Quality Assessment of Edible Plant-Based Fixed Oils Using Different Analytical Techniques and Machine Learning Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Edible Plant-Based Fixed Oil Samples
2.2. Fatty Acid Composition Analysis by Gas Chromatography–Flame Ionization Detector (GC-FID)
2.3. Rancimat Method
2.4. Color Measurement
2.5. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition
3.2. Oxidative Stability (OS) of the Edible Fixed Oils
3.3. Color Measurement of Edible Fixed Oils
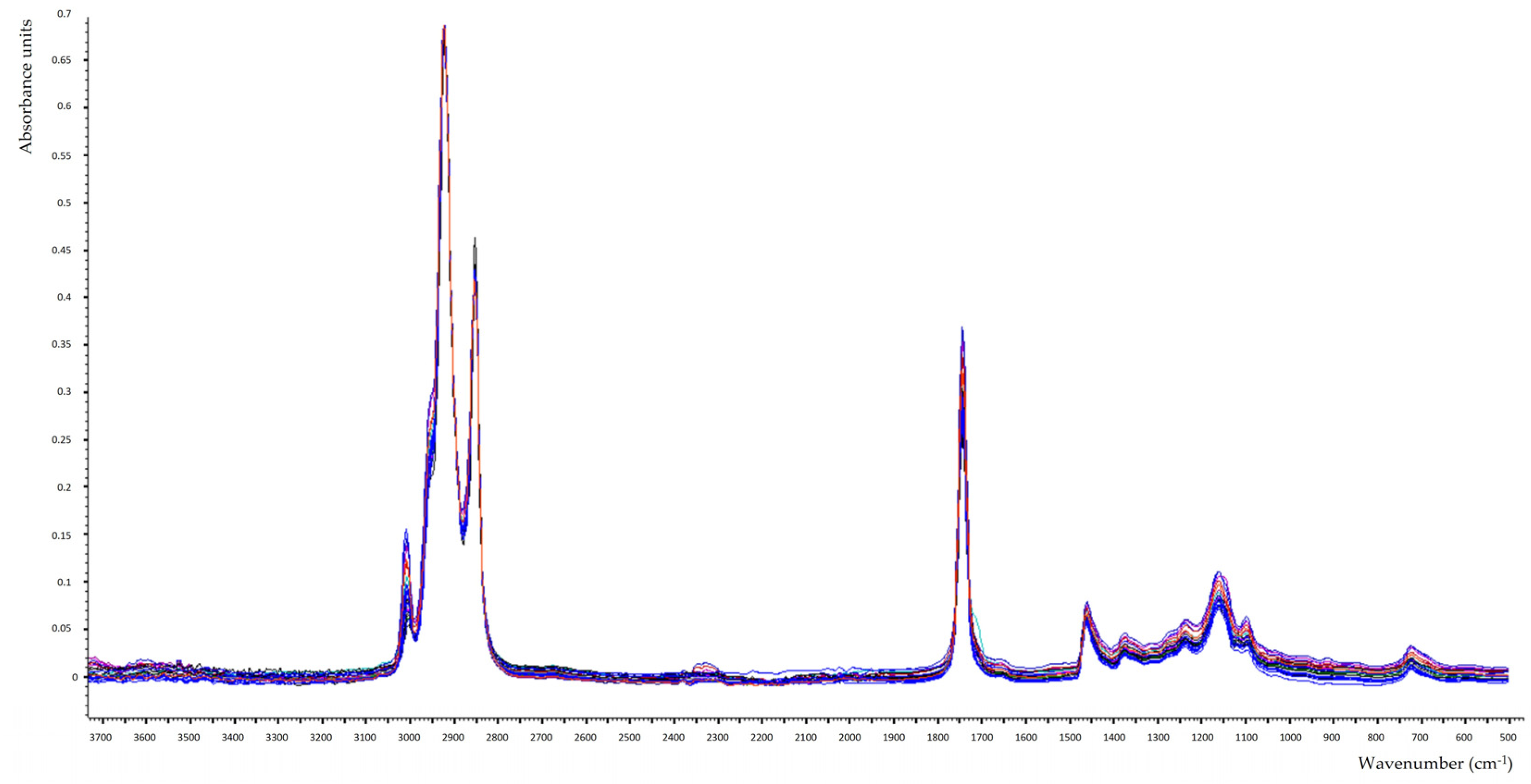
3.4. FTIR Spectra Interpretation of Edible Fixed Oils
3.5. Discriminant Analysis of Edible Fixed Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Athar, M.; Nasir, S.M. Taxonomic Perspective of Plant Species Yielding Vegetable Oils Used in Cosmetics and Skin Care Products. Afr. J. Biotechnol. 2005, 4, 36–44. [Google Scholar]
- Ali, S.; Ekbbal, R.; Salar, S.; Yasheshwar; Ali, S.A.; Jaiswal, A.K.; Singh, M.; Yadav, D.K.; Kumar, S. Gaurav Quality Standards and Pharmacological Interventions of Natural Oils: Current Scenario and Future Perspectives. ACS Omega 2023, 8, 39945–39963. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Begum, N.; Rabbi, F.; Akhtar, N.; Rahman, K.U.; Khan, W.M.; Shah, Z. In-Vitro Antimicrobial, Antioxidant and Enzyme Inhibitory Activities of Fixed Oil Extracted from Stem Bark of Tamarix Aphylla. Pharm. Chem. J. 2022, 56, 1116–1122. [Google Scholar] [CrossRef]
- Savic, I.; Savic Gajic, I.; Gajic, D. Physico-Chemical Properties and Oxidative Stability of Fixed Oil from Plum Seeds (Prunus domestica Linn.). Biomolecules 2020, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yang, R.; Wang, X.; Ma, F.; Yu, L.; Zhang, L.; Li, P. Comparative Advantages of Chemical Compositions of Specific Edible Vegetable Oils. Oil Crop Sci. 2023, 8, 1–6. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, L.; Li, P.; Yu, L.; Mao, J.; Wang, X.; Zhang, Q. A Review of Chemical Composition and Nutritional Properties of Minor Vegetable Oils in China. Trends Food Sci. Technol. 2018, 74, 26–32. [Google Scholar] [CrossRef]
- Toishimanov, M.; Nurgaliyeva, M.; Serikbayeva, A.; Suleimenova, Z.; Myrzabek, K.; Shokan, A.; Myrzabayeva, N. Comparative Analysis and Determination of the Fatty Acid Composition of Kazakhstan’s Commercial Vegetable Oils by GC-FID. Appl. Sci. 2023, 13, 7910. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Miniadis-Meimaroglou, S. Fatty Acid of Neutral and Polar Lipids of (Edible) Mediterranean Cephalopods. Food Res. Int. 1998, 31, 467–473. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Strati, I.F.; Bratakos, S.M.; Proestos, C.; Zoumpoulakis, P.; Miniadis-Meimaroglou, S. On the Combined Application of Iatroscan TLC-FID and GC-FID to Identify Total, Neutral, and Polar Lipids and Their Fatty Acids Extracted from Foods. ISRN Chromatogr. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices-Oil Stability Index, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2017; Method Cd 12b–92. [Google Scholar]
