgrading waste cooking oil (WCO) into organic liquid products (OLPs) was prepared [200]. The acid-treated kaolin (ATK) catalyst showed a high yield of 74.4% and produced high-quality liquid products with 93.8% oxygen removal and 22.5% aromatic content. In comparison, a commercial petroleum catalyst (CC) achieved 90.8% oxygen removal but had a higher aromatic content of 55.8%. The study found that weak Lewis acids were associated with deoxygenation, Brønsted acids were associated with hydrogen transfer, and the total Brønsted acid amount was associated with cracking capability. CC primarily followed a carbonium ion reaction mechanism, while kaolin-based catalysts adhered to a free radical mechanism. Deoxygenation active sites were identified using CH3COOH-TPD. Further innovations in catalyst matrix structures included optimizing the in situ synthesis of zeolite Y from commercial kaolin treated with NaOH solution [201]. It was found that calcination at 1000 °C produced a matrix with enhanced surface area and mesoporosity, leading to improved zeolite Y dispersion and catalytic activity in FCC applications. Additionally, the synthesis of Si-modified pseudo-boehmite@kaolin composites has shown significant improvements in heavy oil catalytic cracking performance [202]. These composites offered a larger surface area, higher pore volume, and better surface acidity than conventional kaolin matrices, resulting in enhanced gasoline and total liquid yields. Additional studies synthesized NaY and Na[B]Y zeolites on alkaline pretreated kaolin microspheres, finding that boron-increased acidity and mesoporosity resulted in more active FCC catalysts [203]. Comparative research on in situ and incorporated FCC catalysts under iron contamination revealed that in situ catalysts maintained higher bottom upgrading and lower dry gas yields, performing better than their incorporated counterparts [204]. The latest research to date in this field reported that optimization of the accessibility of zeolite Y by adjusting crystallization times improved the distribution of zeolite Y in the outer layer of microspheres, which enhanced mass transfer, acid accessibility, and heavy oil cracking performance [205]. In summary, the advancements in FCC catalysts derived from kaolin have led to notable improvements in catalytic performance, including increased yields, enhanced stability, and better product distribution. These developments reflect the ongoing efforts to optimize FCC catalysts for diverse and challenging feedstocks.
7.4.2. Hydrocracking
Hydrocracking is a pivotal process in the petroleum refining industry designed to upgrade heavy oil fractions into lighter, more valuable products [206]. This process involves breaking down large, complex hydrocarbon molecules into simpler ones by reacting them with hydrogen in the presence of a catalyst (Figure 16). Hydrocracking operates under high pressure (typically 1000 to 3000 psi) and elevated temperatures (400 to 450 °C), ensuring the effective conversion of feedstocks into desirable outputs, such as gasoline, kerosene, diesel, and jet fuel [207]. The bifunctional catalysts used in hydrocracking possess both acidic and metallic sites, enabling simultaneous hydrogenation and cracking reactions.
Figure 16.
Schematic representation of the hydrocracking catalytic reaction in industry. Figure adapted from Peng et al. (2019) [207] and Perez-Botella et al. (2022) [208]. Reproduced with the permission of Elsevier and Springer.
In the context of hydrocracking, kaolin-supported catalysts have shown promising results in terms of product selectivity and yield. These characteristics of kaolin enhance the dispersion of active metal sites, improving the overall catalytic performance. Additionally, kaolin’s ability to retain its structure under the harsh conditions of hydrocracking makes it a cost-effective alternative to conventional supports like alumina and silica. The incorporation of kaolin can also influence the acidity of the catalyst, which is a critical factor in determining the pathway of hydrocarbon cracking and the nature of the resulting products. Moreover, the thermal stability of kaolin helps in maintaining catalyst activity over extended periods, reducing the frequency of catalyst regeneration and replacement.
A series of studies have explored the utilization of kaolin-based catalysts in hydrocracking processes, revealing significant advancements and diverse applications. In 2014, Rahayu et al. [209] developed hydrocracking catalyst support from Indonesian kaolin, converting it into a zeolite Y and amorphous alumina–silica phase. This combination demonstrated promising structural and surface properties, achieving a zeolite NaY purity of 86–88% with a specific surface area of 186 m2/g and a total pore volume of 0.107 mL/g. These properties are crucial for effective hydrocracking, providing a solid foundation for subsequent research into kaolin-based catalysts. Building on this foundation, Kumar et al. [210] synthesized hierarchical mesoporous H-ZSM-5 using kaolin clay as a cost-effective alumina source. The resulting catalyst, which was used for the hydrotreatment of jatropha oil, showed high yields of diesel-range hydrocarbons (up to 93% at 375 °C and 50 bar) and kerosene-range hydrocarbons (37.4% at 425 °C and 80 bar). The enhanced stability and performance of this catalyst were attributed to its tailored physicochemical properties, demonstrating the potential of kaolin-based materials in renewable oil processing. In 2017, two significant studies further expanded the application of kaolin in hydrocracking. Wang et al. [211] integrated catalytic cracking and hydrotreating technologies for deoxygenating waste cooking oil, highlighting the superior performance of acid-treated kaolin (ATK) in reducing oxygenate content from 87.6% to 6.6% and hydrogen consumption from 8.8 mol to 2.4 mol per mol of triglyceride. This integrated approach showcased the versatility of kaolin-based catalysts in processing different feedstocks. Concurrently, Bai et al. [212] optimized the synthesis of zeolite NaY from kaolin, improving yield and hydrocracking conversion from 39.58% to 55.93% by refining aging and crystallization conditions. Their work emphasized the importance of precise control over synthesis parameters to enhance catalyst performance. A 2022 study by Wang et al. [213] delved into the mass transfer performance of in situ crystallized Y zeolite on kaolin for vacuum gas oil (VGO) hydrocracking. The modified crystal morphology and pore architecture of the Y zeolite enhanced mass transfer and acidity accessibility, leading to higher aromatic contents in middle distillates and lower Bureau of Mines Correlation Index (BMCI) values of tail oil. This study provided deeper insights into the structural characteristics that influence hydrocracking efficiency. Recently, in 2024, Dhaneswara et al. [214] synthesized bifunctional Ni/Mo-impregnated ZSM-5 catalysts using Badau Belitung kaolin. These catalysts demonstrated exceptional performance in converting heavy petroleum distillates into medium and light fractions, achieving conversions of 92.47% and 92.06% by mass for two optimized formulas, respectively. This performance significantly outperformed commercial catalysts, which yielded conversions of 70.40% and 87.19% by mass under the same conditions. Overall, these studies underscore the potential of kaolin-derived catalysts in enhancing hydrocracking efficiency and product yield, with applications ranging from renewable oil hydrotreatment to heavy petroleum distillate conversion.
7.4.3. Methanol-to-Olefin (MTO) Catalytic Reaction for Olefin Production
The production of olefins is crucial in the petrochemical industry, as olefins serve as fundamental building blocks for numerous chemical products, including plastics, synthetic fibers, and resins. Methanol-to-olefins (MTO) is a key catalytic process that converts methanol into valuable light olefins like ethylene and propylene. These processes employ zeolite-based catalysts, such as ZSM-5 and SAPO-34, which are known for their high selectivity and activity. The catalysts facilitate the efficient conversion of feedstocks through a series of complex reaction mechanisms, including dehydration, oligomerization, and cracking. The choice of catalyst and its optimization are critical for enhancing yield and selectivity, thereby making the production of olefins more efficient and economically viable. The development and improvement of these catalytic processes and catalysts are fundamental for sustaining the supply of essential olefins, meeting the ever-growing demands of the petrochemical industry.
SAPOs, or silicoaluminophosphates, are microporous zeolitic materials with chabazite-like structures that are characterized by a high degree of strong acidity, making them highly suitable for processes like olefin production [215]. Conventional SAPOs are typically synthesized hydrothermally from individual natural compounds containing silica, alumina, and phosphorus or from lamellar aluminophosphates derived from related compounds. These methods, however, are costly. An alternative approach involves using thermally modified raw kaolinite, known as metakaolin, which provides a more economical source of both silica and alumina for the synthesis of SAPO molecular sieves, including SAPO-5, SAPO-11, SAPO-20, SAPO-34, SAPO-44, and SAPO-47, as reported by Wang et al. [216]. The silica and alumina atoms in metakaolin can effectively coordinate with related ligands, facilitating the formation of these valuable SAPO materials. In this study, the authors indicated that not all silica and alumina atoms in metakaolin contribute to the SAPO framework, leading to impurities that hinder catalytic performance. To address this issue, they proposed a three-step crystallization process for synthesizing SAPO-34. The first step involves converting kaolinite to metakaolin at 800 °C to activate it. The second step transforms the metakaolin into primary building units (PBU) through aging and initial heating. The final step is crystallization at 150 °C. This method successfully produced SAPOs free of contaminant phases using kaolinite as an inexpensive precursor. SAPOs, as microporous molecular sieves with a high density of acid sites, are susceptible to rapid deactivation due to coking, which diminishes their catalytic activity. This highlights the necessity for developing SAPOs with enhanced coke suppression capabilities.
In this sense, SAPO-34 catalysts face challenges of rapid deactivation and short lifetimes in methanol-to-olefin (MTO) reactions [217,218]. In a study carried out by Want et al. [219], SAPO-34 was synthesized in situ on fully calcined kaolin microspheres (CKMs) using a hydrothermal method with tetraethylammonium hydroxide (TEAOH) and triethylamine (TEA) as templates. The CKMs were pretreated with 2–14 wt% NaOH aqueous solution to enhance SAPO-34 growth. The impact of NaOH concentration on the physicochemical properties of CKMs and SAPO-34/kaolin microspheres (SCKMs) was analyzed using XRD, XPS, SEM, low-temperature N2 adsorption, and NH3-TPD. The results indicated that NaOH pretreatment significantly influences the chemical composition and morphology of CKMs, affecting the properties of the SAPO-34 formed. The optimal NaOH concentration was found to be 4 wt%. The prepared SCKM catalyst achieved 100% methanol conversion, 89.8% light olefin selectivity, and a 964-min lifetime at 450 °C, outperforming both free SAPO-34 and SAPO-34 supported on metakaolin microspheres. In the same year, other work [220] demonstrated the synthesis of macroporous SAPO-34 microspheres (MAMISAPO-34) using 2 μm polystyrene spheres as a template. These microspheres, which were formed by mixing cubic SAPO-34 with kaolin, silica sol, and aluminum phosphate sol, were spray-dried and then treated hydrothermally and calcined. Compared to non-macroporous SAPO-34 (NOMISAPO-34), MAMISAPO-34 had higher crystallinity and stronger acid sites. In methanol-to-olefin (MTO) reactions, MAMISAPO-34 exhibited better catalytic performance due to its greater crystallinity. Years later, Zhang et al. [221] used β-cyclodextrin (β-CD) as a crystal growth inhibitor during the synthesis of SAPO-34 from metakaolin, leading to smaller crystal sizes and better silicon dispersion. The catalyst with β-CD showed a significantly extended active period in MTO reactions, remaining active for 610 min, compared to 280 min for the sample synthesized without β-CD, indicating enhanced catalytic performance and prolonged lifetime.
Several authors have studied the synthesis of hierarchical mesoporous SAPOs with mild acidity to solve the problem of rapid deactivation [222,223]. Then, Zhang et al. [224] reported a novel strategy that involved synthesizing hierarchical SAPO-34@kaolin composites by thermally treating kaolin microspheres and rearranging the active silicon and aluminum species with an external phosphorus source. This method, which was co-templated by CTAB and TPOAC, produced SAPO-34 with a small crystal size and hierarchical micro-mesoporous structure. The resultant composites exhibited a 20% increase in selectivity to ethylene and propylene and a remarkably prolonged catalyst lifespan, from 50 to 200 min, in MTO reactions. In another approach, a low-cost and efficient MTO catalyst was synthesized via dry gel conversion (DGC) using TEAOH as the structure-directing agent and TEA and H2O vapors [225]. The process yielded nano-SAPO-34 with good pore connectivity and high activity. The prepared microsphere catalysts showed a 14% extension in catalyst lifetime and a 1.55% increase in selectivity for C2 and C3 olefins, which are suitable for industrial applications due to their slow carbon deposition rate and high activity utilization ratio.
