Transforming Petrochemical Processes: Cutting-Edge Advances in Kaolin Catalyst Fabrication
Abstract
:1. Introduction
2. Classification of Kaolin Based on the Origin: A Geological Perspective
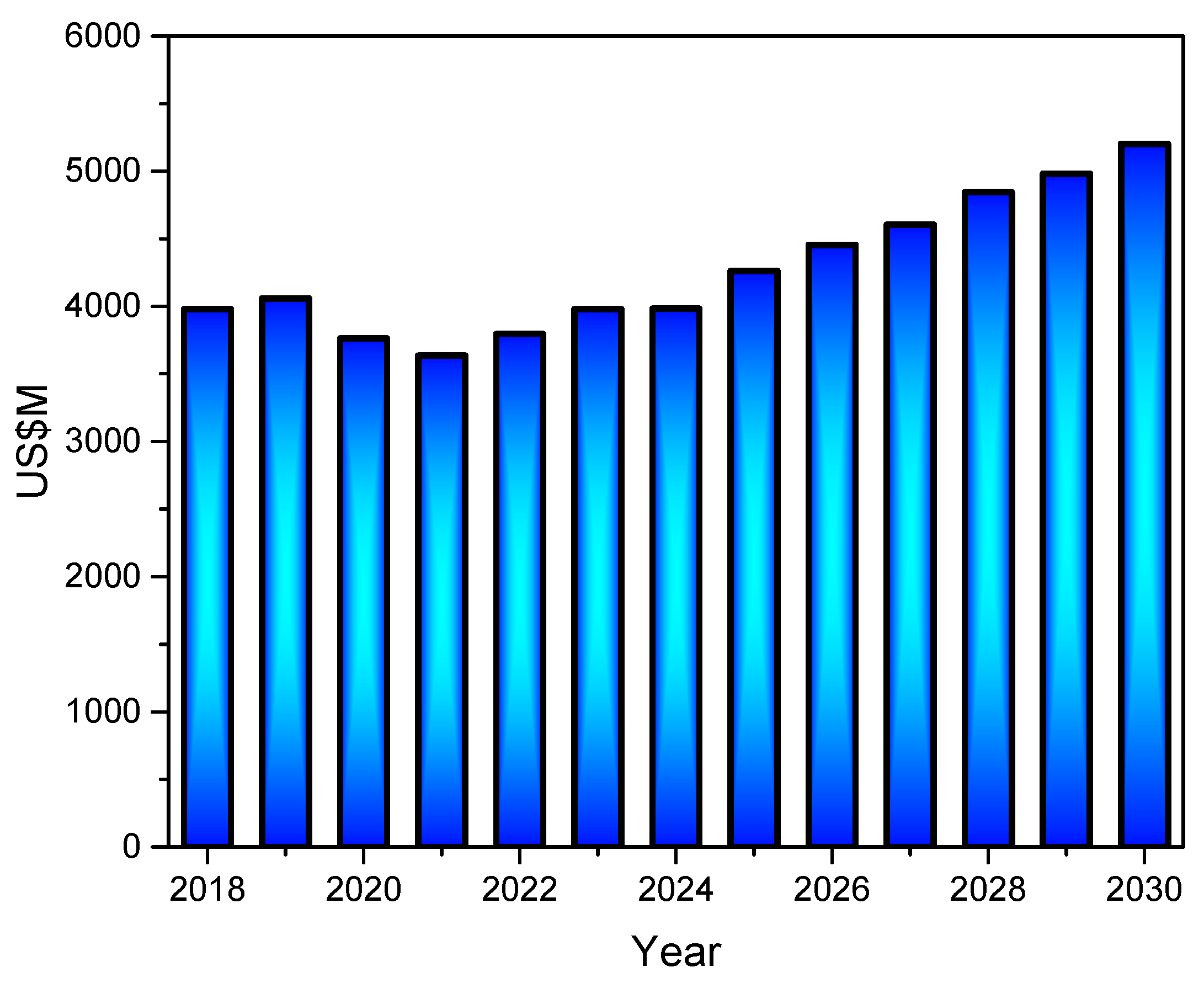
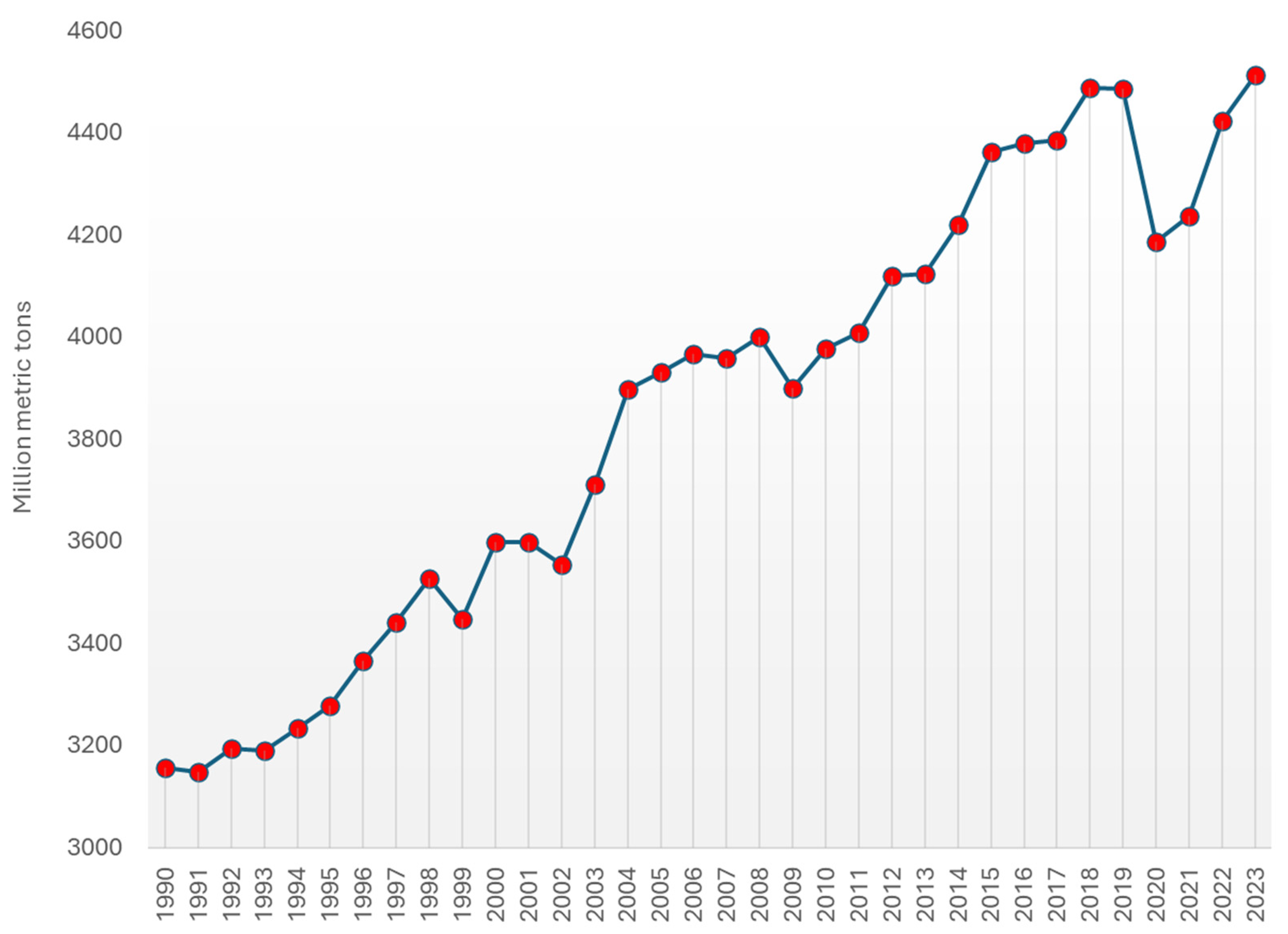
3. Worldwide Kaolin Clay Production, Demand Patterns, and Market Trends
4. Key Properties of Kaolin: Chemical, Structural, and Functional Insights
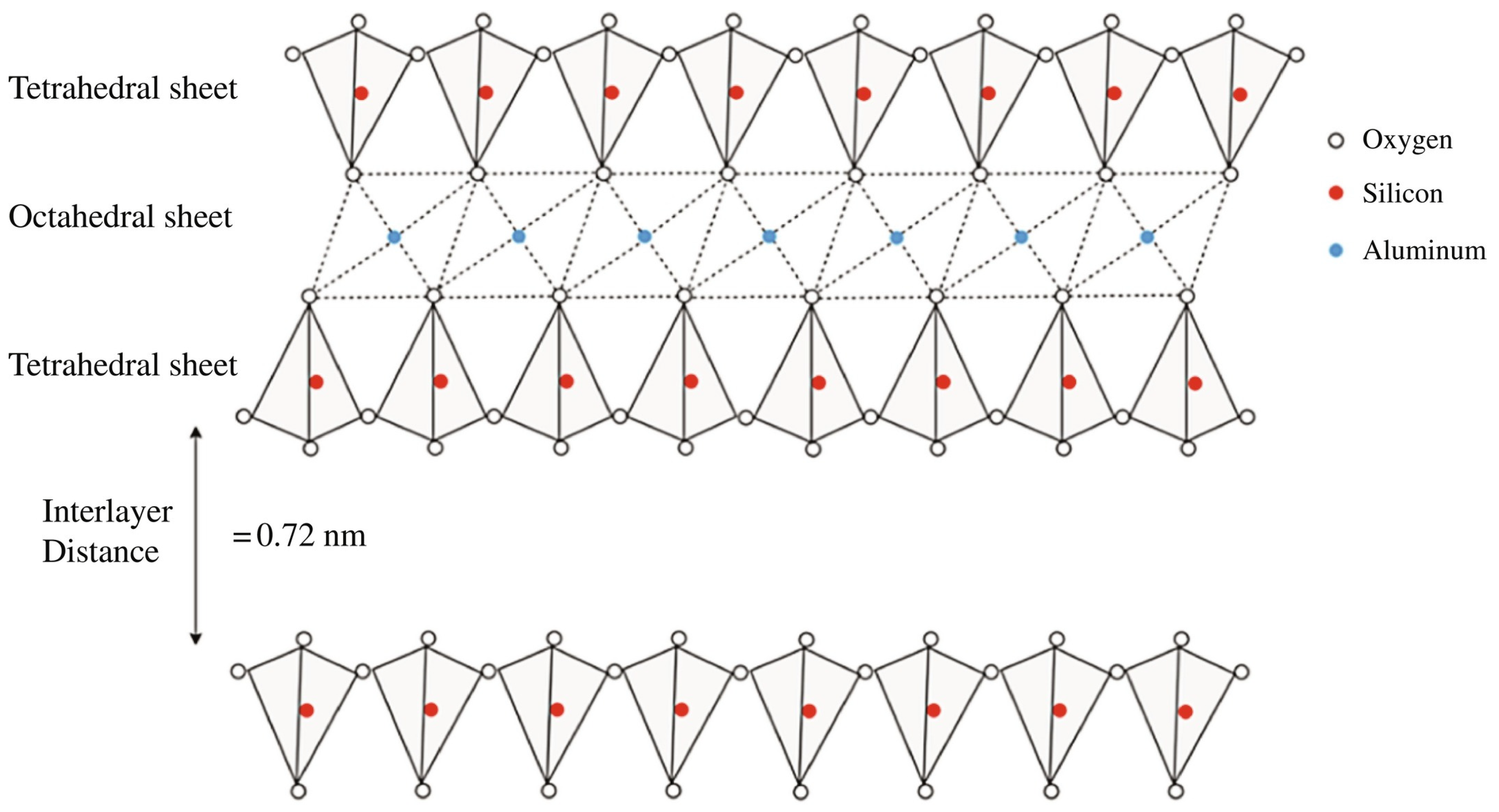
- Chemistry: Unprocessed kaolin has the chemical formula Al2Si2O5(OH)4. Kaolinite, an aluminum silicate mineral, is formed through the chemical weathering of aluminum-rich feldspars found in pegmatites and granites.
- Structure: Kaolin consists of hexagonal crystals ranging from 0.1 to 10 μm in size. The layered structure of these crystals enhances the desirable properties of kaolin.
- Chemically Inert: Kaolin’s lack of reactivity makes it ideal for use in pharmaceuticals, cosmetics, and various industrial applications. Its neutral pH ensures it does not alter the chemical composition of products or promote bacterial growth.
- Adsorbent: Kaolin’s natural absorbency allows it to remove unwanted pollutants, pathogens, and other components from mixtures, thereby enhancing its effectiveness in various applications.
- Non-swelling: The hydrogen bonds between kaolinite crystals prevent water molecules from infiltrating the layers, resulting in kaolin’s non-swelling properties. This makes it a stable additive that does not alter the physical properties of mixtures or products.
- Molecular Stability: Kaolinite exhibits non-expanding behavior and minimal isomorphous replacement due to its high molecular stability. While kaolinite is the least reactive clay, its sensitivity to pH can influence metal adsorption. Metal adsorption occurs through the release of hydrogen (H+) ions at the mineral’s edge sites and on the exposed planar surfaces of the silica and alumina sheets.
- Soothing Properties: Kaolin’s calming properties make it useful for soothing red, irritated skin, benefiting those with sensitive or sunburned skin by reducing redness and irritation.
- Detoxification: Kaolin’s cleansing properties help remove dirt, oil, and impurities from the skin, revitalizing and freshening the complexion by eliminating dead skin cells and debris.
- Gentle Exfoliation: The slightly abrasive texture of kaolin clay enables it to exfoliate the skin gently, promoting a smoother, more luminous complexion by clearing away dead skin cells and unclogging pores.
- Healing Qualities: Kaolin clay is believed to have healing properties and can help treat skin issues such as psoriasis, eczema, and acne. While more research is needed to confirm its efficacy for specific conditions, kaolin clay is widely used in the cosmetic and skincare industries due to its versatility, gentleness, and beneficial properties for various skin issues.
