Designing a Synthetic 3D-Printed Knee Cartilage: FEA Model, Micro-Structure and Mechanical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Data
- -
- Subject 1: group = D, age = 68, sex = M, knee = left, BMI = 27.47;
- -
- Subject 2: group = C, age = 26, sex = M, knee = left, BMI = 22.28.
2.2. Meshes Generation for FEM Analysis and 3D Printing
2.3. Design of Cartilage Tissue Micro-Structure
2.4. Synthetic Materials and 3D Printing
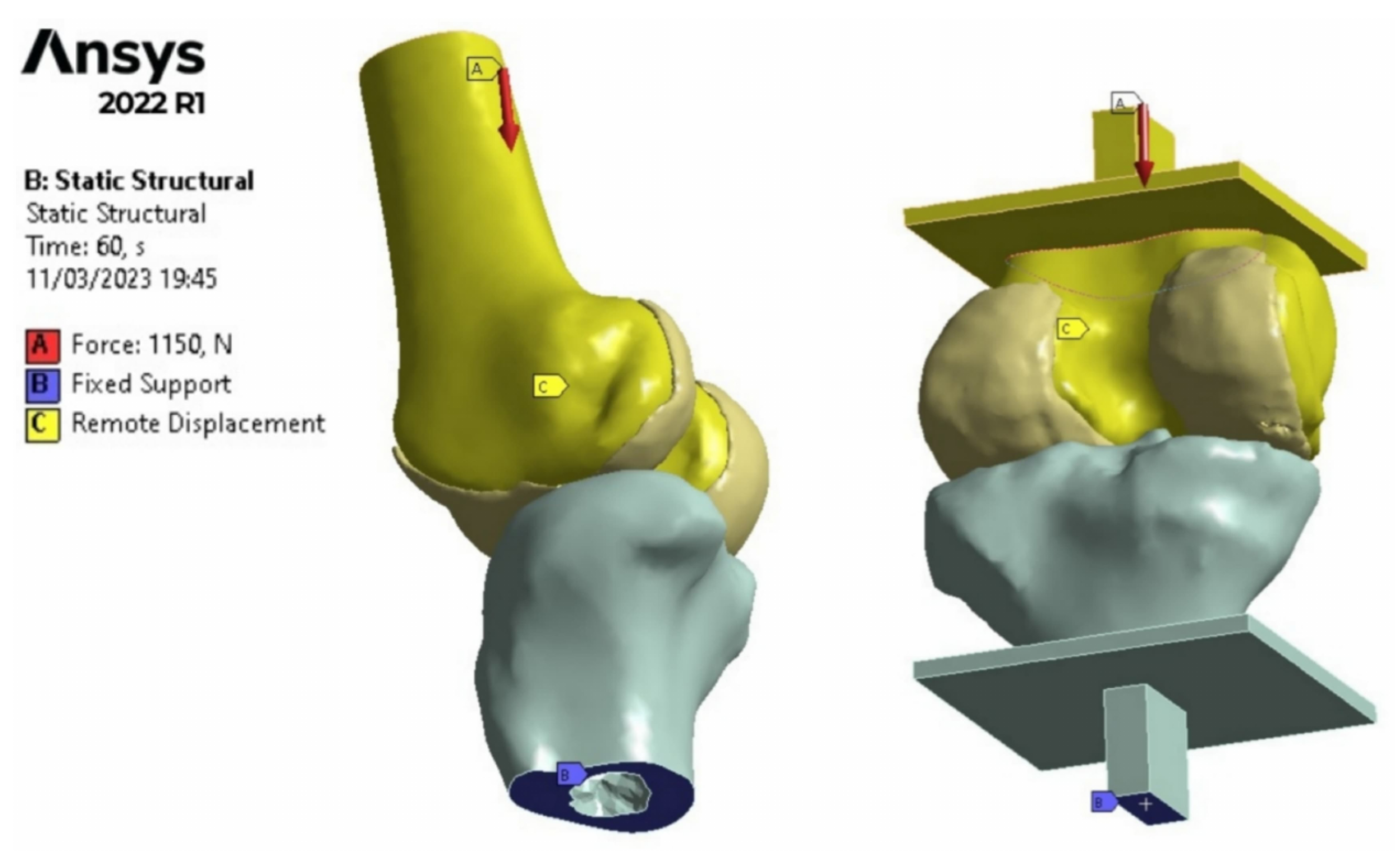
2.5. Finite Element Method Analysis
2.6. Mechanical Tests
3. Results
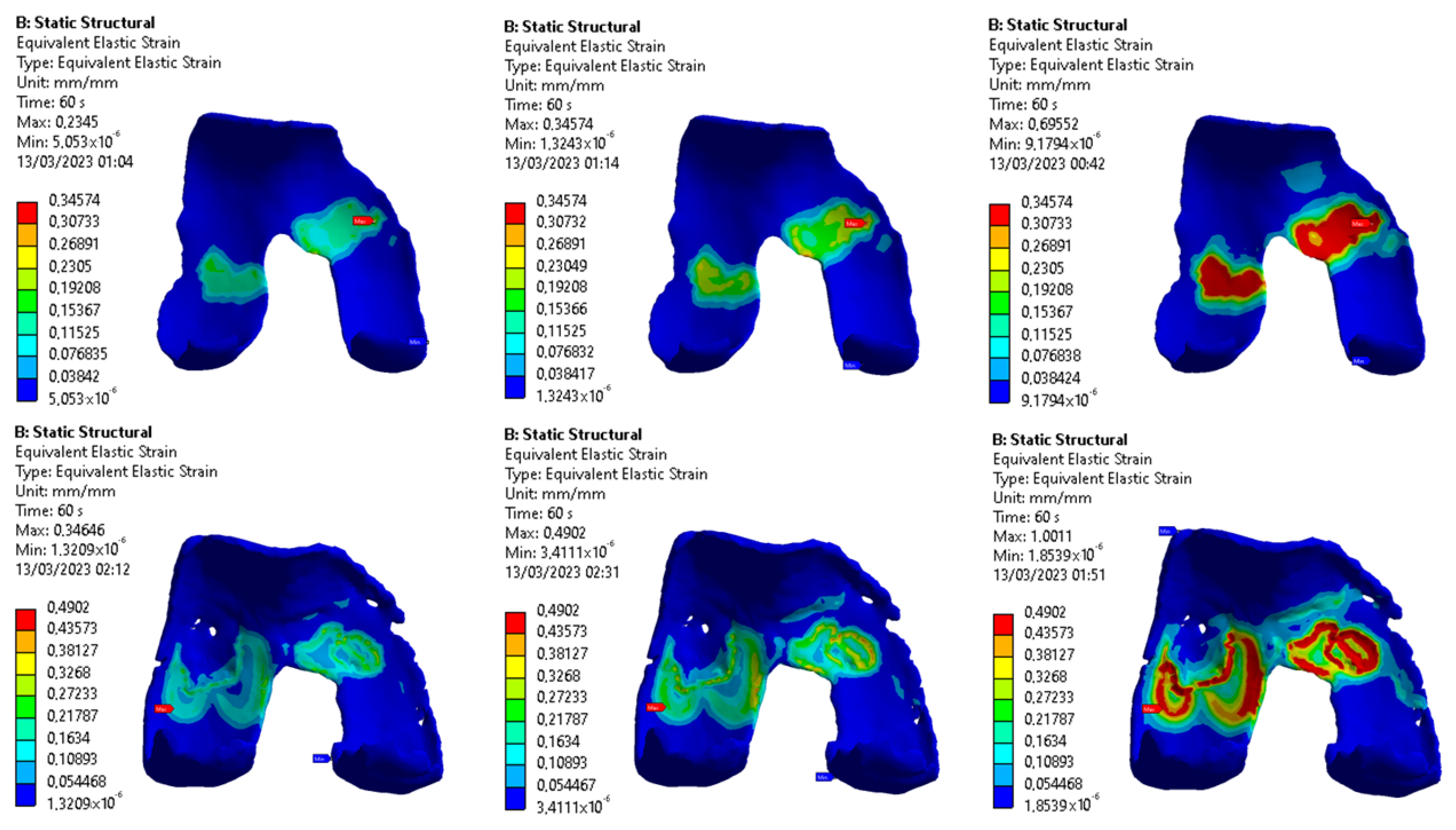
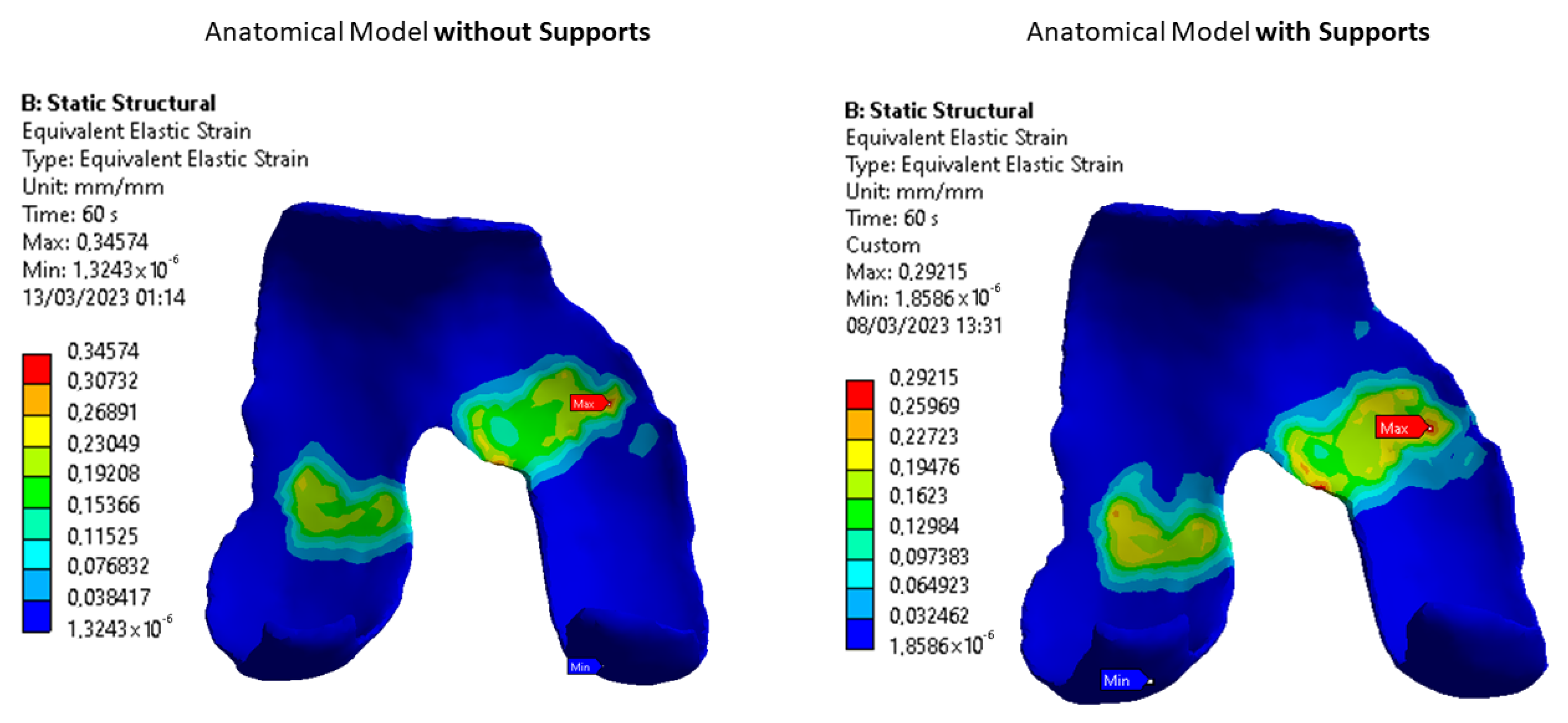
3.1. Images Post-Processing and FEA Results
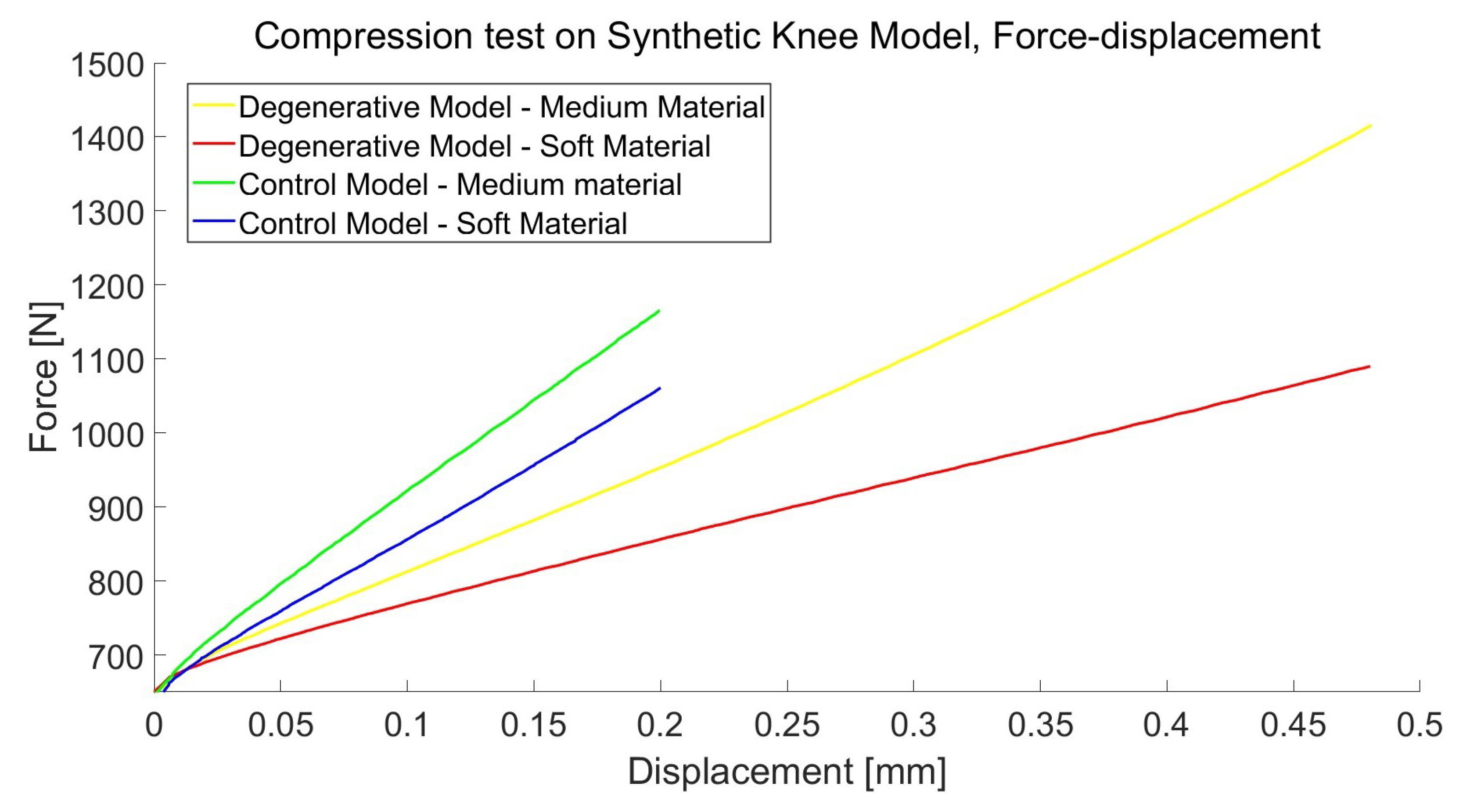
3.2. Mechanical Properties of Synthetic Polymers
4. Discussion
4.1. Radiological Data and FEA Simulation
4.2. Synthetic Knee Joint Testing
4.3. Fibers Contribute Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Mansour, J.M. Biomechanics of cartilage. Kinesiol. Mech. Pathomech. Hum. Mov. 2003, 2, 66–79. [Google Scholar]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Martin, J.A.; Brown, T.D. Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 2006, 43, 603–609. [Google Scholar] [PubMed]
