Eco-Friendly Alternatives in Leather Production: Performance of Biodegradable Alginate-Based Retanned Leather Compared to Conventional Leathers and Plant-Based Materials
Abstract
:1. Introduction
2. Materials and Methods
- Sample #1: Finished ovine leather, chromium-tanned;
- Sample #2: Finished bovine leather, chromium-tanned;
- Sample #3: Finished bovine leather, vegetable-tanned;
- Sample #4: Leatherette;
- Sample #5: Piñatex®;
- Sample #6: Desserto®;
- Sample #7: Bovine leather tanned with SAD;
- Sample #8: Bio-based finished bovine leather tanned with SAD and ZnO nanoparticles;
- Sample #9: Bovine leather tanned with SAD and Tara;
- Sample #10: Bovine leather tanned with SAD, ZnO nanoparticles and Tara.
- -
- Thickness determination according to the standard EN ISO 2589 (mm) [16].
- -
- Tensile strength according to the standard EN ISO 3376 (N/mm2) [17].
- -
- Tear strength according to the standard EN ISO 3377-2 (N/mm) [18].
- -
- Determination of distension (mm) and strength (kg) of surface according to the standard EN ISO 3379 [19].
- -
- Water vapor permeability according to the standard EN ISO 14268 (mg/h·cm2) [20].
- -
- Shrinkage temperature according to the standard EN ISO 3380 (°C) [21].
- -
- Determination of flex resistance according to the standard EN ISO 5402-1 [22].
- -
- Light fastness according to the standard ISO 105:B02 [23].
- -
- Accelerated aging color change according to the standard ISO 17228 [24].
- -
- Color fastness to rubbing according to the standard ISO 11640 [25].
- -
- Finishing adhesion according to the standard ISO 11644 (N/cm) [26].
- -
- Determination of substances soluble in dichloromethane according to the standard EN ISO 4048 (%) [27].
- -
- Water and volatile content according to the standard EN ISO 4684 (%) [28].
- -
- Extractable organic and inorganic matter according to the standard EN ISO 4098 (%) [29].
- -
- Ash and insoluble mineral matter according to the standard EN ISO 4047 (%) [30].
- -
- Nitrogen and leather substance according to the standard ISO 5397 (%) [31].
- -
- pH and difference index according to the standard EN ISO 4045 [32].
- -
- Determination of metal content: total metal content according to the standard EN ISO 17072-2 (mg/kg) [33].
- -
- Determination of formaldehyde content in leather: HPLC quantification, according to the standard EN ISO 17226-1 (mg/kg) [34].
3. Results
3.1. Physicochemical Characterization
3.2. Thermometric Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naviglio, G.; Calvanese, B.G.; Tortora, G.; Cipollaro, L.; Pierri, G. Characterization of tannery chemicals: Retanning agents. Cuoio Pelli Mater. Concia. 2006, 82, 83–102. [Google Scholar]
- Rydin, S.; Black, M.; Canova, M.; Scalet, B.M.; Roudier, S.; Delgado Sancho, S. Best Available Techniques (BAT) Reference Document for the Tanning of Hides and Skins; Europan Commission, Publications Office of the European Union: Luxembourg, 2013; p. 295. [Google Scholar] [CrossRef]
- Buljan, J.; Král, I. The Framework for Sustainable Leather Manufacture; UNIDO: Vienna, Austria, 2019; p. 164. [Google Scholar]
- Marion, P.; Bernela, B.; Piccirilli, A.; Patouillard, N.; Guilbot, J.; Jérôme, F. Sustainable chemistry: How to produce better and more from less? Green Chem. 2017, 19, 4973–4989. [Google Scholar] [CrossRef]
