NMR Analysis of Pulegone in Food Products
Abstract
:Featured Application
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development and Optimization
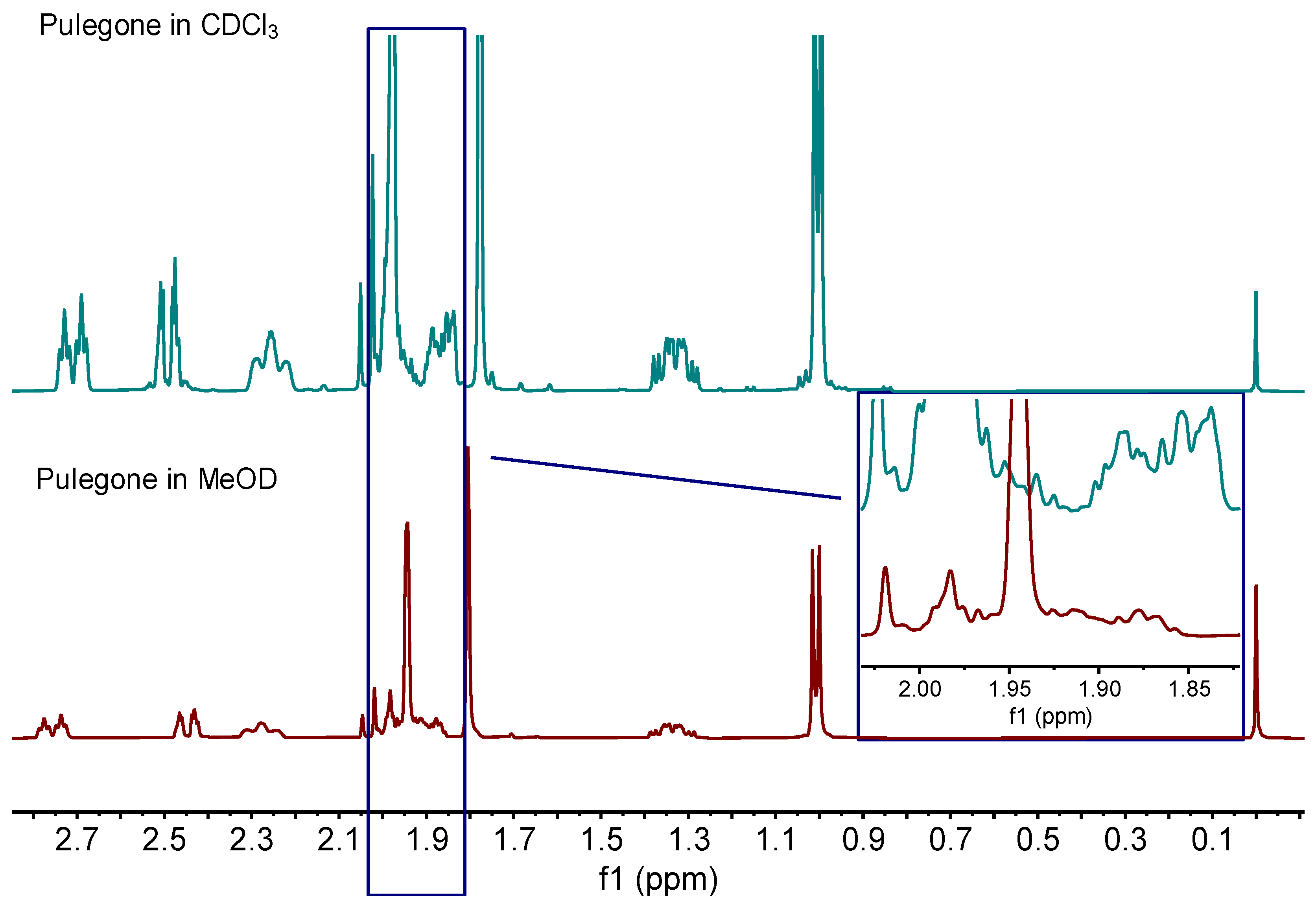
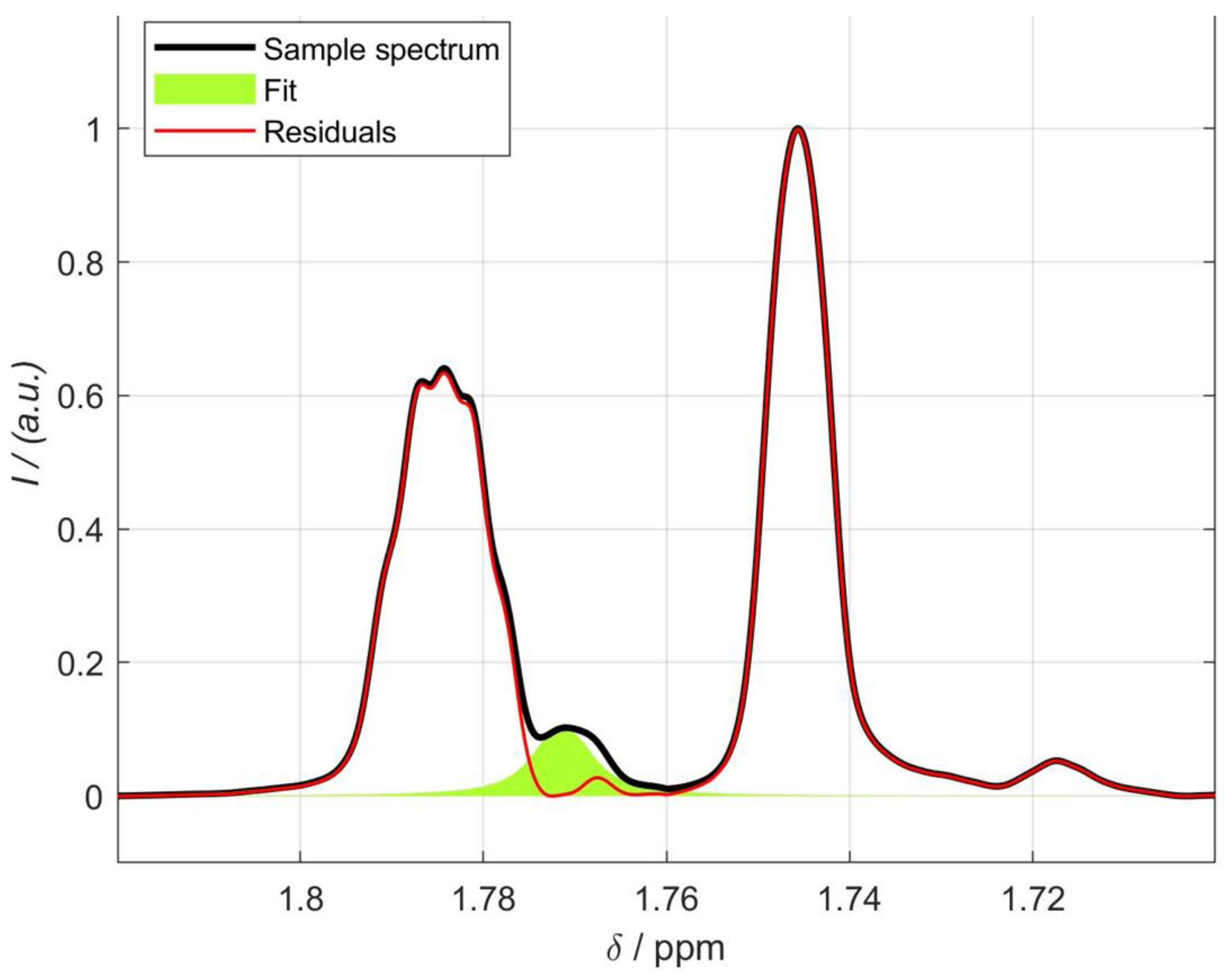
2.1.1. Influence of the Solvent on the Pulegone Signals
2.1.2. Assignment of Signals from Pulegone
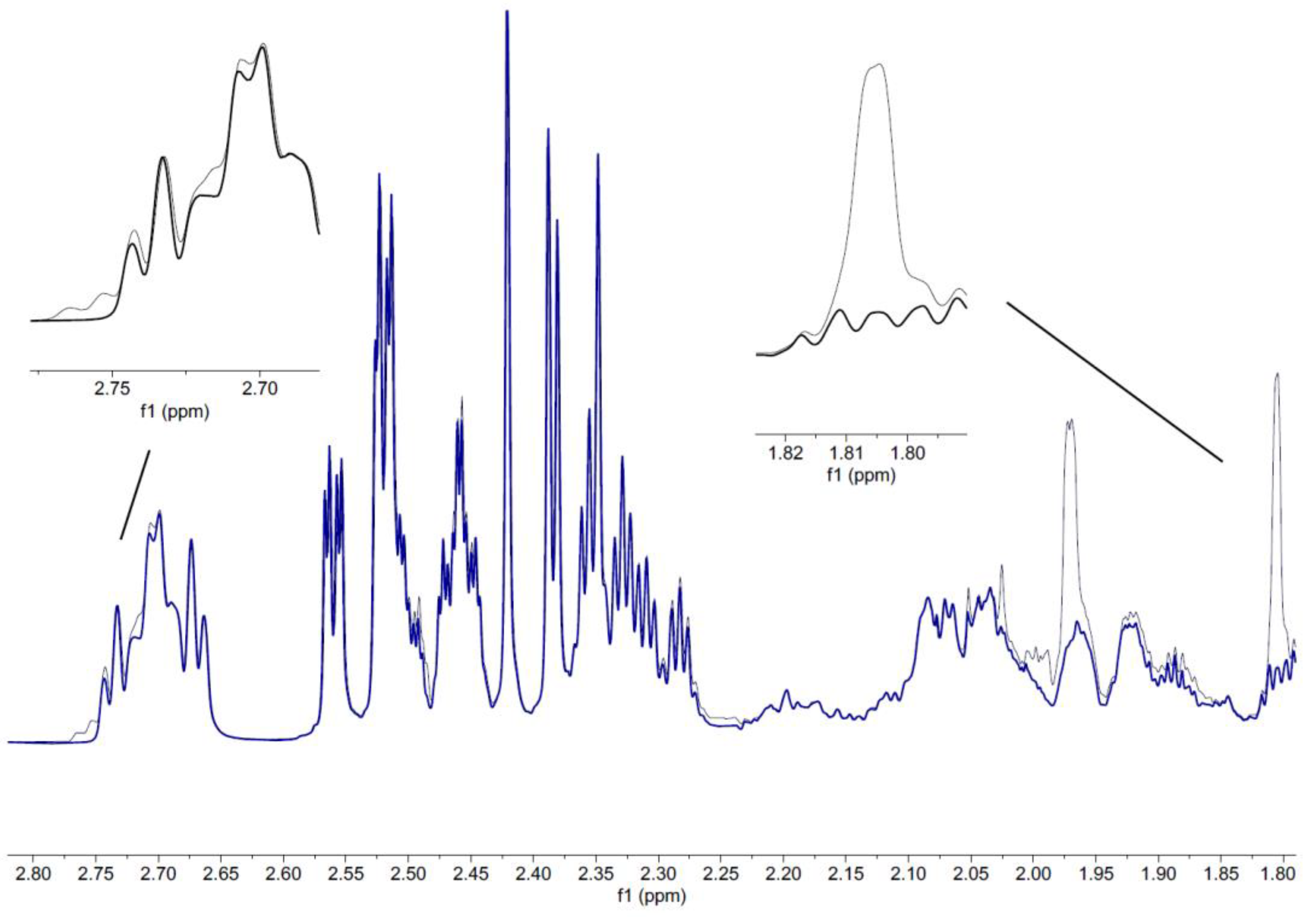
2.1.3. Extraction of Pulegone from Food
2.1.4. Validation of the NMR Method
2.2. Quantification of Pulegone in Essential Oils
2.3. Quantification of Pulegone in Tea, Herbs, and Confectionery Products
2.4. Toxicological Assessment of Pulegone
3. Materials and Methods
3.1. Samples
3.2. Chemicals
3.3. Sample Preparation
3.4. 1H NMR Spectroscopic Measurements
3.5. Quantification
3.6. NMR Spectral Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Pulegone Content [mg/kg] (n = 2) | Extraction Method |
|---|---|---|
| Corn mint EO 1 | 3094 ± 290 | - |
| Corn mint EO 2 | 14,672 ± 569 | - |
| Corn mint EO 3 | 4602 ± 65 | - |
| Corn mint EO 4 | 13,196 ± 890 | - |
| Peppermint EO 1 | 9850 ± 633 | - |
| Peppermint EO 2 | 7396 ± 163 | - |
| Peppermint EO 3 | 16,233 ± 443 | - |
| Peppermint EO 4 | 18,733 ± 170 | - |
| Peppermint EO 5 | 8713 ± 77 | - |
| Peppermint EO 6 | 5581 ± 171 | - |
| Peppermint EO 7 | 18,786 ± 416 | - |
| Peppermint EO 8 | 12,582 ± 41 | - |
| Peppermint EO 9 | 4051 ± 393 | - |
| Peppermint EO 10 | 17,398 ± 959 | - |
| Peppermint EO 11 | 12,768 ± 35 | - |
| Peppermint EO 12 | 8216 ± 442 | - |
| Peppermint EO 13 | 11,720 ± 519 | - |
| Peppermint EO 14 | 5149 ± 82 | - |
| Peppermint EO 15 | n.d. | - |
| Chinese Mint EO | 9566 ± 478 | - |
