Assessment of Surrogate Models for Research on Resistance and Deformation of Repairs of the Human Meniscal Roots: Porcine or Older Human Models?
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
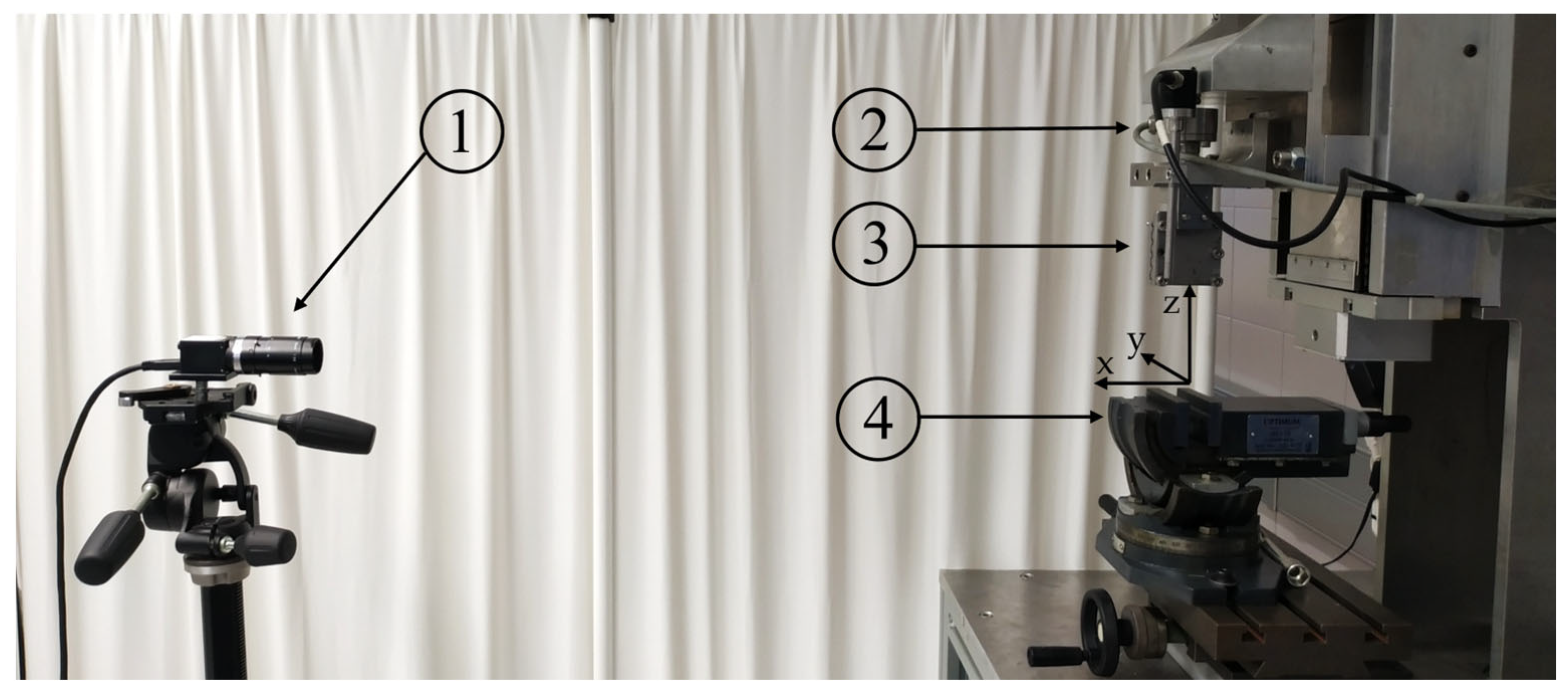
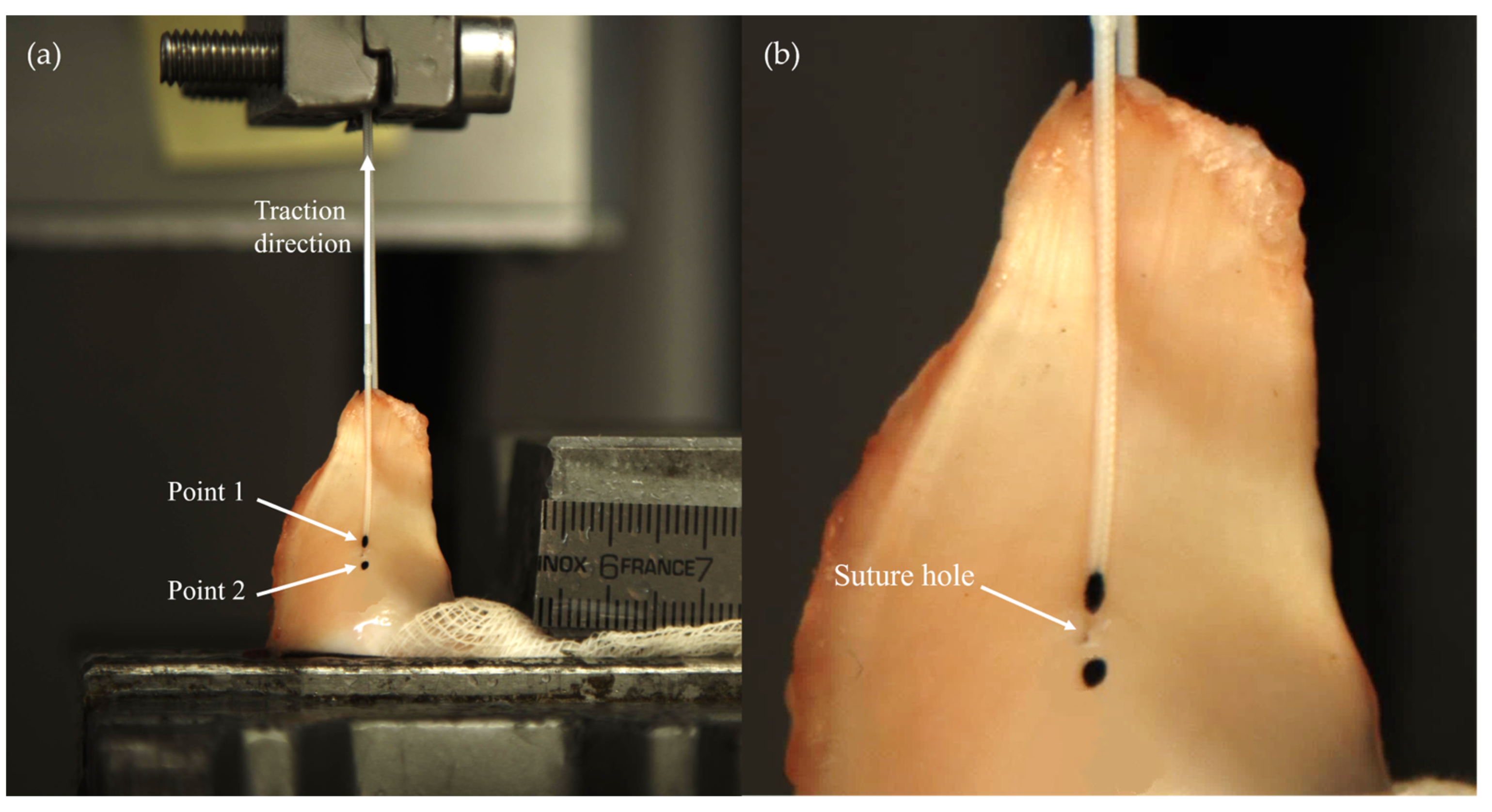
2.2. Biomechanical Testing
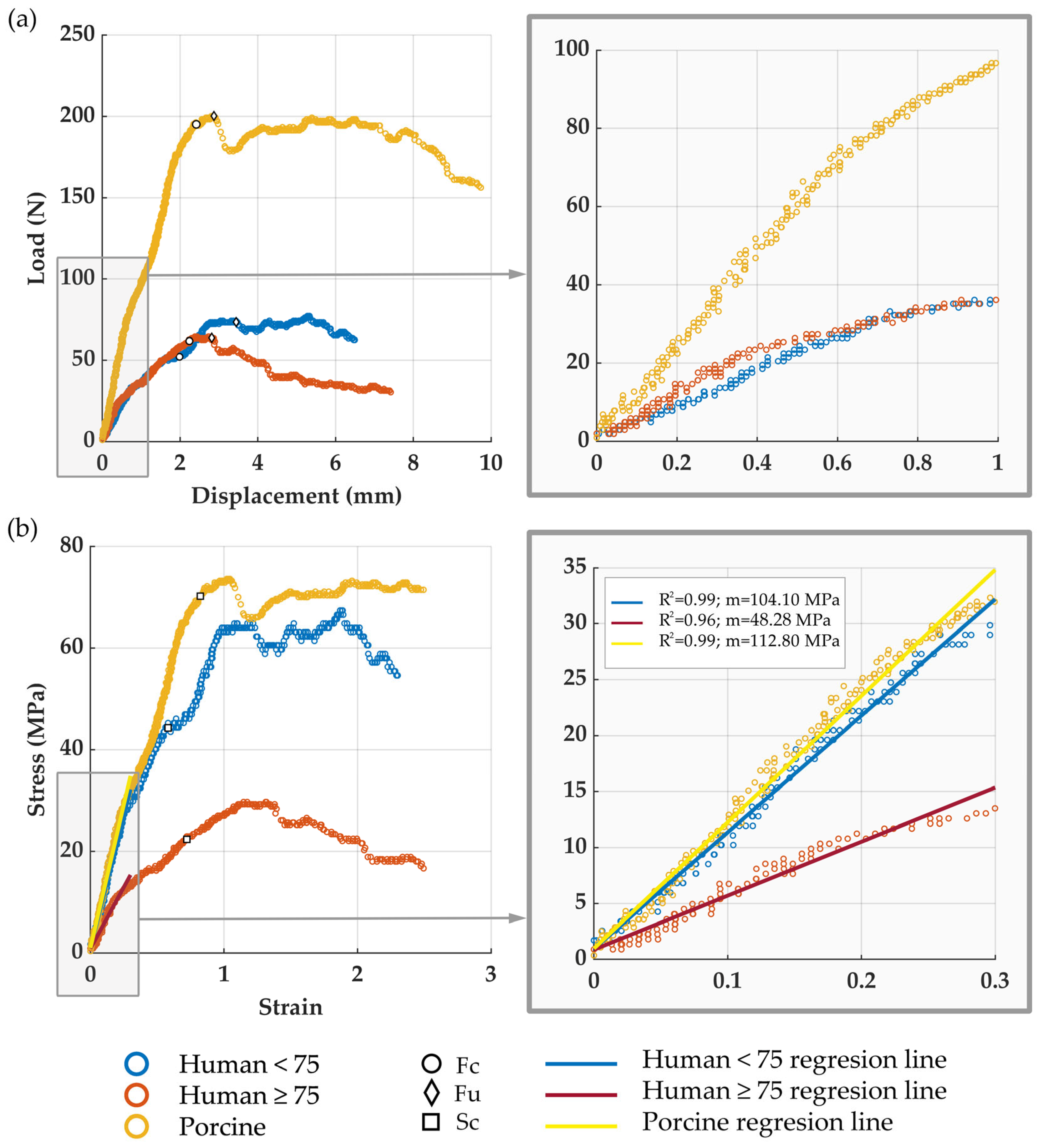
2.3. Data Analysis
2.4. Statistical Analyses
3. Results
- Human <75 years group: 45 younger-than-75-years-old menisci (mean age 51.00 years, SD 13.56, range 28–67 years) obtained from 24 cadaveric knees of 24 donors after discarding 3 menisci due to not meeting the inclusion criteria. The group consisted of 27 menisci from men and 18 from women, 23 medial and 22 lateral menisci, 22 for anterior and 23 for posterior surgery.
- Human ≥75 years group: 21 75-years-old-or-older specimens (mean age 84.86 years, SD 3.82, range 82–95 years) obtained from 14 cadaveric knees of 14 donors after discarding 7 menisci due to not meeting the inclusion criterion. The group consisted of 9 menisci from men and 12 from women, 11 medial and 10 lateral menisci, 9 for anterior and 12 for posterior surgery.
- Porcine group: 21 menisci from 22 6-month-old pigs. All of them met the inclusion criteria. The group consisted of 11 medial and 11 lateral menisci, 10 for anterior and 12 for posterior surgery.
