Data Reconciliation for Assessing Compliance of Physicochemical Properties of Petroleum Products in Commercial Transactions
Abstract
1. Introduction
2. Methodology
2.1. Data Reconciliation General Statistics
2.2. Detailing Data Reconciliation to a Singular Mathematical Model
3. Results and Discussion
3.1. Case Study 1: Dispute Between Supplier and Customer
3.2. Case Study 2: Interlaboratory Study of the Final Boiling Point of Gasoline
3.3. Case Study 3: Metrological Evaluation of Three Distinct Test Methods to Determine Sulfur in Diesel Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resitoglu, I.A.; Altinisik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and aftertreatment systems. Clean Techn. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Cackette, T.A. Diesel engines: Environmental impact and control. J. Air Waste Manag. Assoc. 2001, 51, 809–847. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Harrison, R.; Barratt, B.; Fuller, G. A large reduction in airbone particle number concentration at the time of the introduction of “sulphur free” diesel and the London low emission zone. Atmos. Environ. 2012, 50, 129–138. [Google Scholar] [CrossRef]
- da Costa, L.G.; da Silveira, C.A.A.; de Aguiar, E.V.; Ugulino, R.D.F.; Lima, S.M.C.; de Oliveira, E.C. Statistical compatibility in the flash point by Pensky-Martens closed cup tester of diesel fuel. Pet. Sci. Technol. 2017, 35, 1763–1767. [Google Scholar] [CrossRef]
- Balabin, R.M.; Syunyaev, R.; Karpov, S. Quantitative measurement of ethanol distribution over fractions of ethanol-gasoline fuel. Energy Fuels 2007, 21, 2460–2465. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Wang, J.; He, X. Effects of gasoline research octane number on premixed low-temperature combustion of wide distillation fuel by gasoline/diesel blend. Fuel 2014, 134, 381–388. [Google Scholar] [CrossRef]
- Hibbert, D.B. Compliance of analytical results with regulatory or specification limits: A probabilistic approach. Accred. Qual. Assur. 2001, 6, 346–351. [Google Scholar] [CrossRef]
- Narasimhan, S.; Jordache, C. Data Reconciliation and Gross Error Detection: An Intelligent Use of Process Data; Gulf Publishing Company: Houston, TX, USA, 2000; Chapter 1; pp. 1–32. [Google Scholar]
- Özyurt, D.B.; Pike, R.W. Theory and practice of simultaneous data reconciliation and gross error detection for chemical processes. Comput. Chem. Eng. 2004, 28, 381–402. [Google Scholar] [CrossRef]
- Alhaj-Dibo, M.; Maquin, D.; Ragot, J. Data reconciliation: A robust approach using a contaminated distribution. Control Eng. Pr. 2008, 16, 159–170. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Aguiar, P.F. Data Reconciliation in the Natural Gas Industry: Analytical Applications. Energy Fuels 2009, 23, 3658–3664. [Google Scholar] [CrossRef]
- Mansour, M.; Ellis, J.E. Methodology of on-line optimisation applied to a chemical reactor. Appl. Math. Model. 2008, 32, 170–184. [Google Scholar] [CrossRef]
- Ramamurthi, Y.; Sistu, P.B.; Bequette, B.W. Control-relevant dynamic data reconciliation and parameter estimation. Comput. Chem. Eng. 1993, 17, 41–59. [Google Scholar] [CrossRef]
- Kozioł, M.; Kozioł, J. Application of Data Validation and Reconciliation to Improve Measurement Results in the Determination Process of Emission Characteristics in Co-Combustion of Sewage Sludge with Coal. Sustainability 2021, 13, 5300. [Google Scholar] [CrossRef]
- Thibault, É.; Kelly, J.D.; Lebreux Desilets, F.; Chioua, M.; Poulin, B.; Stuart, P. Industrial Data-Driven Processing Framework Combining Process Knowledge for Improved Decision Making—Part 1: Framework Development. Processes 2023, 11, 2376. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Frota, M.N.; Barreto, G.O. Use of data reconciliation: A strategy for improving the accuracy in gas flow measurements. J. Nat. Gas. Sci. Eng. 2015, 22, 313–320. [Google Scholar] [CrossRef]
