Coumarins in Spirit Beverages: Sources, Quantification, and Their Involvement in Quality, Authenticity and Food Safety
Abstract
1. Introduction
- (i)
- The evolution of analytical methods supported by new technologies (described in Section 5);
- (ii)
- Advancement of knowledge on the ageing factors involved, such as the kind of wood, the ageing time and the ageing technologies (described in Section 6);
- (iii)
- Growing attention paid by the consumer and the scientific community to nutraceutical quality, even in alcoholic beverages, because there is greater awareness that the presence of bioactive compounds, such as coumarins, can partially mitigate the harmful effects of alcohol [49]. From this perspective, scientific outcomes are mainly related to wine [50,51], and only a few works have been devoted to whiskey [52], wine spirit [53,54,55], brandy [54,56], and rum [57] (described in Section 7). This could be an important driver for the six related industries (wine spirit, brandy, whiskey, rum, cachaça, and grape marc spirit);
- (iv)
- Health concerns on the part of the consumer, industries and food science researchers, leading to an interest in food safety. However, little is known about the relationship between coumarins and the food safety of spirits (disclosed in Section 8);
- (v)
- The importance of chemical markers for authenticity purposes, which is a key aspect in the production and trade of spirits, in order to protect crucial players in each value chain: the producers (the Designations of Origin, if applicable) and the consumers [58,59]. Coumarins are potential chemical markers—for example, they are associated with oak wood versus chestnut wood in the ageing of wine spirits [38,40]—but this requires knowledge on their contents and their variation with the ageing conditions for the different spirit beverages (discussed in Section 9).
2. Spirit Beverages: Aged and Unaged
2.1. Wine Spirit
2.2. Brandy
2.3. Whiskey
2.4. Sugar Cane Spirits
2.4.1. Rum
2.4.2. Cachaça
2.5. Grape Marc Spirit
3. Coumarins in the Context of Phenolic Composition
4. Sources of Coumarins
5. Methods for Identification and Quantification of Coumarins
5.1. Sample Preparation
5.2. Analysis
5.2.1. Thin-Layer Chromatography
5.2.2. High-Performance Liquid Chromatography
5.2.3. Gas Chromatography
5.2.4. Spectrometric Detectors
5.3. Analytical Trends
6. Influence of Ageing Factors on the Coumarin Contents of Spirit Beverages
6.1. Wood
6.2. Heat Treatment
6.3. Ageing Time
- Additive—including the ones that introduce or give rise to new compounds in the distillate undergoing ageing, such as extraction of wood compounds and lignin hydroalcoholysis;
- Subtractive—encompassing the ones that remove or modify some constituents of the distillate undergoing ageing, such as evaporation of volatile compounds, adsorption/degradation by the charred surface of the barrel, sorption in wood, and oxidation and hydrolysis reactions, among other chemical transformations.
6.4. Ageing Technology
7. The Role of Coumarins in the Nutraceutical Quality of Spirit Beverages
8. Do Coumarins Have an Impact on Food Safety?
9. Coumarin and Spirit Beverage Authenticity
10. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Reg. EU nº 2019/787 of the European Parliament and of the Council of 17 April 2019 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008; OJEU. L130 European Union. 2019, pp. 1–54. Available online: https://eur-lex.europa.eu/eli/reg/2019/787/2022-08-15 (accessed on 13 September 2023).
- Tsakiris, A.; Kallithrakab, S.; Kourkoutasc, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef]
- Krstić, J.D.; Kostić-Stanković, M.M.; Veljović, S.P. Traditional and innovative aging technologies of distilled beverages: The influence on the quality and consumer preferences of aged spirit drinks. J. Agric. Sci. 2021, 66, 209–230. [Google Scholar] [CrossRef]
- Statista. Alcoholic Drinks—Worldwide. 2023. Available online: https://www.statista.com/outlook/cmo/alcoholic-drinks/worldwide (accessed on 13 September 2023).
- Alcoholic Beverages Global Market Analysis, Insights and Forecast, 2018–2029. Fortune Business Insights 2023, Report FBI 107439. Available online: www.fortunebusinessinsights.com/alcoholic-beverages-market-107439 (accessed on 13 September 2023).
- World Health Organization (WHO). Global Status Report on Alcohol and Health. 2018. Available online: https://www.who.int/publications/i/item/9789241565639 (accessed on 13 September 2023).
- Wardencki, W. Alcoholic Beverages. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 67–76. [Google Scholar] [CrossRef]
- Kang, Q.; Sun, J.; Wang, B.; Sun, B. Wine, beer and Chinese Baijiu in relation to cardiovascular health: The impact of moderate drinking. Food Sci. Hum. Wellness. 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Cravero, M.C.; Laureati, M.; Spinelli, S.; Bonello, F.; Monteleone, E.; Proserpio, C.; Lottero, M.R.; Pagliarini, E.; Dinnella, C. Profiling individual differences in alcoholic beverage preference and consumption: New insights from a large-scale study. Foods 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yang, B.; Dai, M.; Xu, Y.; Li, X.; Sun, B. East meets west in alcoholic beverages: Flavor comparison, microbial metabolism and health effects. Food Biosci. 2023, 56, 103385. [Google Scholar] [CrossRef]
- Pino, J.; Martí, M.P.; Mestres, M.; Pérez, J.; Busto, O.; Guasch, J. Headspace solid-phase microextraction of higher fatty acid ethyl esters in white rum aroma. J. Chromatogr. A 2002, 954, 51–57. [Google Scholar] [CrossRef]
- Faria, B.; Loyola, E.; López, M.G.; Dufour, J.-P. Cachaça, Pisco and Tequila. In Fermented Beverage Production; Lea, A.G.H., Geoffrey, A., Lea, H., Piggott, J.R., Eds.; Springer Science and Business Media: New York, NY, USA, 2003; pp. 335–364. [Google Scholar] [CrossRef]
- Nicol, D.A. Rum. In Fermented Beverage Production; Lea, A.G.H., Geoffrey, A., Lea, H., Piggott, J.R., Eds.; Springer Science and Business Media: New York, NY, USA, 2003; pp. 263–287. [Google Scholar] [CrossRef]
- Russell, I. Whisky. In Technology, Production and Marketing; Elsevier: London, UK, 2003. [Google Scholar]
- López-Vásquez, C.; Bollaín, M.H.; Moser, S.; Orriols, I. Characterization and differentiation of monovarietal grape pomace distillate from native varieties of Galicia. J. Agric. Food Chem. 2010, 58, 9657–9665. [Google Scholar] [CrossRef]
- Da Porto, C. Grappa: Production, sensory properties and market development. In Alcoholic Beverages. Sensory Evaluation and Consumer Research; Piggott, J., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 299–314. [Google Scholar] [CrossRef]
- Louw, L.; Lambrechts, M.G. Grape-based brandies: Production, sensory properties and sensory evaluation. In Alcoholic Beverages. Sensory Evaluation and Consumer Research; Piggott, J., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 281–298. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. First approach to the analytical characterization of barrel-aged grape marc distillates using phenolic compounds and colour parameters. Food Technol. Biotechnol. 2014, 52, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Canas, S. Phenolic composition and related properties of aged wine spirits: Influence of barrel characteristics. A review. Beverages 2017, 3, 55. [Google Scholar] [CrossRef]
- Puentes, C.; Joulia, X.; Vidal, J.-P.; Esteban-Decloux, M. Simulation of spirits distillation for a better understanding of volatile aroma compounds behavior: Application to Armagnac production. Food Bioprod. Process. 2018, 112, 31–62. [Google Scholar] [CrossRef]
- García-Moreno, M.V.; Sánchez-Guillén, M.M.; Ruiz de Mier, M.; Delgado-Gonzaléz, M.J.; Rodríguez-Dodero, M.C.; García-Barroso, C.; Guillén-Sánchez, D.A. Use of alternative wood for the ageing of Brandy de Jerez. Foods 2020, 9, 250. [Google Scholar] [CrossRef]
- Winstel, D.; Gautier, E.; Marchal, A. Role of oak coumarins in the taste of wines and spirits: Identification, quantitation, and sensory contribution through perceptive interactions. J. Agric. Food Chem. 2020, 68, 7434–7443. [Google Scholar] [CrossRef] [PubMed]
- Gollihue, J.; Pook, V.G.; DeBolt, S. Sources of variation in bourbon whiskey barrels: A review. J. Inst. Brew. 2021, 127, 210–223. [Google Scholar] [CrossRef]
- Canas, S.; Caldeira, I.; Fernandes, T.A.; Anjos, A.; Belchior, A.P.; Catarino, S. Sustainable use of wood in wine spirit production. In Improving Sustainable Viticulture and Winemaking Practices; Costa, J.M., Catarino, S., Escalona, J.M., Piergiorgio, C., Eds.; Elsevier: London, UK, 2022; pp. 259–280. [Google Scholar]
- de Aquino, F.W.; Rodrigues, S.; Nascimento, R.F.; Casemiro, A.R.S. Simultaneous determination of aging markers in sugar cane spirits. Food Chem. 2006, 98, 569–574. [Google Scholar] [CrossRef]