3.1. Meniscal Horn Thickness at the Suture Point, h
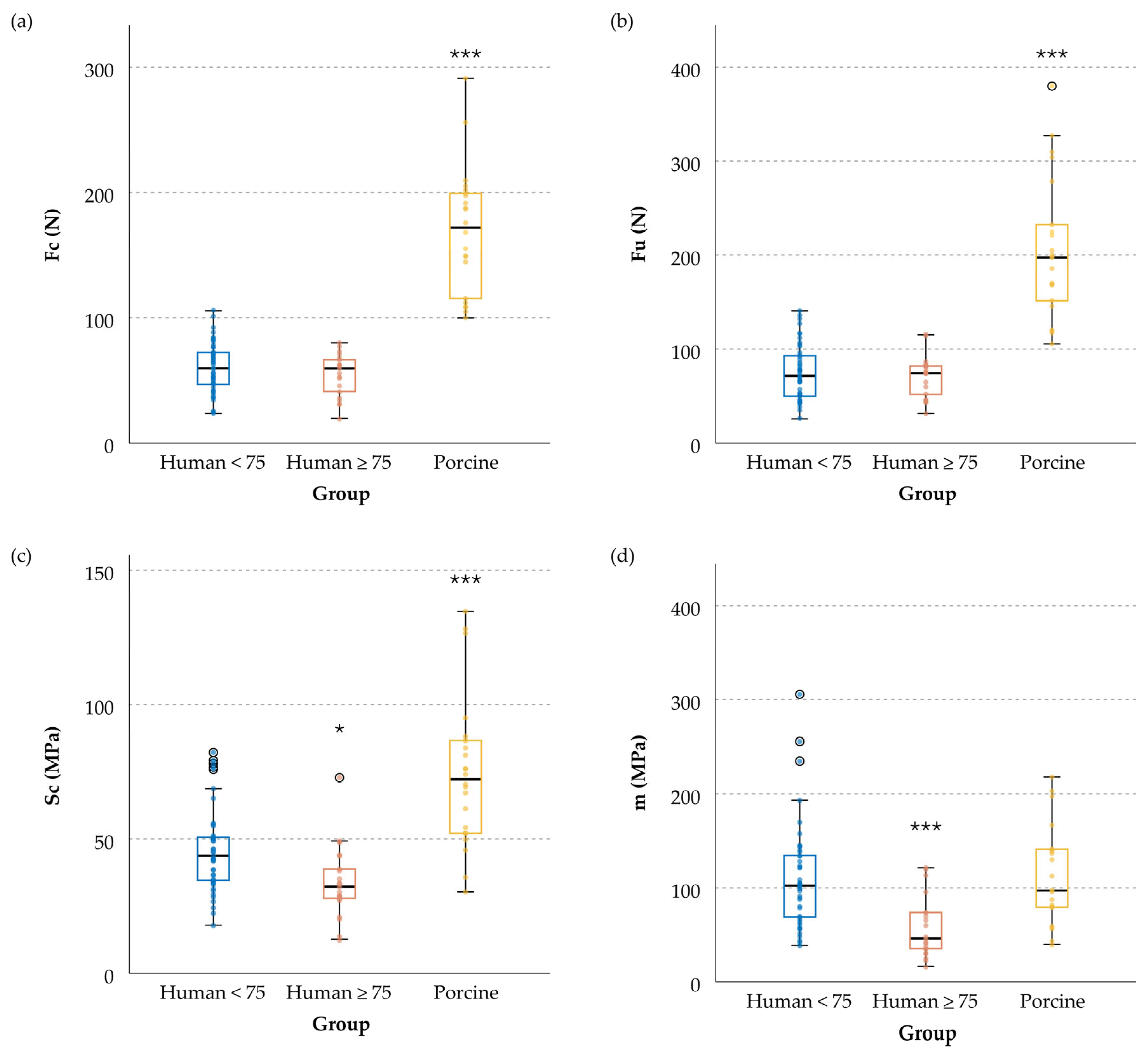
3.2. Meniscal Resistance to Cut-Out Initiation, Fc
3.3. Meniscal Ultimate Force, Fu
3.4. Tissue Cut-Out Resistance, Sc
3.5. Tissue Equivalent Stiffness Modulus, m
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of human knee menisci: Structure, composition, and function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef]
- Perez-Blanca, A.; Espejo-Baena, A.; Amat Trujillo, D.; Prado Nóvoa, M.; Espejo-Reina, A.; Quintero López, C.; Ezquerro Juanco, F. Comparative Biomechanical Study on Contact Alterations after lateral meniscus posterior root avulsion, transosseous reinsertion, and total meniscectomy. Arthroscopy 2016, 32, 624–633. [Google Scholar] [CrossRef]
- Allaire, R.; Muriuki, M.; Gilbertson, L.; Harner, C.D. Biomechanical consequences of a tear of the posterior root of the medial meniscus: Similar to total meniscectomy. J. Bone Jt. Surg. Am. 2008, 90, 1922–1931. [Google Scholar] [CrossRef]
- Espejo-Reina, A.; Prado-Novoa, M.; Espejo-Baena, A.; Peña-Trabalon, A.; Perez-Blanca, A. Biomechanical consequences of anterior root detachment of the lateral meniscus and its reinsertion. Sci. Rep. 2022, 12, 6182. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, K.; Kamiya, T.; Suzuki, D.; Yamakawa, S.; Otsubo, H.; Suzuki, T.; Takahashi, K.; Okada, Y.; Teramoto, A.; Ohnishi, H.; et al. The role of the medial meniscus in anterior knee stability. Orthop. J. Sport Med. 2022, 10, 23259671221132845. [Google Scholar] [CrossRef]
- Musahl, V.; Citak, M.; O’Loughlin, P.; Choi, D.; Bedi, A.; Pearle, A. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am. J. Sports Med. 2010, 38, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, S.; Markolf, K. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Effects of partial versus total excision. J. Bone Jt. Surg. Am. 1986, 68, 71–79. [Google Scholar] [CrossRef]
- Krych, A.; Reardon, P.; Johnson, N.; Mohan, R.; Peter, L.; Levy, B.; Stuart, M.J. Non-operative management of medial meniscus posterior horn root tears is associated with worsening arthritis and poor clinical outcome at 5-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 383–389. [Google Scholar] [CrossRef]
- Hussain, Z.B.; Chahla, J.; Mandelbaum, B.R.; Gomoll, A.H.; LaPrade, R.F. The role of meniscal tears in spontaneous osteonecrosis of the knee: A systematic review of suspected etiology and a call to revisit nomenclature. Am. J. Sports Med. 2019, 47, 501–507. [Google Scholar] [CrossRef]
- Pache, S.; Aman, Z.S.; Kennedy, M.; Nakama, G.Y.; Moatshe, G.; Ziegler, C.; LaPrade, R.F. Meniscal root tears: Current concepts review. Arch. Bone Jt. Surg. 2018, 6, 250–259. [Google Scholar]
- Ahn, J.H.; Wang, J.H.; Yoo, J.C.; Noh, H.K.; Park, J.H. A pull out suture for transection of the posterior horn of the medial meniscus: Using a posterior trans-septal portal. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Feucht, M.J.; Grande, E.; Brunhuber, J.; Burgkart, R.; Imhoff, A.B.; Braun, S. Biomechanical evaluation of different suture techniques for arthroscopic transtibial pull-out repair of posterior medial meniscus root tears. Am. J. Sports Med. 2013, 41, 2784–2790. [Google Scholar] [CrossRef]