- Roemer, F.W.; Demehri, S.; Omoumi, P.; Link, T.M.; Kijowski, R.; Saarakkala, S.; Crema, M.D.; Guermazi, A. State of the art: Imaging of osteoarthritis—Revisited 2020. Radiology 2020, 296, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.; Cole, B.; Dhawan, A.; Kercher, J.; Sherman, S. Clinical cartilage restoration: Evolution and overview. Clin. Orthop. Relat. Res. 2011, 469, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef]
- MacDonald, E.; Wicker, R. Multiprocess 3D printing for increasing component functionality. Science 2016, 353, aaf2093. [Google Scholar] [CrossRef]
- Gargiulo, P.; Giménez, C.C.; Pirozzi, M.A.; Thórdarson, Á.; Ricciardi, C.; Forni, R.; Bjornsson, G.A. 20 years of 3D printing in surgical planning of distraction osteogenesis. In Handbook of Surgical Planning and 3D Printing; Elsevier: Amsterdam, The Netherlands, 2023; pp. 95–123. [Google Scholar]
- Forni, R.; Agnarsdóttir, S.; Torfason, B.; Gargiulo, P. Heart surgery: Septal defect. In Handbook of Surgical Planning and 3D Printing; Elsevier: Amsterdam, The Netherlands, 2023; pp. 143–169. [Google Scholar]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704. [Google Scholar]
- J850 Digital Anatomy Printer Website. Available online: https://www.stratasys.com/en/3d-printers/printer-catalog/polyjet/j850-digital-anatomy/ (accessed on 30 November 2023).