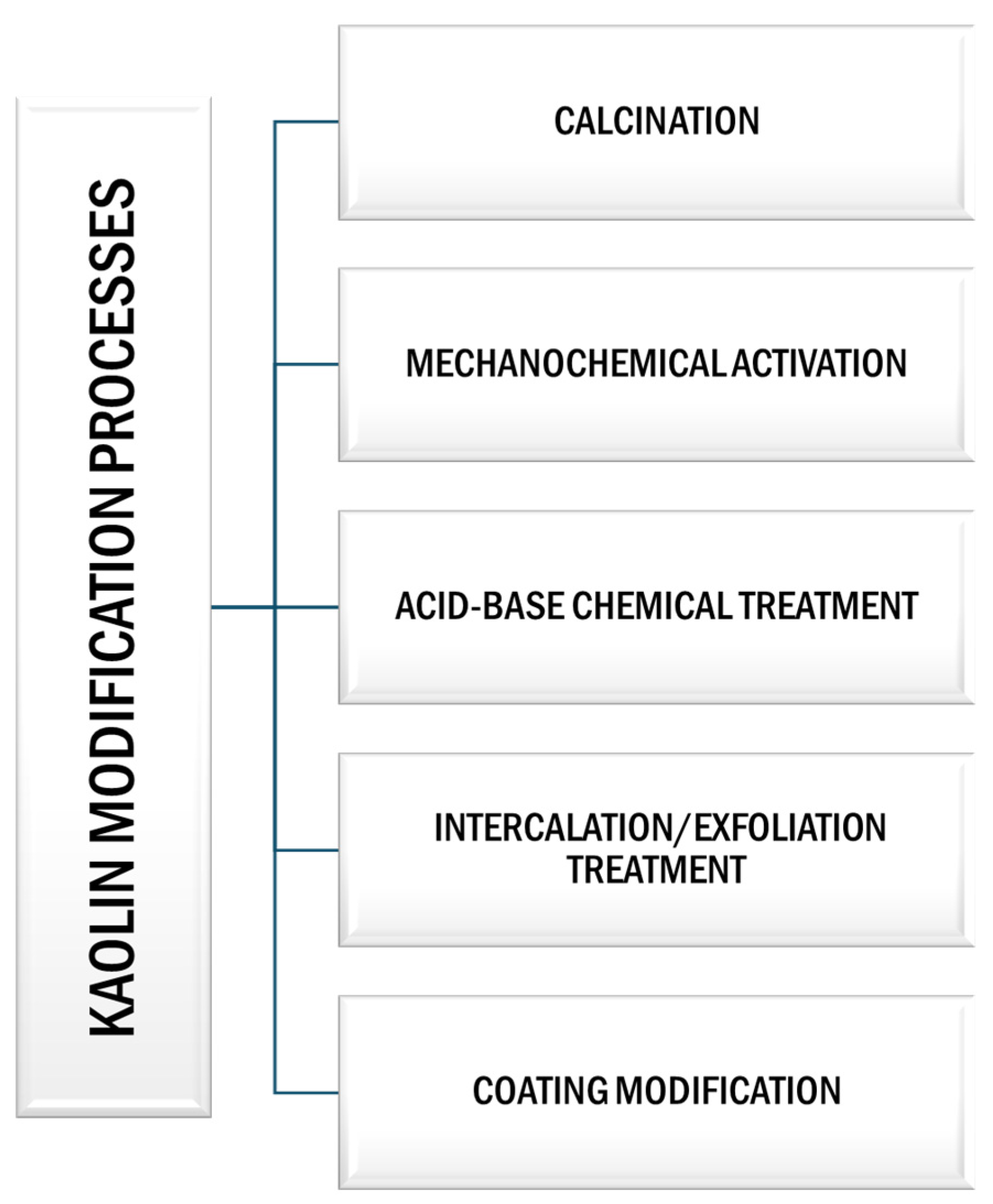
5. Modification Processes of Kaolin: Enhancing Industrial Applicability
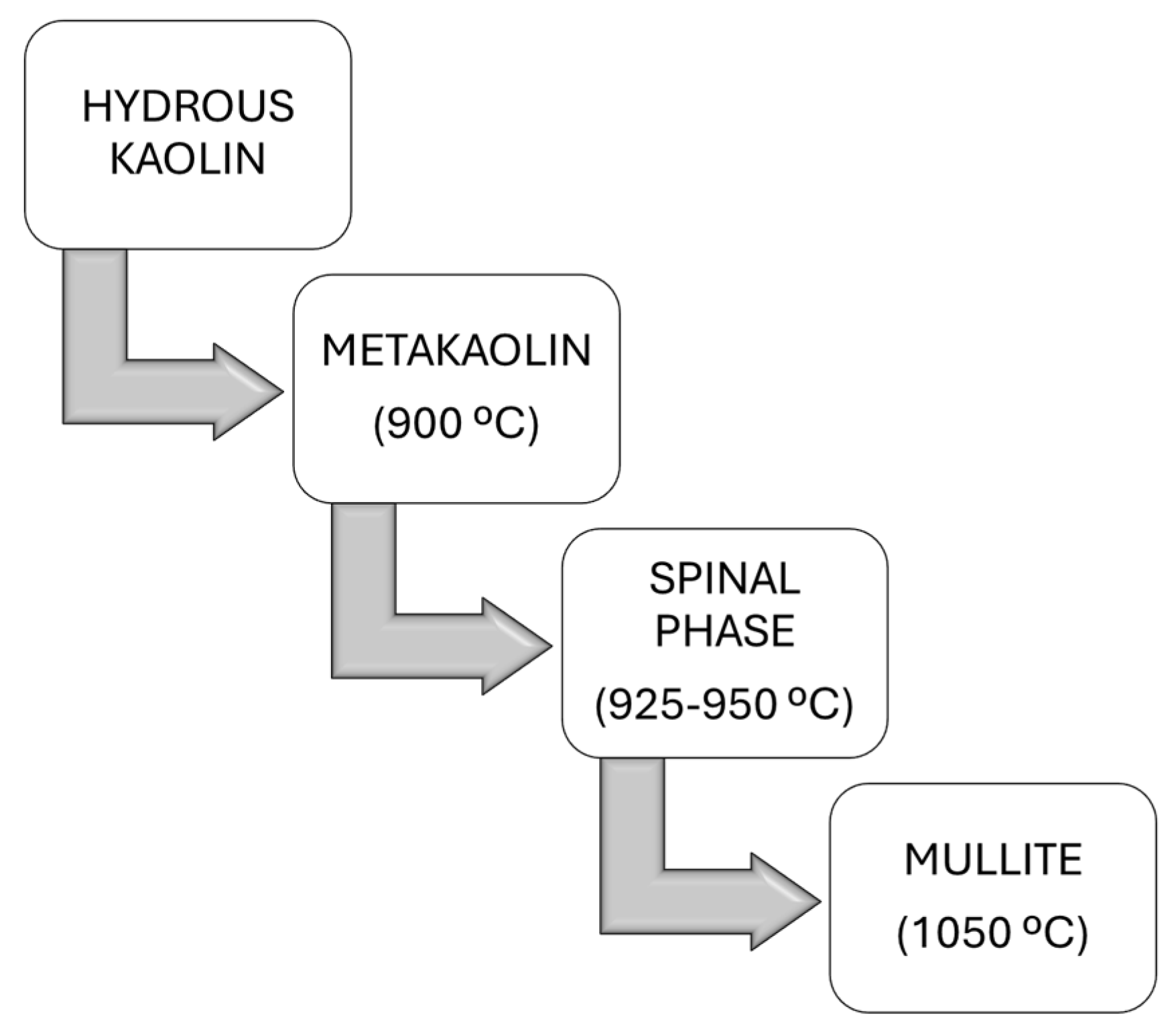
5.1. Calcination Process
5.2. Mechanochemical Activation
5.3. Acid–Base Chemical Treatment
5.3.1. Acid Activation
5.3.2. Alkaline Activation
5.4. Intercalation/Exfoliation Treatment
5.5. Coating Modification
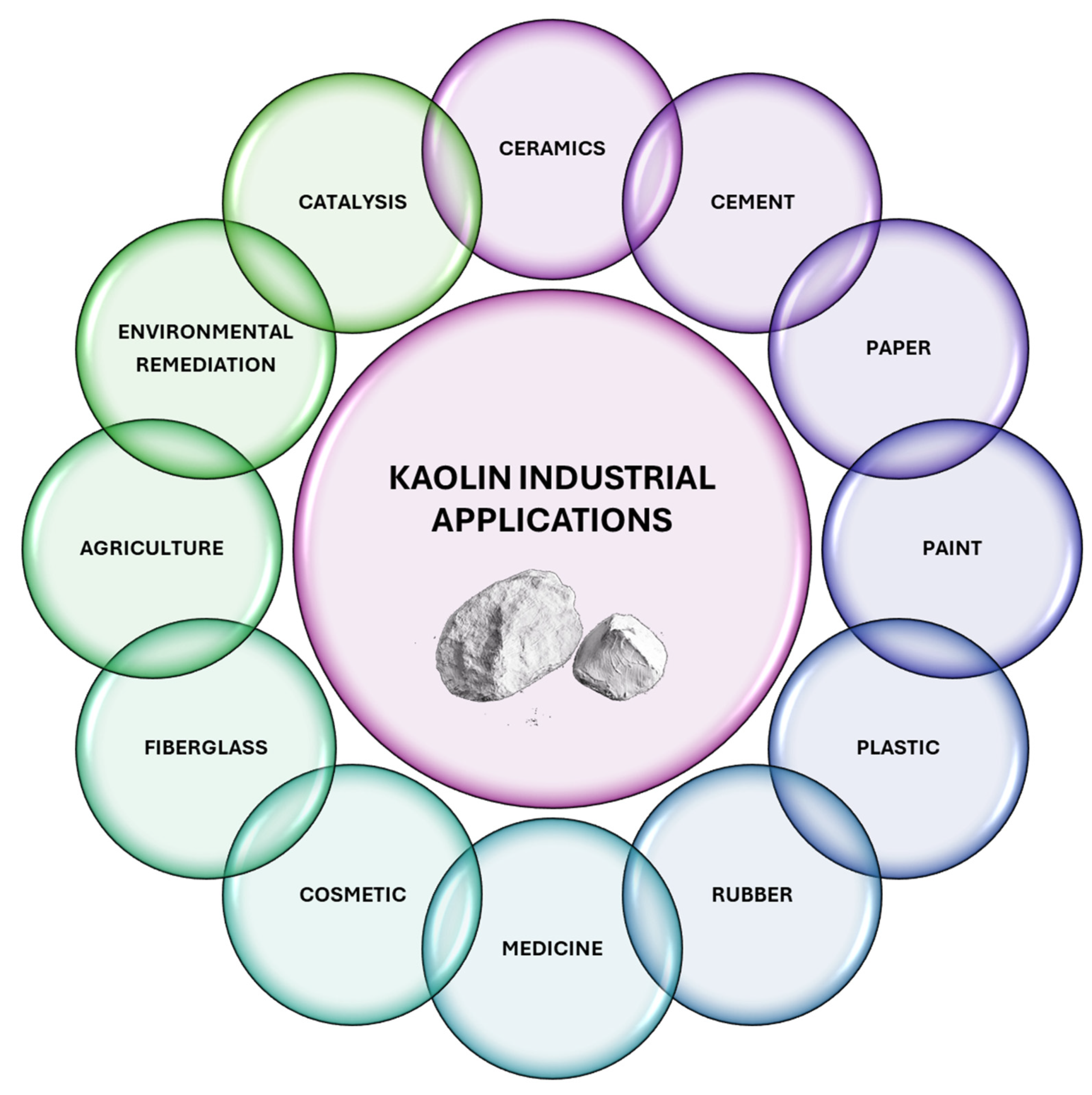
6. Unlocking the Potential: Advanced Applications of Kaolin in Industry
6.1. Ceramic Industry
6.2. Cement-Based Material Industry
6.3. Paper Industry
6.4. Paint Industry
6.5. Plastic Industry
6.6. Rubber Industry
6.7. Medicine Industry
6.8. Cosmetic Industry
6.9. Fiberglass Industry
6.10. Agriculture Industry
6.11. Environmental Decontamination
6.12. Catalysis
7. Kaolin in Petrochemical Catalysis: From Fundamentals to Innovations
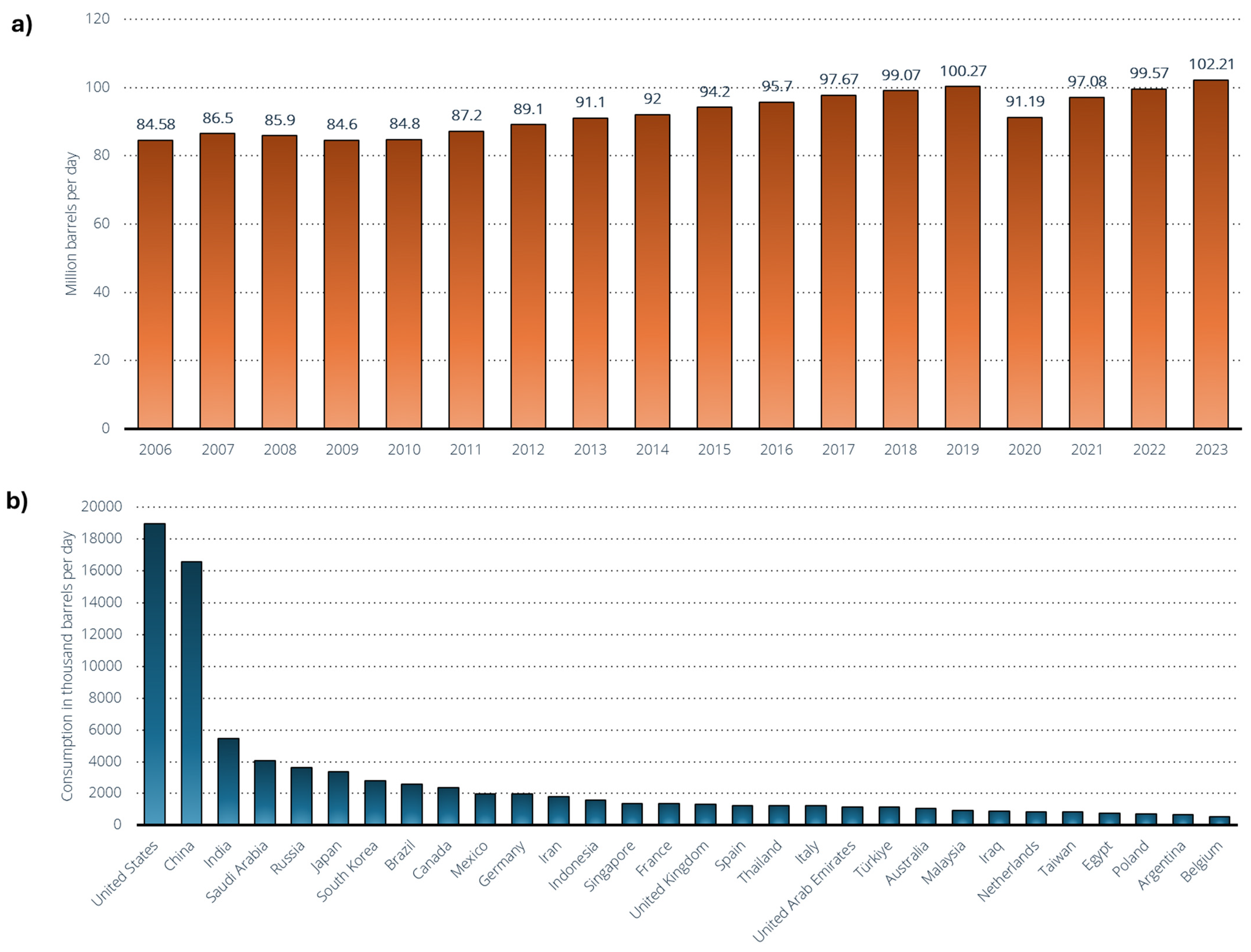
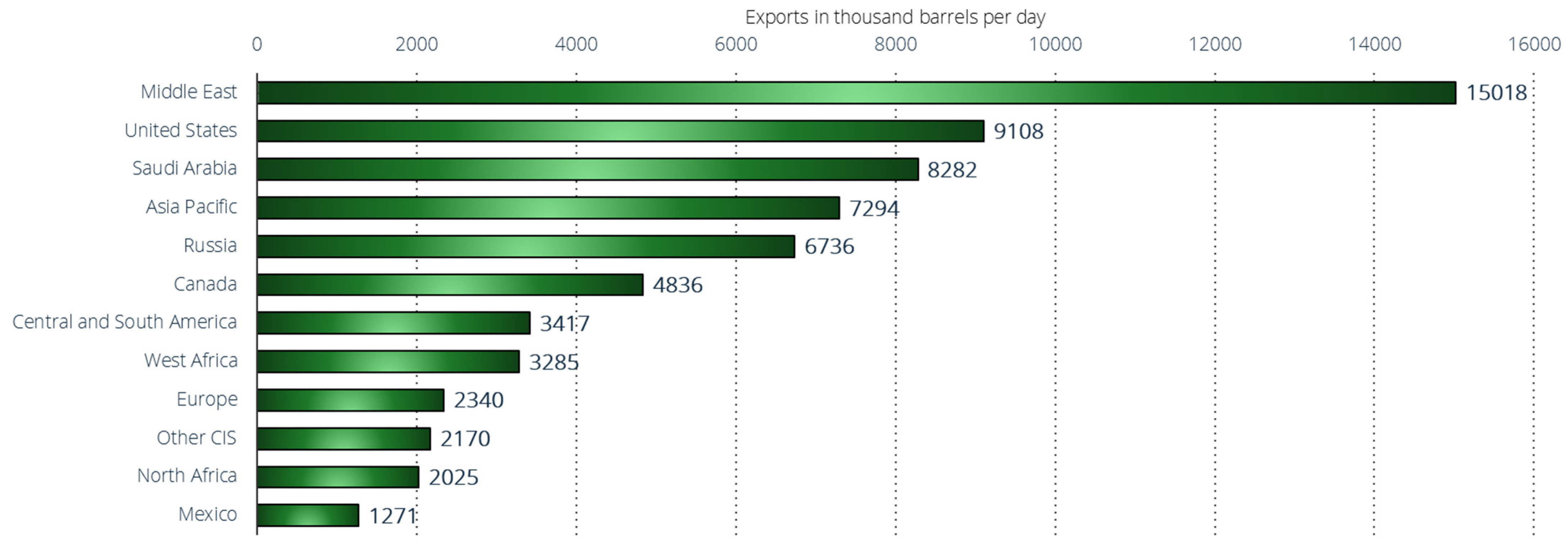
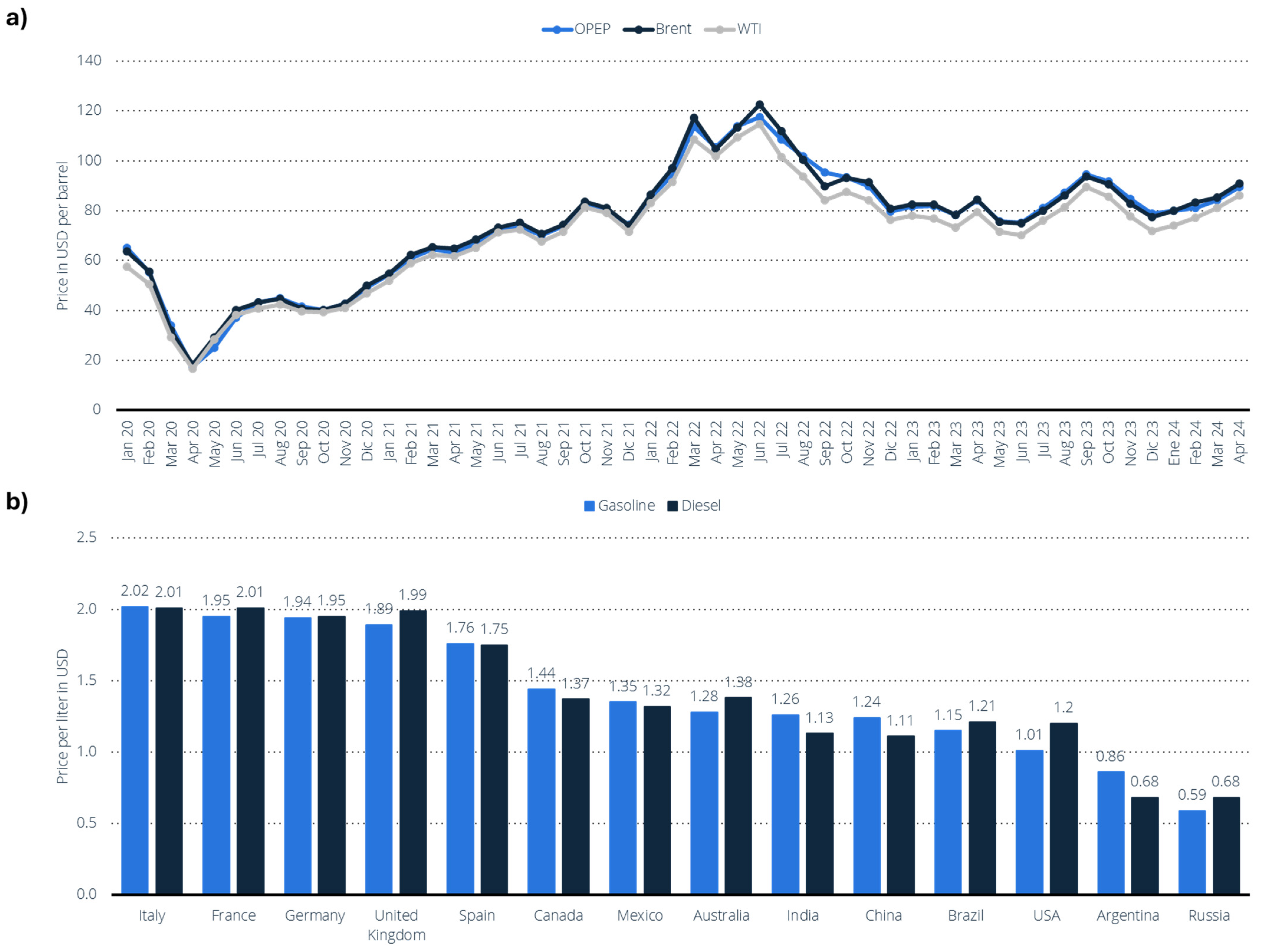
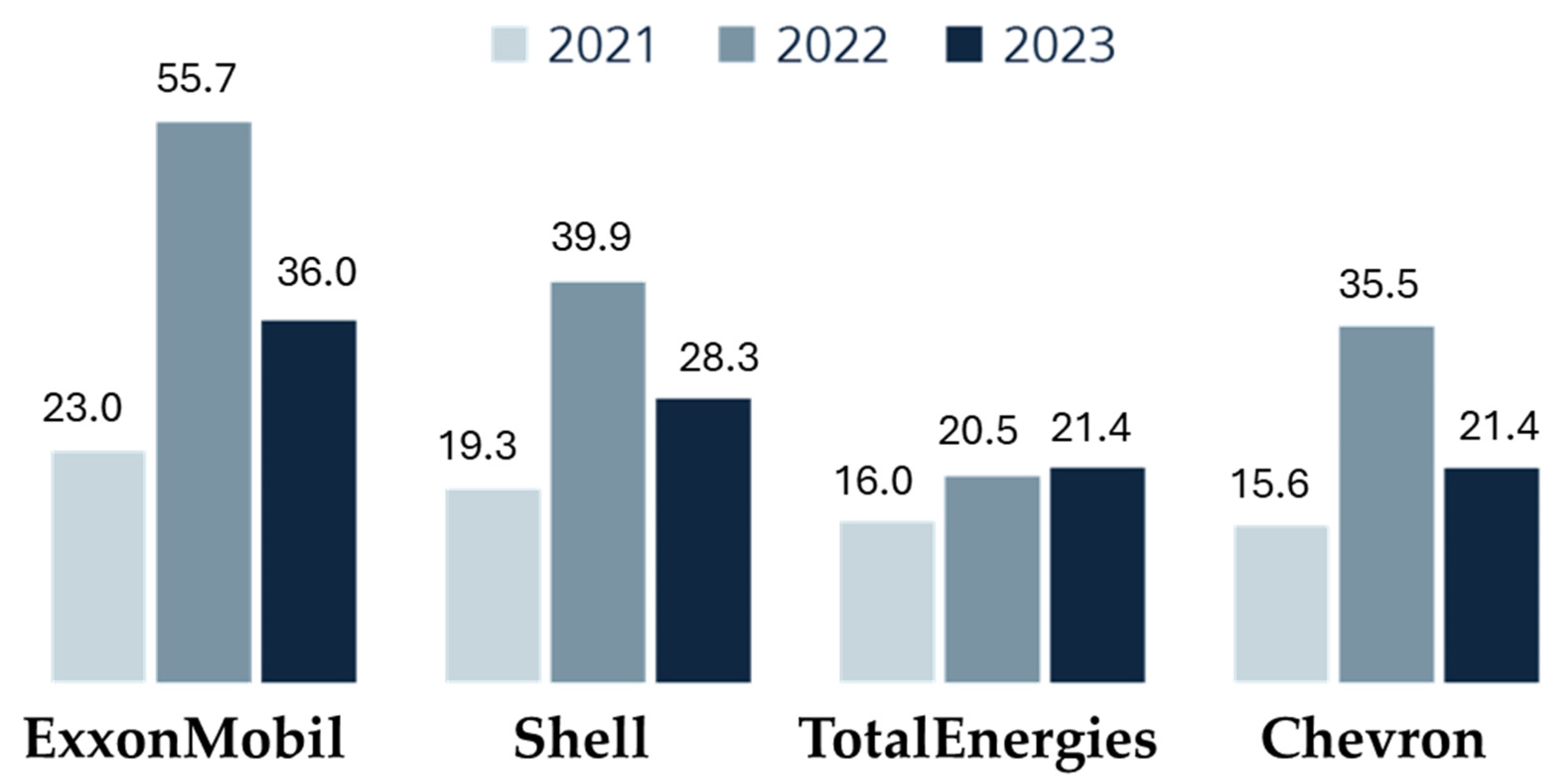
7.1. The Current Global Oil Landscape: Production Giants, Geopolitical Tensions, and Market Volatility
7.2. Petrochemical Processes for Transforming Heavy Feedstocks into Light Distillates
- Cracking: Cracking is a fundamental process in the petrochemical industry, breaking down large hydrocarbon molecules into smaller, more useful fragments. Thermal cracking utilizes high temperatures to achieve this breakdown, while catalytic cracking employs catalysts to enhance the reaction efficiency and selectivity [150]. Catalytic cracking is particularly important for producing high-octane gasoline and other valuable light hydrocarbons [151].
