Yeast-Based Magnetic Biocomposite for Efficient Sorption of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Magnetic Biocomposite
2.2.1. Pyrolysis and Biomass Activation
2.2.2. Obtaining Magnetic Active Carbon
2.2.3. Immobilization of Yarrowia lipolytica on M-L-AC
2.3. Physicochemical Characteristics
2.4. Equilibrium Studies
2.4.1. Langmuir Isotherm
2.4.2. Freundlich Isotherm
2.4.3. Temkin Isotherm
2.4.4. Dubinin–Radushkevich Isotherm
2.5. Kinetic Studies
2.5.1. Pseudo-First-Order Rate Model
2.5.2. Pseudo-Second-Order Rate Model
2.5.3. Elovich Model
2.5.4. Weber–Morris Model
2.6. Thermodynamic Studies
2.7. Desorption Studies
3. Result and Discussion
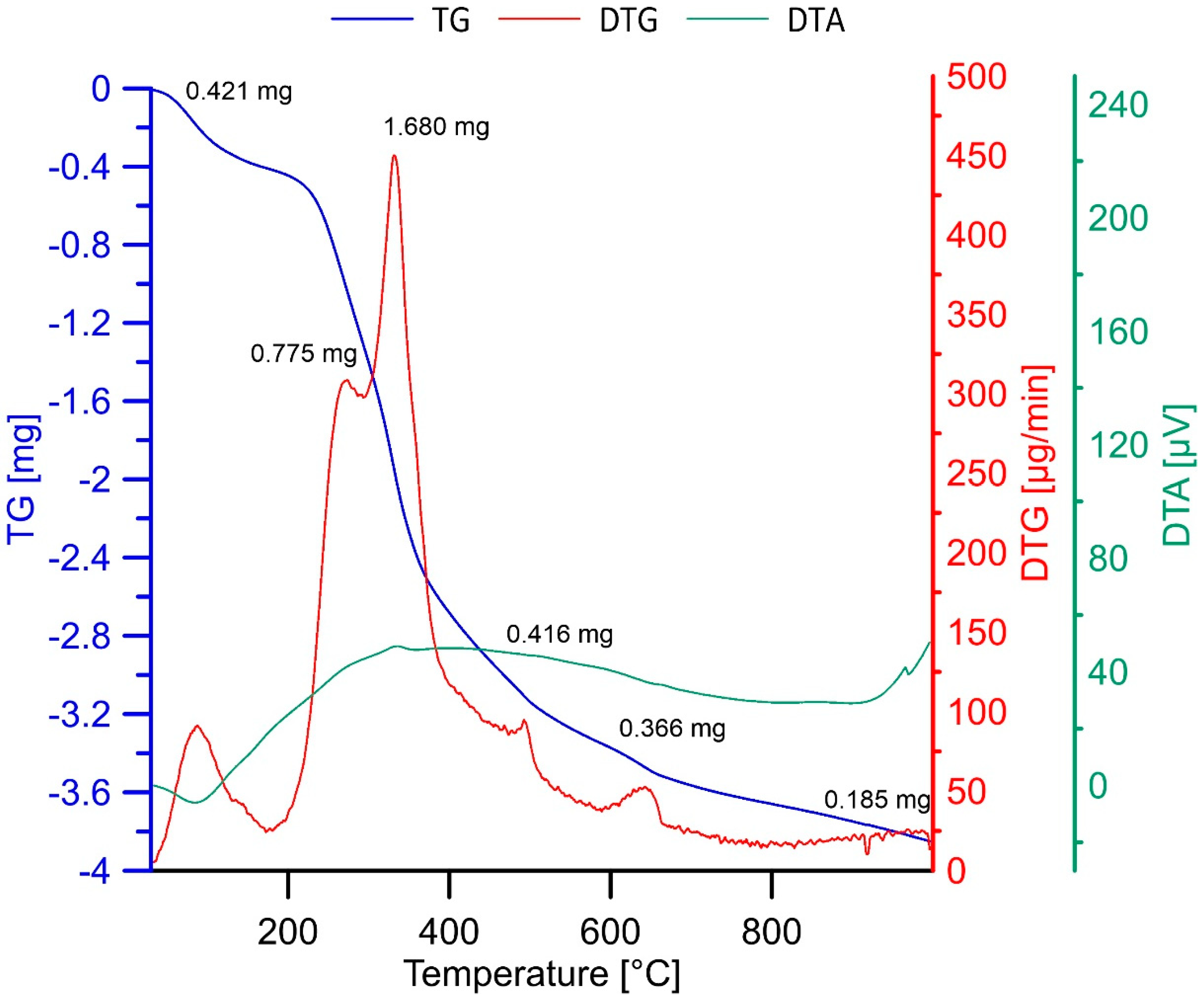
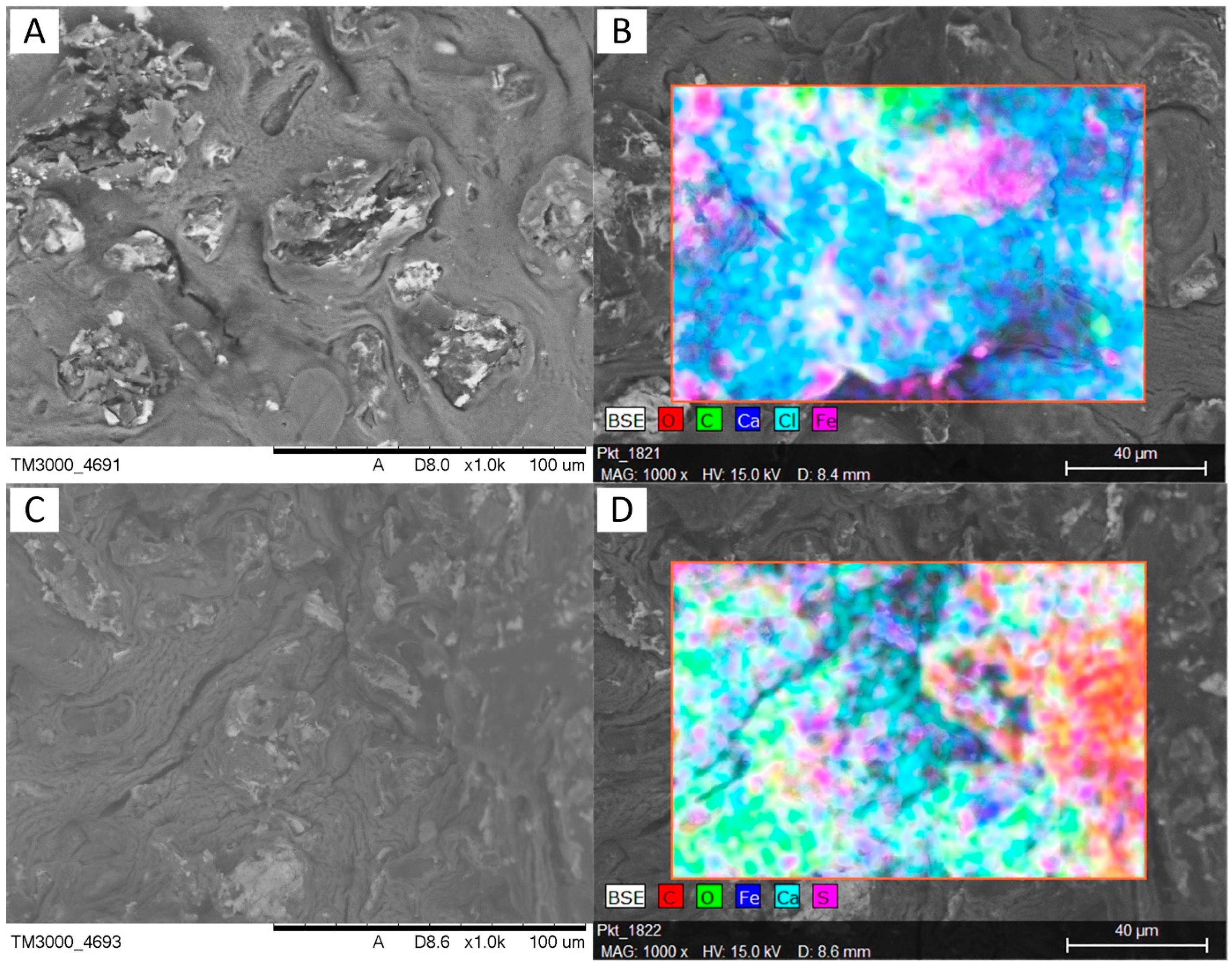
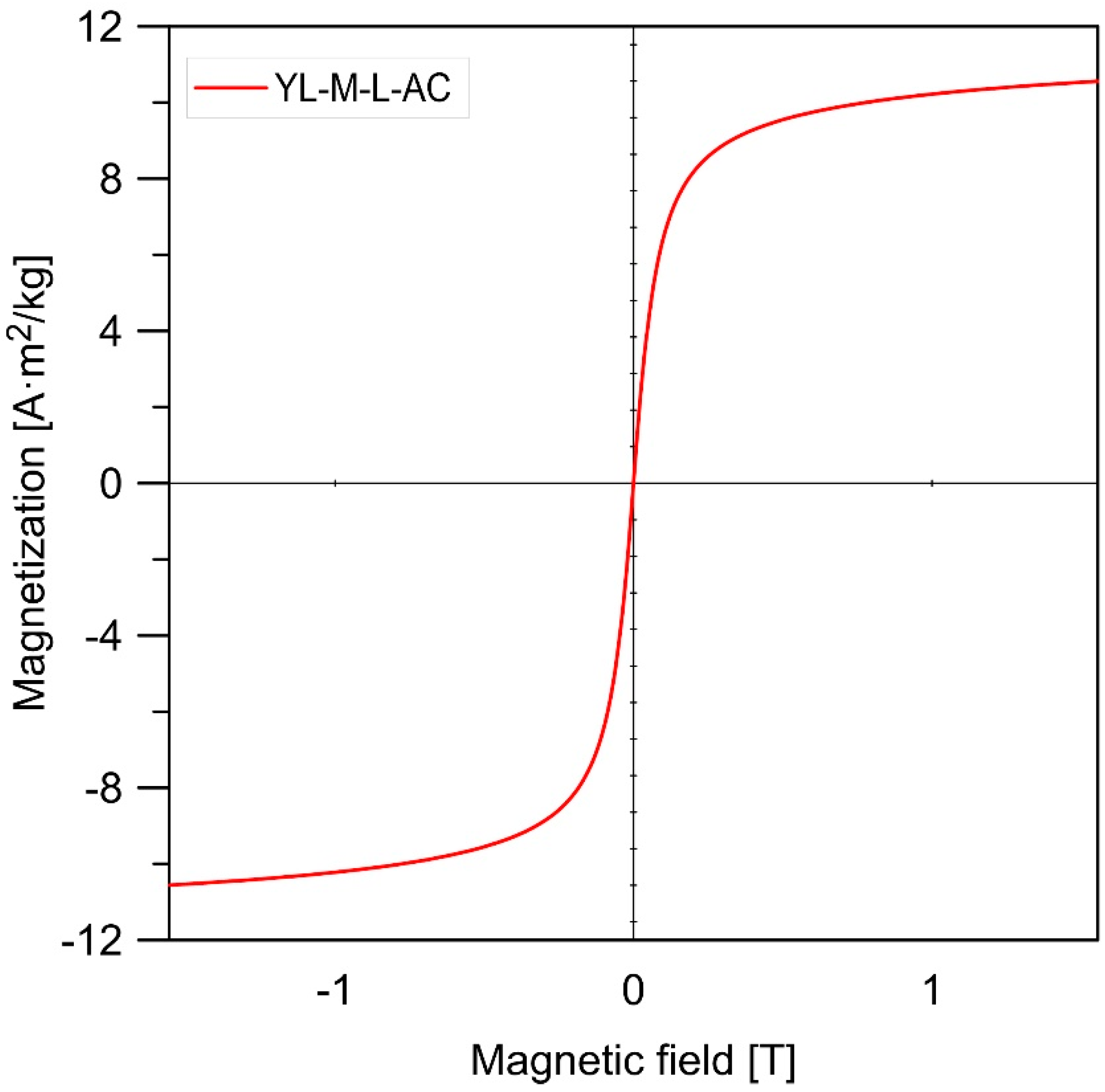
3.1. Characterizations of Materials
3.2. Methylene Blue Adsorption
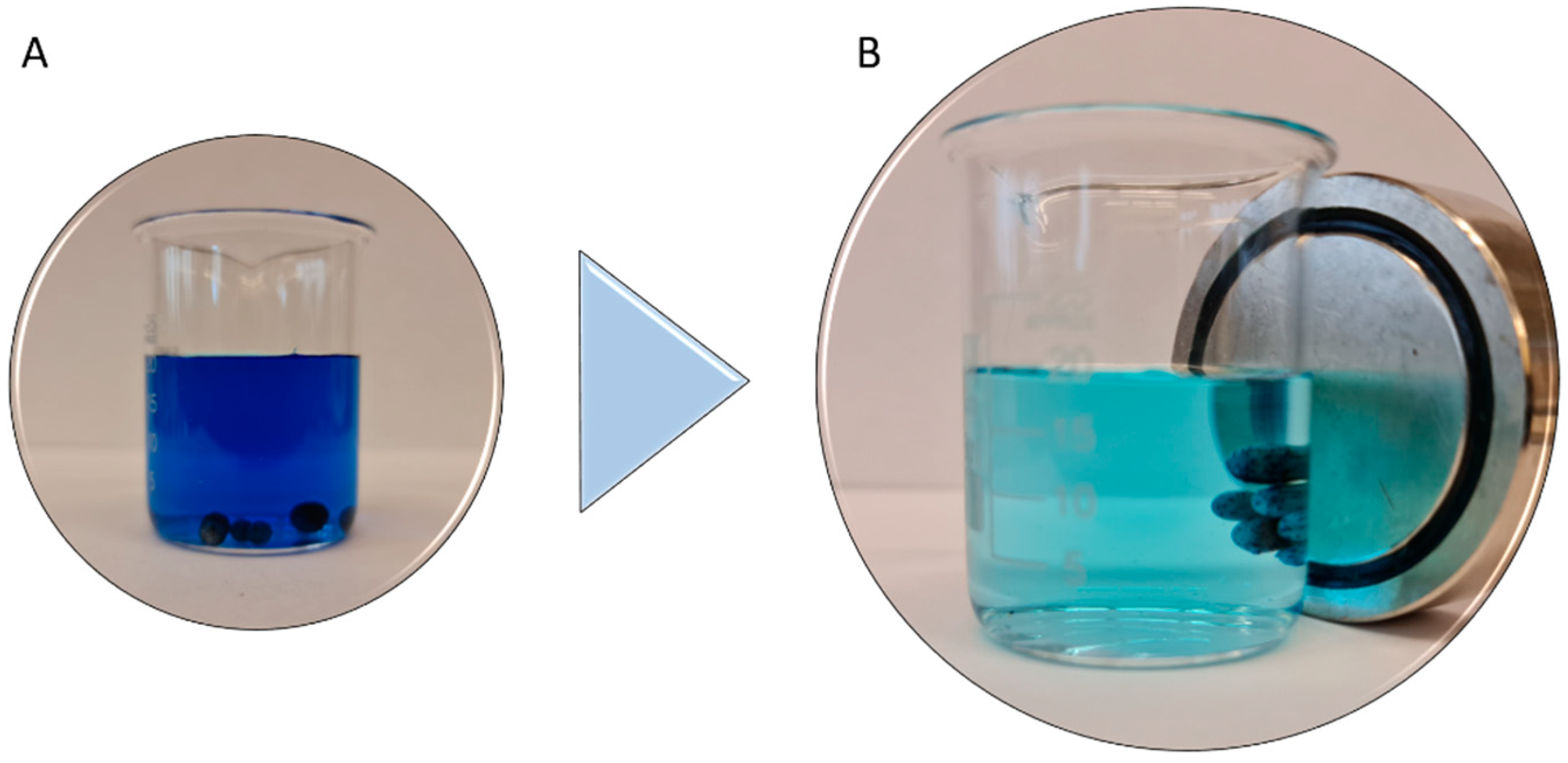
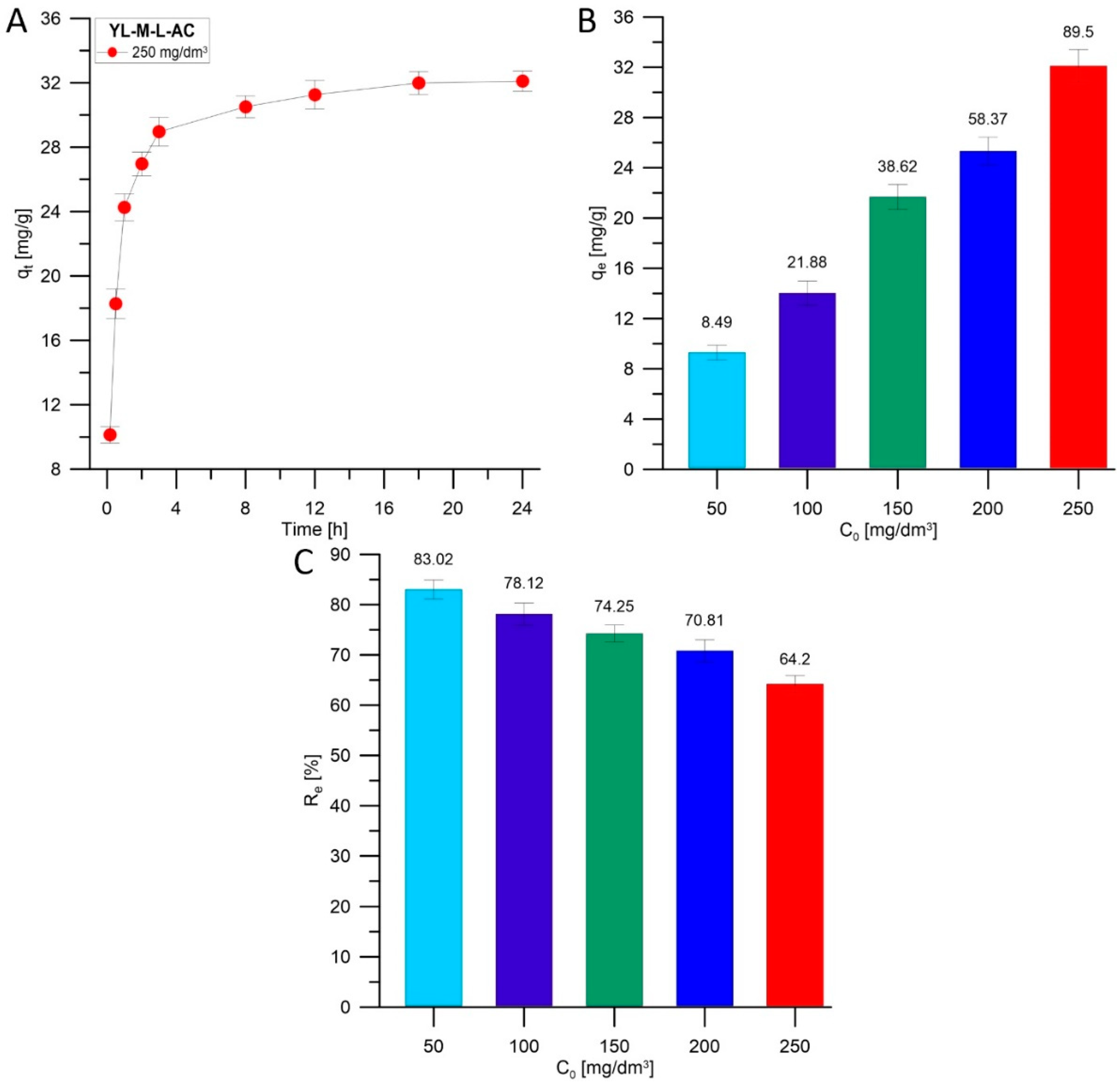
3.2.1. Adsorption of MB onto YL-M-L-AC
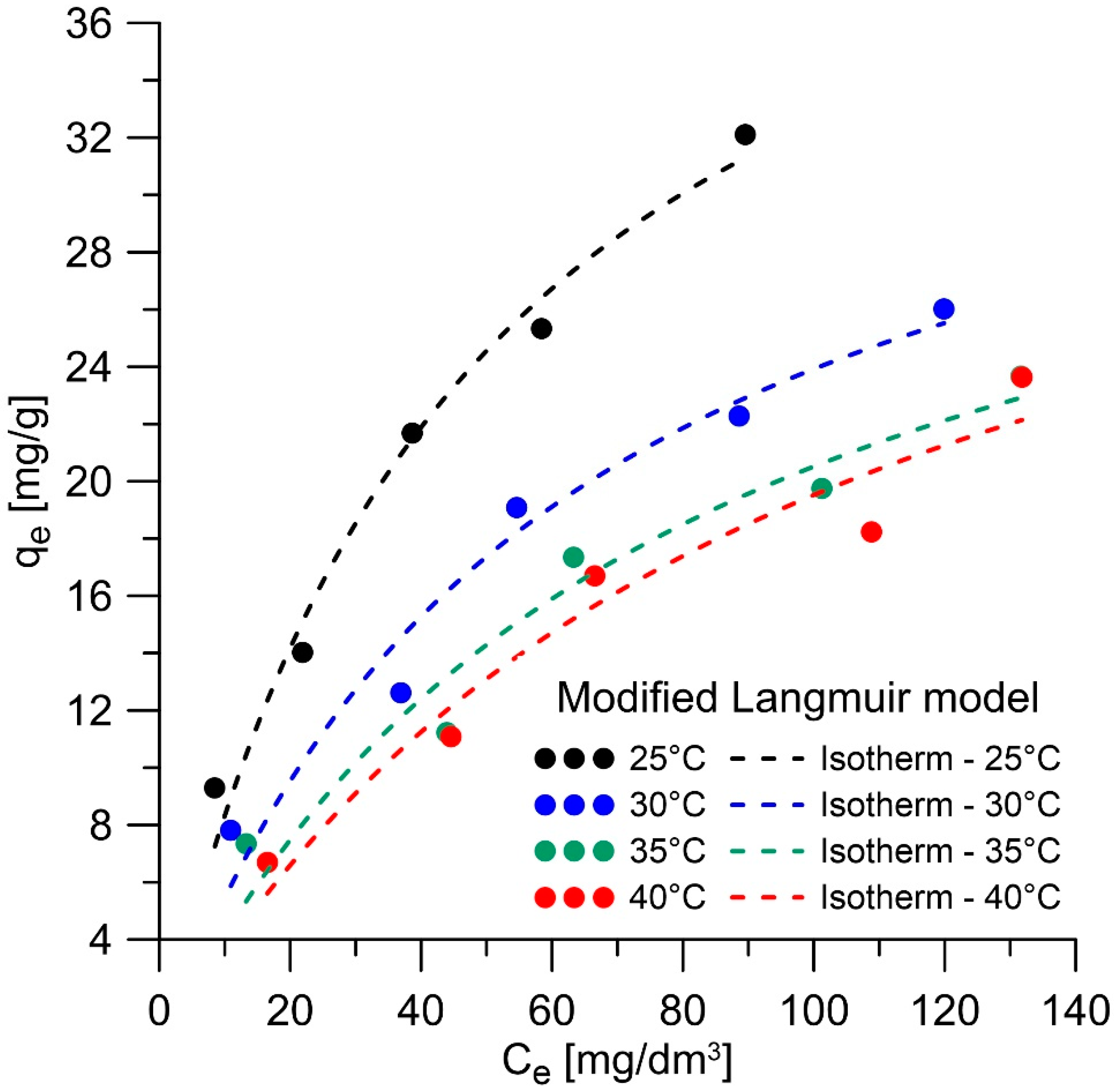
3.2.2. Equilibrium Studies
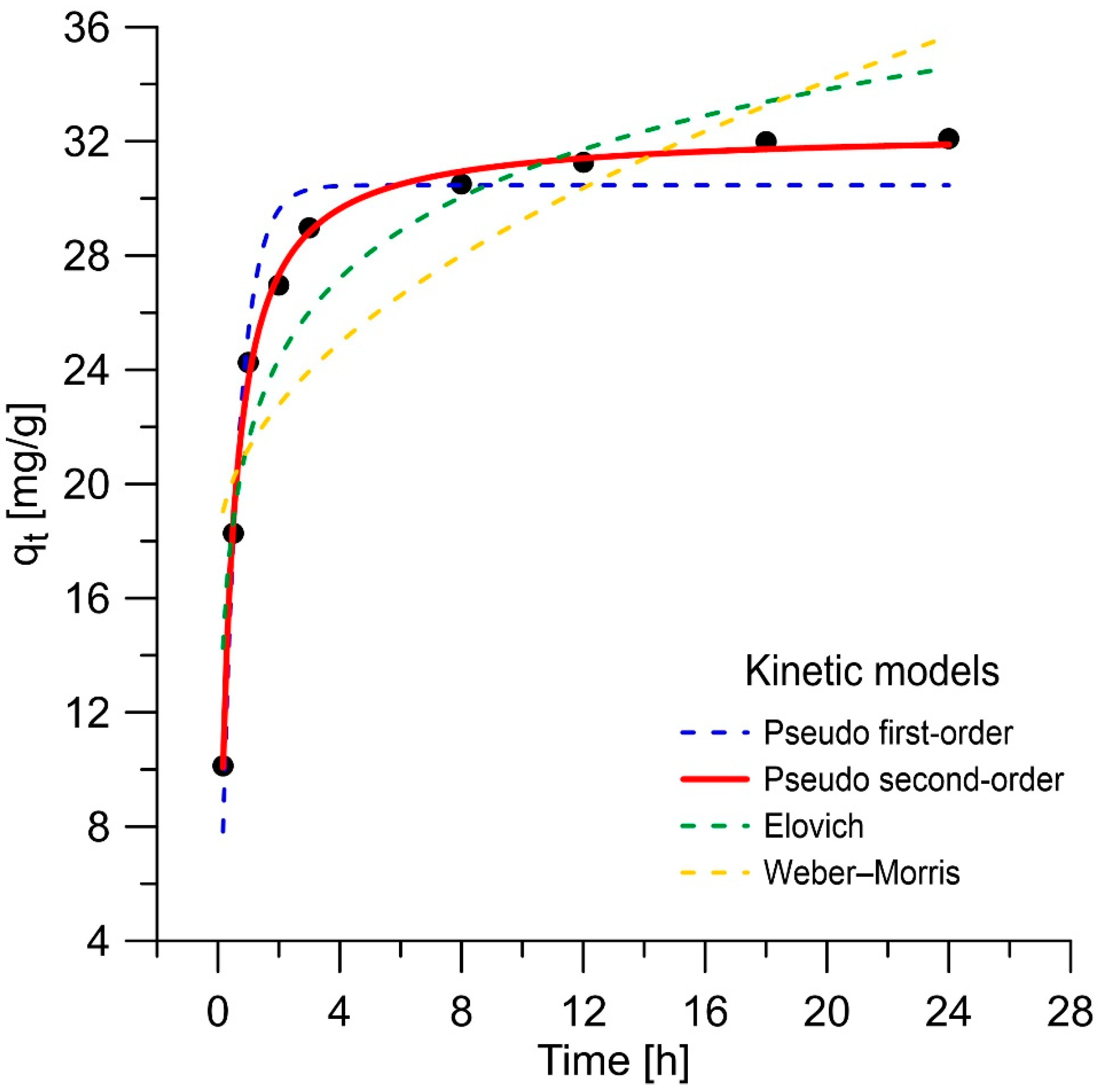
3.2.3. Kinetic Studies
3.2.4. Thermodynamic Studies
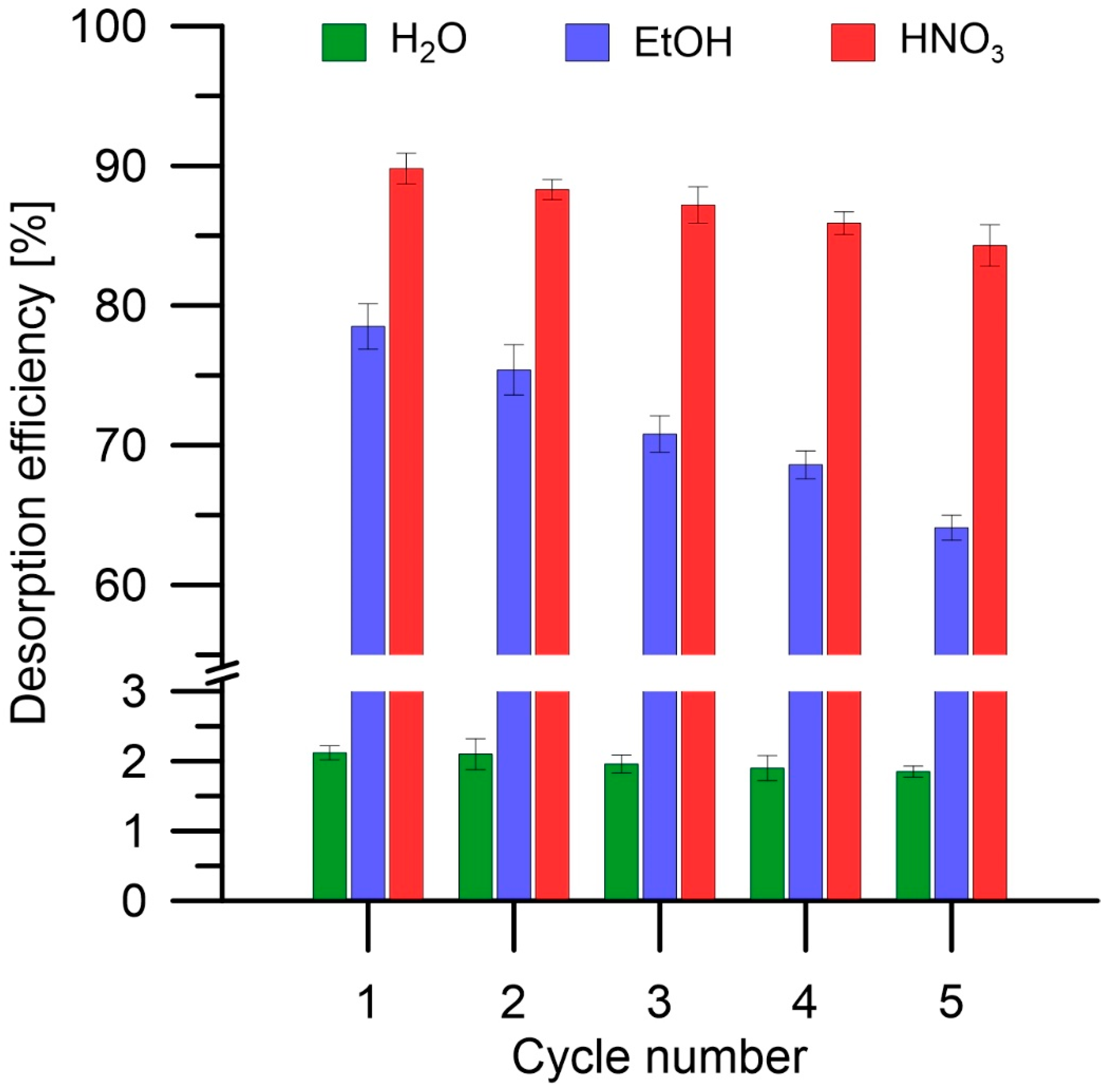
3.2.5. Desorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jameel Rather, L.; Akhter, S.; Parvaiz Hassan, Q.; Rather, L.J.; Akhter, S.; Hassan, Q.P. Bioremediation: Green and Sustainable Technology for Textile Effluent Treatment. In Sustainable Innovations in Textile Chemistry and Dyes; Springer: Berlin/Heidelberg, Germany, 2018; pp. 75–91. [Google Scholar] [CrossRef]
- Abu Talha, M.; Goswami, M.; Giri, B.S.; Sharma, A.; Rai, B.N.; Singh, R.S. Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Bioresour. Technol. 2018, 252, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Treatment of synthetic textile wastewater containing dye mixtures with microcosms. Environ. Sci. Pollut. Res. 2018, 25, 1980–1997. [Google Scholar] [CrossRef]
- Güleç, F.; Williams, O.; Samson, A.; Kostas, E.T.; Stevens, L.A.; Lester, E. Exploring the Utilisation of Natural Biosorbents for Effective Methylene Blue Removal. Appl. Sci. 2024, 14, 81. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Albert, M.; Lessin, M.S.; Gilchrist, B.F. Methylene blue: Dangerous dye for neonates. J. Pediatr. Surg. 2003, 38, 1244–1245. [Google Scholar] [CrossRef]
- Mphuthi, B.R.; Thabede, P.M.; Modise, J.S.; Xaba, T.; Shooto, N.D. Adsorption of Cadmium and Methylene Blue Using Highly Porous Carbon from Hemp Seeds. Appl. Sci. 2023, 13, 9659. [Google Scholar] [CrossRef]
