Application of Active Soil Gas Screening for the Identification of Groundwater Contamination with Chlorinated Hydrocarbons at an Industrial Area—A Case Study of the Former Refrigerator Manufacturer Calex (City of Zlaté Moravce, Western Slovakia)
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
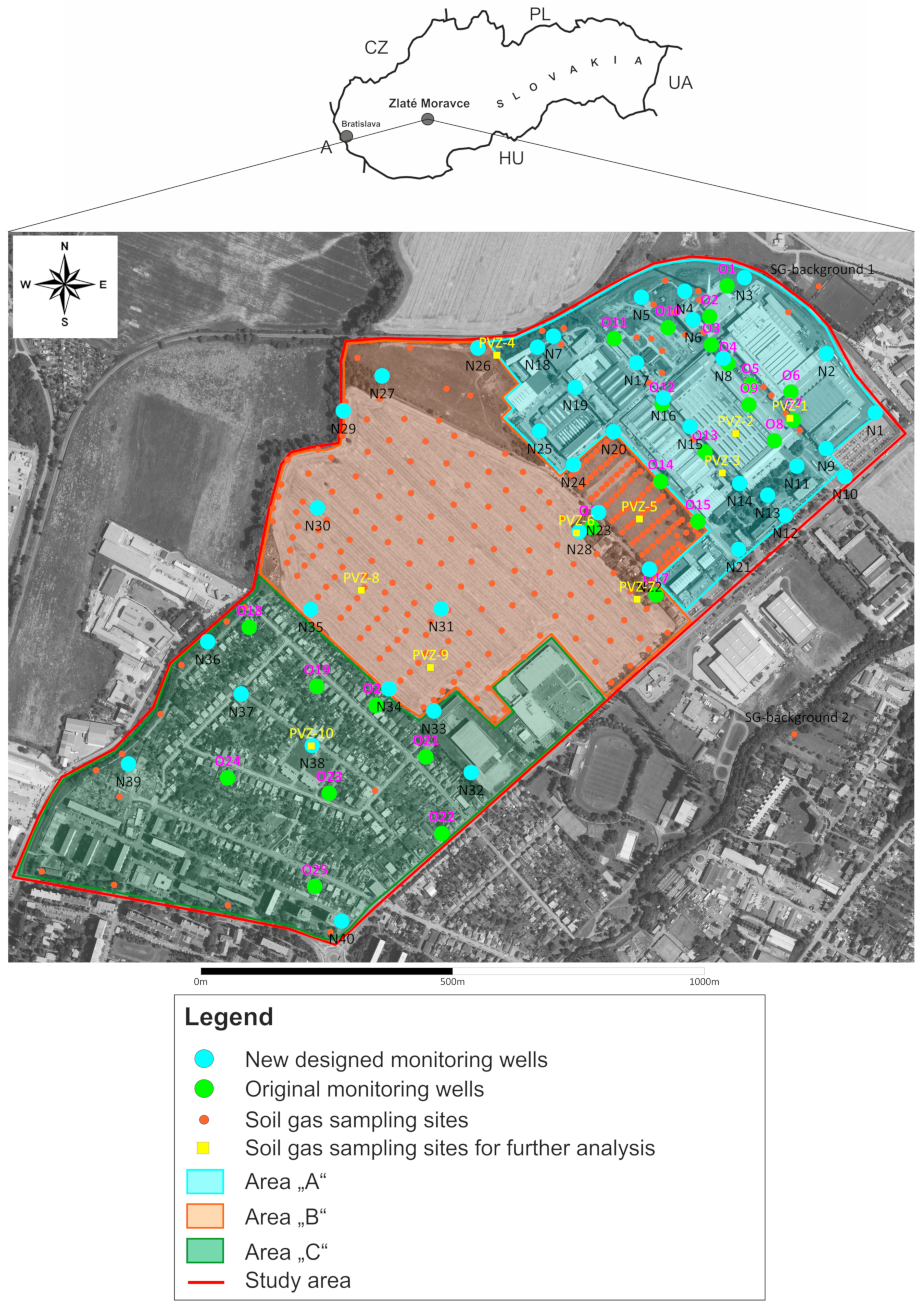
2.1. Area Description
2.2. Soil Gas and Groundwater Sampling and Analysis
2.3. Data Analysis and Human Health Risk Assessment (HHRA)
3. Results and Discussion
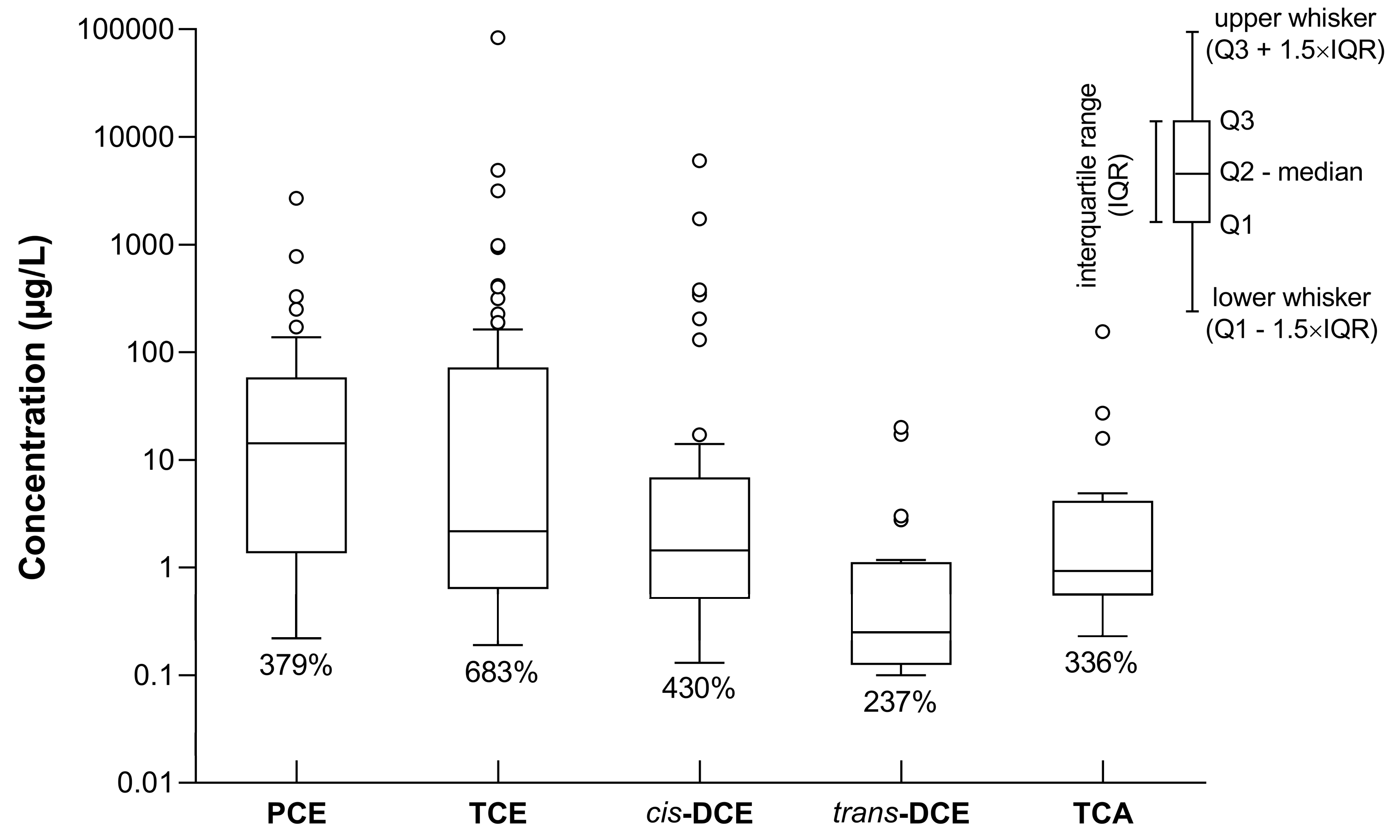
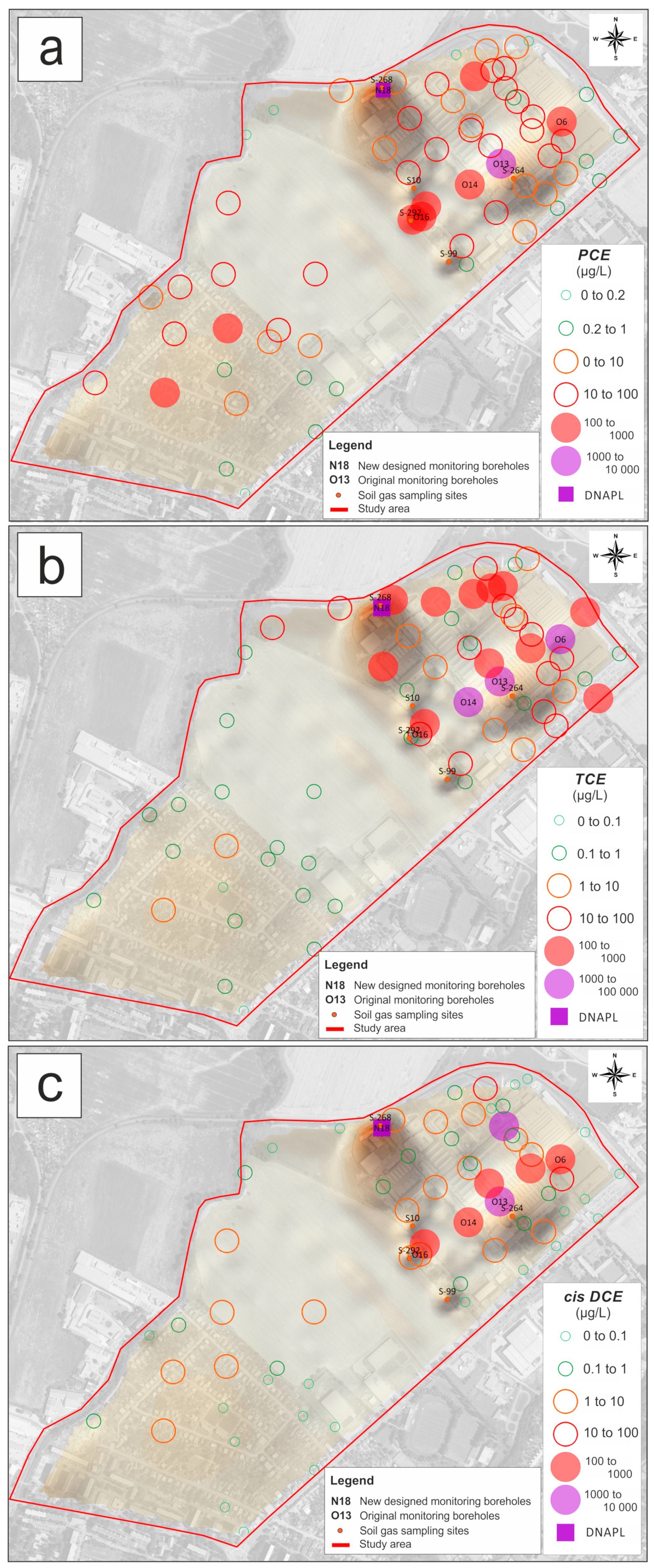
3.1. Occurrence of Chlorinated Hydrocarbons and Their Spatial Distribution in Groundwater
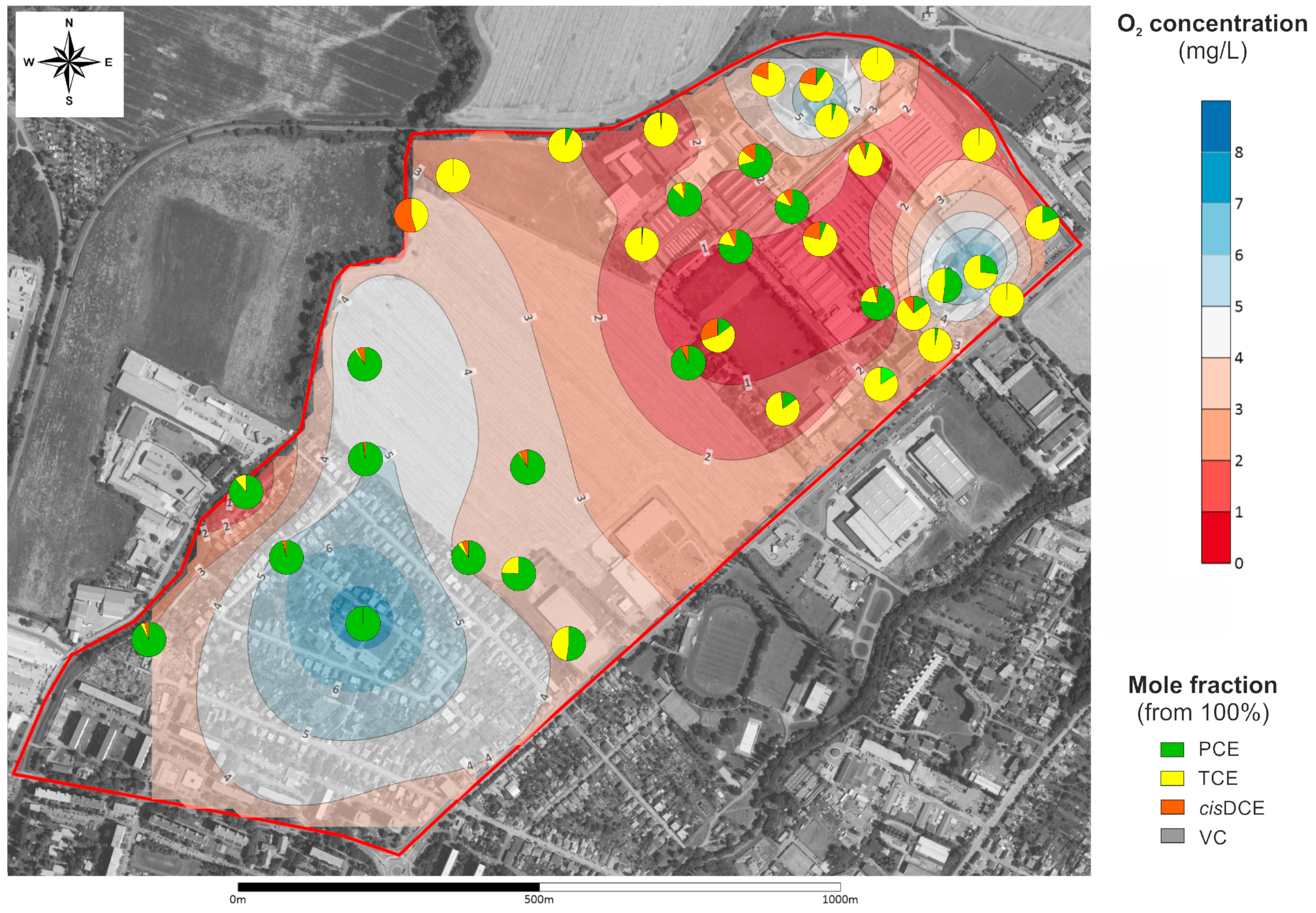
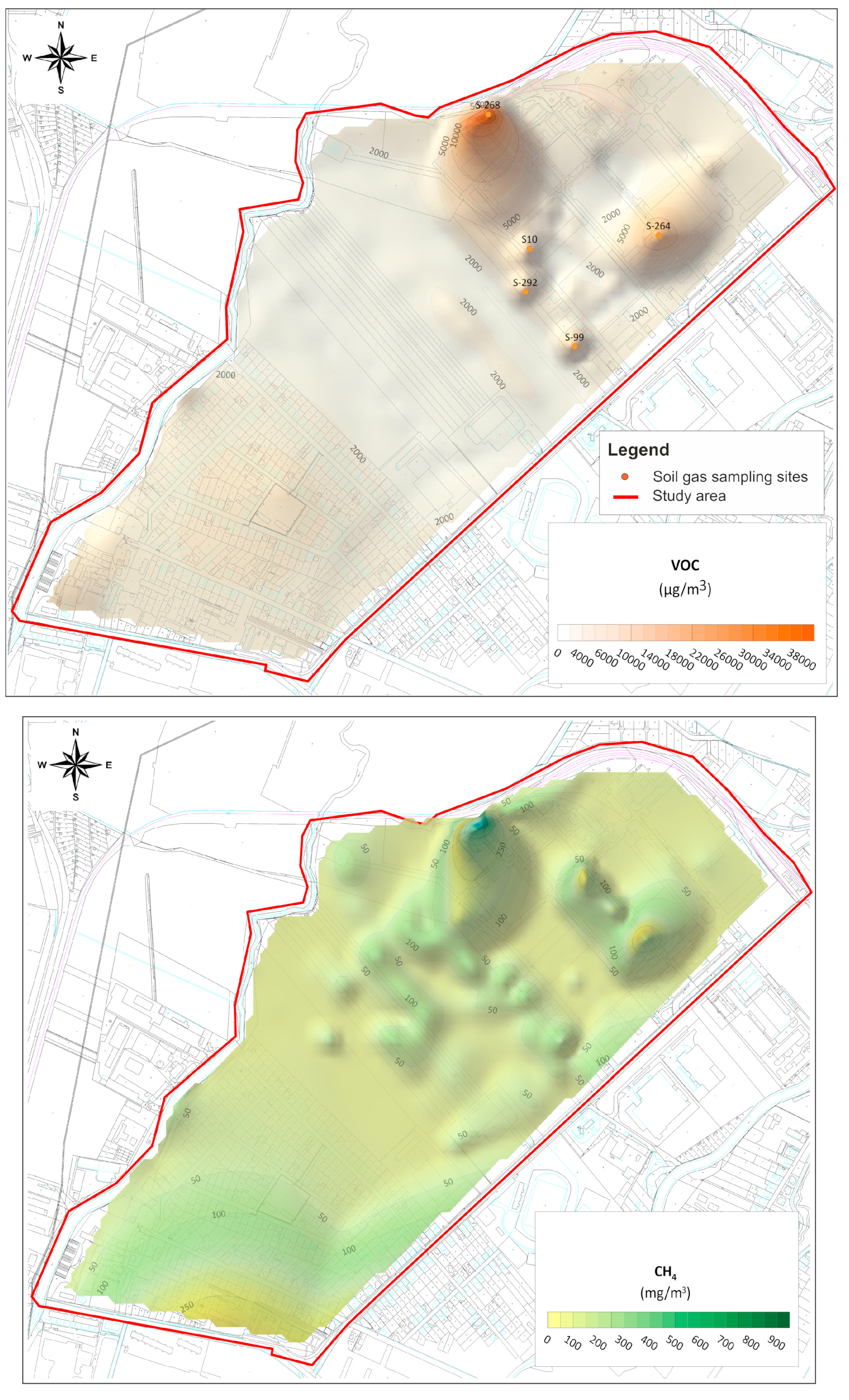
3.2. Spatial Distribution of VOC Concentrations in Soil Gas and Its Relation to Groundwater Contamination with CLHCs
3.3. Human Health Risk Assessment (HHRA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emsbo-Mattingly, S.D.; Flanders, K.L.; Litman, E.R. Integrated differentiation of multiple trichloroethylene and tetrachloroethylene groundwater impacts using spatial concentration, biodegradation indices, chemical fingerprinting and carbon/chlorine isotope patterns. Environ. Foren. 2023, 24, 329–350. [Google Scholar] [CrossRef]
- La Vigna, F.; Sbarbati, C.; Bonfà, I.; Martelli, S.; Ticconi, L.; Aleotti, L.; Covarelli, A.; Petitta, M. First survey on the occurrence of chlorinated solvents in groundwater of eastern sector of Rome. Rend. Lincei. Sci. Fis. Nat. 2019, 30, 297–306. [Google Scholar] [CrossRef]
