Influence of the Salt Addition during the Fermentation Process on the Physical and Chemical Properties of Dried Yellow Beetroot
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Technological Treatment
2.2.1. Fermentation
2.2.2. Freeze-Drying
2.3. Analytical Methods
2.3.1. Determination of the Number of Lactic Acid Bacteria
2.3.2. Dry Matter
2.3.3. pH and Total Acidity
2.3.4. Salt Content
2.3.5. Color Coordinates
2.3.6. Betalain Content
2.3.7. Total Phenolic Content
2.3.8. Antioxidant Activity ABTS Assay
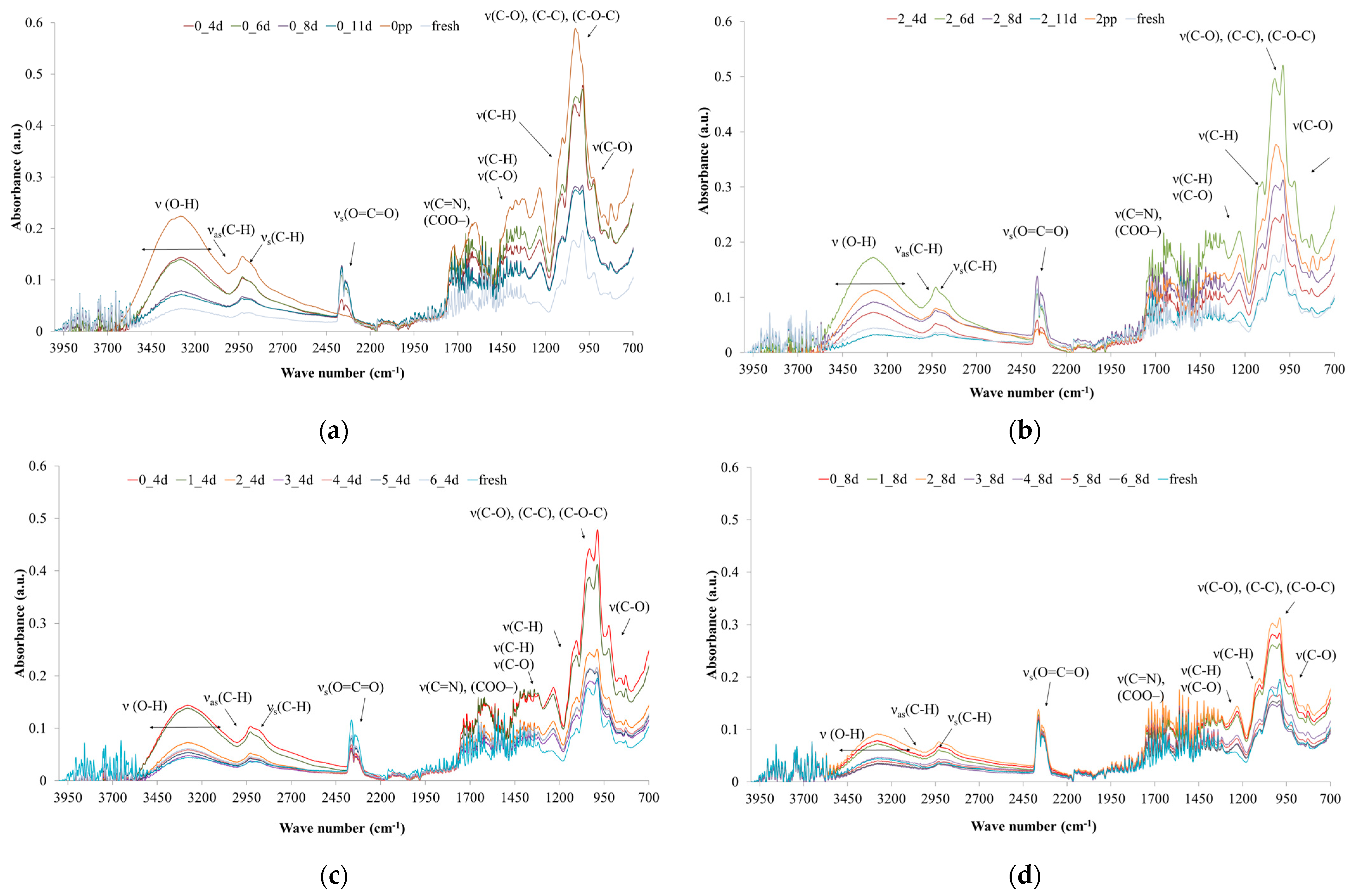
2.3.9. Fourier Infrared Spectroscopy (FTIR)
2.3.10. Thermogravimetric Analysis (TGA)
2.4. Data and Statistical Treatment
3. Results and Discussion
3.1. Microbiology
3.2. Physical Properties
3.2.1. Basic physical properties
3.2.2. Color Coefficients of Fermented Samples
3.3. Chemical Properties of Beetroot Samples
3.3.1. Pigment Content
3.3.2. Antioxidant Activity and Polyphenol Content
3.3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šlosár, M.; Kopta, T.; Hegedűs, O.; Hegedűsová, A.; Mezeyová, I.; Timoracká, M.; Mezey, J. Yield parameters, antioxidant activity, polyphenol and total soluble solids content of beetroot cultivars with different flesh colours. Folia Hortic. 2020, 32, 351–362. [Google Scholar] [CrossRef]
- Ceclu, L.; Nistor, O.-V. Red Beetroot: Composition and Health Effects—A Review. J. Nutr. Med. Diet Care 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Lee, J.-H.; Kim, B.-M.; Baik, S.-J.; Lee, S.-H.; Nam, J.-S. Comparison of Nutritional Components of Beets from Three Different Beta vulgaris L. Cultivars in Korea. J. Korean Soc. Food Sci. Nutr. 2020, 49, 969–976. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Tekieli, A.; Skalicka-Woźniak, K.; Grzegorczyk, A.; Świergosz, T.; Wybraniec, S. Characterization of triterpene saponin composition of white, yellow and red beetroot (Beta vulgaris L.). Pol. J. Food Nutr. Sci. 2022, 72, 159–170. [Google Scholar] [CrossRef]
- Patel, A. Probiotic fruit and vegetable juices-recent advances and future perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- de Oliveira, P.M.; Leite Júnior, B.R.d.C.; Martins, E.M.F.; Martins, M.L.; Vieira, É.N.R.; de Barros, F.A.R.; Cristianini, M.; de Almeida Costa, N.; Ramos, A.M. Mango and carrot mixed juice: A new matrix for the vehicle of probiotic lactobacilli. J. Food Sci. Technol. 2021, 58, 98–109. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Casciano, F.; Mayr, H.; Nissen, L.; Putti, A.; Zoli, F.; Gianotti, A.; Conterno, L. Red Beetroot Fermentation with Different Microbial Consortia to Develop Foods with Improved Aromatic Features. Foods 2022, 11, 3055. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowczyk, A.M.; Drabińska, N. Crossroad of Tradition and Innovation—The Application of Lactic Acid Fermentation to Increase the Nutritional and Health-Promoting Potential of Plant-Based Food Products—A Review. Pol. J. Food Nutr. Sci. 2021, 71, 107–134. [Google Scholar] [CrossRef]
- Jagannath, A.; Kumar, M.; Raju, P. Fermentative stabilization of betanin content in beetroot and its loss during processing and refrigerated storage. J. Food Process. Preserv. 2015, 39, 606–613. [Google Scholar] [CrossRef]
- Ropelewska, E.; Sabanci, K.; Aslan, M.F. The Changes in Bell Pepper Flesh as a Result of Lacto-Fermentation Evaluated Using Image Features and Machine Learning. Foods 2022, 11, 2956. [Google Scholar] [CrossRef] [PubMed]
- Kiczorowski, P.; Kiczorowska, B.; Samolińska, W.; Szmigielski, M.; Winiarska-Mieczan, A. Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Sci. Rep. 2022, 12, 13422. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules 2022, 27, 1008. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Oliveira, K.Á.R.; de Oliveira, M.E.G. Influence of lactic acid bacteria metabolites on physical and chemical food properties. Curr. Opin. Food Sci. 2022, 49, 100981. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation: Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1–4 October 2001 [and] Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30 April –1 May 2002; 0254-4725; World Health Organization: Geneva, Switzerland, 2006.