Over the following 5 years, Sogand Aghamohammadi and colleagues have progressively refined the synthesis and characterization of SAPO-34 catalysts for the methanol-to-olefins (MTO) reaction, focusing on enhancing their physicochemical properties, catalytic performance, and mechanical strength. In their initial research, Aghamohammadi et al. investigated the impact of various ion exchange methods on the assembly of nanostructured kaolin-SAPO-34 catalysts using silica sol as a binder [226]. These catalysts, which were prepared by spray drying, exhibited typical SAPO-34 and kaolinite phases. It was found that using resin as an ion exchange agent resulted in smoother and larger particles. The optimized catalyst demonstrated excellent activity, maintaining nearly 22 h of activity during MTO reactions in a fluidized bed reactor. In subsequent work, the team focused on preparing fluidizable catalysts suitable for fluidized bed reactors by treating kaolin with inorganic acids to enhance its activity [227]. The acid-treated kaolin increased the intensity of acid sites, improving the conversion of methanol to light olefins and reducing the induction period. However, higher reaction rates in the fluidized bed led to faster deactivation due to uniform coke formation. Further studies examined the performance of SAPO-34 catalysts with different particle sizes prepared via spray drying [228]. The smallest particle size resulted in larger and more spherical particles with rapid product diffusion, reducing the formation of heavier byproducts. The optimal catalyst maintained activity for nearly 5 h in a fluidized bed reactor and exhibited lower coking rates and longer lifetimes. Exploring spray drying methods, Aghamohammadi et al. optimized the slurry composition and calcination temperature to produce fluidizable microencapsulated catalysts [229]. They found that kaolinite converted to metakaolinite at temperatures above 550 °C, introducing new Brønsted acid sites. The optimized catalyst, KA-SAPO, demonstrated a lifetime of 900 min in a fluidized bed reactor, highlighting its enhanced performance. Investigating the effects of ultrasound power on solid–liquid slurries, the team found that lower ultrasound power increased pore volume, primarily enhancing mesopore volume and forming spherical, symmetric particles [230]. However, the sonicated catalyst showed a higher attrition rate and a shorter MTO reaction lifetime (432 min) than the non-sonicated catalyst (850 min), which was attributed to lower mechanical strength. In the latest study, the researchers compared single-binder and dual-binder methods for shaping fluidizable MTO catalysts based on Si–Al sol-bound kaolin-matrixed SAPO-34 [231]. The dual-binder catalyst (alumina–silica sol) exhibited a larger surface area and increased acidity, leading to improved selectivity and lifetime in a fluidized bed reactor compared to catalysts using single binders. Additionally, the dual-binder catalyst showed slightly lower coke deposition. Collectively, these studies demonstrate significant advancements in SAPO-34 catalyst synthesis, focusing on improving catalytic activity, selectivity, mechanical strength, and lifetime for efficient methanol-to-olefin conversion.
In 2024, significant advancements were reported in the synthesis and characterization of SAPO-34 catalysts for methanol-to-olefin (MTO) reactions, focusing on enhancing catalytic performance and lifetime. Novel fluidizable micro-mesopore Mn(x)SAPO-34 catalysts were developed using spray drying with Mn-incorporated SAPO-34 powder, kaolin filler, alumina sol binder, and HCl additive [232]. The use of a mixed template of TEAOH/morpholine and varying Mn ion amounts during hydrothermal synthesis was crucial. Characterization revealed that moderate acidity and Mn incorporation into chabazite cages improved selectivity and lifetime, especially for ethylene production due to the restricted diffusion of heavier hydrocarbons. The optimized catalyst, SD-Mn(0.05)SAPO-34 (70–120 µm), exhibited superior selectivity of up to 88.4% for light olefins and a catalytic lifetime of 330 min at 450 °C and WHSV of 4 h−1 in a fluidized bed reactor. Another work systematically explored SAPO-34 molecular sieves synthesized using calcined kaolin in a three-stage high–low–high-temperature hydrothermal process [233]. The focus was on the effect of kaolin calcination temperature on crystallization and MTO performance. The sample synthesized with kaolin calcined at 500 °C (SP-500) demonstrated a clean cubic structure, large surface area, and mild acidity, which was attributed to the well-dispersed structure of MK-500. This provided an optimal Si and Al source, leading to controlled crystallization and a unique Si coordination environment. As a result, SP-500 showed an excellent catalytic lifetime of over 720 min, with a methanol conversion rate above 90% and a stable light olefin yield of 80.6%. In contrast, the SP-800 sample, despite having the highest selectivity of 84.5%, had a shorter lifetime of only 390 min due to stronger acid sites leading to faster deactivation. These studies highlight the importance of precise formulation and synthesis conditions in developing high-performance SAPO-34 catalysts for MTO processes.
Conversely, the ZSM-5 zeolite-catalyzed MTO process is a promising technology for producing light olefins from renewable feedstocks. Tuning the physicochemical properties of ZSM-5, such as acid site characteristics and the impact of metals or non-metals on acidity and porosity, remains challenging. Recent advancements in hierarchical ZSM-5 materials, featuring both meso- and microporosity, enhance molecular transport while maintaining catalytic properties, highlighting their potential to improve MTO process efficiency and selectivity [234]. Modifying kaolin through treatments such as calcination and dealumination enhances its reactivity, facilitating the formation of ZSM-5’s characteristic microporous structure. Initial findings suggest that kaolin-derived ZSM-5 exhibits comparable catalytic properties to conventional methods, offering a sustainable and economical alternative for industrial applications. Further research is needed to optimize these synthesis processes and fully realize kaolin’s potential in producing high-quality ZSM-5 zeolites [235]. Studies from 2014 to 2019 have explored the synthesis and catalytic performance of ZSM-5 from kaolin for the methanol-to-olefin (MTO) reaction, revealing significant insights. Michels et al. (2014) [236] highlighted the importance of binders in shaping zeolite catalysts, noting that kaolin and other clays enhanced macroporosity and mass transfer properties. They found that while binder-induced changes in acid site density and speciation affected intrinsic catalytic activity, they did not directly correlate with selectivity or catalyst lifetime. Notably, kaolin contributed to improved mechanical stability and catalytic performance. In 2017, Pan et al. [237] synthesized nano-/micro-scale ZSM-5 from kaolin, demonstrating that nanosized ZSM-5 exhibited better coke tolerance, longer catalytic lifetime, and higher propylene selectivity compared to submicron and microsized samples. The enhanced performance of kaolin-derived ZSM-5 was attributed to the presence of elements like Fe, P, and Ti, which positively influenced catalytic stability and product distribution. In addition, Shoinkhorova et al. (2019) [238] investigated the effect of spray drying on ZSM-5-based catalysts, incorporating kaolin and other clays. They found that the nature of the selected clay significantly influenced the final acidity of the composite, impacting the MTO reaction pathways. The study underscored the critical role of slurry formulation and processing parameters in achieving optimal particle size and morphological characteristics, with kaolin-based composites showing promising catalytic performance due to their modified acidity profiles. Overall, these studies emphasize the potential of kaolin as a raw material in ZSM-5 synthesis, contributing to enhanced catalytic properties and offering a cost-effective alternative for MTO applications.
7.4.4. Desulfurization
Catalytic desulfurization processes are crucial in the petrochemical industry due to the stringent environmental regulations and the need for cleaner fuels [18,239]. These processes primarily involve the removal of sulfur compounds from petroleum fractions, which helps reduce sulfur dioxide emissions during fuel combustion, thus minimizing air pollution and acid rain. Desulfurization typically includes hydrodesulfurization (HDS), in which sulfur compounds are converted into hydrogen sulfide (H2S) using hydrogen gas in the presence of a catalyst. This reaction generally occurs at elevated temperatures and pressures. The effectiveness of this process largely depends on the catalyst used, with traditional catalysts being based on molybdenum or tungsten, often supported on alumina.
Recent advancements have focused on the use of kaolin-based catalysts due to their unique properties and cost-effectiveness. Kaolin, which is a natural clay, can be transformed into various catalytic forms, including zeolites, which offer high surface areas, appropriate acidity, and thermal stability. Kaolin-based catalysts are particularly advantageous in desulfurization due to their abundant availability, low cost, and versatility in modification. Its properties are essential for effective desulfurization as they enhance the interaction between sulfur compounds and the catalyst, leading to more efficient sulfur removal. They can be engineered to exhibit specific pore structures and acid sites, optimizing their performance for different desulfurization reactions. This adaptability not only improves the desulfurization efficiency but also extends the catalyst’s lifespan and reduces operational costs.
Various studies have explored the effectiveness of kaolin-based catalysts in desulfurization processes, highlighting their advantages and potential industrial applications. The first approach involved the synthesis of a ZSM-5, kaolin, and silica catalyst for the dehydrosulfidation of gasoline [240]. This catalyst demonstrated a higher selectivity for converting ethyl mercaptan (EM) over n-octane, without the need for hydrogen addition, indicating its efficiency in EM conversion with an activation energy of 46.66 kJ/mol. Additionally, the catalytic EM dehydrosulfidation led to higher EM conversions than n-octane, with product species identified during thermal experiments suggesting both intramolecular and intermolecular dehydrosulfidation pathways. Further advancements were made with the synthesis of a multi-pore zeolite beta (MB) from kaolin, which served as a support for NiW catalysts in diesel hydrodesulfurization (HDS) [241]. The NiW/TiO2–Al2O3–MB catalyst achieved an impressive HDS conversion of 99.3%, compared to 97.5% for NiW/γ-Al2O3. This improvement was attributed to the optimal balance of Brønsted and Lewis acids and increased hydrogenation activity, with the incorporation of TiO2 enhancing the catalytic performance further. In 2012, a study by Niu et al. [242] developed a kaolin/Cu2O photocatalyst for photochemical oxidation extraction desulfurization of model fuel. The catalyst achieved a desulfurization rate of over 97% under optimal conditions, which included a 20% kaolin load ratio, 2 h reaction time, and 150 mL·min-1 oxygen volume. This demonstrated the effectiveness of combining photocatalysis with liquid–liquid extraction for sulfur removal. A notable development in 2020 by Huang et al. [243] involved the preparation of a mesoporous kaolin carrier, which significantly increased its surface area from 16.6 m2/g to 303.4 m2/g. The resulting V2O5/mesoporous kaolin catalyst achieved nearly complete oxidative degradation of dibenzothiophene within 10 min. This rapid and efficient sulfur removal was due to the synergistic effects of mesoporous kaolin and well-dispersed V2O5 nanoparticles, exposing more V5+/V4+ Lewis acid sites. Further innovations included the synthesis of alumina metakaolin (AMK) from Iraqi kaolin for gasoil hydrodesulfurization [244]. The CoMo/AMK catalyst, which was tested in a fixed-bed reactor, outperformed commercial CoMo/Al2O3 catalysts by achieving an HDS efficiency of 80.5% compared to 72.3%. This was due to the high surface area, pore size, and accessible active sites of the AMK catalyst. More recently, Hmood et al. [245] utilized a ZSM-5-based catalyst prepared from kaolin for oxidative desulfurization (ODS) of sour heavy naphtha in a three-phase oscillatory baffled reactor. The Fe-impregnated ZSM-5 catalyst, which was coated with a TiO2 nanolayer, maintained a stable sulfur conversion of 90%, demonstrating enhanced stability and environmentally friendly oxidant use, with no coke formation during the process. Simultaneously, Ijadi et al. [246] synthesized tungsten oxide supported on metakaolin for ODS, achieving a maximum sulfur removal of 99.3% under optimized conditions, including a reaction temperature of 60 °C, an oxidant to sulfur molar ratio of 12, a catalyst dosage of 0.04 g, and an oxidation time of 40 min. The catalyst also exhibited excellent durability over five recycling tests, with desulfurization efficiencies of 60% for gasoline and 74% for gas oil, with initial sulfur contents of 300 ppm and 7550 ppm, respectively. Collectively, these studies underscore the significant potential of kaolin-based catalysts in enhancing desulfurization processes. They offer cost-effective, efficient, and environmentally friendly solutions for producing ultra-clean fuels, which is crucial for meeting stringent environmental standards and addressing the growing energy demand.
7.4.5. Deoxygenation
Catalytic deoxygenation processes are pivotal in the petrochemical industry for the conversion of renewable feedstocks into high-value hydrocarbon fuels. These processes, which primarily include hydrodeoxygenation (HDO) and decarboxylation/decarbonylation (DOD/DEC), focus on removing oxygen from organic compounds to enhance fuel quality and energy density. HDO involves the reaction of oxygen-containing compounds with hydrogen to produce water and hydrocarbons, while DOD/DEC eliminates oxygen as carbon dioxide or carbon monoxide. These transformations are essential for upgrading bio-derived oils and fats to meet stringent fuel specifications and improve their overall performance [247]. The efficacy of deoxygenation processes is largely dependent on the catalysts employed, such as nickel (Ni), molybdenum (Mo), or noble metals supported on various materials.