- Kueper, B.H.; Stroo, H.F.; Vogel, C.M.; Ward, C.H. Source zone remediation: The state of the practice. In Chlorinated Solvent Source Zone Remediation, 1st ed.; Kueper, B.H., Stroo, H.F., Vogel, C.M., Ward, C.H., Eds.; Springer: New York, NY, USA, 2014; Chapter 1; pp. 1–27. [Google Scholar]
- Li, P.; Wu, J.; Zhou, W.; LaMoreaux, J.W. Hazard Hydrogeology; Springer Nature: Cham, Switzerland, 2023; pp. 179–256. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Kuchovsky, T.; Sracek, O. Natural attenuation of chlorinated solvents: A comparative study. Environ. Geol. 2007, 53, 147–157. [Google Scholar] [CrossRef]
- Matteucci, F.; Ercole, C.; del Gallo, M. A study of chlorinated solvent contamination of the aquifers of an industrial area in central Italy: A possibility of bioremediation. Front. Microbiol. 2015, 6, 924. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Liu, X.; Chen, J.; Liu, R.; Niu, H. Comparison of the health risks associated with different exposure pathways of multiple volatile chlorinated hydrocarbons in contaminated drinking groundwater. Environ. Pollut. 2019, 255, 113339. [Google Scholar] [CrossRef]
- Moran, M.J.; Zogorski, J.S.; Squillace, P.J. Chlorinated solvents in groundwater of the United States. Environ. Sci. Technol. 2007, 41, 74–81. [Google Scholar] [CrossRef]