- Kandisa, R.V.; Saibaba, K.V.N.; Shaik, K.B.; Gopinath, R. Dye removal by adsorption: A review. J. Bioremediat. Biodegrad. 2016, 7, 371. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Kamarehie, B.; Almasi, A.; Darvishmotevalli, M.; Salari, M.; Moradnia, M.; Azimi, F.; Ghaderpoori, M.; Neyazi, Z.; Karami, M.A. Removal of Rhodamine B from aqueous solution by stalk corn activated carbon: Adsorption and kinetic study. Biomass Convers. Biorefin. 2023, 13, 7927–7936. [Google Scholar] [CrossRef]
- Asgher, M. Biosorption of Reactive Dyes: A Review. Water Air Soil Pollut. 2011, 223, 2417–2435. [Google Scholar] [CrossRef]
- Upendar, G.; Dutta, S.; Bhattacharya, P.; Dutta, A. Bioremediation of methylene blue dye using Bacillus subtilis MTCC 441. Water Sci. Technol. 2017, 75, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Singh, A.P.; Khan, N.; Chowdhary, P.; Giri, B.S.; Varjani, S.; Chaturvedi, P. Bio-composite of Fe-sludge biochar immobilized with Bacillus Sp. in packed column for bio-adsorption of Methylene blue in a hybrid treatment system: Isotherm and kinetic evaluation. Environ. Technol. Innov. 2021, 23, 101734. [Google Scholar] [CrossRef]
- Darvishi, F.; Moradi, M.; Jolivalt, C.; Madzak, C. Laccase production from sucrose by recombinant Yarrowia lipolytica and its application to decolorization of environmental pollutant dyes. Ecotoxicol. Environ. Saf. 2018, 165, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.V.; Kumar, A.R.; Zinjarde, S.S. Environmental and industrial applications of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 84, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, E.; Yonemura, S.; Mitoma, Y.; Harada, H. Methylene Blue Removal by Chitosan Cross-Linked Zeolite from Aqueous Solution and Other Ion Effects: Isotherm, Kinetic, and Desorption Studies. Adsorpt. Sci. Technol. 2022, 2022, 1853758. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Dhedan, S.K. Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilib. 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Baláž, P.; Aláčová, A.; Briančin, J. Sensitivity of Freundlich equation constant 1/n for zinc sorption on changes induced in calcite by mechanical activation. Chem. Eng. J. 2005, 114, 115–121. [Google Scholar] [CrossRef]
- Mousa, S.M.; Ammar, N.S.; Ibrahim, H.A. Removal of lead ions using hydroxyapatite nano-material prepared from phosphogypsum waste. J. Saudi Chem. Soc. 2016, 20, 357–365. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Bai, C.; Wang, L.; Zhu, Z. Adsorption of Cr(III) and Pb(II) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2020, 147, 898–910. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; McKay, G. Sorption kinetics for the removal of copper and zinc from effluents using bone char. Sep. Purif. Technol. 2000, 19, 55–64. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 137, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.d.C.A.; Carrillo-González, R.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.d.C.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1, 950. [Google Scholar] [CrossRef]