Recent advancements demonstrate the significant impact of kaolin-based catalysts on deoxygenation efficiency, showcasing their versatility and effectiveness. In 2019, Wang et al. [200] introduced acid-treated kaolin (ATK) as an effective catalyst for upgrading waste cooking oil (WCO). This catalyst achieved a high deoxygenation efficiency of 93.8% and a yield of 74.4% in organic liquid products (OLPs). Compared to a commercial petroleum catalyst (CC), which reached 90.8% oxygen removal but generated a higher proportion of undesired aromatics, ATK demonstrated superior performance. The effectiveness of ATK was linked to its density of weak Lewis acids, which play a crucial role in the deoxygenation process. Expanding on this, Nugraha et al. (2021) [248] explored the transformation of Indonesian kaolin into aluminosilicates with varying pore structures using a two-step hydrothermal synthesis. They found that the choice of structure-directing agents (SDAs) significantly influenced the formation of micropores and mesopores. Mesoporous Al-MCM-41 exhibited superior catalytic activity for deoxygenating bio-oil into green diesel compared to ZSM-5, which was primarily microporous. This study highlighted the benefits of tailoring pore structures to enhance catalytic performance. Further advancements were made by de Oliveira et al. (2021) [249], who investigated metal-supported zeolite catalysts for the deoxygenation of palm fatty acid distillate (PFAD). Their research showed that the combination of NiO and Co3O4 supported on zeolite achieved the highest organic liquid product (OLP) yield of 67.9% at 300 °C, with hydrocarbon yields reaching 84.8%. This work demonstrated the effectiveness of kaolin-derived zeolite catalysts in converting PFAD into valuable biofuels. In a more recent study, Maharani et al. (2023) [250] further optimized hierarchical ZSM-5 structures synthesized from kaolin. By adjusting hydrothermal conditions, they achieved a balance between micro- and mesopores, which greatly improved catalytic performance for deoxygenation reactions. The optimized catalyst demonstrated a 71.6% selectivity toward diesel hydrocarbons, showcasing enhanced efficiency due to improved reactant diffusion within the hierarchical structure. Lastly, Hou et al. (2023) [49] focused on acid-modified kaolin (AMK) for upgrading coal pyrolysis volatiles. Their study showed that AMK catalysts, which were modified using calcination and HCl leaching, improved tar upgrading performance by reducing the oxygen content in tar by 17.92–31.54%. The AMK800 catalyst, with its high specific surface area and Brønsted acid sites, was particularly effective in enhancing tar conversion and aromatic generation.
Overall, the integration of kaolin-based catalysts into deoxygenation processes represents a significant advancement in the development of efficient and sustainable petrochemical technologies. Their ability to improve catalyst performance while reducing costs and environmental impact underscores their growing importance in the industry. As research continues, kaolin-based materials are expected to play an increasingly vital role in advancing clean energy solutions and enhancing the value of renewable feedstocks.
7.4.6. Isomerization
Catalytic isomerization is a vital process in the petrochemical industry that is essential for converting linear hydrocarbons into branched isomers, thereby improving the octane rating of gasoline. This process addresses the demand for high-performance fuels by preventing engine knocking and enhancing fuel efficiency. The isomerization reaction typically involves the rearrangement of straight-chain alkanes, such as normal butane (n-C4H10) and normal pentane (n-C5H12), into their branched counterparts, isobutane (i-C4H10) and isopentane (i-C5H12), which possess higher octane numbers [161]. This transformation is crucial for producing high-octane gasoline, which is essential for the efficient operation of modern internal combustion engines [251]. In the petrochemical industry, the importance of isomerization extends beyond gasoline production. It also plays a role in the production of high-quality jet fuels and lubricants. The ability to tailor the properties of hydrocarbons through isomerization is crucial for meeting specific performance standards and regulatory requirements.
This process usually takes place in the presence of hydrogen to suppress coke formation and enhance catalyst longevity. The reaction conditions typically involve moderate temperatures and pressures, with hydrogen acting as a protective agent for the catalyst. Catalysts play a fundamental role in isomerization processes. Commonly used catalysts include bifunctional materials that contain both acidic and metallic sites [252]. Platinum (Pt) or palladium (Pd) dispersed on acidic supports, such as zeolites or chlorinated alumina, are frequently employed [253]. The acidic sites facilitate the protonation of alkanes, leading to carbocation intermediates, while the metallic sites aid in hydrogenation and dehydrogenation steps, ensuring the overall stability of the reaction. The isomerization mechanism involves several steps, as follows [254]:
- (a)
- Protonation of alkanes: The acidic sites of the catalyst protonate the alkane, forming a carbocation.
- (b)
- Carbocation rearrangement: The carbocation undergoes a rearrangement to form a more stable, branched isomer.
- (c)
- Deprotonation: The branched carbocation is deprotonated to yield the branched alkane.
- (d)
- Hydrogenation/Dehydrogenation: Metallic sites facilitate the addition or removal of hydrogen atoms, stabilizing the reaction intermediates and products.
The use of kaolin-based catalysts in isomerization offers several advantages, such as cost-effectiveness, environmental sustainability, and enhanced performance, since modifications, such as acid treatment and metal impregnation, can significantly boost the catalytic activity and selectivity of kaolin-based catalysts. Several studies have explored the use of kaolin-based catalysts for isomerization, revealing significant advancements in catalyst preparation and performance.
In 2007, Lenarda et al. [255] investigated the preparation of solid acid catalysts from metakaolin. By treating natural kaolin at high temperatures to produce metakaolin and subsequently acid-treating it, the authors obtained materials with distinct catalytic properties. The acid-treated metakaolin, particularly the sample treated at 850 °C (MK8), exhibited a high surface area and density of acid sites, making it highly effective for the isomerization of 1-butene. This study highlighted the potential of metakaolin as catalyst support with tailored acidity and porosity for isomerization reactions. Building on this foundation, Glotov et al. (2018) [256] synthesized Pt-containing catalysts using halloysite aluminosilicate nanotubes (multilayer aluminosilicate nanotubes formed by rolling kaolin layers) and ZSM-5 zeolite. These catalysts were evaluated for xylene isomerization under elevated hydrogen pressure. The study demonstrated that the Pt/ZSM-5 + HNT/γ-Al2O3 catalyst outperformed others in converting m-xylene and ethylbenzene, achieving conversion rates of 40% and 81%, respectively, at 440 °C. The hierarchical micro-mesoporous structure of the support enhanced the isomerization performance, reducing the yield of undesired hydrogenation products. In 2022, Wu et al. [257] focused on the isomerization of n-hexane using a NiP-Hβ/modified kaolin catalyst. This catalyst was prepared by ball milling NiP nanoparticles with Hβ/modified kaolin composites, resulting in a material with abundant weak-medium acid sites and mesopores. The modified kaolin provided additional active sites for the formation of di-branched isomerized products, achieving a high conversion rate of 78.1% and selectivity toward mono-branched (75.4%) and di-branched (21.4%) i-C6 products. This work underscored the potential of non-noble metal-based catalysts for efficient and environmentally friendly gasoline production. Most recently, Demikhova et al. (2023) [258] synthesized micro-mesoporous ZSM-5 zeolite using natural halloysite aluminosilicate nanotubes as templates. The resulting Pt-containing catalysts were tested for the isomerization of C-8 aromatic fractions. The catalyst prepared with organic templates (ZSM-5(t):HNT) showed superior performance due to its higher surface area and acidity compared to its template-free counterpart. The hierarchical structure facilitated the transformation of xylenes through both intra- and intermolecular isomerization routes, minimizing side reactions and enhancing selectivity.
These studies collectively highlight the advancements in using kaolin-based catalysts for isomerization processes in the petrochemical industry. The ability to tailor the acidity, porosity, and structural properties of kaolin-derived catalysts has led to significant improvements in catalytic performance, making them valuable for producing high-octane fuels and other petrochemical products.
7.4.7. Alkylation
Catalytic alkylation is considered a fundamental reaction in the petrochemical sector, playing a crucial role in the production of high-octane gasoline and various chemical intermediates. This process involves the reaction between light olefins, such as propylene or butylene, and isoparaffins, predominantly isobutane, to form alkylates [259]. Alkylates are highly branched hydrocarbons that significantly improve the octane rating of gasoline by enhancing engine performance and reducing harmful emissions. The importance of alkylation stems from its ability to produce high-quality gasoline components. High-octane fuels are essential for modern high-compression engines as they resist knocking, which can cause severe engine damage. By increasing the octane number of gasoline, alkylation helps meet stringent environmental regulations and fuel standards, contributing to cleaner and more efficient combustion engines.
Alkylation reactions typically occur in the presence of strong acids, such as sulfuric acid or hydrofluoric acid, which act as catalysts [157]. These acids facilitate the protonation of olefins, forming carbocations that react with isoparaffins to produce the desired alkylates. However, the use of liquid acids poses significant safety and environmental risks, including the handling and disposal of corrosive materials and the potential for toxic releases. To mitigate these issues, the industry has been exploring the use of solid acid catalysts as a safer and more sustainable alternative. Solid acids, including those based on zeolites and other porous materials, offer several advantages over liquid acids. They can be easily separated from reaction mixtures, reducing the risk of contamination and simplifying the catalyst recovery process. Additionally, solid acids can be regenerated and reused, lowering operational costs and minimizing environmental impact. Thus, by modifying kaolin solid clays through acid treatments and other chemical processes, it is possible to enhance their acidity and create materials with high surface areas and suitable pore structures for catalytic applications. While the specific role of kaolin-based catalysts in alkylation processes has been less extensively studied than other solid acids, their potential lies in their abundance, low cost, and environmental benignity.
In 2011, Odedairo et al. [260] investigated the gas-phase alkylation of toluene with isopropanol and its transalkylation with cumene using USY zeolite prepared by spray-dried using kaolin as the filler and a dual-zeolite (DZ) catalyst comprising mordenite and ZSM-5. The study revealed that both catalysts showed comparable cymene yields in toluene isopropylation, but the DZ catalyst achieved maximum yield at a lower temperature. In cumene–toluene transalkylation, the DZ catalyst exhibited higher cymene selectivity and conversion than the USY catalyst. This indicates that dual-zeolite catalysts can effectively enhance selectivity and conversion rates in alkylation processes. One year later, Jiang et al. [261] developed a Y/MCM-41 composite molecular sieve from pretreated kaolin, demonstrating high thermal and hydrothermal stability. The composite showed significant catalytic activity in the alkylation of phenol with tert-butyl alcohol, achieving a phenol conversion of 62.3% and a selectivity of 86.2% to 4-tert-butyl-phenol under optimal conditions. The combination of mesoporous MCM-41 and microporous Y-type molecular sieves in the composite contributed to its effectiveness as a catalyst, showcasing the potential of kaolin-based materials in alkylation reactions. Al-Kinany et al. [262], also in 2012, studied a selective BXE ALKCAT zeolite catalyst containing 30% ZSM-5 and kaolinite for the alkylation of benzene with ethylene. The catalyst exhibited high selectivity (85.5%) for ethylbenzene (EB) at a 1:1 benzene-to-ethylene molar ratio at 450 °C. The selectivity decreased at lower temperatures, indicating a strong dependence of reaction efficiency on temperature and reactant ratio. The study demonstrated that the BXE ALKCAT zeolite is an effective catalyst for ethylbenzene production, highlighting the role of kaolinite in enhancing catalytic performance. In 2018, Bok et al. [263] examined the influence of various binders, including kaolin, on the properties of nanocrystalline zeolite beta-based catalysts for benzene alkylation with propylene. They found that the use of kaolin-aluminum hydroxide mixtures as binders increased the number of acid sites without blocking the zeolite’s pore structure. The K-1 sample, with aluminum hydroxide binder, exhibited the best catalytic performance, achieving a cumene selectivity of 89.7% at 100% propylene conversion. This study underscored the significance of binder selection in optimizing catalyst performance. More recently, Gerzeliev et al. [264] explored the effect of different binders on MWW zeolite catalysts for benzene alkylation with propylene. The study found that aluminum hydroxide as a binder precursor provided the best results, enhancing the yield of cumene without adversely affecting the catalyst’s acidic properties. While the addition of kaolin did not impact the strength characteristics, it reduced the number of acid sites, thereby affecting the catalytic performance. This research highlighted the delicate balance between binder selection and catalytic activity in alkylation processes.
These investigations collectively underscore the importance of catalyst development in alkylation processes, with a particular focus on optimizing performance through the use of various zeolite structures and binders based on kaolin-based clays. This ongoing research is vital for advancing sustainable and efficient alkylation processes in the petrochemical industry.
8. Conclusions and Future Outlook
Natural minerals like kaolin present several advantages for industrial applications due to their abundant reserves, low environmental impact, and economic viability. Kaolin’s unique structure, consisting of alumina octahedral and silica tetrahedral sheets, provides a promising foundation for catalyst development. However, its raw form has limitations such as impurities, low surface area, and acidity, which restrict its direct use as an effective catalyst.
To address these limitations, extensive research has focused on enhancing kaolin’s catalytic properties through various modification techniques. Acid and base activation, calcination, and organic compound intercalation, among others, have proven effective in increasing kaolin’s surface area and porosity. These methods have enabled kaolin to serve as a viable catalyst in petrochemical processes, such as the well-known cracking, in which its modified forms have been used to reduce reaction times, lower reaction temperatures, and improve product quality.
Despite these advancements, the application of kaolin in the petrochemical industry faces challenges. The primary hurdle remains the difficulty and cost associated with modifying kaolinite. Current research is directed toward developing more efficient and cost-effective methods for kaolin modification. This includes optimizing activation techniques and exploring alternative modification strategies to enhance the catalytic performance of kaolin.
