Wintering Conditions and Heat Loss during Hibernation in the Brown Long-Eared Bat
Abstract
:1. Introduction
2. Methods
2.1. Body Mass Measurement
2.2. Refugioclimate Parameter Measurement
- –
- Gas parameter metre—for humidity (Rh), range of 0÷100%, resolution of indications 0.1% with an uncertainty of indications ±1.5%; for temperature (Ta), range of −50 ÷ 200 °C, resolution of indications 0.1 °C with an uncertainty of ±0.1 °C; and for atmospheric pressure (p), range of 500 ÷ 1500 hPa, resolution of indications1 hPa with an uncertainty of ±2 hPa;
- –
- Thermo-anemometer (portable digital air speed (v) and temperature metre)—the measurement range was 0.01 ÷ 20m/s, with an uncertainty of indications ±0.01 m/s.
2.3. Heat Loss Calculation
- (a)
- Based on fat burned
- ER—energy reserve, J.
- mfat—mass of fat, g.
- qfat burn—energy according to Poczopko [33] 39.75 kJ/g.
- Qhib—heat, W.
- τ—hibernation time, s.
- qhib—density of heat flux, W/m2.
- S—surface, m2.
- h—height (0.04 m).
- r—radius of the cylinder, calculated as the average of thickness and width (9.75 × 10−3 m).
- (b)
- Based on refugioclimate parameters
- α—heat transfer coefficient, W/m2K.
- Δt—difference between the bat’s body temperature, Tb, and the ambient temperature, Ta(K).
- Re—Reynolds number.
- Nu—Nusselt number.
- w—air velocity, m/s.
- l—characteristic dimensions (diameter of the cylinder), m.
- ν—kinematic viscosity coefficient of the air, m2/s.
- λ—thermal conductivity coefficient of the air, W/m·K.
- α—heat transfer coefficient, W/m2·K.
3. Results
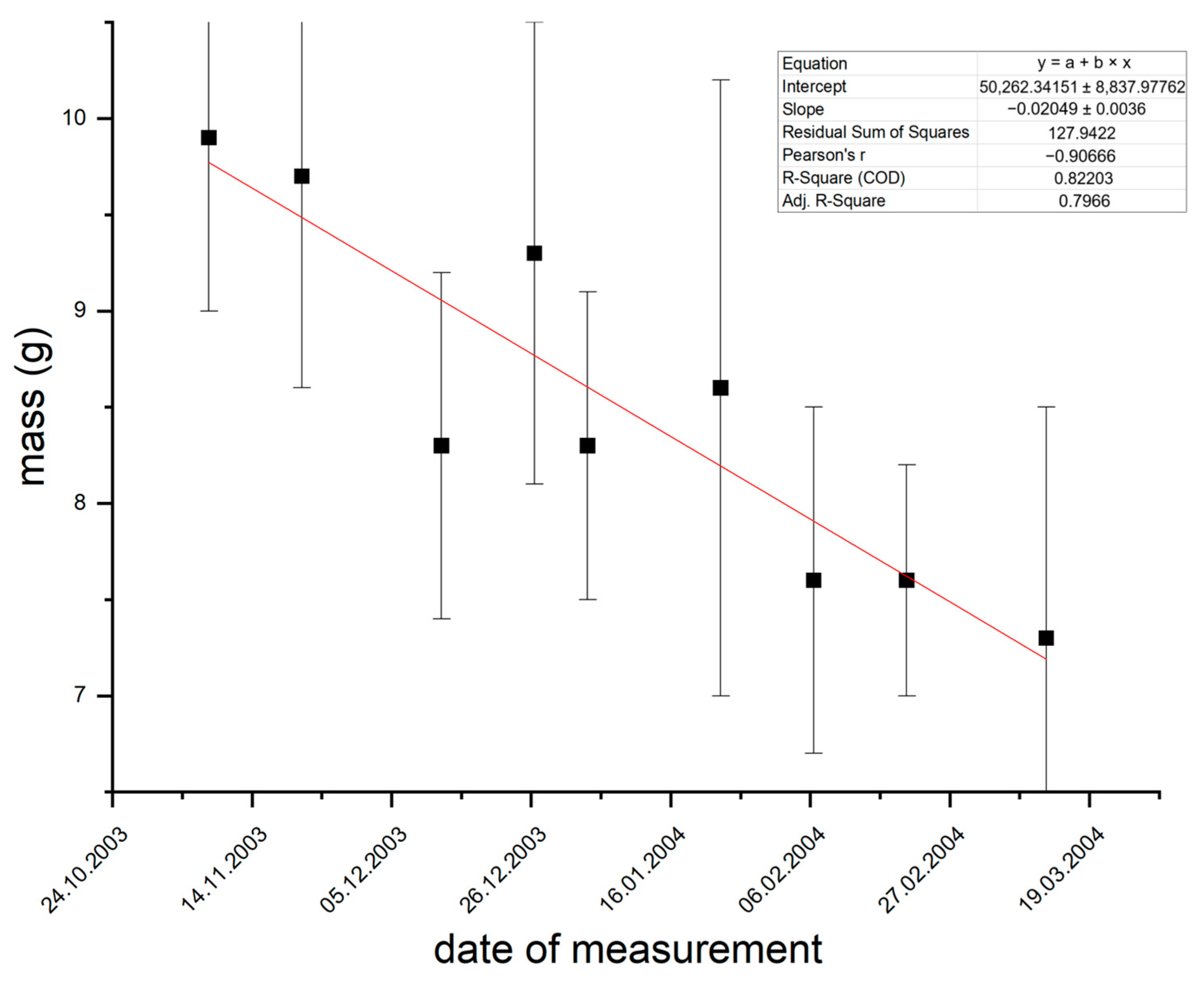
3.1. Body Mass Measurement
3.2. Refugioclimate Parameter Measurement
3.3. Heat Loss Calculation
- (a)
- Based on fat burned
- (b)
- Based on refugioclimate parameters
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyles, J.G.; Dunbar, M.B.; Storm, J.J.; Brack, V., Jr. Energy availability influences microclimate selection of hibernating bats. J. Exp. Biol. 2007, 210, 4345–4350. [Google Scholar] [CrossRef]
- Hranac, C.R.; Haase, C.G.; Fuller, N.W.; McClure, M.L.; Marshall, J.C.; Lausen, C.L.; McGuire, L.P.; Olson, S.H.; David, T.S. What is winter? Modeling spatial variation in bat host traits and hibernation and their implications for overwintering energetics. Ecol. Evol. 2021, 11, 11604–11614. [Google Scholar] [CrossRef]
- Marroquin, C.M.; Lavine, J.O.; Windstam, S.T. Effect of Humidity on Development of Pseudogymnoascus de-structans, the Causal Agent of Bat White-Nose Syndrome. Northeast. Nat. 2017, 24, 54–64. [Google Scholar] [CrossRef]