- Severseike, L.; Lee, V.; Brandon, T.; Bakken, C.; Bhatia, V. Polyjet 3D printing of tissue-mimicking materials: How well can 3D printed synthetic myocardium replicate mechanical properties of organic myocardium? BioRxiv 2019. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kennedy, P.; Bonazza, N.; Yu, Y.; Dhawan, A.; Ozbolat, I. Three-dimensional bioprinting of articular cartilage: A systematic review. Cartilage 2021, 12, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, D.; Liu, Y.; Lueth, T.C. Fem-based mechanics modeling of bio-inspired compliant mechanisms for medical applications. IEEE Trans. Med Robot. Bionics 2020, 2, 364–373. [Google Scholar] [CrossRef]
- Aubonnet, R.; Ramos, J.; Recenti, M.; Jacob, D.; Ciliberti, F.; Guerrini, L.; Gislason, M.K.; Sigurjonsson, O.; Tsirilaki, M.; Jónsson, H., Jr.; et al. Toward New Assessment of Knee Cartilage Degeneration. Cartilage 2023, 14, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, F.K.; Guerrini, L.; Gunnarsson, A.E.; Recenti, M.; Jacob, D.; Cangiano, V.; Tesfahunegn, Y.A.; Islind, A.S.; Tortorella, F.; Tsirilaki, M.; et al. CT-and MRI-based 3D reconstruction of knee joint to assess cartilage and bone. Diagnostics 2022, 12, 279. [Google Scholar] [CrossRef]
- Maas, S.A.; Ellis, B.J.; Rawlins, D.S.; Weiss, J.A. Finite element simulation of articular contact mechanics with quadratic tetrahedral elements. J. Biomech. 2016, 49, 659–667. [Google Scholar] [CrossRef]
- Minns, R.; Steven, F. The collagen fibril organization in human articular cartilage. J. Anat. 1977, 123, 437. [Google Scholar]