- Svilović, S.; Rušić, D.; Bašić, A. Investigations of different kinetic models of copper ions sorption on zeolite 13X. Desalination 2010, 259, 71–75. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the nonlinear and linear forms of the van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Enache, A.C.; Cojocaru, C.; Samoila, P.; Ciornea, V.; Apolzan, R.; Predeanu, G.; Harabagiu, V. Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies. Molecules 2023, 28, 4129. [Google Scholar] [CrossRef]
- Marques, B.S.; Frantz, T.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Dotto, G.L. Adsorption of a textile dye onto piaçava fibers: Kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ. Sci. Pollut. Res. 2019, 26, 28584–28592. [Google Scholar] [CrossRef]
- Marques, J.L.; Lütke, S.F.; Frantz, T.S.; Espinelli, J.B.S.; Carapelli, R.; Pinto, L.A.A.; Cadaval, T.R.S. Removal of Al (III) and Fe (III) from binary system and industrial effluent using chitosan films. Int. J. Biol. Macromol. 2018, 120, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Teh, J.S.; Teoh, Y.H.; How, H.G.; Sher, F. Thermal Analysis Technologies for Biomass Feedstocks: A State-of-the-Art Review. Processes 2021, 9, 1610. [Google Scholar] [CrossRef]
- Novak, J.M.; Johnson, M.G. Elemental and Spectroscopic Characterization of Low-Temperature (350 °C) Lignocellulosic- and Manure-Based Designer Biochars and Their Use as Soil Amendments. In Biochar from Biomass and Waste Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–58. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Chiueh, P.T.; Lo, S.L. Pyrolysis of biomass by thermal analysis–mass spectrometry (TA–MS). Bioresour. Technol. 2011, 102, 3527–3534. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.S.; Rahman, N.A.; Ismail, K.; Alias, A.B.; Rashid, Z.A.; Aris, M.J. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour. Technol. 2010, 101, 4584–4592. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N.; Idris, A. Maghemite/alginate/functionalized multiwalled carbon nanotubes beads for methylene blue removal: Adsorption and desorption studies. J. Mol. Liq. 2019, 275, 431–440. [Google Scholar] [CrossRef]
- Kamakshi, T.; Sundari, G.S.; Erothu, H.; Singh, R.S. Effect of nickel dopant on structural, morphological and optical characteristics of Fe3O4 nanoparticles. Rasayan J. Chem. 2019, 12, 531–536. [Google Scholar] [CrossRef]