- Fujieda, M.; Tanaka, T.; Suwa, Y.; Koshimizu, S.; Kouno, I. Isolation and structure of whiskey polyphenols produced by oxidation of oak wood ellagitannins. J. Agric. Food Chem. 2008, 56, 7305–7310. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Tolle, S.; Gök, R.; Winterhalter, P. Characterisation of odour-active compounds in aged rum. Food Chem. 2012, 132, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Danalache, F.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Santos, N.; Fargeton, L.; Boissier, B.; Catarino, S. Behaviour of low molecular weight compounds, iron and copper of wine spirit aged with chestnut staves under different levels of microoxygenation. Molecules 2020, 25, 5266. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, A.M.; Silvello, G.C.; Alcarde, A.R. Aromatic profiling of flavor active compounds in sugarcane spirits aged in tropical wooden barrels. Braz. J. Food Technol. 2021, 24, e2019071. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Rodríguez-Freigedo, S.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Optimisation of accelerated ageing of grape marc distillate on a micro-scale process using a Box–Benhken design: Influence of oak origin, fragment size and toast level on the composition of the final product. Aust. J. Grape Wine Res. 2017, 23, 5–14. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.; Lourenço, S.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Catarino, S.; Canas, S. Influence of the storage in bottle on the antioxidant activities and related chemical characteristics of wine spirits aged with chestnut staves and micro-oxygenation. Molecules 2022, 27, 106. [Google Scholar] [CrossRef]
- Baldwin, S.; Black, R.A.; Andreasen, A.A.; Adams, S.L. Aromatic congener formation in maturation of alcoholic distillates. J. Agric. Food Chem. 1967, 15, 381–385. [Google Scholar] [CrossRef]
- Bricout, J. Analyse de quelques constituants dérivés du chêne dans les vieilles eaux-de-vie d’armagnac. Ann. Technol. Agric. 1971, 20, 217–224. [Google Scholar]
- Joseph, E.; Marche, M. Contribution à l’étude du vieillissement du cognac. Identification de la scopolétine, del’aesculétine, de l’ombelliférone, de la b-méthyl-ombelliférone, de l’aesculine et de la scopoline, hétérosides provenant du bois. Connaiss. Vigne Vin 1972, 6, 273–330. [Google Scholar] [CrossRef]
- Otsuka, K.I.; Zenibayashi, J. On the Determination of Scopoletin in Aged Distilled Liquors. Agric. Biol. Chem. 1974, 38, 1079–1080. [Google Scholar] [CrossRef]
- Salagoity-Auguste, M.-H.; Tricard, C.; Sudraud, P. Dosage simultané des aldéhydes aromatiques et des coumarines par chromatographie liquide haute performance. J. Chromat. 1987, 392, 379–387. [Google Scholar] [CrossRef]
- Tricard, C.; Salagoity, M.H.; Sudraud, P. La scopolétine: Un marqueur de la conservation en fûts de chêne. Connaiss. Vigne Vin 1987, 21, 33–41. [Google Scholar] [CrossRef]
- Puech, J.-L.; Moutounet, M. Liquid chromatographic determination of scopoletin in hydroalcoholic extract of oak wood and in matured distilled alcoholic beverages. J. Assoc. Off. Anal. Chem. 1988, 71, 512–514. [Google Scholar] [CrossRef]
- Patrício, I.; Canas, S.; Belchior, A.P. Effect of brandies’ agitation on the kinetics of extraction/oxidation and diffusion of wood extractable compounds in experimental model. Ciência Téc Vitiv 2005, 20, 1–15. [Google Scholar]
- Canas, S.; Silva, V.; Belchior, A.P. Wood related chemical markers of aged wine brandies. Ciência Téc Vitiv 2008, 23, 45–52. [Google Scholar]
- Canas, S.; Anjos, O.; Caldeira, I.; Belchior, A.P. Phenolic profile and colour acquired by the wine spirit in the beginning of ageing: Alternative technology using microoxygenation vs traditional technology. LWT—Food Sci. Technol. 2019, 111, 260–269. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Lourenço, S.; Fernandes, T.A.; Anjos, O.; Caldeira, I.; Canas, S.; Catarino, S. Quantification of coumarins in wine spirit aged by different technologies using chestnut wood. In Proceedings of the Livro de Resumos do XV Encontro de Química dos Alimentos: Estratégias para a Excelência, Autenticidade, Segurança e Sustentabilidade Alimentar, Funchal, Portugal, 5–8 September 2021. [Google Scholar]
- da Silva, A.A.; Nascimento, E.S.P.; Cardoso, D.R.; Franco, D.W. Coumarins and phenolic fingerprints of oak and Brazilian woods extracted by sugarcane spirit. J. Sep. Sci. 2009, 32, 3681–3691. [Google Scholar] [CrossRef]
- Santiago, W.D.; Cardoso, M.G.; Nelson, D.L. Cachaça stored in casks newly constructed of oak (Quercus sp.), amburana (Amburana cearensis), jatoba (Hymenaeae carbouril), balsam (Myroxylon peruiferum) and peroba (Paratecoma peroba): Alcohol content, phenol composition, colour intensity and dry extract. J. Inst. Brew. 2017, 123, 232–241. [Google Scholar] [CrossRef]
- Lima, C.M.G.; Benoso, P.; Pierezan, M.D.; Santana, R.F.; de Souza Hassemer, G.; da Rocha, R.A.; Nora, F.M.D.; Verruck, S.; Caetano, D.; Simal-Gandara, J. A state-of-the-art review of the chemical composition of sugarcane spirits and current advances in quality control. J. Food Compos. Anal. 2022, 106, 104338. [Google Scholar] [CrossRef]
- Mignani, A.G.; Ciaccheri, L.; Gordillo, B.; Mencaglia, A.A.; González-Miret, M.L.; Heredia, F.J.; Culshaw, B. Identifying the production region of single-malt Scotch whiskies using optical spectroscopy and pattern recognition techniques. Sens. Actuators B Chem. 2012, 171–172, 458–462. [Google Scholar] [CrossRef]
- Collins, T.S.; Zweigenbaum, J.; Ebeler, S.E. Profiling of nonvolatiles in whiskeys using ultra high pressure liquid chromatography quadrupole time-of-flight mass spectrometry (UHPLC–QTOF MS). Food Chem. 2014, 163, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Sádecká, J.; Uríčková, V.; Májek, P.; Jakubíková, M. Comparison of different fluorescence techniques in brandy classification by region of production. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Li, L.; Sun, B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2017, 59, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Romanet, R.; Sarhane, Z.; Bahut, F.; Uhl, J.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Exploring the chemical space of white wine antioxidant capacity: A combined DPPH, EPR and FT-ICR-MS study. Food Chem. 2021, 355, 129566. [Google Scholar] [CrossRef]
- Koga, K.; Taguchi, A.; Koshimizu, S.; Suwa, Y.; Yamada, Y.; Shirasaka, N.; Yoshizumi, H. Reactive oxygen scavenging activity of matured whiskey and its active polyphenols. J. Food Sci. 2007, 72, S212–S217. [Google Scholar] [CrossRef]
- Canas, S.; Casanova, V.; Belchior, A.P. Antioxidant activity and phenolic content of Portuguese wine aged brandies. J. Food Compos. Anal. 2008, 21, 626–633. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Chronoamperometric estimation of cognac and brandy antioxidant capacity using MWNT modified glassy carbon electrode. Talanta 2014, 125, 378–384. [Google Scholar] [CrossRef]
- Nocera, A.; Ricardo-da-Silva, J.M.; Canas, S. Antioxidant activity and phenolic composition of wine spirit resulting from an alternative ageing technology using microoxygenation: A preliminary study. Oeno One 2020, 54, 485–496. [Google Scholar] [CrossRef]
- Schwarz, M.; Rodríguez, M.C.; Martínez, C.; Bosquet, V.; Guillén, D.; Barroso, C.G. Antioxidant activity of Brandy de Jerez and other distillates, and correlation with their polyphenolic content. Food Chem. 2009, 116, 29–33. [Google Scholar] [CrossRef]
- Regalado, E.L.; Tolle, S.; Pino, J.A.; Winterhalter, P.; Menendez, R.; Morales, A.R.; Rodriguez, J.L. Isolation and identification of phenolic compounds from rum aged in oak barrels by highspeed countercurrent chromatography/high performance liquid chromatography-diode array detection-electrospray ionization mass spectrometry and screening for antioxidant activity. J. Chromatogr. A 2019, 41, 7358–7364. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E. Fluorescence spectroscopy and chemometrics in analysis of beverages. In Quality Control in the Beverage Industry; The Science of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: London, UK, 2019; Volume 17, pp. 161–203. [Google Scholar] [CrossRef]
- Belchior, A.P.; Canas, S.; Caldeira, I.; Carvalho, E. Aguardentes Vinícolas—Tecnologias de Produção e Envelhecimento. Controlo de qualidade; Publindústria, Edições Técnicas: Porto, Portugal, 2015; pp. 1–181. [Google Scholar]
- Cantagrel, R.; Galy, B. From Vine to Cognac. In Fermented Beverage Production; Lea, A.G.H., Geoffrey, A., Lea, H., Piggott, J.R., Eds.; Springer Science and Business Media: New York, NY, USA, 2003; pp. 195–212. [Google Scholar] [CrossRef]
- Lurton, L.; Ferrari, G.; Snakkers, G. Cognac: Production and aromatic characteristics. In Alcoholic Beverages. Sensory Evaluation and Consumer Research; Piggott, J., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 242–266. [Google Scholar] [CrossRef]
- Bertrand, A. Armagnac and Wine-Spirit. In Fermented Beverage Production; Lea, A.G.H., Geoffrey, A., Lea, H., Piggott, J.R., Eds.; Springer Science and Business Media: New York, NY, USA, 2003; pp. 213–238. [Google Scholar]
- Décret nº 2009-1146 du 21 Septembre 2009 Relatif à l’appellation d’origine Contrôlée “Cognac” ou ”Eau-de-vie de Cognac” ou “Eau-de-vie des Charentes”; JOFR nº 0221 du 24 Septembre 2009, p 15619, text nº 25. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000021065172 (accessed on 17 October 2023).