- Gaisler, J. Remarks on the thermopreferendum of Palearctic bats in their natural habits. Bijdr. Dierk. 1970, 40, 33–35. [Google Scholar] [CrossRef]
- Haase, C.G.; Fuller, N.W.; Dzal, Y.A.; Hranac, C.R.; Hayman, D.T.S.; Lausen, C.L.; Silas, K.A.; Olson, S.H.; Plowright, R.K. Body mass and hibernation microclimate may predict bat susceptibility to white-nose syndrome. Ecol. Evol. 2021, 11, 506–515. [Google Scholar] [CrossRef]
- Kłys, G. Multifactor Analysis of Refugioclimate in Places of Hibernation of Chosen Bat Species; Studia Chiropterologica: Annals of the Chiropterological Information Center: Krakó, Poland, 2013; Volume 8. [Google Scholar]
- Czenze, Z.J.; Park, A.D.; Willis, C.K.R. Staying cold through dinner: Cold-climate bats rewarm with conspecifics but not sunset during hibernation. J. Comp. Physiol. B 2013, 183, 859–866. [Google Scholar] [CrossRef]
- Haase, C.G.; Fuller, N.W.; Hranac, C.R.; Hayman, D.T.S.; Olson, S.H.; Plowright, R.K.; McGuire, L.P. Bats are not squirrels: Revisiting the cost of cooling in hibernating mammals. J. Therm. Biol. 2019, 81, 185–193. [Google Scholar] [CrossRef]
- Humphries, M.M.; Thomas, D.W.; Speakman, J.R. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 2002, 418, 313–316. [Google Scholar] [CrossRef]
- McClure, M.L.; Haase, C.G.; Hranac, C.R.; Hayman, D.T.S.; Dickson, B.G.; McGuire, L.P.; Crowley, D.; Fuller, N.W.; Lausen, C.L.; Plowright, R.K.; et al. A hybrid correlative-mechanistic approach for modeling winter distributions of North American bat species. J. Biogeogr. 2021, 48, 2429–2444. [Google Scholar] [CrossRef]
- Perry, R.W. A review of factors affecting cave climates for hibernating bats intemperate North America. Environ. Rev. 2013, 21, 28–39. [Google Scholar] [CrossRef]
- Niedźwiedź, T. Słownik Meteorologiczny; Polskie Towarzystwo Geograficzne: Warszawa, Poland, 2003. [Google Scholar]
- Paszyński, J.; Miara, K.; Skoczek, J. Wymiana energii między atmosferą a podłożem jako podstawa kartowania topoklimatycznego. In Dokumentacja Geograficzna 14; Instytut Geografii i Przestrzennego Zagospodarowania PAN: Warszawa, Poland, 1999. [Google Scholar]
- Pawiński, J.; Roszkowski, J.; Strzemiński, J. Przewietrzanie Kopalń; Śląskie Wydawnictwo Techniczne: Katowice, Poland, 1995. [Google Scholar]
- Whittow, J. Dictionary of Physical Geography; Penguin Books: Great Britain, UK, 1986; p. 591. [Google Scholar]