- RameshKumar, R.; Shaiju, P.; O’Connor, K.E.; Ramesh Babu, P. Bio-based and biodegradable polymers -State-of-the-art, challenges and emerging trends. Curr. Opin. Green Sustain. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Chen, T.L.; Kim, H.; Pan, S.Y.; Tseng, P.C.; Lin, Y.P.; Chiang, P.C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef]
- Tamilselvi, A.; Jayakumar, G.C.; Sri Charan, K.; Sahu, B.; Deepa, P.R.; Kanth, S.V.; Kanagaraj, J. Extraction of cellulose from renewable resources and its application in leather finishing. J. Clean. Prod. 2019, 230, 694–699. [Google Scholar] [CrossRef]
- Ariram, N.; Madhan, B. Development of bio-acceptable leather using bagasse. J. Clean. Prod. 2020, 250, 119441. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Bidgol, N.S.S. Recent progresses in the application of cellulose, starch, alginate, gum, pectin, chitin and chitosan based (nano)catalysts in sustainable and selective oxidation reactions: A review. Carbohydr. Polym. 2020, 241, 116353. [Google Scholar] [CrossRef]
- Ravichandran, V.; Jayakrishnan, A. Synthesis and evaluation of anti-fungal activities of sodium alginate-amphotericin B conjugates. Int. J. Biol. Macromol. 2018, 108, 1101–1109. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Feng, L.; Cao, Y.; Xu, D.; Wang, S.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochem. 2017, 34, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Vicini, S.; Castellano, M. Depolymerization of sodium alginate in saline solutions via ultrasonic treatments: A rheological characterization. Food Hydrocoll. 2020, 109, 106128. [Google Scholar] [CrossRef]
- Ding, W.; Yi, Y.; Wang, Y.; Zhou, J.; Shi, B. Preparation of a Highly Effective Organic Tanning Agent with Wide Molecular Weight Distribution from Bio-Renewable Sodium Alginate. Chem. Sel. 2018, 3, 12330–12335. [Google Scholar] [CrossRef]
- EN ISO 2589:2016; Leather—Physical and Mechanical Tests—Determination of Thickness. AENOR: Madrid, Spain, 2016.
- EN ISO 3376:2021; Leather—Physical and Mechanical Tests—Determination of Tensile Strength and Percentage Elongation. AENOR: Madrid, Spain, 2021.
- EN ISO 3377-2:2016; Leather—Physical and Mechanical Tests—Determination of Tear Load—Part 2: Double Edge Tear. AENOR: Madrid, Spain, 2016.
- EN ISO 3379:2016; Leather—Determination of Distension and Strength of Surface (Ball Burst Method). AENOR: Madrid, Spain, 2016.
- EN ISO 14268:2013; Leather—Physical and Mechanical Tests—Determination of Water Vapour Permeability. AENOR: Madrid, Spain, 2013.
- EN ISO 3380:2015; Leather—Physical and Mechanical Tests—Determination of Shrinkage Temperature Up to 100 °C. AENOR: Madrid, Spain, 2015.
- EN ISO 5402-1:2018; Leather—Determination of Flex Resistance—Part 1: Flexometer Method. AENOR: Madrid, Spain, 2018.
- ISO 105:B02:2014; Textiles—Tests for Colour Fastness—Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp test. AENOR: Madrid, Spain, 2014.
- ISO 17228:2015; Leather—Tests for Colour Fastness—Change in Colour with accelerated Ageing. AENOR: Madrid, Spain, 2015.
- ISO 11640:2019; Leather—Tests for Colour Fastness—Colour Fastness to Cycles of to-and-fro Rubbing. AENOR: Madrid, Spain, 2019.
- ISO 11644:2023; Leather—Test for Adhesion of Finish. AENOR: Madrid, Spain, 2023.
- EN ISO 4048:2019; Leather—Chemical Tests—Determination of Matter Soluble in Dichloromethane and Free Fatty Acid Content. AENOR: Madrid, Spain, 2019.
- EN ISO 4684:2006; Leather—Chemical Tests—Determination of Volatile Matter. AENOR: Madrid, Spain, 2006.
