Preparation of Nano- and Microparticles Obtained from Polymerization Reaction and Their Application to Surface Coating of Woody Materials
Abstract
1. Introduction
2. Materials and Methods
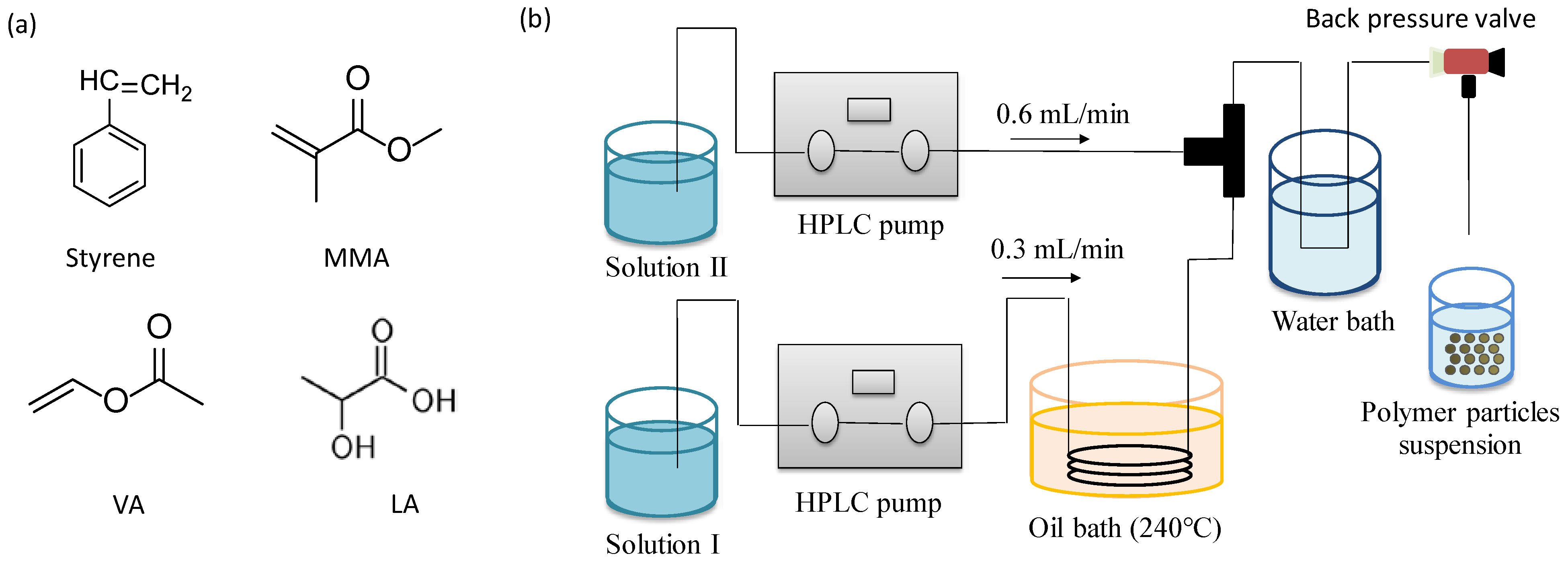
2.1. Materials
2.2. Polymerization Reaction Using Microcapillary
2.3. Preparation of WTBs by Compression Molding
2.4. Immersion Method
2.5. SEM Observation
2.6. AFM Observation
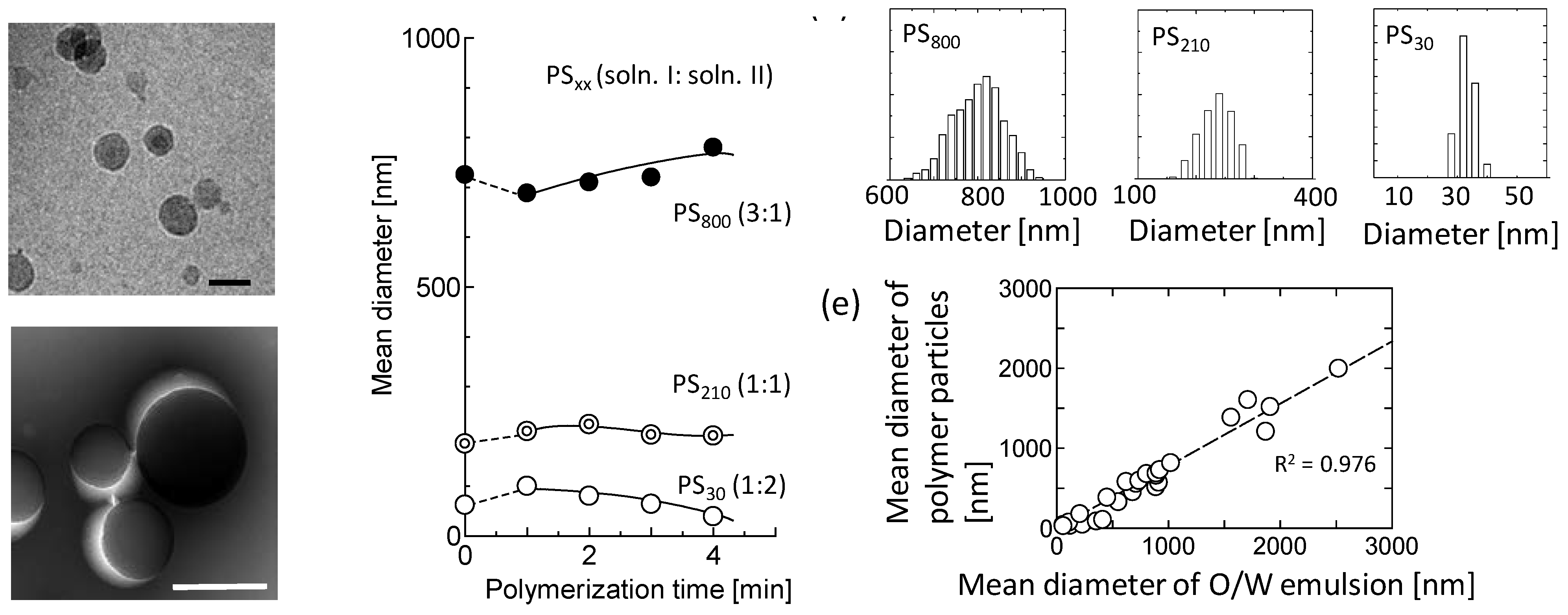
2.7. Determination of Mean Diameter of Polymer Particles
2.8. Measurement of Contact Angle
2.9. Measurement of Long-Term Stability
3. Results
3.1. Simultaneous Polymerization Reaction with Emulsification
3.1.1. Styrene
3.1.2. Other Monomers
3.2. Coatings by Polymer Particles
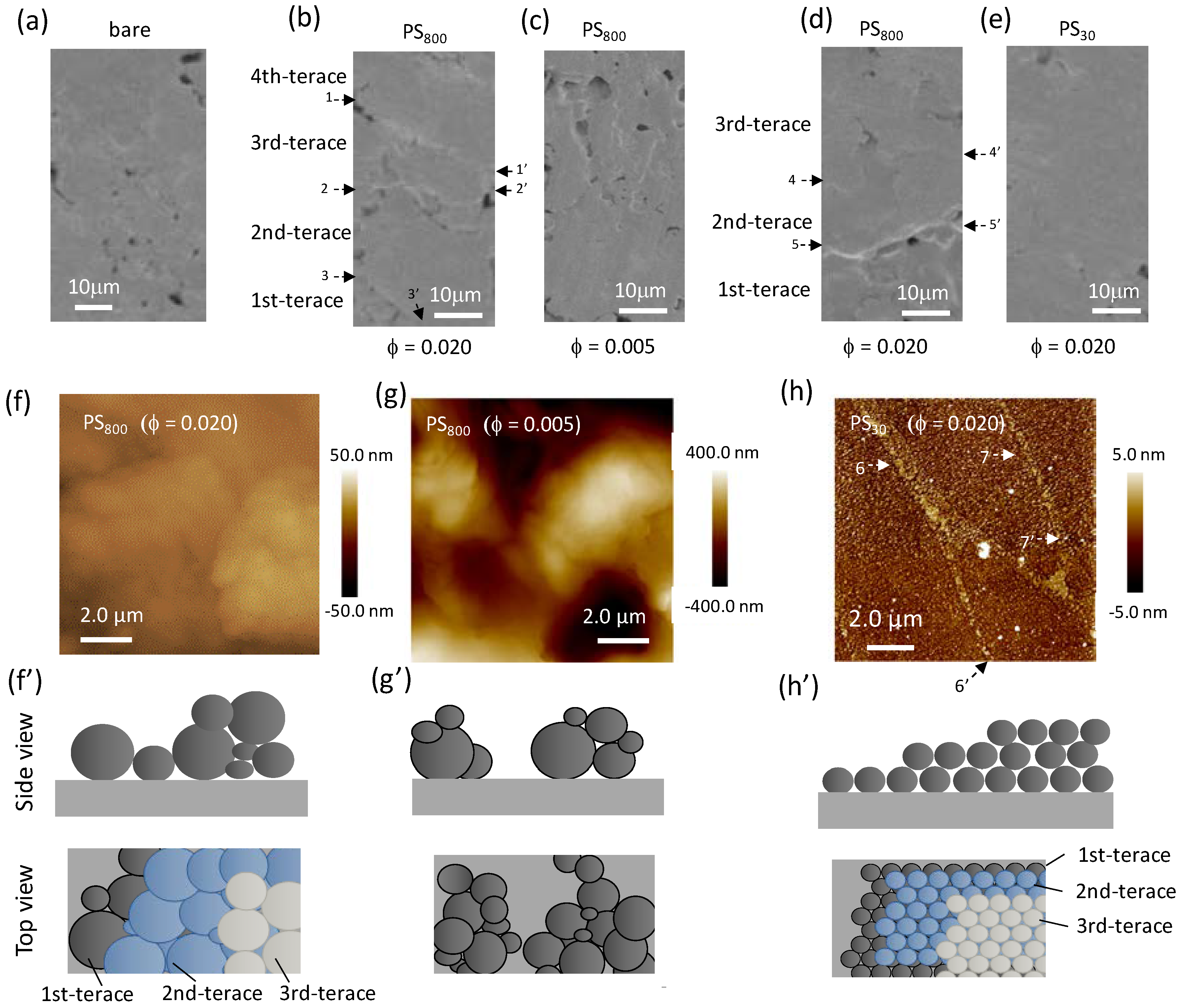
3.2.1. Surface Observation
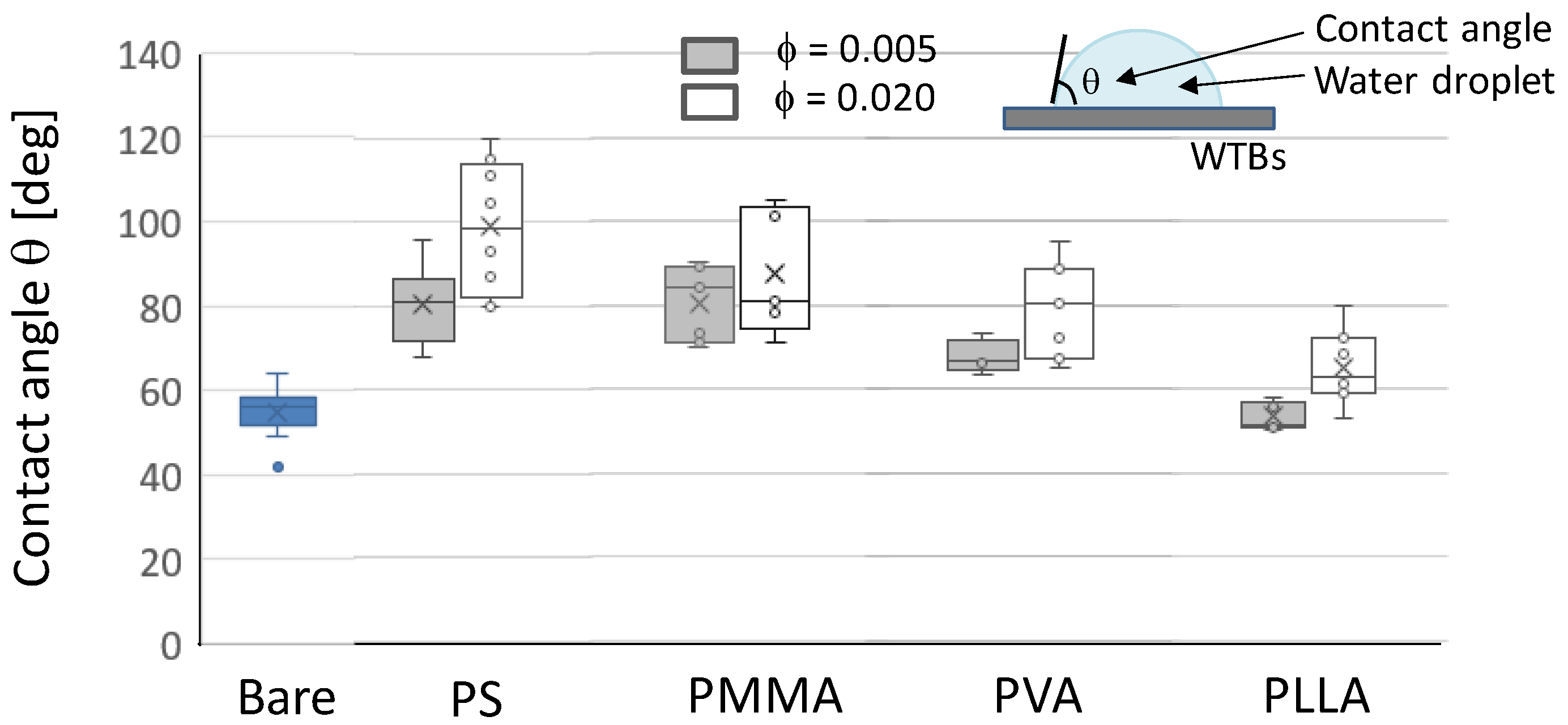
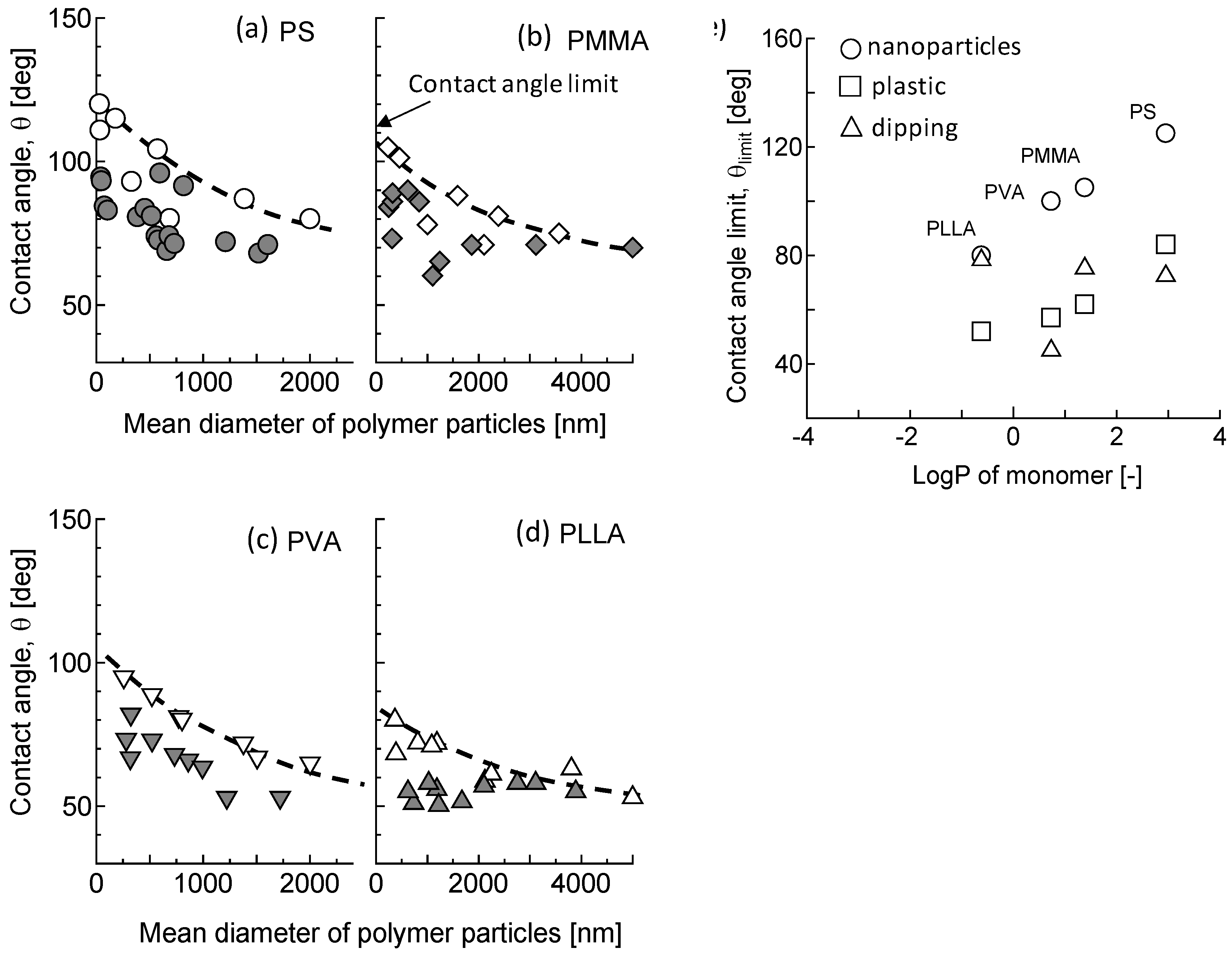
3.2.2. Water Repellency
3.2.3. Comparison of Polymer Particles on Water Repellency
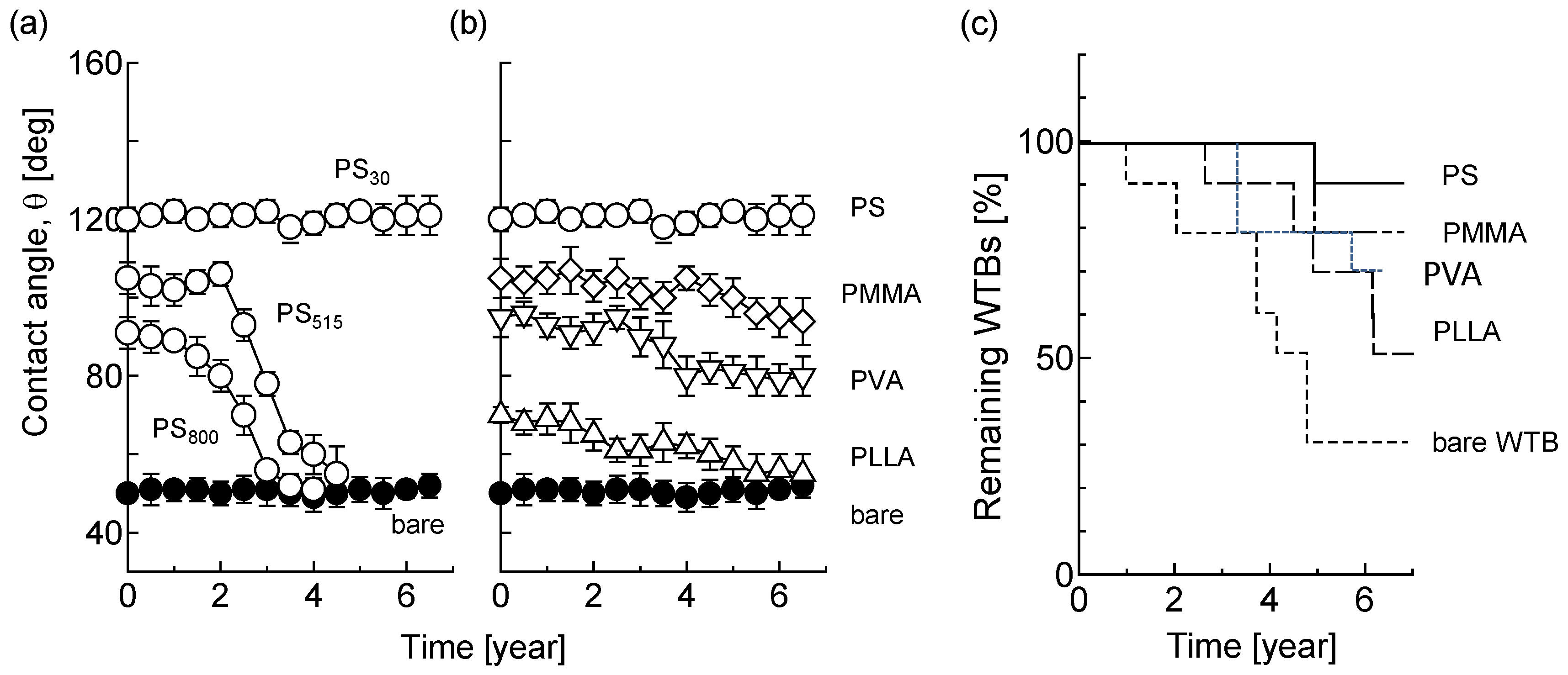
3.3. Long-Term Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Biores. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Biores. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, R.; Zhai, Y.; Song, X.; Xiong, J.; Li, X.; Qiano, Y.; Lu, X.; Yu, Z. Solvent effects enable efficient tandem conversion of cellulose and its monosaccharides towards 5-hydroxymethylfurfural. ChemSusChem 2023, 16, e202201809. [Google Scholar] [CrossRef] [PubMed]