- Reforming: Reforming enhances the quality of lighter hydrocarbons by restructuring their molecular configuration [152]. This process is crucial for producing high-octane gasoline components and aromatic hydrocarbons such as benzene, toluene, and xylenes. Catalytic reforming typically involves the use of a platinum-based catalyst to facilitate the rearrangement of hydrocarbons, improving their performance in various applications.
- Hydrocracking: Hydrocracking combines high pressure, hydrogen, and a catalyst to convert heavy hydrocarbons into lighter, more saturated products [153]. This process effectively produces a wide range of lighter hydrocarbons, including high-quality diesel and jet fuels. Hydrocracking is valued for its ability to produce a high yield of desired products while reducing the sulfur and nitrogen content of the end products.
- Distillation: Distillation is a key separation technique that utilizes differences in boiling points to divide crude oil into various fractions [154]. Fractional distillation is commonly employed to separate the crude oil into light and heavy fractions, which are further processed into specific products. This method is crucial for isolating components such as gasoline, kerosene, and diesel.
- Steam cracking: Steam cracking is a high-temperature process that uses steam to break down hydrocarbons into lighter molecules, including ethylene and propylene [155]. This process is pivotal for producing key building blocks for the petrochemical industry, such as ethylene, which is used to manufacture a wide range of products including plastics and synthetic rubber.
- Extraction and refining: Extraction and refining processes involve the separation of valuable components from heavy feedstocks using physical or chemical methods [156]. Techniques such as solvent extraction and hydroprocessing are employed to purify and enhance the quality of the extracted components, making them suitable for further processing or direct use.