- FAO; WHO. Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO: Rome, Italy; Geneva, Switzerland, 2002.
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and Chemical Properties of Chokeberry Juice Fermented by Novel Lactic Acid Bacteria with Potential Probiotic Properties during Fermentation at 4 degrees C for 4 Weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Pobiega, K.; Nikodem, A.; Gramza-Michałowska, A. Sustainable Production and Characteristics of Dried Fermented Vegetables. Fermentation 2022, 8, 659. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Maróstica Júnior, M.R.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P. Red beetroot juice fermented by water kefir grains: Physicochemical, antioxidant profile and anticancer activity. Eur. Food Res. Technol. 2023, 249, 939–950. [Google Scholar] [CrossRef]
- WHO Europe. Food Based Dietary Guidelines in the WHO European Region; WHO (World Health Organization Regional Office for Europe): Copenhagen, Denmark, 2003.
- Commission, E. Dietary Salt/Sodium. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/dietary-saltsodium_en (accessed on 22 December 2023).
- He, F.J.; MacGregor, G.A. Reducing population salt intake worldwide: From evidence to implementation. Prog. Cardiovasc. Dis. 2010, 52, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Miguel, E.; Marta, L.; Ana Cristina, A.-S.; Maria Eduarda, P. The Role of Salt on Food and Human Health; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Rantsiou, K.; Garrido-Fernandez, A.; Cocolin, L.; Arroyo-Lopez, F.N. Salt reduction in vegetable fermentation: Reality or desire? J. Food Sci. 2013, 78, R1095–R1100. [Google Scholar] [CrossRef] [PubMed]
- Peréz-Dıaz, I.; Breidt, F.; Buescher, R.; Arroyo-López, F.; Jiménez-Dıaz, R.; Garrido-Fernández, A.; Johanningsmeire, S. Fermented and Acidified Vegetables. In Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association: Washington, DC, USA, 2013; pp. 521–532. [Google Scholar]
- Lin, X.; Tang, Y.; Hu, Y.; Lu, Y.; Sun, Q.; Lv, Y.; Zhang, Q.; Wu, C.; Zhu, M.; He, Q. Sodium reduction in traditional fermented foods: Challenges, strategies, and perspectives. J. Agric. Food Chem. 2021, 69, 8065–8080. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yi, J.; Jin, X.; Li, X.; Feng, S.; Bi, J. Freeze-drying of fruits and vegetables in food industry: Effects on phytochemicals and bioactive properties attributes-a comprehensive review. Food Rev. Int. 2023, 39, 6611–6629. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Tracz, K.; Bielińska, P.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Gramza-Michałowska, A. The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Appl. Sci. 2022, 12, 5766. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Galus, S.; Janowicz, M. Freeze-dried snacks obtained from frozen vegetable by-products and apple pomace–Selected properties, energy consumption and carbon footprint. Innov. Food Sci. Emerg. Technol. 2022, 77, 102949. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Nielsen, S.S. Sodium determination using ion-selective electrodes, Mohr titration, and test strips. In Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2017; pp. 161–170. [Google Scholar]