- Millán, G.G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent advances in the catalytic production of platform chemicals from holocellulosic biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Moller, M.; Harnisch, F.; Schrader, U. Microwave-assisted hydrothermal degradation of fructose and glucose in subcritical water. Biomass Bioenergy 2012, 39, 389–398. [Google Scholar] [CrossRef]
- Song, B.; Wu, Z.; Yu, Y.; Wu, H. Hydrothermal Reactions of Biomass-Derived Platform Molecules: Distinct Effect of Aprotic and Protic Solvents on Primary Decomposition of Glucose and Fructose in Hot-Compressed Solvent/Water Mixtures. Ind. Eng. Chem. Res. 2020, 59, 7336–7345. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, Y.; Wu, H. Hydrothermal Reactions of Biomass-Derived Platform Molecules: Mechanistic Insights into 5-Hydroxymethylfurfural (5-HMF) Formation during Glucose and Fructose Decomposition. Energy Fuels 2023, 37, 2115–2126. [Google Scholar] [CrossRef]
- Akbukut, D.; Ozkar, S. A review of the catalytic conversion of glycerol to lactic acid in the presence of aqueous base. RSC Adv. 2022, 12, 18864–18883. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Takahashi, Y.; Yasuhara, K.; Kimura, Y. Conversion of glycerol to lactic acid by using platinum-supported catalyst combined with phosphatidylcholine vesicles. Chem. Lett. 2023, 52, 426–429. [Google Scholar] [CrossRef]