- AOCS. Official Methods and Recommended Practices-Color of Fats and Oil, Lovibond, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2017; Method Cc 13e–92. [Google Scholar]
- Ioannou, A.G.; Kritsi, E.; Sinanoglou, V.J.; Cavouras, D.; Tsiaka, T.; Houhoula, D.; Zoumpoulakis, P.; Strati, I.F. Highlighting the Potential of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy to Characterize Honey Samples with Principal Component Analysis (PCA). Anal. Lett. 2023, 56, 789–806. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of Active Ingredients in Sea-Buckthorn Oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Amit; Jamwal, R.; Kumari, S.; Dhaulaniya, A.S.; Balan, B.; Singh, D.K. Application of ATR-FTIR Spectroscopy along with Regression Modelling for the Detection of Adulteration of Virgin Coconut Oil with Paraffin Oil. LWT 2020, 118, 108754. [Google Scholar] [CrossRef]
- Durmaz, G.; Karabulut, İ.; Topçu, A.; Asiltürk, M.; Kutlu, T. Roasting-Related Changes in Oxidative Stability and Antioxidant Capacity of Apricot Kernel Oil. J. Am. Oil Chem. Soc. 2010, 87, 401–409. [Google Scholar] [CrossRef]
- Maldonado, A.; Riquelme, N.; Muñoz-Fariña, O.; García, O.; Arancibia, C. Stability and Bioaccessibility of α-Tocopherol-Enriched Nanoemulsions Containing Different Edible Oils as Carriers. LWT 2023, 174, 114419. [Google Scholar] [CrossRef]
- Aktas, A.B.; Dastan, T.; Katin, K.P.; Kaya, S. Determination of Oxidative Stability of Different Vegetable Oils by Means of Middle Infrared Spectroscopy and DFT Calculations. Microchem. J. 2023, 194, 109232. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, B.; Kaur, A.; Singh, N. Chemical, Thermal, Rheological and FTIR Studies of Vegetable Oils and Their Effect on Eggless Muffin Characteristics. J. Food Process Preserv. 2019, 43, e1397. [Google Scholar] [CrossRef]
- Vladimír, M.; Matwijczuk, A.P.; Niemczynowicz, A.; Kycia, R.A.; Karcz, D.; Gładyszewska, B.; Ślusarczyk, L.; Burg, P. Chemometric Approach to Characterization of the Selected Grape Seed Oils Based on Their Fatty Acids Composition and FTIR Spectroscopy. Sci. Rep. 2021, 11, 19256. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.; Romani, A.; Lampe, A.; Ferrari Nicoli, S.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef]