| Pennyroyal EO 1 | 630,557 ± 46,305 | - |
| Muña EO 1 | 559,410 ± 17,506 | - |
| Muña EO 2 | 288,603 ± 76,824 | - |
| Buchu EO 1 | 30,181 ± 2,487 | - |
| Buchu EO 2 | n.d. | - |
| Water mint EO | 13,154 ± 530 | - |
| Lesser calamint EO | 271,276 ± 9,476 | - |
| Bergamot mint EO | n.d. | - |
| Balm Mint Bush EO | n.d. | - |
| Spearmint EO 1 | n.d. | - |
| Spearmint EO 2 | n.d. | - |
| Spearmint EO 3 | n.d. | - |
| Horse mint EO | n.d. | - |
| Peppermint tea 1 | 158 ± 18 | hydrodistillation |
| Peppermint tea 2 | 39.9 ± 5.0 | hydrodistillation |
| Peppermint tea 3 (loose) | 639 ± 3 | hydrodistillation |
| Peppermint tea 4 | 443 ± 384 | hydrodistillation |
| Peppermint tea 5 | 48.6 ± 35.2 | hydrodistillation |
| Peppermint tea 6 | n.d. | ultrasonic assisted extraction |
| Spearmint tea | 544 ± 97 | hydrodistillation |
| Apple mint tea | n.d. | hydrodistillation |
| Herbs Mint 1 | 358.5 ± 0.1 | hydrodistillation |
| Herbs Mint 1 | n.d. | ultrasonic assisted extraction |
| Herbal mint 2 | 275 ± 18 | hydrodistillation |
| Herbal mint 3 | 507 ± 58 | hydrodistillation |
| Herbs Mint 4 | n.d. | hydrodistillation |
| Pennyroyal 1 | 1504 ± 409 | hydrodistillation |
| Pennyroyal 2 | n.d. | hydrodistillation |
| Candy 1 (flavor: mint) | 88.3 ± 22.8 | hydrodistillation |
| Candy 2 (flavor: mint) | 46.3 ± 3.8 | hydrodistillation |
| Candy 3 (flavor: peppermint) | 137 ± 25 | hydrodistillation |
| Candy 4 (flavor: Mint) | 29.6 ± 9.2 | hydrodistillation |
| Candy 5 (flavor: Spearmint) | 20.5 ± 18.4 | hydrodistillation |
| Candy 6 (Spearmint flavor) | n.d. | hydrodistillation |
| Dragee mint flavor | 17.6 ± 6.8 | hydrodistillation |
