Optimization of Vacuum Impregnation with Aqueous Extract from Hibiscus sabdariffa Calyces in Apple Slices by Response Surface Methodology: Effect on Soluble Phenols, Flavonoids, Antioxidant Activity, and Physicochemical Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemical Reagents
2.2. Part A
2.2.1. Experimental Design
2.2.2. Vacuum Impregnation Process
2.2.3. Extraction and Quantification of Total Soluble Phenols
2.2.4. Response Surface Methodology
2.2.5. Quantification of Total Flavonoids
2.2.6. Antioxidant Activity
2.2.7. Physicochemical Properties
2.3. Part B
2.3.1. Experimental Validation of Vacuum Impregnation Conditions Obtained by RSM
2.3.2. Impregnation-Dehydration Treatment
2.3.3. Characterization of Dehydrated Apple Slices
2.4. Statistical Analysis
3. Results and Discussions
3.1. Vacuum Impregnation of Soluble Phenols from Hibiscus sabdariffa Aqueous Extract into Apple Slices
3.2. Influence of Vacuum Impregnation Parameters on Soluble Phenols into Apple Slices
3.3. Optimization of VI of H. sabdariffa Calyx Extract in Apple Slices
3.4. Effect of Vacuum Impregnation on Total Flavonoids and Antioxidant Activity
3.5. Effect of Vacuum Impregnation on Physicochemical Parameters
3.6. Vacuum Impregnation Validation and Dehydration of Apple Slices Vacuum Impregnated
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirmiran, P.; Bahadoran, Z.; Delshad, H.; Azizi, F. Effects of Energy-Dense Nutrient-Poor Snacks on the Incidence of Metabolic Syndrome: A Prospective Approach in Tehran Lipid and Glucose Study. Nutrition 2014, 30, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Disegna, M.; Lloyd, T. National Food Consumption Patterns: Converging Trends and the Implications for Health. EuroChoices 2023, 22, 66–73. [Google Scholar] [CrossRef]
- Food and Agriculture Organizations of the United Nations. Fruit and Vegetables—Your Dietary Essentials; The International Year of Fruits and Vegetables; FAO: Rome, Italy, 2021; Available online: https://pubs.acs.org/doi/epdf/10.1021/acsenvironau.2c00050?ref=article_openPDF (accessed on 15 October 2024).
- Aguirre-García, M.; Cortés-Zavaleta, O.; Hernández-Carranza, P.; Ruiz-Espinosa, H.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Modeling the Impregnation of Roselle Antioxidants into Papaya Cubes. J. Food Eng. 2023, 357, 111585. [Google Scholar] [CrossRef]
- Ignazak, A.; Masiarz, E.; Kowalska, H. Nutritional Trends and Methods of Producing Fruit and Vegetable Health-Promoting Snacks. Technol. Progress. Food Process. 2021, 1, 143–155. [Google Scholar]
- Saleena, P.; Jayashree, E.; Anees, K.A. Comprehensive Review on Vacuum Impregnation: Mechanism, Applications and Prospects. Food Bioprocess. Technol. 2024, 17, 1434–1447. [Google Scholar] [CrossRef]