- Vadalà, R.; Nava, V.; Turco, V.L.; Potortì, A.G.; Costa, R.; Rando, R.; Ben Mansour, H.; Ben Amor, N.; Beltifa, A.; Santini, A.; et al. Nutritional and Health Values of Tunisian Edible Oils from Less-Used Plant Sources. Agriculture 2023, 13, 1096. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed]
- Soleimanifar, M.; Niazmand, R.; Jafari, S.M. Evaluation of Oxidative Stability, Fatty Acid Profile, and Antioxidant Properties of Black Cumin Seed Oil and Extract. Food Meas. 2019, 13, 383–389. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A. The Quality Characteristic and Fatty Acid Profile of Cold-Pressed Hazelnut Oils during Nine Months of Storage. Agronomy 2021, 11, 2045. [Google Scholar] [CrossRef]
- WHO. Interim Summary of Conclusions & Dietary Recommendations on Total Fat & Fatty Acids. In The Joint FAO/WHO Expert Consultation on Fats & Fatty Acids in Human Nutri-Tion; WHO: Geneva, Switzerland, 2008; pp. 1–14. [Google Scholar]
- Lamine, M.; Mlikia, A. Nutritional Quality Perceptions through Fatty Acid Profiling, Health Lipid Indices and Antioxidant Potentialities. Open J. Nutr. Food Sci. 2021, 3, 1016. [Google Scholar]
- Simopoulos, A.P.; DiNicolantonio, J.J. The Importance of a Balanced ω-6 to ω-3 Ratio in the Prevention and Management of Obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Gonzalez-Becerra, K.; Barron-Cabrera, E.; Muñoz-Valle, J.F.; Torres-Castillo, N.; Rivera-Valdes, J.J.; Rodriguez-Echevarria, R.; Martinez-Lopez, E. A Balanced Dietary Ratio of N-6:N-3 Polyunsaturated Fatty Acids Exerts an Effect on Total Fatty Acid Profile in RBCs and Inflammatory Markers in Subjects with Obesity. Healthcare 2023, 11, 2333. [Google Scholar] [CrossRef]
- Farag, M.A.; Reda, A.; Nabil, M.; Elimam, D.M.; Zayed, A. Evening Primrose Oil: A Comprehensive Review of Its Bioactives, Extraction, Analysis, Oil Quality, Therapeutic Merits, and Safety. Food Funct. 2023, 14, 8049–8070. [Google Scholar] [CrossRef]
- Yang, C.; Shang, K.; Lin, C.; Wang, C.; Shi, X.; Wang, H.; Li, H. Processing Technologies, Phytochemical Constituents, and Biological Activities of Grape Seed Oil (GSO): A Review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Vever-Bizet, C.; Dellinger, M.; Brault, D.; Rougee, M.; Bensasson, R.V. Singlet molecular oxygen quenching by saturated and unsaturated fatty-acids and by cholesterol. Photochem. Photobiol. 1989, 50, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital Image Colorimetry on Smartphone for Chemical Analysis: A Review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Gao, H.; Sun, D.; Zhou, Y.; Zeng, L.; Shi, X. Experimental Study on Oil Colour Grading Based on Uniform Colour Space. J. Phys. Conf. Ser. 2023, 2428, 012007. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Quality of cold-pressed edible rapeseed oil in Germany. Food/Nahrung 2003, 47, 413–419. [Google Scholar]