- Balke, M.; Akoto, R.; Offerhaus, C.; Hoeher, J. Suture anchor refixation of meniscal root tears without an additional portal. Arthrosc. Tech. 2018, 7, e511–e515. [Google Scholar] [CrossRef] [PubMed]
- Espejo-Reina, A.; Prado-Novoa, M.; Espejo-Reina, M.J.; Gómez-Cáceres, A.; Dalla Rosa-Nogales, J.; Espejo-Baena, A. Non anatomic reinsertion after amputation of the anterior horn of the lateral meniscus. Orthop. Traumatol. Surg. Res. 2019, 105, 1115–1118. [Google Scholar] [CrossRef]
- Robinson, J.R.; Frank, E.G.; Hunter, A.J.; Jermin, P.J.; Gill, H.S. The strength of transosseous medial meniscal root repair using a simple suture technique is dependent on suture material and position. Am. J. Sports Med. 2018, 46, 924–932. [Google Scholar] [CrossRef]
- Wu, J.L.; Lee, C.H.; Yang, C.T.; Chang, C.M.; Li, G.; Cheng, C.K.; Chen, C.H.; Huang, H.S.; Lai, Y.S. Novel technique for repairing posterior medial meniscus root tears using porcine knees and biomechanical study. PLoS ONE 2018, 13, e0192027. [Google Scholar] [CrossRef]
- Feucht, M.J.; Grande, E.; Brunhuber, J.; Rosenstiel, N.; Burgkart, R.; Imhoff, A.B.; Braun, S. Biomechanical evaluation of different suture materials for arthroscopic transtibial pull-out repair of posterior meniscus root tears. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 132–139. [Google Scholar] [CrossRef]
- Chung, K.S.; Choi, C.H.; Bae, T.S.; Ha, J.K.; Jun, D.J.; Wang, J.H.; Kim, J.G. Comparison of tibiofemoral contact mechanics after various transtibial and all-inside fixation techniques for medial meniscus posterior root radial tears in a porcine model. Arthroscopy 2018, 34, 1060–1068. [Google Scholar] [CrossRef]
- Fujii, M.; Furumatsu, T.; Xue, H.; Miyazawa, S.; Kodama, Y.; Hino, T.; Kamatsuki, Y.; Ozaki, T. Tensile strength of the pullout repair technique for the medial meniscus posterior root tear: A porcine study. Int. Orthop. 2017, 41, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Rosslenbroich, S.B.; Borgmann, J.; Herbort, M.; Raschke, M.J.; Petersen, W.; Zantop, T. Root tear of the meniscus: Biomechanical evaluation of an arthroscopic refixation technique. Arch. Orthop. Trauma Surg. 2013, 133, 111–115. [Google Scholar] [CrossRef]
- Okimura, S.; Mae, T.; Tachibana, Y.; Iuchi, R.; Nakata, K.; Yamashita, T.; Shino, K. Biomechanical comparison of meniscus-suture constructs for pullout repair of medial meniscus posterior root tears. J. Exp. Orthop. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Forkel, P.; Foehr, P.; Meyer, J.C.; Herbst, E.; Petersen, W.; Brucker, P.U.; Burgkart, R.; Imhoff, A.B. Biomechanical and viscoelastic properties of different posterior meniscal root fixation techniques. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Yeh, T.-T.; Hsu, W.-C.; Wu, A.T.; Li, G.; Chen, C.-H.; Lee, C.-H.; Wu, J.-L. Biomechanical comparison of four tibial fixation techniques for meniscal root sutures in posterior medial meniscus root repair: A porcine study. J. Orthop. Transl. 2020, 24, 144–149. [Google Scholar] [CrossRef]
- Prado-Novoa, M.; Perez-Blanca, A.; Espejo-Reina, A.; Espejo-Reina, M.J.; Espejo-Baena, A. Initial biomechanical properties of transtibial meniscal root repair are improved by using a knotless anchor as a post-insertion tensioning device. Sci. Rep. 2020, 10, 1748. [Google Scholar] [CrossRef]