- Weigarten, R.; Cho, J.; Conner, C., Jr.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2012, 12, 1423–1429. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Zhu, B.; Zhou, Z.; El-Hout, S.I.; Yang, J.; Zhang, J. 2,5-Furandicarboxylic acid production via catalytic oxidation of 5-hydroxymethylfurfural: Catalysts, processes and reaction mechanism. J. Energy Chem. 2021, 54, 528–554. [Google Scholar] [CrossRef]
- Bian, H.; Wei, L.; Lin, C.; Ma, Q.; Dai, H.; Zhu, J.Y. Lignin-Containing Cellulose Nanofibril-Reinforced Polyvinyl Alcohol Hydrogels. ACS Sustain. Chem. Eng. 2018, 6, 4821–4828. [Google Scholar] [CrossRef]
- Okahisa, Y.; Matsuoka, K.; Yamada, K.; Wataoka, I. Comparison of polyvinyl alcohol films reinforced with cellulose nanofibers derived from oil palm by impregnating and casting methods. Carbhydrate Polym. 2020, 250, 116907. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Kobayashi, Y.; Ito, H. Structural, rheological, and mechanical properties of polyvinyl alcohol composites reinforced with cellulose nanofiber treated by ultrahigh-pressure homogenizer. Mater. Today Commun. 2022, 33, 104316. [Google Scholar] [CrossRef]
- Heise, K.; Delepierre, G.; King, A.W.T.; Kostiainen, M.A.; Zoppe, J.; Weder, C.; Kontturi, E. Chemical Modification of Reducing End-Groups in Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 66–87. [Google Scholar]