- Górnaś, P.; Siger, A.; Juhņeviča, K.; Lācis, G.; Šnē, E.; Segliņa, D. Cold-pressed Japanese quince (Chaenomeles japonica (Thunb.) Lindl. ex Spach) seed oil as a rich source of α-tocopherol, carotenoids and phenolics: A comparison of the composition and antioxidant activity with nine other plant oils. Euro. J. Lipid Sci. Tech. 2014, 116, 563–570. [Google Scholar]
- Erickson, D.R. Edible Fats and Oils Processing Basic Principles and Modern Practices; American Oil Chemists’ Society: Champaign, IL, USA, 1990. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- do Nascimento, T.A.; Lopes, T.I.B.; Nazario, C.E.D.; Oliveira, S.L.; Alcantara, G.B. Vegetable Oils: Are They True? A Point of View from ATR-FTIR, 1H NMR, and Regiospecific Analysis by 13C NMR. Food Res. Int. 2021, 144, 110362. [Google Scholar] [CrossRef]
- Uzun, H.; Kaynak, E.G.; Ibanoglu, E.; Ibanoglu, S. Chemical and Structural Variations in Hazelnut and Soybean Oils after Ozone Treatments. Grasas y Aceites 2018, 69, 253. [Google Scholar] [CrossRef]
- de Souza, T.R.P.; Olenka, L.; Peternella, W.S. A Study of Degradation in Vegetable Oils by Exposure to Sunlight Using Fourier Transform Infrared Spectroscopy. Mater. Sci. Appl. 2020, 11, 678–691. [Google Scholar] [CrossRef]
- Mukhametov, A.; Mamayeva, L.; Kazhymurat, A.; Akhlan, T.; Yerbulekova, M. Study of Vegetable Oils and Their Blends Using Infrared Reflectance Spectroscopy and Refractometry. Food Chem. X 2023, 17, 100386. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Alexa, E.; Munteanu, M.-F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR Spectroscopy to Detect the Changesin Extra Virgin Olive Oil by Adulteration Withsoybean Oil and High Temperature Heat Treatment. Open Chem. 2015, 13, 000010151520150110. [Google Scholar] [CrossRef]
- Arslan, F.N. Atr–ftir spectroscopy combined with chemometrics for rapid classification of extra virgin olive oils and edible oils from different cultivars available on the turkish markets. Eskişehir Tech. Univ. J. Sci. Technol. A-Appl. Sci. Eng. 2018, 19, 926–947. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Ramis-Ramos, G.; Herrero-Martínez, J.M.; Gimeno-Adelantado, J.V.; Simó-Alfonso, E.F. Characterization of the Alcoholic Fraction of Vegetable Oils by Derivatization with Diphenic Anhydride Followed by High-Performance Liquid Chromatography with Spectrophotometric and Mass Spectrometric Detection. J. Chromatogr. A 2009, 1216, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yu, L.; Ma, F.; Li, P. Quantification of Phenolic Compounds in Vegetable Oils by Mixed-Mode Solid-Phase Extraction Isotope Chemical Labeling Coupled with UHPLC-MS/MS. Food Chem. 2021, 334, 127572. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and Safer Clinically-Relevant Activities of Pentadecanoic Acid Compared to Omega-3: Evaluation of an Emerging Essential Fatty Acid across Twelve Primary Human Cell-Based Disease Systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef] [PubMed]
- Vucāne, S.; Cinkmanis, I.; Šabovics, M. Colorimetric Measurements of Vegetable Oils by Smartphone-Based Image Analysis. Proc. Latv. Acad. Sciences. Sect. B Nat. Exact Appl. Sci. 2022, 76, 110–115. [Google Scholar] [CrossRef]

| Edible Fixed Oils | INCI (International Nomenclature of Cosmetic Ingredients) | Plant Family |
|---|---|---|
| Walnut oil (1) * | Juglans regia Seed Oil | Juglandaceae |
| Linseed oil (2) | Linum usitatissimum L. Seed Oil | Linaceae |
| Sesame oil (2) | Sesamum indicum L. Seed Oil | Pedaliaceae |
| Almond oil (1) | Prunus amygdalus var. dulcis Oil | Rosaceae |
| Hazelnut oil (1) | Corylus avellana | Betulaceae |
| Pumpkin seed oil virgin unroasted (2) | Cucurbita pepo seed Oil | Cucurbitaceae |
| Avocado oil (1) | Persea gratissima Oil | Lauraceae |
| Black cumin oil (2) | Nigella sativa L. Seed Oil | Ranunculaceae |
| Coffee bean base oil (1) | Coffea arabica Seed Oil | Rubiaceae |
| Pine cone base oil (2) | Pinus sibirica Seed Oil | Pinaceae |
| Evening Primrose base oil (2) | Oenothera biennis Oil | Onagraceae |
| Grape seed base oil (2) | Vitis vinifera Seed Oil | Vitaceae |
| Plum base oil (1) | Prunus domestica Seed Oil | Rosaceae |
| Pomegranate base oil (2) | Punica granatum L. Seed Oil | Lythraceae |
| Mustard seed base oil (2) | Brassica juncea Seed Extract | Brassicaceae |
| Sea Buckthorn base oil (1) | Hippophae rhamnoides Fruit Extract | Elaeagnaceae |
| Chia seed base oil (2) | Salvia hispanica Seed Oil | Lamiaceae |
| Carrot base oil (1) | Daucus carota subsp. sativus Root Extract | Apiaceae |
| Peanut base oil (1) | Arachis hypogaea L. Oil | Fabaceae |
| Apricot base oil (1) | Prunus armeniaca Kernel Oil | Rosaceae |
| Milk thistle base oil (2) | Silybum marianum Seed Oil | Asteraceae |
| Macadamia base oil kernel (1) | Macadamia integrifolia Seed Oil | Proteaceae |
| Canola base oil (2) | Brassica campestris L. Seed Oil | Brassicaceae |
| Soybean base oil (2) | Glycine soja Oil | Fabaceae |
| Poppy seed base oil (2) | Papaver somniferum Seed Oil | Papaveraceae |