- Wiglej, T.M.L.; Brown, M.C. The Physic of Caves. In The Science of Speleology; Ford, T.D., Cullingford, C.H.D., Eds.; Academic Press: Cambridge, MA, USA, 1976. [Google Scholar]
- Thomas, D.W. Hibernating bats are sensitive to nontactile human disturbance. J. Mammal. 1995, 76, 940–946. [Google Scholar] [CrossRef]
- Thomas, D.W. The physiological ecology of hibernation in vespertilionid bats. Symp. Zool. Soc. Lond. 1995, 67, 219–231. [Google Scholar]
- Turbill, C.; Bieber, C.; Ruf, T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B Biol. Sci. 2011, 278, 3355–3363. [Google Scholar] [CrossRef]
- Zubait, A.; McCracken, G.F.; Kunz, T.H. Functional and Evolutionary Ecology of Bats; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Schmidt-Nielsen, K. Fizjologia Zwierząt; PWN: Warszawa, Poland, 2008. [Google Scholar]
- Boyles, J.G.; Johnson, J.S.; Blomberg, A.; Lilley, T.M. Optimal hibernation theory. Mammal Rev. 2019, 50, 91–100. [Google Scholar] [CrossRef]
- Geise, F. Ecological Physiology of Daily Torpor and Hibernation; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Koteja, P.; Jurczyszyn, M.; Wołoszyn, B. Energy balance of hibernating mouse-eared bat Myotis myotis: A study with a TOBEC instrument. Acta Theriol. 2001, 46, 1–12. [Google Scholar] [CrossRef]
- Ransome, R. The Natural History of Hibernating Bats; Christpher Helm: London, UK, 1990; pp. 1–235. [Google Scholar]
- Speakman, J.R.; Racey, P.A. Hibernal ecology of the pipistrelle bat: Energy expenditure, water requirements and mass loss, implications for survival and the function of winter emergence flights. J. Anim. Ecol. 1989, 58, 797–813. [Google Scholar] [CrossRef]
- Thomas, D.W.; Dorais, M.; Bergeron, J.-M. Winter energy budgets and cost of arousals for hibernating little brown bats, Myotis lucifugus. J. Mammal. 1990, 71, 475–479. [Google Scholar] [CrossRef]
- Harmata, W. The thermopreferendum of some species of bats (Chiroptera). Acta Theriol. 1969, 14, 49–62. [Google Scholar] [CrossRef]
- Drenda, J. Ocena klimatycznych warunków pracy górników w polskich kopalniach węgla kamiennego i rudy miedzi. Górnictwo I Geol. 2012, 7, 19–35. [Google Scholar]
- Wacławik, J. Wentylacja Kopalń; T. 1,2 Wydawnictwa Akademii Górniczo-Hutniczej: Kraków, Poland, 2010. [Google Scholar]
- Kokurewicz, T. Ochrona nietoperzy w obszarze Natura 2000 “Nietoperek” z perspektywy 20 lat doświadczeń. In Materiały Ogólnopolskiej Konferencji Chiropterologicznej; Warchałowski, M., Ed.; Grunwald24: Krynica Zdrój, Poland, 2013; pp. 36–37. [Google Scholar]