- Saleena, P.; Jayashree, E.; Neethu, K.C.; Bhuvaneswari, S.; Alfiya, P.V.; Anees, K. Optimization of Vacuum Impregnated Nutmeg Rind Candy Using RSM Modeling: Effect on Functional and Nutritional Properties. J. Food Sci. Technol. 2024, 61, 2121–2132. [Google Scholar] [CrossRef]
- Akman, P.K.; Uysal, E.; Ozkaya, G.U.; Tornuk, F.; Durak, M.Z. Development of Probiotic Carrier Dried Apples for Consumption as Snack Food with the Impregnation of Lactobacillus Paracasei. LWT Food Sci. Technol. 2019, 103, 60–68. [Google Scholar] [CrossRef]
- Assis, F.R.; Rodrigues, L.G.G.; Tribuzi, G.; de Souza, P.G.; Carciofi, B.A.M.; Laurindo, J.B. Fortified Apple (Malus spp., Var. Fuji) Snacks by Vacuum Impregnation of Calcium Lactate and Convective Drying. LWT Food Sci. Technol. 2019, 113, 108298. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Díaz-Osorio, A.; Osorio-Arias, J.; Sobral, P.J.A.; Vega-Castro, O. Development of Fortified Low-Fat Potato Chips through Vacuum Impregnation and Microwave Vacuum Drying. Innov. Food Sci. Emerg. Technol. 2020, 64, 102437. [Google Scholar] [CrossRef]
- Montalvo-González, E.; Villagrán, Z.; González-Torres, S.; Iñiguez-Muñoz, L.E.; Isiordia-Espinoza, M.A.; Ruvalcaba-Gómez, J.M.; Arteaga-Garibay, R.I.; Acosta, J.L.; González-Silva, N.; Anaya-Esparza, L.M. Physiological Effects and Human Health Benefits of Hibiscus sabdariffa: A Review of Clinical Trials. Pharmaceuticals 2022, 15, 464. [Google Scholar] [CrossRef]
- Dinçer, C. Modeling of Hibiscus Anthocyanins Transport to Apple Tissue during Ultrasound-Assisted Vacuum Impregnation. J. Food Process Preserv. 2022, 46, e15886. [Google Scholar] [CrossRef]
- Tylewicz, U.; Mannozzi, C.; Romani, S.; Castagnini, J.M.; Samborska, K.; Rocculi, P.; Dalla Rosa, M. Chemical and Physicochemical Properties of Semi-Dried Organic Strawberries Enriched with Bilberry Juice-Based Solution. LWT Food Sci. Technol. 2019, 114, 108377. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Pasławska, M.; Stępień, B.; Oziembłowski, M.; Sala, K.; Smorowska, A. Effect of Vacuum Impregnation with Apple-Pear Juice on Content of Bioactive Compounds and Antioxidant Activity of Dried Chokeberry Fruit. Foods 2020, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.V.; Moreira, R.G. Increased Phenolic Compounds in Potato Chips Vacuum Impregnated with Green Tea. J. Food Sci. 2019, 84, 807–817. [Google Scholar] [CrossRef]
- Guz, T.; Rydzak, L.; Domin, M. Influence of Selected Parameters and Different Methods of Implementing Vacuum Impregnation of Apple Tissue on Its Effectiveness. Processes 2020, 8, 428. [Google Scholar] [CrossRef]
- Mierzwa, D.; Szadzińska, J.; Gapiński, B.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R. Assessment of Ultrasound-Assisted Vacuum Impregnation as a Method for Modifying Cranberries’ Quality. Ultrason. Sonochem. 2022, 89, 106117. [Google Scholar] [CrossRef]