- Weber, G.; Di Giuliano, A.; Rauch, R.; Hofbauer, H. Developing a simulation model for a mixed alcohol synthesis reactor and validation of experimental data in IPSEpro. Fuel Process. Technol. 2016, 141, 167–176. [Google Scholar] [CrossRef]
- Kotamreddy, G.; Hughes, R.; Bhattacharyya, D.; Stolaroff, J.; Hornbostel, K.; Matuszewski, M.; Omell, B. Process Modeling and Techno-Economic Analysis of a CO2 Capture Process Using Fixed Bed Reactors with a Microencapsulated Solvent. Energy Fuels 2019, 33, 7534–7549. [Google Scholar] [CrossRef]
- Bai, L.; Pinson, P. Distributed reconciliation in day-ahead wind power forecasting. Energies 2019, 12, 112. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Dong, W. Aeroengine data reconciliation model based on cooperative working equations. Energy 2019, 186, 115914. [Google Scholar] [CrossRef]
- Noriega, M.A.; Narváez, P.C. UNIFAC correlated parameters for liquid-liquid equilibrium prediction of ternary systems related to biodiesel production process. Fuel 2019, 249, 365–378. [Google Scholar] [CrossRef]
- Szega, M. Methodology of advanced data validation and reconciliation application in industrial thermal processes. Energy 2020, 198, 117326. [Google Scholar] [CrossRef]
- Badings, T.S.; van Putten, D.S. Data validation and reconciliation for error correction and gross error detection in multiphase allocation systems. J. Pet. Sci. Eng. 2020, 195, 107567. [Google Scholar] [CrossRef]
- Behroozsarand, A.; Afshari, S. Data reconciliation of an industrial petrochemical plant case study: Olefin plant (Hot section). Comput. Chem. Eng. 2020, 137, 106803. [Google Scholar] [CrossRef]
- da Cunha, A.S.; Peixoto, F.C.; Prata, D.M. Robust data reconciliation in chemical reactors. Comput. Chem. Eng. 2021, 145, 107170. [Google Scholar] [CrossRef]
- Medeiros, K.A.R.; de Matos, A.C.H.; de Oliveira, E.C. Shedding Light on Data Reconciliation Techniques Applied to Analytical Chemistry. Crit. Rev. Anal. Chem. 2023, 53, 975–985. [Google Scholar] [CrossRef]
- de Oliveira, E.C.; Lourenço, F.R. Risk of false conformity assessment applied to automotive fuel analysis: A multiparameter approach. Chemosphere 2021, 263, 128265. [Google Scholar] [CrossRef]
- de Oliveira, E.C.; Lourenço, F.R. Data reconciliation applied to the conformity assessment of fuel products. Fuel 2021, 300, 120936. [Google Scholar] [CrossRef]
- Szymanska, E. Modern data science for analytical chemical data—A comprehensive review. Anal. Chim. Acta 2018, 1018, 1–10. [Google Scholar] [CrossRef]
- Joint Committee for Guides in Metrology. Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement; International Bureau of Weights and Measures (BIPM): Sèvres, France, 2008. [Google Scholar]
- ASTM D-56; Standard Test Method for Flash Point by Tag Closed Cup Tester. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D-86; Standard Test Method for Distillation of Petroleum Products at Atmospheric Pressure. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D-4294; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D-5453; Standard Test Method for Determination of Total Sulfur in Light Hydrocarbons, Spark Ignition Engine Fuel, Diesel Engine Fuel, and Engine Oil by Ultraviolet Fluorescence. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM D-7039-15a; Standard Test Method for Sulfur in Gasoline and Diesel Fuel by Monochromatic Wavelength Dispersive X-ray Fluorescence Spectrometry. ASTM: West Conshohocken, PA, USA, 2020.