| Coconut Virgin oil raw (1) | Cocos nucifera L. Oil | Arecaceae |
| Edible Fixed Oil | SFA | MUFA | PUFA | UFA/SFA | SFA:MUFA:PUFA | n-6/n-3 | OIT (h) |
|---|---|---|---|---|---|---|---|
| Walnut oil | 14.50 ± 0.13 a | 16.54 ± 0.23 | 68.96 ± 0.45 | 5.90 ± 0.02 a | 1:1.1:4.8 | 5.77 | 0.47 ± 0.02 |
| Linseed oil | 14.35 ± 0.15 a | 21.08 ± 0.17 | 64.57 ± 0.42 a | 5.97 ± 0.03 a | 1:1.5:4.5 | 0.33 | 0.12 ± 0.01 |
| Almond oil | 14.98 ± 0.12 | 70.28 ± 0.68 | 14.74 ± 0.36 b | 5.68 ± 0.02 | 1:4.7:1.0 | -- | 3.44 ± 0.01 |
| Hazelnut oil | 12.59 ± 0.08 | 73.36 ± 0.57 | 14.05 ± 0.41 b | 6.94 ± 0.03 | 1:5.8:1.1 | 116 | 2.60 ± 0.01 |
| Pumpkin seed oil | 24.20 ± 0.23 | 27.57 ± 0.22 | 48.23 ± 0.29 | 3.13 ± 0.01 | 1:1.1:2.0 | 123 | 3.14 ± 0.01 |
| Avocado oil | 21.52 ± 0.12 | 69.81 ± 0.38 | 8.84 ± 0.17 | 3.65 ± 0.02 | 1:3.2:0.4 | 13.0 | 10.2 ± 0.01 |
| Black cumin oil | 23.76 ± 0.16 | 23.00 ± 0.16 a | 53.24 ± 0.45 | 3.21 ± 0.01 | 1:1.0:2.2 | 94.1 | 2.15 ± 0.01 ** |
| Coffee bean oil | 20.06 ± 0.24 | 23.35 ± 0.18 a | 56.58 ± 0.26 | 3.98 ± 0.03 | 1:1.2:2.8 | 72.5 | 1.99 ± 0.01 |
| Pine cone oil | 9.39 ± 0.11 c | 25.46 ± 0.19 | 65.12 ± 0.37 ac | 9.65 ± 0.03 c | 1:2.7:6.9 | 232 | 0.33 ± 0.01 |
| Evening primrose | 16.57 ± 0.08 | 7.67 ± 0.12 | 75.77 ± 0.31 e | 5.04 ± 0.02 | 1:0.5:4.6 | -- | 1.15 ± 0.01 * |
| Grape seed oil | 11.16 ± 0.10 b | 23.41 ± 0.26 a | 65.87 ± 0.62 c | 8.00 ± 0.02 b | 1:2.1:5.9 | -- | 1.14 ± 0.02 * |
| Plum oil | 11.05 ± 0.07 b | 68.77 ± 0.35 b | 20.19 ± 0.24 | 8.05 ± 0.03 b | 1:6.2:1.8 | -- | 1.03 ± 0.01 |
| Pomegranate oil | 20.72 ± 0.14 | 68.35 ± 0.41 b | 10.94 ± 0.15 d | 3.83 ± 0.01 | 1:3.3:0.5 | 77.1 | 0.64 ± 0.01 |
| Mustard seed oil | 21.84 ± 0.15 | 67.58 ± 0.48 | 10.59 ± 0.18 d | 3.58 ± 0.03 | 1:3.1:0.5 | 14.8 | 0.82 ± 0.01 |
| Sea buckthorn oil | 36.91 ± 0.22 | 58.54 ± 0.36 c | 4.57 ± 0.11 | 1.71 ± 0.01 | 1:1.6:0.1 | 66.8 | 31.9 ± 0.01 |
| Chia seed oil | 16.00 ± 0.13 | 8.86 ± 0.08 | 75.30 ± 0.85 e | 5.26 ± 0.02 | 1:0.6:4.7 | 0.39 | 0.15 ± 0.02 |
| Carrot oil | 19.87 ± 0.21 | 39.48 ± 0.33 | 40.63 ± 0.34 | 4.03 ± 0.02 | 1:2.0:2.0 | 100 | 5.73 ± 0.01 |
| Peanut oil | 13.96 ± 0.14 | 58.51 ± 0.35 c | 27.53 ± 0.46 | 6.16 ± 0.03 | 1:4.2:2.0 | 26.2 | 1.67 ± 0.01 |
| Apricot oil | 9.42 ± 0.06 c | 66.71 ± 0.42 | 23.85 ± 0.22 | 9.61 ± 0.03 c | 1:7.1:2.5 | 148 | 4.79 ± 0.02 |
| Milk thistle oil | 22.57 ± 0.31 | 21.65 ± 0.27 | 55.79 ± 0.28 | 3.43 ± 0.02 | 1:1.0:2.5 | 27.5 | 2.05 ± 0.01 |
| Macadamia oil | 25.34 ± 0.26 | 71.74 ± 0.51 | 2.94 ± 0.06 | 2.95 ± 0.02 | 1:2.8:0.1 | 3.90 | 7.75 ± 0.01 |
| Canola oil | 11.82 ± 0.09 | 56.19 ± 0.30 | 31.96 ± 0.39 | 7.45 ± 0.01 | 1:4.8:2.7 | 2.08 | 1.50 ± 0.01 |