- EN ISO 4098:2019; Leather—Chemical Tests—Determination of Water-Soluble Matter, Water-Soluble Inorganic Matter and Water-Soluble Organic Matter. AENOR: Madrid, Spain, 2019.
- EN ISO 4047:1999; Leather—Determination of Sulphated Total Ash and Sulphated Water-Insoluble Ash. AENOR: Madrid, Spain, 1999.
- ISO 5397:1984; Leather—Determination of Nitrogen Content and “Hide Substance”—Titrimetric Method. AENOR: Madrid, Spain, 1984.
- EN ISO 4045:2019; Leather—Chemical Tests—Determination of pH and Difference Figure. AENOR: Madrid, Spain, 2019.
- EN ISO 17072-2:2023; Leather—Chemical Determination of Metal Content—Part 2: Total Metal Content. AENOR: Madrid, Spain, 2023.
- EN ISO 17226-1:2019; Leather—Chemical Determination of Formaldehyde Content—Part 1: Method Using High Performance Liquid Chromatography. AENOR: Madrid, Spain, 2019.
- Zhang, J.; Chen, W. A faster and more effective chrome tanning process assisted by microwave. RSC Adv. 2020, 10, 23503–23509. [Google Scholar] [CrossRef]
- Badea, E.; Carşote, C.; Hadîmbu, E.; Șendrea, C.; Lupaș, M.C. The effect of halloysite nanotubes dispersions on vegetable-tanned leather thermal stability. Herit. Sci. 2019, 7, 68. [Google Scholar] [CrossRef]
- Vidal, A.R.; Cansian, R.L.; de Oliveira Mello, R.; Demiate, I.M.; Kempka, A.P.; Dornelles, R.C.P.; Rodriguez, J.M.L.; Campagnol, P.C.B. Production of Collagens and Protein Hydrolysates with Antimicrobial and Antioxidant Activity from Sheep Slaughter By-Products. Antioxidants 2022, 11, 1173. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Shen, L.; Li, G. Factors affecting thermal stability of collagen from the aspects of extraction, processing and modification. J. Leather Sci. Eng. 2020, 2, 19. [Google Scholar] [CrossRef]
- Odabaş, E.; Akarsu, E. Improving interface interactions in PLA/HAP composites with hydroxyapatite surface modification. Compos. Mater. 2023, 30, 1305–1319. [Google Scholar]