- Shimanouchi, T.; Yoshida, M.; Yang, W.; Kimra, Y. Effect of cellulose-g-poly(L-lactide) on the properties of woody thin boards made of lignin/cellulose biphasic system: Water repellency and long-term stability. Poly. Sci. Peer Rev. J. 2021, 3, 536–542. [Google Scholar]
- Rana, A.K.; Gupta, V.K.; Saini, A.K.; Voicu, S.I.; Abdellattifaand, M.H.; Thakur, V.K. Water desalination using nanocelluloses/cellulose derivatives based membranes for sustainable future. Desalination 2021, 520, 115359. [Google Scholar] [CrossRef]
- Kim, N.H.; Imai, T.; Wada, M.; Sugiyama, J. Molecular Directionality in Cellulose Polymorphs. Biomacromolecules 2006, 7, 274–280. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Kamba, T.; Yang, W.; Aoyagi, S.; Kimura, Y. Surface properties of woody thin boards composed of commercially available lignin and cellulose: Relationship between the orientation of lignin and water repellency. Appl. Surf. Sci. 2015, 347, 406–413. [Google Scholar] [CrossRef]
- Nakagawa, A.; Steiniger, F.; Richter, W.; Koschella, A.; Heinze, T.; Kamitakahara, H. Thermoresponsive Hydrogel of Diblock Methylcellulose: Formation of Ribbonlike Supramolecular Nanostructures by Self-Assembly. Langmuir 2012, 28, 12609–12618. [Google Scholar] [CrossRef]
- Barhoum, A.; Rastogi, V.K.; Mahur, B.K.; Rastogi, A.; Abdel-Haleem, F.M.; Samyn, P. Nanocelluloses as new generation materials: Natural resources, structure-related properties, engineering nanostructures, and technical challenges. Mater. Today Chem. 2022, 26, 101247. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Freire, C.S.R.; Pinto, R.J.B.; Fernandes, S.C.M.; Pascoal Neto, C.; Silvestre, A.J.D.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 2012, 19, 1425–1436. [Google Scholar] [CrossRef]
- Kamitakahara, H.; Enomoto, Y.; Hasegawa, C.; Nakatsubo, F. Synthesis of Diblock Copolymers with Cellulose Derivatives. 2. Characterization and Thermal Properties of Cellulose Triacetate-Block-Oligoamide-15. Cellulose 2005, 12, 527–541. [Google Scholar] [CrossRef]
- Kong, D.C.; Yang, M.H.; Zhang, X.S.Z.; Du, C.; Fu, Q.; Gao, X.Q.; Gong, J.W. Control of Polymer Properties by Entanglement: A Review. Macromol. Mater. Eng. 2021, 306, 2100536. [Google Scholar] [CrossRef]
- Lovell, P.A.; Schork, F.J. Fundamentals of emulsion polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef] [PubMed]
- Nagao, D.; Hashimoto, M.; Hayasaka, K.; Konno, M. Synthesis of anisotropic polymer particles with soap-free emulsion polymerization in the presence of a reactive silane coupling agent. Macromol. Rapid Commun. 2008, 29, 1484. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Tange, T.; Kimura, Y. Subcritical water-assisted emulsification of decane/water: Influence of surfactants. Solvent Extr. Res. Dev. Jpn. 2014, 21, 103–110. [Google Scholar] [CrossRef][Green Version]