Significant progress has been made in integrating metals such as nickel, cerium, and cobalt into kaolin to create more acidic sites and increase the surface area available for reactions. These metal-enhanced kaolin catalysts show promise in improving the production of light distillates from heavy oils, which are typically rich in heavy distillates like bitumen and asphalt. By increasing the yield of valuable lighter fractions, these advanced kaolin-based catalysts could play a crucial role in addressing the growing demand for transportation fuels and petrochemicals.
Looking ahead, the future of kaolin-based catalysts in petrochemical processes will likely involve further innovation in several key areas. Enhanced methods for the synthesis and modification of kaolin will be essential for improving catalytic efficiency and reducing costs. Additionally, the development of hybrid catalysts that combine kaolin with other materials or metals could lead to more effective and versatile catalytic systems. The industry will also benefit from ongoing research into the environmental impacts of kaolin-based catalysis. Sustainable practices, including the recycling and reusability of catalysts, will be important in mitigating the environmental footprint of petrochemical processes.
Future research should prioritize (i) energy efficiency: developing catalysts that operate effectively at lower temperatures and with reduced energy consumption, (ii) cost-effectiveness: streamlining modification processes to lower costs while maintaining high performance, (iii) enhanced properties: increasing the surface area, acidity, and catalytic activity of kaolin-based materials, and (iv) sustainability: incorporating environmentally friendly practices and exploring the recyclability of catalysts.
In summary, kaolin’s potential in the petrochemical industry is substantial, particularly through its use as a precursor for zeolite synthesis and as a support for advanced catalytic systems. Advances in modification techniques and metal incorporation are paving the way for more efficient and sustainable processes. Continued research and innovation will be crucial in overcoming current limitations and unlocking the full potential of kaolin-based catalysts, thereby contributing to the development of more sustainable and economically viable petrochemical technologies.
Author Contributions
Conceptualization, J.A.-G., M.A., E.B.-G. and F.C.-M.; methodology, J.A.-G., M.A., E.B.-G. and F.C.-M.; investigation O.B.A.-A., M.A. and J.A.-G.; writing—original draft preparation, O.B.A.-A. and J.A.-G.; writing—review and editing, O.B.A.-A., E.B.-G. and J.A.-G.; supervision, M.A. and F.C.-M.; project administration, J.A.-G., M.A., E.B.-G. and F.C.-M.; funding acquisition, M.A., J.A.-G. and E.B.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejería de Universidad, Investigación e Innovación and the ERDF Andalusia Program 2021–2027 under Grant C-EXP-247-UGR23, as well as by MICIU/AEI/10.13039/501100011033, the “European Union NextGenerationEU/PRTR” program, and “ERDF A way of making Europe” under Projects PID2021-127803OB-I00 and CNS2023-144680.
Data Availability Statement
Data are contained within the article.
Acknowledgments
J.A.-G. offers thanks for the funding from MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU/PRTR” for the Juan de la Cierva contract (JDC 2022-048903-I). E.B.-G. acknowledges MICINN for her postdoctoral fellowship (RYC2020-029301-I). O.B.A.-A. and M.A. would like to thank the University of Technology-Iraq for all the service and support provided.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El-Aal, M.A.; Said, A.E.; Abdallah, M.H.; Goda, M.N. Modified natural kaolin clay as an active, selective, and stable catalyst for methanol dehydration to dimethyl ether. Sci. Rep. 2022, 12, 9407. [Google Scholar] [CrossRef] [PubMed]
- Ulfiati, R.; Dhaneswara, D.; Harjanto, S.; Fatriansyah, J.F. Synthesis and Characterization ZSM-5 Based on Kaolin as a Catalyst for Catalytic Cracking of Heavy Distillate. Int. J. Technol. 2022, 13, 860. [Google Scholar] [CrossRef]
- Custodis, V.B.F.; Karakoulia, S.A.; Triantafyllidis, K.S.; van Bokhoven, J.A. Catalytic Fast Pyrolysis of Lignin over High-Surface-Area Mesoporous Aluminosilicates: Effect of Porosity and Acidity. ChemSusChem 2016, 9, 1134–1145. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Mahmoud, M. Introduction to reservoir fluids and rock properties. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 2023; Volume 78, pp. 1–19. [Google Scholar]
- Hamdi Karaoğlu, M.; Doğan, M.; Alkan, M. Removal of cationic dyes by kaolinite. Microporous Mesoporous Mater. 2009, 122, 20–27. [Google Scholar] [CrossRef]
- Murray, H.H. Chapter 5 Kaolin Applications. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 85–109. [Google Scholar]
- Prasad, M.S.; Reid, K.J.; Murray, H.H. Kaolin: Processing, properties and applications. Appl. Clay Sci. 1991, 6, 87–119. [Google Scholar] [CrossRef]
- Liu, Y.; Pinnavaia, T.J. Metakaolin as a reagent for the assembly of mesoporous aluminosilicates with hexagonal, cubic and wormhole framework structures from proto-faujasitic nanoclusters. J. Mater. Chem. 2004, 14, 3416. [Google Scholar] [CrossRef]
- Shen, B.; Wang, P.; Yi, Z.; Zhang, W.; Tong, X.; Liu, Y.; Guo, Q.; Gao, J.; Xu, C. Synthesis of Zeolite β from Kaolin and Its Catalytic Performance For FCC Naphtha Aromatization. Energy Fuels 2009, 23, 60–64. [Google Scholar] [CrossRef]
- Holmes, S.M.; Khoo, S.H.; Kovo, A.S. The direct conversion of impure natural kaolin into pure zeolite catalysts. Green Chem. 2011, 13, 1152. [Google Scholar] [CrossRef]
- Belviso, C.; Giannossa, L.C.; Huertas, F.J.; Lettino, A.; Mangone, A.; Fiore, S. Synthesis of zeolites at low temperatures in fly ash-kaolinite mixtures. Microporous Mesoporous Mater. 2015, 212, 35–47. [Google Scholar] [CrossRef]
- Eman, E.A. Clays as catalysts in petroleum refining industry. ARPN J. Sci. Technol. 2013, 3, 356–375. [Google Scholar]
- Liu, J.; Yun, Z.; Gui, X. Ce/kaolin clay as an active catalyst for fatty acid methyl esters production from cottonseed oil in a new integrated apparatus. Braz. J. Chem. Eng. 2018, 35, 147–154. [Google Scholar] [CrossRef]
- Elfadly, A.M.; Zeid, I.F.; Yehia, F.Z.; Abouelela, M.M.; Rabie, A.M. Production of aromatic hydrocarbons from catalytic pyrolysis of lignin over acid-activated bentonite clay. Fuel Process. Technol. 2017, 163, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, C.; Yue, J.; Xu, G. Synthesis, characterization and catalytic methanation performance of modified kaolin-supported Ni-based catalysts. Chin. J. Chem. Eng. 2019, 27, 2953–2959. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Ashri Wan Daud, W.M. Kaolinite properties and advances for solid acid and basic catalyst synthesis. RSC Adv. 2015, 5, 101127–101147. [Google Scholar] [CrossRef]
- He, Y.; Tang, S.; Yin, S.; Li, S. Research progress on green synthesis of various high-purity zeolites from natural material-kaolin. J. Clean. Prod. 2021, 306, 127248. [Google Scholar] [CrossRef]
- Abdelwahab Emam, E. Clay Adsorption Perspective on Petroleum Refining Industry. Ind. Eng. 2018, 2, 19. [Google Scholar] [CrossRef]
- Liang, F.; Sayed, M.; Al-Muntasheri, G.A.; Chang, F.F.; Li, L. A comprehensive review on proppant technologies. Petroleum 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Pangilinan, K.D.; de Leon, A.C.C.; Advincula, R.C. Polymers for proppants used in hydraulic fracturing. J. Pet. Sci. Eng. 2016, 145, 154–160. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Haldar, S.K. Basic mineralogy. In Introduction to Mineralogy and Petrology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 109–143. ISBN 978-0-12-820585-3. [Google Scholar]
- Kerr, C.S.R.P.F. Dickite, a kaolin mineral. Am. Mineral. J. Earth Planet. Mater. 1930, 15, 34–39. [Google Scholar]
- Mehmel, M. Über die Struktur von Halloysit und Metahalloysit. Z. Krist.—Cryst. Mater. 1935, 90, 35–43. [Google Scholar] [CrossRef]
- Hofmann, U.; Endell, K.; Wilm, D. Röntgenographische und kolloidchemische Untersuchungen über Ton. Angew. Chem. Int. Ed. 1934, 47, 539–547. [Google Scholar] [CrossRef]
- Gysau, D. 5. Eigenschaften von Füllstoffen. In Füllstoffe; Vincentz Network: Hannover, Germany, 2019; pp. 97–154. [Google Scholar]
- Varga, G. The structure of kaolinite and metakaolinite. Epitoanyag—J. Silic. Based Compos. Mater. 2007, 59, 6–9. [Google Scholar] [CrossRef]
- Dill, H.G. Kaolin: Soil, rock and ore: From the mineral to the magmatic, sedimentary and metamorphic environments. Earth-Sci. Rev. 2016, 161, 16–129. [Google Scholar] [CrossRef]
- Bristow, C.M. Kaolin deposits of the United Kingdom of Great Britain and Northern Ireland. In Kaolin Deposits of the World: A-Europe; Proceedings of Symposium 1; Malkovsky, M., Vachtl, J., Eds.; International Geological Congress. Report of the TwentyThird Session, Prague, Czechoslovakia; 1969; pp. 215–288. Available online: https://books.google.com.hk/books/about/Kaolin_Deposits_of_the_world.html?id=nbi4tQEACAAJ&redir_esc=y (accessed on 19 July 2024).
- Murray, H.H. Major kaolin processing developments. Int. J. Miner. Process. 1980, 7, 263–274. [Google Scholar] [CrossRef]
- Murray, H.H.; Bundy, W.M.; Harvey, C.C. Kaolin Genesis and Utilization; Clay Minerals Society: Chantilly, VA, USA, 1993; ISBN 978-1-8812-0838-9. [Google Scholar]
- Research, P. Kaolin Market Size to Attain around USD 6.74 Billion by 2032. Available online: https://www.precedenceresearch.com/kaolin-market (accessed on 19 July 2024).
- Blengini, G.A.; Latunussa, C.E.L.; Eynard, U.; Torres de Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s list of Critical Raw Materials (2020); Final Report; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Research, G.V. Horizon—Global Kaolin Market Size & Outlook, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/kaolin-market (accessed on 19 July 2024).
- Research, G.V. Horizon—Global Kaolin Market Statistics, by Application, 2018–2030. Available online: https://www.grandviewresearch.com/horizon/statistics/worldwide-kaolin-market-117532 (accessed on 19 July 2024).