- Le, D.; Konsue, N. Mass Transfer Behavior during Osmotic Dehydration and Vacuum Impregnation of “Phulae” Pineapple and the Effects on Dried Fruit Quality. Curr. Res. Nutr. Food Sci. 2021, 9, 308–319. [Google Scholar] [CrossRef]
- González-Pérez, J.E.; Jiménez-González, O.; Ramírez-Corona, N.; Guerrero-Beltrán, J.A.; López-Malo, A. Vacuum Impregnation on Apples with Grape Juice Concentrate: Effects of Pressure, Processing Time, and Juice Concentration. Innov. Food Sci. Emerg. Technol. 2022, 77, 102981. [Google Scholar] [CrossRef]
- Barat, J.M.; Chiralt, A.; Fito, P. Effect of Osmotic Solution Concentration, Temperature and Vacuum Impregnation Pretreatment on Osmotic Dehydration Kinetics of Apple Slices. Food Sci. Technol. Int. 2001, 7, 451–456. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Ersus Bilek, S. Natural Colorant Enrichment of Apple Tissue with Black Carrot Concentrate Using Vacuum Impregnation. Int. J. Food Sci. Technol. 2017, 52, 1508–1516. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression of Results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Montreau, F.R. Sur Le Dosage Des Composés Phénoliques Totaux Dans Les Vins Par La Méthode Folin-Ciocalteu. OENO One 1972, 1, 397–404. [Google Scholar] [CrossRef]
- Esmaeili, A.K.; Taha, R.M.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from in Vivo and in Vitro Grown Trifolium Pratense L. (Red clover). Biomed. Res. Int. 2015, 2015, 643285. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pasławska, M.; Stȩpień, B.; Nawirska-Olszańska, A.; Maślankowski, R.; Rydzak, L. Effect of Vacuum Impregnation on Drying Kinetics and Selected Quality Factors of Apple Cubes. Int. J. Food Eng. 2017, 13, 20160309. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemist Official Method of Analysis of Association of Official Analytical Chemists; AOAC: Arlington, VA, USA, 2005. [Google Scholar]
- Xiao, M.; Bi, J.; Yi, J.; Zhao, Y.; Peng, J.; Zhou, L.; Chen, Q. Osmotic Pretreatment for Instant Controlled Pressure Drop Dried Apple Chips: Impact of the Type of Saccharides and Treatment Conditions. Dry. Technol. Int. J. 2019, 37, 896–905. [Google Scholar] [CrossRef]
- González-Pérez, J.E.; Jiménez-González, O.; Ramírez-Corona, N.; López-Malo, A. Use of Response Surface Methodology to Optimise Vacuum Impregnation of B-Carotene from Daucus carota in Pachyrhizus erosus. Sustain. Food Technol. 2023, 1, 404–414. [Google Scholar] [CrossRef]
- Ramos-Morales, M.; Aguirre-García, M.; Cortés-Zavaleta, O.; Ruiz-Espinosa, H.; Estévez-Sánchez, K.H.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Improving the Bioactive Content in Honeydew Melon by Impregnation with Hibiscus Extract/Sucrose Solutions: A Coupled Mass Transfer Analysis. Food Biop Proces. 2024, 144, 1–12. [Google Scholar] [CrossRef]