- Kłys, G. Bats in the Tarnowskie Góry-Bytom Underground system. In The Influence of Environmental Conditions on the Bat Hibernaculum Choice; Kłys, G., Wołoszyn, B.W., Jagt-Yazykowa, E., Kuśnierz, A., Eds.; ZPW Plik: Bytom, Poland, 2008. [Google Scholar]
- Poczopko, P. Ciepło a życie. In Zarys Termofizjologii Zwierząt; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1990. [Google Scholar]
- Kowalski, K.; Ruprecht, L. Rząd nietoperze. In Klucz Dooznaczania Ssaków Polski; Pucek, Z., Ed.; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1984; pp. 85–139. [Google Scholar]
- Henshaw, R.E.; Folk, G.E. Relation of termoregulation toseasonally changing microclimate in two species of bats Myotis lucifugus and M. sodalis. Physiol. Zool. 1966, 39, 223–236. [Google Scholar] [CrossRef]
- McNab, B.K. The behavior of temperature cave bats in a subtropical environment. Ecology 1974, 55, 943–958. [Google Scholar] [CrossRef]
- McNab, B.K. Evolutionary alternatives in the physiological ecology of bats. In Ecology of Bats; Kunz, T.H., Ed.; Plenum Press: New York, NY, USA; London, UK, 1982; pp. 151–200. [Google Scholar]
- Bohdal, T.; Charun, H. Zasady Transportu Ciepła, cz. 2; Wydawnictwo Uczelniane Politechniki Koszalińskiej: Koszalin, Poland, 2013. [Google Scholar]
- Harrje, C. Etho-ökologische Untersuchung der ganzjährigen Aktivitt von Wasserfledermäusen (Myotis daubentoni Kuhl, 1819) am Winterquartier. Mitteilungen Naturwissenschaftlichen Ges. Schaffhausen 1994, 39, 15–52. [Google Scholar]
- Pistole, D.H. Sexual differences in the annual lipid cycle of the big Brown bat Eptesicus fuscus. Can. J. Zool. 1988, 67, 1891–1894. [Google Scholar] [CrossRef]
- Hayman, D.T.S.; Cryan, P.M.; Fricker, P.D.; Dannemiller, N.G. Long-term video surveillance and automated analyses revealarousal patterns in groups of hibernating bats. Methods Ecol. Evol. 2017, 8, 1813–1821. [Google Scholar] [CrossRef]
- Cheng, T.L.; Gerson, A.; Moore, M.S.; Reichard, J.D.; DeSimone, J.; Willis, C.K.R.; Frick, W.F.; Kilpatrick, A.M. Higher fat stores contribute to persistence of little brown bat populations with white-nose syndrome. J. Anim. Ecol. 2019, 88, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Bachorec, E.; Bartonicka, T.; Heger, T.; Pikula, J.; Zukal, J. Cold arousal—A mechanism used by hibernating bats to reduce the energetic costs of disturbance. J. Therm. Biol. 2021, 101, 103–107. [Google Scholar] [CrossRef]
