An Oxygenated Layer Coating to Enhance the Healing Process of Non-Healing Wounds—A Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polyelectrolyte Layer Coating
2.2.2. PFC Labeling
2.2.3. Bandage Modification
2.2.4. AFM Evaluation
2.2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
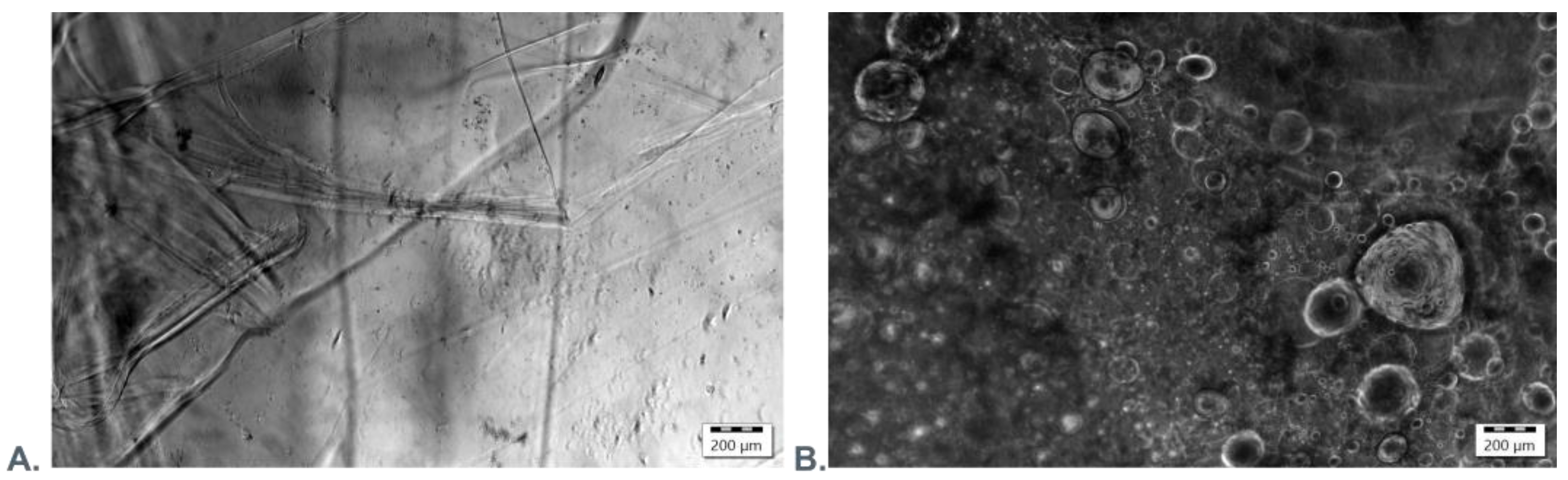