- Evora, M.C.; Gonçalez, O.L.; Dutra, R.C.L.; Diniz, M.F.; Wiebeck, H.; de Andrade e Silva, L.G. Comparação de Técnicas FTIR de Transmissão, Reflexão e Fotoacústica na Análise de Poliamida-6, Reciclada e Irradiada. Polímeros 2002, 12, 60–68. [Google Scholar] [CrossRef]
- Cavagna, M.; Dell’Anna, R.; Monti, F.; Rossi, F.; Torriani, S. Use of ATR-FTIR Microspectroscopy to Monitor Autolysis of Saccharomyces cerevisiae Cells in a Base Wine. J. Agric. Food Chem. 2009, 58, 39–45. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef]
- Mitter, E.K.; Corso, C.R. FT-IR analysis of acid black dye biodegradation using saccharomyces cerevisiae immobilized with treated sugarcane bagasse. Water. Air. Soil Pollut. 2013, 224, 1–9. [Google Scholar] [CrossRef]
- El-Kabbany, F.; Taha, S.; Hafez, M. Infrared spectroscopic anaylsis of polymorphism in diphenyl carbazide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 78, 981–988. [Google Scholar] [CrossRef]
- Pistorius, A.M.A.; DeGrip, W.J.; Egorova-Zachernyuk, T.A. Monitoring of biomass composition from microbiological sources by means of FT-IR spectroscopy. Biotechnol. Bioeng. 2009, 103, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, A.N.A.; Mulok, T.E.T.Z.; Nor, N.H.M.; Yahya, M.F.Z.R. Ftir Spectral Changes in Candida albicans Biofilm Following Exposure to Antifungals. Malays. Appl. Biol. 2022, 51, 57–66. [Google Scholar] [CrossRef]
- Olatunji, O.O.; Akinlabi, S.; Madushele, N.; Adedeji, P.A.; Ndolomingo, M.J.; Meshack, T. Blended tropical almond residue for fuel production: Characteristics, energy benefits, and emission reduction potential. J. Clean. Prod. 2020, 267, 122013. [Google Scholar] [CrossRef]
- Dong, X.; Guo, S.; Wang, H.; Wang, Z.; Gao, X. Physicochemical characteristics and FTIR-derived structural parameters of hydrochar produced by hydrothermal carbonisation of pea pod (Pisum sativum Linn.) waste. Biomass Convers. Biorefin. 2019, 9, 531–540. [Google Scholar] [CrossRef]
- Wahab, M.A.; Boubakri, H.; Jellali, S.; Jedidi, N. Characterization of ammonium retention processes onto Cactus leaves fibers using FTIR, EDX and SEM analysis. J. Hazard. Mater. 2012, 241–242, 101–109. [Google Scholar] [CrossRef]
- Abbas, O.; Rebufa, C.; Dupuy, N.; Kister, J. FTIR—Multivariate curve resolution monitoring of photo-Fenton degradation of phenolic aqueous solutions: Comparison with HPLC as a reference method. Talanta 2008, 77, 200–209. [Google Scholar] [CrossRef]
- Cherdoud-Chihani, A.; Mouzali, M.; Abadie, M.J.M. Study of crosslinking acid copolymer/DGEBA systems by FTIR. J. Appl. Polym. Sci. 2003, 87, 2033–2051. [Google Scholar] [CrossRef]