- Panayampadan, A.S.; Alam, M.S.; Aslam, R.; Kaur, J. Vacuum Impregnation Process and Its Potential in Modifying Sensory, Physicochemical and Nutritive Characteristics of Food Products. Food Eng. Rev. 2022, 14, 229–256. [Google Scholar] [CrossRef]
- Blanda, G.; Cerretani, L.; Bendini, A.; Cardinali, A.; Scarpellini, A.; Lercker, G. Effect of Vacuum Impregnation on the Phenolic Content of Granny Smith and Stark Delicious Frozen Apple Cvv. Eur. Food Res. Technol. 2008, 226, 1229. [Google Scholar] [CrossRef]
- Zeng, J.; Dou, Y.; Yan, N.; Li, N.; Zhang, H.; Tan, J.N. Optimizing Ultrasound-Assisted Deep Eutectic Solvent Extraction of Bioactive Compounds from Chinese Wild Rice. Molecules 2019, 24, 2718. [Google Scholar] [CrossRef] [PubMed]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-Sustainable Recovery of Phenolic and Antioxidant Compounds from Industrial Chestnut Shells Using Ultrasound-Assisted Extraction: Optimization and Evaluation of Biological Activities In Vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Durán-Castañeda, A.C.; González-Moya, S.; Sánchez-Burgos, J.A.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. Applications of Vacuum Impregnation as a Technology to Incorporate Functional Components in Vegetal Matrices. Food Chem. Adv. 2024, 4, 100579. [Google Scholar] [CrossRef]
- González-Moya, S.; Durán-Castañeda, A.C.; Velázquez-Estrada, R.M.; Sánchez-Burgos, J.A.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. Vacuum Impregnation of Polyphenols in Yam Bean: Effect on Sensory Acceptability, Antioxidant Capacity, and Potential Absorption Ability. ACS Food Sci. Technol. 2023, 3, 1155–1164. [Google Scholar] [CrossRef]
- Moreira, R.G.; Almohaimeed, S. Technology for Processing of Potato Chips Impregnated with Red Rootbeet Phenolic Compounds. J. Food Eng. 2018, 228, 57–68. [Google Scholar] [CrossRef]
- Diamante, L.M.; Hironaka, K.; Yamaguchi, Y.; Nademude, E. Optimisation of Vacuum Impregnation of Blackcurrant-Infused Apple Cubes: Application of Response Surface Methodology. Int. J. Food Sci. Technol. 2014, 49, 689–695. [Google Scholar] [CrossRef]
- Schulze, B.; Hubbermann, E.M.; Schwarz, K. Stability of Quercetin Derivatives in Vacuum Impregnated Apple Slices after Drying (Microwave Vacuum Drying, Air Drying, Freeze Drying) and Storage. LWT Food Sci. Technol. 2014, 57, 426. [Google Scholar] [CrossRef]
- Betoret, E.; Sentandreu, E.; Betoret, N.; Codoñer-Franch, P.; Valls-Bellés, V.; Fito, P. Technological Development and Functional Properties of an Apple Snack Rich in Flavonoid from Mandarin Juice. Innov. Food Sci. Emerg. Technol. 2012, 16, 298–304. [Google Scholar] [CrossRef]