| Soybean oil | 18.63 ± 0.11 | 26.38 ± 0.21 | 55.00 ± 0.34 | 4.37 ± 0.01 | 1:1.4:3.0 | 7.78 | 2.16 ± 0.01 ** |
| Poppy seed oil | 21.21 ± 0.16 | 42.27 ± 0.27 | 36.53 ± 0.41 | 3.72 ± 0.00 | 1:2.0:1.7 | 364 | 0.81 ± 0.01 |
| Sesame oil | 18.06 ± 0.18 | 37.20 ± 0.17 | 44.72 ± 0.53 | 4.54 ± 0.01 | 1:2.1:2.5 | 80.3 | 2.75 ± 0.01 |
| Virgin coconut oil | 94.10 ± 0.42 | 4.95 ± 0.06 | 0.94 ± 0.05 | 0.06 ± 0.00 | 1:0.1:0.0 | 22.5 | 69.3 ± 0.02 |
| Edible Fixed Oil | L* | a* | b* | Hue (°) | R | Y | B | N | Chlorophyll (ppb) | b-carotene (ppb) |
|---|---|---|---|---|---|---|---|---|---|---|
| Walnut oil | 56.05 ± 0.12 ef | −3.04 ± 0.02 h | 13.21 ± 0.20 h | 102.99 ± 0.15 ef | 1.0 | 3.9 | 0.0 | 0.0 | 256.0 | 12,891.0 |
| Linseed oil | 60.04 ± 0.22 h | −5.92 ± 0.10 a | 35.76 ± 0.20 o | 99.38 ± 0.12 de | 2.3 | 60.0 | 0.0 | 1.9 | 294.0 | 60,421.0 |
| Almond oil | 64.17 ± 0.12 mn | −2.26 ± 0.04 ij | 8.56 ± 0.05 g | 104.80 ± 0.22 fg | 0.4 | 1.2 | 0.0 | 0.2 | 0.0 | 4054.0 |
| Hazelnut oil | 55.62 ± 0.04 e | −3.44 ± 0.05 g | 14.95 ± 0.41 ij | 102.96 ± 0.23 ef | 1.2 | 7.2 | 0.0 | 0.0 | 161.0 | 19,119.0 |
| Pumpkin seed oil | 41.46 ± 0.05 c | 3.26 ± 0.11 o | 4.73 ± 0.19 c | 55.43 ± 1.92 b | 10.9 | 8.6 | 6.1 | 0.0 | 0.0 | 15,324.0 |
| Avocado oil | 46.01 ± 0.32 d | 0.01 ± 0.08 n | 15.83 ± 0.54 k | 89.97 ± 0.28 c | 2.3 | 60.0 | 1.4 | 0.0 | 17,611.0 | 55,022.0 |
| Black cumin oil | 60,05 ± 0.05 h | −5.19 ± 0.05 b | 23.46 ± 0.13 n | 102.48 ± 0.17 ef | 1.6 | 13.5 | 0.0 | 0.8 | 2440.0 | 36,582.0 |
| Coffee bean oil | 59.89 ± 0.17 h | −2.52 ± 0.07 i | 19.80 ± 0.16 m | 97.27 ± 0.22 d | 2.0 | 10.8 | 2.1 | 0.0 | 0.0 | 31,241.0 |
| Pine cone oil | 67.07 ± 0.27 p | −2.24 ± 0.05 ij | 7.32 ± 0.21 f | 107.05 ± 0.22 fg | 0.3 | 1.4 | 0.0 | 0.0 | 0.0 | 5853.0 |
| Evening Primrose oil | 63.91 ± 0.20 lm | −5.22 ± 0.04 b | 23.94 ± 0.17 n | 103.30 ± 1.69 ef | 1.8 | 21.0 | 0.0 | 0.9 | 2945.0 | 36,740.0 |
| Grape seed oil | 64.85 ± 0.54 no | −4.45 ± 0.02 d | 14.30 ± 0.23 i | 107.26 ± 0.19 fg | 0.7 | 7.1 | 0.0 | 0.0 | 3922.0 | 23,654.0 |
| Plum oil | 62.71 ± 0.18 k | −3.80 ± 0.07 f | 15.77 ± 0.15 jk | 103.54 ± 0.13 ef | 1.2 | 6.4 | 0.0 | 0.5 | 226.0 | 18,269.0 |
| Pomegranate oil | 55.61 ± 0.14 e | −2.43 ± 0.07 ij | 7.20 ± 0.26 f | 108.68 ± 0.70 g | 0.4 | 2.4 | 0.0 | 0.0 | 1140.0 | 8569.0 |
| Mustard seed oil | 56.64 ± 0,12 f | −1.69 ± 0.06 k | 6.18 ± 0.08 e | 105.33 ± 0.43 fg | 0.4 | 1.6 | 1.0 | 0.2 | 0.0 | 5843.0 |
| Sea buckthorn oil | 39,90 ± 0.43 b | 8.17 ± 0.15 p | 5.01 ± 0.04 cd | 31.55 ± 0.57 a | 18.2 | 2.4 | 10.1 | 0.0 | 30,000.0 | 30,000.0 |