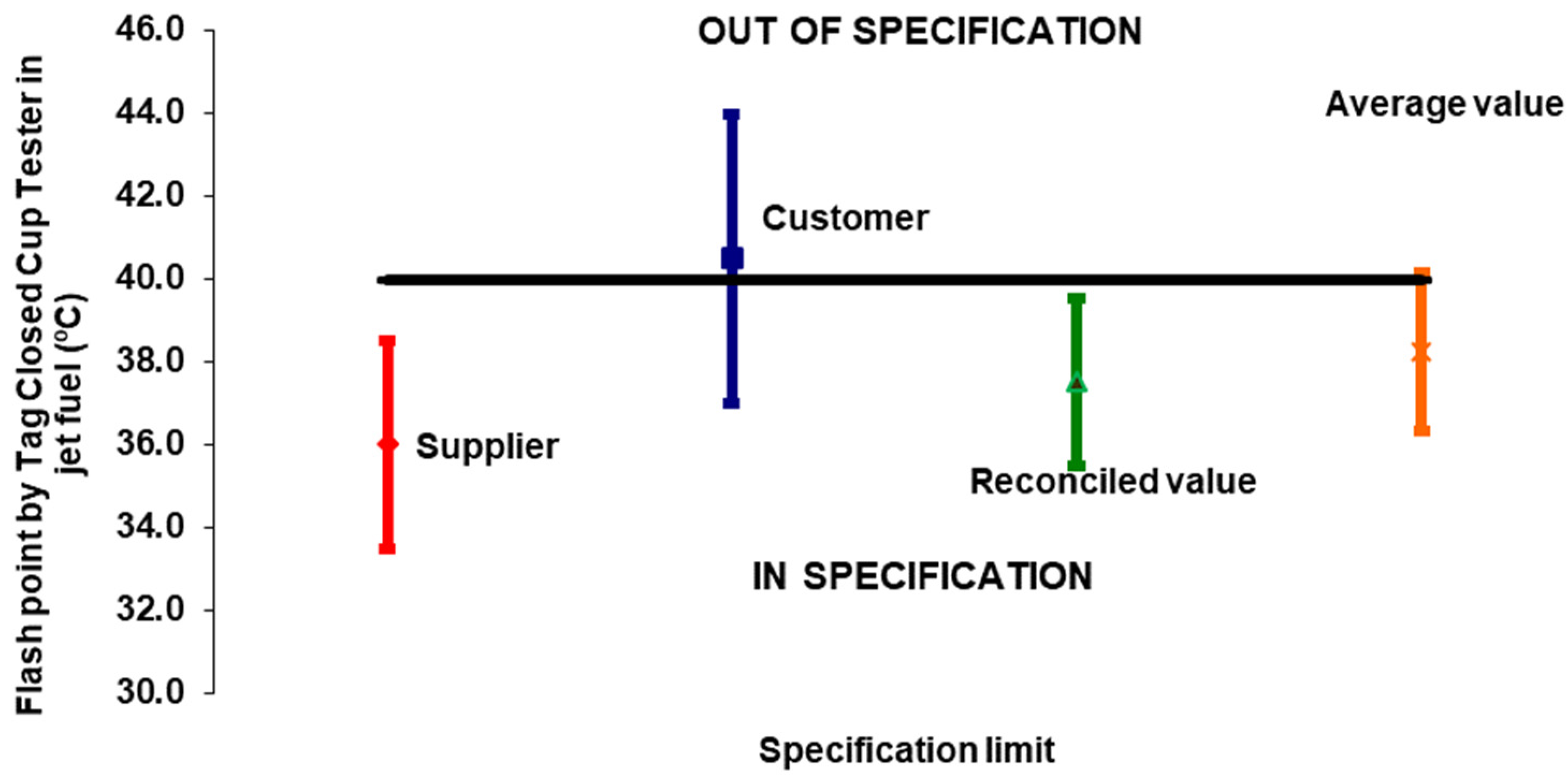
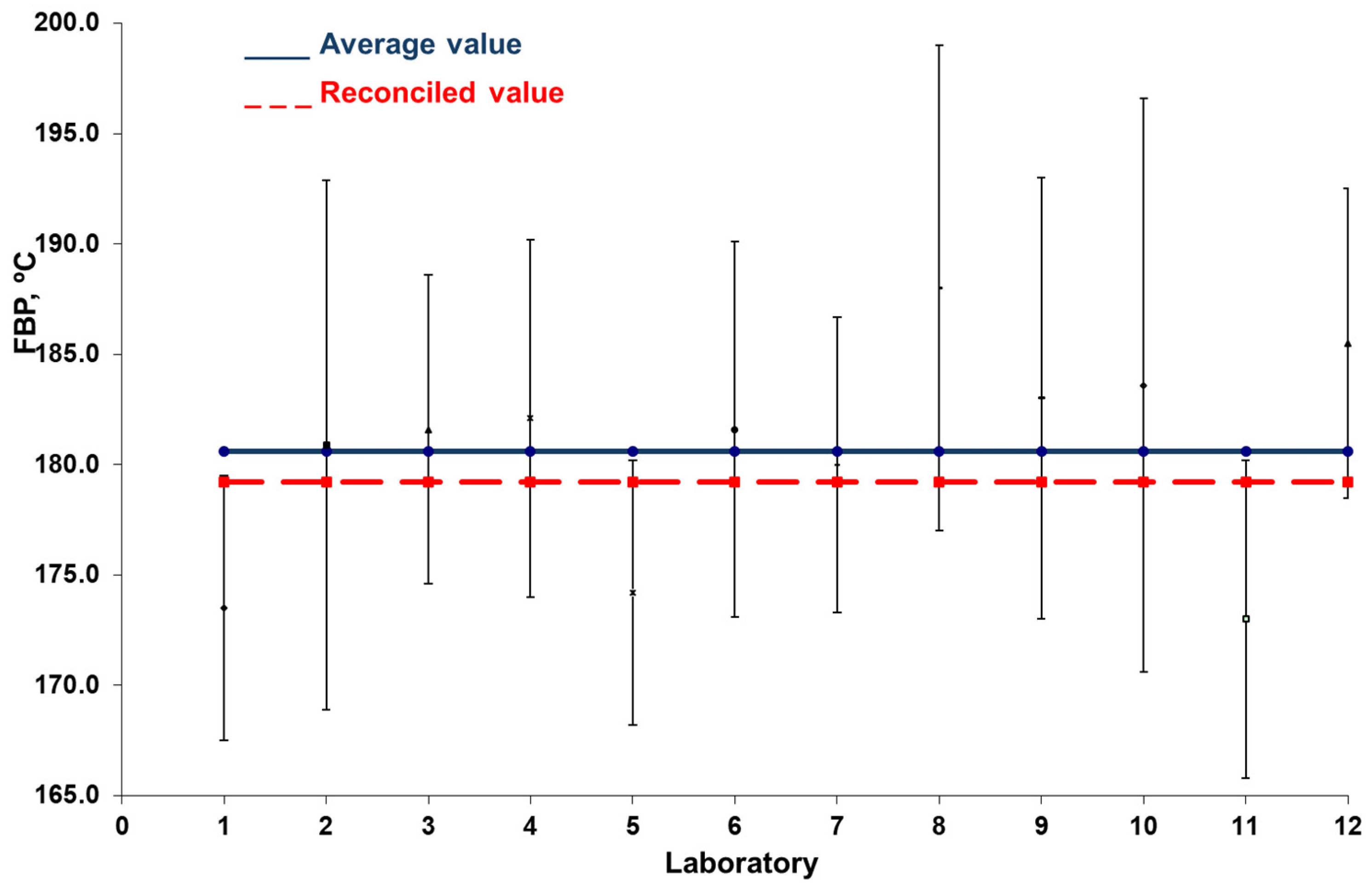
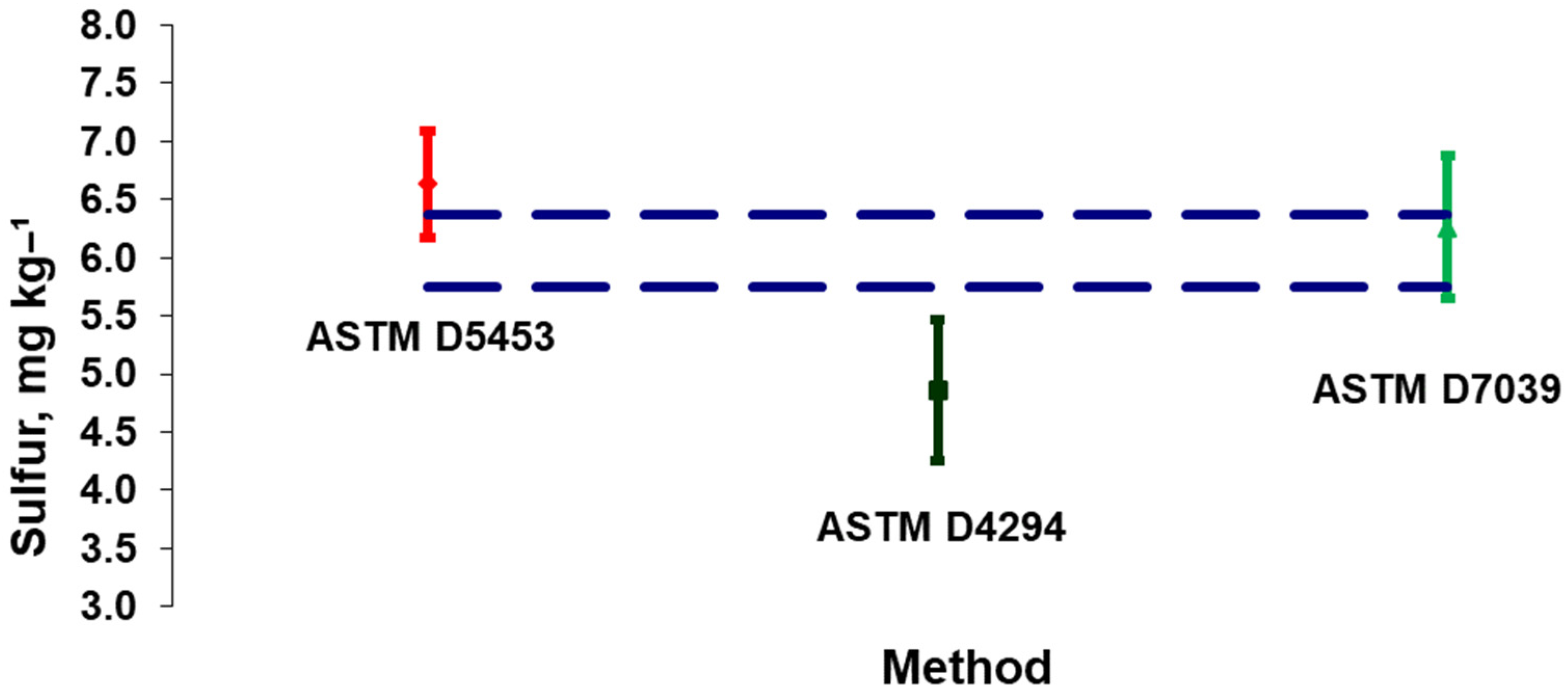
| Flash Point | Expanded Uncertainty | Specification | |
|---|---|---|---|
| Supplier | 36.0 °C | 2.5 °C | In |
| Customer | 40.5 °C | 3.5 °C | Out |
| Laboratory | FBP (°C) | Expanded Uncertainty (°C) |
|---|---|---|
| 1 | 173.5 | 6.0 |
| 2 | 180.9 | 12.0 |
| 3 | 181.6 | 7.0 |
| 4 | 182.1 | 8.1 |
| 5 | 174.2 | 6.0 |
| 6 | 181.6 | 8.5 |
| 7 | 180.0 | 6.7 |
| 8 | 188.0 | 11.0 |
| 9 | 183.0 | 10.0 |
| 10 | 183.6 | 13.0 |
| 11 | 173.0 | 7.2 |
| 12 | 185.5 | 7.0 |
| Average value | 180.6 |
| ASTM D5453 [34] | ASTM D4294 [33] | ASTM D7039 [35] | |
|---|---|---|---|
| 6.2 | 5.4 | 6.3 | |
| 6.9 | 4.9 | 5.2 | |