- Decreto-Lei nº 323/94, de 29 de Dezembro, do Ministério da Agricultura, Estatuto da Região Demarcada das Aguardentes Vínicas da Lourinhã. Diário da República nº 300/1994, Série I-A. 7486–7489. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/323-1994-322096 (accessed on 17 October 2023).
- Décret nº 2009-1285 du 23 Octobre Relatif aux Appellations d’origine Contrôlées “Armagnac”, ”Blanche Armagnac”, “Bas Armagnac”, “Haut Armagnac”et “Armagnac Ténarèze”; NOR: AGRT0912388D Version Consolidée au 25 Octobre 2009. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000021190654 (accessed on 17 October 2023).
- Bamforth, C.W. Fermented Beverages. In Encyclopedia of Agriculture and Food Systems; Alfen, N.K.V., Ed.; Elsevier: London, UK, 2014; pp. 124–136. [Google Scholar] [CrossRef]
- Schwarz, M.; Rodríguez, M.C.; Sanchez, M.; Guillen, D.A.; Barroso, C.G. Development of an accelerated aging method for Brandy. LWT—Food Sci. 2014, 59, 108–114. [Google Scholar] [CrossRef]
- Schwarz, M.; Rodríguez-Dodero, M.C.; Soledad Jurado, M.; Puertas, B.; Barroso, C.G.; Guillén, D.A. Analytical characterization and sensory analysis of distillates of different varieties of grapes aged by an accelerated method. Foods 2020, 9, 277. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Castro, R.; García-Moreno, M.V.; Rodríguez-Dodero, M.C.; Schwarz, M.; Guillén-Sánchez, D. Aroma of Sherry products: A review. Foods 2021, 10, 753. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Chemical composition analysis and authentication of whisky. J. Sci. Food Agric. 2015, 95, 2159–2166. [Google Scholar] [CrossRef]
- Piggott, J.R.; Conner, J.M. Whiskies. In Fermented Beverage Production; Lea, A.G.H., Geoffrey, A., Lea, H., Piggott, J.R., Eds.; Springer Science and Business Media: New York, NY, USA, 2003; pp. 239–262. [Google Scholar] [CrossRef]
- Jack, F.R. Whiskies: Composition, sensory properties and sensory analysis. In Alcoholic Beverages. Sensory Evaluation and Consumer Research; Piggott, J., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 379–392. [Google Scholar] [CrossRef]
- Authenticated U.S.; Government Information GPO. Title 27—Alcohol, Tobacco Products and Firearms. 27 CFR Ch. I (4–1–11 Edition). Available online: https://www.govinfo.gov/content/pkg/CFR-2011-title27-vol1/pdf/CFR-2011-title27-vol1-chapI.pdf (accessed on 17 October 2023).
- Faria, J.B. Sugar cane spirits: Cachaça and rum production and sensory properties. In Alcoholic Beverages. Sensory Evaluation and Consumer Research; Piggott, J., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 348–358. [Google Scholar] [CrossRef]
- Decreto Legislativo Regional nº 18/2021/M, Região Autónoma da Madeira, Confirma, Define e Caracteriza o «Rum da Madeira» e Estabelece as Regras Relativas à sua Produção e Comercialização; Diário da República nº 145 de 28 de Julho de 2021, 1ª Série, 76–83. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-legislativo-regional/18-2021-168475297 (accessed on 24 October 2023).
- Portaria MAPA Nº 539, de 26 de Dezembro de 2022, do Ministério de Estado da Agricultura, Pecuária e Abastecimento, Estabelece os Padrões de Identidade e Qualidade da Aguardente de Cana e da Cachaça, DOU—Diário Oficial da União, Edição 243, Seção 1. p. 12. Available online: https://www.in.gov.br/en/web/dou/-/portaria-mapa-n-539-de-26-de-dezembro-de-2022-453828778 (accessed on 30 October 2023).
- Da Porto, C. Grappa and grape-spirit production. Crit. Rev. Biotechnol. 1998, 18, 13–24. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manage. 2017, 72, 99–118. [Google Scholar] [CrossRef]
- Belchior, A.P.; Carvalho, E.C. Factores que condicionam o teor de metanol dos bagaços. Vin. Port. Doc. 1980, 10, 1–9. [Google Scholar]
- Belchior, A.P. Qualidade e composição química de aguardentes de bagaço. Vin. Port. Doc. 1977, 7, 1–15. [Google Scholar]
- Silva, M.L.; Macedo, A.C.; Malcata, F.X. Review: Steam distilled spirits from fermented grape pomace. Food Sci. Technol. Int. 2000, 6, 285–300. [Google Scholar] [CrossRef]
- Orriols, I.; Cortés, S.M.; Fornos, D. Carcactéristiques des distillats de marc du commerce “Orujo de galicia” d’Espagne. In Les eaux-de-vie Traditionelles d’origine Viticole; Bertrand, A., Ed.; Lavoisier Tec & Doc: Paris, France, 2008; pp. 173–179. [Google Scholar]
- Reg. EU nº 110/2008 of the European Parliament and of the Council of 15 January 2008 on the on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC) No 1576/89; OJEU. L39 European Union. 2008, pp. 16–54. Available online: https://eur-lex.europa.eu/eli/reg/2008/110(1)/oj/por (accessed on 30 October 2023).
- Vogel, A. Darstellung von Benzoesaure aus der Tonka-Boline und aus den MeliIoten-oder Steinklee-Blumen. Ann. Phys. 1820, 64, 161–166. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Uriarte, E.; Santana, L. Simple coumarins: Privileged scaffolds in medicinal chemistry. Front. Med. Chem. Biol. Inter. 2009, 4, 23–85. [Google Scholar] [CrossRef]
- Dean, F.M. Naturally occurring coumarins. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products/Progrès Dans La Chimie Des Substances Organiques Naturelles. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products/Progrès Dans La Chimie Des Substances Organiques Naturelles; Zechmeister, L., Ed.; Springer: Wien, Austria, 1952; Volume 9, pp. 225–291. [Google Scholar] [CrossRef]
- Murray, R.D.H.; Mendez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry; John Wiley & Sons: New York, USA, 1982. [Google Scholar]
- Murray, R.D.H. Naturally Occurring Plant Coumarins. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Steglich, W., Tamm, C., Eds.; Springer: Wien, Austria, 1991; Volume 58, pp. 83–316. [Google Scholar] [CrossRef]
- Keating, G.J.; O’Kennedy, R. The Chemistry and Occurrence of Coumarins, 1st ed.; John Wiley & Sons: West Sussex, UK, 1997. [Google Scholar]
- Iranshahi, M.; Askari, M.; Sahebkar, A.; Hadjipavlou-Litina, D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU 2009, 17, 99–103. Available online: https://ikee.lib.auth.gr/record/226183/files/Litina.pdf (accessed on 10 November 2023).