- Shimanouchi, T.; Tange, T.; Kimura, Y. Subcritical Water-Assisted Emulsification of Oil/Water: The Effect of Solubility of Organic Solvent and Surfactants. Solvent Extr. Res. Dev. Jpn. 2014, 21, 223–230. [Google Scholar] [CrossRef][Green Version]
- Prevo, B.G.; Velev, O.D. Controlled, rapid deposition of structured coatings from micro- and nanoparticle suspensions. Langmuir 2004, 20, 2099–2107. [Google Scholar] [CrossRef]
- Dimitrov, A.S.; Nagayama, K. Continuous convective assembling of fine particles into two-dimensional arrays on solid surfaces. Langmuir 1996, 12, 1303–1311. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Poly. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Santangelo, P.G.; Roland, C.M. Molecular Weight Dependence of Fragility in Polystyrene. Macromolecules 1998, 31, 4581–4585. [Google Scholar] [CrossRef]
- Wu, S. Chain structure and entanglement. J. Polym. Sci. B Polym. Phys. 1989, 27, 723–741. [Google Scholar] [CrossRef]
- Senanayake, K.K.; Fakhrabadi, E.A.; Liberatore, M.W.; Mukhopadhyay, A. Diffusion of Nanoparticles in Entangled Poly(vinyl alcohol) Solutions and Gels. Macromolecules 2019, 52, 787–795. [Google Scholar] [CrossRef]
- Wu, C.-S. Handbook of Size Exclusion Chromatography and Related Techniques: Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003; Volume 91. [Google Scholar]
- Dorgan, J.R.; Williams, J.S. Melt rheology of poly(lactic acid): Entanglement and chain architecture effects. J. Rheol. 1999, 43, 1141–1155. [Google Scholar] [CrossRef]