| Dragee spearmint flavor | n.d. | hydrodistillation |
| Chewing dragee 1 | n.d. | hydrodistillation |
| Chewing dragee 1 | n.d. | ultrasonic assisted extraction |
| Chewing gum 2 | n.d. | ultrasonic assisted extraction |
| Candy 7 | n.d. | hydrodistillation |
| Measurement Parameters | Methanol-d4/Chloroform-d1 (1:1; v/v) |
|---|---|
| Pulse program | zg |
| TD | 65,788 |
| SI | 131,072 |
| NS | 32 |
| DS | 4 |
| D1 [s] | 30 |
| SW [ppm] | 20.5617 |
| O1P [ppm] | 4800 |
| O1 [Hz] | 1919.76 |
| AQ [s] | 3.9999 |
| Temperature [K] | 300 |
References
- Amalich, S.; Zerkani, H.; Tagnaout, I.; Ali, C.; Kamal, F.; Zair, T. Chemical composition of the essential oil and isolation of two main constituents of Mentha pulegium L. Vegetos 2024, 37, 82–93. [Google Scholar] [CrossRef]
- Nazarova, D.V.; Temerdashev, Z.A.; Vinitskaya, E.A.; Kiseleva, N.V.; Nagalevskii, M.V. Comparative Analysis of Chemical Compositions of Mentha L. Plant Extracts by Gas Chromatography–Mass Spectrometry after Hydrodistillation and Subcritical Extraction. J. Anal. Chem. 2023, 78, 1174–1183. [Google Scholar] [CrossRef]
- Telci, I.; Demirtas, I.; Bayram, E.; Arabaci, O.; Kacar, O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.). Ind. Crops Prod. 2010, 32, 588–592. [Google Scholar] [CrossRef]
- Collins, N.F.; Graven, E.H.; van Beek, T.A.; Lelyveld, G.P. Chemotaxonomy of Commercial Buchu Species (Agathosma betulina and A. crenulata). J. Essent. Oil Res. 1996, 8, 229–235. [Google Scholar] [CrossRef]