- O’Kennedy, R.; Thornes, R.D. (Eds.) Coumarins: Biology, Applications, and Mode of Action; JohnWiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Cai, Y.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Murray, R.D.H. Naturally Occurring Plant Coumarins. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer: Wien, Austria, 1997; Volume 72, pp. 1–119. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Tagliapietra, S.; Martina, K.; Palmisanob, G.; Cravotto, G. Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Adv. 2016, 6, 46394–46405. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Progress in the chemistry of naturally occurring coumarins. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer International Publishing: Cham, Swiss, 2017; Volume 106, pp. 241–304. [Google Scholar] [CrossRef]
- Zou, Y.; Teng, Y.; Li, J.; Yan, Y. Recent advances in the biosynthesis of coumarin and its derivatives. Green Chem. Eng. 2023, in press. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie -Phytochimie Plantes Médicinales. Tecnique et Documentation, 2nd ed.; Lavoisier: Paris, France, 1993. [Google Scholar]
- Kai, K.; Mizutani, M.; Kawamura, N.; Yamamoto, R.; Tamai, M.; Yamaguchi, H.; Skata, K.; Shimizu, B. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxolutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 2008, 55, 989–999. [Google Scholar] [CrossRef]
- Kai, K.; Shimizu, B.; Mizutani, M.; Watanabe, K.; Sakata, K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 2006, 67, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Hussain, H.; Hussain, J.; Al-Harrasi, A.; Krohn, K. The chemistry and biology of bicoumarins. Tetrahedron 2012, 68, 2553–2578. [Google Scholar] [CrossRef]
- Basa, S.C. Natural bicoumarins. Phylochemistry 1988, 27, 1933–1941. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2002. [Google Scholar] [CrossRef]
- Hussain, M.I.; Qamar Abbas, S.; Reigosa, M.J. Activities and novel applications of secondary metabolite coumarins. Planta Daninha 2018, 36, e018174040. [Google Scholar] [CrossRef]
- Arndt, F.; Loewe, L.; Ün, R.; Ayça, E. Cumarindiol und Cumarin-Chromon Tautomerie. Chem. Ber. 1951, 84, 319–329. [Google Scholar] [CrossRef]
- Farmer, V.C. Spectra and structure of 4-hydroxycoumarins. Spectrochim. Acta 1959, 15, 870–882. [Google Scholar] [CrossRef]
- Lake, B.G.; Gaudin, H.; Price, R.J.; Walters, D.G. Metabolism of (3-carbon-14) coumarin to polar and covalently bound products by hepatic microsomes from the rat, Syrian hamster, gerbil and humans. Food Chem. Toxicol. 1992, 30, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Katsutani, N.; Maruyama, T.; Kawamura, T.; Yamazaki, H.; Murayama, N.; Tong, W.; Yamazoe, Y.; Hirose, A. Combined risk assessment of food-derived coumarin with in Silico approaches. Food Safety 2022, 10, 73–82. [Google Scholar] [CrossRef]
- Catarino, S.; Thanasi, V.; Morin, G.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Fargeton, L.; Boissier, B.; Canas, S. Shedding light on metals release from chestnut wood to wine spirit using ICP-MS. Foods 2022, 11, 3617. [Google Scholar] [CrossRef]
- Prusty, J.S.; Kumar, A. Coumarins: Antifungal effectiveness and future therapeutic scope. Mol. Divers 2020, 24, 1367–1383. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Fernandes, T.A.; André, V.; Kirillov, A.M. Mild C−H functionalization of alkanes catalyzed by bioinspired copper(II) cores. Org. Biomol. Chem. 2019, 17, 7706–7714. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochemicals—Isolation, characterisation and role in human health. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTechOpen: London, UK, 2015; pp. 113–140. [Google Scholar] [CrossRef]
- Fraissinet-Tachet, L.; Baltz, R.; Chong, J.; Kauffmann, S.; Fritig, B.; Saindrenan, P. Two tobacco genes induced by infection, elicitor and salicylic acid encode glucosyltransferases acting on phenylpropanoids and benzoic acid derivatives, including salicylic acid. FEBS Lett. 1998, 437, 319–323. [Google Scholar] [CrossRef]
- Nitao, J.K.; Zangerl, A.R. Floral development and chemical defense allocation in wild parsnip (Pastinaca sativa). Ecology 1987, 68, 521–529. Available online: http://www.jstor.org/stable/1938457 (accessed on 12 November 2023). [CrossRef]
- Tiwari, R.; Mishra, S.; Danaboina, G.; Jadaun, G.P.S.; Kalaivani, M.; Kalaiselvan, V.; Dhobi, M.; Raghuvanshi, R.S. Comprehensive chemo-profiling of coumarins enriched extract derived from Aegle marmelos (L.) Correa fruit pulp, as an anti-diabetic and anti-inflammatory agent. Saudi Pharm. J. 2023, 31, 101708. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, D.; Pfister, M.; Bork, W.-R.; Toffel-Nadolny, P. HPLC analysis of tonka bean extracts. Z. Lebensm.-Unters. Forsch. 1995, 201, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; van Doorsselaere, J.; Joseleau, J.-P.; Vuylsteke, M.; et al. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Versini, G.; Sarti, S. La presenza di scopoletina come indicatore della modalità di invecchiamento dei distillati. Riv. Vitic. Enol. 1989, 2, 3–10. Available online: https://openpub.fmach.it/retrieve/e1dbfeaa-784b-4ac9-e053-1705fe0a1c61/RIV.%20ENOLOGIA%201989.pdf (accessed on 1 September 2023).
- Canas, S.; Leandro, M.C.; Spranger, M.I.; Belchior, A.P. Influence of botanical species and geographical origin on the content of low molecular weight phenolic compounds of woods used in Portuguese cooperage. Holzforschung 2000, 54, 255–261. [Google Scholar] [CrossRef]
- Latté, K.P.; Kayser, O.; Tan, N.; Kaloga, M.; Kolodziej, H. Unusual coumarin patterns of Pelargonium species forming the origin of the traditional herbal medicine umckaloabo. Z. Naturforsch. C J. Biosci. 2000, 55, 528–533. [Google Scholar] [CrossRef]
- Maier, W.; Schmidt, J.; Nimtz, M.; Wray, V.; Strack, D. Secondary products in mycorrhizal roots of tobacco and tomato. Phytochemistry 2000, 54, 473–479. [Google Scholar] [CrossRef]
- Eckey-Kaltenbach, H.; Ernst, D.; Heller, W.; Sandermann, H., Jr. Biochemical plant responses to ozone (IV. Cross-induction of defensive pathways in parsley (Petroselinum crispum L.) plants). Plant Physiol. 1994, 104, 67–74. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Parry, A.; Tena, M.; Jorrin, J.; Edwards, R. Abiotic elicitation of coumarin phytoalexins in sunflower. Phytochemistry 1995, 38, 1185–1191. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy. A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Sharan, M.; Taguchi, G.; Gonda, K.; Jouke, Y.; Shimosaka, M.; Hayashida, N.; Okazaki, M. Effects of methyl jasmonate and elicitor on the activation of phenylalanine ammonia-lyase and the accumulation of scopoletin and scopolin in tobacco cell cultures. Plant Sci. 1998, 132, 13–19. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. [Google Scholar] [CrossRef]
- de Lira, S.P.; Seleghim, M.H.R.; Williams, D.E.; Marion, F.; Hamill, P.; Jean, F.; Andersen, R.J.; Hajdu, E.; Berlinck, R.G.S. A SARS-coronovirus 3CL protease inhibitor isolated from the marine sponge Axinella cf. corrugata: Structure elucidation and synthesis. J. Braz. Chem. Soc. 2007, 18, 440–443. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Hroboňová, K.; Sádecká, J. Coumarins content in wine: Application of HPLC, fluorescence spectrometry, and chemometric approach. J. Food Sci. Technol. 2020, 57, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, M.R.; Cacco, G.; Sorgonà, A.; Marabottini, R.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. The inhibitory effects of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, cv. Simeto) seeds. J. Chem. Ecol. 2006, 32, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.M.; Kebeish, R. Coumarin impairs redox homeostasis in wheat aleurone layers. J. Plant Res 2018, 131, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bini, A.P.; Rossi, G.D.; Poeschl, Y.; Serra, M.C.D.; Camargo, L.E.A.; Monteiro-Vitorello, C.B.; van Sluys, M.-A.; van Dam, N.M.; Uthe, H.; Creste, S. Molecular, biochemical and metabolomics analyses reveal constitutive and pathogen-induced defense responses of two sugarcane contrasting genotypes against leaf scald disease. Plant Physiol. Biochem. 2023, 203, 108033. [Google Scholar] [CrossRef] [PubMed]