- Joyce Hellen Sathya, D.; Manohar Turakhia, A.; Anil Kumar, M.; Balaji, N.; Selvanaveen, S.; Vinodhini, G.; Seenuvasan, M. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects Bioethanol from saccharificed lignocellulosic rich Aloe vera rinds using Saccharomyces cerevisiae MTCC 4779 Bioethanol from saccharificed lignocellulosic rich Aloe vera rinds using Saccharomyces cerevisiae MTCC 4779. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1347–1352. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Renganayaki, V.; Natarajan, S. Vibrational spectra and thermodynamic analysis of metformin. Indian J. Pure Appl. Phys. 2006, 44, 495–500. [Google Scholar]
- Zhou, W.; He, W.; Zhong, S.; Wang, Y.; Zhao, H.; Li, Z.; Yan, S. Biosynthesis and magnetic properties of mesoporous Fe3O4 composites. J. Magn. Magn. Mater. 2009, 321, 1025–1028. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Alvarez-Silva, M.; Uribe-Salas, A.; Mirnezami, M.; Finch, J.A. The point of zero charge of phyllosilicate minerals using the Mular–Roberts titration technique. Miner. Eng. 2010, 23, 383–389. [Google Scholar] [CrossRef]
- Haq, A.u.; Saeed, M.; Usman, M.; Zahoor, A.F.; Anjum, M.N.; Maqbool, T.; Naheed, S.; Kashif, M. Mechanisms of halosulfuron methyl pesticide biosorption onto neem seeds powder. Sci. Rep. 2021, 11, 9960. [Google Scholar] [CrossRef]
- Maqbool, M.; Bhatti, H.N.; Sadaf, S.; Mana AL-Anazy, M.; Iqbal, M. Biocomposite of polyaniline and sodium alginate with Oscillatoria biomass: A potential adsorbent for the removal of basic blue 41. J. Mater. Res. Technol. 2020, 9, 14729–14741. [Google Scholar] [CrossRef]
- Heybet, E.N.; Ugraskan, V.; Isik, B.; Yazici, O. Adsorption of methylene blue dye on sodium alginate/polypyrrole nanotube composites. Int. J. Biol. Macromol. 2021, 193, 88–99. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Abd El-Latif, M.M.; El-Kady, M.F.; Ibrahim, A.M.; Ossman, M.E. Alginate/Polyvinyl Alcohol - Kaolin Composite for Removal of Methylene Blue from Aqueous Solution in a Batch Stirred Tank Reactor. J. Am. Sci. 2010, 6, 280–292. [Google Scholar]
- Liu, F.; Li, W.; Zhou, Y. Preparation and characterization of magnetic sodium alginate-modified zeolite for the efficient removal of methylene blue. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 629, 127403. [Google Scholar] [CrossRef]
- Biswas, S.; Mohapatra, S.S.; Kumari, U.; Meikap, B.C.; Sen, T.K. Batch and continuous closed circuit semi-fluidized bed operation: Removal of MB dye using sugarcane bagasse biochar and alginate composite adsorbents. J. Environ. Chem. Eng. 2020, 8, 103637. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Shen, L.; Wang, X.; Chen, J.; Ma, A.; Jiang, W. Lead(II) and methylene blue removal using a fully biodegradable hydrogel based on starch immobilized humic acid. Chem. Eng. J. 2015, 268, 348–355. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Wen, H.Y.; Gollakota, A.R.K.; Wen, J.C.; Lin, K.Y.A.; Shu, C.M.; Yarramuthi, V.; Basivi, P.K.; Reddy, G.M.; Zyryanov, G.V. Magnetic Fe3O4 nanoparticles loaded guava leaves powder impregnated into calcium alginate hydrogel beads (Fe3O4-GLP@CAB) for efficient removal of methylene blue dye from aqueous environment: Synthesis, characterization, and its adsorption performance. Int. J. Biol. Macromol. 2023, 246, 125675. [Google Scholar] [CrossRef]
- Noori, M.; Tahmasebpoor, M.; Foroutan, R. Enhanced adsorption capacity of low-cost magnetic clinoptilolite powders/beads for the effective removal of methylene blue: Adsorption and desorption studies. Mater. Chem. Phys. 2022, 278, 125655. [Google Scholar] [CrossRef]
- Erfani, M.; Javanbakht, V. Methylene Blue removal from aqueous solution by a biocomposite synthesized from sodium alginate and wastes of oil extraction from almond peanut. Int. J. Biol. Macromol. 2018, 114, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Haghshenasfard, M.; Moheb, A. Optimization of dye adsorption using Fe3O4 nanoparticles encapsulated with alginate beads by Taguchi method. Adsorpt. Sci. Technol. 2017, 35, 55–71. [Google Scholar] [CrossRef]
- E, T.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Xu, J.Y.; Yang, X.L. MXene/sodium alginate gel beads for adsorption of methylene blue. Mater. Chem. Phys. 2021, 260, 124123. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Mohmmadi, L.; Ahmadi, S.; Rahdar, A.; Khadkhodaiy, D.; Dehghani, R.; Rahdar, S. Modeling of adsorption of Methylene Blue dye on Ho-CaWO4 nanoparticles using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) techniques. MethodsX 2019, 6, 1779–1797. [Google Scholar] [CrossRef]
- Mu, T.-H.; Sun, H.-N. Sweet Potato Leaf Polyphenols: Preparation, Individual Phenolic Compound Composition and Antioxidant Activity. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 365–380. [Google Scholar]
- Özacar, M.; Şengil, I.A. Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour. Technol. 2005, 96, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Kongsune, P.; Rattanapan, S.; Chanajaree, R. The removal of Pb2+ from aqueous solution using mangosteen peel activated carbon: Isotherm, kinetic, thermodynamic and binding energy calculation. Groundw. Sustain. Dev. 2021, 12, 100524. [Google Scholar] [CrossRef]
- Ünlü, N.; Ersoz, M. Removal of heavy metal ions by using dithiocarbamated-sporopollenin. Sep. Purif. Technol. 2007, 52, 461–469. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Dai, G. Silkworm exuviae—A new non-conventional and low-cost adsorbent for removal of methylene blue from aqueous solutions. J. Hazard. Mater. 