- van Baren, C.M.; Di Leo Lira, P.; Elechosa, M.A.; Molina, A.M.; Juárez, M.A.; Martínez, A.; Perelman, S.; Bandoni, A.L. New insights into the chemical biodiversity of Minthostachys mollis in Argentina. Biochem. Syst. Ecol. 2014, 57, 374–383. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Moolia, A.; van Vuuren, S.F.; van Zyl, R.L.; Başer, K.H.C.; Demirci, B.; Özek, T.; Trinder-Smith, T.H. The Biological Activity and Essential Oil Composition of 17 Agathosma (Rutaceae) Species. J. Essent. Oil Res. 2006, 18, 2–16. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Kimbaris, A.C.; Pappas, C.S.; Tarantilis, P.A.; Polissiou, M.G. Quantitative determination of pulegone in pennyroyal oil by FT-IR spectroscopy. J. Agric. Food Chem. 2009, 57, 10044–10048. [Google Scholar] [CrossRef]
- Benabdallah, A.; Boumendjel, M.; Aissi, O.; Rahmoune, C.; Boussaid, M.; Messaoud, C. Chemical composition, antioxidant activity and acetylcholinesterase inhibitory of wild Mentha species from northeastern Algeria. S. Afr. J. Bot. 2018, 116, 131–139. [Google Scholar] [CrossRef]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Siano, F.; Catalfamo, M.; Cautela, D.; Servillo, L.; Castaldo, D. Analysis of pulegone and its enanthiomeric distribution in mint-flavoured food products. Food Addit. Contam. 2005, 22, 197–203. [Google Scholar] [CrossRef]
- Araghi, A.M.; Nemati, H.; Azizi, M.; Moshtaghi, N.; Shoor, M.; Hadian, J. Assessment of phytochemical and agro-morphological variability among different wild accessions of Mentha longifolia L. cultivated in field condition. Ind. Crops Prod. 2019, 140, 111698. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Chemical composition and biological assays of essential oils of Calamintha nepeta (L.) Savi subsp. nepeta (Lamiaceae). Nat. Prod. Res. 2010, 24, 1734–1742. [Google Scholar] [CrossRef]