| Chia seed oil | 65.15 ± 0.16 o | −1.59 ± 0.05 kl | 5.34 ± 0.08 cde | 106.59 ± 0.27 fg | 0.5 | 1.8 | 0.0 | 0.0 | 0.0 | 6691.0 |
| Carrot oil | 60.94 ± 0.10 i | −4.17 ± 0.03 de | 17.95 ± 0.09 l | 103.07 ± 0.03 ef | 1.5 | 11.8 | 0.0 | 0.8 | 789.0 | 25,196.0 |
| Peanut oil | 64.23 ± 0.16 mn | −0.63 ± 0.34 m | 3.83 ± 0.20 b | 99.38 ± 5.39 de | 0.0 | 0.3 | 0.0 | 0.0 | 3.0 | 1443.0 |
| Apricot oil | 56.61 ± 0.35 f | −1.41 ± 0.04 kl | 5.72 ± 0.19 de | 103.83 ± 0.80 efg | 0.8 | 1.9 | 0.0 | 0.4 | 0.0 | 6321.0 |
| Milk thistle oil | 61.80 ± 0.22 j | −3.92 ± 0.01 ef | 14.80 ± 0.09 i | 104.85 ± 0.08 fg | 1.4 | 6.0 | 0.0 | 0.4 | 179.0 | 17,211.0 |
| Macadamia oil | 63.30 ± 0.18 kl | −1.35 ± 0.08 l | 4.99 ± 0.11 cd | 105.09 ± 0.73 fg | 0.3 | 0.8 | 0.0 | 0.0 | 0.0 | 3472.0 |
| Canola oil | 63.17 ± 0.59 kl | −4.79 ± 0.11 c | 43.69 ± 0.74 q | 96.25 ± 0.18 d | 2.5 | 69.0 | 0.0 | 1.7 | 1103.0 | 60,121.0 |
| Soybean oil | 58.68 ± 0.19 g | −4.03 ± 0.07 ef | 36.86 ± 0.61 p | 96.23 ± 0.04 d | 2.8 | 68.0 | 0.0 | 1.8 | 1822.0 | 61,176.0 |
| Poppy seed oil | 65.43 ± 0.33 o | −4.11 ± 0.12 ef | 14.97 ± 0.17 ijk | 105.34 ± 0.27 fg | 1.0 | 7.1 | 0.0 | 0.1 | 2843.0 | 19,001.0 |
| Sesame oil | 56.56 ± 0.25 f | −2.18 ± 0.11 j | 8.58 ± 0.19 g | 104.26 ± 0.39 efg | 0.8 | 2.4 | 0.0 | 0.2 | 54.0 | 8049.0 |
| Virgin Coconut oil | 26.03 ± 0.01 a | −0.14 ± 0.05 n | −0.55 ± 0.02 a | 255.77 ± 5.24 h | 0.4 | 0.5 | 1.5 | 0.0 | 0.0 | 2293.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strati, I.F.; Tsiantas, K.; Psouni, A.; Ladika, G.; Cavouras, D.; Sinanoglou, V.J. Quality Assessment of Edible Plant-Based Fixed Oils Using Different Analytical Techniques and Machine Learning Approaches. Appl. Sci. 2024, 14, 10305. https://doi.org/10.3390/app142210305
Strati IF, Tsiantas K, Psouni A, Ladika G, Cavouras D, Sinanoglou VJ. Quality Assessment of Edible Plant-Based Fixed Oils Using Different Analytical Techniques and Machine Learning Approaches. Applied Sciences. 2024; 14(22):10305. https://doi.org/10.3390/app142210305
Chicago/Turabian StyleStrati, Irini F., Konstantinos Tsiantas, Angeliki Psouni, Georgia Ladika, Dionisis Cavouras, and Vassilia J. Sinanoglou. 2024. "Quality Assessment of Edible Plant-Based Fixed Oils Using Different Analytical Techniques and Machine Learning Approaches" Applied Sciences 14, no. 22: 10305. https://doi.org/10.3390/app142210305
APA StyleStrati, I. F., Tsiantas, K., Psouni, A., Ladika, G., Cavouras, D., & Sinanoglou, V. J. (2024). Quality Assessment of Edible Plant-Based Fixed Oils Using Different Analytical Techniques and Machine Learning Approaches. Applied Sciences, 14(22), 10305. https://doi.org/10.3390/app142210305