- Muir, H.; Bullough, P.; Maroudas, A. The distribution of collagen in human articular cartilage with some of its physiological implications. J. Bone Jt. Surg. Br. Vol. 1970, 52, 554–563. [Google Scholar] [CrossRef]
- Responte, D.J.; Natoli, R.M.; Athanasiou, K.A. Collagens of articular cartilage: Structure, function, and importance in tissue engineering. Crit. Rev. Biomed. Eng. 2007, 35, 363–411. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Momot, K. Biophysics and Biochemistry of Cartilage by NMR and MRI; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Trad, Z.; Barkaoui, A.; Chafra, M.; Tavares, J.M.R. FEM Analysis of the Human Knee Joint: A Review; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Armstrong, C.; Lai, W.; Mow, V. An analysis of the unconfined compression of articular cartilage. J. Biomech. Eng. 1984, 106, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, P.S.; Spilker, R.L.; Ateshian, G.A.; Mow, V.C. Contact analysis of biphasic transversely isotropic cartilage layers and correlations with tissue failure. J. Biomech. 1999, 32, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Lai, W.M.; Mow, V.C. Biphasic indentation of articular cartilage—I. Theoretical analysis. J. Biomech. 1987, 20, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lopez, O.; Rubash, H. Variability of a three-dimensional finite element model constructed using magnetic resonance images of a knee for joint contact stress analysis. J. Biomech. Eng. 2001, 123, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Pena, E.; Calvo, B.; Martinez, M.; Doblare, M. A three-dimensional finite element analysis of the combined behavior of ligaments and menisci in the healthy human knee joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Blankevoort, L.; Huiskes, R. Validation of a three-dimensional model of the knee. J. Biomech. 1996, 29, 955–961. [Google Scholar] [CrossRef]
- Bachtar, F.; Chen, X.; Hisada, T. Finite element contact analysis of the hip joint. Med. Biol. Eng. Comput. 2006, 44, 643–651. [Google Scholar] [CrossRef]
- Haut Donahue, T.L.; Hull, M.; Rashid, M.M.; Jacobs, C.R. A finite element model of the human knee joint for the study of tibio-femoral contact. J. Biomech. Eng. 2002, 124, 273–280. [Google Scholar] [CrossRef]
- Yang, Z. Finite Element Analysis for Biomedical Engineering Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Bendjaballah, M.; Shirazi-Adl, A.; Zukor, D. Finite element analysis of human knee joint in varus-valgus. Clin. Biomech. 1997, 12, 139–148. [Google Scholar] [CrossRef]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. The effect of ageing and osteoarthritis on the mechanical properties of cartilage and bone in the human knee joint. Sci. Rep. 2018, 8, 5931. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. Tissue material properties and computational modelling of the human tibiofemoral joint: A critical review. PeerJ 2018, 6, e4298. [Google Scholar] [CrossRef] [PubMed]
- Thambyah, A.; Nather, A.; Goh, J. Mechanical properties of articular cartilage covered by the meniscus. Osteoarthr. Cartil. 2006, 14, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.; Seedhom, B. The ’instantaneous’ compressive modulus of human articular cartilage in joints of the lower limb. Rheumatology 1999, 38, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.; Hasler, E.; Hagg, R.; Weiler, C.; Jakob, R.; Mainil-Varlet, P. In situ compressive stiffness, biochemical composition, and structural integrity of articular cartilage of the human knee joint. Osteoarthr. Cartil. 2001, 9, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.; Seedhom, B. A technique for measuring the compressive modulus of articular cartilage under physiological loading rates with preliminary results. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1997, 211, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hori, R.Y.; Mockros, L. Indentation tests of human articular cartilage. J. Biomech. 1976, 9, 259–268. [Google Scholar] [CrossRef]
- Patel, J.M.; Wise, B.C.; Bonnevie, E.D.; Mauck, R.L. A systematic review and guide to mechanical testing for articular cartilage tissue engineering. Tissue Eng. Part C Methods 2019, 25, 593–608. [Google Scholar] [CrossRef]