- Sutour, S.; Bradesi, P.; Casanova, J.; Tomi, F. Composition and chemical variability of Mentha suaveolens ssp. suaveolens and M. suaveolens ssp. insularis from Corsica. Chem. Biodivers. 2010, 7, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, Y.W.; Yang, G.L.; Husaini, A.M.; Wu, W. Variation of essential oil of Mentha haplocalyx Briq. and Mentha spicata L. from China. Ind. Crops Prod. 2013, 42, 251–260. [Google Scholar] [CrossRef]
- Statista. Absatz von Kräuter- und Früchtetee in Deutschland nach Art in den Jahren 2016 bis 2018 (in Tonnen). Available online: https://de.statista.com/statistik/daten/studie/38232/umfrage/fruechtetee-und-kraeutertee-konsum-in-deutschland/ (accessed on 17 February 2024).
- Taneja, S.C.; Chandra, S. Mint. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 366–387. [Google Scholar] [CrossRef]
- Lopez, P.; van Sisseren, M.; de Marco, S.; Jekel, A.; de Nijs, M.; Mol, H.G.J. A straightforward method to determine flavouring substances in food by GC-MS. Food Chem. 2015, 174, 407–416. [Google Scholar] [CrossRef] [PubMed]
- NTP. Toxicological and Carcinogensis Studies of Pulegone (CAS No. 89-82-7) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2011, 563, 1–201. [Google Scholar]
- European Commission. Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Off. J. EU 2008, L354, 34–50. [Google Scholar]
- Speijers, G.J.A. IPCS 2001 WHO Food Additives Series 46: Pulegone and Related Substances. Available online: http://www.inchem.org/documents/jecfa/jecmono/v46je10.htm (accessed on 2 July 2024).