- Bexfield, L.M.; Belitz, K.; Fram, M.S.; Lindsey, B.D. Volatile organic compounds in groundwater used for public supply across the United States: Occurrence, explanatory factors, and human health context. Sci. Total Environ. 2022, 827, 154313. [Google Scholar] [CrossRef]
- Guha, N.; Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol. 2012, 13, 1192–1193. [Google Scholar] [CrossRef]
- Bolt, H.M. Vinyl chloride—A classical industrial toxicant of new interest. Crit. Rev. Toxicol. 2005, 35, 307–323. [Google Scholar] [CrossRef]
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Alcántara, C.; Nägele, N.; Antón-Herrero, R.; Escolástico, C.; Eymar, E. Mycoremediation of soils polluted with trichloroethylene: First evidence of Pleurotus genus effectiveness. Appl. Sci. 2021, 11, 1354. [Google Scholar] [CrossRef]
- Goodman, R.S.; Mittal, L.; Parker, E.R. Public health risks, dermatological manifestations, and environmental justice associated with vinyl chloride exposure: Narrative review. JMIR Dermatol. 2023, 6, e48998. [Google Scholar] [CrossRef] [PubMed]
- European Union (EU). Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Off. J. Eur. Un. 2020, L435, 1–62. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 (accessed on 15 September 2024).
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda. 2022. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 8 November 2024).
- United States Environmental Protection Agency (USEPA). 2018 Edition of the Drinking Water Standards and Health Advisories Tables. Available online: https://www.epa.gov/system/files/documents/2022-01/dwtable2018.pdf (accessed on 8 November 2024).