- Valapa, R.; Pugazhenthi, G.; Katiyar, V. Hydrolytic degradation behaviour of sucrose palmitate reinforced poly(lactic acid) nanocomposites. Int. J. Biol. Macromol. 2016, 89, 70–80. [Google Scholar] [CrossRef]
- Hezma, A.M.; Elashmawi, I.S.; Rajeh, A.; Kamal, M. Change Spectroscopic, thermal and mechanical studies of PU/PVC blends. Phys. B 2016, 495, 4–10. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, J.; Han, G.; Li, X.; Zhang, Z.; Zheng, X.; Wang, F.; Pei, Y.; Lei, Y.; Tang, K. Artificial deterioration of vegetable-tanned leather under synergistic effect of temperature and humidity. J. Cult. Herit. 2022, 53, 118–126. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Jakab, E.; Badea, E.; Barta-Rajnai, E.; Şendrea, C.; Czégény, Z. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J. Anal. Appl. Pyrol. 2019, 138, 178–187. [Google Scholar] [CrossRef]
- Czirok, I.S.; Jakab, E.; Czégény, Z.; Badea, E.; Babinszki, B.; Tömösközi, S.; May, Z.; Sebestyén, Z. Thermal characterization of leathers tanned by metal salts and vegetable tannins. J. Anal. Appl. Pyrol. 2023, 173, 106035. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.D.; Crudu, A.M.; Rosu, D. Influence of different tanning agents on bovine leather thermal degradation. J. Therm. Anal. Calorim. 2018, 134, 583–594. [Google Scholar] [CrossRef]
- Barba BJ, D.; Causapin CG, V.; Cabalar PJ, E.; Luna JA, A.; Seko, N.; Omichi, M.; Kakuchi, R.; Madrid, J.F. Pineapple Fiber Hybrids Prepared by the Fusion of radiation-induced Graft Polymerization and Kabachnik-Fields three-component Reaction (RIGP-KF3CR). J. Nat. Fibers 2022, 19, 13550–13562. [Google Scholar] [CrossRef]