2.2.6. Scanning Electron Microscopy and Fluorescence Microscopy Evaluation
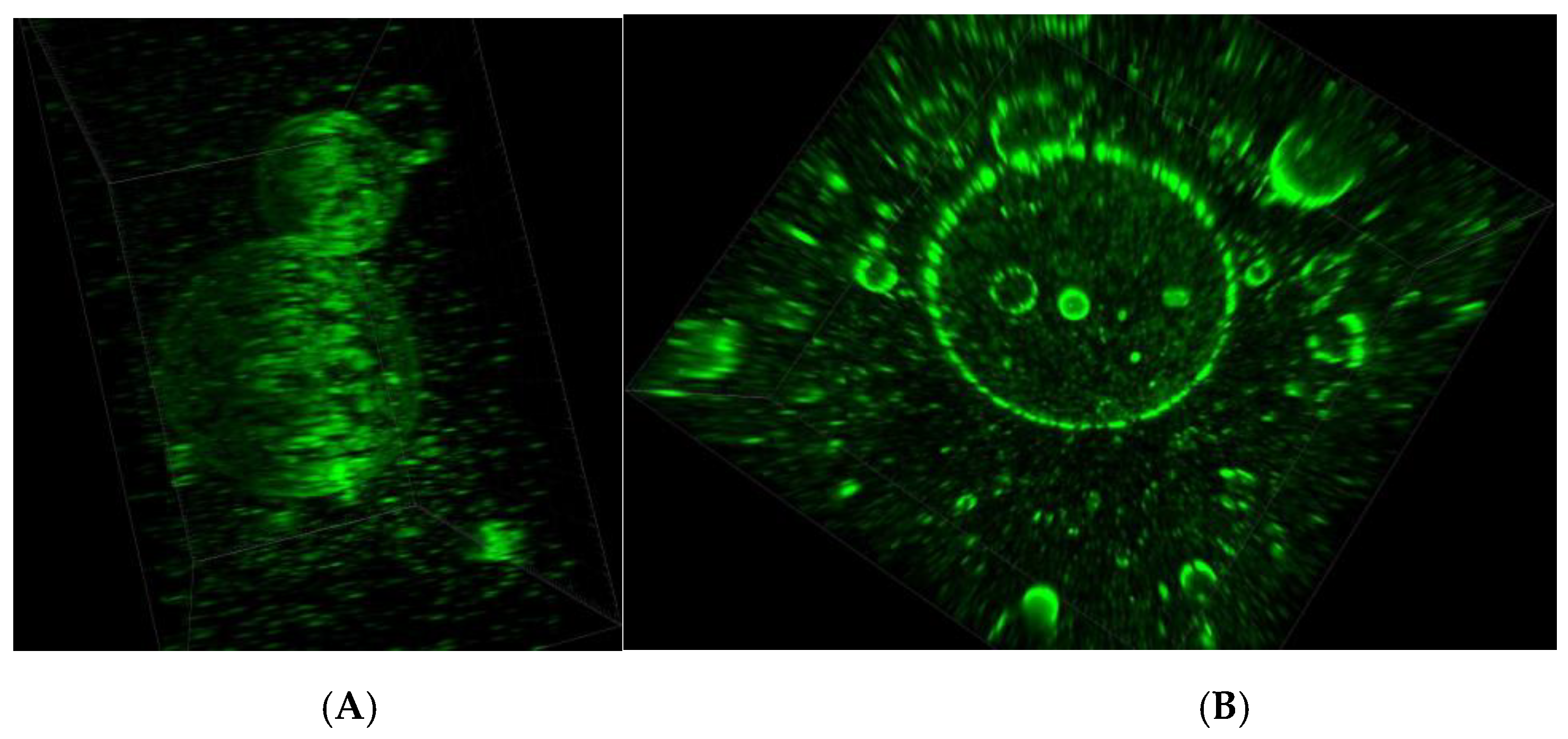
2.2.7. Confocal Microscopy
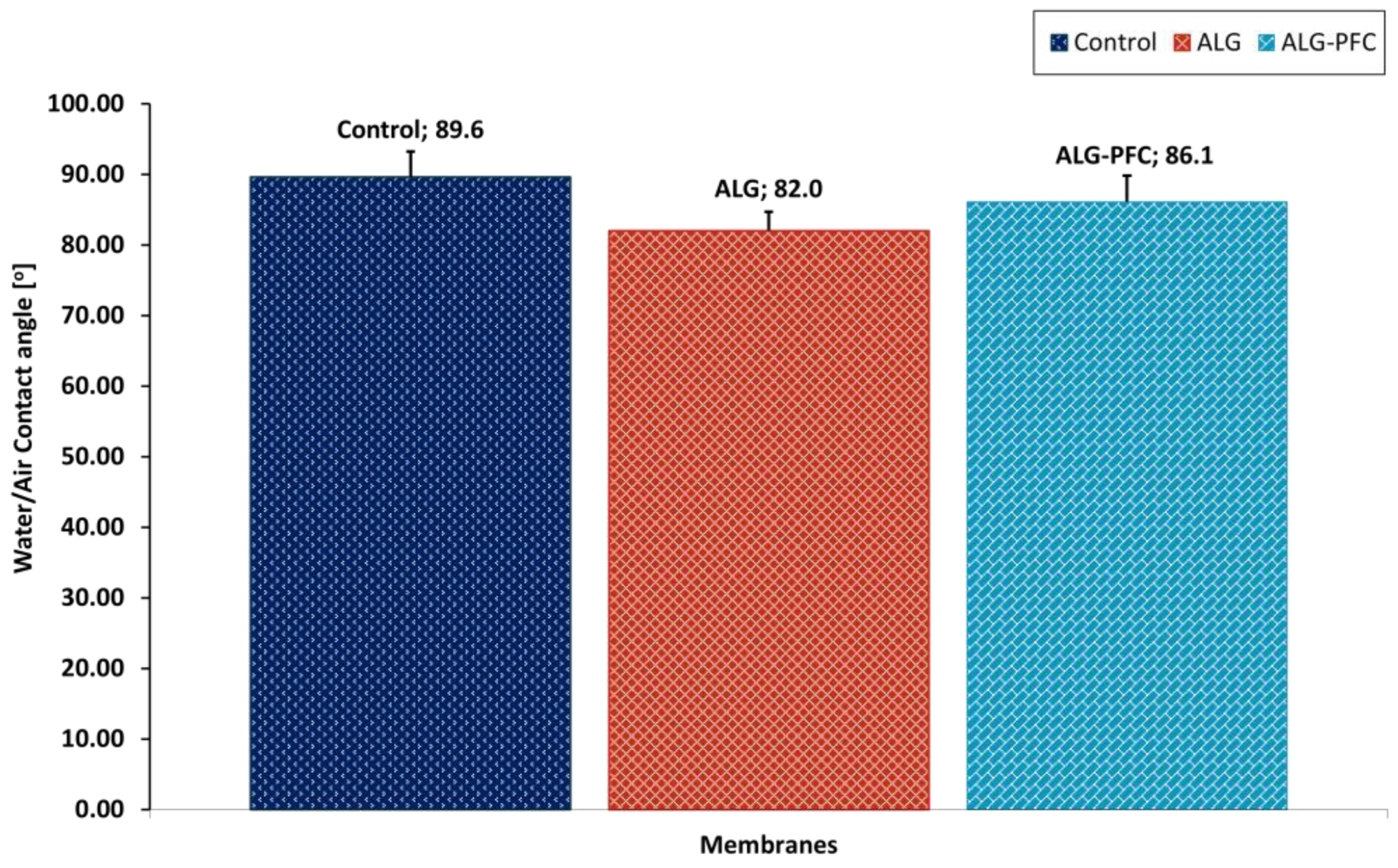
2.2.8. Evaluation of the Wettability Angle of Polyelectrolyte Shells
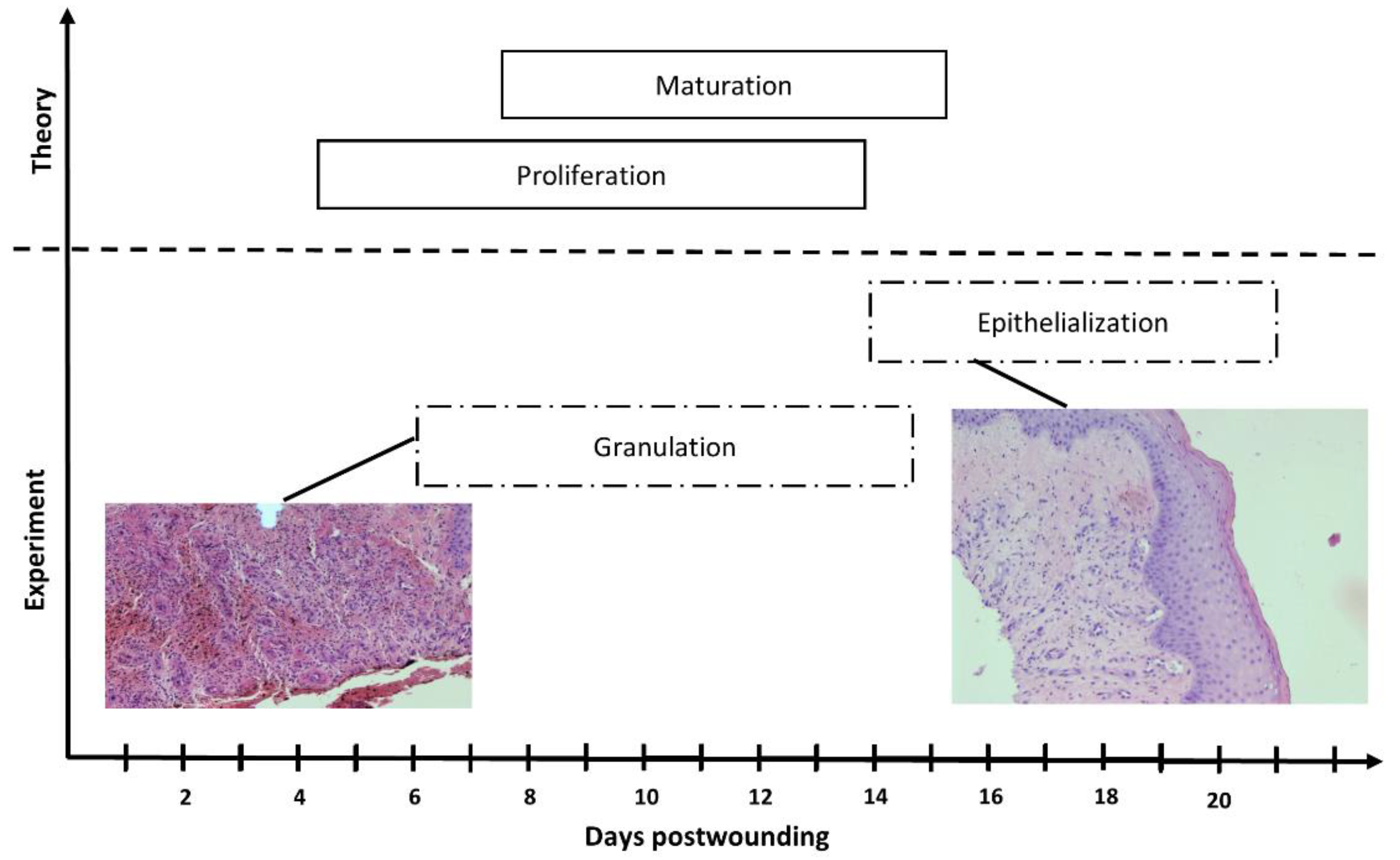
2.2.9. Histopathology
- (1)
- The skin sample of a square shape of about 1 cm2 was immersed in 10% formalin.