- Johnson, H.E.; Lewis, D.L.; Verzuh, T.L.; Wallace, C.F.; Much, R.M.; Willmarth, L.K.; Breck, S.W. Human development and climate affect hibernation in a large carnivore with implications for human–carnivoreconflicts. J. Appl. Ecol. 2017, 55, 663–672. [Google Scholar] [CrossRef]
- Nagel, A.; Nagel, R. How do bats choose optimal hibernation temperatures for hibernation? Comp. Bio-Chem. Physiol. 1991, 99A, 323–326. [Google Scholar] [CrossRef]
- Klüg-Baerwald, J.; Gower, L.E.; Lausen, C.L.; Brigham, R.M. Environmental correlates and energetics of winter flight by bats in southern Alberta, Canada. Can. J. Zool. 2016, 94, 829–836. [Google Scholar] [CrossRef]
- Bogdanowicz, W. Community structure and interspecific interactions in bats hibernating in Poznań. Acta Theriol. 1983, 28, 357–370. [Google Scholar] [CrossRef]
- Bogdanowicz, W.; Urbańczyk, Z. Some ecological aspects of bats hibernating in city of Poznań. Acta Theriol. 1983, 28, 371–385. [Google Scholar] [CrossRef]
- Daan, S.; Wichers, H.J. Habitat selection of bats hibernating in a limestone cave. Z. Saugetierk. 1968, 33, 262–287. [Google Scholar]
- Harmata, W. Nietoperze zimujące w fortyfikacjach twierdzy Kraków. In Zimowe Spisy Nietoperzy w Polsce 1988–1992. Wyniki i Ocena Skuteczności; Wołoszyn, B.W., Ed.; Publikacje Centrum Informacji Chiropterologicznej ISEZ PAN Kraków: Warszawa, Poland, 1994; pp. 69–90. [Google Scholar]
- Lesiński, G. Ecology of bats hibernating underground in Central Poland. Acta Theriol. 1986, 31, 507–521. [Google Scholar] [CrossRef]
- Webb, P.I.; Speakman, J.R.; Racey, P. How hot is a hibernaculum? A review of the temperatures at which bats hibernate. Can. J. Zool. 1996, 74, 761–765. [Google Scholar] [CrossRef]
- Boyles, J.G.; Boyles, E.; Dunlap, R.K.; Johnson, S.A.; Brack, V. Long-term microclimate measurements add further evidence that there is no “optimal” temperature for bat hibernation. Mamm. Biol. 2017, 86, 9–16. [Google Scholar] [CrossRef]
- Humphries, M.M.; Kramer, D.L.; Thomas, D.W. The Role of Energy Availability in Mammalian Hibernation: An Experimental Test in Free-Ranging Eastern Chipmunks. Physiol. Biochem. Zool. 2003, 76, 180–186. [Google Scholar] [CrossRef]
- Nowack, J.; Levesque, D.L.; Reher, S.; Dausmann, K.H. Variable Climates Lead to Varying Phenotypes: “Weird” Mammalian Torpor and Lessons from Non-Holarctic Species. Front. Ecol. Evol. 2020, 8, 60. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. Mammalian hibernation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 326, 669–986. [Google Scholar]