7.3. Complementary Processes in the Petrochemical Industry
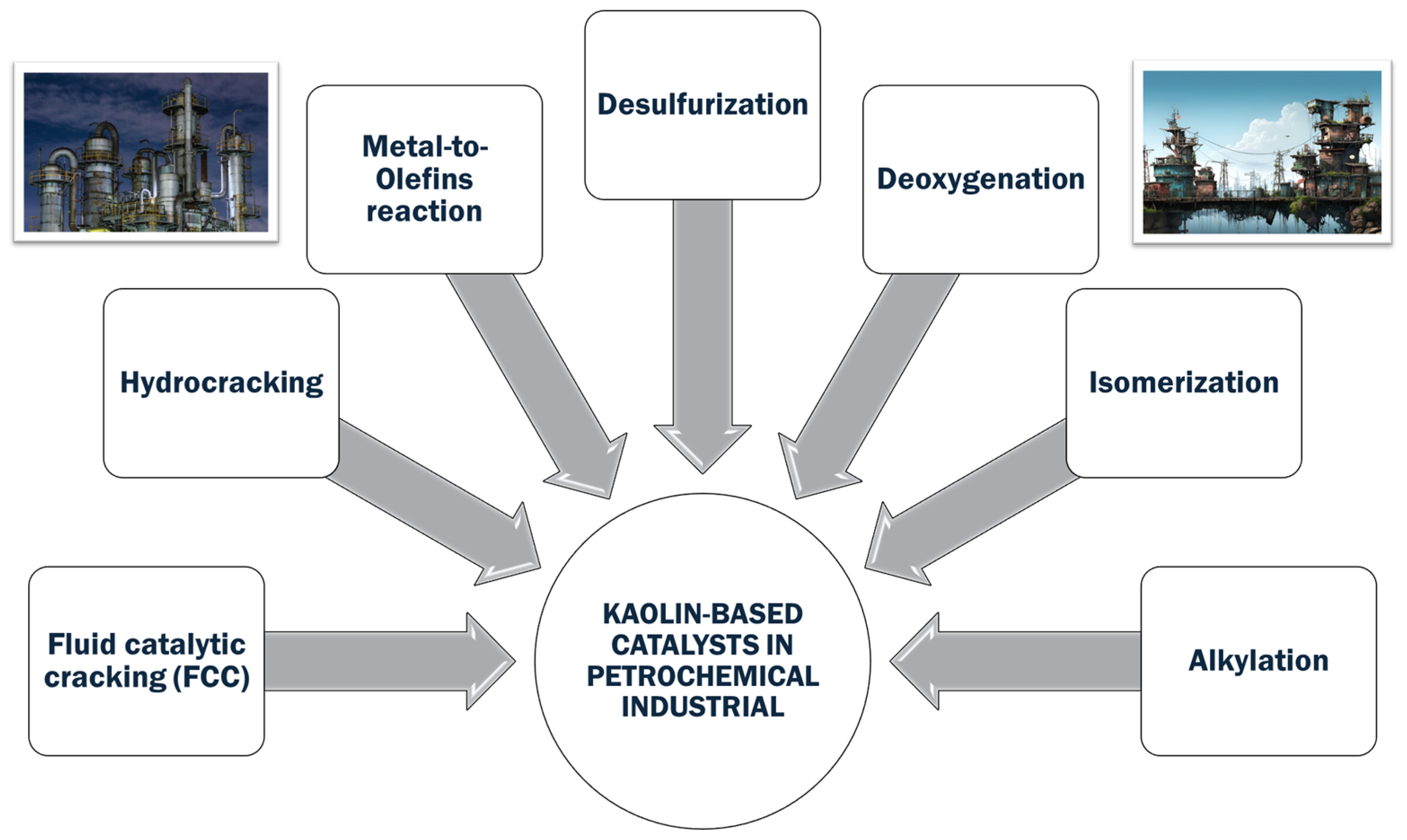
7.4. Kaolin-Driven Catalysis in Petrochemical Processes
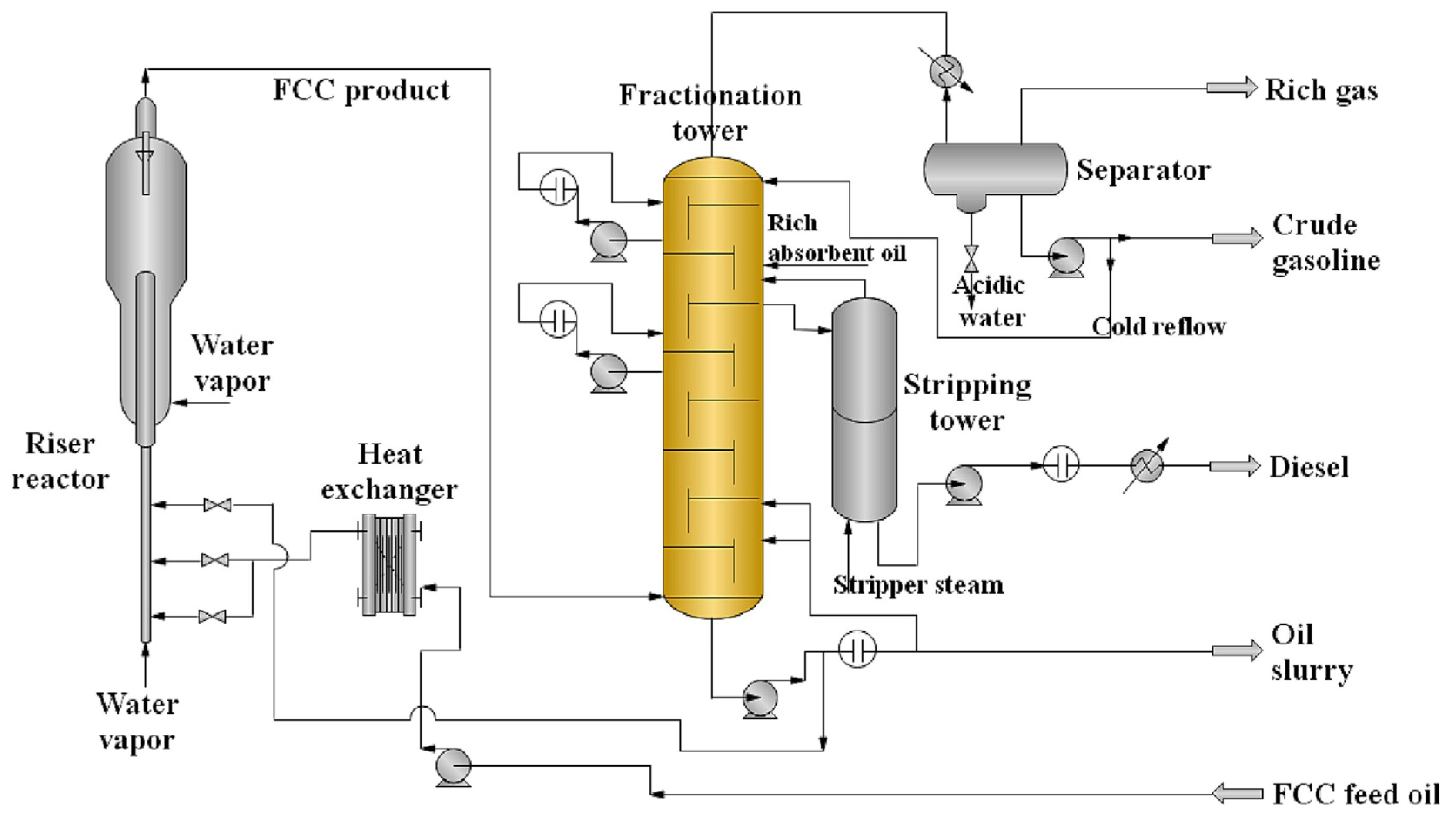
7.4.1. Fluid Catalytic Cracking (FCC)
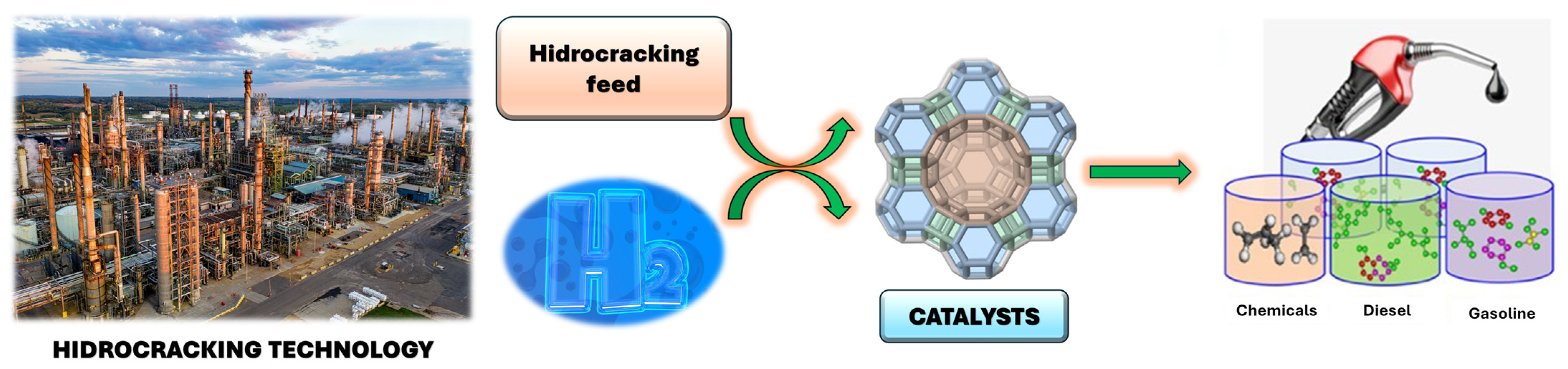
7.4.2. Hydrocracking
7.4.3. Methanol-to-Olefin (MTO) Catalytic Reaction for Olefin Production
7.4.4. Desulfurization
7.4.5. Deoxygenation
7.4.6. Isomerization
- (a)
- Protonation of alkanes: The acidic sites of the catalyst protonate the alkane, forming a carbocation.
- (b)
- Carbocation rearrangement: The carbocation undergoes a rearrangement to form a more stable, branched isomer.
- (c)
- Deprotonation: The branched carbocation is deprotonated to yield the branched alkane.
- (d)
- Hydrogenation/Dehydrogenation: Metallic sites facilitate the addition or removal of hydrogen atoms, stabilizing the reaction intermediates and products.
7.4.7. Alkylation
8. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Aal, M.A.; Said, A.E.; Abdallah, M.H.; Goda, M.N. Modified natural kaolin clay as an active, selective, and stable catalyst for methanol dehydration to dimethyl ether. Sci. Rep. 2022, 12, 9407. [Google Scholar] [CrossRef] [PubMed]
- Ulfiati, R.; Dhaneswara, D.; Harjanto, S.; Fatriansyah, J.F. Synthesis and Characterization ZSM-5 Based on Kaolin as a Catalyst for Catalytic Cracking of Heavy Distillate. Int. J. Technol. 2022, 13, 860. [Google Scholar] [CrossRef]
- Custodis, V.B.F.; Karakoulia, S.A.; Triantafyllidis, K.S.; van Bokhoven, J.A. Catalytic Fast Pyrolysis of Lignin over High-Surface-Area Mesoporous Aluminosilicates: Effect of Porosity and Acidity. ChemSusChem 2016, 9, 1134–1145. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Mahmoud, M. Introduction to reservoir fluids and rock properties. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 2023; Volume 78, pp. 1–19. [Google Scholar]
- Hamdi Karaoğlu, M.; Doğan, M.; Alkan, M. Removal of cationic dyes by kaolinite. Microporous Mesoporous Mater. 2009, 122, 20–27. [Google Scholar] [CrossRef]
- Murray, H.H. Chapter 5 Kaolin Applications. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 85–109. [Google Scholar]
- Prasad, M.S.; Reid, K.J.; Murray, H.H. Kaolin: Processing, properties and applications. Appl. Clay Sci. 1991, 6, 87–119. [Google Scholar] [CrossRef]
- Liu, Y.; Pinnavaia, T.J. Metakaolin as a reagent for the assembly of mesoporous aluminosilicates with hexagonal, cubic and wormhole framework structures from proto-faujasitic nanoclusters. J. Mater. Chem. 2004, 14, 3416. [Google Scholar] [CrossRef]
- Shen, B.; Wang, P.; Yi, Z.; Zhang, W.; Tong, X.; Liu, Y.; Guo, Q.; Gao, J.; Xu, C. Synthesis of Zeolite β from Kaolin and Its Catalytic Performance For FCC Naphtha Aromatization. Energy Fuels 2009, 23, 60–64. [Google Scholar] [CrossRef]
- Holmes, S.M.; Khoo, S.H.; Kovo, A.S. The direct conversion of impure natural kaolin into pure zeolite catalysts. Green Chem. 2011, 13, 1152. [Google Scholar] [CrossRef]
- Belviso, C.; Giannossa, L.C.; Huertas, F.J.; Lettino, A.; Mangone, A.; Fiore, S. Synthesis of zeolites at low temperatures in fly ash-kaolinite mixtures. Microporous Mesoporous Mater. 2015, 212, 35–47. [Google Scholar] [CrossRef]
- Eman, E.A. Clays as catalysts in petroleum refining industry. ARPN J. Sci. Technol. 2013, 3, 356–375. [Google Scholar]
- Liu, J.; Yun, Z.; Gui, X. Ce/kaolin clay as an active catalyst for fatty acid methyl esters production from cottonseed oil in a new integrated apparatus. Braz. J. Chem. Eng. 2018, 35, 147–154. [Google Scholar] [CrossRef]
- Elfadly, A.M.; Zeid, I.F.; Yehia, F.Z.; Abouelela, M.M.; Rabie, A.M. Production of aromatic hydrocarbons from catalytic pyrolysis of lignin over acid-activated bentonite clay. Fuel Process. Technol. 2017, 163, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, C.; Yue, J.; Xu, G. Synthesis, characterization and catalytic methanation performance of modified kaolin-supported Ni-based catalysts. Chin. J. Chem. Eng. 2019, 27, 2953–2959. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Ashri Wan Daud, W.M. Kaolinite properties and advances for solid acid and basic catalyst synthesis. RSC Adv. 2015, 5, 101127–101147. [Google Scholar] [CrossRef]
- He, Y.; Tang, S.; Yin, S.; Li, S. Research progress on green synthesis of various high-purity zeolites from natural material-kaolin. J. Clean. Prod. 2021, 306, 127248. [Google Scholar] [CrossRef]
- Abdelwahab Emam, E. Clay Adsorption Perspective on Petroleum Refining Industry. Ind. Eng. 2018, 2, 19. [Google Scholar] [CrossRef]
- Liang, F.; Sayed, M.; Al-Muntasheri, G.A.; Chang, F.F.; Li, L. A comprehensive review on proppant technologies. Petroleum 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Pangilinan, K.D.; de Leon, A.C.C.; Advincula, R.C. Polymers for proppants used in hydraulic fracturing. J. Pet. Sci. Eng. 2016, 145, 154–160. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Haldar, S.K. Basic mineralogy. In Introduction to Mineralogy and Petrology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 109–143. ISBN 978-0-12-820585-3. [Google Scholar]
- Kerr, C.S.R.P.F. Dickite, a kaolin mineral. Am. Mineral. J. Earth Planet. Mater. 1930, 15, 34–39. [Google Scholar]
- Mehmel, M. Über die Struktur von Halloysit und Metahalloysit. Z. Krist.—Cryst. Mater. 1935, 90, 35–43. [Google Scholar] [CrossRef]
- Hofmann, U.; Endell, K.; Wilm, D. Röntgenographische und kolloidchemische Untersuchungen über Ton. Angew. Chem. Int. Ed. 1934, 47, 539–547. [Google Scholar] [CrossRef]
- Gysau, D. 5. Eigenschaften von Füllstoffen. In Füllstoffe; Vincentz Network: Hannover, Germany, 2019; pp. 97–154. [Google Scholar]
- Varga, G. The structure of kaolinite and metakaolinite. Epitoanyag—J. Silic. Based Compos. Mater. 2007, 59, 6–9. [Google Scholar] [CrossRef]
- Dill, H.G. Kaolin: Soil, rock and ore: From the mineral to the magmatic, sedimentary and metamorphic environments. Earth-Sci. Rev. 2016, 161, 16–129. [Google Scholar] [CrossRef]
- Bristow, C.M. Kaolin deposits of the United Kingdom of Great Britain and Northern Ireland. In Kaolin Deposits of the World: A-Europe; Proceedings of Symposium 1; Malkovsky, M., Vachtl, J., Eds.; International Geological Congress. Report of the TwentyThird Session, Prague, Czechoslovakia; 1969; pp. 215–288. Available online: https://books.google.com.hk/books/about/Kaolin_Deposits_of_the_world.html?id=nbi4tQEACAAJ&redir_esc=y (accessed on 19 July 2024).