- Rybak, K.; Wiktor, A.; Kaveh, M.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave-Convective Methods. Molecules 2022, 27, 2164. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Turak, E.; Pobiega, K.; Rybak, K.; Synowiec, A.; Woźniak, Ł.; Trych, U.; Gniewosz, M.; Witrowa-Rajchert, D. Changes in Physical and Chemical Parameters of Beetroot and Carrot Juices Obtained by Lactic Fermentation. Appl. Sci. 2023, 13, 6113. [Google Scholar] [CrossRef]
- Kruszewski, B.; Domian, E.; Nowacka, M. Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice. Molecules 2023, 28, 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluis, A.A.; Dekker, M.; Skrede, G.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J. Agric. Food Chem. 2002, 50, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Ropelewska, E.; Wrzodak, A.; Sabanci, K.; Aslan, M.F. Effect of lacto-fermentation and freeze-drying on the quality of beetroot evaluated using machine vision and sensory analysis. Eur. Food Res. Technol. 2021, 284, 153–161. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Moraes, I.C.F.; Favaro-Trindade, C.S. Reducing carotenoid loss during storage by co-encapsulation of pequi and buriti oils in oil-in-water emulsions followed by freeze-drying: Use of heated and unheated whey protein isolates as emulsifiers. Food Res. Int. 2020, 130, 108901. [Google Scholar] [CrossRef]
- Kale, R.; Sawate, A.; Kshirsagar, R.; Patil, B.; Mane, R. Studies on evaluation of physical and chemical composition of beetroot (Beta vulgaris L.). Int. J. Chem. Stud. 2018, 6, 2977–2979. [Google Scholar]
- Carrillo, C.; Wilches-Pérez, D.; Hallmann, E.; Kazimierczak, R.; Rembiałkowska, E. Organic versus conventional beetroot. Bioactive compounds and antioxidant properties. LWT-Food Sci. Technol. 2019, 116, 108552. [Google Scholar] [CrossRef]
- Kuria, M.W.; Matofari, J.W.; Nduko, J.M. Physicochemical, antioxidant, and sensory properties of functional mango (Mangifera indica L.) leather fermented by lactic acid bacteria. J. Agric. Food Res. 2021, 6, 100206. [Google Scholar] [CrossRef]
- Niakousari, M.; Razmjooei, M.; Nejadmansouri, M.; Barba, F.J.; Marszałek, K.; Koubaa, M. Current Developments in Industrial Fermentation Processes. In Fermentation Processes: Emerging and Conventional Technologies; Koubaa, M., Barba, F.J., Roohinejad, S., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 23–96. [Google Scholar]
- Dimakopoulou-Papazoglou, D.; Lazaridou, A.; Biliaderis, C.G.; Katsanidis, E. Effect of Process Temperature on the Physical State of Beef Meat Constituents—Implications on Diffusion Kinetics during Osmotic Dehydration. Food Bioprocess Technol. 2022, 15, 706–716. [Google Scholar] [CrossRef]
- Kim, M.; Kerr, W.L. Vacuum-belt drying of rabbiteye blueberry (Vaccinium ashei) slurries: Influence of drying conditions on physical and quality properties of blueberry powder. Food Bioprocess Technol. 2013, 6, 3227–3237. [Google Scholar] [CrossRef]