| 6.1 | 5.3 | 6.3 | |
| 6.1 | 4.6 | 5.8 | |
| 7.5 | 4.9 | 6.3 | |
| 7.2 | 5.8 | 6.9 | |
| 7.0 | 4.9 | 5.6 | |
| 6.9 | 5.9 | 6.3 | |
| 6.5 | 4.9 | 6.2 | |
| 6.8 | 4.7 | 7.2 | |
| 6.6 | 3.8 | 6.2 | |
| 6.5 | 3.9 | 7.7 | |
| 6.7 | 4.4 | 5.9 | |
| 6.1 | 4.6 | 5.9 | |
| 6.0 | 6.3 | ||
| 7.0 | |||
| Arithmetic average | 6.6 | 4.9 | 6.3 |
| Standard deviation | 0.46 | 0.60 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, R.M.; Silva Rocha, A.M.; de Oliveira, E.C. Data Reconciliation for Assessing Compliance of Physicochemical Properties of Petroleum Products in Commercial Transactions. Appl. Sci. 2024, 14, 10295. https://doi.org/10.3390/app142210295
Moreira RM, Silva Rocha AM, de Oliveira EC. Data Reconciliation for Assessing Compliance of Physicochemical Properties of Petroleum Products in Commercial Transactions. Applied Sciences. 2024; 14(22):10295. https://doi.org/10.3390/app142210295
Chicago/Turabian StyleMoreira, Rosana Medeiros, Ariadne Mayra Silva Rocha, and Elcio Cruz de Oliveira. 2024. "Data Reconciliation for Assessing Compliance of Physicochemical Properties of Petroleum Products in Commercial Transactions" Applied Sciences 14, no. 22: 10295. https://doi.org/10.3390/app142210295
APA StyleMoreira, R. M., Silva Rocha, A. M., & de Oliveira, E. C. (2024). Data Reconciliation for Assessing Compliance of Physicochemical Properties of Petroleum Products in Commercial Transactions. Applied Sciences, 14(22), 10295. https://doi.org/10.3390/app142210295