- Cai, W.; Xin, W.; Zhang, H.; Luo, Y. Synthesis and Application of a Low Dye Absorption Waterborne Polyurethane for Microfiber Synthetic Leather. Coatings 2022, 12, 728. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Abd-El-All, A.S.; Masoud, R.A.; ElSayed, H. Activity of inorganic salts on different properties of synthetic leather. Egypt. J. Chem. 2021, 64, 3971–3979. [Google Scholar] [CrossRef]
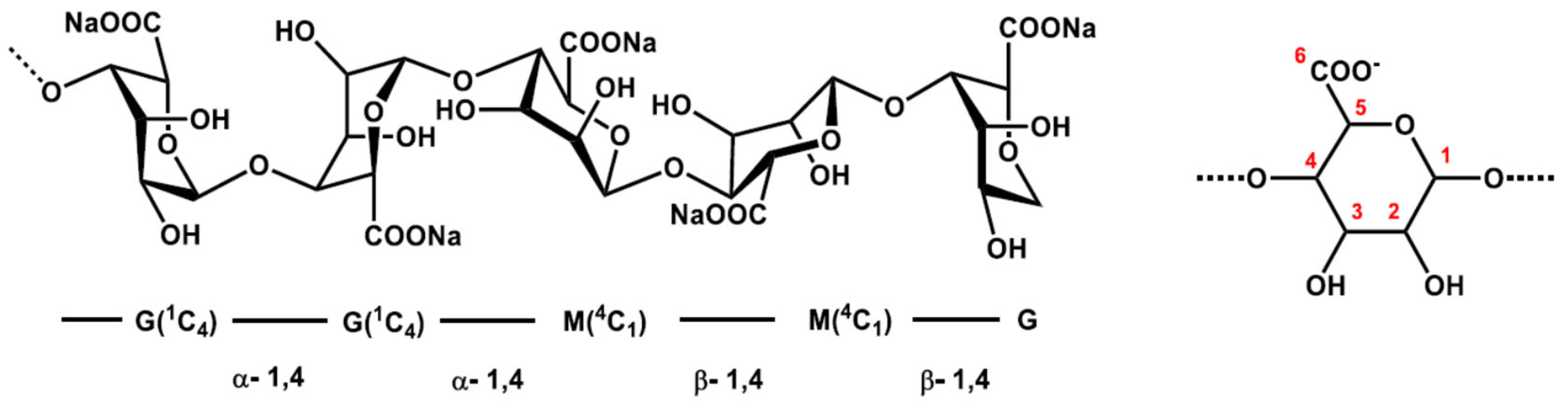
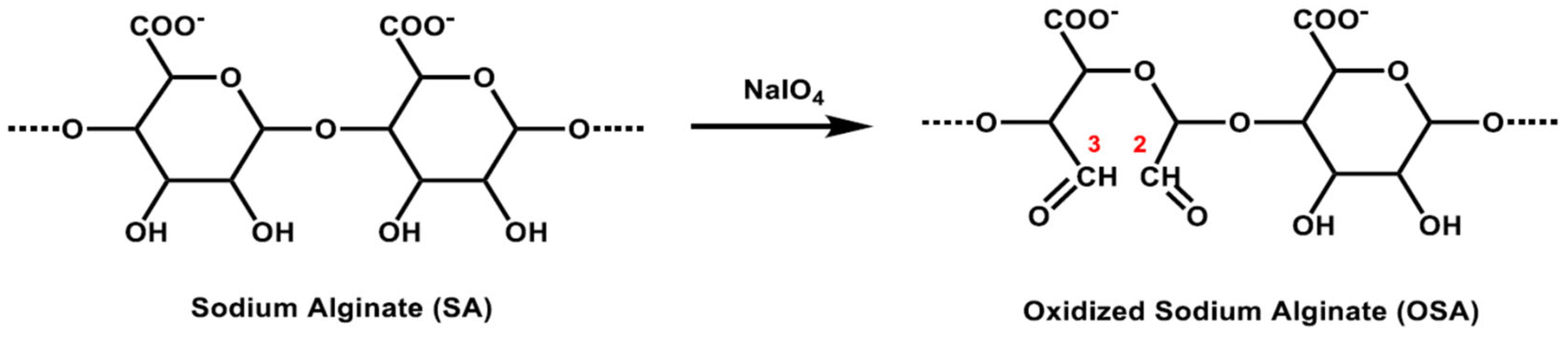
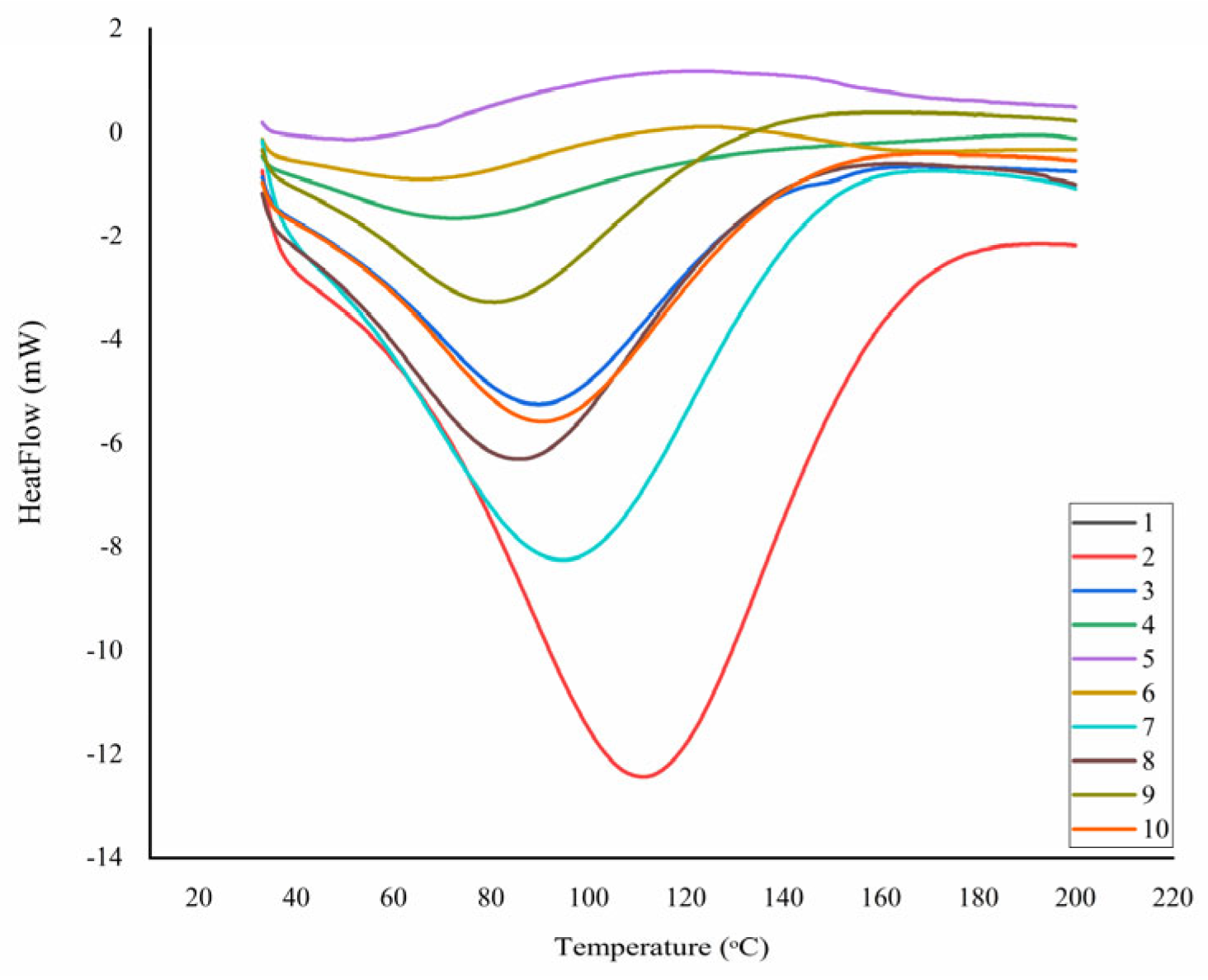

| Step | °C | % | Product | Time | Remarks |
|---|---|---|---|---|---|
| Washing | 30 | 200 | Water | 10′ | |
| Drain | |||||
| Neutralization | 30 | 200 | Water | ||
| 0.4 | Sodium Formate | 90′ | pH = 4–5 | ||
| Retanning | 35 | 32 | SA/SAD + ZnO | 120′ | |
| Dyeing | 3 | Dyeing | 240′ | ||
| 3 | Formic acid (1:10) | 60′ | pH = 3.87 | ||
| Drain | |||||
| Washing | 50 | 200 | Water | 15′ | |
| Drain | |||||
| Fatliquoring | 50 | 200 | Water | ||
| 4 | Sulphited oil | ||||
| 8 | Sulphated oil | 60′ | |||
| 1.5 | Formic acid (1:10) | 30′ | pH = 3.10 | ||
| Drain | |||||
| Washing | 40 | 200 | Water | 10′ | |
| Drain | |||||
| Horsing 24 h | |||||
| Sammying, drying, conditioning, staking, and milling | |||||
| Step | °C | % | Product | Time | Remarks |
|---|---|---|---|---|---|
| Washing | 30 | 200 | Water | 10′ | |
| Drain | |||||
| Neutralization | 30 | 200 | Water | ||
| 0.4 | Sodium Formate | ||||
| 0.4 | Sodium Bicarbonate | 120′ | pH = 5 | ||
| Retanning | 10 | SA/SAD + ZnO | 60′ | ||
| 5 | Tara | ||||
| 10 | SA/SAD + ZnO | 60′ | |||
| 5 | Tara | ||||
| Dyeing | 3 | Dyeing | 240′ | ||
| 3 | Formic acid (1:10) | 60′ | pH = 3.87 | ||
| Drain | |||||
| Washing | 50 | 200 | Water | 15′ | |
| Drain | |||||
| Fatliquoring | 50 | 200 | Water | ||
| 4 | Sulphited oil | ||||
| 8 | Sulphated oil | 60′ | |||
| 1.5 | Formic acid (1:10) | 30′ | pH = 3.10 | ||
| Drain | |||||
| Washing | 40 | 200 | Water | 10′ | |
| Drain | |||||