- Setton, L.A.; Zhu, W.; Mow, V.C. The biphasic poroviscoelastic behavior of articular cartilage: Role of the surface zone in governing the compressive behavior. J. Biomech. 1993, 26, 581–592. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The biomechanics of cartilage—An overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Petitjean, N.; Canadas, P.; Royer, P.; Noël, D.; Le Floc’h, S. Cartilage biomechanics: From the basic facts to the challenges of tissue engineering. J. Biomed. Mater. Res. Part A 2023, 111, 1067–1089. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.; Bourban, P.; Pioletti, D. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Laasanen, M.; Töyräs, J.; Korhonen, R.; Rieppo, J.; Saarakkala, S.; Nieminen, M.; Hirvonen, J.; Jurvelin, J. Biomechanical properties of knee articular cartilage. Biorheology 2003, 40, 133–140. [Google Scholar] [PubMed]





| Layer | Length | Diameter |
|---|---|---|
| Superficial | 6 | 0.06 |
| Middle | 6 | 0.12 |
| Deep | 6 | 0.18 |
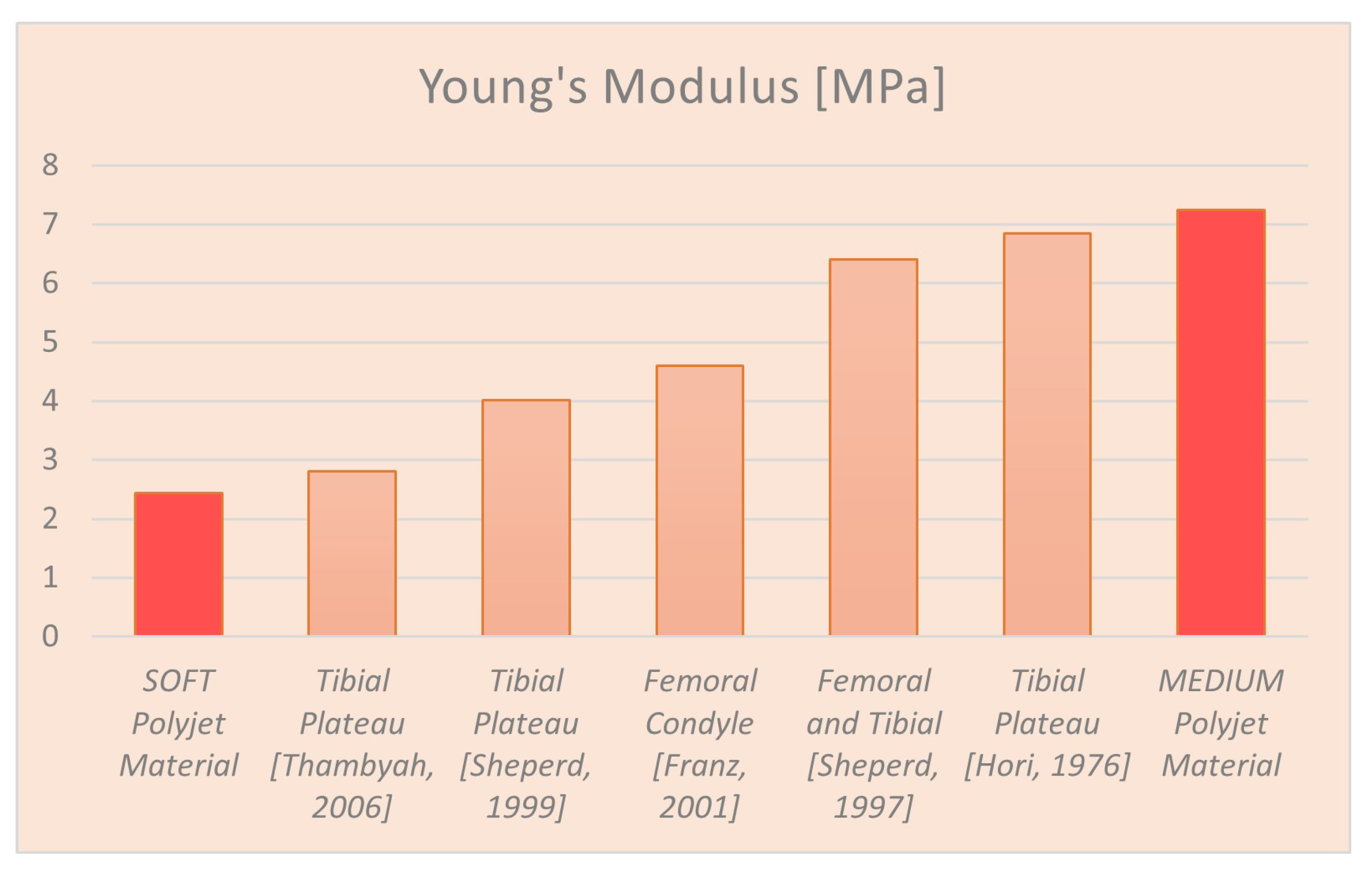
| Name | Young’s Modulus (MPa) |
|---|---|
| Cartilage Literature | 5.00 |
| Printed Cartilage Soft | 2.43 |
| Printed Cartilage Medium | 7.24 |
| Bone Literature | 17,000.00 |
| Printed Bone | 2500.00 |
| Layer | 1% Volume | 5% Volume |