- Stoll, D.A.; Müller, A.; Meinhardt, A.-K.; Dötsch, A.; Greiner, R.; Kulling, S.E.; Huch, M. Influence of salt concentration and iodized table salt on the microbiota of fermented cucumbers. Food Microbiol. 2020, 92, 103552. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Krzyżanowska, J.; Woźniak, Ł.; Skąpska, S. Kinetic modelling of polyphenol oxidase, peroxidase, pectin esterase, polygalacturonase, degradation of the main pigments and polyphenols in beetroot juice during high pressure carbon dioxide treatment. LWT-Food Sci. Technol. 2017, 85, 412–417. [Google Scholar] [CrossRef]
- Czyżowska, A.; Klewicka, E.; Libudzisz, Z. The influence of lactic acid fermentation process of red beet juice on the stability of biologically active colorants. Eur. Food Res. Technol. 2006, 223, 110–116. [Google Scholar] [CrossRef]
- Shaik, L.; Chakraborty, S. Nonthermal pasteurization of pineapple juice: A review on the potential of achieving microbial safety and enzymatic stability. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4716–4737. [Google Scholar] [CrossRef]
- Kozielska, E.; Bienia, B. Roślinne produkty fermentacji mlekowej w tradycyjnej kuchni polskiej. In Turystyka wiejska i dziedzictwo wsi w kontekście pandemii SARS-CoV-2; Kimta-Dziasek, E., Szanduła, M., Eds.; Instytut Zootechniki, Państwowy Instytut Badawczy: Kraków, Poland, 2021; pp. 89–94. [Google Scholar]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Characterization of Beet Root Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin. Molecules 2020, 25, 5498. [Google Scholar] [CrossRef]
- Kaderides, K.; Solomakou, N.; Mourtzinos, I.; Goula, A.M. Spray drying encapsulation of natural food colorants. In Spray Drying for the Food Industry: Unit Operations and Processing Equipment in the Food Industry; Jafari, S.M., Samborska, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 303–337. [Google Scholar]
- Nowacka, M.; Dadan, M.; Janowicz, M.; Wiktor, A.; Witrowa-Rajchert, D.; Mandal, R.; Pratap-Singh, A.; Janiszewska-Turak, E. Effect of nonthermal treatments on selected natural food pigments and color changes in plant material. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5097–5144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hu, L. High efficient freeze-drying technology in food industry. Crit. Rev. Food Sci. Nutr. 2021, 62, 3370–3388. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Lyu, Y.; Bi, J.; Chen, Q.; Wu, X.; Gou, M.; Yang, X. Color enhancement mechanisms analysis of freeze-dried carrots treated by ultrasound-assisted osmosis (ascorbic acid-CaCl2) dehydration. Food Chem. 2022, 381, 132255. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, S.; Lele, S. Dehydration of red beet root (Beta vulgaris) by hot air drying: Process optimization and mathematical modeling. Food Sci. Biotechnol. 2011, 20, 955–964. [Google Scholar] [CrossRef]
