Developmental Toxicity of C10 Massoia Lactone, the Main Constituent of Cryptocarya massoia, on Zebrafish (Danio rerio) Embryos
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Maintenance
2.3. Acute Toxicity of C10 Massoia Lactone to Zebrafish Embryos
2.4. Assessment of the Developmental Defects in Heart
2.5. Analysis Gene Expression Using RT-qPCR
2.6. Statistical Analysis
3. Results
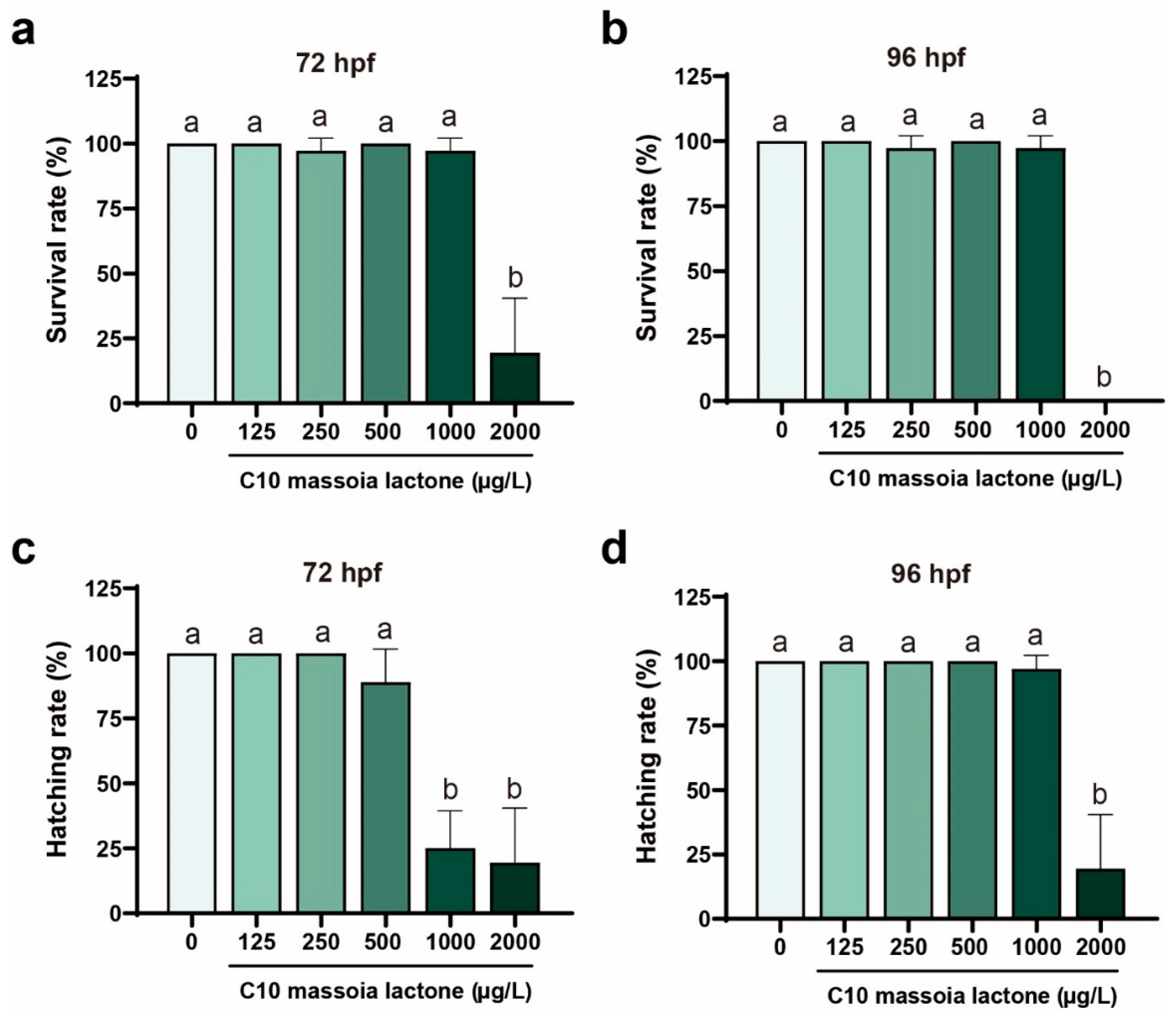
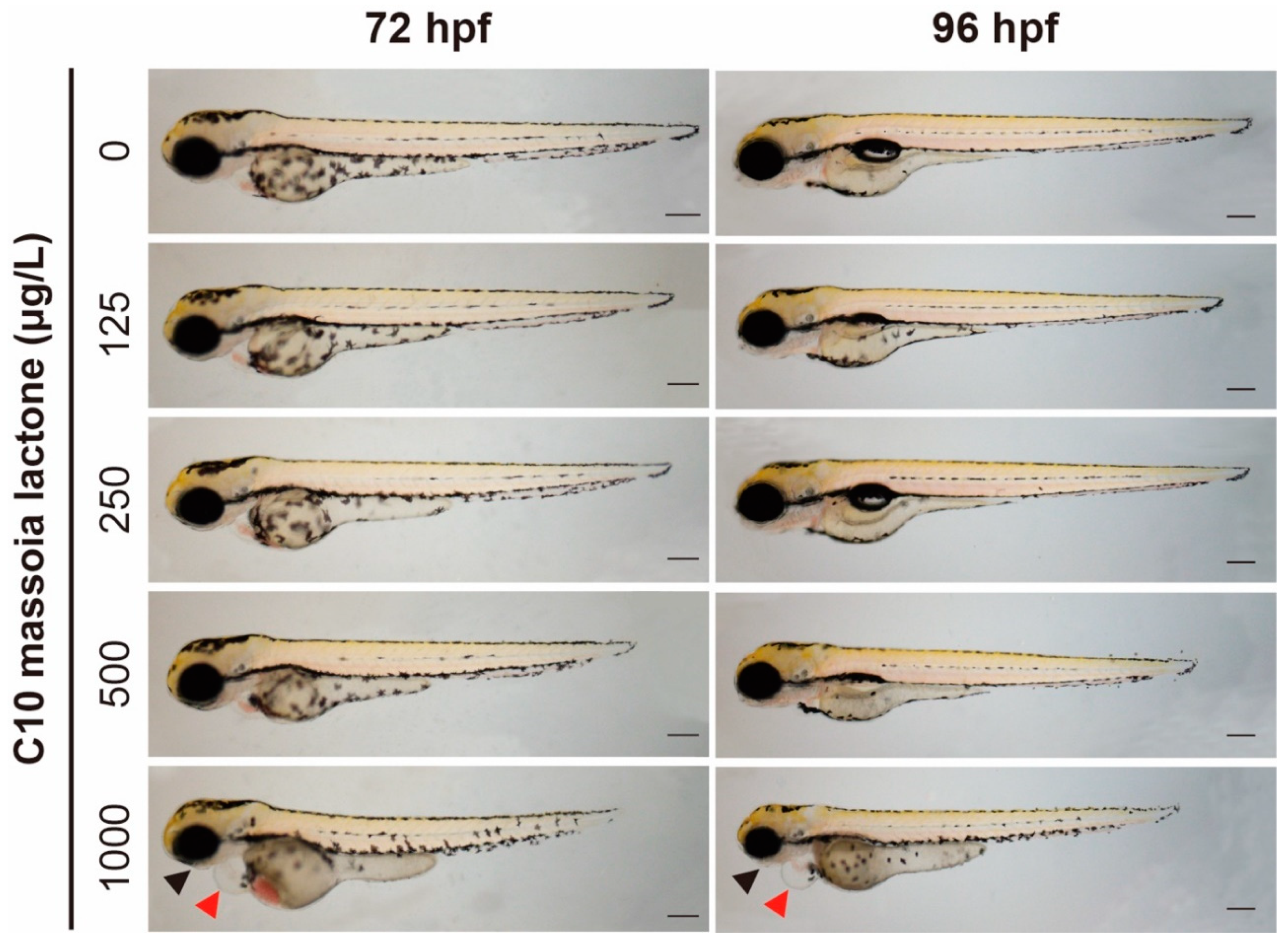
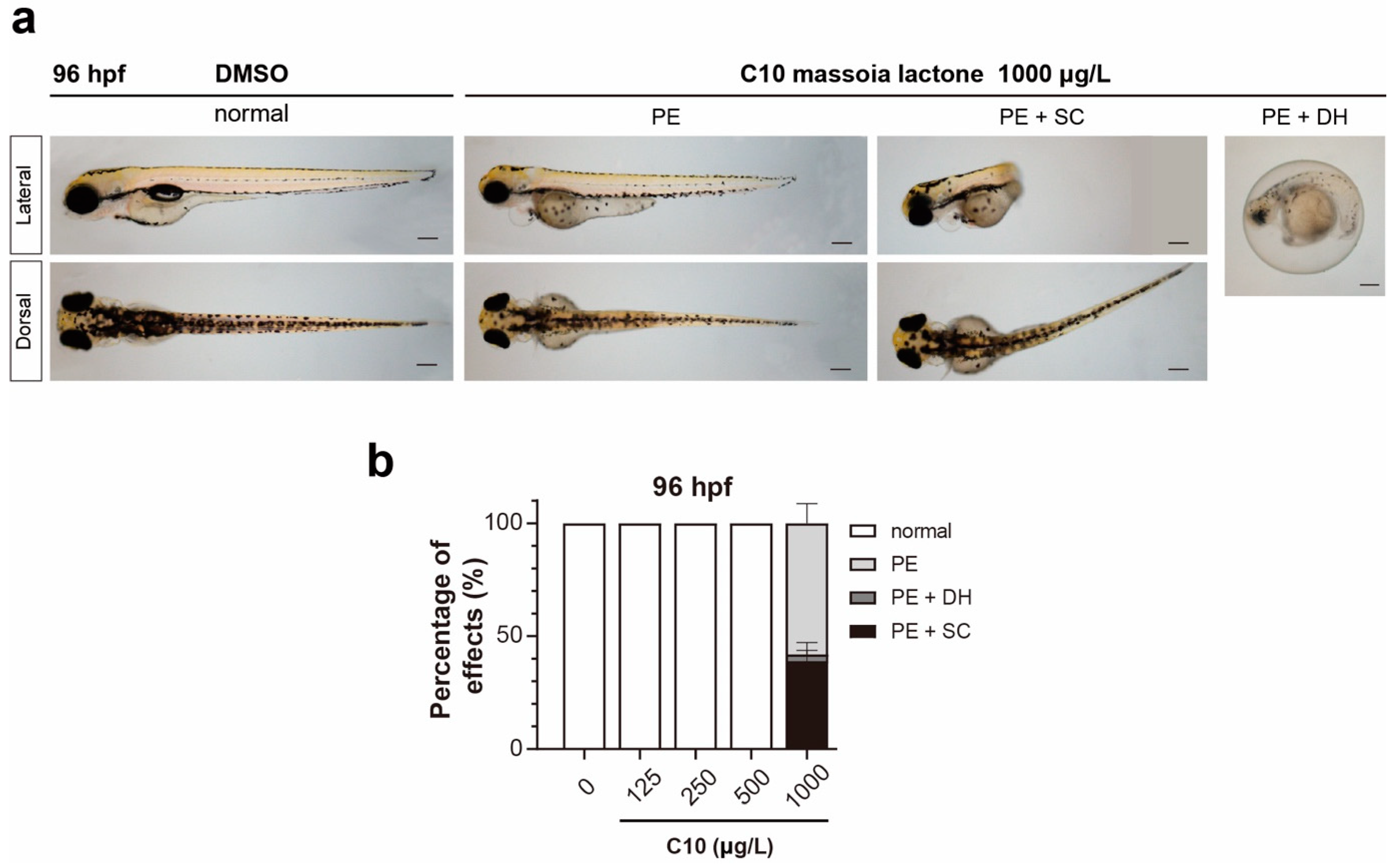
3.1. Acute Toxicity of C10 on Zebrafish Embryos and Phenotypic Alterations
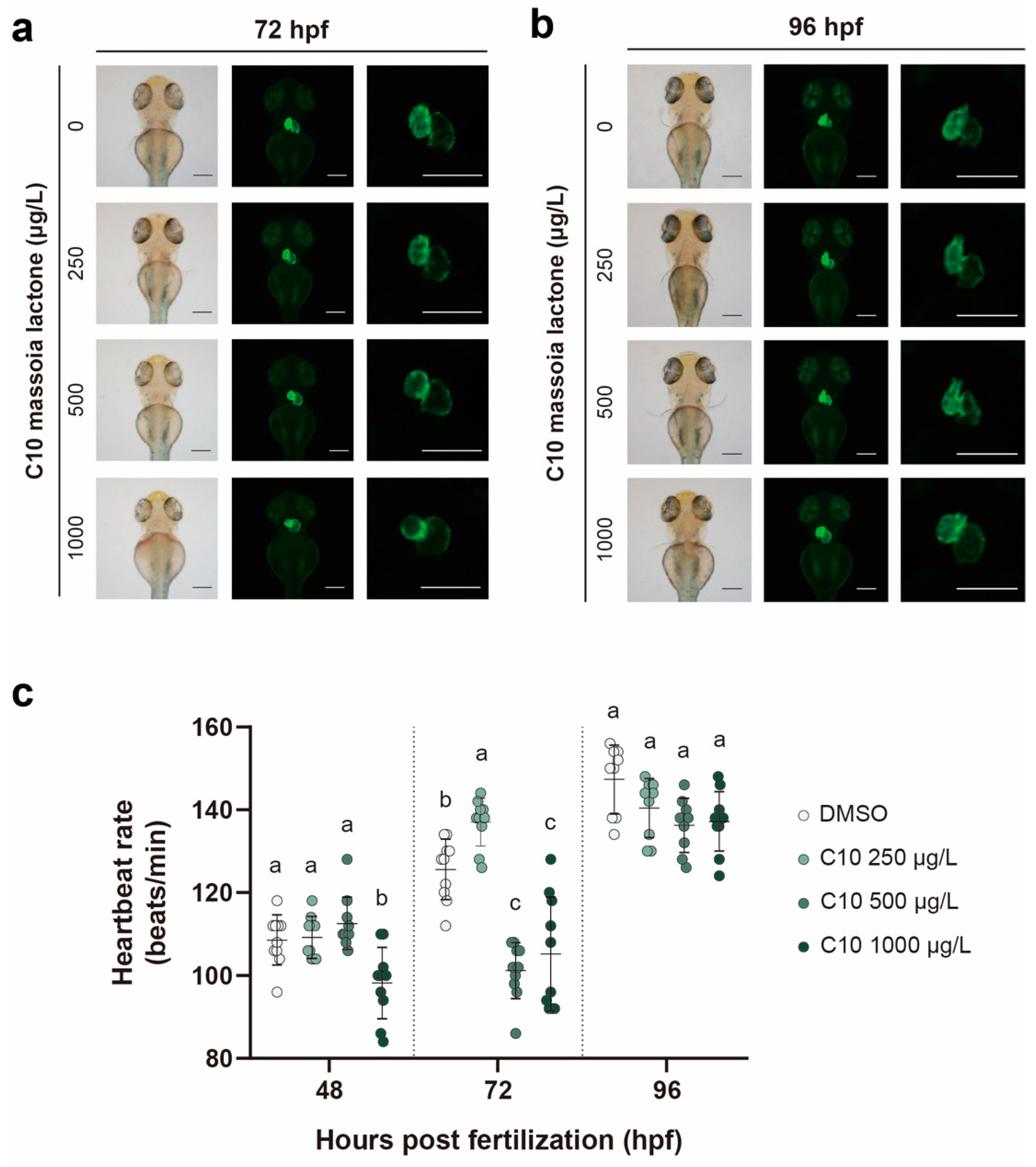
3.2. Heart Development with the Measurement of the Heartbeat Rate
3.3. Heart Malformation and the Relative mRNA Expression
4. Discussion
4.1. Lethal Effect and Inhibition of the Hatching Rates by C10 toward Zebrafish Embryos
4.2. Morphological Changes in C10-Treated Zebrafish Embryos
4.3. Heart Development in C10-Treated Zebrafish Embryos
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosol, T.J.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS Assessment of Natural Flavor Complexes: Lemongrass Oil, Chamomile Oils, Citronella Oil and Related Flavoring Ingredients. Food Chem. Toxicol. 2023, 175, 113697. [Google Scholar] [CrossRef] [PubMed]