- Feucht, M.J.; Grande, E.; Brunhuber, J.; Rosenstiel, N.; Burgkart, R.; Imhoff, A.B.; Braun, S. Biomechanical comparison between suture anchor and transtibial pull-out repair for posterior medial meniscus root tears. Am. J. Sports Med. 2014, 42, 187–193. [Google Scholar] [CrossRef]
- Kim, Y.M.; Joo, Y.B.; Noh, C.K.; Park, I.Y. The optimal suture site for the repair of posterior horn root tears: Biomechanical evaluation of pullout strength in porcine menisci. Knee Surg. Relat. Res. 2016, 28, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Li, G.; Shetty, G.M.; Kim, J.H.; Bae, J.H.; Jo, M.L.; Kim, J.S.; Lee, S.J.; Nha, K.W. Effect of Repair of Radial Tears at the Root of the Posterior Horn of the Medial meniscus with the pullout suture technique: A biomechanical study using porcine knees. Arthroscopy 2009, 25, 1281–1287. [Google Scholar] [CrossRef]
- Stärke, C.; Kopf, S.; Gröbel, K.H.; Becker, R. The Effect of a Nonanatomic repair of the meniscal horn attachment on meniscal tension: A biomechanical study. Arthroscopy 2010, 26, 358–365. [Google Scholar] [CrossRef]
- Perez-Blanca, A.; Prado Nóvoa, M.; Lombardo Torre, M.; Espejo-Reina, A.; Ezquerro Juanco, F.; Espejo-Baena, A. The role of suture cutout in the failure of meniscal root repair during the early post-operative period: A biomechanical study. Int. Orthop. 2018, 42, 811–818. [Google Scholar] [CrossRef]
- Takroni, T.; Laouar, L.; Adesida, A.; Elliott, J.A.W.; Jomha, N.M. Anatomical study: Comparing the human, sheep and pig knee meniscus. J. Exp. Orthop. 2016, 3, 35. [Google Scholar] [CrossRef]
- Deponti, D.; Di Giancamillo, A.; Scotti, C.; Peretti, G.M.; Martin, I. Animal models for meniscus repair and regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Kyle Martin, R.; Gillis, D.; Leiter, J.; Shantz, J.S.; MacDonald, P. A porcine knee model is valid for use in the evaluation of arthroscopic skills: A pilot study. Clin. Orthop. Relat. Res. 2016, 474, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Sweigart, M.A.; Zhu, C.F.; Burt, D.M.; Deholl, P.D.; Agrawal, C.M.; Clanton, T.O.; Athanasiou, K.A. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann. Biomed. Eng. 2004, 32, 1569–1579. [Google Scholar] [CrossRef]
- Polito, U.; Andreis, M.; Di Giancamillo, A.; Modina, S.; Scurati, R.; Marmotti, A.; Michielon, G.; Domenicucci, M.; Lombardo, M.; Di Giancamillo, M.; et al. Clinical anatomy of the meniscus in animal models: Pros and cons. J. Biol. Regul. Homeost. Agents 2020, 34, 197. [Google Scholar]
- Camarda, L.; Bologna, E.; Pavan, D.; Morello, F.; Monachino, F.; Giacco, F.; Zingales, M. Posterior meniscal root repair: A biomechanical comparison between human and porcine menisci. Muscle Ligaments Tendons J. 2019, 9, 76–81. [Google Scholar] [CrossRef]
- Espejo-Reina, A.; Prado-Novoa, M.; Espejo-Baena, A.; Estebanez, B.; Perez-Blanca, A. Improved tibiofemoral contact restoration after transtibial reinsertion of the anterior root of the lateral meniscus compared to in situ repair: A biomechanical study. Int. Orthop. 2023, 47, 2419–2427. [Google Scholar] [CrossRef]
- Anz, A.W.; Branch, E.A.; Saliman, J.D. Biomechanical comparison of arthroscopic repair constructs for meniscal root tears. Am. J. Sports Med. 2014, 42, 2699–2706. [Google Scholar] [CrossRef]