- Sawicki, T.; Wiczkowski, W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018, 259, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Płatosz, N.; Sawicki, T.; Wiczkowski, W. Profile of Phenolic Acids and Flavonoids of Red Beet and Its Fermentation Products. Does Long-Term Consumption of Fermented Beetroot Juice Affect Phenolics Profile in Human Blood Plasma and Urine? Pol. J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]
- Czyżowska, A.; Siemianowska, K.; Śniadowska, M.; Nowak, A. Bioactive Compounds and Microbial Quality of Stored Fermented Red Beetroots and Red Beetroot Juice. Pol. J. Food Nutr. Sci. 2020, 70, 35–44. [Google Scholar] [CrossRef]
- Malta, L.G.; Liu, R.H. Analyses of Total Phenolics, Total Flavonoids, and Total Antioxidant Activities in Foods and Dietary Supplements. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Elsevier, Academic Press: London, UK, 2014; pp. 305–314. [Google Scholar]
- Carmo, E.L.D.; Teodoro, R.A.R.; Felix, P.H.C.; Fernandes, R.V.B.; Oliveira, E.R.; Veiga, T.; Borges, S.V.; Botrel, D.A. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018, 249, 51–59. [Google Scholar] [CrossRef]
- Otalora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Cheng, X.-C.; Cui, X.-Y.; Qin, Z.; Liu, H.-M.; Wang, X.-D.; Liu, Y.-L. Effect of drying pretreatment methods on structural features and antioxidant activities of Brauns native lignin extracted from Chinese quince fruit. Process Biochem. 2021, 106, 70–77. [Google Scholar] [CrossRef]
- Quijano-Ortega, N.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.; Diaz-Moreno, C.; Ortiz-Grisales, S.; García-Mahecha, M.; Grassi, S. FTIR-ATR Spectroscopy Combined with Multivariate Regression Modeling as a Preliminary Approach for Carotenoids Determination in Cucurbita spp. Appl. Sci. 2020, 10, 3722. [Google Scholar] [CrossRef]
- Nesakumar, N.; Baskar, C.; Kesavan, S.; Rayappan, J.B.B.; Alwarappan, S. Analysis of Moisture Content in Beetroot using Fourier Transform Infrared Spectroscopy and by Principal Component Analysis. Sci. Rep. 2018, 8, 7996. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Hu, S. FT-IR spectra of NaCl–H2O in the region from near to beyond the critical state. Res. Chem. Intermed. 2011, 37, 405–413. [Google Scholar] [CrossRef]
- Janković, B.; Marinović-Cincović, M.; Janković, M. TG-DTA-FTIR analysis and isoconversional reaction profiles for thermal and thermo-oxidative degradation processes in black chokeberry (Aroniamelanocarpa). Chem. Pap. 2016, 70, 1094–1105. [Google Scholar] [CrossRef]
- Aztatzi-Rugerio, L.; Granados-Balbuena, S.Y.; Zainos-Cuapio, Y.; Ocaranza-Sánchez, E.; Rojas-López, M. Analysis of the degradation of betanin obtained from beetroot using Fourier transform infrared spectroscopy. J. Food Sci. Technol. 2019, 56, 3677–3686. [Google Scholar] [CrossRef]
- Mocanu, G.D.; Chirila, A.C.; Vasile, A.M.; Andronoiu, D.G.; Nistor, O.V.; Barbu, V.; Stanciuc, N. Tailoring the Functional Potential of Red Beet Purees by Inoculation with Lactic Acid Bacteria and Drying. Foods 2020, 9, 1611. [Google Scholar] [CrossRef]
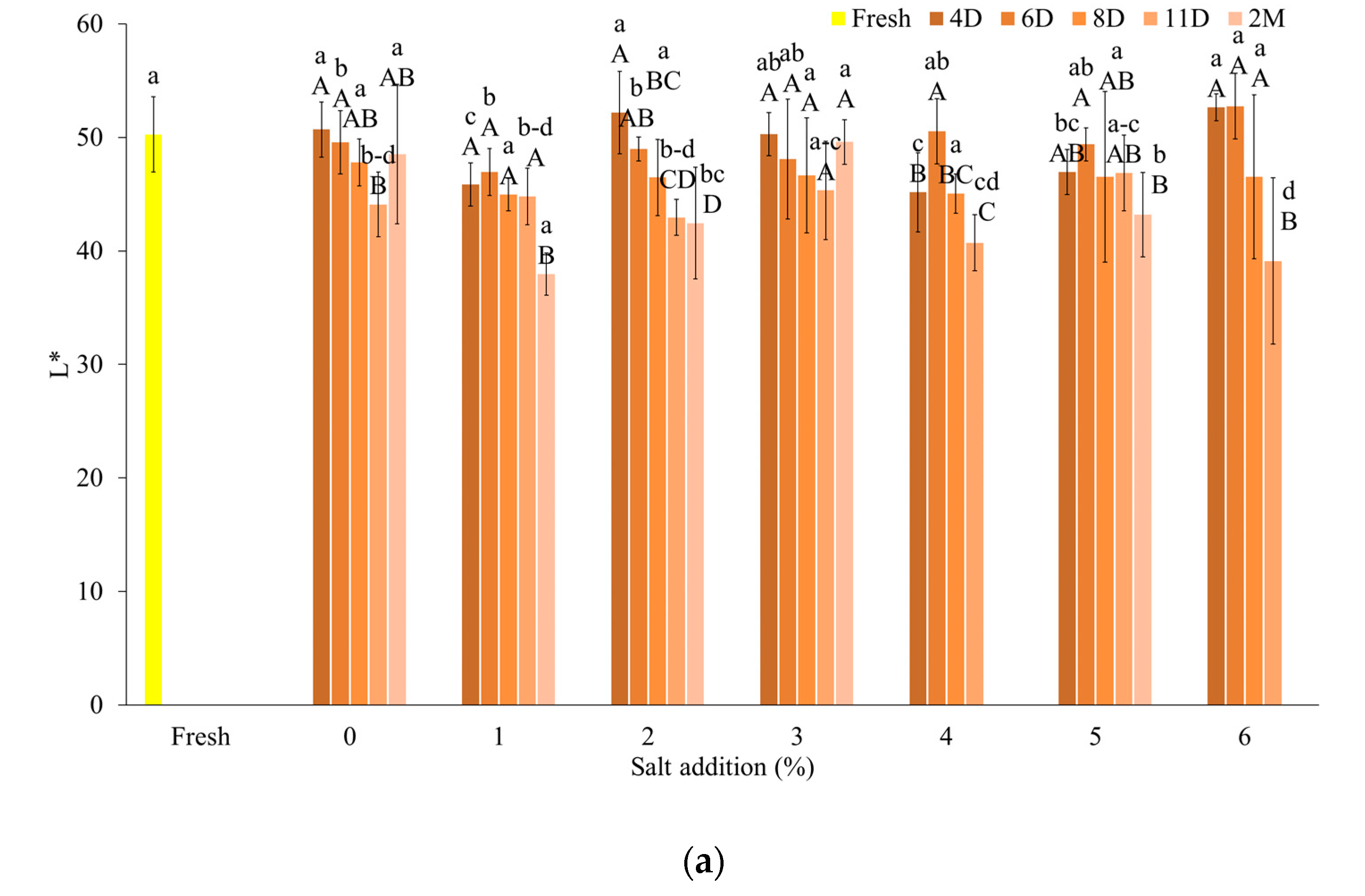
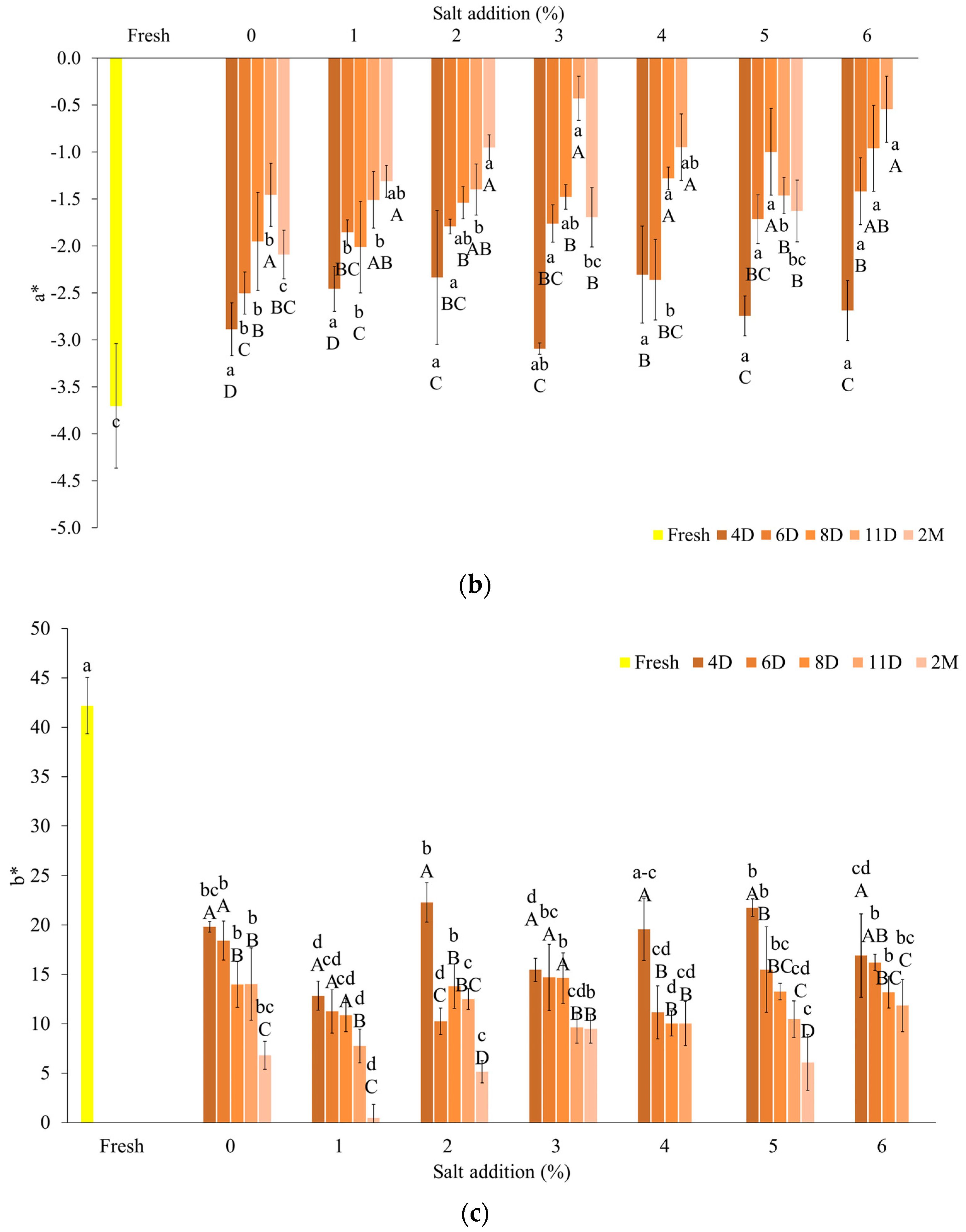