- Murray, H.H. Major kaolin processing developments. Int. J. Miner. Process. 1980, 7, 263–274. [Google Scholar] [CrossRef]
- Murray, H.H.; Bundy, W.M.; Harvey, C.C. Kaolin Genesis and Utilization; Clay Minerals Society: Chantilly, VA, USA, 1993; ISBN 978-1-8812-0838-9. [Google Scholar]
- Research, P. Kaolin Market Size to Attain around USD 6.74 Billion by 2032. Available online: https://www.precedenceresearch.com/kaolin-market (accessed on 19 July 2024).
- Blengini, G.A.; Latunussa, C.E.L.; Eynard, U.; Torres de Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s list of Critical Raw Materials (2020); Final Report; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Research, G.V. Horizon—Global Kaolin Market Size & Outlook, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/kaolin-market (accessed on 19 July 2024).
- Research, G.V. Horizon—Global Kaolin Market Statistics, by Application, 2018–2030. Available online: https://www.grandviewresearch.com/horizon/statistics/worldwide-kaolin-market-117532 (accessed on 19 July 2024).
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Cheng, Y.; Xing, J.; Bu, C.; Zhang, J.; Piao, G.; Huang, Y.; Xie, H.; Wang, X. Dehydroxylation and Structural Distortion of Kaolinite as a High-Temperature Sorbent in the Furnace. Minerals 2019, 9, 587. [Google Scholar] [CrossRef]
- Bellotto, M.; Gualtieri, A.; Artioli, G.; Clark, S.M. Kinetic study of the kaolinite-mullite reaction sequence. Part I: Kaolinite dehydroxylation. Phys. Chem. Miner. 1995, 22, 207–217. [Google Scholar] [CrossRef]
- Moya, J.S.; Cabal, B.; Lopez-Esteban, S.; Bartolomé, J.F.; Sanz, J. Significance of the formation of pentahedral aluminum in the reactivity of calcined kaolin/metakaolin and its applications. Ceram. Int. 2024, 50, 1329–1340. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; Riley, D.P.; van Deventer, J.S.J. Combining density functional theory (DFT) and pair distribution function (PDF) analysis to solve the structure of metastable materials: The case of metakaolin. Phys. Chem. Chem. Phys. 2010, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.R.; Jia, D.C.; He, P.G.; Zhou, Y. Influence of calcination temperature of kaolin on the structure and properties of final geopolymer. Mater. Lett. 2010, 64, 2551–2554. [Google Scholar] [CrossRef]
- Horváth, E.; Frost, R.L.; Makó, É.; Kristóf, J.; Cseh, T. Thermal treatment of mechanochemically activated kaolinite. Thermochim. Acta 2003, 404, 227–234. [Google Scholar] [CrossRef]
- Dellisanti, F.; Valdrè, G. The role of microstrain on the thermostructural behaviour of industrial kaolin deformed by ball milling at low mechanical load. Int. J. Miner. Process. 2012, 102–103, 69–77. [Google Scholar] [CrossRef]
- Tarì, G.; Bobos, I.; Gomes, C.S.F.; Ferreira, J.M.F. Modification of Surface Charge Properties during Kaolinite to Halloysite-7Å Transformation. J. Colloid Interface Sci. 1999, 210, 360–366. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Pb(II) uptake by kaolinite and montmorillonite in aqueous medium: Influence of acid activation of the clays. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 277, 191–200. [Google Scholar] [CrossRef]
- Jabar, T.A.; Alzuhairi, M.A.; Abed, M.S. Acidic Influence on Geopolymerization: A Thorough Study Using HCl and Iraqi Kaolin. Russ. J. Appl. Chem. 2024, 97, 104–113. [Google Scholar] [CrossRef]
- Sabu, K.; Sukumar, R.; Rekha, R.; Lalithambika, M. A comparative study on H2SO4, HNO3 and HClO4 treated metakaolinite of a natural kaolinite as Friedel–Crafts alkylation catalyst. Catal. Today 1999, 49, 321–326. [Google Scholar] [CrossRef]
- Hou, Y.; Bai, Z.; Lu, H.; Feng, Z.; Zhang, T.; Jia, Y.; Guo, Z.; Kong, L.; Bai, J.; Li, W. In-situ catalytic upgrading of Hami coal pyrolysis volatiles over acid-modified kaolin. Fuel 2023, 331, 125660. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Influence of Acid Activation of Kaolinite and Montmorillonite on Adsorptive Removal of Cd(II) from Water. Ind. Eng. Chem. Res. 2007, 46, 3734–3742. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Influence of acid activation on adsorption of Ni(II) and Cu(II) on kaolinite and montmorillonite: Kinetic and thermodynamic study. Chem. Eng. J. 2008, 136, 1–13. [Google Scholar] [CrossRef]
- Panda, A.K.; Mishra, B.G.; Mishra, D.K.; Singh, R.K. Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 363, 98–104. [Google Scholar] [CrossRef]
- Mahmoud, S.; Saleh, S. Effect of Acid Activation on the De-Tert-Butylation Activity of Some Jordanian Clays. Clays Clay Miner. 1999, 47, 481–486. [Google Scholar] [CrossRef]
- Hartati; Prasetyoko, D.; Santoso, M.; Qoniah, I.; Leaw, W.L.; Firda, P.B.D.; Nur, H. A review on synthesis of kaolin-based zeolite and the effect of impurities. J. Chin. Chem. Soc. 2020, 67, 911–936. [Google Scholar] [CrossRef]
- Feng, M.; Kou, Z.; Tang, C.; Shi, Z.; Tong, Y.; Zhang, K. Recent progress in synthesis of zeolite from natural clay. Appl. Clay Sci. 2023, 243, 107087. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Len, T.; de Oliveira, A.D.; Costa, A.A.; Souza, A.R.; Costa, C.E.; Luque, R.; Rocha Filho, G.N.; Noronha, R.C.; Santos do Nascimiento, L.A. Zeolites: A Theoretical and Practical Approach with Uses in (Bio)Chemical Processes. Appl. Sci. 2023, 13, 1897. [Google Scholar] [CrossRef]
- Lussier, R.J. A novel clay-based catalytic material—Preparation and properties. J. Catal. 1991, 129, 225–237. [Google Scholar] [CrossRef]
- Belver, C.; Bañares Muñoz, M.A.; Vicente, M.A. Chemical Activation of a Kaolinite under Acid and Alkaline Conditions. Chem. Mater. 2002, 14, 2033–2043. [Google Scholar] [CrossRef]
- Slaty, F.; Khoury, H.; Wastiels, J.; Rahier, H. Characterization of alkali activated kaolinitic clay. Appl. Clay Sci. 2013, 75–76, 120–125. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer—An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Murat, M.; Amokrane, A.; Bastide, J.P.; Montanaro, L. Synthesis of zeolites from thermally activated kaolinite. Some observations on nucleation and growth. Clay Miner. 1992, 27, 119–130. [Google Scholar] [CrossRef]
- Mackinnon, I.D.R.; Millar, G.J.; Stolz, W. Hydrothermal syntheses of zeolite N from kaolin. Appl. Clay Sci. 2012, 58, 1–7. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X-type zeolites synthesised from kaolinite at low temperature. Appl. Clay Sci. 2013, 80–81, 162–168. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, C.; Alshameri, A.; Qiu, X.; Zhou, C.; Li, D. Synthesis and characterization of 13X zeolite from low-grade natural kaolin. Adv. Powder Technol. 2014, 25, 495–499. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Daud, W.M.A.W. Synthesis and characterization of hierarchical nanoporous HY zeolites from acid-activated kaolin. Chin. J. Catal. 2015, 36, 1846–1851. [Google Scholar] [CrossRef]
- EL-Mekkawi, D.M.; Ibrahim, F.A.; Selim, M.M. Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar] [CrossRef]
- Saito, Y.; Motohashi, T.; Hayashi, S.; Yasumori, A.; Okada, K. High thermal resistance of γ-Al2O3 prepared by the selective leaching of calcined kaolin minerals. J. Mater. Chem. 1997, 7, 1615. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part II Application of a new theory to silicates, aluminosilicates and quartz. Hydrometallurgy 2014, 149, 265–275. [Google Scholar] [CrossRef]
- Sivapullaiah, P.V. Manju Kaolinite—Alkali interaction and effects on basic properties. Geotech. Geol. Eng. 2005, 23, 601–614. [Google Scholar] [CrossRef]
- Wang, Y.H.; Siu, W.K. Structure characteristics and mechanical properties of kaolinite soils. I. Surface charges and structural characterizations. Can. Geotech. J. 2006, 43, 587–600. [Google Scholar] [CrossRef]
- Elbokl, T.A.; Detellier, C. Aluminosilicate nanohybrid materials. Intercalation of polystyrene in kaolinite. J. Phys. Chem. Solids 2006, 67, 950–955. [Google Scholar] [CrossRef]
- Deng, Y.; White, G.N.; Dixon, J.B. Effect of Structural Stress on the Intercalation Rate of Kaolinite. J. Colloid Interface Sci. 2002, 250, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Matusik, J.; Kłapyta, Z. Characterization of kaolinite intercalation compounds with benzylalkylammonium chlorides using XRD, TGA/DTA and CHNS elemental analysis. Appl. Clay Sci. 2013, 83–84, 433–440. [Google Scholar] [CrossRef]
- Letaief, S.; Tonle, I.K.; Diaco, T.; Detellier, C. Nanohybrid materials from interlayer functionalization of kaolinite. Application to the electrochemical preconcentration of cyanide. Appl. Clay Sci. 2008, 42, 95–101. [Google Scholar] [CrossRef]
- Makó, É.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Mechanochemical intercalation of low reactivity kaolinite. Appl. Clay Sci. 2013, 83–84, 24–31. [Google Scholar] [CrossRef]
- Bundy, W.M.; Ishley, J.N. Kaolin in paper filling and coating. Appl. Clay Sci. 1991, 5, 397–420. [Google Scholar] [CrossRef]
- Elton, N.J.; Gate, L.F.; Hooper, J.J. Texture and orientation of kaolin in coatings. Clay Miner. 1999, 34, 89–98. [Google Scholar] [CrossRef]
- Morris, J.D.; Daood, S.S.; Nimmo, W. The use of kaolin and dolomite bed additives as an agglomeration mitigation method for wheat straw and miscanthus biomass fuels in a pilot-scale fluidized bed combustor. Renew. Energy 2022, 196, 749–762. [Google Scholar] [CrossRef]
- Conceição, S.; Santos, N.F.; Velho, J.; Ferreira, J.M.F. Properties of paper coated with kaolin: The influence of the rheological modifier. Appl. Clay Sci. 2005, 30, 165–173. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Zhang, M.; Liu, Y.; Yu, Y.; Yu, H. Electrical and Mechanical Properties of Epoxy Composites Reinforced with Kaolin-Coated Basalt Fibers. Fibers Polym. 2023, 24, 95–108. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Mohd Mortar, N.A.; Abdullah, M.M.A.B.; Abdul Razak, R.; Abd Rahim, S.Z.; Aziz, I.H.; Nabiałek, M.; Jaya, R.P.; Semenescu, A.; Mohamed, R.; Ghazali, M.F. Geopolymer Ceramic Application: A Review on Mix Design, Properties and Reinforcement Enhancement. Materials 2022, 15, 7567. [Google Scholar] [CrossRef] [PubMed]
- Grim, R.E. Applied Clay Mineralogy. Geol. Föreningen Stockh. Förhandlingar 1962, 84, 533. [Google Scholar] [CrossRef]
- Chaudhuri, S.P. A Review on the Kaolinite-Mullite Transformation. Trans. Indian Ceram. Soc. 1977, 36, 71–81. [Google Scholar] [CrossRef]
- Bourret, J.; Michot, A.; Tessier-Doyen, N.; Naït-Ali, B.; Pennec, F.; Alzina, A.; Vicente, J.; Peyratout, C.S.; Smith, D.S. Thermal Conductivity of Very Porous Kaolin-Based Ceramics. J. Am. Ceram. Soc. 2014, 97, 938–944. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Ismail, A.F.; Gani, P. Effect of kaolin particle size and loading on the characteristics of kaolin ceramic support prepared via phase inversion technique. J. Asian Ceram. Soc. 2016, 4, 164–177. [Google Scholar] [CrossRef]
- Johns, W.D. High-temperature phase changes in kaolinites. Mineral. Mag. J. Mineral. Soc. 1953, 30, 186–198. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, M.C.; Hon, M.H. Phase transformation and growth of mullite in kaolin ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- Alves, H.P.A.; Silva, J.B.; Campos, L.F.A.; Torres, S.M.; Dutra, R.P.S.; Macedo, D.A. Preparation of mullite based ceramics from clay–kaolin waste mixtures. Ceram. Int. 2016, 42, 19086–19090. [Google Scholar] [CrossRef]
- Shafiq, N.; Nuruddin, M.F.; Khan, S.U.; Ayub, T. Calcined kaolin as cement replacing material and its use in high strength concrete. Constr. Build. Mater. 2015, 81, 313–323. [Google Scholar] [CrossRef]
- Bundy, W.M. The Diverse Industrial Applications of Kaolin; Special Publication No. 1; Clay Minerals Society: Boulder, CO, USA, 1993; pp. 43–73. [Google Scholar]
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in Cosmetics and Personal-Care Products. Clays Clay Miner. 2021, 69, 561–575. [Google Scholar] [CrossRef]
- Murray, H.H. Applied rheology. Proc. Porc. Enamel Inst. Tech. Forum 1975, 37, 1–9. [Google Scholar]
- Murray, H.H.; Kogel, J.E. Engineered clay products for the paper industry. Appl. Clay Sci. 2005, 29, 199–206. [Google Scholar] [CrossRef]
- Buyondo, K.A.; Kasedde, H.; Kirabira, J.B. A comprehensive review on kaolin as pigment for paint and coating: Recent trends of chemical-based paints, their environmental impacts and regulation. Case Stud. Chem. Environ. Eng. 2022, 6, 100244. [Google Scholar] [CrossRef]
- Ruszala, M.J.A.; Rowson, N.A.; Grover, L.M.; Choudhery, R.A. Low Carbon Footprint TiO2 Substitutes in Paint: A Review. Int. J. Chem. Eng. Appl. 2015, 6, 331–340. [Google Scholar] [CrossRef]
- Narayan, R.; Raju, K.V.S.N. The use of calcined clay as part replacement of titanium dioxide in latex paint formulations. J. Appl. Polym. Sci. 2000, 77, 1029–1036. [Google Scholar] [CrossRef]
- Ahmed, N.M. Comparative study on the role of kaolin, calcined kaolin and chemically treated kaolin in alkyd-based paints for protection of steel. Pigment Resin Technol. 2013, 42, 3–14. [Google Scholar] [CrossRef]