- Akamatsu, M. Hydrophobicity of chemical compounds including pesticides in the environment. J. Environ. Sci. Sustain. Soc. 2021, 10, 27–30. [Google Scholar] [CrossRef]
- Kunstche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar]
- Shimanouchi, T.; Hayashi, T.; Toramoto, K.; Fukuma, S.; Hayashi, K.; Yasuhara, K.; Kimura, Y. Microfluidic and hydrothermal preparation of vesicles using sorbitan monolaurate/polyoxyethylene (20) sorbitan monolaurate (Span 20/Tween 20). Colloids Surf. B 2021, 205, 111836. [Google Scholar] [CrossRef]
- Guo, F.; Chen, B. Numerical study on Taylor bubble formation in a micro-channel T-junction using VOF method. Microgravity Sci. Technol. 2009, 21, S51–S58. [Google Scholar] [CrossRef]
- Ayrilmis, N. Effect of fire retardants on surface roughness and wettability of wood plastic composites panels. BioResources 2011, 6, 3178–3187. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Zhao, Y.; Lu, Q. Control over the hydrophobic behavior of polystyrene surface by annealing temperature based on capillary template wetting method. Colloids Surf. A 2007, 302, 136–140. [Google Scholar] [CrossRef]
- Marmur, A. The Lotus Effect: Superhydrophobicity and Metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Monomer | Hydrophobicity of Monomer, logP a | Entanglement Molecular Weight, Me b | Softening Temperature, Ts [deg] |
|---|---|---|---|---|
| Polystyrene (PS) | styrene | 2.95 | 18,700 c | ~90 |