- European Medicines Agency. Committee on Herbal Medicinal Products. In Public Statement on the Use of Herbal Medicinal Products Containing Pulegone and Menthofuran; European Medicines Agency: Amsterdam, The Netherlands, 2016; pp. 1–24. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/public-statement-use-herbal-medicinal-products-containing-pulegone-and-menthofuran-revision-1_en.pdf (accessed on 29 March 2024).
- Grosch, S.; Monakhova, Y.B.; Kuballa, T.; Ruge, W.; Kimmich, R.; Lachenmeier, D.W. Comparison of GC/MS and NMR for quantification of methyleugenol in food. Eur. Food Res. Technol. 2013, 236, 267–275. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 15th ed.; Merck Sharp & Dohme Corp., RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Safety Data Sheet: (+)-Pulegon. Available online: https://www.carlroth.com/medias/SDB-5168-MT-EN.pdf (accessed on 29 March 2024).
- Sicherheitsdatenblatt–Cyclohexan. Available online: https://www.sigmaaldrich.com/DE/de/sds/sial/28920 (accessed on 27 March 2024).
- Sicherheitsdatenblatt–Chloroform-d. Available online: https://www.sigmaaldrich.com/DE/de/sds/aldrich/151823 (accessed on 27 March 2024).
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- ISO 6571:2008/Amd 1:2017; Spices, Condiments and Herbs—Determination of Volatile Oil Content (Hydrodistillation Method). International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Tsitlakidou, P.; Tasopoulos, N.; Chatzopoulou, P.; Mourtzinos, I. Current status, technology, regulation and future perspectives of essential oils usage in the food and drink industry. J. Sci. Food Agric. 2023, 103, 6727–6751. [Google Scholar] [CrossRef]