| Horsing 24 h | |||||
| Sammying, drying, conditioning, staking, and milling | |||||
| TEST | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Substances soluble in dichloromethane | 3.8 | 4.0 | 7.1 | 23.0 | 29.1 | 2.4 | 10.2 | 8.1 | 8.7 | 11.7 |
| Water and volatile content | 11.3 | 12.8 | 9.8 | 6.1 | 3.0 | 1.3 | 10.7 | 9.9 | 10.5 | 10.4 |
| Extractable organic and inorganic matter | ||||||||||
| Inorganic matter | 1.8 | 0.08 | 0.06 | - | - | - | 0.08 | 0.09 | 0.2 | 0.3 |
| Organic matter | 0.1 | 0.06 | 0.5 | - | - | - | 0.08 | 0.08 | 0.2 | 0.2 |
| Ash and insoluble mineral matter | 12.8 | 5.5 | 5.5 | 15.1 | 15.1 | 2.4 | 0.5 | 0.6 | 1.3 | 1.5 |
| Nitrogen and leather substance | ||||||||||
| Nitrogen | 11.3 | 13.0 | 13.0 | 5.3 | 5.3 | 1.9 | 12.8 | 13.2 | 12.1 | 11.8 |
| Leather substance | 63.5 | 72.8 | 72.81 | 29.9 | 29.9 | 10.4 | 71.9 | 74.2 | 68.06 | 66.1 |
| pH and difference index | ||||||||||
| pH | 4.5 | 3.7 | 3.7 | 4.2 | 4.2 | 5.8 | 3.0 | 3.0 | 3.2 | 3.3 |
| Difference index | - | - | - | - | - | - | - | - | - | - |
| Formaldehyde | 10.5 | <10 | <10 | <10 | <10 | <10 | 9.0 | <5 | <10 | <10 |
| Titanium (Ti) | <12 | <12 | 12.2 | 449 | <12 | 133 | <12 | <12 | <12 | <12 |
| Aluminum (Al) | 102 | 339 | 247 | 3948 | 244 | 153 | 102 | 38.0 | 66.9 | 77.1 |
| Zirconium (Zr) | <12 | <12 | <12 | <12 | <12 | <12 | <12 | <12 | <12 | <12 |
| Chromium (Cr) | 25,079 | 18,034 | 215 | 9179 | <3 | <3 | 11.3 | 11.1 | 1127 | 1121 |
| Zinc (Zn) | <3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 |
| Iron (Fe) | 38.6 | 60.7 | 217 | 26,377 | 48.7 | 10.8 | 38.3 | 31.3 | 57.4 | 71.5 |
| Tensile strength | ||||||||||
| Strength | 25.4 | 25.39 | 32.32 | 5.50 | 5.10 | 9.48 | 38.37 | 35.86 | 18.35 | 21.04 |
| Elongation | 79.8 | 79.75 | 45.45 | 4.63 | 33.47 | 15.77 | 70.35 | 62.0 | 64.50 | 68.20 |