- Thomas, D.W.; Geiser, F. Periodic arousals in hibernating mammals: Is evaporative water loss involved? Funct. Ecol. 1997, 11, 585–591. [Google Scholar] [CrossRef]
- Wojciechowski, M.S.; Jefimow, M.; Tęgowska, E. Environmental conditions, rather than season, determine torpor use and temperature selection in large mouse-eared bats (Myotis myotis). Comp. Biochem. Physiol. 2007, 147A, 828–840. [Google Scholar] [CrossRef]
- McGuire, L.P.; Mayberry, H.W.; Willis, C.K. White-nose syndrome increases torpid metabolic rate and evaporative water loss in hibernating bats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 313, R680–R686. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.W.; Cloutier, D. Evaporative water by hibernating little brown bats, Myotis lucifugus. Physiol. Zool. 1992, 65, 443–456. [Google Scholar] [CrossRef]
- Speakman, J.R. Position of the pinnae and thermoregulatory status in litlie brown bats (Plecotus auritus). J. Therm. Biol. 1988, 13, 25–29. [Google Scholar] [CrossRef]

| Data | m | sd | Cv | n | N |
|---|---|---|---|---|---|
| 11 October 2003 | 8.2 | 1.34 | 16 | 91 | 0 |
| 25 October 2003 | 9.8 | 1.31 | 13 | 38 | 14 |
| 8 November 2003 | 9.9 | 0.86 | 9 | 38 | 14 |
| 22 November 2003 | 9.7 | 1.07 | 11 | 15 | 14 |
| 13 December 2003 | 8.3 | 0.94 | 11 | 10 | 21 |
| 27 December 2003 | 9.3 | 1.24 | 13 | 7 | 14 |
| 4 January 2004 | 8.3 | 0.82 | 10 | 5 | 8 |
| 24 January 2004 | 8.6 | 1.56 | 18 | 8 | 20 |
| 7 February 2004 | 7.6 | 0.93 | 12 | 14 | 14 |
| 21 February 2004 | 7.6 | 0.57 | 8 | 16 | 14 |
| 13 March 2004 | 7.3 | 1.22 | 17 | 25 | 21 |
| 27 March 2004 | 8.8 | 0.95 | 11 | 37 | 14 |
| 10 April 2004 | 7.9 | 0.80 | 10 | 44 | 14 |
| 24 April 2004 | 7.7 | 0.75 | 10 | 22 | 14 |
| 8 May 2004 | 9.3 | 0.58 | 6 | 3 | 14 |
| Date | T, °C | sd T, °C | Cv, % | Rh, % | sd Hr, % | Cv, % | v, m/s | sd v, m/s | Cv, % | P, hPa | n, - |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 January 2002 | 3.0 | 0.96 | 30 | 98.0 | 0.57 | 1 | 0.08 | 0.01 | 11 | 1000 | 30 |
| 19 January 2002 | 3.0 | 1.23 | 47 | 97.0 | 0.89 | 1 | 0.07 | 0.02 | 28 | 1000 | 30 |
| 1 February 2002 | 5.0 | 1.15 | 22 | 97.0 | 0.65 | 1 | 0.06 | 0.02 | 26 | 1000 | 34 |
| 22 February 2002 | 5.0 | 1.35 | 27 | 97.0 | 0.84 | 1 | 0.06 | 0.01 | 19 | 1000 | 28 |
| 8 December 2002 | 5.0 | 1.53 | 31 | 97.0 | 0.64 | 1 | 0.07 | 0.01 | 17 | 1000 | 28 |
| 21 December 2002 | 6.0 | 1.26 | 23 | 97.0 | 0.50 | 1 | 0.07 | 0.01 | 13 | 1000 | 26 |