- Verma, S.K.; Goswami, P.; Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R.; Darokar, M.P. Chemical composition and antimicrobial activity of bergamot-mint (Mentha citrata Ehrh.) essential oils isolated from the herbage and aqueous distillate using different methods. Ind. Crops Prod. 2016, 91, 152–160. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef] [PubMed]
- BBC. Rack of Lamb with Sauce Paloise. Available online: https://www.bbc.co.uk/food/recipes/rack_of_lamb_with_sauce_31777 (accessed on 2 April 2024).
- BBC. Beef Shin Stew with Spiced Sweet Potato Dumplings and Couscous. Available online: https://www.bbc.co.uk/food/recipes/beef_shin_couscous_with_51409 (accessed on 2 April 2024).
- Voigt, V.; Franke, H.; Lachenmeier, D.W. Risk Assessment of Pulegone in Foods Based on Benchmark Dose–Response Modeling. Foods 2024, 13, 2906. [Google Scholar] [CrossRef]
- Wider, G.; Dreier, L. Measuring protein concentrations by NMR spectroscopy. J. Am. Chem. Soc. 2006, 128, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Claridge, T.D. High-Resolution NMR Techniques in Organic Chemistry, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Kromidas, S. Handbuch Validierung in der Analytik, 2nd ed.; Wiley-VCH-Verlag: Weinheim, Germany, 2011; ISBN 978-3-527-32938-0. [Google Scholar]


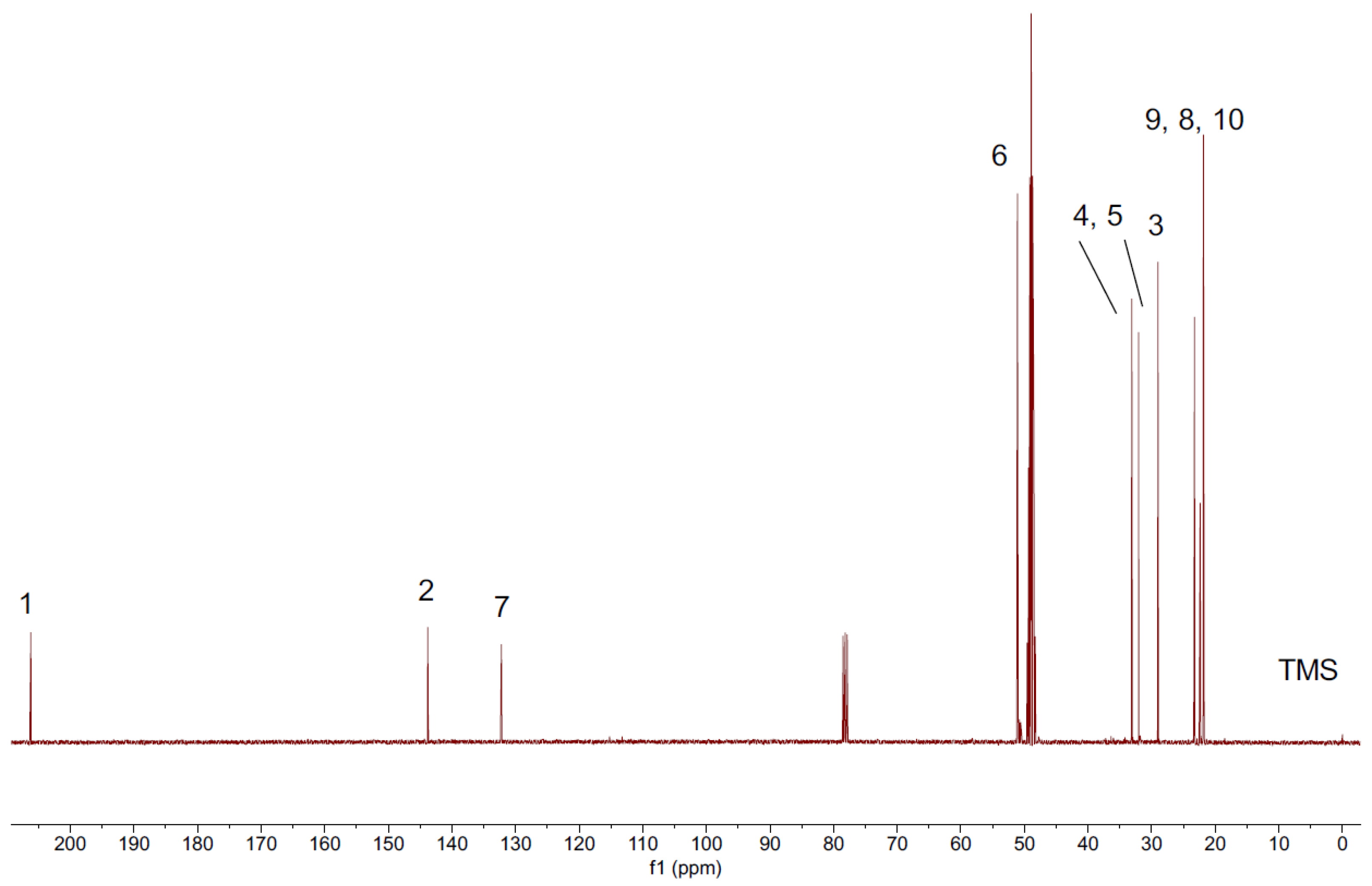
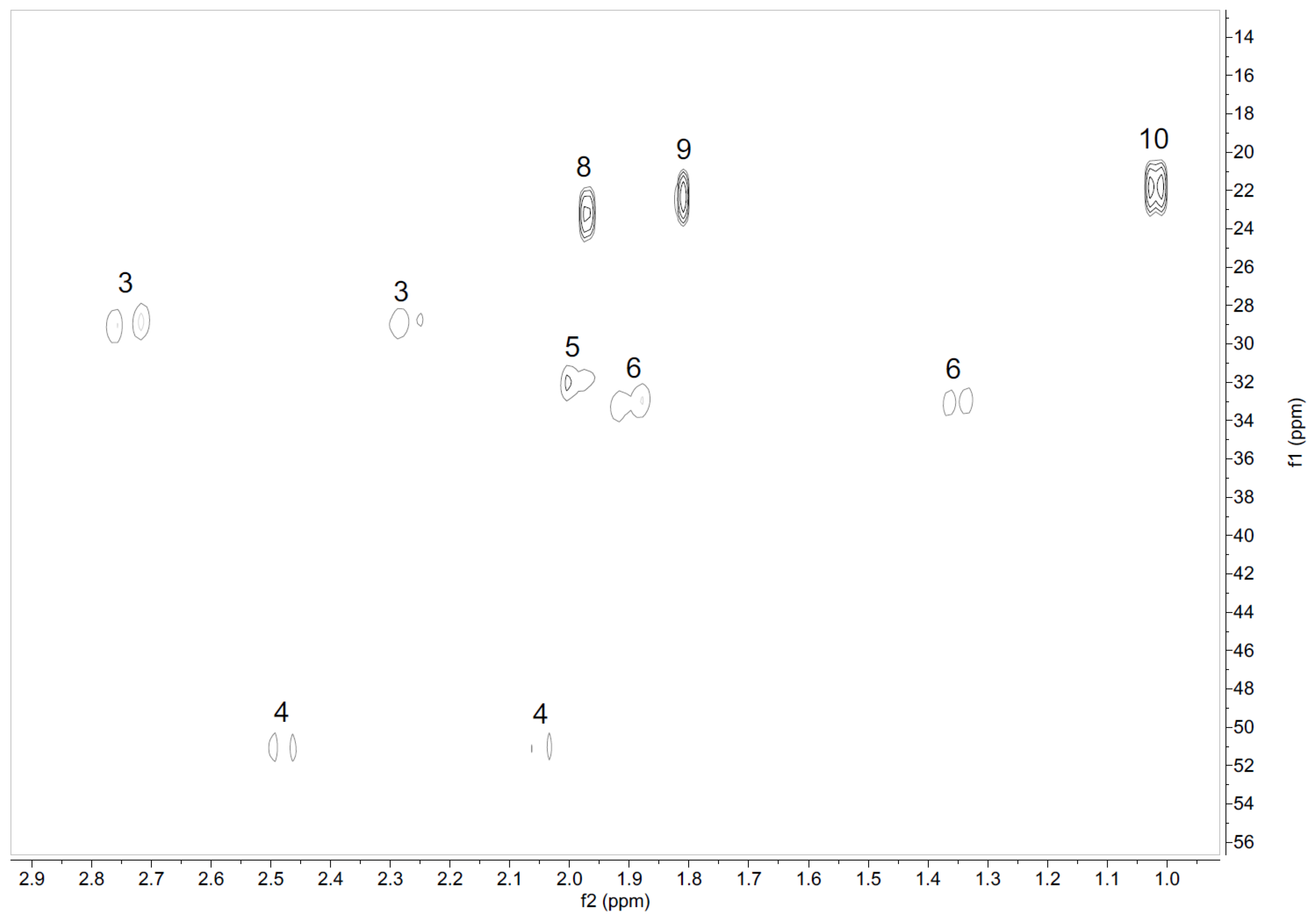