- (2)
- The samples were embedded in paraffin.
- (3)
- The material was cut into pieces with a thickness of 4 μm.
- (4)
- Paraffin sections were stained by the routine hematoxylin–eosin method.
2.2.10. Patients and Methods
- Inclusion criteria
- Exclusion criteria
- Patients with leg ulcers during atherosclerosis of the lower limbs and chronic venous insufficiency before treatment of the underlying disease.
- Patients with chronic end-stage heart failure NYHA4 addicted to alcohol and psychoactive substances.
- Patients with severe mental disorders were not promising to comply with medical recommendations.
- Patients’ characteristics
- Preliminary examination
- Procedure
- Ethics approval and consent to participate
2.2.11. Statistical Analysis
3. Results and Discussion
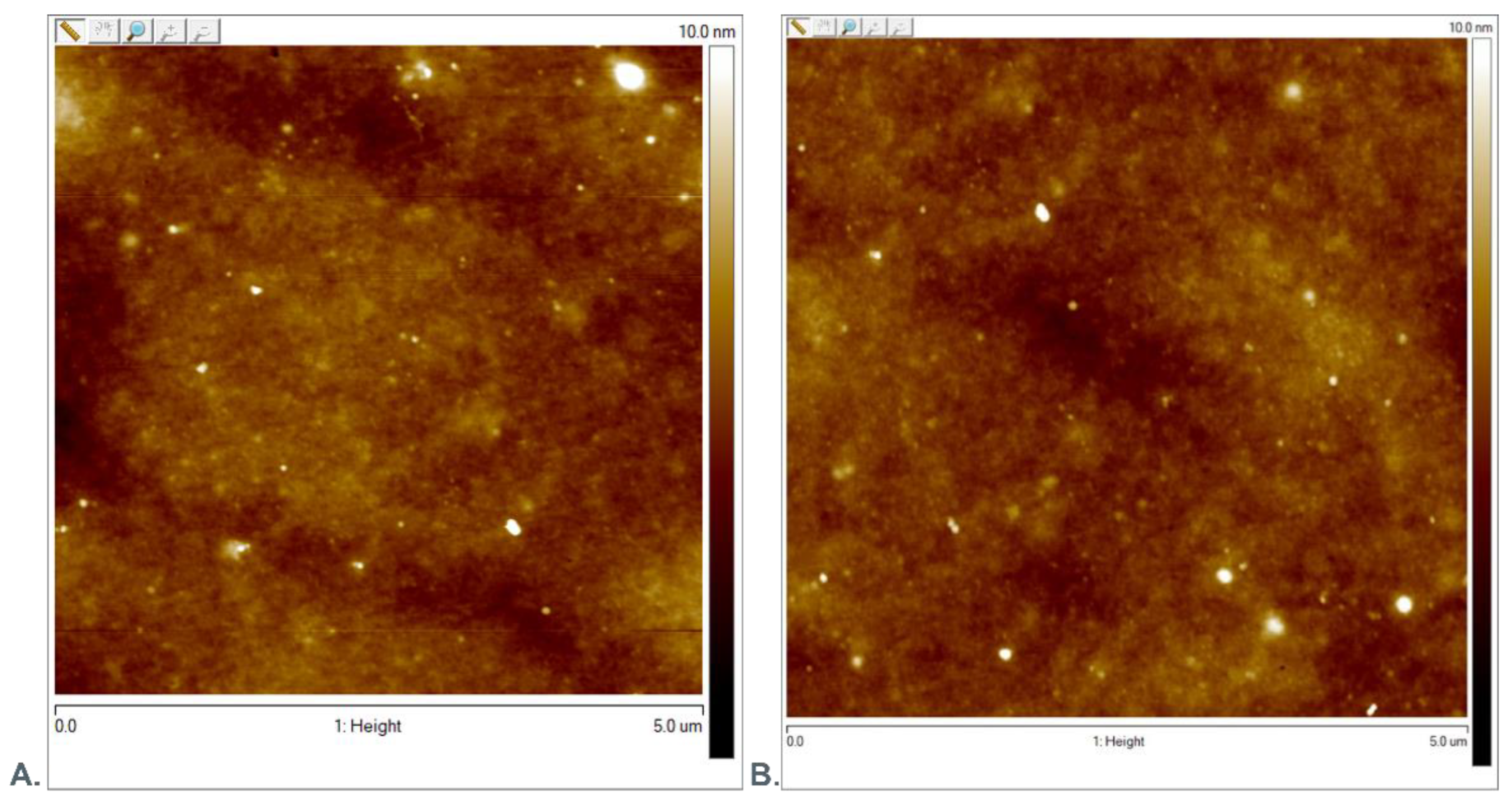
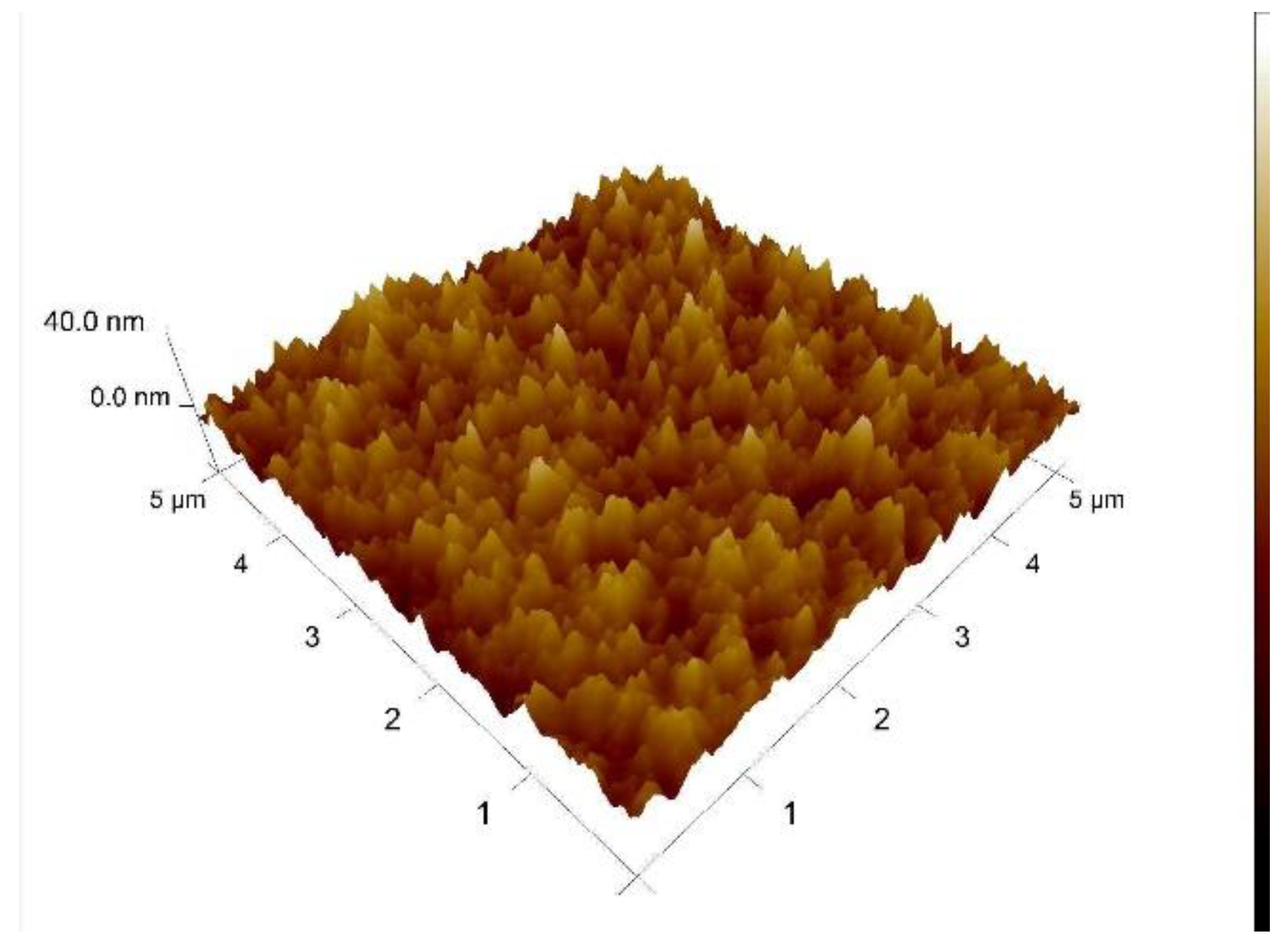
3.1. AFM Evaluation of Morphology of Oxygenated Coating Layer
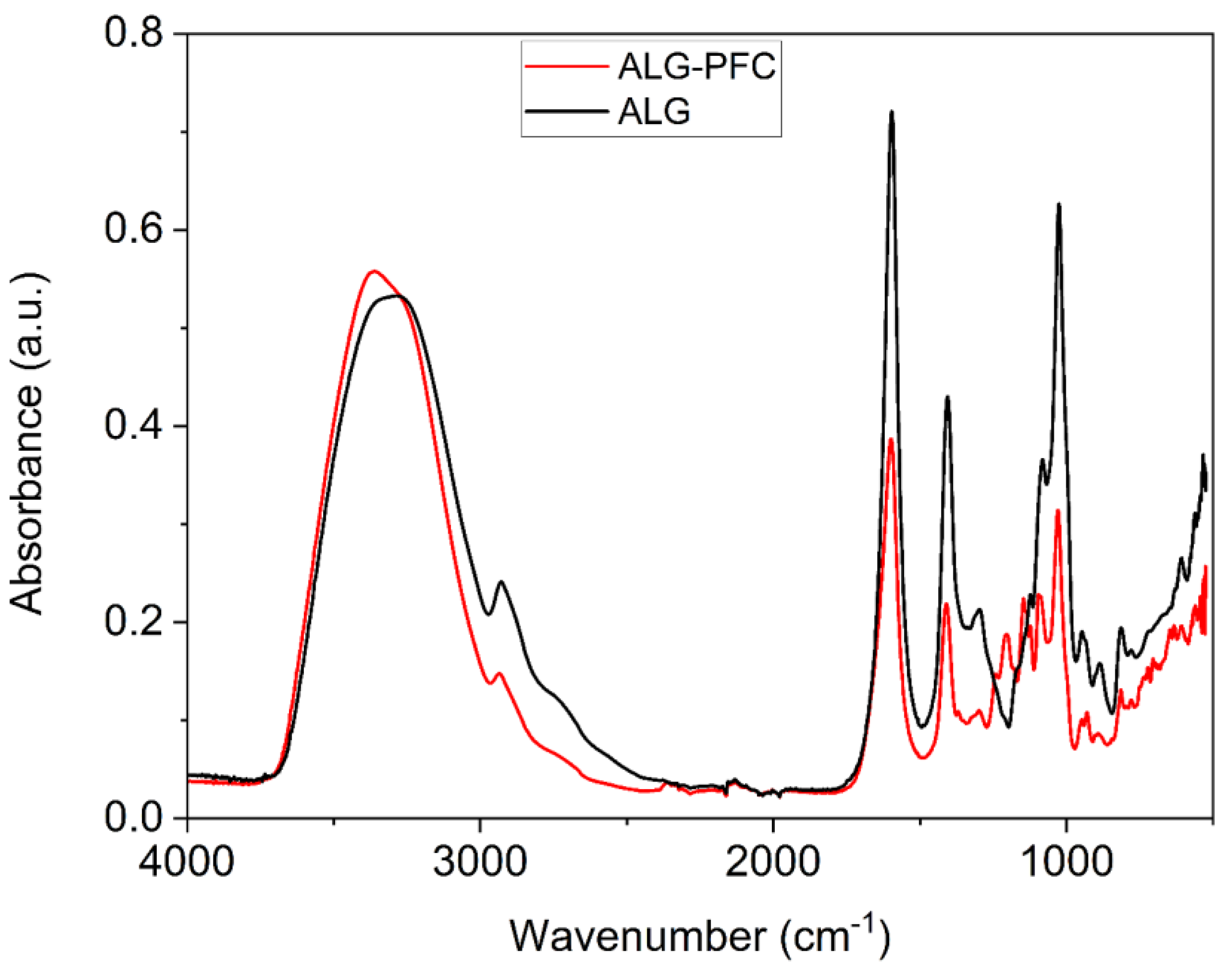
3.2. Fourier Transform Infrared (FT-IR) Spectroscopic Evaluation
3.3. Microscopic Evaluation of Morphology of Coating and Oxygenating Elements Presence
3.4. Water Contact Angle Evaluation
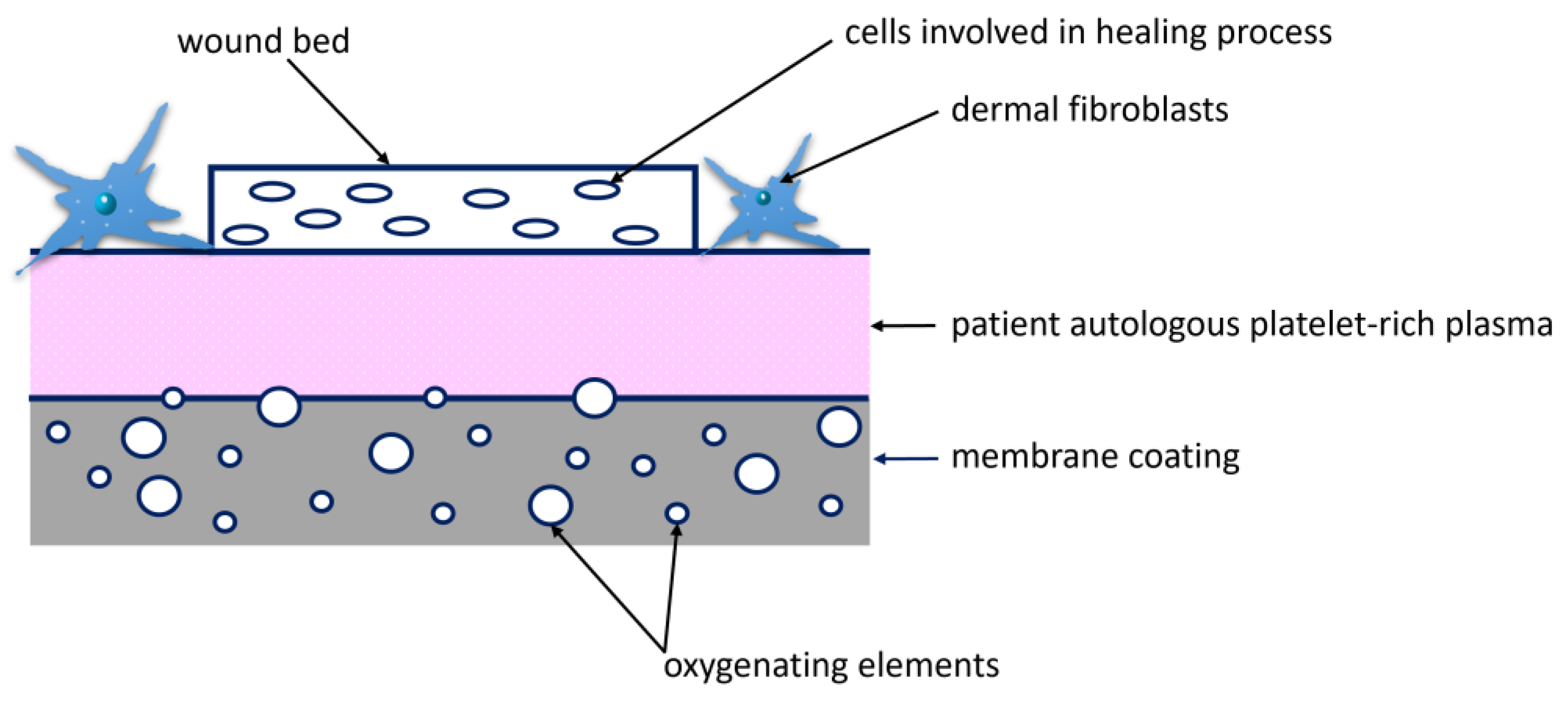
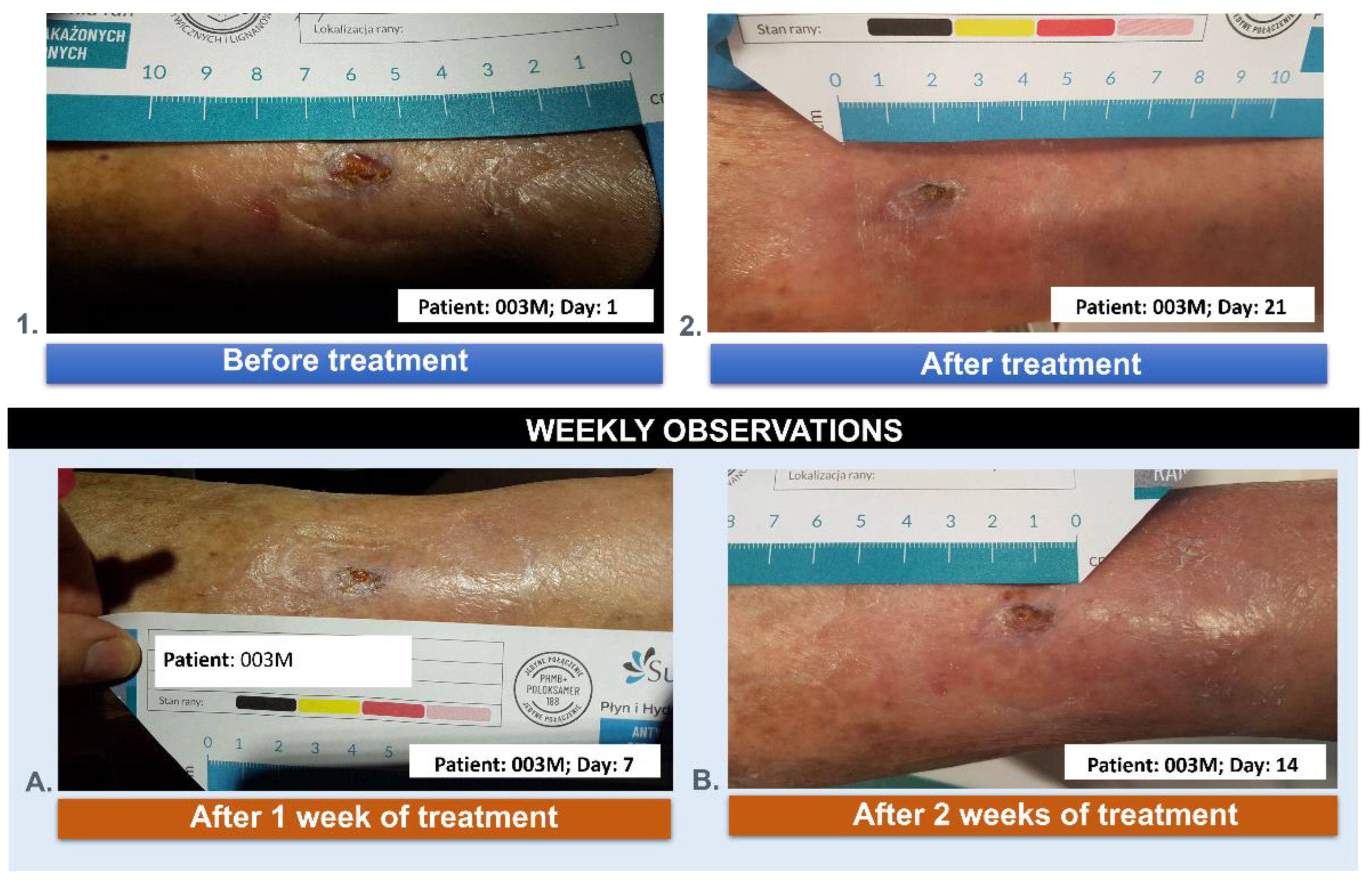
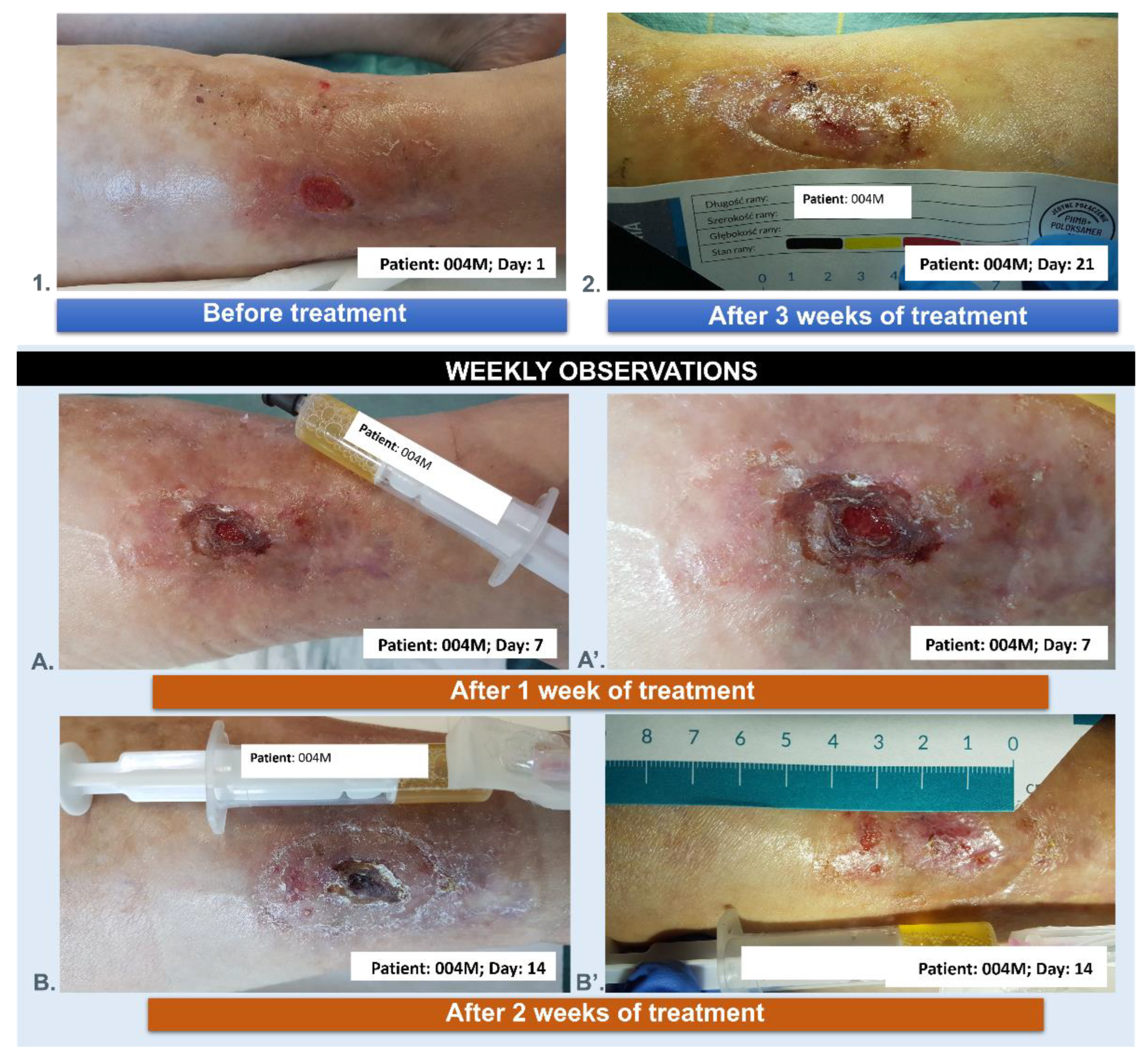
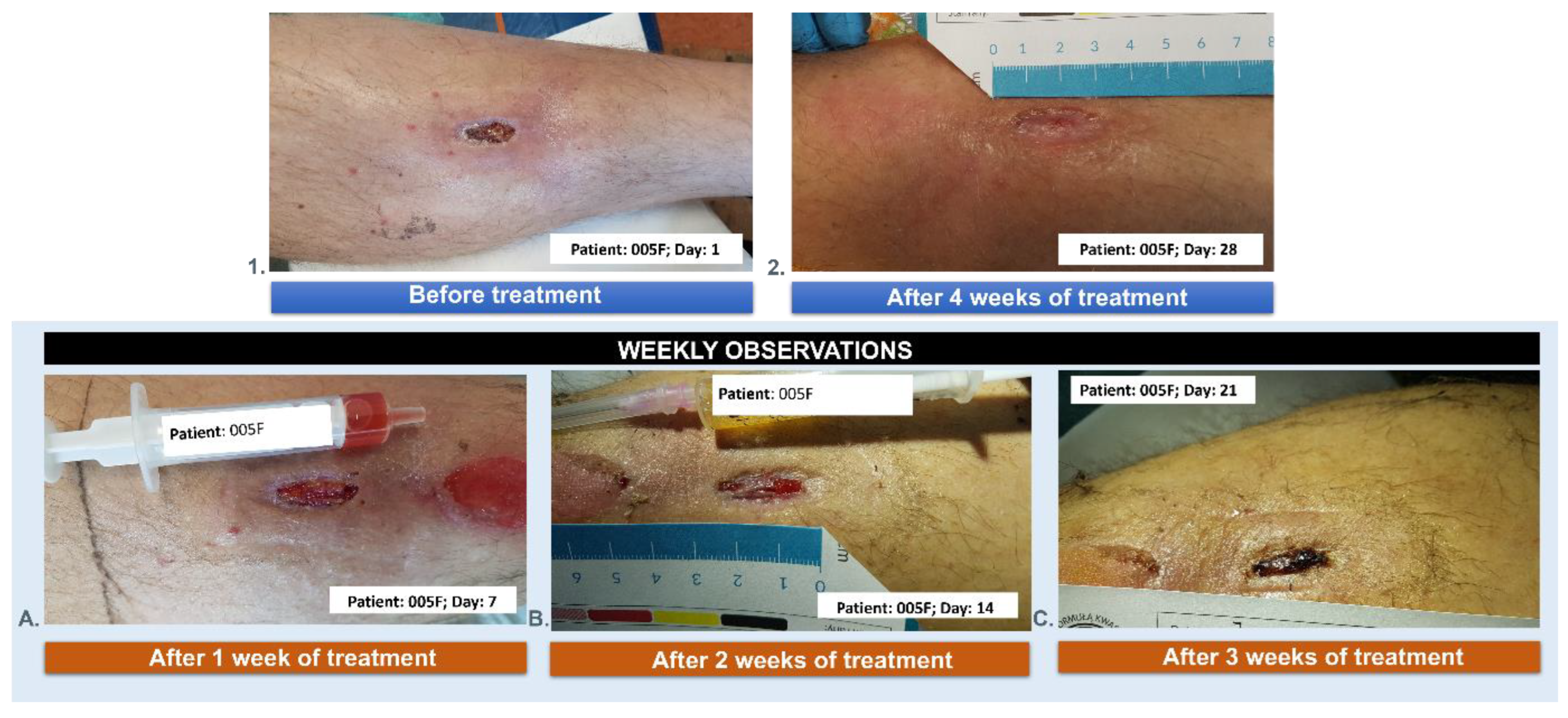
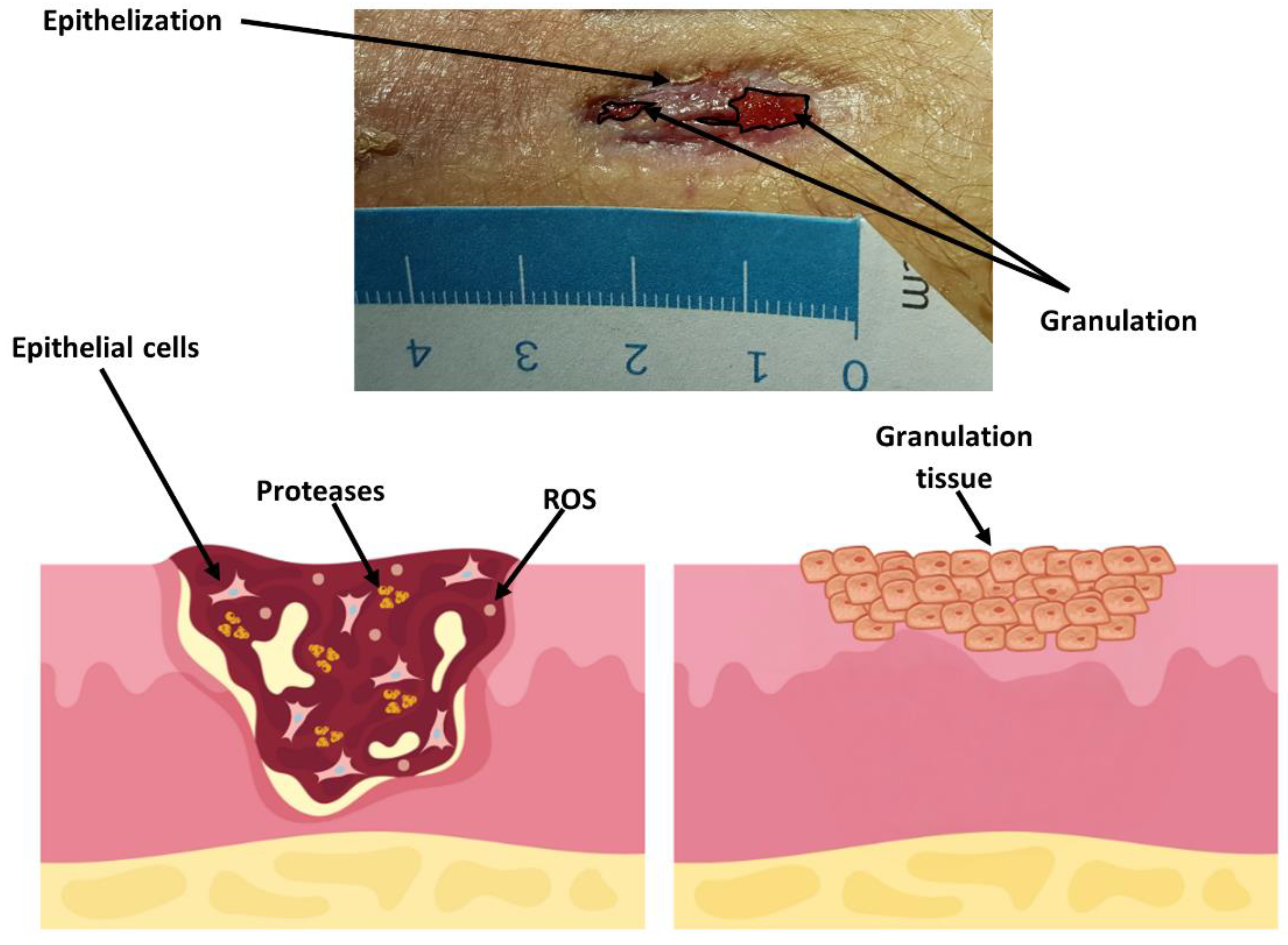
3.5. Wound Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Chan, B.; Cadarette, S.; Wodchis, W.; Wong, J.; Mittmann, N.; Krahn, M. Cost-of-illness studies in chronic ulcers: A systematic review. J. Wound Care 2017, 26 (Suppl. 4), S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Prim. 2022, 8, 50. [Google Scholar] [CrossRef]