- Park, S.; Kodihalli, I.; Zhao, Y. Nutritional, Sensory, and Physicochemical Properties of Vitamin E-and Mineral-Fortified Fresh-Cut Apples by Use of Vacuum Impregnation. J. Food Sci. 2006, 12, 4745–4760. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, Y. Nutritional Enrichment of Fresh Apple (Royal Gala) by Vacuum Impregnation. Int. J. Food Sci. Nutr. 2003, 54, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Tappi, S.; Velickova, E.; Mannozzi, C.; Tylewicz, U.; Laghi, L.; Rocculi, P. Multi-Analytical Approach to Study Fresh-Cut Apples Vacuum Impregnated with Different Solutions. Foods 2022, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa, D.; Szadzińska, J.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Kidoń, M.; Gapiński, B. Effectiveness of Cranberry (Vaccinium macrocarpon, Cv. Pilgrim) Vacuum Impregnation: The Effect of Sample Pretreatment, Pressure, and Processing Time. Food Bioprod. Proces. 2022, 134, 223–234. [Google Scholar] [CrossRef]
- Joshi, A.; Rupasinghe, H.; Pitts, N. Sensory and Nutritional Quality of the Apple Snacks Prepared by Vacuum Impregnation Process. J. Food Qual. 2010, 33, 758. [Google Scholar] [CrossRef]



| Run | Predictors 1 | Response Variables | Relative Error (%) | Impregnation Degree (%) | |||
|---|---|---|---|---|---|---|---|
| XVP (bar) | XIT (min) | XRT (min) | Experimental TSP 2 | Predicted TSP 3 | |||
| Untreated control | 34.99 ± 0.01 j | ||||||
| T1 | −0.2 | 10 | 2 | 100.34 ± 3.13 def | 103.86 | −3.38 | 11.72 |
| T2 | −0.3 | 10 | 1 | 113.44 ± 1.96 c | 107.59 | −5.43 | 12.19 |
| T3 | −0.3 | 10 | 3 | 135.21 ± 5.13 b | 134.04 | −8.27 | 13.38 |
| T4 | −0.3 | 6 | 2 | 105.73 ± 0.99 d | 104.59 | −1.08 | 10.53 |
| T5 | −0.2 | 2 | 3 | 63.63 ± 1.34 i | 60.11 | −5.80 | 9.31 |
| T6 | −0.4 | 2 | 2 | 68.66 ± 1.00 hi | 65.14 | −5.40 | 10.29 |
| T7 | −0.3 | 6 | 2 | 104.82 ± 0.59 d | 104.59 | −0.21 | 10.61 |
| T8 | −0.2 | 6 | 3 | 97.05 ± 4.25 ef | 95.63 | −1.48 | 11.33 |
| T9 | −0.3 | 2 | 3 | 95.06 ± 2.33 f | 99.16 | 4.13 | 10.74 |
| T10 | −0.4 | 6 | 3 | 142.73 ± 1.81 a | 141.21 | −1.07 | 16.17 |
| T11 | −0.4 | 6 | 1 | 113.67 ± 1.79 c | 115.19 | 1.31 | 11.40 |
| T12 | −0.4 | 10 | 2 | 86.51 ± 1.22 g | 90.03 | 3.90 | 10.37 |
| T13 | −0.3 | 2 | 1 | 70.64 ± 1.59 h | 73.58 | 3.99 | 9.53 |
| T14 | −0.3 | 6 | 2 | 103.21 ± 0.79 de | 104.59 | 1.31 | 10.75 |
| T15 | −0.2 | 6 | 1 | 68.19 ± 1.01 hi | 69.61 | 2.03 | 10.07 |
| Source 1 | Analysis of Variance | Regression Coefficients Soluble Phenolic Content β-Coefficient | |||
|---|---|---|---|---|---|
| SS 2 | DF 3 | MS 4 | F-Value | ||
| Mean/intercept | - | - | - | - | 48.12 * |
| XIT | 7291.45 | 1 | 7291.44 | 1409.37 * | −42.82 * |
| XIT2 | 1963.00 | 1 | 1963.00 | 379.42 * | 4.52 * |
| XRT | 4063.72 | 1 | 4063.72 | 785.42 * | −21.61 * |
| XRT2 | 1679.09 | 1 | 1679.09 | 324.55 * | 6.43 * |