- Li, X.; Shang, X.; Luo, T.; Du, X.; Wang, Y.; Xie, Q.; Matsuura, N.; Chen, J.; Kadokami, K. Screening and health risk of organic micropollutants in rural groundwater of Liaodong Peninsula, China. Environ. Pollut. 2016, 218, 739–748. [Google Scholar] [CrossRef]
- Yang, X.; Du, J.; Jia, C.; Yang, T.; Shao, S. Groundwater pollution risk, health effects and sustainable management of halocarbons in typical industrial parks. Environ. Res. 2024, 250, 118422. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Lu, H.; Fan, X. Stochastic goal programming based groundwater remediation management under human-health-risk uncertainty. J. Hazard. Mater. 2014, 279, 257–267. [Google Scholar] [CrossRef]
- Han, L.; Qian, L.; Yan, J.; Liu, R.; Du, Y.; Chen, M. A comparison of risk modeling tools and a case study for human health risk assessment of volatile organic compounds in contaminated groundwater. Environ. Sci. Pollut. Res. 2016, 23, 1234–1245. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, S.; Zhao, X.; Li, X.; Qiao, X.; Dionysiou, D.D.; Zheng, B. Distribution characteristics and health risk assessment of volatile organic compounds in the groundwater of Lanzhou City, China. Environ. Geochem. Health 2020, 42, 3609–3622. [Google Scholar] [CrossRef]
- Lamé, F.P.J. A practical approach for site investigation. In Dealing with Contaminated Sites: From Theory Towards Practical Application; Swartjes, F.A., Ed.; Springer: Dordrecht, The Netherlands, 2011; Chapter 3; pp. 139–163. [Google Scholar]
- Döberl, G.; Müller, D.; Dörrie, T.; Kästner, M. Section 1—Guideline. In Model-Driven Soil Probing, Site Assessment and Evaluation, Guidance on Technologies; Kästner, M., Braeckevelt, M., Döberl, G., Cassiani, G., Papini, M.P., Leven-Pfister, C., van Ree, D., Eds.; Sapienza Università Editrice: Rome, Italy, 2012; pp. 1–16. [Google Scholar]
- Deiana, R.; Cassiani, G.; Kemna, A.; Villa, A.; Bruno, V.; Bagliani, A. An experiment of non-invasive characterization of the vadose zone via water injection and cross-hole time-lapse geophysical monitoring. Near. Surf. Geophys. 2007, 5, 183–194. [Google Scholar] [CrossRef]
- Algreen, M.; Trapp, S.; Jensen, P.R.; Broholm, M.M. Tree coring as a complement to soil gas screening to locate PCE and TCE source zones and hot spots. Groundw. Monit. Remediat. 2015, 35, 57–66. [Google Scholar] [CrossRef]
- Mccall, W.; Christy, T.M.; Pipp, D.A.; Jaster, B.; White, J.; Goodrich, J.; Fontana, J.; Doxtader, S. Evaluation and application of the optical image profiler (OIP) a direct push probe for photo-logging UV-induced fluorescence of petroleum hydrocarbons. Environ. Earth Sci. 2018, 77, 374. [Google Scholar] [CrossRef]
- Lutes, C.; Stewart, L.; Truesdale, R.; De Loera, J.; Zimmerman, J.H.; Schumacher, B. Cost comparison of soil vapor extraction and subslab depressurization for vapor intrusion mitigation. Groundw. Monit. Remediat. 2022, 42, 43–45. [Google Scholar] [CrossRef]
- Rein, A.; Popp, S.; Zacharias, S.; Leven, C.; Bittens, M.; Dietrich, P. Comparison of approaches for the characterization of contamination at rural megasites. Environ. Earth Sci. 2011, 63, 1239–1249. [Google Scholar] [CrossRef]
- Wycisk, P.; Stollberg, R.; Neumann, C.; Gossel, W.; Weiss, H.; Weber, R. Integrated methodology for assessing the HCH groundwater pollution at the multi-source contaminated mega-site Bitterfeld/Wolfen. Environ. Sci. Pollut. Res. 2013, 20, 1907–1917. [Google Scholar] [CrossRef]
- Ciampi, P.; Esposito, C.; Cassiani, G.; Deidda, G.P.; Flores-Orozco, A.; Rizzetto, P.; Chiappa, A.; Bernabei, M.; Gardon, A.; Papini, M.P. Contamination presence and dynamics at a polluted site: Spatial analysis of integrated data and joint conceptual modeling approach. J. Contam. Hydrol. 2022, 248, 104026. [Google Scholar] [CrossRef]
- Wittlingerova, Z.; Machackova, J.; Petruzelkova, A.; Trapp, S.; Vlk, K.; Zima, J. One-year measurements of chloroethenes in tree cores and groundwater at the SAP Mimo Site, Northern Bohemia. Environ. Sci. Pollut. Res. 2013, 20, 834–847. [Google Scholar] [CrossRef]
- Algreen, M.; Trapp, S. Guideline for Application of Tree Coring as an Initial Screening Tool for Typical Pollutants in the Subsurface. TIMBRE Project. 2014, FP7-ENV-2010.3.1.5-2, Contract no: 265364. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/104826872/timbre_265364_D4.2_TC_guideline.pdf (accessed on 5 November 2024).
- Hood, G.R.; Papuga, S.A.; Socrates, C.; Rankin, K.; Hwang, K. On the phytoscreening potential of insect-induced plant galls. Plant Soil 2021, 467, 569–578. [Google Scholar] [CrossRef]
- Shao, S.; Guo, X.; Gao, C. Fresh underground light non-aqueous liquid (LNAPL) pollution source zone monitoring in an outdoor experiment using cross-hole electrical resistivity tomography. Environ. Sci. Pollut. Res. 2019, 26, 18316–18328. [Google Scholar] [CrossRef]
- Xia, T.; Dong, Y.; Mao, D.; Meng, J. Delineation of LNAPL contaminant plumes at a former perfumery plant using electrical resistivity tomography. Hydrogeol. J. 2021, 29, 1189–1201. [Google Scholar] [CrossRef]
- Dietrich, P.; Leven, C. Direct-push technologies. In Groundwater Geophysics; Kirsch, R., Ed.; Springer: Berlin, Germany, 2006; pp. 321–340. [Google Scholar]
- Algreen, M.; Kalisz, M.; Stalder, M.; Martac, E.; Krupanek, J.; Trapp, S.; Bartke, S. Using pre-screening methods for an effective and reliable site characterization at megasites. Environ. Sci. Pollut. Res. 2015, 22, 14673–14686. [Google Scholar] [CrossRef]
- Teramoto, E.H.; Isler, E.; Polese, L.; Baessa, M.P.M.; Chang, H.K. LNAPL saturation derived from laser induced fluorescence method. Sci. Total Environ. 2019, 683, 762–772. [Google Scholar] [CrossRef]
- Rivett, M.O.; Weathall, G.P.; Dearden, R.A.; McAlary, T.A. Review of unsaturated-zone transport and attenuation of volatile organic compound (VOC) plumes leached from shallow source zones. J. Contam. Hydrol. 2011, 123, 130–156. [Google Scholar] [CrossRef]
- Rein, A.; Holm, O.; Trapp, S.; Popp-Hofmann, S.; Leven, C.; Dietrich, P. Comparison of phytoscreening and direct push based site investigations at a rural megasite contaminated with chlorinated ethenes. Groundw. Monit. Remediat. 2015, 35, 45–56. [Google Scholar] [CrossRef]
- Hamamin, D.F. Passive soil gas technique for investigating soil and groundwater plume emanating from volatile organic hydrocarbon at Bazian oil refinery site. Sci. Total Environ. 2018, 622–623, 1485–1498. [Google Scholar] [CrossRef]
- Unnithan, A.; Bekele, D.N.; Chadalavada, S.; Naidu, R. Insights into vapour intrusion phenomena: Current outlook and preferential pathway scenario. Sci. Total Environ. 2021, 796, 148885. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Negri, M.C.; Minsker, B.S.; Werth, C.J. Monitoring subsurface contamination using tree branches. Groundw. Monit. Remediat. 2007, 27, 65–74. [Google Scholar] [CrossRef]
- Wilson, J.; Bartz, R.; Limmer, M.; Burken, J. Plants as bio-indicators of subsurface conditions: Impact of groundwater level on BTEX concentrations in trees. Int. J. Phytoremediat. 2013, 15, 900–910. [Google Scholar] [CrossRef]
- Cassiani, G.; Binley, A.; Kemna, A.; Wehrer, M.; Flores Orozco, A.; Deiana, R.; Boaga, J.; Rossi, M.; Dietrich, P.; Werban, U.; et al. Noninvasive characterization of the Trecate (Italy) crude-oil contaminated site: Links between contamination and geophysical signals. Environ. Sci. Pollut. Res. 2014, 21, 8914–8931. [Google Scholar] [CrossRef]
- Ramalho, A.M.Z.; de Aquino Sobrinho, H.L.; dos Anjos, H.L.; de Castro Dantas, T.N.; da Silva, D.R. Study of contamination by benzene due to diesel and gasoline leaks at a gas station in Natal/Brazil. Int. J. Eng. Technol. 2014, 14, 49–54. [Google Scholar]
- Lawrence, S.J. Description, Properties, and Degradation of Selected Volatile Organic Compounds Detected in Ground Water—A Review of Selected Literature; Open-File Report 2006-1338; U.S. Geological Survey: Atlanta, GA, USA, 2006; 62p. Available online: https://pubs.usgs.gov/of/2006/1338/pdf/ofr2006-1338.pdf (accessed on 25 July 2024).