- Ahmed, N.M.; El-Sabbagh, S.H. The influence of kaolin and calcined kaolin on SBR composite properties. Polym. Compos. 2014, 35, 570–580. [Google Scholar] [CrossRef]
- Meite, N.; Konan, L.K.; Bamba, D.; Goure-Doubi, B.I.H.; Oyetola, S. Structural and Thermomechanical Study of Plastic Films Made from Cassava-Starch Reinforced with Kaolin and Metakaolin. Mater. Sci. Appl. 2018, 9, 41–54. [Google Scholar] [CrossRef]
- Hussein, Z.A.; Shakor, Z.M.; Alzuhairi, M.; Al-Sheikh, F. Kinetic and Thermodynamic Study of the Pyrolysis of Plastic Waste. Environ. Process. 2023, 10, 27. [Google Scholar] [CrossRef]
- Vesely, D.; Kalendova, A.; Manso, M.V. Properties of calcined kaolins in anticorrosion paints depending on PVC, chemical composition and shape of particles. Prog. Org. Coat. 2012, 74, 82–91. [Google Scholar] [CrossRef]
- Mallem, O.K.; Zouai, F.; Gumus, O.Y.; Benabid, F.Z.; Bedeloglu, A.C.; Benachour, D. Synergistic effect of talc/calcined kaolin binary fillers on rigid PVC: Improved properties of PVC composites. J. Vinyl Addit. Technol. 2021, 27, 881–893. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, S.; Gu, X.; Sun, J.; Jin, X.; Li, H. Effects of kaolinite nanoroll on the flammability of polypropylene nanocomposites. Appl. Clay Sci. 2016, 132–133, 579–588. [Google Scholar] [CrossRef]
- Ogbebor, O.; Okieimen, F.; Ogbeifun, D.; Okwu, U. Organomodified kaolin as filler for natural rubber. Chem. Ind. Chem. Eng. Q. 2015, 21, 477–484. [Google Scholar] [CrossRef]
- Arroyo, M.; López-Manchado, M.; Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Susanto, T.; Rahmaniar; Putra, V.G.V.; Sari, T.I.; Farida; Roni, K.A.; Puspitasari, G. Effect carbon black and modified kaolin hybrid filler on the curing and physic-mechanical properties of natural rubber-styrene butadiene rubber blends. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 963, p. 012029. [Google Scholar] [CrossRef]
- Yadav, J.S.; Tiwari, S.K. A study on the potential utilization of crumb rubber in cement treated soft clay. J. Build. Eng. 2017, 9, 177–191. [Google Scholar] [CrossRef]
- El-Nashar, D.; Khalaf, A. A review on reinforcing rubber goods for different valuable applications. Egypt. J. Chem. 2022, 66, 503–529. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical industry. Appl. Clay Sci. 2009, 46, 73–80. [Google Scholar] [CrossRef]
- Christian, Z.C. Chemical investigation of kankara kaolin for its pharmaceutical applications. Open Access J. Transl. Med. Res. 2018, 2, 11–13. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in pharmaceutics and biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Yarangsee, C.; Wattanaarsakit, P.; Sirithunyalug, J.; Leesawat, P. Particle Engineering of Chitosan and Kaolin Composite as a Novel Tablet Excipient by Nanoparticles Formation and Co-Processing. Pharmaceutics 2021, 13, 1844. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.S.; Subha, M.C.; Jithendra, T.; Madhavi, C.; Rao, K.C. Emerging Novel Drug Delivery System for Control Release of Curcumin through Sodium Alginate/Poly(ethylene glycol) Semi IPN Microbeads-Intercalated with Kaolin Nanoclay. J. Drug Deliv. Ther. 2019, 9, 324–333. [Google Scholar] [CrossRef]
- Dissanayake, N.U.S.; Pupulewatte, P.G.H.; Jayawardana, D.T.; Senevirathne, D.M. Characterization of Kaolin-rich Laterite Soil for Applications for the Development of Soil-based Cosmetic Products. J. Drug Deliv. Ther. 2022, 12, 31–40. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin care and rejuvenation by cosmeceutical facial mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef]
- Pinheiro, G.; Carvalho, T.; Michel, B.; Arjona, J.; Bobadilha, M.; Silva-Valenzuela, M.; Costa, T.; Valenzuela-Diaz, F. Characterization of a Brazilian Kaolin and Its Sorption Ability to Mineral Oils. In Minerals, Metals and Materials Series; Springer: Berlin/Heidelberg, Germany, 2020; Volume PartF1, pp. 367–373. [Google Scholar]
- Watkins, E.C. Mineral Raw Materials for Fiberglass Manufacturing; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 1986; p. 6. Available online: https://onemine.org/documents/mineral-raw-materials-for-fiber-glass-manufacturing (accessed on 19 July 2024).
- Pascual, S.; Cobos, G.; Seris, E.; González-Núñez, M. Effects of processed kaolin on pests and non-target arthropods in a Spanish olive grove. J. Pest Sci. 2010, 83, 121–133. [Google Scholar] [CrossRef]
- Gilkes, R.J.; Prakongkep, N. How the unique properties of soil kaolin affect the fertility of tropical soils. Appl. Clay Sci. 2016, 131, 100–106. [Google Scholar] [CrossRef]
- Cantore, V.; Pace, B.; Albrizio, R. Kaolin-based particle film technology affects tomato physiology, yield and quality. Environ. Exp. Bot. 2009, 66, 279–288. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Unuabonah, I.E.; Olu-Owolabi, B.I. Adsorption of some heavy metal ions on sulfate- and phosphate-modified kaolin. Appl. Clay Sci. 2005, 29, 145–148. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Li, J.; Jiao, S.; Zhong, L.; Pan, J.; Ma, Q. Optimizing coagulation and flocculation process for kaolinite suspension with chitosan. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 428, 100–110. [Google Scholar] [CrossRef]
- Ferasat, Z.; Panahi, R.; Mokhtarani, B. Natural polymer matrix as safe flocculant to remove turbidity from kaolin suspension: Performance and governing mechanism. J. Environ. Manag. 2020, 255, 109939. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.; Tijani, J.; Ndamitso, M.; Abdulkareem, A.; Shuaib, D.; Mohammed, A. Adsorptive removal of pollutants from industrial wastewater using mesoporous kaolin and kaolin/TiO2 nanoadsorbents. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100414. [Google Scholar] [CrossRef]
- Kang, W.; Cui, Y.; Yang, Y.; Guo, M.; Zhao, Z.; Wang, X.; Liu, X. Preparation of nitrogen-doped hollow carbon nanosphere/graphene composite aerogel for efficient removal of quinoline from wastewater. J. Hazard. Mater. 2021, 417, 126160. [Google Scholar] [CrossRef]
- Cai, W.; Bordoloi, S.; Ng, C.W.W.; Sarmah, A.K. Influence of pore fluid salinity on shrinkage and water retention characteristics of biochar amended kaolin for landfill liner application. Sci. Total Environ. 2022, 838, 156493. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Zhao, T.; Gui, K.; Wang, J.; Thomas, H.R. Superior activity of iron–manganese supported on kaolin for NO abatement at low temperature. J. Environ. Sci. 2020, 88, 237–247. [Google Scholar] [CrossRef]
- Selim, M.M.; Abd El-Maksoud, I.H. Hydrogenation of edible oil over zeolite prepared from local kaolin. Microporous Mesoporous Mater. 2004, 74, 79–85. [Google Scholar] [CrossRef]
- Li, X.; Tang, A. Pd modified kaolinite nanocomposite as a hydrogenation catalyst. RSC Adv. 2016, 6, 15585–15591. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, L.; Chen, J.; Luo, H.; Liu, J. In situ growth of g-C3N4 on clay minerals of kaolinite, sepiolite, and talc for enhanced solar photocatalytic energy conversion. Appl. Clay Sci. 2022, 216, 106337. [Google Scholar] [CrossRef]
- Maj, I.; Matus, K. Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies 2023, 16, 4359. [Google Scholar] [CrossRef]
- Ferreira, B.F.; do Prado, M.V.; Marçal, L.; Ciuffi, K.J.; Vicente, M.A.; Gil, A.; de Faria, E.H. Manganese-sulfonato porphyrin adsorbed on amino kaolinite as heterogeneous catalyst for oxidation and polymerization reactions. Appl. Clay Sci. 2023, 235, 106871. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, L.; Cheng, H. Rational design of kaolinite-based photocatalytic materials for environment decontamination. Appl. Clay Sci. 2021, 208, 106098. [Google Scholar] [CrossRef]
- Chen, M.; Yang, T.; Han, J.; Zhang, Y.; Zhao, L.; Zhao, J.; Li, R.; Huang, Y.; Gu, Z.; Wu, J. The Application of Mineral Kaolinite for Environment Decontamination: A Review. Catalysts 2023, 13, 123. [Google Scholar] [CrossRef]
- Statista Fossil Fuels. Available online: www.statista.com (accessed on 25 July 2024).
- Energy Institute. Energy Institute Statistical Review of World Energy 2024. Available online: https://www.energyinst.org/statistical-review (accessed on 25 July 2024).
- BP. BP Statistical Review of World Energy 2021. Available online: https://www.bp.com/ (accessed on 25 July 2024).
- OPEC OPEC Monthly Oil Market Report 2024. Available online: https://www.opec.org/opec_web/en/ (accessed on 25 July 2024).
- Energy Institute. Statistical Review of World Energy 2024, Tab “Oil Consumption–Barrels”. Available online: https://www.energyinst.org/ (accessed on 25 July 2024).
- Energy Institute. Statistical Review of World Energy 2024, Tab “Oil Trade Movements”. Available online: https://www.energyinst.org/statistical-review (accessed on 25 July 2024).
- Gelder, A.; Mitchell, E. Assessing the Refineries at Risk of Closure. Available online: https://www.woodmac.com/news/opinion/assessing-the-refineries-at-risk-of-closure/ (accessed on 25 July 2024).
- Datosmacro. Economic and Sociodemographic Information. Available online: https://datosmacro.expansion.com/ (accessed on 25 July 2024).
- GlobalPetrolPrices. Energy Prices around the World. Available online: https://www.globalpetrolprices.com/ (accessed on 25 July 2024).
- Statista. Oil Giants Make Record Profits. Available online: https://es.statista.com/grafico/27899/ganancias-netas-de-companias-de-petroleo-y-gas/ (accessed on 25 July 2024).
- Rana, M.S.; Sámano, V.; Ancheyta, J.; Diaz, J.A.I. A review of recent advances on process technologies for upgrading of heavy oils and residua. Fuel 2007, 86, 1216–1231. [Google Scholar] [CrossRef]
- Castañeda, L.C.; Muñoz, J.A.D.; Ancheyta, J. Current situation of emerging technologies for upgrading of heavy oils. Catal. Today 2014, 220–222, 248–273. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of hydrocarbons for the production of olefins: A state-of-the-art review I: Thermal cracking review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Al-Samhan, M.; Al-Fadhli, J.; Al-Otaibi, A.M.; Al-Attar, F.; Bouresli, R.; Rana, M.S. Prospects of refinery switching from conventional to integrated: An opportunity for sustainable investment in the petrochemical industry. Fuel 2022, 310, 122161. [Google Scholar] [CrossRef]
- Weitkamp, J. Catalytic Hydrocracking—Mechanisms and Versatility of the Process. ChemCatChem 2012, 4, 292–306. [Google Scholar] [CrossRef]
- Kinsara, R.A.; Demirbas, A. Upgrading of crude oil via distillation processes. Pet. Sci. Technol. 2016, 34, 1300–1306. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Vakili, M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies 2021, 14, 8190. [Google Scholar] [CrossRef]
- Ragothaman, A.; Anderson, W. Air Quality Impacts of Petroleum Refining and Petrochemical Industries. Environments 2017, 4, 66. [Google Scholar] [CrossRef]
- Perego, C.; Ingallina, P. Recent advances in the industrial alkylation of aromatics: New catalysts and new processes. Catal. Today 2002, 73, 3–22. [Google Scholar] [CrossRef]
- Pan, Y.; Bai, J.; Yang, G.; Li, Z. Progress in the Research on Branched Polymers with Emphasis on the Chinese Petrochemical Industry. Molecules 2023, 28, 7934. [Google Scholar] [CrossRef]
- Bahri, M.; Mahdavi, A.; Mirzaei, A.; Mansouri, A.; Haghighat, F. Integrated oxidation process and biological treatment for highly concentrated petrochemical effluents: A review. Chem. Eng. Process. 2018, 125, 183–196. [Google Scholar] [CrossRef]
- Matar, S.; Mirbach, M.J.; Tayim, H.A. Hydrogenation—Dehydrogenation Processes. In Catalysis in Petrochemical Processes; Springer: Dordrecht, The Netherlands, 1989; pp. 35–65. ISBN 978-94-009-1177-2. [Google Scholar]
- Valavarasu, G.; Sairam, B. Light Naphtha Isomerization Process: A Review. Pet. Sci. Technol. 2013, 31, 580–595. [Google Scholar] [CrossRef]
- Hettinger, W.P. Contribution to catalytic cracking in the petroleum industry. Appl. Clay Sci. 1991, 5, 445–468. [Google Scholar] [CrossRef]
- Chang, Y.F.; Vaughn, S.N.; Martens, L.R.; Baumgartner, J.E.; Soled, S.L.; Clem, K.R. Molecular Sieve Catalyst Composition, Its Making and Use in Conversion Processes. U.S. Patent No. 6,872,680, 24 June 2002. [Google Scholar]
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Appl. Petrochem. Res. 2015, 5, 377–392. [Google Scholar] [CrossRef]
- Qin, X.; Ye, L.; Hou, L.; Wang, T.; Ma, M.; Pu, X.; Han, X.; Liu, J.; Luan, B.; Liu, P. A coupling model of fluid catalytic cracking and diesel hydrotreating processes to study the effects of reaction temperature on the composition of diesel. Chem. Eng. J. 2023, 466, 143078. [Google Scholar] [CrossRef]
- Fujiyama, Y.; Al-Tayyar, M.H.; Dean, C.F.; Aitani, A.; Redhwi, H.H. Chapter 1 Development of high-severity FCC process: An overview. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 166, pp. 1–12. ISBN 0444530606. [Google Scholar]