| XVP | 811.60 | 1 | 811.59 | 156.87 * | −425.84 * |
| XVP2 | 1462.70 | 1 | 1460.69 | 282.72 * | −1149.13 * |
| XIT * XRT | 5.25 | 1 | 5.20 | 1.015 ** | −0.56 ** |
| XIT * XRT2 | 302.15 | 1 | 302.15 | 58.40 * | 0.98 * |
| XIT2 * XRT | 51.77 | 1 | 51.765 | 10.00 * | −0.33 * |
| XIT * XVP | 266.94 | 1 | 266.94 | 51.59 * | −175.64 * |
| XIT2 * XVP | 3747.13 | 1 | 3747.13 | 724.28 * | 15.62 * |
| XRT * XVP | 0.03 | 1 | 0.031 | 0.06 ** | −0.51 ** |
| Lack of fit | 57.05 | 3 | 19.01 | 3.676 ** | |
| Pure error | 165.55 | 32 | 5.174 | ||
| R-square | 0.9905 | ||||
| R-Adjust | 0.9881 | ||||
| Total SS | 23,643.64 | ||||
| Parameter | Optimal Experimental Conditions | Prediction of Soluble Polyphenols (mg GAE/100 g Fresh Weight) |
|---|---|---|
| Vacuum pressure (bar) | −0.4 | |
| Impregnation time (min) | 6.73 | |
| Restoration time (min) | 3 | |
| Optimal response | 142.39 | |
| −95% Confidence limit | 139.2 | |
| +95% Confidence limit | 149.52 |
| Treatment | Flavonoids (mg CE/100 g Fresh Weight) | ABTS (mmol TE/100 g Fresh Weight) | DPPH (mmol TE/100 g Fresh Weight) |
|---|---|---|---|
| Untreated control | 25.36 ± 0.43 | 67.98 ± 0.64 f | 82.68 ± 1.96 f |
| T1 | 64.81 ± 2.11 cde | 99.74 ± 0.67 cd | 166.74 ± 0.94 cd |
| T2 | 53.38 ± 4.29 h | 135.36 ± 0.52 ab | 170.11 ± 2.74 abcd |
| T3 | 61.34 ± 2.55 def | 142.19 ± 0.38 ab | 175.21 ± 4.13 a |
| T4 | 53.68 ± 2.26 h | 106.32 ± 0.34 c | 134.20 ± 1.04 e |
| T5 | 54.30 ± 1.26 h | 58.17 ± 0.35 e | 132.87 ± 1.77 e |
| T6 | 60.44 ± 0.57 efg | 57.74 ± 0.50 e | 171.65 ± 1.11 abc |
| T7 | 55.23 ± 0.98 gh | 92.96 ± 0.17 d | 169.93 ± 2.25 abcd |
| T8 | 67.01 ± 0.93 cd | 101.20 ± 0.88 cd | 168.07 ± 0.41 bcd |
| T9 | 68.31 ± 0.96 bc | 99.67 ± 0.59 cd | 164.65 ± 1.48 d |
| T10 | 78.05 ± 1.16 a | 147.30 ± 1.29 a | 172.84 ± 2.07 ab |
| T11 | 69.69 ± 0.22 bc | 131.04 ± 1.85 b | 167.34 ± 1.16 bcd |
| T12 | 73.88 ± 2.51 ab | 111.57 ± 1.05 c | 165.86 ± 2.08 cd |
| T13 | 55.96 ± 2.66 fgh | 102.33 ± 0.92 cd | 169.95 ± 2.27 abcd |
| T14 | 51.93 ± 0.57 h | 131.30 ± 0.19 c | 167.82 ± 0.34 bcd |
| T15 | 52.54 ± 0.78 h | 101.24 ± 0.41 cd | 134.99 ± 1.20 e |
| Treatment | pH | TA (% Citric Acid) | TSS (°Brix) | Water Activity (aw) | Moisture (%) |
|---|---|---|---|---|---|
| Untreated control | 4.01 ± 0.01 a | 0.18 ± 0.01 c | 15.03 ± 0.35 a | 0.94 ± 0.01 bcde | 86.08 ± 0.39 ab |
| T1 | 3.29 ± 0.21 d | 0.39 ± 0.04 ab | 9.97 ± 0.61 bcd | 0.94 ± 0.01 bcde | 87.81 ± 0.34 a |
| T2 | 3.31 ± 0.02 d | 0.37 ± 0.03 ab | 8.83 ± 1.65 e | 0.93 ± 0.01 de | 85.58 ± 0.60 ab |
| T3 | 3.38 ± 0.15 cd | 0.43 ± 0.01 ab | 8.20 ± 0.10 de | 0.93 ± 0.01 de | 84.36 ± 0.88 ab |
| T4 | 3.32 ± 0.01 d | 0.39 ± 0.01 ab | 8.23 ± 0.23 de | 0.94 ± 0.01 cde | 85.53 ± 0.04 ab |
| T5 | 3.54 ± 0.02 bcd | 0.45 ± 0.01 a | 9.93 ± 0.35 bbcde | 0.92 ± 0.01 e | 87.20 ± 0.34 a |