- Kroes, R.; Galli, C.; Munro, I.; Schilter, B.; Tran, L.A.; Walker, R.; Würtzen, G. Threshold of Toxicological Concern for Chemical Substances Present in the Diet: A Practical Tool for Assessing the Need for Toxicity Testing. Food Chem. Toxicol. 2000, 38, 255–312. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Davidsen, J.M.; Harman, C.L.; Taylor, S.V. Updated Procedure for the Safety Evaluation of Natural Flavor Complexes Used as Ingredients in Food. Food Chem. Toxicol. 2018, 113, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hertiani, T.; Pratiwi, S.U.T.; Yuswanto, A.; Permanasari, P. Potency of Massoia Bark in Combating Immunosuppressed-Related Infection. Pharmacogn. Mag. 2016, 12, S363. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, S.J.; Kim, K.; Kim, T.O.; Lee, S.E. Antifungal and Antiaflatoxigenic Activities of Massoia Essential Oil and C10 Massoia Lactone against Aflatoxin-Producing Aspergillus Flavus. Toxins 2023, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, Z.C.; Chi, Z.; Wang, Z.; Liu, G.L.; Li, X.F.; Hu, Z.; Chi, Z.M. Massoia Lactone Displays Strong Antifungal Property Against Many Crop Pathogens and Its Potential Application. Microb. Ecol. 2022, 84, 376–390. [Google Scholar] [CrossRef]
- Seo, S.M.; Lee, J.W.; Shin, J.; Tak, J.H.; Hyun, J.; Park, I.K. Development of Cellulose Nanocrystal-Stabilized Pickering Emulsions of Massoia and Nutmeg Essential Oils for the Control of Aedes Albopictus. Sci. Rep. 2021, 11, 12038. [Google Scholar] [CrossRef] [PubMed]