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Cheng, Y.; Xing, J.; Bu, C.; Zhang, J.; Piao, G.; Huang, Y.; Xie, H.; Wang, X. Dehydroxylation and Structural Distortion of Kaolinite as a High-Temperature Sorbent in the Furnace. Minerals 2019, 9, 587. [Google Scholar] [CrossRef]
- Bellotto, M.; Gualtieri, A.; Artioli, G.; Clark, S.M. Kinetic study of the kaolinite-mullite reaction sequence. Part I: Kaolinite dehydroxylation. Phys. Chem. Miner. 1995, 22, 207–217. [Google Scholar] [CrossRef]
- Moya, J.S.; Cabal, B.; Lopez-Esteban, S.; Bartolomé, J.F.; Sanz, J. Significance of the formation of pentahedral aluminum in the reactivity of calcined kaolin/metakaolin and its applications. Ceram. Int. 2024, 50, 1329–1340. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; Riley, D.P.; van Deventer, J.S.J. Combining density functional theory (DFT) and pair distribution function (PDF) analysis to solve the structure of metastable materials: The case of metakaolin. Phys. Chem. Chem. Phys. 2010, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.R.; Jia, D.C.; He, P.G.; Zhou, Y. Influence of calcination temperature of kaolin on the structure and properties of final geopolymer. Mater. Lett. 2010, 64, 2551–2554. [Google Scholar] [CrossRef]
- Horváth, E.; Frost, R.L.; Makó, É.; Kristóf, J.; Cseh, T. Thermal treatment of mechanochemically activated kaolinite. Thermochim. Acta 2003, 404, 227–234. [Google Scholar] [CrossRef]
- Dellisanti, F.; Valdrè, G. The role of microstrain on the thermostructural behaviour of industrial kaolin deformed by ball milling at low mechanical load. Int. J. Miner. Process. 2012, 102–103, 69–77. [Google Scholar] [CrossRef]
- Tarì, G.; Bobos, I.; Gomes, C.S.F.; Ferreira, J.M.F. Modification of Surface Charge Properties during Kaolinite to Halloysite-7Å Transformation. J. Colloid Interface Sci. 1999, 210, 360–366. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Pb(II) uptake by kaolinite and montmorillonite in aqueous medium: Influence of acid activation of the clays. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 277, 191–200. [Google Scholar] [CrossRef]
- Jabar, T.A.; Alzuhairi, M.A.; Abed, M.S. Acidic Influence on Geopolymerization: A Thorough Study Using HCl and Iraqi Kaolin. Russ. J. Appl. Chem. 2024, 97, 104–113. [Google Scholar] [CrossRef]
- Sabu, K.; Sukumar, R.; Rekha, R.; Lalithambika, M. A comparative study on H2SO4, HNO3 and HClO4 treated metakaolinite of a natural kaolinite as Friedel–Crafts alkylation catalyst. Catal. Today 1999, 49, 321–326. [Google Scholar] [CrossRef]
- Hou, Y.; Bai, Z.; Lu, H.; Feng, Z.; Zhang, T.; Jia, Y.; Guo, Z.; Kong, L.; Bai, J.; Li, W. In-situ catalytic upgrading of Hami coal pyrolysis volatiles over acid-modified kaolin. Fuel 2023, 331, 125660. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Influence of Acid Activation of Kaolinite and Montmorillonite on Adsorptive Removal of Cd(II) from Water. Ind. Eng. Chem. Res. 2007, 46, 3734–3742. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Influence of acid activation on adsorption of Ni(II) and Cu(II) on kaolinite and montmorillonite: Kinetic and thermodynamic study. Chem. Eng. J. 2008, 136, 1–13. [Google Scholar] [CrossRef]
- Panda, A.K.; Mishra, B.G.; Mishra, D.K.; Singh, R.K. Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 363, 98–104. [Google Scholar] [CrossRef]
- Mahmoud, S.; Saleh, S. Effect of Acid Activation on the De-Tert-Butylation Activity of Some Jordanian Clays. Clays Clay Miner. 1999, 47, 481–486. [Google Scholar] [CrossRef]
- Hartati; Prasetyoko, D.; Santoso, M.; Qoniah, I.; Leaw, W.L.; Firda, P.B.D.; Nur, H. A review on synthesis of kaolin-based zeolite and the effect of impurities. J. Chin. Chem. Soc. 2020, 67, 911–936. [Google Scholar] [CrossRef]
- Feng, M.; Kou, Z.; Tang, C.; Shi, Z.; Tong, Y.; Zhang, K. Recent progress in synthesis of zeolite from natural clay. Appl. Clay Sci. 2023, 243, 107087. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Len, T.; de Oliveira, A.D.; Costa, A.A.; Souza, A.R.; Costa, C.E.; Luque, R.; Rocha Filho, G.N.; Noronha, R.C.; Santos do Nascimiento, L.A. Zeolites: A Theoretical and Practical Approach with Uses in (Bio)Chemical Processes. Appl. Sci. 2023, 13, 1897. [Google Scholar] [CrossRef]
- Lussier, R.J. A novel clay-based catalytic material—Preparation and properties. J. Catal. 1991, 129, 225–237. [Google Scholar] [CrossRef]
- Belver, C.; Bañares Muñoz, M.A.; Vicente, M.A. Chemical Activation of a Kaolinite under Acid and Alkaline Conditions. Chem. Mater. 2002, 14, 2033–2043. [Google Scholar] [CrossRef]
- Slaty, F.; Khoury, H.; Wastiels, J.; Rahier, H. Characterization of alkali activated kaolinitic clay. Appl. Clay Sci. 2013, 75–76, 120–125. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer—An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Murat, M.; Amokrane, A.; Bastide, J.P.; Montanaro, L. Synthesis of zeolites from thermally activated kaolinite. Some observations on nucleation and growth. Clay Miner. 1992, 27, 119–130. [Google Scholar] [CrossRef]
- Mackinnon, I.D.R.; Millar, G.J.; Stolz, W. Hydrothermal syntheses of zeolite N from kaolin. Appl. Clay Sci. 2012, 58, 1–7. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X-type zeolites synthesised from kaolinite at low temperature. Appl. Clay Sci. 2013, 80–81, 162–168. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, C.; Alshameri, A.; Qiu, X.; Zhou, C.; Li, D. Synthesis and characterization of 13X zeolite from low-grade natural kaolin. Adv. Powder Technol. 2014, 25, 495–499. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Daud, W.M.A.W. Synthesis and characterization of hierarchical nanoporous HY zeolites from acid-activated kaolin. Chin. J. Catal. 2015, 36, 1846–1851. [Google Scholar] [CrossRef]
- EL-Mekkawi, D.M.; Ibrahim, F.A.; Selim, M.M. Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar] [CrossRef]
- Saito, Y.; Motohashi, T.; Hayashi, S.; Yasumori, A.; Okada, K. High thermal resistance of γ-Al2O3 prepared by the selective leaching of calcined kaolin minerals. J. Mater. Chem. 1997, 7, 1615. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part II Application of a new theory to silicates, aluminosilicates and quartz. Hydrometallurgy 2014, 149, 265–275. [Google Scholar] [CrossRef]
- Sivapullaiah, P.V. Manju Kaolinite—Alkali interaction and effects on basic properties. Geotech. Geol. Eng. 2005, 23, 601–614. [Google Scholar] [CrossRef]
- Wang, Y.H.; Siu, W.K. Structure characteristics and mechanical properties of kaolinite soils. I. Surface charges and structural characterizations. Can. Geotech. J. 2006, 43, 587–600. [Google Scholar] [CrossRef]
- Elbokl, T.A.; Detellier, C. Aluminosilicate nanohybrid materials. Intercalation of polystyrene in kaolinite. J. Phys. Chem. Solids 2006, 67, 950–955. [Google Scholar] [CrossRef]
- Deng, Y.; White, G.N.; Dixon, J.B. Effect of Structural Stress on the Intercalation Rate of Kaolinite. J. Colloid Interface Sci. 2002, 250, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Matusik, J.; Kłapyta, Z. Characterization of kaolinite intercalation compounds with benzylalkylammonium chlorides using XRD, TGA/DTA and CHNS elemental analysis. Appl. Clay Sci. 2013, 83–84, 433–440. [Google Scholar] [CrossRef]
- Letaief, S.; Tonle, I.K.; Diaco, T.; Detellier, C. Nanohybrid materials from interlayer functionalization of kaolinite. Application to the electrochemical preconcentration of cyanide. Appl. Clay Sci. 2008, 42, 95–101. [Google Scholar] [CrossRef]
- Makó, É.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Mechanochemical intercalation of low reactivity kaolinite. Appl. Clay Sci. 2013, 83–84, 24–31. [Google Scholar] [CrossRef]
- Bundy, W.M.; Ishley, J.N. Kaolin in paper filling and coating. Appl. Clay Sci. 1991, 5, 397–420. [Google Scholar] [CrossRef]
- Elton, N.J.; Gate, L.F.; Hooper, J.J. Texture and orientation of kaolin in coatings. Clay Miner. 1999, 34, 89–98. [Google Scholar] [CrossRef]
- Morris, J.D.; Daood, S.S.; Nimmo, W. The use of kaolin and dolomite bed additives as an agglomeration mitigation method for wheat straw and miscanthus biomass fuels in a pilot-scale fluidized bed combustor. Renew. Energy 2022, 196, 749–762. [Google Scholar] [CrossRef]
- Conceição, S.; Santos, N.F.; Velho, J.; Ferreira, J.M.F. Properties of paper coated with kaolin: The influence of the rheological modifier. Appl. Clay Sci. 2005, 30, 165–173. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Zhang, M.; Liu, Y.; Yu, Y.; Yu, H. Electrical and Mechanical Properties of Epoxy Composites Reinforced with Kaolin-Coated Basalt Fibers. Fibers Polym. 2023, 24, 95–108. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Mohd Mortar, N.A.; Abdullah, M.M.A.B.; Abdul Razak, R.; Abd Rahim, S.Z.; Aziz, I.H.; Nabiałek, M.; Jaya, R.P.; Semenescu, A.; Mohamed, R.; Ghazali, M.F. Geopolymer Ceramic Application: A Review on Mix Design, Properties and Reinforcement Enhancement. Materials 2022, 15, 7567. [Google Scholar] [CrossRef] [PubMed]
- Grim, R.E. Applied Clay Mineralogy. Geol. Föreningen Stockh. Förhandlingar 1962, 84, 533. [Google Scholar] [CrossRef]
- Chaudhuri, S.P. A Review on the Kaolinite-Mullite Transformation. Trans. Indian Ceram. Soc. 1977, 36, 71–81. [Google Scholar] [CrossRef]
- Bourret, J.; Michot, A.; Tessier-Doyen, N.; Naït-Ali, B.; Pennec, F.; Alzina, A.; Vicente, J.; Peyratout, C.S.; Smith, D.S. Thermal Conductivity of Very Porous Kaolin-Based Ceramics. J. Am. Ceram. Soc. 2014, 97, 938–944. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Ismail, A.F.; Gani, P. Effect of kaolin particle size and loading on the characteristics of kaolin ceramic support prepared via phase inversion technique. J. Asian Ceram. Soc. 2016, 4, 164–177. [Google Scholar] [CrossRef]
- Johns, W.D. High-temperature phase changes in kaolinites. Mineral. Mag. J. Mineral. Soc. 1953, 30, 186–198. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, M.C.; Hon, M.H. Phase transformation and growth of mullite in kaolin ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- Alves, H.P.A.; Silva, J.B.; Campos, L.F.A.; Torres, S.M.; Dutra, R.P.S.; Macedo, D.A. Preparation of mullite based ceramics from clay–kaolin waste mixtures. Ceram. Int. 2016, 42, 19086–19090. [Google Scholar] [CrossRef]
- Shafiq, N.; Nuruddin, M.F.; Khan, S.U.; Ayub, T. Calcined kaolin as cement replacing material and its use in high strength concrete. Constr. Build. Mater. 2015, 81, 313–323. [Google Scholar] [CrossRef]
- Bundy, W.M. The Diverse Industrial Applications of Kaolin; Special Publication No. 1; Clay Minerals Society: Boulder, CO, USA, 1993; pp. 43–73. [Google Scholar]
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in Cosmetics and Personal-Care Products. Clays Clay Miner. 2021, 69, 561–575. [Google Scholar] [CrossRef]
- Murray, H.H. Applied rheology. Proc. Porc. Enamel Inst. Tech. Forum 1975, 37, 1–9. [Google Scholar]
- Murray, H.H.; Kogel, J.E. Engineered clay products for the paper industry. Appl. Clay Sci. 2005, 29, 199–206. [Google Scholar] [CrossRef]
- Buyondo, K.A.; Kasedde, H.; Kirabira, J.B. A comprehensive review on kaolin as pigment for paint and coating: Recent trends of chemical-based paints, their environmental impacts and regulation. Case Stud. Chem. Environ. Eng. 2022, 6, 100244. [Google Scholar] [CrossRef]
- Ruszala, M.J.A.; Rowson, N.A.; Grover, L.M.; Choudhery, R.A. Low Carbon Footprint TiO2 Substitutes in Paint: A Review. Int. J. Chem. Eng. Appl. 2015, 6, 331–340. [Google Scholar] [CrossRef]
- Narayan, R.; Raju, K.V.S.N. The use of calcined clay as part replacement of titanium dioxide in latex paint formulations. J. Appl. Polym. Sci. 2000, 77, 1029–1036. [Google Scholar] [CrossRef]
- Ahmed, N.M. Comparative study on the role of kaolin, calcined kaolin and chemically treated kaolin in alkyd-based paints for protection of steel. Pigment Resin Technol. 2013, 42, 3–14. [Google Scholar] [CrossRef]
- Ahmed, N.M.; El-Sabbagh, S.H. The influence of kaolin and calcined kaolin on SBR composite properties. Polym. Compos. 2014, 35, 570–580. [Google Scholar] [CrossRef]
- Meite, N.; Konan, L.K.; Bamba, D.; Goure-Doubi, B.I.H.; Oyetola, S. Structural and Thermomechanical Study of Plastic Films Made from Cassava-Starch Reinforced with Kaolin and Metakaolin. Mater. Sci. Appl. 2018, 9, 41–54. [Google Scholar] [CrossRef][Green Version]
- Hussein, Z.A.; Shakor, Z.M.; Alzuhairi, M.; Al-Sheikh, F. Kinetic and Thermodynamic Study of the Pyrolysis of Plastic Waste. Environ. Process. 2023, 10, 27. [Google Scholar] [CrossRef]
- Vesely, D.; Kalendova, A.; Manso, M.V. Properties of calcined kaolins in anticorrosion paints depending on PVC, chemical composition and shape of particles. Prog. Org. Coat. 2012, 74, 82–91. [Google Scholar] [CrossRef]
- Mallem, O.K.; Zouai, F.; Gumus, O.Y.; Benabid, F.Z.; Bedeloglu, A.C.; Benachour, D. Synergistic effect of talc/calcined kaolin binary fillers on rigid PVC: Improved properties of PVC composites. J. Vinyl Addit. Technol. 2021, 27, 881–893. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, S.; Gu, X.; Sun, J.; Jin, X.; Li, H. Effects of kaolinite nanoroll on the flammability of polypropylene nanocomposites. Appl. Clay Sci. 2016, 132–133, 579–588. [Google Scholar] [CrossRef]
- Ogbebor, O.; Okieimen, F.; Ogbeifun, D.; Okwu, U. Organomodified kaolin as filler for natural rubber. Chem. Ind. Chem. Eng. Q. 2015, 21, 477–484. [Google Scholar] [CrossRef]
- Arroyo, M.; López-Manchado, M.; Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Susanto, T.; Rahmaniar; Putra, V.G.V.; Sari, T.I.; Farida; Roni, K.A.; Puspitasari, G. Effect carbon black and modified kaolin hybrid filler on the curing and physic-mechanical properties of natural rubber-styrene butadiene rubber blends. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 963, p. 012029. [Google Scholar] [CrossRef]
- Yadav, J.S.; Tiwari, S.K. A study on the potential utilization of crumb rubber in cement treated soft clay. J. Build. Eng. 2017, 9, 177–191. [Google Scholar] [CrossRef]
- El-Nashar, D.; Khalaf, A. A review on reinforcing rubber goods for different valuable applications. Egypt. J. Chem. 2022, 66, 503–529. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical industry. Appl. Clay Sci. 2009, 46, 73–80. [Google Scholar] [CrossRef]
- Christian, Z.C. Chemical investigation of kankara kaolin for its pharmaceutical applications. Open Access J. Transl. Med. Res. 2018, 2, 11–13. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in pharmaceutics and biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Yarangsee, C.; Wattanaarsakit, P.; Sirithunyalug, J.; Leesawat, P. Particle Engineering of Chitosan and Kaolin Composite as a Novel Tablet Excipient by Nanoparticles Formation and Co-Processing. Pharmaceutics 2021, 13, 1844. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.S.; Subha, M.C.; Jithendra, T.; Madhavi, C.; Rao, K.C. Emerging Novel Drug Delivery System for Control Release of Curcumin through Sodium Alginate/Poly(ethylene glycol) Semi IPN Microbeads-Intercalated with Kaolin Nanoclay. J. Drug Deliv. Ther. 2019, 9, 324–333. [Google Scholar] [CrossRef]
- Dissanayake, N.U.S.; Pupulewatte, P.G.H.; Jayawardana, D.T.; Senevirathne, D.M. Characterization of Kaolin-rich Laterite Soil for Applications for the Development of Soil-based Cosmetic Products. J. Drug Deliv. Ther. 2022, 12, 31–40. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin care and rejuvenation by cosmeceutical facial mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef]
- Pinheiro, G.; Carvalho, T.; Michel, B.; Arjona, J.; Bobadilha, M.; Silva-Valenzuela, M.; Costa, T.; Valenzuela-Diaz, F. Characterization of a Brazilian Kaolin and Its Sorption Ability to Mineral Oils. In Minerals, Metals and Materials Series; Springer: Berlin/Heidelberg, Germany, 2020; Volume PartF1, pp. 367–373. [Google Scholar]
- Watkins, E.C. Mineral Raw Materials for Fiberglass Manufacturing; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 1986; p. 6. Available online: https://onemine.org/documents/mineral-raw-materials-for-fiber-glass-manufacturing (accessed on 19 July 2024).