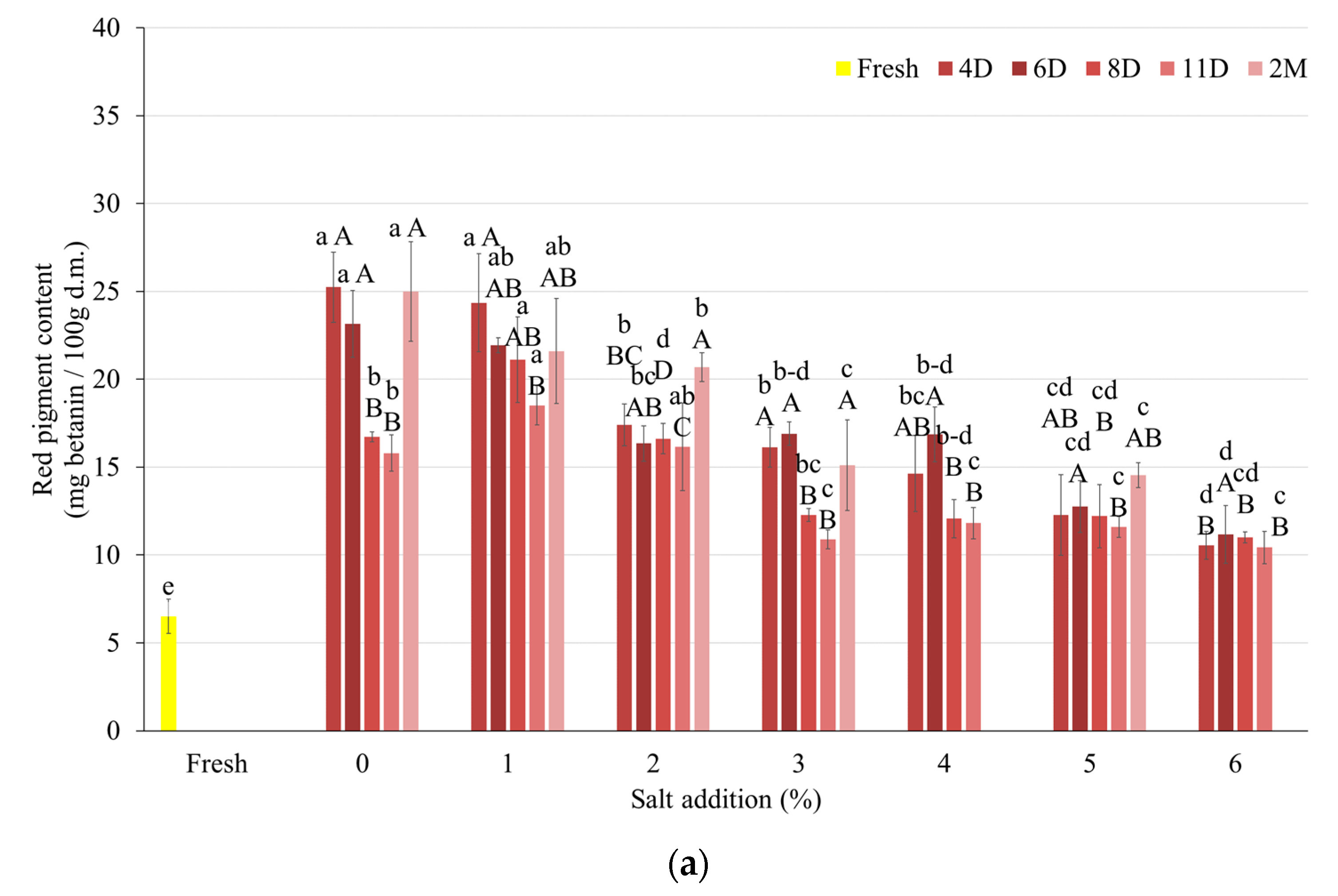
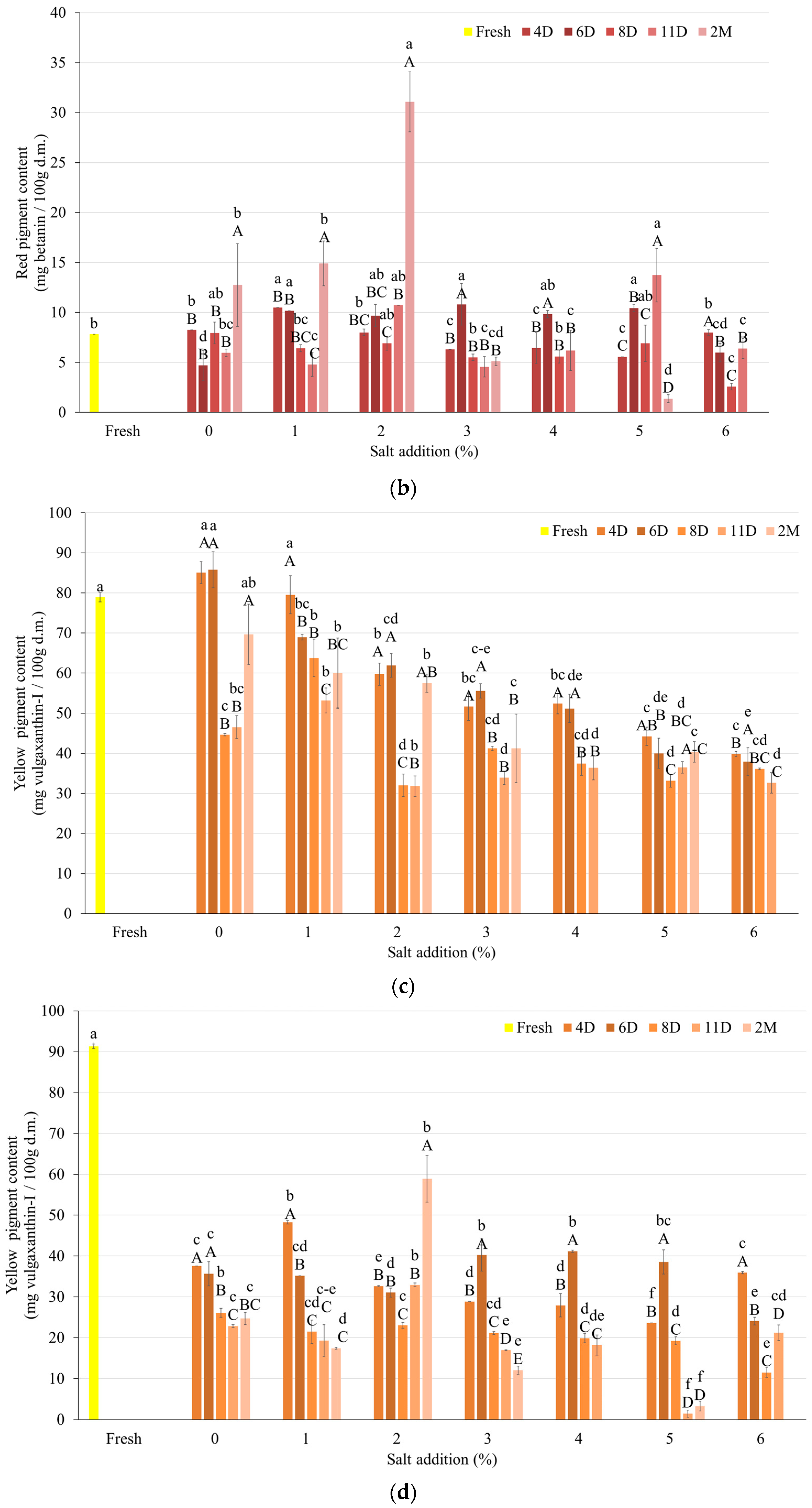


| Salt Content (%) | Day | After Fermentation (log CFU/cm3) | After FD (log CFU/cm3) |
|---|---|---|---|
| Fresh | 0 | 0 | 0 |
| 0 | 4 | 8.87 ± 0.24 BC | 7.36 ± 0.37 I–K |
| 1 | 10.07 ± 0.35 A | 6.45 ± 0.12 LM | |
| 2 | 8.3 ± 0.02 C–F | 6.61 ± 0 L | |
| 3 | 8.68 ± 0.31 B–D | 6.35 ± 0.03 LM | |
| 4 | 8.18 ± 0.1 C–F | 6.46 ± 0.05 LM | |
| 5 | 8.22 ± 0.01 C–F | 6.37 ± 0.05 LM | |
| 6 | 8.03 ± 0.26 D–H | 6.32 ± 0.03 LM | |
| 0 | 8 | 9.2 ± 0.47 B | 7.42 ± 0.38 LM |
| 1 | 8.14 ± 0.05 D–F | 6.51 ± 0.12 LM | |
| 2 | 8.46 ± 0.06 C–F | 6.67 ± 0 L | |
| 3 | 7.83 ± 0.02 F–I | 6.41 ± 0.03 LM | |
| 4 | 8.06 ± 0.02 D–H | 6.51 ± 0.05 LM | |
| 5 | 8.07 ± 0.01 D–H | 6.43 ± 0.05 LM | |
| 6 | 8.11 ± 0.04 D–G | 6.38 ± 0.04 LM | |
| 0 | 11 | 8.5 ± 0.24 C–E | 6.75 ± 0.21 KL |
| 1 | 8.04 ± 0.01 D–H | 6.53 ± 0.01 LM | |
| 2 | 8.45 ± 0 C–F | 6.8 ± 0.09 J–L | |
| 3 | 7.47 ± 0.24 G–I | 5.93 ± 0.23 M | |
| 4 | 7.9 ± 0.03 E–I | 6.5 ± 0.07 LM | |
| 5 | 8.17 ± 0.01 D–F | 6.65 ± 0.06 L | |
| 6 | 7.42 ± 0.16 H–J | 5.94 ± 0.16 M | |
| 0 | 2M | 5.2 ± 0.15 N | 4.65 ± 0.13 N–P |
| 1 | 4.92 ± 0.01 NO | 4.34 ± 0.02 OP | |
| 2 | 5.17 ± 0 N | 4.56 ± 0.03 N–P | |
| 3 | 4.57 ± 0.15 N–P | 4.17 ± 0.16 P | |
| 5 | 5 ± 0.01 NO | 4.44 ± 0.02 OP |
| Salt Addition (%) | Day | Dry Matter after Fermentation [%] | Dry Matter of after FD [%] | pH of Brine (-) | Measured Salt Content (%) | Total Acidity (g Lactic Acid/ 100 g Sample) |
|---|---|---|---|---|---|---|
| Fresh | 0 | 10.92 ± 0.13 A | 96.75 ± 0.15 ABC | 6.28 ± 0.06 A | 0.25 ± 0.01 T | 4.1 ± 0.0 R |
| 0 | 4 | 5.7 ± 0.13 L–O | 95.57 ± 0.45 B–F | 3.48 ± 0.00 BC | 0.21 ± 0.01 UW | 15 ± 0.1 A |
| 1 | 6.14 ± 0.02 J–M | 95.56 ± 1.35 B–F | 3.45 ± 0.01 C | 0.67 ± 0.02 PR | 7.7 ± 0.1 F–K | |
| 2 | 6.41 ± 0.04 I–L | 96 ± 0.98 B–E | 3.26 ± 0.00 H–L | 1.15 ± 0.02 MN | 7.2 ± 0.1 I–L | |
| 3 | 6.18 ± 0.07 J–M | 96.87 ± 0.74 ABC | 3.19 ± 0.00 O–S | 1.84 ± 0.04 I | 5.4 ± 0.2 OP | |
| 4 | 7.1 ± 1.04 GHI | 97.41 ± 0.5 AB | 3.24 ± 0.00 J–M | 1.94 ± 0.03 H | 8.6 ± 0.1 C–G | |
| 5 | 8.11 ± 0.09 D–F | 96.83 ± 0.99 ABC | 3.29 ± 0.01 FGH | 2.53 ± 0.01 D | 5.6 ± 0.2 NOP | |
| 6 | 9.03 ± 0.1 B | 97.1 ± 0.56 AB | 3.28 ± 0.00 G–J | 3.17 ± 0.02 B | 6.4 ± 0.1 L–O | |
| 0 | 6 | 4.48 ± 0.33 P | 95.96 ± 0.61 B–E | 3.51 ± 0.00 B | 0.56 ± 0.05 ST | 7.8 ± 0.0 F–J |
| 1 | 5.92 ± 0.05 K–M | 95.87 ± 0.7 B–E | 3.32 ± 0.01 EF | 0.62 ± 0.00 RS | 8.6 ± 0.1 C–F | |
| 2 | 5.58 ± 0.11 M–O | 96.44 ± 0.16 A–D | 3.26 ± 0.00 H–L | 1.15 ± 0.02 MN | 8 ± 0.1 E–I | |
| 3 | 6.78 ± 0.3 HIJ | 97.42 ± 0.08 AB | 3.22 ± 0.00 L–O | 1.5 ± 0.04 J | 7.4 ± 0.1 H–L | |
| 4 | 6.46 ± 0.08 IJK | 97.38 ± 0.25 AB | 3.21 ± 0.00 M–P | 1.99 ± 0.03 GH | 6.9 ± 0.2 J–M | |
| 5 | 7.47 ± 0.12 FGH | 98.03 ± 0.15 A | 3.25 ± 0.00 I–L | 2.68 ± 0.01 C | 6.9 ± 0.5 J–M | |
| 6 | 8.03 ± 0.43 D–F | 97.3 ± 0.7 AB | 3.27 ± 0.01 G–K | 3.29 ± 0.03 A | 7.4 ± 0 H–L | |
| 0 | 8 | 6.08 ± 0.08 J–M | 92.35 ± 0.53 GH | 3.4 ± 0.00 D | 0.11 ± 0.02 X | 9.3 ± 0.5 BCD |