- Woltermann, G.M.; Magee, J.S.; Griffith, S.D. Chapter 4 Commercial Preparation and Characterization of FCC Catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1993; Volume 76, pp. 105–144. [Google Scholar]
- Milton, R.M. Molecular Sieve Adsorbents. U.S. Patent 2,882,244, 14 April 1959. [Google Scholar]
- Maher, P.K.; Albers, E.W.; Mcdaniel, C.V. Preparation of High Silica Synthetic Faujasite. U.S. Patent US3,671,191A, 20 June 1972. [Google Scholar]
- Brown, S.M.; Woltermann, G.M. Zeolitized Composite Bodies and Manufacture Thereof. U.S. Patent 4,235,753, 25 November 1980. [Google Scholar]
- Atta, A.Y.; Ajayi, O.A.; Adefila, S.S. Synthesis of Faujasite Zeolites from Kankara Kaolin Clay. J. Appl. Sci. Res. 2007, 3, 1017–1021. [Google Scholar]
- Haden, W.L.; Dzierzanowski, F.J.; Somerset, N.J. Microspherical Zeolitic Molecular Sieve Composite Catalyst and Preparation Thereof. U.S. Patent No. 3,402,996, 20 June 1968. [Google Scholar]
- Haden, W.L., Jr.; Dzierzanowski, F.J. Microspherical Zeolitic Molecular Sieve Composite Catalyst and Preparation Thereof. U.S. Patent 3,624,718, 7 March 1972. [Google Scholar]
- Speronello, B.K. Porous Mullite. U.S. Patent 4,628,042, 1986. [Google Scholar]
- Howell, P.A. Process for Preparing Crystalline Zeolitic Molecular Sieves. U.S. Patent 3,390,958, 2 July 1968. [Google Scholar]
- Chandrasekhar, S.; Pramada, P.N. Kaolin-based zeolite Y, a precursor for cordierite ceramics. Appl. Clay Sci. 2004, 27, 187–198. [Google Scholar] [CrossRef]
- Ajayi, O.A.; Adefila, S.S.; Ityokumbul, M.T. Monitoring zeolite NaY formation from potassium-rich Nigerian kaolinite clay. Ain Shams Eng. J. 2018, 9, 1653–1661. [Google Scholar] [CrossRef]
- Plank, C.J.; Rosinski, E.J. Catalytic Conversion of Hydrocarbons with a Crystalline Alumino-Silicate in a Silica-Alumina Matrix. U.S. Patent 3,271,418, 6 September 1966. [Google Scholar]
- Secor, R.B. Method for Producing Catalysts. U.S. Patent 3,446,727, 1968. [Google Scholar]
- Danforth, J.D. 57 The Structure of Silica-Alumina Cracking Catalysts. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 1957; Volume 9, pp. 558–568. [Google Scholar]
- Joseph, C.S.; Shi, E.W.; Albers, G.R.W. Process for Improving the Physical and Catalytic Properties of a Fluid Cracking Catalyst. U.S. Patent 5,739,072, 1998. [Google Scholar]
- Engelhardt, G.; Lohse, U.; Samoson, A.; Mägi, M.; Tarmak, M.; Lippmaa, E. High resolution 29Si n.m.r. of dealuminated and ultrastable Y-zeolites. Zeolites 1982, 2, 59–62. [Google Scholar] [CrossRef]
- Brown, S.M.; Durante, V.A.; Reagan, W.J.; Speronello, B.K. Fluid Catalytic Cracking Catalyst Comprising Microspheres Containing More Than about 40 Percent by Weight Y-Faujasite and Methods for Making. U.S. Patent 4,601,997, 1986. [Google Scholar]
- Absil, P.L.; Robert, K.A.J. Catalyst and Catalytic Conversion Therewith. U.S. Patent No. 5,366,948, 1994. [Google Scholar]
- Absil, R.P.; Herbst, J.A. Cracking Catalysts Comprising Phosphorus and Method of Preparing and Using the Same. U.S. Patent 5,231,064, 1993. [Google Scholar]
- Komvokis, V.; Tan, L.X.L.; Clough, M.; Pan, S.S.; Yilmaz, B. Zeolites in Fluid Catalytic Cracking (FCC). In Zeolites in Sustainable Chemistry: Synthesis, Characterization and Catalytic Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–297. ISBN 9783662473955. [Google Scholar]
- Patrylak, L.; Likhnyovskyi, R.; Vypyraylenko, V.; Leboda, R.; Skubiszewska-Zięba, J.; Patrylak, K. Adsorption Properties of Zeolite-Containing Microspheres and FCC Catalysts Based on Ukrainian Kaolin. Adsorpt. Sci. Technol. 2001, 19, 525–540. [Google Scholar] [CrossRef]
- Al-Khattaf, S. The influence of Y-zeolite unit cell size on the performance of FCC catalysts during gas oil catalytic cracking. Appl. Catal. A 2002, 231, 293–306. [Google Scholar] [CrossRef]
- Zheng, S.-Q.; Sun, S.-H.; Wang, Z.-F.; Gao, X.-H.; Xu, X.-L. Suzhou kaolin as a FCC catalyst. Clay Miner. 2005, 40, 303–310. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Gao, X. Synthesis, characterization and evaluation of a novel resid FCC catalyst based on in situ synthesis on kaolin microspheres. Catal. Lett. 2006, 110, 229–234. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Gao, X.; Ma, J. A novel FCC catalyst synthesized via in situ overgrowth of NaY zeolite on kaolin microspheres for maximizing propylene yield. Catal. Today 2007, 125, 163–168. [Google Scholar] [CrossRef]
- Wang, P.; Shen, B.; Shen, D.; Peng, T.; Gao, J. Synthesis of ZSM-5 zeolite from expanded perlite/kaolin and its catalytic performance for FCC naphtha aromatization. Catal. Commun. 2007, 8, 1452–1456. [Google Scholar] [CrossRef]
- Gonçalves, M.L.A.; Barreto, J.R.C.; Cerqueira, W.V.; Teixeira, A.M.R.F. Effect of zeolite, kaolin and alumina during cracking of heavy petroleum residue evaluated by thermogravimetry. J. Therm. Anal. Calorim. 2009, 97, 515–519. [Google Scholar] [CrossRef]
- Wan, G.; Duan, A.; Zhang, Y.; Zhao, Z.; Jiang, G.; Zhang, D.; Gao, Z.; Liu, J.; Chung, K.H. Hydrodesulfurization of Fluidized Catalytic Cracking Diesel Oil over NiW/AMB Catalysts Containing H-Type β-Zeolite in Situ Synthesized from Kaolin Material. Energy Fuels 2009, 23, 3846–3852. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Zheng, S.-Q.; Qian, D. Sulphur reduction in fluid catalytic cracking using a kaolin in situ crystallization catalyst modified with vanadium. Clay Miner. 2009, 44, 281–288. [Google Scholar] [CrossRef]
- Duan, A.; Wan, G.; Zhang, Y.; Zhao, Z.; Jiang, G.; Liu, J. Optimal synthesis of micro/mesoporous beta zeolite from kaolin clay and catalytic performance for hydrodesulfurization of diesel. Catal. Today 2011, 175, 485–493. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, R.; Li, X.; Liu, X.; Yan, Z. In situ synthesis, characterization and catalytic activity of ZSM-5 zeolites on kaolin microspheres from amine-free system. J. Porous Mater. 2013, 20, 137–141. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Machado Júnior, H.F.; Costa, D.A. Kaolin and commercial fcc catalysts in the cracking of loads of polypropylene under refinary conditions. Braz. J. Chem. Eng. 2013, 30, 825–834. [Google Scholar] [CrossRef]
- Ghrib, Y.; Frini-Srasra, N.; Srasra, E.; Martínez-Triguero, J.; Corma, A. Synthesis of cocrystallized USY/ZSM-5 zeolites from kaolin and its use as fluid catalytic cracking catalysts. Catal. Sci. Technol. 2018, 8, 716–725. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Zheng, Y.; Ng, S.; Brown, H.; Xia, Y. Kaolin-based catalyst as a triglyceride FCC upgrading catalyst with high deoxygenation, mild cracking, and low dehydrogenation performances. Catal. Today 2019, 319, 164–171. [Google Scholar] [CrossRef]
- Padilla, J.; Guzman, A.; Molina, D.; Poveda-Jaramillo, J.C. Structural transformation of kaolin as an active matrix for the in situ synthesis of zeolite Y. Clay Miner. 2020, 55, 293–302. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Z.; Zhou, L.; Ju, G. Synthesis of Si-Modified Pseudo-Boehmite@kaolin Composite and Its Application as a Novel Matrix Material for FCC Catalyst. Materials 2022, 15, 2169. [Google Scholar] [CrossRef]
- Padilla, J.; Guzman, A.; Poveda-Jaramillo, J.C. Synthesis and catalytic behavior of FCC catalysts obtained from kaolin by the in-situ method. Braz. J. Chem. Eng. 2023, 40, 803–816. [Google Scholar] [CrossRef]
- Kharas, K.; Mastry, M.C.; Thompson, A.; Yilmaz, B. Comparison of an in situ and an incorporated FCC catalyst under iron contamination. Catal. Commun. 2022, 171, 106483. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Q.; Qin, Y.; Liu, H.; Liu, H.; Cao, G.; Gao, X.; Song, L.; Sun, Z. Optimizing the accessibility of zeolite Y on FCC catalyst to improve heavy oil conversion capacity. Microporous Mesoporous Mater. 2023, 359, 112627. [Google Scholar] [CrossRef]
- Saab, R.; Polychronopoulou, K.; Zheng, L.; Kumar, S.; Schiffer, A. Synthesis and performance evaluation of hydrocracking catalysts: A review. J. Ind. Eng. Chem. 2020, 89, 83–103. [Google Scholar] [CrossRef]
- Peng, C.; Du, Y.; Feng, X.; Hu, Y.; Fang, X. Research and development of hydrocracking catalysts and technologies in China. Front. Chem. Sci. Eng. 2018, 12, 867–877. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Samadhi, T.W.; Subagjo, S.; Gunawan, M.L. Development of Hydrocracking Catalyst Support from Kaolin of Indonesian Origin. Adv. Mater. Res. 2014, 896, 532–536. [Google Scholar] [CrossRef]
- Kumar, R.; Rana, B.S.; Verma, D.; Rayaroth, S.; Prasad, V.S.; Sinha, A.K. Hydrotreatment of renewable oils using hierarchical mesoporous H-ZSM-5 synthesized from kaolin clay. RSC Adv. 2015, 5, 39342–39349. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Feng, P.; Han, X.; Zheng, Y. Integration of catalytic cracking and hydrotreating technology for triglyceride deoxygenation. Catal. Today 2017, 291, 172–179. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, W.; Bian, X. Dynamic synthesis route of zeolite Y with kaolin to improve yield. Green Process. Synth. 2018, 7, 23–29. [Google Scholar] [CrossRef]
- Wang, H.; Mao, W.; Wang, K.; Qin, Y.; Li, Q.; Peng, C.; Wang, J.; Sun, Z.; Song, L. Insight into the Effects of Mass Transfer Performance of Y Zeolite on the Product Selectivity of VGO Hydrocracking. Ind. Eng. Chem. Res. 2022, 61, 18270–18281. [Google Scholar] [CrossRef]
- Dhaneswara, D.; Fatriansyah, J.F.; Sudiro, T.; Harjanto, S.; Mastuli, M.S.; Federico, A.; Ulfiati, R. Synthesis and optimization of Ni/Mo-impregnated kaolin-based ZSM-5 as a catalytic hydrocracking catalyst for heavy petroleum distillates. Sustain. Energy Fuels 2024, 8, 2695–2703. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Cheng, B.; Tao, T.; Min, X.; Mi, R.; Huang, Z.; Fang, M.; Liu, Y. Synthesis and Applications of SAPO-34 Molecular Sieves. Chem.—A Eur. J. 2022, 28, e202102787. [Google Scholar] [CrossRef]
- Wang, T.; Lu, X.; Yan, Y. Synthesis, characterization and crystallization mechanism of SAPOs from natural kaolinite. Microporous Mesoporous Mater. 2010, 136, 138–147. [Google Scholar] [CrossRef]
- Fatourehchi, N.; Sohrabi, M.; Royaee, S.J.; Mirarefin, S.M. Preparation of SAPO-34 catalyst and presentation of a kinetic model for methanol to olefin process (MTO). Chem. Eng. Res. Des. 2011, 89, 811–816. [Google Scholar] [CrossRef]
- Taheri Najafabadi, A.; Fatemi, S.; Sohrabi, M.; Salmasi, M. Kinetic modeling and optimization of the operating condition of MTO process on SAPO-34 catalyst. J. Ind. Eng. Chem. 2012, 18, 29–37. [Google Scholar] [CrossRef]
- Wang, P.; Lv, A.; Hu, J.; Xu, J.; Lu, G. In Situ Synthesis of SAPO-34 Grown onto Fully Calcined Kaolin Microspheres and Its Catalytic Properties for the MTO Reaction. Ind. Eng. Chem. Res. 2011, 50, 9989–9997. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhang, J.; Chen, F.; Anpo, M. Preparation of macroporous SAPO-34 microspheres by a spray drying method using polystyrene spheres as hard template. Res. Chem. Intermed. 2011, 37, 949–959. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Wang, T. Synthesis of SAPO-34 using metakaolin in the presence of β-cyclodextrin. J. Energy Chem. 2015, 24, 401–406. [Google Scholar] [CrossRef]
- Zhu, J.; Cui, Y.; Wang, Y.; Wei, F. Direct synthesis of hierarchical zeolite from a natural layered material. Chem. Commun. 2009, 22, 3282–3284. [Google Scholar] [CrossRef]
- Yang, S.-T.; Kim, J.-Y.; Chae, H.-J.; Kim, M.; Jeong, S.-Y.; Ahn, W.-S. Microwave synthesis of mesoporous SAPO-34 with a hierarchical pore structure. Mater. Res. Bull. 2012, 47, 3888–3892. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Yue, Y.; Olsbye, U.; Bao, X. Design and in situ synthesis of hierarchical SAPO-34@kaolin composites as catalysts for methanol to olefins. Catal. Sci. Technol. 2019, 9, 6438–6451. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, X.-F.; Di, C.-Y.; Dou, T.; Chen, S.-L. A Green and Cost-Effective Synthesis of Hierarchical SAPO-34 through Dry Gel Conversion and Its Performance in a Methanol-to-Olefin Reaction. Ind. Eng. Chem. Res. 2021, 60, 15380–15390. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M. Application of resin and NH4NO3 as an ion exchange agent for microspherical preparation of nanostructured kaolin-SAPO-34 catalyst for methanol to light olefins reaction in a fluidized bed reactor. Res. Chem. Intermed. 2019, 45, 355–378. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M. Spray-dried zeotype/clay nanocatalyst for methanol to light olefins in fluidized bed reactor: Comparison of active and non-active filler. Appl. Clay Sci. 2019, 170, 70–85. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Ebrahimi, A. Fabrication of attrition-resistant nanostructured catalyst by spray dryer for methanol to light olefins reaction in a fluid bed reactor and coke formation. Microporous Mesoporous Mater. 2019, 279, 371–386. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Haghighi, M.; Aghamohammadi, S. Effect of calcination temperature and composition on the spray-dried microencapsulated nanostructured SAPO-34 with kaolin for methanol conversion to ethylene and propylene in fluidized bed reactor. Microporous Mesoporous Mater. 2020, 297, 110046. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Ebrahimi, A. Pathways in particle assembly by ultrasound-assisted spray-drying of kaolin/SAPO-34 as a fluidized bed catalyst for methanol to light olefins. Ultrason. Sonochem. 2019, 53, 237–251. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Haghighi, M.; Aghamohammadi, S. Single vs. dual-binder surface design of spray-dried Si–Al-sol-bound kaolin-matrixed SAPO-34 nanocatalyst for conversion of methanol to light-olefins in fluidized bed reactor. Microporous Mesoporous Mater. 2022, 332, 111714. [Google Scholar] [CrossRef]