- Pascual, S.; Cobos, G.; Seris, E.; González-Núñez, M. Effects of processed kaolin on pests and non-target arthropods in a Spanish olive grove. J. Pest Sci. 2010, 83, 121–133. [Google Scholar] [CrossRef]
- Gilkes, R.J.; Prakongkep, N. How the unique properties of soil kaolin affect the fertility of tropical soils. Appl. Clay Sci. 2016, 131, 100–106. [Google Scholar] [CrossRef]
- Cantore, V.; Pace, B.; Albrizio, R. Kaolin-based particle film technology affects tomato physiology, yield and quality. Environ. Exp. Bot. 2009, 66, 279–288. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Unuabonah, I.E.; Olu-Owolabi, B.I. Adsorption of some heavy metal ions on sulfate- and phosphate-modified kaolin. Appl. Clay Sci. 2005, 29, 145–148. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Li, J.; Jiao, S.; Zhong, L.; Pan, J.; Ma, Q. Optimizing coagulation and flocculation process for kaolinite suspension with chitosan. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 428, 100–110. [Google Scholar] [CrossRef]
- Ferasat, Z.; Panahi, R.; Mokhtarani, B. Natural polymer matrix as safe flocculant to remove turbidity from kaolin suspension: Performance and governing mechanism. J. Environ. Manag. 2020, 255, 109939. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.; Tijani, J.; Ndamitso, M.; Abdulkareem, A.; Shuaib, D.; Mohammed, A. Adsorptive removal of pollutants from industrial wastewater using mesoporous kaolin and kaolin/TiO2 nanoadsorbents. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100414. [Google Scholar] [CrossRef]
- Kang, W.; Cui, Y.; Yang, Y.; Guo, M.; Zhao, Z.; Wang, X.; Liu, X. Preparation of nitrogen-doped hollow carbon nanosphere/graphene composite aerogel for efficient removal of quinoline from wastewater. J. Hazard. Mater. 2021, 417, 126160. [Google Scholar] [CrossRef]
- Cai, W.; Bordoloi, S.; Ng, C.W.W.; Sarmah, A.K. Influence of pore fluid salinity on shrinkage and water retention characteristics of biochar amended kaolin for landfill liner application. Sci. Total Environ. 2022, 838, 156493. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Zhao, T.; Gui, K.; Wang, J.; Thomas, H.R. Superior activity of iron–manganese supported on kaolin for NO abatement at low temperature. J. Environ. Sci. 2020, 88, 237–247. [Google Scholar] [CrossRef]
- Selim, M.M.; Abd El-Maksoud, I.H. Hydrogenation of edible oil over zeolite prepared from local kaolin. Microporous Mesoporous Mater. 2004, 74, 79–85. [Google Scholar] [CrossRef]
- Li, X.; Tang, A. Pd modified kaolinite nanocomposite as a hydrogenation catalyst. RSC Adv. 2016, 6, 15585–15591. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, L.; Chen, J.; Luo, H.; Liu, J. In situ growth of g-C3N4 on clay minerals of kaolinite, sepiolite, and talc for enhanced solar photocatalytic energy conversion. Appl. Clay Sci. 2022, 216, 106337. [Google Scholar] [CrossRef]
- Maj, I.; Matus, K. Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies 2023, 16, 4359. [Google Scholar] [CrossRef]
- Ferreira, B.F.; do Prado, M.V.; Marçal, L.; Ciuffi, K.J.; Vicente, M.A.; Gil, A.; de Faria, E.H. Manganese-sulfonato porphyrin adsorbed on amino kaolinite as heterogeneous catalyst for oxidation and polymerization reactions. Appl. Clay Sci. 2023, 235, 106871. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, L.; Cheng, H. Rational design of kaolinite-based photocatalytic materials for environment decontamination. Appl. Clay Sci. 2021, 208, 106098. [Google Scholar] [CrossRef]
- Chen, M.; Yang, T.; Han, J.; Zhang, Y.; Zhao, L.; Zhao, J.; Li, R.; Huang, Y.; Gu, Z.; Wu, J. The Application of Mineral Kaolinite for Environment Decontamination: A Review. Catalysts 2023, 13, 123. [Google Scholar] [CrossRef]
- Statista Fossil Fuels. Available online: www.statista.com (accessed on 25 July 2024).
- Energy Institute. Energy Institute Statistical Review of World Energy 2024. Available online: https://www.energyinst.org/statistical-review (accessed on 25 July 2024).
- BP. BP Statistical Review of World Energy 2021. Available online: https://www.bp.com/ (accessed on 25 July 2024).
- OPEC OPEC Monthly Oil Market Report 2024. Available online: https://www.opec.org/opec_web/en/ (accessed on 25 July 2024).
- Energy Institute. Statistical Review of World Energy 2024, Tab “Oil Consumption–Barrels”. Available online: https://www.energyinst.org/ (accessed on 25 July 2024).
- Energy Institute. Statistical Review of World Energy 2024, Tab “Oil Trade Movements”. Available online: https://www.energyinst.org/statistical-review (accessed on 25 July 2024).
- Gelder, A.; Mitchell, E. Assessing the Refineries at Risk of Closure. Available online: https://www.woodmac.com/news/opinion/assessing-the-refineries-at-risk-of-closure/ (accessed on 25 July 2024).
- Datosmacro. Economic and Sociodemographic Information. Available online: https://datosmacro.expansion.com/ (accessed on 25 July 2024).
- GlobalPetrolPrices. Energy Prices around the World. Available online: https://www.globalpetrolprices.com/ (accessed on 25 July 2024).
- Statista. Oil Giants Make Record Profits. Available online: https://es.statista.com/grafico/27899/ganancias-netas-de-companias-de-petroleo-y-gas/ (accessed on 25 July 2024).
- Rana, M.S.; Sámano, V.; Ancheyta, J.; Diaz, J.A.I. A review of recent advances on process technologies for upgrading of heavy oils and residua. Fuel 2007, 86, 1216–1231. [Google Scholar] [CrossRef]
- Castañeda, L.C.; Muñoz, J.A.D.; Ancheyta, J. Current situation of emerging technologies for upgrading of heavy oils. Catal. Today 2014, 220–222, 248–273. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of hydrocarbons for the production of olefins: A state-of-the-art review I: Thermal cracking review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Al-Samhan, M.; Al-Fadhli, J.; Al-Otaibi, A.M.; Al-Attar, F.; Bouresli, R.; Rana, M.S. Prospects of refinery switching from conventional to integrated: An opportunity for sustainable investment in the petrochemical industry. Fuel 2022, 310, 122161. [Google Scholar] [CrossRef]
- Weitkamp, J. Catalytic Hydrocracking—Mechanisms and Versatility of the Process. ChemCatChem 2012, 4, 292–306. [Google Scholar] [CrossRef]
- Kinsara, R.A.; Demirbas, A. Upgrading of crude oil via distillation processes. Pet. Sci. Technol. 2016, 34, 1300–1306. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Vakili, M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies 2021, 14, 8190. [Google Scholar] [CrossRef]
- Ragothaman, A.; Anderson, W. Air Quality Impacts of Petroleum Refining and Petrochemical Industries. Environments 2017, 4, 66. [Google Scholar] [CrossRef]
- Perego, C.; Ingallina, P. Recent advances in the industrial alkylation of aromatics: New catalysts and new processes. Catal. Today 2002, 73, 3–22. [Google Scholar] [CrossRef]
- Pan, Y.; Bai, J.; Yang, G.; Li, Z. Progress in the Research on Branched Polymers with Emphasis on the Chinese Petrochemical Industry. Molecules 2023, 28, 7934. [Google Scholar] [CrossRef]
- Bahri, M.; Mahdavi, A.; Mirzaei, A.; Mansouri, A.; Haghighat, F. Integrated oxidation process and biological treatment for highly concentrated petrochemical effluents: A review. Chem. Eng. Process. 2018, 125, 183–196. [Google Scholar] [CrossRef]
- Matar, S.; Mirbach, M.J.; Tayim, H.A. Hydrogenation—Dehydrogenation Processes. In Catalysis in Petrochemical Processes; Springer: Dordrecht, The Netherlands, 1989; pp. 35–65. ISBN 978-94-009-1177-2. [Google Scholar]
- Valavarasu, G.; Sairam, B. Light Naphtha Isomerization Process: A Review. Pet. Sci. Technol. 2013, 31, 580–595. [Google Scholar] [CrossRef]
- Hettinger, W.P. Contribution to catalytic cracking in the petroleum industry. Appl. Clay Sci. 1991, 5, 445–468. [Google Scholar] [CrossRef]
- Chang, Y.F.; Vaughn, S.N.; Martens, L.R.; Baumgartner, J.E.; Soled, S.L.; Clem, K.R. Molecular Sieve Catalyst Composition, Its Making and Use in Conversion Processes. U.S. Patent No. 6,872,680, 24 June 2002. [Google Scholar]
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Appl. Petrochem. Res. 2015, 5, 377–392. [Google Scholar] [CrossRef]
- Qin, X.; Ye, L.; Hou, L.; Wang, T.; Ma, M.; Pu, X.; Han, X.; Liu, J.; Luan, B.; Liu, P. A coupling model of fluid catalytic cracking and diesel hydrotreating processes to study the effects of reaction temperature on the composition of diesel. Chem. Eng. J. 2023, 466, 143078. [Google Scholar] [CrossRef]
- Fujiyama, Y.; Al-Tayyar, M.H.; Dean, C.F.; Aitani, A.; Redhwi, H.H. Chapter 1 Development of high-severity FCC process: An overview. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 166, pp. 1–12. ISBN 0444530606. [Google Scholar]
- Woltermann, G.M.; Magee, J.S.; Griffith, S.D. Chapter 4 Commercial Preparation and Characterization of FCC Catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1993; Volume 76, pp. 105–144. [Google Scholar]
- Milton, R.M. Molecular Sieve Adsorbents. U.S. Patent 2,882,244, 14 April 1959. [Google Scholar]
- Maher, P.K.; Albers, E.W.; Mcdaniel, C.V. Preparation of High Silica Synthetic Faujasite. U.S. Patent US3,671,191A, 20 June 1972. [Google Scholar]
- Brown, S.M.; Woltermann, G.M. Zeolitized Composite Bodies and Manufacture Thereof. U.S. Patent 4,235,753, 25 November 1980. [Google Scholar]
- Atta, A.Y.; Ajayi, O.A.; Adefila, S.S. Synthesis of Faujasite Zeolites from Kankara Kaolin Clay. J. Appl. Sci. Res. 2007, 3, 1017–1021. [Google Scholar]
- Haden, W.L.; Dzierzanowski, F.J.; Somerset, N.J. Microspherical Zeolitic Molecular Sieve Composite Catalyst and Preparation Thereof. U.S. Patent No. 3,402,996, 20 June 1968. [Google Scholar]
- Haden, W.L., Jr.; Dzierzanowski, F.J. Microspherical Zeolitic Molecular Sieve Composite Catalyst and Preparation Thereof. U.S. Patent 3,624,718, 7 March 1972. [Google Scholar]
- Speronello, B.K. Porous Mullite. U.S. Patent 4,628,042, 1986. [Google Scholar]
- Howell, P.A. Process for Preparing Crystalline Zeolitic Molecular Sieves. U.S. Patent 3,390,958, 2 July 1968. [Google Scholar]
- Chandrasekhar, S.; Pramada, P.N. Kaolin-based zeolite Y, a precursor for cordierite ceramics. Appl. Clay Sci. 2004, 27, 187–198. [Google Scholar] [CrossRef]