| 1 | 5.54 ± 0.07 M–O | 94.47 ± 0.35 D–F | 3.27 ± 0.01 G–K | 0.55 ± 0.01 ST | 9.6 ± 0.8 BC | |
| 2 | 7.5 ± 0.12 FG | 95.04 ± 0.76 C–F | 3.23 ± 0.00 K–N | 0.98 ± 0.02 O | 8 ± 0.5 E–I | |
| 3 | 7.46 ± 0.03 FGH | 96.27 ± 0.74 A–E | 3.21 ± 0.00 M–P | 1.27 ± 0.02 KL | 7.5 ± 0.6 H–L | |
| 4 | 8.27 ± 0.12 C–E | 97.06 ± 0.35 AB | 3.19 ± 0.00 O–S | 1.56 ± 0.08 J | 7.6 ± 0.4 F–K | |
| 5 | 8.62 ± 0.05 B–D | 96.81 ± 0.7 ABC | 3.24 ± 0.00 J–M | 2.33 ± 0.04 E | 7.0 ± 0.2 I–M | |
| 6 | 8.89 ± 0.05 BC | 96.82 ± 0.99 ABC | 3.3 ± 0.00 FG | 2.53 ± 0.04 D | 4.6 ± 0.4 PR | |
| 0 | 11 | 5.29 ± 0.09 N–O | 92.09 ± 0.8 GH | 3.4 ± 0.00 D | 0.14 ± 0.01 WX | 8.4 ± 1.0 D–H |
| 1 | 5.88 ± 0.05 K–M | 94.42 ± 0.71 EF | 3.25 ± 0.00 H–L | 0.49 ± 0.01 S | 7.7 ± 0.2 F–K | |
| 2 | 6.66 ± 0.05 IJ | 94.5 ± 0.54 D–F | 3.21 ± 0.00 M–P | 0.72 ± 0.01 P | 7.8 ± 0.1 F–J | |
| 3 | 7.69 ± 0.03 EFG | 97.02 ± 0.3 ABC | 3.19 ± 0.00 OPR | 1.08 ± 0.04 N | 7.1 ± 0.1 I–L | |
| 4 | 8.02 ± 0.06 D–F | 97.02 ± 0.2 ABC | 3.2 ± 0.01 NOP | 1.35 ± 0.02 K | 7.4 ± 0.2 H–L | |
| 5 | 8.11 ± 0.11 D–F | 97.38 ± 0.25 AB | 3.26 ± 0.00 G–K | 2.1 ± 0.02 F | 6.6 ± 0.2 K–N | |
| 6 | 8.89 ± 0.09 BC | 96.07 ± 0.72 A–E | 3.28 ± 0.00 G–J | 2.24 ± 0.04 E | 5.9 ± 0.2 MNO | |
| 0 | 2M | 5.12 ± 0.04 OP | 90.88 ± 0.3 H | 3.34 ± 0.00 D | 0.17 ± 0.02 UWX | 9 ± 0.1 B–E |
| 1 | 5.88 ± 0.04 K–M | 90.42 ± 0.35 H | 3.18 ± 0.00 PRS | 0.47 ± 0.02 S | 9.3 ± 0.5 BCD | |
| 2 | 6.52 ± 0.13 IJK | 92.19 ± 0.59 GH | 3.12 ± 0.01 T | 1.22 ± 0 LM | 10.1 ± 0.3 B | |
| 3 | 7.5 ± 0.13 FG | 93.66 ± 0.4 FG | 3.16 ± 0.01 RS | 1.29 ± 0.01 KL | 8.1 ± 0.3 E–I | |
| 5 | 8.71 ± 0.06 B–D | 94.67 ± 0.39 DEF | 3.15 ± 0.01 ST | 2.04 ± 0.03 FG | 7.5 ± 0.6 F–L |
| Salt Content (%) | Day | Step 1 | Step 2 | Step 3 | Step 4 | Sum (%) | Decomposition Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp Range (°C) | Mass Loss (%) | Temp Range (°C) | Mass Loss (%) | Temp Range (°C) | Mass Loss (%) | Temp. Range (°C) | Mass Loss (%) | 1 | 2 | 3 | |||||
| Fresh | 0 | 30–110 | 1.98 | 110–230 | 33.73 | 230–380 | 24.67 | 380–600 | 8.03 | 68.4 | - | 162.81 | 200.75 | - | 303.39 |
| 0 | 4 | 5.50 | 34.95 | 25.74 | 12.82 | 79.0 | - | 153.49 | 197.13 | - | 303.97 | ||||
| 1 | 3.46 | 27.55 | 25.57 | 7.17 | 63.8 | - | 158.41 | 194.75 | - | 298.14 | |||||
| 2 | 2.90 | 23.28 | 24.53 | 7.06 | 57.8 | - | 159.51 | 191.82 | 252.48 | 302.20 | |||||
| 3 | 2.41 | 16.71 | 26.93 | 6.02 | 52.1 | - | 156.29 | 193.28 | 253.53 | 299.53 | |||||
| 4 | 1.92 | 18.74 | 21.77 | 5.73 | 48.2 | - | 158.88 | 197.09 | 253.96 | 300.81 | |||||
| 5 | 1.81 | 16.33 | 21.03 | 5.25 | 44.4 | - | 160.68 | 197.46 | 251.79 | 298.49 | |||||
| 6 | 1.49 | 16.10 | 19.84 | 4.94 | 42.4 | - | 159.07 | 198.32 | 255.82 | 299.23 | |||||
| 0 | 6 | 30–110 | 1.02 | 110–230 | 25.36 | 230–380 | 35.16 | 380–600 | 7.11 | 68.7 | - | 156.07 | 198.49 | 251.75 | 299.71 |
| 1 | 1.22 | 28.91 | 24.35 | 7.70 | 62.2 | - | 155.72 | 188.39 | 253.23 | 305.15 | |||||
| 2 | 0.50 | 28.19 | 22.71 | 6.38 | 57.8 | - | 154.26 | 196.75 | - | 300.07 | |||||
| 3 | 0.64 | 24.64 | 22.76 | 6.31 | 54.4 | - | 154.55 | 193.60 | 253.63 | 301.38 | |||||