| T6 | 3.46 ± 0.09 bcd | 0.45 ± 0.01 a | 9.20 ± 0.62 cde | 0.98 ± 0.01 a | 88.01 ± 0.15 a |
| T7 | 3.55 ± 0.04 bcd | 0.40 ± 0.01 ab | 9.97 ± 1.11 bcd | 0.97 ± 0.01 abc | 87.80 ± 0.01 a |
| T8 | 3.53 ± 0.07 bcd | 0.42 ± 0.01 ab | 9.90 ± 0.10 bcde | 0.97 ± 0.01 abc | 87.51 ± 0.03 a |
| T9 | 3.52 ± 0.04 bcd | 0.37 ± 0.02 ab | 9.33 ± 0.30 bcde | 0.96 ± 0.02 abcd | 86.13 ± 1.33 a |
| T10 | 3.64 ± 0.06 bc | 0.43 ± 0.02 ab | 9.53 ± 0.25 bcde | 0.96 ± 0.01 abc | 87.09 ± 0.35 a |
| T11 | 3.62 ± 0.12 bc | 0.41 ± 0.05 ab | 9.83 ± 0.70 bcde | 0.97 ± 0.02 abc | 85.88 ± 0.40 ab |
| T12 | 3.49 ± 0.09 bcd | 0.38 ± 0.02 ab | 9.17 ± 0.49 cde | 0.97 ± 0.01 abc | 86.52 ± 8.44 b |
| T13 | 3.65 ± 0.09 bc | 0.40 ± 0.01 ab | 9.70 ± 0.45 bcde | 0.96 ± 0.01 abcd | 86.41 ± 0.20 a |
| T14 | 3.55 ± 0.03 bcd | 0.40 ± 0.01 ab | 9.50 ± 0.17 bc | 0.97 ± 0.01 ab | 86.86 ± 0.55 a |
| T15 | 3.67 ± 0.07 b | 0.36 ± 0.02 ab | 9.07 ± 0.05 b | 0.86 ± 0.01 abc | 87.30 ± 0.13 a |
| Treatment | L* | a* | b* | TCD | Color |
|---|---|---|---|---|---|
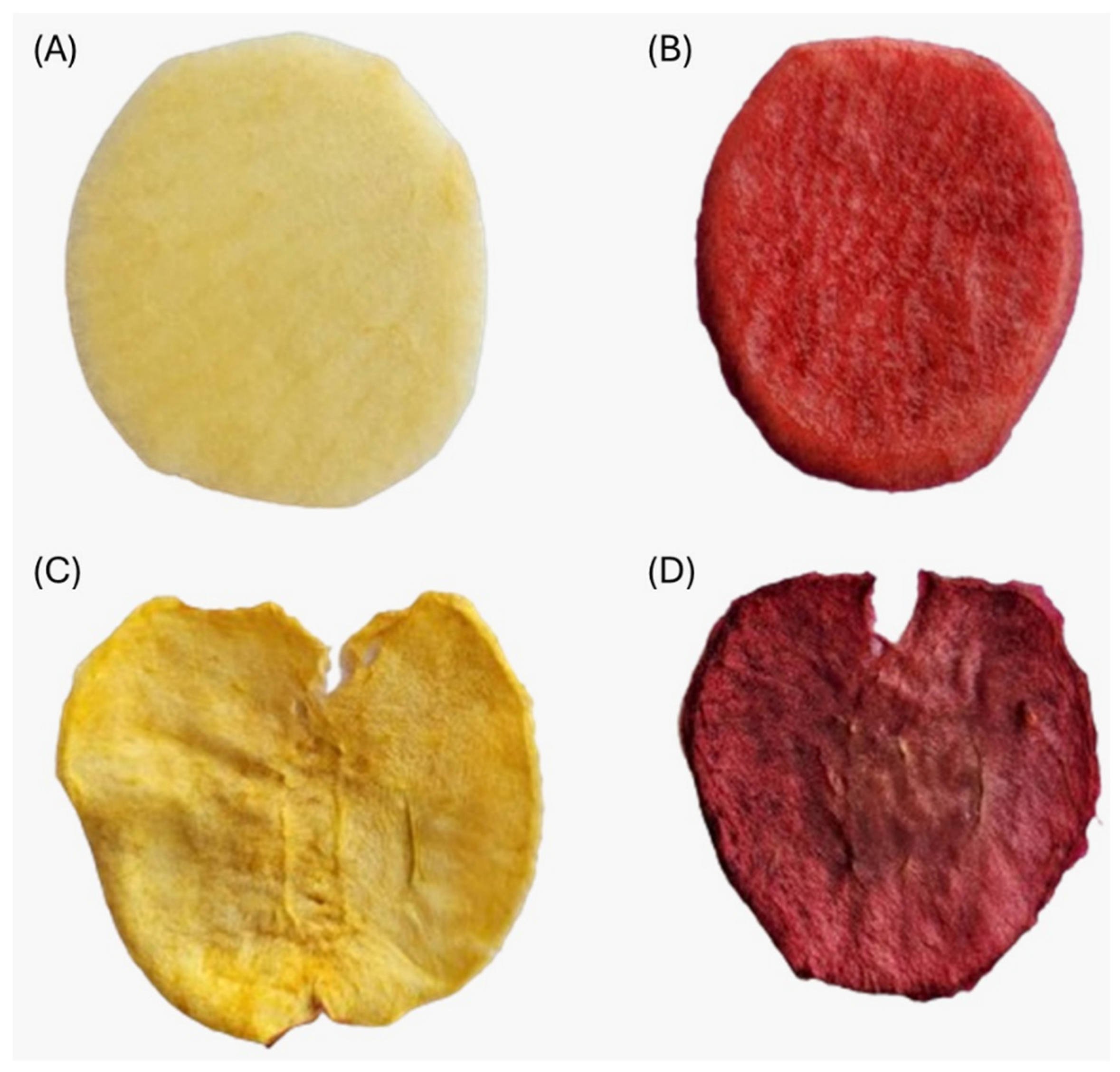
| Untreated control | 72.22 ± 0.56 a | −3.41 ± 0.18 e | 21.30 ± 1.08 bc | - |  |
| T1 | 48.25 ± 0.23 b | 15.48 ± 0.38 cd | 20.08 ± 0.78 cd | 30.59 |  |
| T2 | 43.00 ± 0.67 e | 15.55 ± 0.57 cd | 23.24 ± 0.24 b | 34.84 |  |
| T3 | 39.54 ± 0.47 fg | 14.17 ± 0.59 cd | 28.38 ± 0.49 a | 38.44 |  |
| T4 | 46.29 ± 0.28 jk | 16.27 ± 0.58 d | 17.82 ± 0.66 ef | 41.35 |  |
| T5 | 40.34 ± 0.64 c | 15.46 ± 0.89 bc | 17.57 ± 0.28 efg | 32.76 |  |
| T6 | 44.53 ± 0.78 f | 15.58 ± 0.42 cd | 11.80 ± 0.72 i | 38.23 |  |
| T7 | 40.60 ± 0.52 de | 17.28 ± 0.22 cd | 11.56 ± 0.36 i | 34.96 |  |
| T8 | 44.63 ± 0.11 f | 16.88 ± 0.19 ab | 19.16 ± 0.80 de | 37.84 |  |
| T9 | 34.44 ± 0.46 d | 15.24 ± 0.23 a | 15.60 ± 0.54 gh | 35.51 |  |
| T10 | 36.86 ± 0.8 k | 16.55 ± 0.45 cd | 17.12 ± 0.61 fg | 42.34 |  |
| T11 | 36.16 ± 0.11 hi | 15.36 ± 0.51 bc | 16.98 ± 0.61 fg | 40.83 |  |
| T12 | 37.99 ± 0.52 ij | 15.35 ± 0.28 cd | 16.72 ± 0.68 fg | 40.91 |  |
| T13 | 40.38 ± 0.52 gh | 15.58 ± 0.58 cd | 12.22 ± 0.54 i | 38.76 |  |
| T14 | 49.36 ± 0.26 f | 15.41 ± 0.46 cd | 15.98 ± 0.78 fgh | 37.46 |  |
| T15 | 48.24 ± 0.26 b | 15.53 ± 0.34 cd | 14.26 ± 1.08 h | 30.44 |  |
| Treatments | ||||
|---|---|---|---|---|
| Parameter | Fresh Sample (Fresh Weight) | Dehydrated Sample (Dry Weight) | ||
| Untreated Sample | * Vacuum Impregnated Sample | Untreated Sample | * Vacuum Impregnated Sample | |
| pH | 3.94 ± 0.03 a | 3.26 ± 0.02 b | 3.80 ± 0.01 a | 3.38 ± 0.01 b |
| Titratable acidity (% citric acid) | 0.18 ± 0.01 b | 0.41 ± 0.01 a | 0.026 ± 0.02 b | 0.032 ± 0.001 a |
| Total soluble solids (°Brix) | 14.13 ± 0.05 a | 10.16 ± 0.40 b | 18.48 ± 0.17 a | 20.05 ± 0.30 b |
| Water activity | 0.94 ± 0.01 b | 0.98 ± 0.01 b | 0.36 ± 0.01 a | 0.37 ± 0.01 a |