- Ajayi, O.A.; Adefila, S.S.; Ityokumbul, M.T. Monitoring zeolite NaY formation from potassium-rich Nigerian kaolinite clay. Ain Shams Eng. J. 2018, 9, 1653–1661. [Google Scholar] [CrossRef]
- Plank, C.J.; Rosinski, E.J. Catalytic Conversion of Hydrocarbons with a Crystalline Alumino-Silicate in a Silica-Alumina Matrix. U.S. Patent 3,271,418, 6 September 1966. [Google Scholar]
- Secor, R.B. Method for Producing Catalysts. U.S. Patent 3,446,727, 1968. [Google Scholar]
- Danforth, J.D. 57 The Structure of Silica-Alumina Cracking Catalysts. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 1957; Volume 9, pp. 558–568. [Google Scholar]
- Joseph, C.S.; Shi, E.W.; Albers, G.R.W. Process for Improving the Physical and Catalytic Properties of a Fluid Cracking Catalyst. U.S. Patent 5,739,072, 1998. [Google Scholar]
- Engelhardt, G.; Lohse, U.; Samoson, A.; Mägi, M.; Tarmak, M.; Lippmaa, E. High resolution 29Si n.m.r. of dealuminated and ultrastable Y-zeolites. Zeolites 1982, 2, 59–62. [Google Scholar] [CrossRef]
- Brown, S.M.; Durante, V.A.; Reagan, W.J.; Speronello, B.K. Fluid Catalytic Cracking Catalyst Comprising Microspheres Containing More Than about 40 Percent by Weight Y-Faujasite and Methods for Making. U.S. Patent 4,601,997, 1986. [Google Scholar]
- Absil, P.L.; Robert, K.A.J. Catalyst and Catalytic Conversion Therewith. U.S. Patent No. 5,366,948, 1994. [Google Scholar]
- Absil, R.P.; Herbst, J.A. Cracking Catalysts Comprising Phosphorus and Method of Preparing and Using the Same. U.S. Patent 5,231,064, 1993. [Google Scholar]
- Komvokis, V.; Tan, L.X.L.; Clough, M.; Pan, S.S.; Yilmaz, B. Zeolites in Fluid Catalytic Cracking (FCC). In Zeolites in Sustainable Chemistry: Synthesis, Characterization and Catalytic Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–297. ISBN 9783662473955. [Google Scholar]
- Patrylak, L.; Likhnyovskyi, R.; Vypyraylenko, V.; Leboda, R.; Skubiszewska-Zięba, J.; Patrylak, K. Adsorption Properties of Zeolite-Containing Microspheres and FCC Catalysts Based on Ukrainian Kaolin. Adsorpt. Sci. Technol. 2001, 19, 525–540. [Google Scholar] [CrossRef]
- Al-Khattaf, S. The influence of Y-zeolite unit cell size on the performance of FCC catalysts during gas oil catalytic cracking. Appl. Catal. A 2002, 231, 293–306. [Google Scholar] [CrossRef]
- Zheng, S.-Q.; Sun, S.-H.; Wang, Z.-F.; Gao, X.-H.; Xu, X.-L. Suzhou kaolin as a FCC catalyst. Clay Miner. 2005, 40, 303–310. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Gao, X. Synthesis, characterization and evaluation of a novel resid FCC catalyst based on in situ synthesis on kaolin microspheres. Catal. Lett. 2006, 110, 229–234. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Gao, X.; Ma, J. A novel FCC catalyst synthesized via in situ overgrowth of NaY zeolite on kaolin microspheres for maximizing propylene yield. Catal. Today 2007, 125, 163–168. [Google Scholar] [CrossRef]
- Wang, P.; Shen, B.; Shen, D.; Peng, T.; Gao, J. Synthesis of ZSM-5 zeolite from expanded perlite/kaolin and its catalytic performance for FCC naphtha aromatization. Catal. Commun. 2007, 8, 1452–1456. [Google Scholar] [CrossRef]
- Gonçalves, M.L.A.; Barreto, J.R.C.; Cerqueira, W.V.; Teixeira, A.M.R.F. Effect of zeolite, kaolin and alumina during cracking of heavy petroleum residue evaluated by thermogravimetry. J. Therm. Anal. Calorim. 2009, 97, 515–519. [Google Scholar] [CrossRef]
- Wan, G.; Duan, A.; Zhang, Y.; Zhao, Z.; Jiang, G.; Zhang, D.; Gao, Z.; Liu, J.; Chung, K.H. Hydrodesulfurization of Fluidized Catalytic Cracking Diesel Oil over NiW/AMB Catalysts Containing H-Type β-Zeolite in Situ Synthesized from Kaolin Material. Energy Fuels 2009, 23, 3846–3852. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Zheng, S.-Q.; Qian, D. Sulphur reduction in fluid catalytic cracking using a kaolin in situ crystallization catalyst modified with vanadium. Clay Miner. 2009, 44, 281–288. [Google Scholar] [CrossRef]
- Duan, A.; Wan, G.; Zhang, Y.; Zhao, Z.; Jiang, G.; Liu, J. Optimal synthesis of micro/mesoporous beta zeolite from kaolin clay and catalytic performance for hydrodesulfurization of diesel. Catal. Today 2011, 175, 485–493. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, R.; Li, X.; Liu, X.; Yan, Z. In situ synthesis, characterization and catalytic activity of ZSM-5 zeolites on kaolin microspheres from amine-free system. J. Porous Mater. 2013, 20, 137–141. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Machado Júnior, H.F.; Costa, D.A. Kaolin and commercial fcc catalysts in the cracking of loads of polypropylene under refinary conditions. Braz. J. Chem. Eng. 2013, 30, 825–834. [Google Scholar] [CrossRef]
- Ghrib, Y.; Frini-Srasra, N.; Srasra, E.; Martínez-Triguero, J.; Corma, A. Synthesis of cocrystallized USY/ZSM-5 zeolites from kaolin and its use as fluid catalytic cracking catalysts. Catal. Sci. Technol. 2018, 8, 716–725. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Zheng, Y.; Ng, S.; Brown, H.; Xia, Y. Kaolin-based catalyst as a triglyceride FCC upgrading catalyst with high deoxygenation, mild cracking, and low dehydrogenation performances. Catal. Today 2019, 319, 164–171. [Google Scholar] [CrossRef]
- Padilla, J.; Guzman, A.; Molina, D.; Poveda-Jaramillo, J.C. Structural transformation of kaolin as an active matrix for the in situ synthesis of zeolite Y. Clay Miner. 2020, 55, 293–302. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Z.; Zhou, L.; Ju, G. Synthesis of Si-Modified Pseudo-Boehmite@kaolin Composite and Its Application as a Novel Matrix Material for FCC Catalyst. Materials 2022, 15, 2169. [Google Scholar] [CrossRef]
- Padilla, J.; Guzman, A.; Poveda-Jaramillo, J.C. Synthesis and catalytic behavior of FCC catalysts obtained from kaolin by the in-situ method. Braz. J. Chem. Eng. 2023, 40, 803–816. [Google Scholar] [CrossRef]
- Kharas, K.; Mastry, M.C.; Thompson, A.; Yilmaz, B. Comparison of an in situ and an incorporated FCC catalyst under iron contamination. Catal. Commun. 2022, 171, 106483. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Q.; Qin, Y.; Liu, H.; Liu, H.; Cao, G.; Gao, X.; Song, L.; Sun, Z. Optimizing the accessibility of zeolite Y on FCC catalyst to improve heavy oil conversion capacity. Microporous Mesoporous Mater. 2023, 359, 112627. [Google Scholar] [CrossRef]
- Saab, R.; Polychronopoulou, K.; Zheng, L.; Kumar, S.; Schiffer, A. Synthesis and performance evaluation of hydrocracking catalysts: A review. J. Ind. Eng. Chem. 2020, 89, 83–103. [Google Scholar] [CrossRef]
- Peng, C.; Du, Y.; Feng, X.; Hu, Y.; Fang, X. Research and development of hydrocracking catalysts and technologies in China. Front. Chem. Sci. Eng. 2018, 12, 867–877. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Samadhi, T.W.; Subagjo, S.; Gunawan, M.L. Development of Hydrocracking Catalyst Support from Kaolin of Indonesian Origin. Adv. Mater. Res. 2014, 896, 532–536. [Google Scholar] [CrossRef]
- Kumar, R.; Rana, B.S.; Verma, D.; Rayaroth, S.; Prasad, V.S.; Sinha, A.K. Hydrotreatment of renewable oils using hierarchical mesoporous H-ZSM-5 synthesized from kaolin clay. RSC Adv. 2015, 5, 39342–39349. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Feng, P.; Han, X.; Zheng, Y. Integration of catalytic cracking and hydrotreating technology for triglyceride deoxygenation. Catal. Today 2017, 291, 172–179. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, W.; Bian, X. Dynamic synthesis route of zeolite Y with kaolin to improve yield. Green Process. Synth. 2018, 7, 23–29. [Google Scholar] [CrossRef]
- Wang, H.; Mao, W.; Wang, K.; Qin, Y.; Li, Q.; Peng, C.; Wang, J.; Sun, Z.; Song, L. Insight into the Effects of Mass Transfer Performance of Y Zeolite on the Product Selectivity of VGO Hydrocracking. Ind. Eng. Chem. Res. 2022, 61, 18270–18281. [Google Scholar] [CrossRef]
- Dhaneswara, D.; Fatriansyah, J.F.; Sudiro, T.; Harjanto, S.; Mastuli, M.S.; Federico, A.; Ulfiati, R. Synthesis and optimization of Ni/Mo-impregnated kaolin-based ZSM-5 as a catalytic hydrocracking catalyst for heavy petroleum distillates. Sustain. Energy Fuels 2024, 8, 2695–2703. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Cheng, B.; Tao, T.; Min, X.; Mi, R.; Huang, Z.; Fang, M.; Liu, Y. Synthesis and Applications of SAPO-34 Molecular Sieves. Chem.—A Eur. J. 2022, 28, e202102787. [Google Scholar] [CrossRef]
- Wang, T.; Lu, X.; Yan, Y. Synthesis, characterization and crystallization mechanism of SAPOs from natural kaolinite. Microporous Mesoporous Mater. 2010, 136, 138–147. [Google Scholar] [CrossRef]
- Fatourehchi, N.; Sohrabi, M.; Royaee, S.J.; Mirarefin, S.M. Preparation of SAPO-34 catalyst and presentation of a kinetic model for methanol to olefin process (MTO). Chem. Eng. Res. Des. 2011, 89, 811–816. [Google Scholar] [CrossRef]
- Taheri Najafabadi, A.; Fatemi, S.; Sohrabi, M.; Salmasi, M. Kinetic modeling and optimization of the operating condition of MTO process on SAPO-34 catalyst. J. Ind. Eng. Chem. 2012, 18, 29–37. [Google Scholar] [CrossRef]
- Wang, P.; Lv, A.; Hu, J.; Xu, J.; Lu, G. In Situ Synthesis of SAPO-34 Grown onto Fully Calcined Kaolin Microspheres and Its Catalytic Properties for the MTO Reaction. Ind. Eng. Chem. Res. 2011, 50, 9989–9997. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhang, J.; Chen, F.; Anpo, M. Preparation of macroporous SAPO-34 microspheres by a spray drying method using polystyrene spheres as hard template. Res. Chem. Intermed. 2011, 37, 949–959. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Wang, T. Synthesis of SAPO-34 using metakaolin in the presence of β-cyclodextrin. J. Energy Chem. 2015, 24, 401–406. [Google Scholar] [CrossRef]
- Zhu, J.; Cui, Y.; Wang, Y.; Wei, F. Direct synthesis of hierarchical zeolite from a natural layered material. Chem. Commun. 2009, 22, 3282–3284. [Google Scholar] [CrossRef]