| Tear load | 41.1 | 41.11 | 67.50 | 7.12 | 68.12 | 47.74 | 145.67 | 140.83 | 77.36 | 78.65 |
| Distension and strength of surface | ||||||||||
| Thickness | 1.14 | 1.27 | 2.20 | 0.34 | 1.59 | 1.28 | 1.75 | 1.83 | 1.81 | 1.86 |
| Break | ||||||||||
| Strength | 34.8 | 20.5 | 55.0 | 1.1 | 23.5 | 17.6 | 62.9 | 51.9 | 25.1 | 26.9 |
| Distension | 12.0 | 6.2 | 8.5 | 4.1 | 10.6 | 10.9 | 11.0 | 9.6 | 9.0 | 9.5 |
| Burst | ||||||||||
| Strength | 45.9 | 52.3 | 102.1 | 1.1 | 23.5 | 17.6 | 105.6 | 97.5 | 49.8 | 64.8 |
| Distension | 14.3 | 11.1 | 13.2 | 5.7 | 10.6 | 11.6 | 14.3 | 13.3 | 12.8 | 14.1 |
| Water vapor permeability | 4.9 | 4.0 | 1.1 | 0.4 | 6.8 | 0 | 5.9 | 7.7 | 10.1 | 9.9 |
| Shrinkage temperature | 106 | 107 | 81 | >140 | >140 | >140 | 68 | 69 | 72 | 71 |
| Flex resistance | ||||||||||
| Dry, 100,000 cycles | Small wrinkles | Wrinkles | Wrinkles | Breaks | No changes | Wrinkles | Wrinkles | Wrinkles | Wrinkles | Wrinkles |
| Wet, 50,000 cycles | Wrinkles | Wrinkles | Wrinkles | Breaks | No changes | Wrinkles | Breaks | Big wrinkles | Wrinkles | Wrinkles |
| Light fastness | 7 | >7 | >7 | >7 | >7 | >7 | 3–2 | 2–3 | 4–5 | 4–5 |
| Accelerated aging color change | ||||||||||
| 50 °C, 90%HR for 96 h | 5 | 5 | 4–5 | 5 | 5 | 5 | 4 | 3–4 | 4–5 | 4 |
| Rub fastness | ||||||||||
| Dry, 100 cycles | 4–5 | 1–2 | 3–4 | 5 | - | 5 | 4 | 4 | 4 | 4–5 |
| Wet, 20 cycles | 4 | 3 | 3–4 | 4–5 | - | 4–5 | 3 | 2–3 | 4–5 | 4–5 |
| Finishing adhesion | ||||||||||
| Dry | 18.3 | 6.9 | 14.2 | Leather breaks | Leather breaks | 41.8 | 51.1 | 64.7 | 37.3 | 39.5 |
| Wet | 17.5 | 6.5 | 14.2 | Leather breaks | 46.1 | 42.7 | 44.4 | 50.9 | 23.4 | 27.4 |
| Td °C | ΔH J g−1 | T1 °C | W1 % | T2 °C | Residue Mass % | |
|---|---|---|---|---|---|---|
| 1 | 87.3 | −214.90 | 77.93 | −8.63 | 314.40 | 12.91 |
| 2 | 106.83 | −813.27 | 83.91 | −3.77 | 352.64 | 4.56 |
| 3 | 85.19 | −270.24 | 75.38 | −8.30 | 329.41 | 0.80 |