- Thomson, N.R.; Sykes, J.F.; Van Vliet, D. A numerical investigation into factors affecting gas and aqueous phase plumes in the subsurface. J. Contam. Hydrol. 1997, 28, 39–70. [Google Scholar] [CrossRef]
- Ma, J.; McHugh, T.; Beckley, L.; Lahvis, M.; DeVaull, G.; Jiang, L. Vapor intrusion investigations and decision-making: A critical review. Environ. Sci. Technol. 2020, 54, 7050–7069. [Google Scholar] [CrossRef]
- Pitchford, A.M.; Mazzella, A.T.; Scarbrough, K.R. Soil-Gas and Geophysical Techniques for Detection of Subsurface Organic Contamination; United States Environmental Protection Agency, Environmental Monitoring Systems Laboratory: Las Vegas, NV, USA, 1989; 186p, Available online: https://clu-in.org/download/technology/geophysical_methods/EPA-600-4-88-019.PDF (accessed on 28 July 2024).
- Arato, A.; Wehrer, M.; Biró, B.; Godio, A. Integration of geophysical, geochemical and microbiological data for a comprehensive small-scale characterization of an aged LNAPL-contaminated site. Environ. Sci. Pollut. Res. 2014, 21, 8948–8963. [Google Scholar] [CrossRef]
- Lapalla, E.G.; Thompson, G.M. Detection of Ground Water Contamination by Shallow Soil Gas Sampling in the Vadose Zone: Theory and Applications. In Proceedings of the 5th National Conference on Management of Uncontrolled Hazardous Waste Sites, Silver Spring, MD, USA, 7–9 November 1984; pp. 20–28. [Google Scholar]
- Marrin, D.L. Delineation of Gasoline Hydrocarbons in Ground Water by Soil Gas Analysis. In Proceedings of the Hazardous Materials Management Conference Wast ’85; Tower Conference Management Company: Wheaton, IL, USA, 1985; pp. 112–119. [Google Scholar]
- Wilson, L.H.; Johnson, P.C.; Rocco, J.R. Collecting and Interpreting Soil Gas Samples from the Vadose Zone: A Practical Strategy for Assessing the Subsurface Vapor-to-Indoor Air Migration Pathway at Petroleum Hydrocarbon Sites; Publication number 4741; American Petroleum Institute: Washington, DC, USA, 2005; 106p, Available online: https://www.api.org/~/media/files/ehs/clean_water/ground_water_quality/pub4741_vi_assessment_2005.pdf (accessed on 13 September 2024).
- Kerfoot, H.B.; Barrows, L.J. Soil-Gas Measurement for Detection of Subsurface Organic Contamination; Report No. EPA/600/2-87/027; United States Environmental Protection Agency, Environmental Monitoring Systems Laboratory: Las Vegas, NV, USA, 1987; 61p. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/940060Q2.PDF?Dockey=940060Q2.PDF (accessed on 5 August 2024).
- Kerfoot, H.B. Soil-gas surveys for detection and delineation of groundwater contamination. Trends Anal. Chem. 1990, 9, 157–163. [Google Scholar] [CrossRef]
- Moseley, C.L.; Meyer, M.R. Petroleum contamination of an elementary school: A case history involving air, soil-gas, and groundwater monitoring. Environ. Sci. Technol. 1992, 26, 185–192. [Google Scholar] [CrossRef]
- Marrin, D.L.; Kerfoot, H.B. Soil-gas surveying techniques. Environ. Sci. Technol. 1988, 22, 740–745. [Google Scholar] [CrossRef]
- Pankow, J.F.; Cherry, J.A. Dense Chlorinated Solvents and Other DNAPLs in Groundwater: History, Behavior, and Remediation; Waterloo Press: Portland, OR, USA, 1996; pp. 429–431. [Google Scholar]
- Schubert, M.; Peña, P.; Balcázar, M.; Meissner, R.; Lopez, A.; Flores, J.H. Determination of radon distribution patterns in the upper soil as a tool for the localization of subsurface NAPL contamination. Radiat. Meas. 2005, 40, 633–637. [Google Scholar] [CrossRef]
- Cvetković, M.; Kapuralić, J.; Pejić, M.; Močilac, K.I.; Rukavina, D.; Smirčić, D.; Kamenski, A.; Matoš, B.; Špelić, M. Soil gas measurements of radon, CO2 and hydrocarbon concentrations as indicators of subsurface hydrocarbon accumulation and hydrocarbon seepage. Sustainability 2021, 13, 3840. [Google Scholar] [CrossRef]
- Mattia, M.; Tuccimei, P.; Ciotoli, G.; Soligo, M.; Carusi, C.; Rainaldi, E.; Voltaggio, M. Combining radon deficit, NAPL concentration, and groundwater table dynamics to assess soil and groundwater contamination by NAPLs and related attenuation processes. Appl. Sci. 2023, 13, 12813. [Google Scholar] [CrossRef]
- NJDEP. Guidance: Field Sampling Procedures Manual; New Jersey Department of Environmental Protection, State of New Jersey: Trenton, NJ, USA, 2005; 574p. Available online: https://www.nj.gov/dep/srp/guidance/fspm/manual_edition/2005/fsmp2005.pdf (accessed on 8 August 2024).
- USEPA. Expedited Site Assessment Tools for Underground Storage Tank Sites. A Guide for Regulators; EPA 510-B-16-004; United States Environmental Protection Agency, Land and Emergency Management 5401R: Washington, DC, USA, 1997; 31p. Available online: https://www.epa.gov/ust/expedited-site-assessment-tools-underground-storage-tank-sites-guide-regulators (accessed on 8 August 2024).
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Basheer, S.; Wang, X.; Farooque, A.A.; Nawaz, R.A.; Pang, T.; Neokye, E.O. A review of greenhouse gas emissions from agricultural soil. Sustainability 2024, 16, 4789. [Google Scholar] [CrossRef]
- Bannov, A.G.; Trubin, I.V.; Zakharov, I.K.; Maksimovskiy, E.A.; Kurmashov, P.B. A critical review on soil gas analysis: Modern technologies and problems. Agronomy 2024, 14, 2374. [Google Scholar] [CrossRef]
- Jánová, V. Environmental burdens—State of solution in Europe and Slovakia. Enviromagazín 2009, 2, 4–7. [Google Scholar]
- Kordík, J.; Slaninka, I.; Bodiš, D.; Bottlik, F.; Černák, R.; Dananaj, I.; Demko, R.; Fričovská, J.; Fričovský, B.; Gluch, A.; et al. Monitorovanie environmentálnych záťaží na vybraných lokalitách Slovenskej republiky. Záverečná správa. Lokalita MEZ č. 35, Zlaté Moravce—Calex [Monitoring of Environmental Burdens in Selected Locations of the Slovak Republic. Final Report. Locality MEZ no. 35, Zlaté Moravce—Calex]; Ministerstvo životného prostredia Slovenskej republiky and Štátny geologický ústav Dionýza Štúra: Bratislava, Slovakia, 2015; 252p. [Google Scholar]
- Auxt, A.; Ingár, K.; Šuchová, M.; Masiar, R.; Zajacová, A.; Musil, J. Čerpanie podzemných vôd znečistených chlórovanými uhľovodíkmi v Zlatých Moravciach v areáli Danfoss—Vyhodnotenie výsledkov a analýza rizika [Pumping of Groundwater Contaminated by Chlorinated Hydrocarbons in Zlaté Moravce in the Danfoss Area—Assessment of Results and Risk Analysis]; Záverečná správa. HES-COMGEO s.r.o.: Banská Bystrica, Slovakia, 2005. [Google Scholar]
- Auxt, A.; Klačanová, Z. Zlaté Moravce—Danfoss Compressors—Monitorovanie chlóretylénového znečistenia v podzemnej vode v roku 2008, monitorovanie GF ŽP [Zlaté Moravce—Danfoss Compressors—Monitoring of Chlorethylene Contamination in Groundwater in 2008, Monitoring of GF ŽP]; HES-COMGEO s.r.o.: Banská Bystrica, Slovakia, 2009; 11p. [Google Scholar]
- Wyatt, D.E.; Richers, D.M.; Pirkle, R.J. Barometric pumping effects on soil-gas studies for geological and environmental characterization. Environ. Geol. 1995, 25, 243–250. [Google Scholar] [CrossRef]
- Mills, W.B.; Liu, S.; Rigby, M.C.; Brenner, D. Time variable simulation of soil vapor intrusion into a building with a combined crawl space and basement. Environ. Sci. Technol. 2007, 41, 4993–5001. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Srinivasa Rao, P.L.; Annapurna, B.; Hasan, S.Z. Implication of soil gas method for prospecting of hydrocarbon microseepage. Int. J. Petrol. Petrochem. Eng. 2015, 1, 34–41. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Kibet, E.; Musafiri, C.M.; Kiboi, M.; Macharia, J.; Ngetich, O.K.; Kosgei, D.K.; Mulianga, B.; Okoti, M.; Zeila, A.; Ngetich, F.K. Soil greenhouse gas emissions from different land utilization types in Western Kenya. Front. Soil Sci. 2022, 2, 956634. [Google Scholar] [CrossRef]
- Bastviken, D.; Treat, C.C.; Pangala, S.R.; Gauci, V.; Enrich-Prast, A.; Karlson, M.; Galfalk, M.; Romano, M.B.; Sawakuchi, H.O. The importance of plants for methane emission at the ecosystem scale. Aquat. Bot. 2023, 184, 103596. [Google Scholar] [CrossRef]
- ISO 18400-204:2017; Soil Quality—Sampling—Part 204: Guidance on Sampling of Soil Gas. The International Organization for Standardization: Geneva, Switzerland, 2017.