- Hoyberghs, J.; Bars, C.; Pype, C.; Foubert, K.; Ayuso Hernando, M.; Van Ginneken, C.; Ball, J.; Van Cruchten, S. Refinement of the Zebrafish Embryo Developmental Toxicity Assay. MethodsX 2020, 7, 101087. [Google Scholar] [CrossRef]
- Porretti, M.; Arrigo, F.; Di Bella, G.; Faggio, C. Impact of Pharmaceutical Products on Zebrafish: An Effective Tool to Assess Aquatic Pollution. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 261, 109439. [Google Scholar] [CrossRef]
- Song, Y.S.; Dai, M.Z.; Zhu, C.X.; Huang, Y.F.; Liu, J.; Zhang, C.D.; Xie, F.; Peng, Y.; Zhang, Y.; Li, C.Q.; et al. Validation, Optimization, and Application of the Zebrafish Developmental Toxicity Assay for Pharmaceuticals Under the ICH S5(R3) Guideline. Front. Cell Dev. Biol. 2021, 9, 721130. [Google Scholar] [CrossRef]
- Wang, R.; Liu, K.; Zhang, Y.; Chen, X.; Wang, X. Evaluation of the Developmental Toxicity Induced by E804 in Zebrafish Embryos. Front. Pharmacol. 2020, 11, 493657. [Google Scholar] [CrossRef] [PubMed]
- Hema, T.; Poopal, R.K.; Ramesh, M.; Ren, Z.; Li, B. Developmental Toxicity of the Emerging Contaminant Cyclophosphamide and the Integrated Biomarker Response (IBRv2) in Zebrafish. Environ. Sci. Process Impacts 2023, 25, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kim, C.; Kim, K.; Lee, S.E. Piperlongumine Treatment Impacts Heart and Liver Development and Causes Developmental Delay in Zebrafish (Danio rerio) Embryos. Ecotoxicol. Environ. Saf. 2023, 258, 114995. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Biswal, B.K. Piperlongumine, a Potent Anticancer Phytotherapeutic: Perspectives on Contemporary Status and Future Possibilities as an Anticancer Agent. Pharmacol. Res. 2020, 156, 104772. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Kim, C.; Choe, H.; Park, J.; Kim, G.; Kim, K.; Jeon, H.J.; Moon, J.K.; Kim, M.J.; Lee, S.E. Molecular Mechanisms of Developmental Toxicities of Azoxystrobin and Pyraclostrobin toward Zebrafish (Danio rerio) Embryos: Visualization of Abnormal Development Using Two Transgenic Lines. Environ. Pollut. 2021, 270, 116087. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.R.; Andrade-Porto, S.M.; Oliveira, M.I.B.; Brandão, F.R.; Matos, L.V.; Velásquez, J.G.R.; Farias, C.F.S.; Carpio, K.C.R.; Chaves, F.C.M.; Chagas, E.C. Acute Toxicity of Essential Oils of Aloysia triphylla (L’Hér.) Britton, Lippia Gracilis Schauer, and Piper aduncum L. in Colossoma macropomum (Cuvier, 1818). Braz. J. Biol. 2023, 83, 272853. [Google Scholar] [CrossRef]