- Murray, R.Z.; West, Z.E.; McGuiness, W. The multifactorial formation of chronic wounds. Wound Pract. Res. 2018, 26, 38–46. [Google Scholar]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME concept: What have we learned in the past 10 years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Monti, D.; Tampucci, S.; Tiwari, R.; Pathak, K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef]
- Andrade, S.M.; Santos, I.C. Hyperbaric oxygen therapy for wound care. Rev. Gauch. Enferm. 2016, 37, e59257. [Google Scholar] [CrossRef]
- Vinkel, J.; Holm, N.F.R.; Jakobsen, J.C.; Hyldegaard, O. Effects of adding adjunctive hyperbaric oxygen therapy to standard wound care for diabetic foot ulcers: A protocol for a systematic review with meta-analysis and trial sequential analysis. BMJ Open 2020, 10, e031708. [Google Scholar] [CrossRef] [PubMed]
- Rosadi Seswandhana, M.; Anzhari, S.; Dachlan, I.; Widodo Wirohadidjojo, Y.; Aryandono, T. A case series of negative pressure wound therapy as a promising treatment in patients with burn injury. Int. J. Surg. Case Rep. 2020, 69, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra Artificial Skin® for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A short history of skin grafting in burns: From the gold standard of autologous skin grafting to the possibilities of allogeneic skin grafting with immunomodulatory approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef]
- Driver, V.R.; Hanft, J.; Fylling, C.P.; Beriou, J.M.; Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manag. 2006, 52, 68–87. [Google Scholar]