2011, 186, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Işık, B.; Uğraşkan, V. Adsorption of methylene blue on sodium alginate–flax seed ash beads: Isotherm, kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2021, 167, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.A.S.; El-Sakhawy, M.; Kamel, S. Microwave-assisted synthesis of amphoteric fluorescence carbon quantum dots and their chromium adsorption from aqueous solution. Sci. Rep. 2023, 13, 11306. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.S.; Chen, M.L. Application of the Elovich Equation to the Kinetics of Metal Sorption with Solvent-Impregnated Resins. Ind. Eng. Chem. Res. 1997, 36, 813–820. [Google Scholar] [CrossRef]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium uptake, isotherm and kinetic studies of Cd(II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Ravi; Pandey, L.M. Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl. Clay Sci. 2019, 169, 102–111. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Esmaeili, H.; Foroutan, R. Adsorptive Behavior of Methylene Blue onto Sawdust of Sour Lemon, Date Palm, and Eucalyptus as Agricultural Wastes. J. Dispers. Sci. Technol. 2019, 40, 990–999. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Al-Rawajfeh, A.E.; Al-Itawi, H.I.; Sharadqah, S.; Al-Qazaqi, S.; Abu Hashish, E.; Al-Qatatsheh, M.; Sillanpaa, M. Utilization of Calcined Gypsum in Water and Wastewater Treatment: Removal of Phenol. J. Ecol. Eng. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Vilela, P.B.; Matias, C.A.; Dalalibera, A.; Becegato, V.A.; Paulino, A.T. Polyacrylic acid-based and chitosan-based hydrogels for adsorption of cadmium: Equilibrium isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2019, 7, 103327. [Google Scholar] [CrossRef]
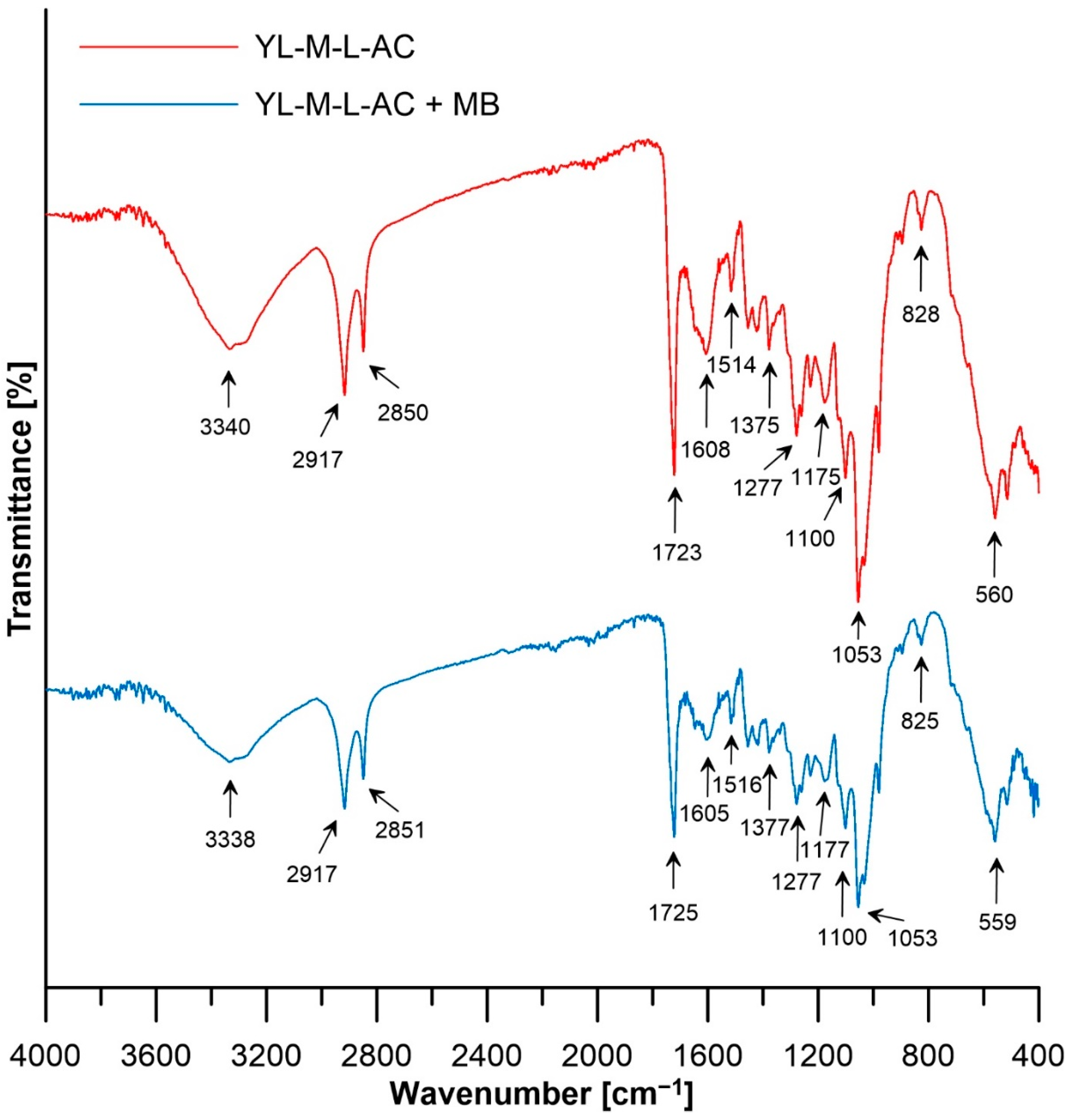


| Wavenumber (cm−1) | Functional Group | Reference |
|---|---|---|
| 3000–3675 | Stretching vibrations of –OH groups | [41] |
| 2916 | νasym CH2 | [42] |
| 2850–2851 | νsym CH2 | [43] |
| 1723–1725 | Stretching C=O | [44] |
| 1648 | C–N axial deformation and C–O bonding | [44] |
| 1605–1608 | C=O vibrations | [45] |
| 1514–1516 | C=C | [46] |
| 1375–1378 | C-N stretching vibration/N-H bending vibration | [47] |
| 1277 | Aromatic ring vibration and aromatic C-O stretching | [48] |
| 1175–1177 | Alcohol C–O stretches, ethers, and carboxylic acids | [48] |
| 1100 | The stretching of the C–O–C bond | [49] |
| 1053 | The stretching vibrations of C–O | [49] |
| 1053–1025 | OH groups in sugars or C–O–C stretching vibrations in lignin or hemicellulose, δ C–H bonding of aromatic ring, S=O stretching | [50,51,52] |
| 828–825 | Represent the presence of sugars, mainly monosaccharides and deformation band δ(C–H) | [53] |
| 600–400 | Deforming vibrations from C-N-C | [54] |