- USEPA. EPA Method 8260D (SW-846): Volatile Organic Compounds by Gas Chromatography-Mass Spectrometry (GC/MS); USEPA: Washington, DC, USA, 2017. Available online: https://www.epa.gov/sites/default/files/2017-04/documents/method_8260d_update_vi_final_03-13-2017.pdf (accessed on 9 August 2024).
- MESR. Smernica Ministerstva životného prostredia Slovenskej republiky z 28. januára 2015 č. 1/2015—7. na vypracovanie analýzy rizika znečisteného územia [Directive of the Ministry of Environment of the Slovak Republic dated January 28, 2015 no. 1/2015—7. to Elaborate a Risk Analysis of the Contaminated Area]; Ministry of Environment of the Slovak Republic: Bratislava, Slovakia, 2015; 96p, Available online: https://www.minzp.sk/files/sekcia-geologie-prirodnych-zdrojov/ar_smernica_final.pdf (accessed on 9 August 2024).
- Guerra, P.; Bauer, A.; Reiss, R.A.; McCord, J. In situ bioremediation of a chlorinated hydrocarbon plume: A superfund site field pilot test. Appl. Sci. 2021, 11, 10005. [Google Scholar] [CrossRef]
- Dutta, N.; Usman, M.; Ashraf, M.A.; Luo, G.; Zhang, S. A critical review of recent advances in the bio-remediation of chlorinated substances by microbial dechlorinators. Chem. Eng. J. Adv. 2022, 12, 100359. [Google Scholar] [CrossRef]
- Mattes, T.E.; Alexander, A.K.; Coleman, N.V. Aerobic biodegradation of the chloroethenes: Pathways, enzymes, ecology, and evolution. FEMS Microbiol. Rev. 2010, 34, 445–475. [Google Scholar] [CrossRef]
- Bradley, P.M.; Chapelle, F.H. Effect of contaminant concentration on aerobic microbial mineralization of DCE and VC in stream-bed sediments. Environ. Sci. Technol. 1998, 32, 553–557. [Google Scholar] [CrossRef]
- Newell, C.J.; Kueper, B.H.; Wilson, J.T.; Johnson, P.C. Natural attenuation of chlorinated solvent source zones. In Chlorinated Solvent Source Zone Remediation, 1st ed.; Kueper, B.H., Stroo, H.F., Vogel, C.M., Ward, C.H., Eds.; Springer: New York, NY, USA, 2014; Chapter 13; pp. 459–503. [Google Scholar]
- Broholm, K.; Feenstra, S.; Cherry, J.A. Solvent release into a sandy aquifer. 1. Overview of source distribution and dissolution behavior. Environ. Sci. Technol. 1999, 33, 681–690. [Google Scholar] [CrossRef]
- Seyedabbasi, M.A.; Newell, C.J.; Adamson, D.T.; Sale, T.C. Relative contribution of DNAPL dissolution and matrix diffusion to the long-term persistence of chlorinated solvent source zones. J. Contam. Hydrol. 2012, 134–135, 69–81. [Google Scholar] [CrossRef]
- Patil, P.; Jeppu, G.P.; Vasudevan, M.; Girish, C.R. A review on dissolution of multi-component non-aqueous phase liquids: Recent studies, mechanisms and mass transfer limitations. Desalin. Water Treat. 2023, 283, 164–184. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Evans, C.E.; Hayes, E.C. Porosity and pore size distribution in a sedimentary rock: Implications for the distribution of chlorinated solvents. J. Contam. Hydrol. 2017, 203, 70–84. [Google Scholar] [CrossRef]
- Němeček, J.; Marková, K.; Špánek, R.; Antoš, V.; Kozubek, P.; Lhotský, O.; Černík, M. Hydrochemical conditions for aerobic/anaerobic biodegradation of chlorinated ethenes—A multi-site assessment. Water 2020, 12, 322. [Google Scholar] [CrossRef]
- Tiehm, A.; Schmidt, K.R. Sequential anaerobic/aerobic biodegradation of chloroethenes—Aspects of field application. Curr. Opin. Biotechnol. 2011, 22, 415–421. [Google Scholar] [CrossRef]
- Polák, R. Zlaté Moravce—Calex—Prieskum obsahu chlórovaných uhľovodíkov v zeminách a podzemných vodách, hydrogeologický prieskum [Zlaté Moravce—Calex—Monitoring of the Content of Chlorinated Hydrocarbons in Soils and Groundwater, Hydrogeological Investigation]; Hydropol Bratislava, Geofond Bratislava (77971): Bratislava, Slovakia, 1992. [Google Scholar]
- Kminiaková, K.; Šimák, J. Aktualizácia rozsahu znečistenia alifatickými chlórovanými uhľovodíkmi Calex a.s., Zlaté Moravce [Update of the Extent of Pollution by Aliphatic Chlorinated Hydrocarbons Calex Plc., Zlaté Moravce]; ATE Slovakia, Geofond Bratislava (82334): Bratislava, Slovakia, 1999. [Google Scholar]
- Gao, X.; Wang, Y.; Wu, L.; Zheng, F.; Sun, N.; Liu, G.; Liu, Y.; Meng, P.; Sun, L.; Jing, B. The impact of anthropogenic VOC emissions on atmospheric pollution: A case study of a typical industrialized area in China. Atmosphere 2023, 14, 1586. [Google Scholar] [CrossRef]
- Raysoni, A.U.; Stock, T.H.; Sarnat, J.A.; Chavez, M.C.; Sarnat, S.E.; Montoya, T.; Holguin, F.; Li, W.W. Evaluation of VOC concentrations in indoor and outdoor microenvironments at near-road schools. Environ. Pollut. 2017, 231, 681–693. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, W. Assessment of ambient volatile organic compounds (VOCs) near major roads in urban Nanjing, China. Atmos. Res. 2008, 89, 289–297. [Google Scholar] [CrossRef]
- Kram, M.L.; Keller, A.A.; Rossabi, J.; Everett, L.G. DNAPL characterization methods and approaches, part 2: Cost comparisons. Groundw. Monitor. Remediat. 2002, 22, 46–61. [Google Scholar] [CrossRef]
- Rivett, M.O.; Turner, R.J.; Glibbery, P.; Cuthbert, M.O. The legacy of chlorinated solvents in the Birmingham aquifer, UK: Observations spanning three decades and the challenge of future urban groundwater development. J. Contam. Hydrol. 2012, 140–141, 107–123. [Google Scholar] [CrossRef]
- Essaid, H.I.; Bekins, B.A.; Cozzarelli, I.M. Organic contaminant transport and fate in the subsurface: Evolution of knowledge and understanding. Water Resour. Res. 2015, 51, 4861–4902. [Google Scholar] [CrossRef]
- Guo, Y.; Dahlen, P.; Johnson, P. Temporal variability of chlorinated volatile organic compound vapour concentrations in a residential sewer and land drain system overlying a dilute groundwater plume. Sci. Total Environ. 2018, 702, 134756. [Google Scholar] [CrossRef]