- Mitchell, R.; Pitts, R.; Kim, Y.M.; Matava, M.J. Medial meniscal root avulsion: A biomechanical comparison of 4 different repair constructs. Arthroscopy 2016, 32, 111–119. [Google Scholar] [CrossRef]
- Krych, A.J.; Johnson, N.R.; Wu, I.T.; Smith, P.A.; Stuart, M.J. A simple cinch is superior to a locking loop for meniscus root repair: A human biomechanical comparison of suture constructs in a transtibial pull-out model. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Poland, S.; Everhart, J.S.; Kim, W.; Axcell, K.; Magnussen, R.A.; Flanigan, D.C. Age of 40 years or older does not affect meniscal repair failure risk at 5 years. Arthroscopy 2019, 35, 1527–1532. [Google Scholar] [CrossRef]
- Ventura, M.; Seabra, P.; Oliveira, J.; Sousa, P.; Quesado, M.; Sousa, H.; Pereira, R.; Costa, A.; Carvalho, P. Meniscal Injuries in Patients Aged 40 Years or Older: A comparative study between meniscal repair and partial meniscectomy. Cureus 2023, 15, e33270. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Beaufils, P.; Hirschmann, M.T.; Rotigliano, N.; Ollivier, M.; Pereira, H.; Verdonk, R.; Darabos, N.; Ntagiopoulos, P.; Dejour, D.; et al. Management of traumatic meniscus tears: The 2019 ESSKA meniscus consensus. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Pauli, C.; Grogan, S.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.; D’Lima, D.D. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Rhee, K.J.; Lee, J.K.; Hwang, D.S.; Yang, J.Y.; Kim, S.J. Arthroscopic pullout repair of a complete radial tear of the tibial attachment site of the medial meniscus posterior horn. Arthroscopy 2006, 22, 795.e1–795.e4. [Google Scholar] [CrossRef] [PubMed]
- Menge, T.J.; Chahla, J.; Dean, C.S.; Mitchell, J.J.; Moatshe, G.; LaPrade, R.F. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc. Tech. 2016, 5, e679–e684. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Blanca, A. Biomecánica de la Reinserción Transtibial de la raíz Posterior del Menisco Lateral de la Rodilla: Avances en la Técnica de Reparación. Ph.D. Thesis, University of Málaga, Málaga, Spain, 2019. [Google Scholar]
- Cerminara, A.J.; LaPrade, C.M.; Smith, S.D.; Ellman, M.B.; Wijdicks, C.A.; LaPrade, R.F. Biomechanical evaluation of a transtibial pull-out meniscal root repair challenging the bungee effect. Am. J. Sports Med. 2014, 42, 2988–2995. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Peña-Trabalon, A.; Perez-Blanca, A.; Moreno-Vegas, S.; Estebanez Campos, M.B.; Prado-Novoa, M. Age influence on resistance and deformation of the human sutured meniscal horn in the immediate postoperative period. Front. Bioeng. Biotechnol. 2024, 11, 1249982. [Google Scholar] [CrossRef]
- Bansal, S.; Keah, N.M.; Neuwirth, A.L.; O’Reilly, O.; Qu, F.; Seiber, B.N.; Mandalapu, S.; Mauck, R.L.; Zgonis, M.H. large animal models of meniscus repair and regeneration: A systematic review of the state of the field. Tissue Eng. Part C Methods 2017, 23, 661. [Google Scholar] [CrossRef]
- Husen, M.; Kennedy, N.I.; Till, S.; Reinholz, A.; Stuart, M.J.; Krych, A.J.; Saris, D.B. Benefits of meniscal repair in selected patients aged 60 years and older. Orthop. J. Sport Med. 2022, 10, 23259671221117492. [Google Scholar] [CrossRef]