- Cadahia, E.; Muñoz, L.; Fernández de Simón, B.; García-Vallejo, M.C. Changes in low molecular weight phenolic compounds in Spanish, French, and American oak woods during natural seasoning and toasting. J. Agric. Food Chem. 2001, 49, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Smailagić, A.; Veljović, S.; Gašić, U.; Zagorac, D.D.; Stanković, M.; Radotić, K.; Natić, M. Phenolic profile, chromatic parameters and fluorescence of different woods used in Balkan cooperage. Ind. Crops Prod. 2019, 132, 156–167. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Sanz, M.; Cadahía, E.; Poveda, P.; Broto, M. Chemical characterization of oak heartwood from Spanish forests of Quercus pyrenaica (Wild.). Ellagitannins, low molecular weight phenolic, and volatile compounds. J. Agric. Food Chem. 2006, 54, 8314–8321. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahia, E.; Esteruelas, E.; Muñoz, A.M.; Fernández de Simón, B.; Hernández, T.; Estrella, I.; Pinto, E. Phenolic compounds in chestnut (Castanea sativa Mill.) heartwood. Effect of toasting at cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahia, E.; Esteruelas, E.; Muñoz, A.M.; Fernández de Simón, B.; Hernández, T.; Estrella, I. Phenolic compounds in cherry (Prunus avium) heartwood with a view to their use in cooperage. J. Agric. Food Chem. 2010, 58, 4907–4914. [Google Scholar] [CrossRef]
- Masson, G.; Guichard, E.; Fournier, N.; Puech, J.-L. Stereoisomers of b-methyl-g-octalactone. II. Contents in the wood of French (Quercus robur and Quercus petraea) and American (Quercus alba) oaks. Am. J. Enol. Vitic. 1995, 46, 424–428. [Google Scholar] [CrossRef]
- Masson, G.; Moutounet, M.; Puech, J.-L. Ellagitannin content of oak wood as a function of species and of sampling position in the tree. Am. J. Enol. Vit. 1995, 46, 262–268. [Google Scholar] [CrossRef]
- Snakkers, G.; Nepveu, G.; Guilley, E.; Cantagrel, R. Variabilités géographique, sylvicole et individuelle de la teneur en extractibles de chênes sessiles français (Quercus petraea Liebl.): Polyphénols, octalactones et phénols volatils. Ann. For. Sci. 2000, 57, 251–260. [Google Scholar] [CrossRef]
- Doussot, F.; De Jeso, B.; Quideau, S.; Pardon, P. Extractives content in cooperage oak wood during natural seasoning and toasting; influence of tree species, geographic location, and single-tree effects. J. Agric. Food Chem. 2002, 50, 5955–5961. [Google Scholar] [CrossRef] [PubMed]
- Jordão, A.M.; Ricardo da Silva, J.M.; Laureano, O. Ellagitannins from Portuguese oak wood (Quercus pyrenaica Willd.) used in cooperage: Influence of geographical origin, coarseness of the grain and toasting level. Holzforschung 2007, 61, 155–160. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pérez-Coello, M.; Díaz-Maroto, I.J.; Martín-Alvarez, P.J.; Vila-Lameiro, P.; Díaz-Maroto, M.C. Influence of geographical location, site and silvicultural parameters, on volatile composition of Quercus pyrenaica Willd. Wood used in wine aging. For. Ecol. Manag. 2011, 262, 124–130. [Google Scholar] [CrossRef]
- Peng, S.; Scalbert, A.; Monties, B. Insoluble ellagitannins in Castanea sativa and Quercus petraea woods. Phytochemistry 1991, 30, 775–778. [Google Scholar] [CrossRef]
- Hamada, J.; Pétrissans, A.; Mothe, F.; Ruelle, J.; Pétrissans, M.; Gérardin, P. Intraspecific variation of European oak wood thermal stability according to radial position. Wood Sci. Technol. 2017, 51, 785–794. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Variation of wood color and chemical composition in the stem cross-section of oak (Quercus spp.) trees, with special attention to the sapwood-heartwood transition zone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121893. [Google Scholar] [CrossRef]
- Canas, S.; Belchior, A.P.; Spranger, M.I.; Bruno de Sousa, R. HPLC method for the quantification of phenolic acids, phenolic aldehydes, coumarins and furanic derivatives in different kinds of toasted wood used for the ageing of brandies. Anal. Methods 2011, 3, 186–191. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Olvera-Aguirre, G.; Piñeiro-Vázquez, Á.T.; Sanginés-García, J.R.; Sánchez Zárate, A.; Ochoa-Flores, A.A.; Segura-Campos, M.R.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Using plant-based compounds as preservatives for meat products: A review. Heliyon 2023, 9, e17071. [Google Scholar] [CrossRef] [PubMed]
- Piller, N.B. A comparison of the effectiveness of some anti-inflammatory drugs on thermal oedema. Br. J. Exp. Pathol. 1975, 56, 554–560. [Google Scholar] [PubMed]
- Huang, G.J.; Deng, J.S.; Liao, J.C.; Hou, W.C.; Wang, S.Y.; Sung, P.J.; Kuo, Y.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis. J. Agric. Food Chem. 2012, 60, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.I.; Ahn, E.M.; Kim, H.Y.; Park, Y.D. Furanocoumarins from the root of Angelica dahurica. Arch. Pharm. Res. 2000, 23, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, S.; Maggio, A.M.; Faraone, N.; Spadaro, V.; Morris-Natschke, S.L.; Bastow, K.F.; Lee, K.H.; Bruno, M. The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat. Prod. Commun. 2009, 4, 1701–1706. [Google Scholar]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R.; Johnson, R.K.; et al. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.E.F.; Granados, J.Q.; Mir, M.V.; Martinez, M.C.L. Comparison of methods for determining coumarins in distilled beverages. Food Chem. 2000, 70, 251–258. [Google Scholar] [CrossRef]
- Lozhkin, A.V.; Sakanyan, E.I. Structure of chemical compounds, methods of analysis and process control. Natural coumarins: Methods of isolation and analysis. Pharm. Chem. J. 2006, 40, 337–346. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Głowniak, K. Coumarins—Analytical and preparative techniques. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd: Chichester, UK, 2014; pp. 1–26. [Google Scholar] [CrossRef]
- Canas, S.; Belchior, A.P.; Falcão, A.; Gonçalves, J.A.; Spranger, M.I.; Bruno de Sousa, R. Effect of heat treatment on the thermal and chemical modifications of oak and chestnut wood used in brandy ageing. Ciência Téc Vitiv 2007, 22, 5–14. [Google Scholar]
- Lourenço, S.; Anjos, O.; Caldeira, I.; Oliveira Alves, S.; Santos, N.; Canas, S. Natural blending as a novel technology for the production process of aged wine spirits: Potential impact on their quality. Appl. Sci. 2022, 12, 10055. [Google Scholar] [CrossRef]
- Zacaroni, L.M.; Cardoso, M.G.; Saczk, A.A.; de Moraes, A.R.; dos Anjos, J.P.; Machado, A.M.R.; Nelson, D.L. Determination of phenolic compounds and coumarins in sugar cane spirit aged in different species of wood. Anal. Lett. 2011, 44, 2061–2073. [Google Scholar] [CrossRef]
- Bernardes, C.D.; Barbeira, P.J.S. Different chemometric methods for the discrimination of commercial aged cachaças. Food Anal. Methods 2016, 9, 1053–1059. [Google Scholar] [CrossRef]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Služek, V.B.; Cindrić, I.; Molnar, M. Coumarins in food and methods of their determination: Review. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Abu-Mustafa, E.A.; El-Bay, F.K.A.; Fayez, M.B.E. Natural coumarins XI. The distribution of coumarins in the Ammi Majus plant and a possible pattern for their biogenetic evolution. Planta Med. 1970, 18, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Krieger, S.; Hayen, H.; Scmitz, O.J. Quantification of coumarin in cinnamon and woodruff beverages using DIP-APCI-MS and LC-MS. Anal. Bioanal. Chem. 2013, 405, 8337–8345. [Google Scholar] [CrossRef] [PubMed]
- Ballin, N.Z.; Sorensen, A.T. Coumarin content in cinnamon containing food products on the danish market. Food Control 2014, 38, 198–203. [Google Scholar] [CrossRef]
- Moreira, L.M.; de Melo, M.M.; Martis, P.A.; Lyon, J.P.; Romani, A.P.; Codognoto, L.; dos Santos, S.C.; de Oliveira, H.P.M. Photophysical properties of coumarin compounds in neat and binary solvent mixtures: Evaluation and correlation between solvatochromism and solvent polarity parameters. J. Braz. Chem. Soc. 2014, 25, 873–881. [Google Scholar] [CrossRef]