- Bozkurt, O.; Pennell, K.G.; Suuberg, E.M. Simulation of the vapor intrusion process for nonhomogeneous soils using a three-dimensional numerical model. Groundw. Monitor. Remediat. 2009, 29, 92–104. [Google Scholar] [CrossRef]
- Yao, Y.; Xiao, Y.; Mao, F.; Chen, H.; Verginelli, I. Examining the role of subfoundation soil texture in chlorinated vapor intrusion from groundwater sources with a two-layer numerical model. J. Hazard. Mater. 2018, 359, 544–553. [Google Scholar] [CrossRef]
- Verginelli, I.; Yao, Y.; Suuberg, E.M. Risk assessment tool for chlorinated vapor intrusion based on a two-dimensional analytical model involving vertical heterogeneity. Environ. Eng. Sci. 2019, 36, 969–980. [Google Scholar] [CrossRef]
- Bekele, D.N.; Naidu, R.; Chadalavada, S. Influence of soil properties on vapor-phase sorption of trichloroethylene. J. Hazard. Mater. 2016, 306, 34–40. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Slater, G.F.; Sleep, B.; Witt, M.; Klecka, G.M.; Harkness, M.; Spivack, J. Stable carbon isotope evidence for intrinsic bioremediation of tetrachloroethene and trichloroethene at area 6, Dover Air Force Base. Environ. Sci. Technol. 2001, 35, 261–269. [Google Scholar] [CrossRef]
- Ferrey, M.L.; Wilkin, R.T.; Ford, R.G.; Wilson, J.T. Nonbiological removal of cis-dichloroethylene and 1,1-dichloroethylene in aquifer sediment containing magnetite. Environ. Sci. Technol. 2004, 38, 1746–1752. [Google Scholar] [CrossRef]
- Kawabe, Y.; Komai, T. A case study of natural attenuation of chlorinated solvents under unstable groundwater conditions in Takahata, Japan. Bull. Environ. Contam. Toxicol. 2019, 102, 280–286. [Google Scholar] [CrossRef]
- Yager, R.M.; Bilotta, S.E.; Mann, C.L.; Madsen, E.L. Metabolic and in situ attenuation of chlorinated ethenes by naturally occurring microorganisms in a fractured dolomite aquifer near Niagara Falls, New York. Environ. Sci. Technol. 1997, 31, 3138–3147. [Google Scholar] [CrossRef]
- McGuire, T.M.; Newell, C.J.; Looney, B.B.; Vangelas, K.M.; Sink, C.H. Historical analysis of monitored natural attenuation: A survey of 191 chlorinated solvent sites and 45 solvent plumes. Remediat. J. 2004, 15, 99–112. [Google Scholar] [CrossRef]
- He, Y.T.; Wilson, J.T.; Wilkin, R.T. Impact of iron sulfide transformation on trichloroethylene degradation. Geochim. Cosmochim. Acta 2010, 74, 2025–2039. [Google Scholar] [CrossRef]
- DeVaull, G.E. Indoor vapor intrusion with oxygen-limited biodegradation for a subsurface gasoline source. Environ. Sci. Technol. 2007, 41, 3241–3248. [Google Scholar] [CrossRef]
- Davis, G.B.; Patterson, B.M.; Trefry, M. Evidence for instantaneous oxygen-limited biodegradation of petroleum hydrocarbon vapors in the subsurface. Groundw. Monit. Remediat. 2009, 29, 126–137. [Google Scholar] [CrossRef]
- Gossett, J.M. Sustained aerobic oxidation of vinyl chloride at low oxygen concentrations. Environ. Sci. Technol. 2010, 44, 1405–1411. [Google Scholar] [CrossRef]
- Conrad, M.E.; Templeton, A.S.; Daley, P.F.; Alvarez-Cohen, L. Seasonaly-induced fluctuations in microbial production and consumption of methane during bioremediation of aged subsurface refinery contamination. Environ. Sci. Technol. 1999, 33, 4061–4068. [Google Scholar] [CrossRef]
- Tassi, F.; Venturi, S.; Cabassi, J.; Vaselli, O.; Gelli, I.; Cinti, D.; Capacchiacci, F. Biodegradation of CO2, CH4 and volatile organic compounds (VOCs) in soil gas from the Vicano–Cimino hydrothermal system (central Italy). Org. Geochem. 2015, 86, 81–93. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial degradation of aromatic compounds—From one strategy to four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Deyo, B.G.; Robbins, G.A.; Binkhorst, G.K. Use of portable oxygen and carbon dioxine detectors to screen soil gas for subsurface gasoline contamination. Groundwater 1993, 31, 598–604. [Google Scholar] [CrossRef]
- Zhang, W.; Sheng, R.; Zhang, M.; Xiong, G.; Hou, H.; Li, S.; Wei, W. Effects of continuous manure application on methanogenic and methanotrophic communities and methane production potentials in rice paddy soil. Agric. Ecosyst. Environ. 2018, 258, 121–128. [Google Scholar] [CrossRef]
- Cárdenas, A.; Ammon, C.; Schumacher, B.; Stinner, W.; Herrmann, C.; Schneider, M.; Weinrich, S.; Fischer, P.; Amon, T.; Amon, B. Methane emissions from the storage of liquid dairy manure: Influences of season, temperature and storage duration. Waste Manage. 2021, 121, 393–402. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, H. Mitigating carbon emissions: The impact of peat moss feeding on CH4 and CO2 emissions during pig slurry storage. Appl. Sci. 2023, 13, 10492. [Google Scholar] [CrossRef]
- Kerfoot, H.B. Soil-gas measurement for detection of groundwater contamination by volatile organic compounds. Environ. Sci. Technol. 1987, 21, 1022–1024. [Google Scholar] [CrossRef]
- Abreu, L.D.V.; Ettinger, R.; McAlary, T. Simulated soil vapor intrusion attenuation factors including biodegradation for petroleum hydrocarbons. Groundw. Monitor. Remediat. 2009, 29, 105–117. [Google Scholar] [CrossRef]
- Bekele, D.N.; Naidu, R.; Chadalavada, S. Development of a modular vapor intrusion model with variably saturated and non-isothermal vadose zone. Environ. Geochem. Health 2018, 40, 887–902. [Google Scholar] [CrossRef]
- Eberle, C.S.; Wade, W.M.; Tharp, T.; Brinkman, J. Comparison of Passive Soil Vapor Survey Techniques at a Tijeras Arroyo Site; Sandia National Laboratories: Albuquerque, NM, USA, 1996; 6p, Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/27/070/27070295.pdf (accessed on 13 September 2024).