- Vertullo, C.J.; Cadman, J.; Dabirrahmani, D.; Appleyard, R. Biomechanical comparison of an all-inside meniscal repair device construct versus pullout sutures for arthroscopic transtibial repair of posterior medial meniscus root tears a matched-pair cadaveric study. Orthop. J. Sport Med. 2021, 9, 23259671211000464. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Colvin, A.C.; Muriuki, M.; Zhang, X.; Harner, C.D. Meniscal root suturing techniques implications for root fixation. Am. J. Sports Med. 2011, 39, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Prado-Novoa, M.; Perez-Sanchez, L.; Estebanez, B.; Moreno-Vegas, S.; Perez-Blanca, A. influence of loading conditions on the mechanical performance of multifilament coreless UHMWPE sutures used in orthopaedic surgery. Materials 2022, 15, 2573. [Google Scholar] [CrossRef] [PubMed]
- Norberg, C.; Filippone, G.; Andreopoulos, F.; Best, T.M.; Baraga, M.; Jackson, A.R.; Travascio, F. Viscoelastic and equilibrium shear properties of human meniscus: Relationships with tissue structure and composition. J. Biomech. 2021, 120, 110343. [Google Scholar] [CrossRef]
- Morejon, A.; Mantero, A.M.A.; Best, T.M.; Jackson, A.R.; Travascio, F. Mechanisms of energy dissipation and relationship with tissue composition in human meniscus. Osteoarthr. Cartil. 2022, 30, 605–612. [Google Scholar] [CrossRef]
- de Roy, L.; Morejon, A.; Jackson, A.R.; Travascio, F.; Seitz, A.M. A comparative study on the biomechanical properties of porcine meniscus in confined compression. In Proceedings of the 28th Congress of the European Society of Biomechanics, Maastricht, The Netherlands, 9–12 July 2023. [Google Scholar]

| Human <75 Years | Human ≥75 Years | Porcine | |
|---|---|---|---|
| Mean | 2.71 mm | 3.38 mm ‡ | 4.98 mm *** |
| SD | 0.60 mm | 0.85 mm | 1.13 mm |
| Human <75 Years | Human ≥75 Years | Porcine | |||
|---|---|---|---|---|---|
| (a) | Fc (N) | Mean | 60.1 | 54.5 | 168.9 *** |
| SD | 19.9 | 16.9 | 50.4 | ||
| (b) | Fu (N) | Mean | 75.1 | 70.5 | 205.8 *** |
| SD | 29.6 | 21.9 | 74.3 | ||
| (c) | Sc (MPa) | Mean | 47.4 | 33.8 * | 74.5 *** |
| SD | 14.1 | 13.1 | 27.5 | ||
| (d) | m (MPa) | Mean | 110.7 | 57.5 *** | 111.3 |
| SD | 55.6 | 32.0 | 52.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Trabalon, A.; Perez-Blanca, A.; Moreno-Vegas, S.; Estebanez-Campos, M.B.; Prado-Novoa, M. Assessment of Surrogate Models for Research on Resistance and Deformation of Repairs of the Human Meniscal Roots: Porcine or Older Human Models? Appl. Sci. 2024, 14, 670. https://doi.org/10.3390/app14020670
Peña-Trabalon A, Perez-Blanca A, Moreno-Vegas S, Estebanez-Campos MB, Prado-Novoa M. Assessment of Surrogate Models for Research on Resistance and Deformation of Repairs of the Human Meniscal Roots: Porcine or Older Human Models? Applied Sciences. 2024; 14(2):670. https://doi.org/10.3390/app14020670
Chicago/Turabian StylePeña-Trabalon, Alejandro, Ana Perez-Blanca, Salvador Moreno-Vegas, M. Belen Estebanez-Campos, and Maria Prado-Novoa. 2024. "Assessment of Surrogate Models for Research on Resistance and Deformation of Repairs of the Human Meniscal Roots: Porcine or Older Human Models?" Applied Sciences 14, no. 2: 670. https://doi.org/10.3390/app14020670