- Mousavi, Y.S.; Akbari, A.; Omidkhah, M.; Safari, P. Formulated Mn-promoted SAPO-34/kaolin/alumina sol micro-size catalyst with a superior performance for methanol to light olefins conversion in a fluidized bed reactor. J. Ind. Eng. Chem. 2024, 129, 403–412. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Z.; Ding, Z.; Zhang, J.; Liu, W.; He, Q.; Li, Z.; Liu, C. Effect of kaolin calcined temperature on the preparation and crystallization mechanism of SAPO-34 molecular sieve for methanol-to-olefins performance. Microporous Mesoporous Mater. 2024, 369, 113037. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, X.; Jiang, C.; Tian, C.; Yang, Z.; Zhang, J. Hierarchical ZSM-5/SiC nano-whisker/SiC foam composites: Preparation and application in MTP reactions. J. Catal. 2015, 332, 70–76. [Google Scholar] [CrossRef]
- Sharbini Kamaluddin, H.; Gong, X.; Ma, P.; Narasimharao, K.; Dutta Chowdhury, A.; Mokhtar, M. Influence of zeolite ZSM-5 synthesis protocols and physicochemical properties in the methanol-to-olefin process. Mater. Today Chem. 2022, 26, 101061. [Google Scholar] [CrossRef]
- Michels, N.-L.; Mitchell, S.; Pérez-Ramírez, J. Effects of Binders on the Performance of Shaped Hierarchical MFI Zeolites in Methanol-to-Hydrocarbons. ACS Catal. 2014, 4, 2409–2417. [Google Scholar] [CrossRef]
- Pan, F.; Lu, X.; Yan, Y.; Wang, T. Synthesis of nano/micro scale ZSM-5 from kaolin and its catalytic performance. Kinet. Catal. 2017, 58, 541–548. [Google Scholar] [CrossRef]
- Shoinkhorova, T.; Dikhtiarenko, A.; Ramirez, A.; Dutta Chowdhury, A.; Caglayan, M.; Vittenet, J.; Bendjeriou-Sedjerari, A.; Ali, O.S.; Morales-Osorio, I.; Xu, W.; et al. Shaping of ZSM-5-Based Catalysts via Spray Drying: Effect on Methanol-to-Olefins Performance. ACS Appl. Mater. Interfaces 2019, 11, 44133–44143. [Google Scholar] [CrossRef]
- Javadli, R.; de Klerk, A. Desulfurization of heavy oil. Appl. Petrochem. Res. 2012, 1, 3–19. [Google Scholar] [CrossRef]
- de Lasa, H.; Hernandez Enriquez, R.; Tonetto, G. Catalytic Desulfurization of Gasoline via Dehydrosulfidation. Ind. Eng. Chem. Res. 2006, 45, 1291–1299. [Google Scholar] [CrossRef]
- Wan, G.; Duan, A.; Zhang, Y.; Zhao, Z.; Jiang, G.; Zhang, D.; Gao, Z. Zeolite beta synthesized with acid-treated metakaolin and its application in diesel hydrodesulfurization. Catal. Today 2010, 149, 69–75. [Google Scholar] [CrossRef]
- Niu, F.X.; Fu, F.; Gao, X.M.; Zhang, X.M.; Wu, Y.F. Preparation of Kaolin/Cu2O Photocatalyst and its Application in the Photocatalytic Oxidation-Extraction Desulfurization. Adv. Mater. Res. 2012, 518–523, 878–883. [Google Scholar] [CrossRef]
- Huang, C.; Wu, P.; Guo, Y.; Guo, Y. Facile synthesis of mesoporous kaolin catalyst carrier and its application in deep oxidative desulfurization. Microporous Mesoporous Mater. 2020, 306, 110415. [Google Scholar] [CrossRef]
- AlKhafaji, K.S.; Shakor, Z.M.; Al-Zaidi, B.Y.; Hussein, S.J. Preparation and Characterization of Metakaolin-Based Catalysts for Gasoil Hydrodesulfurization Purposes. Arab. J. Sci. Eng. 2022, 47, 6283–6296. [Google Scholar] [CrossRef]
- Hmood, H.M.; Gheni, S.A.; Ahmed, S.M.R.; Ali, M.M.; Saleh, H.Y.; Mohammed, M.H.; Mohammed, A.E.; Mahomood, M.A.; Mohammed, H.R.; Hassan, A.A.; et al. Kaoline-based catalyst for a high stability desulfurization of sour heavy naphtha in a three-phase oscillatory baffled reactor. Particuology 2024, 84, 249–260. [Google Scholar] [CrossRef]
- Ijadi, S.; Moradi, G.; Ghanadi, T. Efficient catalytic oxidative desulfurization of liquid fuels over tungsten oxide supported on metakaolin surface. ChemistrySelect 2024, 9, e202303715. [Google Scholar] [CrossRef]
- Gadin, J.M.; Afolabi, E.A.; Kovo, A.S.; Abdulkareem, A.S.; Oloruntoba James, M. A retrospect on recent research works in the preparation of zeolites catalyst from kaolin for biodiesel production. Biofuels 2023, 14, 315–332. [Google Scholar] [CrossRef]
- Nugraha, R.E.; Prasetyoko, D.; Asikin-Mijan, N.; Bahruji, H.; Suprapto, S.; Taufiq-Yap, Y.H.; Jalil, A.A. The effect of structure directing agents on micro/mesopore structures of aluminosilicates from Indonesian kaolin as deoxygenation catalysts. Microporous Mesoporous Mater. 2021, 315, 110917. [Google Scholar] [CrossRef]
- Honorato de Oliveira, B.F.; de França, L.F.; Fernandes Corrêa, N.C.; Ribeiro, N.F.d.P.; Velasquez, M. Renewable Diesel Production from Palm Fatty Acids Distillate (PFAD) via Deoxygenation Reactions. Catalysts 2021, 11, 1088. [Google Scholar] [CrossRef]
- Maharani, D.K.; Kusumawati, Y.; Safitri, W.N.; Nugraha, R.E.; Holilah, H.; Sholeha, N.A.; Jalil, A.A.; Bahruji, H.; Prasetyoko, D. Optimization of hierarchical ZSM-5 structure from kaolin as catalysts for biofuel production. RSC Adv. 2023, 13, 14236–14248. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Bibi, A.; Naqvi, M.; Noor, T.; Nizami, A.-S.; Rehan, M.; Ayoub, M. New trends in improving gasoline quality and octane through naphtha isomerization: A short review. Appl. Petrochem. Res. 2018, 8, 131–139. [Google Scholar] [CrossRef]
- Khalaf, Y.H.; Sherhan, B.Y.; Shakor, Z.M.; Al-Sheikh, F. Bimetallic Catalysts for Isomerization of Alkanes (A Review). Pet. Chem. 2023, 63, 829–843. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, W.; Du, Y.; Li, J. Species and impacts of metal sites over bifunctional catalyst on long chain n-alkane hydroisomerization: A review. Appl. Catal. A 2021, 611, 117916. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Liu, Q. Base-Metal-Catalyzed Olefin Isomerization Reactions. Synthesis 2019, 51, 1293–1310. [Google Scholar] [CrossRef]
- Lenarda, M.; Storaro, L.; Talon, A.; Moretti, E.; Riello, P. Solid acid catalysts from clays: Preparation of mesoporous catalysts by chemical activation of metakaolin under acid conditions. J. Colloid Interface Sci. 2007, 311, 537–543. [Google Scholar] [CrossRef]
- Glotov, A.P.; Roldugina, E.A.; Artemova, M.I.; Smirnova, E.M.; Demikhova, N.R.; Stytsenko, V.D.; Egazar’yants, S.V.; Maksimov, A.L.; Vinokurov, V.A. Isomerization of Xylenes in the Presence of Pt-Containing Catalysts Based on Halloysite Aluminosilicate Nanotubes. Russ. J. Appl. Chem. 2018, 91, 1353–1362. [Google Scholar] [CrossRef]
- Wu, X.; Lei, X.; Wang, S.; Zhang, H.; Cui, Q.; Bao, X.; Wang, T.; Yuan, P. Tuning the acidity and pore structures of NiP based bifunctional catalyst for efficient n-hexane isomerization. Fuel 2022, 324, 124396. [Google Scholar] [CrossRef]
- Demikhova, N.R.; Rubtsova, M.I.; Kireev, G.A.; Cherednichenko, K.A.; Vinokurov, V.A.; Glotov, A.P. Micro-mesoporous catalysts based on ZSM-5 zeolite synthesized from natural clay nanotubes: Preparation and application in the isomerization of C-8 aromatic fraction. Chem. Eng. J. 2023, 453, 139581. [Google Scholar] [CrossRef]
- Jones, E.K. Commercial Alkylation of Paraffins and Aromatics. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 1958; Volume 10, pp. 165–195. [Google Scholar]
- Odedairo, T.; Al-Khattaf, S. Alkylation and transalkylation of alkylbenzenes in cymene production over zeolite catalysts. Chem. Eng. J. 2011, 167, 240–254. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, L.; Ji, M.; Ding, H.; Li, Y.; Tao, Z.; Zhao, Q. Characterization of Y/MCM-41 composite molecular sieve with high stability from Kaolin and its catalytic property. Appl. Clay Sci. 2012, 62–63, 32–40. [Google Scholar] [CrossRef]
- Al-Kinany, M.C.; Al-Megren, H.A.; Al-Ghilan, E.A.; Edwards, P.P.; Xiao, T.; Al-Shammari, A.S.; Al-Drees, S.A. Selective zeolite catalyst for alkylation of benzene with ethylene to produce ethylbenzene. Appl. Petrochem. Res. 2012, 2, 73–83. [Google Scholar] [CrossRef]
- Bok, T.O.; Andriako, E.P.; Knyazeva, E.E.; Konnov, S.V.; Ivanova, I.I. Influence of the Binder Type on the Properties of Nanocrystalline Zeolite Beta-Based Catalysts for Benzene Alkylation with Propylene. Pet. Chem. 2018, 58, 833–840. [Google Scholar] [CrossRef]
- Gerzeliev, I.M.; Zhmylev, V.P.; Khusaimova, D.O.; Shkuropatov, A.V.; Knyazeva, E.E.; Ponomareva, O.A.; Ivanova, I.I.; Maksimov, A.L. Effect of Binder on the Properties of MWW Zeolite Catalysts in Benzene Alkylation with Propylene. Pet. Chem. 2019, 59, 695–700. [Google Scholar] [CrossRef]


| Year | Middle East | Central and South America | North America | Europe | Africa | Pacific Asia |
|---|---|---|---|---|---|---|
| 1992 | 63.70 | 7.60 | 11.70 | 7.50 | 5.90 | 3.60 |
| 2002 | 56.10 | 7.60 | 17.30 | 8.30 | 7.70 | 3.10 |
| 2012 | 48.40 | 19.70 | 13.20 | 8.40 | 7.80 | 2.50 |
| 2020 | 48.10 | 18.70 | 14.10 | 9.20 | 7.20 | 2.60 |
| 2024 | 48.50 | 20.00 | 14.10 | 7.50 | 7.30 | 2.30 |
| Catalytic Process | General Description | Role of Kaolin | Key Benefits of Kaolin |
|---|---|---|---|
| Fluid Catalytic Cracking (FCC) | Converts heavy petroleum fractions into lighter, more valuable products like gasoline and diesel | Kaolin is used as a support material for zeolite catalysts (e.g., Zeolite Y) in FCC, providing thermal stability and aiding cracking efficiency. | - Enhances catalyst durability - Reduces coke formation - Improves product yields and selectivity |
| Hydrocracking | Upgrades heavy oil fractions into lighter products by reacting with hydrogen under high pressure and temperature | Kaolin improves the dispersion of active metal sites and retains structure under harsh conditions. | - Cost-effective support - Improved catalytic activity and product selectivity - Higher thermal stability |
| Methanol-to-Olefins (MTO) | Converts methanol into light olefins like ethylene and propylene using zeolite-based catalysts (ZSM-5 and SAPO-34). | Kaolin serves as a raw material in synthesizing microporous SAPO molecular sieves. | - Enhanced mass transfer - Lower production costs - High selectivity and catalyst lifetime |
| Desulfurization | Removes sulfur compounds from petroleum fractions to reduce sulfur dioxide emissions and meet environmental standards | Kaolin-based catalysts improve desulfurization efficiency due to high surface area and customizable acidity profiles. | - Cost-effective and environmentally friendly - Efficient sulfur removal - Long catalyst life |
| Deoxygenation | Removes oxygen from organic compounds to upgrade renewable feedstocks into high-value hydrocarbon fuels | Acid-treated kaolin shows high deoxygenation efficiency in transforming renewable oils into hydrocarbons. | - High deoxygenation efficiency - Reduction of undesired aromatic compounds - Lower production of byproducts |
| Isomerization | Rearranges hydrocarbons to improve fuel quality, often applied to convert linear alkanes into branched ones, improving octane rating | Kaolin-derived catalysts enhance the dispersion of active sites, improving isomerization reactions. | - Increases fuel quality - High thermal and mechanical stability - Enhanced activity under reaction conditions |
| Alkylation | Combines isobutane with alkenes to produce high-octane gasoline components | Kaolin serves as a component in zeolitic catalysts, providing a high surface area and favorable acidity for the reaction. | - High selectivity for desired products - Improved catalyst lifespan - Increased production efficiency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ameri, O.B.; Alzuhairi, M.; Bailón-García, E.; Carrasco-Marín, F.; Amaro-Gahete, J. Transforming Petrochemical Processes: Cutting-Edge Advances in Kaolin Catalyst Fabrication. Appl. Sci. 2024, 14, 9080. https://doi.org/10.3390/app14199080
Al-Ameri OB, Alzuhairi M, Bailón-García E, Carrasco-Marín F, Amaro-Gahete J. Transforming Petrochemical Processes: Cutting-Edge Advances in Kaolin Catalyst Fabrication. Applied Sciences. 2024; 14(19):9080. https://doi.org/10.3390/app14199080
Chicago/Turabian StyleAl-Ameri, Osamah Basil, Mohammed Alzuhairi, Esther Bailón-García, Francisco Carrasco-Marín, and Juan Amaro-Gahete. 2024. "Transforming Petrochemical Processes: Cutting-Edge Advances in Kaolin Catalyst Fabrication" Applied Sciences 14, no. 19: 9080. https://doi.org/10.3390/app14199080