|---|---|---|
| Superficial | 0.839 | 1.740 |
| Middle | 0.794 | 1.707 |
| Deep | 0.784 | 1.829 |
| Layer | 1% Volume | 5% Volume |
|---|---|---|
| Superficial | 0.233 | 0.409 |
| Middle | 0.221 | 0.379 |
| Deep | 0.337 | 1.266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolino, G.; Coato, D.; Forni, R.; Boretti, G.; Ciliberti, F.K.; Gargiulo, P. Designing a Synthetic 3D-Printed Knee Cartilage: FEA Model, Micro-Structure and Mechanical Characteristics. Appl. Sci. 2024, 14, 331. https://doi.org/10.3390/app14010331
Dolino G, Coato D, Forni R, Boretti G, Ciliberti FK, Gargiulo P. Designing a Synthetic 3D-Printed Knee Cartilage: FEA Model, Micro-Structure and Mechanical Characteristics. Applied Sciences. 2024; 14(1):331. https://doi.org/10.3390/app14010331
Chicago/Turabian StyleDolino, Gianmarco, Damiano Coato, Riccardo Forni, Gabriele Boretti, Federica Kiyomi Ciliberti, and Paolo Gargiulo. 2024. "Designing a Synthetic 3D-Printed Knee Cartilage: FEA Model, Micro-Structure and Mechanical Characteristics" Applied Sciences 14, no. 1: 331. https://doi.org/10.3390/app14010331
APA StyleDolino, G., Coato, D., Forni, R., Boretti, G., Ciliberti, F. K., & Gargiulo, P. (2024). Designing a Synthetic 3D-Printed Knee Cartilage: FEA Model, Micro-Structure and Mechanical Characteristics. Applied Sciences, 14(1), 331. https://doi.org/10.3390/app14010331