| 4 | 1.24 | 21.35 | 20.98 | 5.81 | 49.4 | - | 154.85 | 195.12 | 253.11 | 301.09 | |||||
| 5 | 0.49 | 21.57 | 20.29 | 5.62 | 48.0 | - | 156.06 | 194.20 | 253.28 | 298.91 | |||||
| 6 | 0.52 | 20.06 | 19.63 | 4.99 | 45.2 | - | 156.38 | 194.32 | 253.00 | 298.63 | |||||
| 0 | 8 | 30–110 | 1.15 | 110–230 | 26.97 | 230–380 | 34.93 | 380–600 | 7.33 | 70.4 | - | 154.50 | 198.09 | 250.45 | 300.89 |
| 1 | 1.35 | 28.48 | 24.95 | 7.01 | 61.8 | - | 154.85 | - | 249.32 | 297.31 | |||||
| 2 | 1.28 | 25.41 | 24.53 | 7.39 | 58.6 | - | 154.51 | 189.31 | 253.17 | 306.48 | |||||
| 3 | 1.13 | 24.13 | 22.27 | 6.08 | 53.6 | - | 154.88 | 193.52 | 252.92 | 300.44 | |||||
| 4 | 0.86 | 23.68 | 20.55 | 5.93 | 51.0 | - | 153.91 | 194.35 | 252.75 | 300.45 | |||||
| 5 | 0.81 | 19.07 | 21.43 | 5.32 | 46.6 | - | 156.74 | 193.85 | 252.69 | 298.28 | |||||
| 6 | 0.62 | 18.50 | 19.30 | 5.09 | 43.5 | - | 156.74 | 197.54 | 252.77 | 301.67 | |||||
| 0 | 11 | 30–110 | 1.07 | 110–230 | 32.70 | 230–380 | 29.64 | 380–600 | 6.95 | 70.4 | - | 153.26 | 199.12 | 248.21 | 297.98 |
| 1 | 1.24 | 29.22 | 24.12 | 6.96 | 61.5 | - | 154.26 | 190.34 | 252.06 | 298.81 | |||||
| 2 | 1.49 | 28.82 | 21.58 | 6.76 | 58.7 | - | 153.76 | 189.48 | 252.86 | 303.90 | |||||
| 3 | 1.25 | 25.70 | 22.04 | 6.37 | 55.4 | - | 154.43 | 189.30 | 252.78 | 302.71 | |||||
| 4 | 1.04 | 24.44 | 20.10 | 5.71 | 51.3 | - | 153.95 | 195.12 | 252.87 | 303.60 | |||||
| 5 | 0.70 | 20.23 | 19.97 | 3.78 | 44.7 | - | 154.77 | 198.66 | 248.91 | 295.55 | |||||
| 6 | 0.63 | 20.81 | 18.98 | 5.18 | 45.6 | - | 151.85 | 197.32 | 252.88 | 302.28 | |||||
| 0 | 2M | 30–90 | 4.51 | 90–380 | 60.15 | 380–360 | 10.54 | - | - | 75.20 | - | 149.59 | 204.81 | 292.42 | - |
| 1 | 2.49 | 52.01 | 7.65 | - | 62.2 | - | 152.04 | - | 295 | - | |||||
| 2 | 2.79 | 43.89 | 8.01 | - | 54.7 | - | 161.01 | - | 301.77 | - | |||||
| 3 | 1.57 | 45.00 | 6.97 | - | 53.5 | - | 153.79 | 190.69 | 298.77 | - | |||||
| 5 | 1.24 | 40.90 | 6.70 | - | 48.8 | - | - | 191.32 | 300.75 | - | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, A.; Janiszewska-Turak, E. Influence of the Salt Addition during the Fermentation Process on the Physical and Chemical Properties of Dried Yellow Beetroot. Appl. Sci. 2024, 14, 524. https://doi.org/10.3390/app14020524
Wierzbicka A, Janiszewska-Turak E. Influence of the Salt Addition during the Fermentation Process on the Physical and Chemical Properties of Dried Yellow Beetroot. Applied Sciences. 2024; 14(2):524. https://doi.org/10.3390/app14020524
Chicago/Turabian StyleWierzbicka, Anna, and Emilia Janiszewska-Turak. 2024. "Influence of the Salt Addition during the Fermentation Process on the Physical and Chemical Properties of Dried Yellow Beetroot" Applied Sciences 14, no. 2: 524. https://doi.org/10.3390/app14020524
APA StyleWierzbicka, A., & Janiszewska-Turak, E. (2024). Influence of the Salt Addition during the Fermentation Process on the Physical and Chemical Properties of Dried Yellow Beetroot. Applied Sciences, 14(2), 524. https://doi.org/10.3390/app14020524