| 1H | 3 | 4 | 5 | 6 | 8 | 9 | 10 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Integral start [ppm] | 2.79 | 2.34 | 1.94 | 1.41 | 2.01 | 2.55 | 2.08 | 1.83 | 1.98 | 1.05 |
| Integral end [ppm] | 2.69 | 2.24 | 1.87 | 1.29 | 1.98 | 2.42 | 2.01 | 1.79 | 1.96 | 0.99 |
| Center point [ppm] | 2.74 | 2.29 | 1.90 | 1.35 | 2.00 | 2.49 | 2.04 | 1.81 | 1.97 | 1.02 |
| Number of protons | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 |
| Multiplicity | dt | m | m | ddd | m | dd | d | d | d | d |
| Coupling constant [Hz] | 15.52 4.33 | - | - | 17.57 10.62 4.82 | - | 11.06 2.20 | 11.06 | 0.85 | 1.18 | 6.21 |
| Matrix | LOD [mg/kg] | LOQ [mg/kg] |
|---|---|---|
| Peppermint | 59.9 | 214 |
| Pennyroyal | 134 | 491 |
| Spearmint | 371 | 1363 |
| Pastille | 14.5 | 51.5 |
| Dragee | 7.15 | 26.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Kuballa, T.; Lachenmeier, D.W. NMR Analysis of Pulegone in Food Products. Appl. Sci. 2024, 14, 10838. https://doi.org/10.3390/app142310838
Yu Y, Kuballa T, Lachenmeier DW. NMR Analysis of Pulegone in Food Products. Applied Sciences. 2024; 14(23):10838. https://doi.org/10.3390/app142310838
Chicago/Turabian StyleYu, Yifei, Thomas Kuballa, and Dirk W. Lachenmeier. 2024. "NMR Analysis of Pulegone in Food Products" Applied Sciences 14, no. 23: 10838. https://doi.org/10.3390/app142310838
APA StyleYu, Y., Kuballa, T., & Lachenmeier, D. W. (2024). NMR Analysis of Pulegone in Food Products. Applied Sciences, 14(23), 10838. https://doi.org/10.3390/app142310838