- Tabarraei, H.; Hassan, J.; Mosavi, S.S. Determination of LD50 of Some Essential Oils and Histopathological Changes in Short-Term Exposure to One of Them in Rainbow Trout (Oncorhynchus mykiss). Toxicol. Res. Appl. 2019, 3, 239784731882071. [Google Scholar] [CrossRef]
- Bullangpoti, V.; Mujchariyakul, W.; Laksanavilat, N.; Junhirun, P. Acute Toxicity of Essential Oil Compounds (thymol and 1,8-cineole) to Insectivorous Guppy, Poecilia reticulata Peters, 1859. Agric. Nat. Resour. 2018, 52, 190–194. [Google Scholar] [CrossRef]
- Mektrirat, R.; Yano, T.; Okonogi, S.; Katip, W.; Pikulkaew, S. Phytochemical and Safety Evaluations of Volatile Terpenoids from Zingiber cassumunar Roxb. on Mature Carp Peripheral Blood Mononuclear Cells and Embryonic Zebrafish. Molecules 2020, 25, 613. [Google Scholar] [CrossRef]
- Duringer, J.M.; Swan, L.R.; Walker, D.B.; Craig, A.M. Acute Aquatic Toxicity of Western Juniper (Juniperus Occidentalis) Foliage and Port Orford Cedar (Chamaecyparis lawsoniana) Heartwood Oils. Environ. Monit. Assess 2010, 170, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, S.E. Developmental Toxicity of Fipronil and Its Two Metabolites towards Zebrafish (Danio rerio) Embryos. Environ. Pollut. 2023, 333, 122119. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac Natriuretic Peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Salo, P.P.; Havulinna, A.S.; Tukiainen, T.; Raitakari, O.; Lehtimäki, T.; Kähönen, M.; Kettunen, J.; Männikkö, M.; Eriksson, J.G.; Jula, A.; et al. Genome-Wide Association Study Implicates Atrial Natriuretic Peptide Rather Than B-Type Natriuretic Peptide in the Regulation of Blood Pressure in the General Population. Circ. Cardiovasc. Genet. 2017, 10, e001713. [Google Scholar] [CrossRef]
- Arrokhman, S.; Luo, Y.H.; Lin, P. Additive Cardiotoxicity of a Bisphenol Mixture in Zebrafish Embryos: The Involvement of Calcium Channel and Pump. Ecotoxicol. Environ. Saf. 2023, 263, 115225. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Kim, C.; Kim, T.-O.; Lee, S.-E. Developmental Toxicity of C10 Massoia Lactone, the Main Constituent of Cryptocarya massoia, on Zebrafish (Danio rerio) Embryos. Appl. Sci. 2024, 14, 538. https://doi.org/10.3390/app14020538
Lee Y, Kim C, Kim T-O, Lee S-E. Developmental Toxicity of C10 Massoia Lactone, the Main Constituent of Cryptocarya massoia, on Zebrafish (Danio rerio) Embryos. Applied Sciences. 2024; 14(2):538. https://doi.org/10.3390/app14020538
Chicago/Turabian StyleLee, Yubin, Chaeeun Kim, Tae-Oh Kim, and Sung-Eun Lee. 2024. "Developmental Toxicity of C10 Massoia Lactone, the Main Constituent of Cryptocarya massoia, on Zebrafish (Danio rerio) Embryos" Applied Sciences 14, no. 2: 538. https://doi.org/10.3390/app14020538