- Gilligan, A.M.; Waycaster, C.R.; Motley, T.A. Cost-effectiveness of becaplermin gel on wound healing of diabetic foot ulcers. Wound Repair Regen. 2015, 23, 353–360. [Google Scholar] [CrossRef]
- Mazzucco, L.; Medici, D.; Serra, M.; Panizza, R.; Rivara, G.; Orecchia, S.; Libener, R.; Cattana, E.; Levis, A.; Betta, P.G.; et al. The use of autologous platelet gel to treat difficult-to-heal wounds: A pilot study. Transfusion 2004, 44, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Grigore, T.V.; Cozma, C. Platelet-rich plasma as a site-targeted approach in wound healing: A molecular perspective. Discoveries 2018, 6, e87. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The Role of Oxygen in Wound Healing: A Review of the Literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Castilla, D.M.; Liu, Z.-J.; Velazquez, O.C. Oxygen: Implications for Wound Healing. Adv. Wound Care 2012, 1, 225–230. [Google Scholar] [CrossRef]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Ross, C.L.; Smith, L.C.; Jeong, S.S.; Kim, J.; Yoo, J.J.; Harrison, B.S. Peroxide-based oxygen generating topical wound dressing for enhancing healing of dermal wounds. Wound Repair Regen. 2015, 23, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Rahimi, R.; Zhou, J.; Jiang, H.; Yoon, C.K.; Maddipatla, D.; Narakathu, B.B.; Jain, V.; Oscai, M.M.; Morken, T.J.; et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pang, K.; Zhu, S.; Pate, K.; Yin, J. Perfluorodecalin-based oxygenated emulsion as a topical treatment for chemical burn to the eye. Nat. Commun. 2022, 13, 7371. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.H.; Lai, C.W.; Gholami, A.; Omidifar, N.; Shokripour, M. Asymmetric membranes: A potential scaffold for wound healing applications. Symmetry 2020, 12, 1100. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Drabik, M.; Lipko, A.; Grzeczkowicz, A.; Stachowiak, R.; Marszalik, A.; Granicka, L.H. Composite Membrane Dressings System with Metallic Nanoparticles as an Antibacterial Factor in Wound Healing. Membranes 2022, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, H.; Yu, F.; Pei, Y.; Luo, X. Superclear, Porous Cellulose Membranes with Chitosan-Coated Nanofibers for Visualized Cutaneous Wound Healing Dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef]
- Bagrov, V.V.; Bukhtiyarov, I.V.; Volodin, L.Y.; Zibarev, E.V.; Kamrukov, A.S.; Kondratiev, A.V.; Krylov, V.I.; Nikonova, S.M.; Novikov, D.O.; Semenov, K.A. Preclinical Studies of the Antimicrobial and Wound-Healing Effects of the High-Intensity Optical Irradiation “Zarnitsa-A” Apparatus. Appl. Sci. 2023, 13, 10794. [Google Scholar] [CrossRef]
- Miłek, T.; Grzeczkowicz, A.; Lipko, A.; Oleksinski, L.; Kwiatkowska, A.; Strawski, M.; Drabik, M.; Stachowiak, R.; Goliszewski, J.; Granicka, L.H. A Functionalized Membrane Layer as Part of a Dressing to Aid Wound Healing. Membranes 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Ferenz, K.B. Perfluorocarbons for the treatment of decompression illness: How to bridge the gap between theory and practice. Eur. J. Appl. Physiol. 2019, 119, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflügers Arch.—Eur. J. Physiol. 2020, 473, 139–150. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cheng, H.; Zhang, Z.; Chen, K.; Zhang, Q.; Zhang, C.; Gao, W.; Zheng, Y. Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends. Gels 2024, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Lemnaru, G.M.; Motelica, L.; Trusca, R.D.; Ilie, C.I.; Croitoru, A.M.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Ditu, L.M.; et al. Antimicrobial Wound Dressings based on Bacterial Cellulose and Independently Loaded with Nutmeg and Fir Needle Essential Oils. Polymers 2023, 15, 3629. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, L. Growth factors in wound healing—A review. Biomed. Pharmacol. J. 2021, 14, 1469–1480. [Google Scholar] [CrossRef]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Twu, O.; Mednik, S.; Scumpia, P.; Doaty, S.; Worswick, S. Use of Becaplermin for nondiabetic ulcers: Pyoderma gangrenosum and calciphylaxis. Dermatol. Ther. 2016, 29, 104–108. [Google Scholar] [CrossRef]
- Papanas, N.; Maltezos, E. Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf. 2010, 33, 455–461. [Google Scholar] [CrossRef]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Simcock, J.; May, B.C.H. Ovine Forestomach Matrix as a Substrate for Single-Stage Split-Thickness Graft Reconstruction. Eplasty 2013, 13, e58. [Google Scholar] [PubMed]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. General principles of wound healing. Surg. Clin. N. Am. 1997, 77, 509–528. [Google Scholar] [CrossRef] [PubMed]






| Code | Age | Ulceration Time [Months] | Gender | Diabetes | Treatment [Weeks] |
|---|---|---|---|---|---|
| 001M | 54 | 4 | male | YES | 2 |
| 002M | 62 | 3 | male | NO | 4 |
| 003M | 84 | 8 | male | NO | 3 |
| 004M | 71 | 5 | male | YES | 3 |
| 005F | 58 | 3 | female | NO | 4 |
| Code | Age | Gender | Wound Characteristics |