- Yang, S.-T.; Kim, J.-Y.; Chae, H.-J.; Kim, M.; Jeong, S.-Y.; Ahn, W.-S. Microwave synthesis of mesoporous SAPO-34 with a hierarchical pore structure. Mater. Res. Bull. 2012, 47, 3888–3892. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Yue, Y.; Olsbye, U.; Bao, X. Design and in situ synthesis of hierarchical SAPO-34@kaolin composites as catalysts for methanol to olefins. Catal. Sci. Technol. 2019, 9, 6438–6451. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, X.-F.; Di, C.-Y.; Dou, T.; Chen, S.-L. A Green and Cost-Effective Synthesis of Hierarchical SAPO-34 through Dry Gel Conversion and Its Performance in a Methanol-to-Olefin Reaction. Ind. Eng. Chem. Res. 2021, 60, 15380–15390. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M. Application of resin and NH4NO3 as an ion exchange agent for microspherical preparation of nanostructured kaolin-SAPO-34 catalyst for methanol to light olefins reaction in a fluidized bed reactor. Res. Chem. Intermed. 2019, 45, 355–378. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M. Spray-dried zeotype/clay nanocatalyst for methanol to light olefins in fluidized bed reactor: Comparison of active and non-active filler. Appl. Clay Sci. 2019, 170, 70–85. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Ebrahimi, A. Fabrication of attrition-resistant nanostructured catalyst by spray dryer for methanol to light olefins reaction in a fluid bed reactor and coke formation. Microporous Mesoporous Mater. 2019, 279, 371–386. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Haghighi, M.; Aghamohammadi, S. Effect of calcination temperature and composition on the spray-dried microencapsulated nanostructured SAPO-34 with kaolin for methanol conversion to ethylene and propylene in fluidized bed reactor. Microporous Mesoporous Mater. 2020, 297, 110046. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Ebrahimi, A. Pathways in particle assembly by ultrasound-assisted spray-drying of kaolin/SAPO-34 as a fluidized bed catalyst for methanol to light olefins. Ultrason. Sonochem. 2019, 53, 237–251. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Haghighi, M.; Aghamohammadi, S. Single vs. dual-binder surface design of spray-dried Si–Al-sol-bound kaolin-matrixed SAPO-34 nanocatalyst for conversion of methanol to light-olefins in fluidized bed reactor. Microporous Mesoporous Mater. 2022, 332, 111714. [Google Scholar] [CrossRef]
- Mousavi, Y.S.; Akbari, A.; Omidkhah, M.; Safari, P. Formulated Mn-promoted SAPO-34/kaolin/alumina sol micro-size catalyst with a superior performance for methanol to light olefins conversion in a fluidized bed reactor. J. Ind. Eng. Chem. 2024, 129, 403–412. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Z.; Ding, Z.; Zhang, J.; Liu, W.; He, Q.; Li, Z.; Liu, C. Effect of kaolin calcined temperature on the preparation and crystallization mechanism of SAPO-34 molecular sieve for methanol-to-olefins performance. Microporous Mesoporous Mater. 2024, 369, 113037. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, X.; Jiang, C.; Tian, C.; Yang, Z.; Zhang, J. Hierarchical ZSM-5/SiC nano-whisker/SiC foam composites: Preparation and application in MTP reactions. J. Catal. 2015, 332, 70–76. [Google Scholar] [CrossRef]
- Sharbini Kamaluddin, H.; Gong, X.; Ma, P.; Narasimharao, K.; Dutta Chowdhury, A.; Mokhtar, M. Influence of zeolite ZSM-5 synthesis protocols and physicochemical properties in the methanol-to-olefin process. Mater. Today Chem. 2022, 26, 101061. [Google Scholar] [CrossRef]
- Michels, N.-L.; Mitchell, S.; Pérez-Ramírez, J. Effects of Binders on the Performance of Shaped Hierarchical MFI Zeolites in Methanol-to-Hydrocarbons. ACS Catal. 2014, 4, 2409–2417. [Google Scholar] [CrossRef]
- Pan, F.; Lu, X.; Yan, Y.; Wang, T. Synthesis of nano/micro scale ZSM-5 from kaolin and its catalytic performance. Kinet. Catal. 2017, 58, 541–548. [Google Scholar] [CrossRef]
- Shoinkhorova, T.; Dikhtiarenko, A.; Ramirez, A.; Dutta Chowdhury, A.; Caglayan, M.; Vittenet, J.; Bendjeriou-Sedjerari, A.; Ali, O.S.; Morales-Osorio, I.; Xu, W.; et al. Shaping of ZSM-5-Based Catalysts via Spray Drying: Effect on Methanol-to-Olefins Performance. ACS Appl. Mater. Interfaces 2019, 11, 44133–44143. [Google Scholar] [CrossRef]
- Javadli, R.; de Klerk, A. Desulfurization of heavy oil. Appl. Petrochem. Res. 2012, 1, 3–19. [Google Scholar] [CrossRef]
- de Lasa, H.; Hernandez Enriquez, R.; Tonetto, G. Catalytic Desulfurization of Gasoline via Dehydrosulfidation. Ind. Eng. Chem. Res. 2006, 45, 1291–1299. [Google Scholar] [CrossRef]
- Wan, G.; Duan, A.; Zhang, Y.; Zhao, Z.; Jiang, G.; Zhang, D.; Gao, Z. Zeolite beta synthesized with acid-treated metakaolin and its application in diesel hydrodesulfurization. Catal. Today 2010, 149, 69–75. [Google Scholar] [CrossRef]
- Niu, F.X.; Fu, F.; Gao, X.M.; Zhang, X.M.; Wu, Y.F. Preparation of Kaolin/Cu2O Photocatalyst and its Application in the Photocatalytic Oxidation-Extraction Desulfurization. Adv. Mater. Res. 2012, 518–523, 878–883. [Google Scholar] [CrossRef]
- Huang, C.; Wu, P.; Guo, Y.; Guo, Y. Facile synthesis of mesoporous kaolin catalyst carrier and its application in deep oxidative desulfurization. Microporous Mesoporous Mater. 2020, 306, 110415. [Google Scholar] [CrossRef]
- AlKhafaji, K.S.; Shakor, Z.M.; Al-Zaidi, B.Y.; Hussein, S.J. Preparation and Characterization of Metakaolin-Based Catalysts for Gasoil Hydrodesulfurization Purposes. Arab. J. Sci. Eng. 2022, 47, 6283–6296. [Google Scholar] [CrossRef]
- Hmood, H.M.; Gheni, S.A.; Ahmed, S.M.R.; Ali, M.M.; Saleh, H.Y.; Mohammed, M.H.; Mohammed, A.E.; Mahomood, M.A.; Mohammed, H.R.; Hassan, A.A.; et al. Kaoline-based catalyst for a high stability desulfurization of sour heavy naphtha in a three-phase oscillatory baffled reactor. Particuology 2024, 84, 249–260. [Google Scholar] [CrossRef]
- Ijadi, S.; Moradi, G.; Ghanadi, T. Efficient catalytic oxidative desulfurization of liquid fuels over tungsten oxide supported on metakaolin surface. ChemistrySelect 2024, 9, e202303715. [Google Scholar] [CrossRef]
- Gadin, J.M.; Afolabi, E.A.; Kovo, A.S.; Abdulkareem, A.S.; Oloruntoba James, M. A retrospect on recent research works in the preparation of zeolites catalyst from kaolin for biodiesel production. Biofuels 2023, 14, 315–332. [Google Scholar] [CrossRef]
- Nugraha, R.E.; Prasetyoko, D.; Asikin-Mijan, N.; Bahruji, H.; Suprapto, S.; Taufiq-Yap, Y.H.; Jalil, A.A. The effect of structure directing agents on micro/mesopore structures of aluminosilicates from Indonesian kaolin as deoxygenation catalysts. Microporous Mesoporous Mater. 2021, 315, 110917. [Google Scholar] [CrossRef]
- Honorato de Oliveira, B.F.; de França, L.F.; Fernandes Corrêa, N.C.; Ribeiro, N.F.d.P.; Velasquez, M. Renewable Diesel Production from Palm Fatty Acids Distillate (PFAD) via Deoxygenation Reactions. Catalysts 2021, 11, 1088. [Google Scholar] [CrossRef]
- Maharani, D.K.; Kusumawati, Y.; Safitri, W.N.; Nugraha, R.E.; Holilah, H.; Sholeha, N.A.; Jalil, A.A.; Bahruji, H.; Prasetyoko, D. Optimization of hierarchical ZSM-5 structure from kaolin as catalysts for biofuel production. RSC Adv. 2023, 13, 14236–14248. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Bibi, A.; Naqvi, M.; Noor, T.; Nizami, A.-S.; Rehan, M.; Ayoub, M. New trends in improving gasoline quality and octane through naphtha isomerization: A short review. Appl. Petrochem. Res. 2018, 8, 131–139. [Google Scholar] [CrossRef]
- Khalaf, Y.H.; Sherhan, B.Y.; Shakor, Z.M.; Al-Sheikh, F. Bimetallic Catalysts for Isomerization of Alkanes (A Review). Pet. Chem. 2023, 63, 829–843. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, W.; Du, Y.; Li, J. Species and impacts of metal sites over bifunctional catalyst on long chain n-alkane hydroisomerization: A review. Appl. Catal. A 2021, 611, 117916. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Liu, Q. Base-Metal-Catalyzed Olefin Isomerization Reactions. Synthesis 2019, 51, 1293–1310. [Google Scholar] [CrossRef]
- Lenarda, M.; Storaro, L.; Talon, A.; Moretti, E.; Riello, P. Solid acid catalysts from clays: Preparation of mesoporous catalysts by chemical activation of metakaolin under acid conditions. J. Colloid Interface Sci. 2007, 311, 537–543. [Google Scholar] [CrossRef]
- Glotov, A.P.; Roldugina, E.A.; Artemova, M.I.; Smirnova, E.M.; Demikhova, N.R.; Stytsenko, V.D.; Egazar’yants, S.V.; Maksimov, A.L.; Vinokurov, V.A. Isomerization of Xylenes in the Presence of Pt-Containing Catalysts Based on Halloysite Aluminosilicate Nanotubes. Russ. J. Appl. Chem. 2018, 91, 1353–1362. [Google Scholar] [CrossRef]
- Wu, X.; Lei, X.; Wang, S.; Zhang, H.; Cui, Q.; Bao, X.; Wang, T.; Yuan, P. Tuning the acidity and pore structures of NiP based bifunctional catalyst for efficient n-hexane isomerization. Fuel 2022, 324, 124396. [Google Scholar] [CrossRef]
- Demikhova, N.R.; Rubtsova, M.I.; Kireev, G.A.; Cherednichenko, K.A.; Vinokurov, V.A.; Glotov, A.P. Micro-mesoporous catalysts based on ZSM-5 zeolite synthesized from natural clay nanotubes: Preparation and application in the isomerization of C-8 aromatic fraction. Chem. Eng. J. 2023, 453, 139581. [Google Scholar] [CrossRef]
- Jones, E.K. Commercial Alkylation of Paraffins and Aromatics. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 1958; Volume 10, pp. 165–195. [Google Scholar]
- Odedairo, T.; Al-Khattaf, S. Alkylation and transalkylation of alkylbenzenes in cymene production over zeolite catalysts. Chem. Eng. J. 2011, 167, 240–254. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, L.; Ji, M.; Ding, H.; Li, Y.; Tao, Z.; Zhao, Q. Characterization of Y/MCM-41 composite molecular sieve with high stability from Kaolin and its catalytic property. Appl. Clay Sci. 2012, 62–63, 32–40. [Google Scholar] [CrossRef]
- Al-Kinany, M.C.; Al-Megren, H.A.; Al-Ghilan, E.A.; Edwards, P.P.; Xiao, T.; Al-Shammari, A.S.; Al-Drees, S.A. Selective zeolite catalyst for alkylation of benzene with ethylene to produce ethylbenzene. Appl. Petrochem. Res. 2012, 2, 73–83. [Google Scholar] [CrossRef]
- Bok, T.O.; Andriako, E.P.; Knyazeva, E.E.; Konnov, S.V.; Ivanova, I.I. Influence of the Binder Type on the Properties of Nanocrystalline Zeolite Beta-Based Catalysts for Benzene Alkylation with Propylene. Pet. Chem. 2018, 58, 833–840. [Google Scholar] [CrossRef]
- Gerzeliev, I.M.; Zhmylev, V.P.; Khusaimova, D.O.; Shkuropatov, A.V.; Knyazeva, E.E.; Ponomareva, O.A.; Ivanova, I.I.; Maksimov, A.L. Effect of Binder on the Properties of MWW Zeolite Catalysts in Benzene Alkylation with Propylene. Pet. Chem. 2019, 59, 695–700. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).