| Water content (%) | 87.90 ± 0.22 a | 88.23 ± 0.12 a | 6.83 ± 1.90 a | 6.97 ± 0.35 a |
| L* | 66.98 ± 1.29 a | 31.38 ± 0.79 b | 74.61 ± 1.33 a | 39.69 ± 1.08 b |
| a* | −2.13 ± 0.12 b | 18.66 ± 1.22 a | 5.01 ± 0.86 b | 21.47 ± 0.62 a |
| b* | 19.71 ± 1.98 a | 19.11 ± 0.28 a | 30.79 ± 0.57 a | 7.73 ± 1.52 b |
| Total soluble phenols (mg GAE/100 g) | 43.27 ± 4.10 b | 143.17 ± 2.72 a | 593.94 ± 73.94 b | 855.85 ± 80.63 a |
| Total flavonoids (mg CE/100 g) | 37.61 ± 4.63 b | 56.04 ± 1.52 a | 331.84 ± 4.63 b | 525.29 ± 28.05 a |
| DPPH (mmol TE/100 g) | 122.51 ± 1.68 b | 230.07 ± 3.14 a | 433.44 ± 7.70 b | 560.49 ± 11.34 a |
| ABTS (mmol TE/100 g) | 104.29 ± 0.77 b | 147.99 ± 0.70 a | 125.16 ± 1.54 b | 150.51 ± 1.93 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Esparza, L.M.; Rodríguez-Lafitte, E.; Villagrán, Z.; Aurora-Vigo, E.F.; Ruvalcaba-Gómez, J.M.; Símpalo-López, W.B.; Martínez-Esquivias, F.; Sarango-Córdova, C.H. Optimization of Vacuum Impregnation with Aqueous Extract from Hibiscus sabdariffa Calyces in Apple Slices by Response Surface Methodology: Effect on Soluble Phenols, Flavonoids, Antioxidant Activity, and Physicochemical Parameters. Appl. Sci. 2024, 14, 10850. https://doi.org/10.3390/app142310850
Anaya-Esparza LM, Rodríguez-Lafitte E, Villagrán Z, Aurora-Vigo EF, Ruvalcaba-Gómez JM, Símpalo-López WB, Martínez-Esquivias F, Sarango-Córdova CH. Optimization of Vacuum Impregnation with Aqueous Extract from Hibiscus sabdariffa Calyces in Apple Slices by Response Surface Methodology: Effect on Soluble Phenols, Flavonoids, Antioxidant Activity, and Physicochemical Parameters. Applied Sciences. 2024; 14(23):10850. https://doi.org/10.3390/app142310850
Chicago/Turabian StyleAnaya-Esparza, Luis Miguel, Ernesto Rodríguez-Lafitte, Zuamí Villagrán, Edward F. Aurora-Vigo, José Martín Ruvalcaba-Gómez, Walter Bernardo Símpalo-López, Fernando Martínez-Esquivias, and Cristhian Henry Sarango-Córdova. 2024. "Optimization of Vacuum Impregnation with Aqueous Extract from Hibiscus sabdariffa Calyces in Apple Slices by Response Surface Methodology: Effect on Soluble Phenols, Flavonoids, Antioxidant Activity, and Physicochemical Parameters" Applied Sciences 14, no. 23: 10850. https://doi.org/10.3390/app142310850
APA StyleAnaya-Esparza, L. M., Rodríguez-Lafitte, E., Villagrán, Z., Aurora-Vigo, E. F., Ruvalcaba-Gómez, J. M., Símpalo-López, W. B., Martínez-Esquivias, F., & Sarango-Córdova, C. H. (2024). Optimization of Vacuum Impregnation with Aqueous Extract from Hibiscus sabdariffa Calyces in Apple Slices by Response Surface Methodology: Effect on Soluble Phenols, Flavonoids, Antioxidant Activity, and Physicochemical Parameters. Applied Sciences, 14(23), 10850. https://doi.org/10.3390/app142310850