|---|---|---|---|
| 001M | 54 | male | generalized scleroderma wound of the wrist |
| 002M | 62 | male | the postoperative wound of liposarcoma of the lower leg |
| 003M | 84 | male | the post-traumatic wound of the lower leg |
| 004M | 71 | male | chronic venous ulcer wound of the lower leg |
| 005F | 58 | female | the post-traumatic wound of the lower leg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłek, T.; Grzeczkowicz, A.; Lipko, A.; Mesjasz, M.; Oklesiński, L.; Kwiatkowska, A.; Strawski, M.; Drabik, M.; Granicka, L.H. An Oxygenated Layer Coating to Enhance the Healing Process of Non-Healing Wounds—A Case Report. Appl. Sci. 2024, 14, 11200. https://doi.org/10.3390/app142311200
Miłek T, Grzeczkowicz A, Lipko A, Mesjasz M, Oklesiński L, Kwiatkowska A, Strawski M, Drabik M, Granicka LH. An Oxygenated Layer Coating to Enhance the Healing Process of Non-Healing Wounds—A Case Report. Applied Sciences. 2024; 14(23):11200. https://doi.org/10.3390/app142311200
Chicago/Turabian StyleMiłek, Tomasz, Anna Grzeczkowicz, Agata Lipko, Maria Mesjasz, Leszek Oklesiński, Angelika Kwiatkowska, Marcin Strawski, Monika Drabik, and Ludomira H. Granicka. 2024. "An Oxygenated Layer Coating to Enhance the Healing Process of Non-Healing Wounds—A Case Report" Applied Sciences 14, no. 23: 11200. https://doi.org/10.3390/app142311200
APA StyleMiłek, T., Grzeczkowicz, A., Lipko, A., Mesjasz, M., Oklesiński, L., Kwiatkowska, A., Strawski, M., Drabik, M., & Granicka, L. H. (2024). An Oxygenated Layer Coating to Enhance the Healing Process of Non-Healing Wounds—A Case Report. Applied Sciences, 14(23), 11200. https://doi.org/10.3390/app142311200