- Clarke, J.N.; Goodwin, D.F.; O’Neill, H.; Odencrantz, J.E. Application of passive soil gas technology to determine the source and extent of a PCE groundwater plume in an urban environment. Remediat. J. 2008, 18, 55–62. [Google Scholar] [CrossRef]
- Fan, C.; Wang, G.S.; Chen, Y.C.; Ko, C.H. Risk assessment of exposure to volatile organic compounds in groundwater in Taiwan. Sci Total Environ. 2009, 407, 2165–2174. [Google Scholar] [CrossRef]
- Rybak, J.; Wróbel, M.; Pieśniewska, A.; Rogula-Kozłowska, W.; Majewski, G. Possible health effects of road dust in winter: Studies in Poland. Appl. Sci. 2023, 13, 7444. [Google Scholar] [CrossRef]
- Wei, J.; Liu, S.; Chu, T.; Yuan, G.; Xie, M.; Huang, Y.; Sun, Q.; Ma, C.; Xue, Q. The distribution and health risk assessment of potential toxic elements in atmospheric deposition from ion-adsorption rare earth mining areas in the Ganzhou City of southeast China. Appl. Sci. 2024, 14, 3585. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Zafar, M.; Lettenberger, S.E.; Pawlik, M.E.; Kinel, D.; Frissen, M.; Schneider, R.B.; Kieburtz, K.; Tanner, C.M.; De Miranda, B.R.; et al. Trichloroethylene: An invisible cause of Parkinson’s disease? J. Parkinson. Dis. 2023, 13, 203–218. [Google Scholar] [CrossRef]
- Francisco, L.F.V.; da Silva, R.N.; Oliveira, M.A.; dos Santos Neto, M.F.; Gonçalves, I.Z.; Marques, M.M.C.; Silveira, H.C.S. Occupational exposures and risks of non-Hodgkin lymphoma: A meta-analysis. Cancers 2023, 15, 2600. [Google Scholar] [CrossRef]
- Muntyanu, A.; Milan, R.; Rahme, E.; Baron, M.; Netchiporouk, E.; the Canadian Scleroderma Research Group. Organic solvent exposure and systemic sclerosis: A retrospective cohort study based on the Canadian Scleroderma Research Group registry. J. Am. Acad. Dermatol. 2024, 90, 605–607. [Google Scholar] [CrossRef]
- Westra, S.; Goldberg, M.S.; Labrèche, F.; Baumgartner, J.; Ho, V. The association between the incidence of postmenopausal breast cancer and occupational exposure to selected organic solvents, Montreal, Canada, 2008–2011. Am. J. Ind. Med. 2023, 66, 911–927. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Borgoni, R.; Ambrosini, R.; Cifoni, M.; Galassi, D.M.P.; Petitta, M. Occurrence of volatile organic compounds in shallow alluvial aquifers of a Mediterranean region: Baseline scenario and ecological implications. Sci. Total Environ. 2015, 538, 712–723. [Google Scholar] [CrossRef]
- Holton, C.; Luo, H.; Dahlen, P.; Gorder, K.; Dettenmaier, E.; Johnson, P.C. Temporal variability of indoor air concentrations under natural conditions in a house overlying a dilute chlorinated solvent groundwater plume. Environ. Sci. Technol. 2013, 47, 13347–13354. [Google Scholar] [CrossRef]
- Guo, Y.; Holton, C.; Luo, H.; Dahlen, P.; Johnson, P.C. Influence of fluctuating groundwater table on volatile organic chemical emission flux at a dissolved chlorinated-solvent plume site. Groundw. Monit. Remediat. 2019, 39, 43–52. [Google Scholar] [CrossRef]


| Receptor | Adults | Children | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure Route a | Exposure Route a | ||||||||||||||
| Contaminant | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |||
| Area B | HI = ΣHQ or IELCR = ΣIELCR | HI = ΣHQ or IELCR = ΣIELCR | |||||||||||||
| PCE | HQ | 1.2 × 10−2 | 9.9 × 10−6 | 1.9 × 10−4 | 2.1 × 10−3 | 2.4 × 10−5 | 5.0 × 10−4 | 1.5 × 10−2 | 4.2 × 10−2 | 3.5 × 10−5 | 6.0 × 10−4 | 2.3 × 10−3 | 5.7 × 10−5 | 5.0 × 10−4 | 4.5 × 10−2 |
| IELCR | 4.1 × 10−8 | 3.4 × 10−11 | 8.4 × 10−10 | 9.2 × 10−9 | 8.5 × 10−11 | 2.2 × 10−9 | 3.3 × 10−8 | 3.6 × 10−8 | 3.0 × 10−11 | 6.5 × 10−10 | 2.5 × 10−9 | 4.9 × 10−11 | 2.2 × 10−9 | 4.1 × 10−8 | |
| TCE | HQ | 3.6 × 10−2 | 3.9 × 10−5 | 5.8 × 10−3 | 3.0 × 10−1 | 1.3 × 10−3 | 1.6 × 10−2 | 3.6 × 10−1 | 1.3 × 10−1 | 1.3 × 10−4 | 1.8 × 10−2 | 3.3 × 10−1 | 3.0 × 10−3 | 7.2 × 10−2 | 5.5 × 10−1 |
| IELCR | 1.0 × 10−6 | 1.1 × 10−9 | 4.6 × 10−8 | 2.4 × 10−6 | 3.6 × 10−9 | 1.3 × 10−7 | 3.4 × 10−6 | 9.0 × 10−7 | 9.5 × 10−10 | 3.5 × 10−8 | 6.4 × 10−7 | 2.0 × 10−9 | 1.4 × 10−7 | 1.7 × 10−6 | |
| cis-DCE | HQ | NA | NA | 7.2 × 10−4 | 1.7 × 10−3 | NA | 4.8 × 10−4 | 2.9 × 10−3 | NA | NA | 2.3 × 10−3 | 1.8 × 10−3 | NA | 2.2 × 10−3 | 6.3 × 10−3 |
| IELCR | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| HItotal | 3.8 × 10−1 | 6.1 × 10−1 | |||||||||||||
| IELCRtotal | 3.4 × 10−6 | 1.7 × 10−6 | |||||||||||||
| Area C | |||||||||||||||
| PCE | HQ | 2.8 × 10−3 | 1.9 × 10−6 | 4.6 × 10−3 | 1.4 × 10−2 | 6.0 × 10−4 | 1.1 × 10−2 | 3.3 × 10−2 | 1.3 × 10−2 | 1.9 × 10−6 | 8.1 × 10−2 | 6.8 × 10−1 | 7.9 × 10−3 | 2.7 × 10−2 | 8.1 × 10−1 |
| IELCR | 9.7 × 10−9 | 6.7 × 10−12 | 2.0 × 10−8 | 6.2 × 10−8 | 2.1 × 10−9 | 4.6 × 10−8 | 1.4 × 10−7 | 1.1 × 10−8 | 5.6 × 10−11 | 8.7 × 10−8 | 7.3 × 10−7 | 6.8 × 10−9 | 2.9 × 10−8 | 8.7 × 10−7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, R.; Hiller, E.; Špirová, V.; Jurkovič, Ľ.; Ševčíková, Ľ.; Macek, J.; Čičáková, C.; Kovács, T.; Auxt, A. Application of Active Soil Gas Screening for the Identification of Groundwater Contamination with Chlorinated Hydrocarbons at an Industrial Area—A Case Study of the Former Refrigerator Manufacturer Calex (City of Zlaté Moravce, Western Slovakia). Appl. Sci. 2024, 14, 10842. https://doi.org/10.3390/app142310842
Tóth R, Hiller E, Špirová V, Jurkovič Ľ, Ševčíková Ľ, Macek J, Čičáková C, Kovács T, Auxt A. Application of Active Soil Gas Screening for the Identification of Groundwater Contamination with Chlorinated Hydrocarbons at an Industrial Area—A Case Study of the Former Refrigerator Manufacturer Calex (City of Zlaté Moravce, Western Slovakia). Applied Sciences. 2024; 14(23):10842. https://doi.org/10.3390/app142310842
Chicago/Turabian StyleTóth, Roman, Edgar Hiller, Veronika Špirová, Ľubomír Jurkovič, Ľubica Ševčíková, Juraj Macek, Claudia Čičáková, Tibor Kovács, and Anton Auxt. 2024. "Application of Active Soil Gas Screening for the Identification of Groundwater Contamination with Chlorinated Hydrocarbons at an Industrial Area—A Case Study of the Former Refrigerator Manufacturer Calex (City of Zlaté Moravce, Western Slovakia)" Applied Sciences 14, no. 23: 10842. https://doi.org/10.3390/app142310842
APA StyleTóth, R., Hiller, E., Špirová, V., Jurkovič, Ľ., Ševčíková, Ľ., Macek, J., Čičáková, C., Kovács, T., & Auxt, A. (2024). Application of Active Soil Gas Screening for the Identification of Groundwater Contamination with Chlorinated Hydrocarbons at an Industrial Area—A Case Study of the Former Refrigerator Manufacturer Calex (City of Zlaté Moravce, Western Slovakia). Applied Sciences, 14(23), 10842. https://doi.org/10.3390/app142310842