- Shen, Y.; Han, C.; Liu, B.; Lin, Z.; Zhou, X.; Wang, C.; Zhu, Z. Determination of vanillin, ethyl vanillin, and coumarin in infant formula by liquid chromatography-quadrupole linear ion trap mass spectrometry. J. Dairy Sci. 2014, 97, 679–686. [Google Scholar] [CrossRef]
- McAdam, K.; Enos, T.; Goss, C.; Kimpton, H.; Faizi, A.; Edwards, S.; Wright, C.; Porter, A.; Rodu, B. Analysis of coumarin and angelica lactones in smokeless tobacco products. Chem. Cent. J. 2018, 12, 142. [Google Scholar] [CrossRef]
- Arigó, A.; Rigano, F.; Ruuso, M.; Trovato, E.; Dugo, P.; Mondello, L. Dietary intake of coumarins and furocoumarins through citrus beverages: A detailed estimation by HPLC-MS/MS method combined with the linear retention index system. Foods 2021, 10, 1533. [Google Scholar] [CrossRef]
- Aznar, R.; Rodríguez-Pérez, C.; Rai, D.K. Comprehensive characterization and quantification of antioxidant compounds in finger lime (Citrus australasica L.) by HPLC-QTof_MS and UPLC-MS/MS. Appl. Sci. 2022, 12, 1712. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Antunes, A.M.M.; Caldeira, I.; Anjos, O.; de Freitas, V.; Fargeton, L.; Boissier, B.; Catarino, S.; Canas, S. Identification of gallotannins and ellagitannins in aged wine spirits: A new perspective using alternative ageing technology and high-resolution mass spectrometry. Food Chem. 2022, 382, 132322. [Google Scholar] [CrossRef] [PubMed]
- Razuvaeva, Y.G.; Toropova, A.A.; Salchak, S.M.; Olennikov, D.N. Coumarins of Ferulopsis hystrix: LC-MS profiling and gastroprotective and antioxidant activities of skimmin and peucenidin. Appl. Sci. 2023, 13, 9653. [Google Scholar] [CrossRef]
- Ren, Z.; Nie, B.; Liu, T.; Yuan, F.; Feng, F.; Zhang, Y.; Zhou, W.; Xu, X.; Yao, M.; Zhang, F. Simultaneous determination of coumarin and its derivatives in tobacco products by liquid chromatography-tandem mass spectrometry. Molecules 2016, 21, 1511. [Google Scholar] [CrossRef] [PubMed]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.M. Comparative metabolic profiling of olive leaf extracts from twelve different cultivars collected in both fruiting and flowering seasons. Sci. Rep. 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ma, X.; Fedotov, D.; Kjaerulff, L.; Frydenvang, K.; Coriani, S.; Hansen, P.R.; Kongstad, K.T.; Staerk, D. Structure Elucidation of Prenyl- and Geranyl-Substituted Coumarins in Gerbera piloselloides by NMR Spectroscopy, Electronic Circular Dichroism Calculations, and Single Crystal X-ray Crystallography. Molecules 2020, 25, 1706. [Google Scholar] [CrossRef]
- Tabago, M.K.A.G.; Calingacion, M.N.; Garcia, J. Recent advances in NMR-based metabolomics of alcoholic beverages. Food Chem. Mol. Sci. 2021, 2, 100009. [Google Scholar] [CrossRef] [PubMed]
- Hartner, N.T.; Wink, K.; Raddatz, C.R.; Thoben, C.; Schirmer, M.; Zimmermann, S.; Belder, D. Coupling droplet microfluidics with ion mobility spectrometry for monitoring chemical conversions at nanoliter scale. Anal. Chem. 2021, 93, 13615–13623. [Google Scholar] [CrossRef]
- Faleh, A.B.; Warnke, S.; Rizzo, T.R. Combining ultrahigh-resolution ion-mobility spectrometry with cryogenic infrared spectroscopy for the analysis of glycan mixtures. Anal. Chem. 2019, 91, 4876–4882. [Google Scholar] [CrossRef]
- Salgado, A. Recent advances in nuclear magnetic resonance spectroscopy detection compatible with on-flow operational regimes: New uses of NMR detectors hyphenated to LCs and other separation techniques and in reaction monitoring. In Handbooks in Separation Science, Liquid Chromatography, 3rd ed.; Fanali, S., Chankvetadze, B., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: London, UK, 2023; Volume 1, pp. 743–794. [Google Scholar] [CrossRef]
- Gawad, S.A.A.; Sakr, M.A.S. Spectroscopic investigation, DFT and TD-DFT calculations of 7-(Diethylamino) Coumarin (C466). J. Mol. Struct. 2022, 1248, 131413. [Google Scholar] [CrossRef]
- Rampogu, S.; Shaik, M.R.; Khan, M.; Khan, M.; Hwan Oh, T.; Shaik, B. CBPDdb: A curated database of compounds derived from Coumarin–Benzothiazole–Pyrazole. Database 2023, 2023, baad062. [Google Scholar] [CrossRef]
- Chen, H.M.; Nasseri, S.A.; Rahfeld, P.; Wardman, J.F.; Kohsiek, M.; Withers, S.G. Synthesis and evaluation of sensitive coumarin-based fluorogenic substrates for discovery of α-N-acetyl galactosaminidases through droplet-based screening. Org. Biomol. Chem. 2021, 19, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Kapoor, A.; Kumar, P.S.; Ponnuchamy, M.; Sivasamy, B.; Vo, D.N. Lab-on-a-chip technologies for food safety, processing, and packaging applications: A review. Environ. Chem. Lett. 2022, 20, 901–927. [Google Scholar] [CrossRef]
- Corbion, C.; Smith-Ravin, J.; Marcelin, O.; Bouajila, J. An overview of spirits made from sugarcane juice. Molecules 2023, 28, 6810. [Google Scholar] [CrossRef]
- Dhiman, A.K.; Attri, S. Production of brandy. In Handbook of Enology: Principles, Practices and Recent Innovations; Josh, V.K., Ed.; Asiatech Publisher, Inc.: New Delhi, India, 2011. [Google Scholar]
- Roullier-Gall, C.; Signoret, J.; Hemmler, D.; Witting, M.A.; Kanawati, B.; Schäfer, B.; Gougeon, R.D.; Schmitt-Koppli, P. Usage of FT-ICR-MS metabolomics for characterizing the chemical signatures of barrel-aged whisky. Front. Chem. 2018, 6, 29. [Google Scholar] [CrossRef]
- Quesada Granados, J.; Merelo Guervós, J.J.; Oliveras López, M.J.; González Penãlver, J.; Olalla Herrera, M.; Blanca Herrera, R.; López Martinez, M.C. Application of artificial aging techniques to samples of rum and comparison with traditionally aged rums by analysis with artificial neural nets. J. Agric. Food Chem. 2002, 50, 1470–1477. [Google Scholar] [CrossRef]
- Aylot, R.I.; Clyne, A.H.; Fox, A.P.; Walker, D.A. Analytical strategies to confirm Scotch whisky authenticity. Analyst 1994, 119, 1741–1746. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Puech, J.-L. Wood maturation of distilled beverages. Trends Food Sci. Technol. 1998, 9, 95–101. [Google Scholar] [CrossRef]
- Canas, S.; Vaz, M.; Belchior, A.P. Influence de la dimension du fût dans les cinétiques d’extraction/oxydation des composés phénoliques du bois pour les eaux-de-vie Lourinhã. In Les Eaux-de-vie Traditionnelles D’origine Viticole; Bertrand, A., Ed.; Lavoisier—Tec & Doc: Paris, France, 2008; pp. 143–146. [Google Scholar]
- Bettin, S.; Isique, W.; Franco, D.; Andersen, M.; Knudsen, S.; Skibsted, L. Phenols and metals in sugar-cane spirits. Quantitative analysis and effect on radical formation and radical scavenging. Eur. Food Res. Technol. 2002, 215, 169–175. [Google Scholar] [CrossRef]
- Dos Anjos, J.P.; das Graças Cardoso, M.; Saczk, A.A.; Dórea, H.S.; Santiago, W.D.; Machado, A.M.R.; Zacaroni, L.M.; Nelson, D.L. Evolution of the concentration of phenolic compounds in cachaça during aging in an oak (Quercus sp.) barrel. J. Braz. Chem. Soc. 2011, 22, 1307–1314. [Google Scholar] [CrossRef]
- Nishimura, K.; Matsuyama, R. Maturation and maturation chemistry. In The science and Technology of Whiskies; Piggott, J.R., Sharp, R., Duncan, R.E.B., Eds.; Longman Scientific & Technical: Essex, UK, 1989; pp. 235–263. [Google Scholar]
- Sarni, F.; Moutounet, M.; Puech, J.-L.; Rabier, P. Effect of heat treatment of oak wood extractable compounds. Holzforschung 1990, 44, 461–466. [Google Scholar] [CrossRef]
- Le Floch, A.; Jourdes, M.; Teissedre, P.-L. Polysaccharides and lignin from oak wood used in cooperage: Composition, interest, assays: A review. Carbohydr. Res. 2015, 417, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.D.; McCafferty, K.; Larmie, E.; Newton, J.; Swan, J.S. The influence of oak seasoning and toasting parameters on the composition and quality of wine. Am. J. Enol. Vitic. 1999, 50, 495–502. [Google Scholar] [CrossRef]
- Acuña, L.; Gonzalez, D.; de la Fuente, J.; Moya, L. Influence of toasting treatment on permeability of six wood species for enological use. Holzforschung 2014, 68, 447–454. [Google Scholar] [CrossRef]
- Collins, T.S.; Miles, J.L.; Boulton, R.B.; Ebeler, S.E. Targeted volatile composition of oak wood samples taken during toasting at a commercial cooperage. Tetrahedron 2015, 71, 2971–2982. [Google Scholar] [CrossRef]
- Clyne, J.; Conner, J.M.; Paterson, A.; Piggott, J.R. The effect of cask charring on Scotch whisky maturation. Int. J. Food Sci. Technol. 1993, 28, 69–81. [Google Scholar] [CrossRef]