| 4 | 186 | 605.80 | - | - | 391.39 | 10.66 |
| 5 | 43.94 | −16.15 | - | - | 355.75 | 1.16 |
| 6 | 58.24 | −43.70 | - | - | 372.04 | 2.51 |
| 7 | 98.78 | −585.77 | 73.84 | −9.70 | 330.29 | 1.86 |
| 8 | 87.07 | −334.04 | 87.36 | −9.36 | 331.15 | 1.84 |
| 9 | 78.5 | −183.19 | 80.62 | −11.03 | 315.99 | 2.74 |
| 10 | 87.08 | −323.47 | 88.35 | −8.09 | 341.20 | 2.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaratesi, I.; Badea, E.; Călinescu, I.; Sardroudi, N.P.; Zengin, G.; Casas, C.; Bacardit, A. Eco-Friendly Alternatives in Leather Production: Performance of Biodegradable Alginate-Based Retanned Leather Compared to Conventional Leathers and Plant-Based Materials. Appl. Sci. 2024, 14, 10263. https://doi.org/10.3390/app142210263
Quaratesi I, Badea E, Călinescu I, Sardroudi NP, Zengin G, Casas C, Bacardit A. Eco-Friendly Alternatives in Leather Production: Performance of Biodegradable Alginate-Based Retanned Leather Compared to Conventional Leathers and Plant-Based Materials. Applied Sciences. 2024; 14(22):10263. https://doi.org/10.3390/app142210263
Chicago/Turabian StyleQuaratesi, Ilaria, Elena Badea, Ioan Călinescu, Nima Pourrasoul Sardroudi, Gökhan Zengin, Concepció Casas, and Anna Bacardit. 2024. "Eco-Friendly Alternatives in Leather Production: Performance of Biodegradable Alginate-Based Retanned Leather Compared to Conventional Leathers and Plant-Based Materials" Applied Sciences 14, no. 22: 10263. https://doi.org/10.3390/app142210263
APA StyleQuaratesi, I., Badea, E., Călinescu, I., Sardroudi, N. P., Zengin, G., Casas, C., & Bacardit, A. (2024). Eco-Friendly Alternatives in Leather Production: Performance of Biodegradable Alginate-Based Retanned Leather Compared to Conventional Leathers and Plant-Based Materials. Applied Sciences, 14(22), 10263. https://doi.org/10.3390/app142210263