| Poly(methyl methacrylate) (PMMA) | methyl methacrylate | 1.38 | 9200 d | 85–165 |
| Poly(vinyl alcohol) (PVA) | vinyl alcohol | 0.73 | 5100 e | 85 |
| Poly(lactic acid) (PLLA) | L-lactic acid | −0.62 | 9000 f | 60–65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimanouchi, T.; Hirota, D.; Yoshida, M.; Yasuhara, K.; Kimura, Y. Preparation of Nano- and Microparticles Obtained from Polymerization Reaction and Their Application to Surface Coating of Woody Materials. Appl. Sci. 2024, 14, 11326. https://doi.org/10.3390/app142311326
Shimanouchi T, Hirota D, Yoshida M, Yasuhara K, Kimura Y. Preparation of Nano- and Microparticles Obtained from Polymerization Reaction and Their Application to Surface Coating of Woody Materials. Applied Sciences. 2024; 14(23):11326. https://doi.org/10.3390/app142311326
Chicago/Turabian StyleShimanouchi, Toshinori, Daichi Hirota, Masafumi Yoshida, Kazuma Yasuhara, and Yukitaka Kimura. 2024. "Preparation of Nano- and Microparticles Obtained from Polymerization Reaction and Their Application to Surface Coating of Woody Materials" Applied Sciences 14, no. 23: 11326. https://doi.org/10.3390/app142311326
APA StyleShimanouchi, T., Hirota, D., Yoshida, M., Yasuhara, K., & Kimura, Y. (2024). Preparation of Nano- and Microparticles Obtained from Polymerization Reaction and Their Application to Surface Coating of Woody Materials. Applied Sciences, 14(23), 11326. https://doi.org/10.3390/app142311326