- Whiters, S.J.; Piggott, J.R.; Leroy, G.; Conner, J.M.; Paterson, A. Factors affecting pungency of malt distillates and ethanol-water mixtures. J. Sens. Studies 1995, 10, 273–283. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; MacNamara, K. Characterization of sulfur compounds in whisky by full evaporation dynamic headspace and selectable one-dimensional retention time locked gas chromatography-mass. J. Chromat. A 2012, 1270, 296–304. [Google Scholar] [CrossRef]
- Fengel, D.; Wegner, G. Wood. Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989; pp. 1–612. [Google Scholar]
- Gagić, T.; Knez, Z.; Skerget, M. Hydrothermal hydrolysis of sweet chestnut (Castanea sativa) tannins. J. Serbian Chem. Soc. 2019, 84, 1–14. [Google Scholar] [CrossRef]
- Sampaio, P.A.; Serafim, S.C.; Nascimento Menezes, P.M.; Valenca Pereira, E.C.; Sousa de Sá, P.G.; Teixeira de Alencar Filho, J.M.; Gonçalves de Oliveira Júnior, R.; Rolim Neto, P.J.; da Silva, J.M.; Rolim, L.A. Development and characterization of the zeolite imidazolate framework for a modified release of the drug scopoletin. J. Drug Deliv. Sci. Technol. 2022, 61, 102131. [Google Scholar] [CrossRef]
- Sun, C.; Huang, Z.; Liu, L.; Li, M.; Zheng, H. Umbelliferone as a Small Molecular Peroxidase Mimic towards Sensitive Detection of H2O2 and Glucose. Anal. Sci. 2018, 34, 933–938. [Google Scholar] [CrossRef]
- Canas, S.; Belchior, A.P.; Mateus, A.M.; Spranger, M.I.; Bruno de Sousa, R. Kinetics of impregnation/evaporation and release of phenolic compounds from wood to brandy in experimental model. Ciência Téc Vitiv 2002, 17, 1–14. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Salagoity-Auguste, M.-H. La scopolétine: Un marqueur de la conservation en fûts de chêne. Vigne Vin Publ. Intern. 1992, 105–113. [Google Scholar]
- Ceccherini, G.; Duveiller, G.; Grassi, G.; Lemoine, G.; Avitabile, V.; Pilli, R.; Cescatti, A. Abrupt increase in harvested forest area over Europe after 2015. Nature 2020, 583, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Caldeira, I.; Belchior, A.P. Comparison of alternative systems for the ageing of wine brandy. Wood shape and wood botanical species effect. Ciência Tec. Vitiv. 2009, 24, 91–99. [Google Scholar]
- Bortoletto, A.M.; Alcarde, A.R. Aging marker profile in cachaça is influenced by toasted oak chips. J. Inst. Brew. 2015, 121, 70–77. [Google Scholar] [CrossRef]
- Caldeira, I.; Anjos, O.; Belchior, A.P.; Canas, S. Sensory impact of alternative ageing technology for the production of wine brandies. Ciência Tec. Vitiv. 2017, 32, 12–22. [Google Scholar] [CrossRef]
- Taloumi, T.; Makris, D.P. Accelerated aging of the traditional Greek distillate Tsipouro using wooden chips. Part I: Effect of static maceration vs. ultrasonication on the polyphenol extraction and antioxidant activity. Beverages 2017, 3, 5. [Google Scholar] [CrossRef]
- Granja-Soares, J.; Roque, R.; Cabrita, M.J.; Anjos, O.; Belchior, A.P.; Caldeira, I.; Canas, S. Effect of innovative technology using staves and micro-oxygenation on the sensory and odorant profile of aged wine spirit. Food Chem. 2020, 333, 127450. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Jain, S.; Singh, J. Nutraceutical—Medicine of the future. J. Glob. Biosci. 2015, 4, 2790–2794. [Google Scholar]
- McClements, D.J.; Xiao, H. Designing food structure and composition to enhance nutraceutical bioactivity to support cancer inhibition. Semin. Cancer Biol. 2017, 46, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Alves, S.C.; Serra, A.T.; Bronze, M.R. Food matrices that improve the oral bioavailability of pharmaceuticals and nutraceuticals. In Nutraceuticals and Natural Product Pharmaceuticals; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 197–233. [Google Scholar] [CrossRef]
- Holmes, M.V.; Dale, C.E.; Zuccolo, L.; Silverwood, R.J.; Guo, Y.; Ye, Z.; Prieto-Merino, D.; Dehghan, A.; Trompet, S.; Wong, A.; et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014, 349, 4164. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, S2057–S2069. [Google Scholar] [CrossRef]
- Da Porto, C.; Calligaris, S.; Celotti, E.; Nicoli, M.C. Antiradical properties of commercial cognacs assessed by the DPPH(.) test. J. Agric. Food Chem. 2000, 48, 4241–4245. [Google Scholar] [CrossRef] [PubMed]
- Duthie, G.G.; Pedersen, M.W.; Gardner, P.T.; Morrice, P.C.; Jenkinson, A.; McPhail, D.B.; Steele, G.M. The effect of whisky and wine consumption on total phenol content and antioxidant capacity of plasma from healthy volunteers. Eur. J. Clin. Nutr. 1998, 52, 733–736. [Google Scholar] [CrossRef]
- Suzuki, K.; Nemoto, A.; Tanaka, I.; Koshimizu, S.; Suwa, Y.; Ishihara, H. Induction of heme oxygenase-1 by whisky congeners in human endothelial cells. J. Food Sci. 2010, 75, H163–H166. [Google Scholar] [CrossRef]
- Owuor, E.D.; Kong, A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002, 64, 765–770. [Google Scholar] [CrossRef]
- Piggott, J.R. Whisky. In Current Developments in Biotechnology and Bioengineering. Food and Beverages Industry; Pandey, A., Sanromán, M.A., Du, G., Soccol, C.R., Dussap, C.-G., Eds.; Elsevier: London, UK, 2017; pp. 435–450. [Google Scholar] [CrossRef]
- Duriez, P.; Cren, C.; Luc, G.; Fruchart, J.C.; Rolando, C.; Teissier, E. Ingestion of cognac significantly increases plasma phenolic and ellagic acid concentrations and plasma antioxidant capacity in humans. In Proceedings of 26th World Congress of OIV—Section Wine and Health; International Organisation of Vine and Wine: Paris, France, 2001; pp. 358–369. [Google Scholar]
- Umar, A.; Guerin, V.; Renard, M.; Boisseau, M.; Garreau, C.; Begaud, B.; Molimard, M.; Moore, N. Effects of Armagnac extracts on human platelet function in vitro and on rat arteriovenous shunt thrombosis in vivo. Thromb. Res. 2003, 110, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.P.; Pereira, T.M.; Vitorio, F.; Nadur, N.F.; Lacerda, R.B.; Kümmerle, A.E. A importância das cumarinas para a química medicinal e o desenvolvimento de compostos bioativos nos últimos anos. Quim. Nova 2021, 44, 180–197. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ding, Y.; Yan, X.T.; Kim, Y.-H.; Jan, H.-D. Scopoletin and scopolin isolated from Artemisia iwayomogi suppress differentiation of osteoclastic macrophage RAW 264.7 cells by scavenging reactive oxygen species. J. Nat. Prod. 2013, 76, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Fang, F.; Song, M.; Li, R.; Ma, Z.; Ma, S. Umbelliferone reverses depression-like behavior in chronic unpredictable mild stress-induced rats by attenuating neuronal apoptosis via regulating ROCK/Akt pathway. Behav. Brain Res. 2017, 317, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Tong, W.; Cui, Y.; Li, X.; Sun, H. Umbelliferone alleviates lipopolysaccharide-induced inflammatory responses in acute lung injury by down-regulating TLR4/MyD88/NF-κB signaling. Inflammation 2019, 42, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Umbelliferone modulates depression-like symptoms by altering monoamines in a rat post-traumatic stress disorder model. J. Nat. Med. 2020, 74, 377–386. [Google Scholar] [CrossRef]
- Naowaboot, J.; Somparn, N.; Saentaweesuk, S.; Pannangpetch, P. Umbelliferone improves an impaired glucoseand lipid metabolism in high-fat diet/streptozotocin-induced type 2 diabetic rats. Phytother. Res. 2015, 29, 1388–1395. [Google Scholar] [CrossRef]
- Ramesh, B.; Pugalendi, K.V. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J. Med. Food 2006, 9, 562–566. [Google Scholar] [CrossRef]
- Yu, S.M.; Hu, D.H.; Zhang, J.J. Umbelliferone exhibits anticancer activity via the induction of apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 3869–3873. [Google Scholar] [CrossRef]
- Jesus, R.L.C.; Silva, I.L.P.; Araújo, F.A.; Moraes, R.A.; Silva, L.B.; Brito, D.S.; Lima, G.B.C.; Alves, Q.L.; Silva, D.F. 7-Hydroxycoumarin induces vasorelaxation in animals with essential hypertension: Focus on potassium channels and intracellular Ca2+ mobilization. Molecules 2022, 27, 7324. [Google Scholar] [CrossRef]