| Adsorbent | qe [mg/g] | Reference |
|---|---|---|
| Alginate/polyvinyl alcohol–kaolin composite | 30.8 | [62] |
| SA/zeolite/Fe3O4 composite | 181.8 | [63] |
| Sugarcane bagasse biochar and alginate composite | 30.1 | [64] |
| Starch–humic acid composite hydrogel beads | 110.0 | [65] |
| Magnetic Fe3O4-nanoparticle-loaded guava leaves powder impregnated into calcium alginate hydrogel beads | 136.7 | [66] |
| Alginate/Clin/Fe3O4 nanocomposite bead | 12.5 | [67] |
| Alginate/almond peanut biocomposite | 22.8 | [68] |
| Magnetic alginate beads | 106.4 | [69] |
| Graphene oxide–montmorillonite/sodium alginate (GO–MMT/SA) aerogel beads | 150.7 | [70] |
| MXene/sodium alginate gel beads | 92.2 | [71] |
| YL-M-L-AC | 32.1 | This study |
| Isotherm Model | Parameters | |||
|---|---|---|---|---|
| Langmuir | ARE [%] | R2 | qm [mg/g] | KL [dm3/mg] |
| 7.47 | 0.9889 | 47.94 | 0.021 | |
| Freundlich | ARE [%] | R2 | KF (mg1−(1/n)(dm3)1/ng−1) | 1/n |
| 3.41 | 0.9959 | 2.865 | 0.538 | |
| Temkin | ARE [%] | R2 | KT [dm3/g] | B [kJ/mol] |
| 9.76 | 0.9777 | 0.256 | 9.643 | |
| D–R | ARE [%] | R2 | Kad [mol2/kJ2] | qd [mg/g] |
| 15.91 | 0.9482 | 0.0069 | 35.18 | |
| Kinetic Model | Parameters | |||
|---|---|---|---|---|
| Pseudo-first order rate | ARE [%] | R2 | q1 [mg/g] | k1 [1/min] |
| 6.22 | 0.9761 | 30.46 | 1.783 | |
| Pseudo-second order rate | ARE [%] | R2 | q2 [mg/g] | k2 [g/(mg·min)] |
| 1.14 | 0.9989 | 32.37 | 0.0837 | |
| Elovich | ARE [%] | R2 | α [mg/(g·min)] | β [g/mg] |
| 9.90 | 0.9439 | 765.8 | 0.243 | |
| Weber–Morris | ARE [%] | R2 | I | Kid |
| 18.86 | 0.8057 | 17.5 | 3.70 | |
| T [°C] | qe [mg/g] (C0 = 250 mg/dm3) | KML | ΔG° [kJ/mol] | ΔH° [kJ/mol] | ΔS° [J/molK] |
|---|---|---|---|---|---|
| 25 | 32.10 | 914.63 | −16.894 | −36.447 | −65.616 |
| 30 | 26.02 | 719.04 | −16.566 | ||
| 35 | 23.67 | 564.12 | −16.238 | ||
| 40 | 23.64 | 453.15 | −15.910 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staroń, P.; Chwastowski, J. Yeast-Based Magnetic Biocomposite for Efficient Sorption of Organic Pollutants. Appl. Sci. 2024, 14, 655. https://doi.org/10.3390/app14020655
Staroń P, Chwastowski J. Yeast-Based Magnetic Biocomposite for Efficient Sorption of Organic Pollutants. Applied Sciences. 2024; 14(2):655. https://doi.org/10.3390/app14020655
Chicago/Turabian StyleStaroń, Paweł, and Jarosław Chwastowski. 2024. "Yeast-Based Magnetic Biocomposite for Efficient Sorption of Organic Pollutants" Applied Sciences 14, no. 2: 655. https://doi.org/10.3390/app14020655
APA StyleStaroń, P., & Chwastowski, J. (2024). Yeast-Based Magnetic Biocomposite for Efficient Sorption of Organic Pollutants. Applied Sciences, 14(2), 655. https://doi.org/10.3390/app14020655