| 5 January 2007 | 8.0 | 0.41 | 5 | 82.0 | 2.87 | 4 | 0.36 | 0.13 | 38 | 1000 | 58 |
| 6 January 2007 | 10.0 | 0.55 | 6 | 74.0 | 25.64 | 35 | 0.21 | 0.01 | 47 | 1000 | 55 |
| 7 January 2007 | 9.0 | 0.60 | 7 | 81.0 | 1.93 | 2 | 0.06 | 0.04 | 60 | 1000 | 3 |
| 8 January 2007 | 9.0 | 0.22 | 3 | 82.0 | 1.76 | 2 | 0.06 | 0.04 | 59 | 1000 | 24 |
| 9 January 2007 | 10.0 | 0.39 | 4 | 91.0 | 0.63 | 1 | 0.50 | 0.29 | 58 | 1000 | 5 |
| 7 February 2007 | 7.0 | 0.77 | 12 | 89.0 | 3.36 | 4 | 0.95 | 0.35 | 36 | 1000 | 25 |
| 8 February 2007 | 8.0 | 0 | 0 | 84.0 | 0 | 0 | 0.61 | 0.04 | 7 | 1000 | 4 |
| 9 February 2007 | 7.0 | 0.45 | 6 | 85.0 | 10.32 | 12 | 0.54 | 0.03 | 6 | 1000 | 8 |
| 11 February 2007 | 10.0 | 1.15 | 11 | - | - | - | 0.53 | 0.53 | 100 | 1000 | 8 |
| 13 February 2007 | 7.0 | 1.35 | 18 | 84.0 | 6.03 | 7 | 0.22 | 0.19 | 83 | 1000 | 74 |
| 14 February 2007 | 9.0 | 0.41 | 4 | 88.0 | 0.37 | 0 | 0.20 | 0.04 | 18 | 1000 | 6 |
| 15 February 2007 | 9.0 | 1.13 | 12 | 78.0 | 5.94 | 8 | 0.08 | 0.05 | 66 | 1000 | 86 |
| Measurement Date | qhib, W/m2 | sdqhib, W/m2 | Cv, % | n, - |
|---|---|---|---|---|
| 4 January 2002 | 2 | 0.2 | 7 | 30 |
| 19 January 2002 | 2 | 0.4 | 18 | 30 |
| 1 February 2002 | 2 | 0.3 | 15 | 34 |
| 22 February 2002 | 2 | 0.2 | 11 | 28 |
| 8 December 2002 | 2 | 0.2 | 11 | 28 |
| 21 December 2002 | 2 | 0.2 | 8 | 26 |
| 5 January 2007 | 5 | 1.6 | 28 | 58 |
| 6 January 2007 | 4 | 1.2 | 31 | 55 |
| 7 January 2007 | 2 | 0.7 | 37 | 3 |
| 8 January 2007 | 2 | 0.7 | 36 | 24 |
| 9 January 2007 | 6 | 3.3 | 51 | 5 |
| 7 February 2007 | 10 | 2.5 | 26 | 25 |
| 8 February 2007 | 8 | 0.3 | 4 | 4 |
| 9 February 2007 | 7 | 0.3 | 4 | 8 |
| 11 February 2007 | 6 | 4.1 | 65 | 8 |
| 13 February 2007 | 4 | 2.1 | 54 | 74 |
| 14 February 2007 | 4 | 0.4 | 12 | 6 |
| 15 February 2007 | 2 | 1.1 | 54 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłys, G.; Makuchowska-Fryc, J. Wintering Conditions and Heat Loss during Hibernation in the Brown Long-Eared Bat. Appl. Sci. 2024, 14, 716. https://doi.org/10.3390/app14020716
Kłys G, Makuchowska-Fryc J. Wintering Conditions and Heat Loss during Hibernation in the Brown Long-Eared Bat. Applied Sciences. 2024; 14(2):716. https://doi.org/10.3390/app14020716
Chicago/Turabian StyleKłys, Grzegorz, and Joanna Makuchowska-Fryc. 2024. "Wintering Conditions and Heat Loss during Hibernation in the Brown Long-Eared Bat" Applied Sciences 14, no. 2: 716. https://doi.org/10.3390/app14020716