- Hajime, M.; Shuichi, Y.; Makoto, N.; Masanori, Y.; Ikuko, K.; Atsushi, K.; Mutsuo, S.; Keiichi, T. Inhibitory effect of 4-methylesculetin on hyaluronan synthesis slows the development of human pancreatic cancer in vitro and in nude mice. Int. J. Cancer 2007, 120, 2704–2709. [Google Scholar] [CrossRef]
- Urakawa, H.; Nishida, Y.; Wasa, J.; Arai, E.; Zhuo, L.; Kimata, K.; Kozawa, E.; Futamura, N.; Ishiguro, N. Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int. J. Cancer 2012, 130, 454–466. [Google Scholar] [CrossRef]
- Edward, M.; Quinn, J.A.; Pasonen-Seppänen, S.M.; McCann, B.A.; Tammi, R.H. 4-Methylumbelliferone inhibits tumour cell growth and the activation of stromal hyaluronan synthesis by melanoma cell-derived factors. Br. J. Dermatol. 2010, 162, 1224–1232. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Nikolskaya, A.I.; Goriainov, S.V.; Astakhova, A.A.; Sergeeva, M.G. Inhibitor of hyaluronic acid synthesis 4-methylumbelliferone as an anti-inflammatory modulator of LPS-mediated astrocyte responses. Int. J. Mol. Sci. 2020, 21, 8203. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.-O.; Ham, J.R.; Lee, H.-I.; Seo, K.-I.; Lee, M.-K. Long-term supplementation of umbelliferone and 4-methylumbelliferone alleviates high-fat diet induced hypertriglyceridemia and hyperglycemia in mice. Chem. Biol. Interact. 2014, 216, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Tosun, F.; Sener, B. Coumarin, anthroquinone and stilbene derivatives with anticholinesterase activity. Z. Naturforsch. C J. Biosci. 2008, 63, 366–370. [Google Scholar] [CrossRef]
- Shin, E.; Choi, K.-M.; Yoo, H.-S.; Lee, C.-K.; Hwang, B.Y.; Lee, M.K. Inhibitory effects of coumarins from the stem barks of Fraxinus rhynchophylla on adipocyte differentiation in 3T3-L1 cells. Biol. Pharm. Bull. 2010, 33, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, D.; Ashokkumar, N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 2013, 95, 366–373. [Google Scholar] [CrossRef]
- Hsia, C.-W.; Lin, K.-C.; Lee, T.-Y.; Hsia, C.-H.; Chou, D.-S.; Jayakumar, T.; Velusamy, M.; Chang, C.-C.; Sheu, J.-R. Esculetin, a coumarin derivative, prevents thrombosis: Inhibitory signaling on PLCγ2–PKC–AKT activation in human platelets. Int. J. Mol. Sci. 2019, 20, 2731. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Lee, H.-J.; Hu, C.-C.; Shun, H.-I.; Tseng, T.-H. Enhancement of esculetin on Taxol-induced apoptosis in human hepatoma HepG2 cells. Toxicol. Appl. Pharmacol. 2006, 210, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kok, S.H.; Yeh, C.C.; Chen, M.L.; Kuo, M.Y.P. Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation in human oral cancer SAS cells. Oral Oncol. 2009, 45, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; Di Stasi, L.C. Intestinal antiinflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chem. Biol. Interact. 2010, 186, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Li, L.; Yu, H.; Wu, Y.; Li, H. Coumarins ameliorate diabetogenic action of dexamethasone via Akt activation and AMPK signaling in skeletal muscle. J. Pharmacol. Sci. 2019, 139, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Qin, S.; Zhou, S.; Li, J.; Gao, Y. Esculin ameliorates cognitive impairment in experimental diabetic nephropathy and induces anti-oxidative stress and anti-inflammatory effects via the MAPK pathway. Mol. Med. Rep. 2018, 17, 7395–7402. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, W.; Jung, Y.; Lee, J.H.; Lee, S.; Eom, D.W.; Jeon, Y.; Yoo, H.H.; Jin, M.J.; Song, K.I.; et al. Protective effect of esculin on streptozotocin-induced diabetic renal damage in mice. J. Agric. Food Chem. 2014, 62, 2069–2076. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wang, X.; Zhang, H.; He, Z.; Zhi, W.; Liu, F.; Niu, X. Gastroprotective effect of esculin on ethanol-induced gastric lesion in mice Weifeng. Fundam. Clin. Pharmacol. 2017, 31, 174–184. [Google Scholar] [CrossRef]
- Zhang, M.; Xin, X.; Lai, F.; Zhang, X.; Li, X.; Wu, H. Cellular tansport of esculin and its acylated derivatives in Caco-2 Cell monolayers and their antioxidant properties in vitro. J. Agric. Food Chem. 2017, 65, 7424–7432. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Y.; Li, W.; Zhang, H.; Wang, X.; Mu, Q.; He, Z.; Yao, H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int. Immunopharmacol. 2015, 29, 779–786. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wang, X.; He, Z.; Liu, F.; Zhi, W.; Zhang, H.; Niu, X. Esculin attenuates endotoxin shock induced by lipopolysaccharide in mouse and NO production in vitro through inhibition of NF-κB activation. Eur. J. Pharmacol. 2016, 791, 726–734. [Google Scholar] [CrossRef]
- Liu, A.; Shen, Y.; Du, Y.; Chen, J.; Pei, F.; Fu, W.; Qiao, J. Esculin prevents lipopolysaccharide/D-galactosamine-induced acute liver injury in mice. Microb. Pathog. 2018, 125, 418–422. [Google Scholar] [CrossRef]
- Pari, L.; Rajarajeswari, N. Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chem. Biol. Interact. 2009, 181, 292–296. [Google Scholar] [CrossRef]
- Abul Qais, F.; Ahmad, I. Mechanism of non-enzymatic antiglycation action by coumarin: A biophysical study. New J. Chem. 2019, 43, 12823. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Huang, Y.F.; Lu, H.F.; Ho, H.C.; Yang, J.S.; Li, T.M.; Chang, N.W.; Chung, J.G. Coumarin induces cell cycle arrest and apoptosis in human cervical cancer HeLa cells through a mitochondria and caspase-3 dependent mechanism and NF-κB down-regulation. In Vivo 2007, 21, 1003–1009. Available online: https://iv.iiarjournals.org/content/invivo/21/6/1003.full.pdf (accessed on 2 December 2023).
- Elinos-Báez, C.M.; León, F.; Santos, E. Effects of coumarin and 7OH-coumarin on bcl-2 and Bax expression in two human lung cancer cell lines in vitro. Cell Biol Int. 2005, 29, 703–708. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, J.S.; Prado-Garcia, H.; Aguilar-Cazares, D.; Molina-Guarneros, J.A. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer 2004, 43, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Seito, L.N.; Witaicenis, A.; Pellizzon, C.H.; Di Stasi, L.C. Intestinal anti-inflammatory activity of coumarin and 4-hydroxycoumarin in the trinitrobenzenesulphonic acid model of rat colitis. Biol. Pharm. Bull. 2008, 31, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Napiroon, T.; Bacher, M.; Balslev, H.; Tawaitakham, K.; Santimaleeworagun, W.; Vajrodaya, S. Scopoletin from Lasianthus lucidus Blume (Rubiaceae): A potential antimicrobial against multidrug-resistant Pseudomonas aeruginosa. J. Appl. Pharmaceut. Sci. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Armenia, A.; Hidayat, R.; Meiliani, M.; Yuliandra, Y. Blood pressure lowering effect of scopoletin on oxidative stress-associated hypertensive rats. J. Res. Pharm. 2019, 23, 249–258. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, J.E.; Han, J.S. Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur. J. Pharmacol. 2018, 834, 152–156. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, J.E.; Han, J.S. Scopoletin increases glucose uptake through activation of PI3K and AMPK signaling pathway and improves insulin sensitivity in 3T3-L1 cells. Nutr. Res. 2020, 74, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jamuna, S.; Karthika, K.; Paulsamy, S.; Thenmozhi, K.; Kathiravan, S.; Venkatesh, R. Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Ind. Crop Prod. 2015, 70, 221–230. [Google Scholar] [CrossRef]
- Nam, H.; Kim, M.-M. Scopoletin has a potential activity for anti-aging via autophagy in human lung fibroblasts. Phytomedicine 2015, 22, 362–368. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural coumarins: Exploring the pharmacological complexity and underlying molecular mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, X.; Wang, X.; Jiang, Z.; Qiu, Z. Design, synthesis, and bioavailability evaluation of coumarin-based prodrug of meptazinol. Bioorganic Med. Chem. Lett. 2005, 15, 4953–4956. [Google Scholar] [CrossRef]
- Escudero, G.E.; Laino, C.H.; Echeverría, G.A.; Piro, O.E.; Martini, N.; Rodríguez, A.N.; Martínez Medina, J.J.; López Tévez, L.L.; Ferrer, E.G.; Williams, P.A.M. Improving the antidepressant action and the bioavailability of sertraline by co-crystallization with coumarin 3-carboxylate. Structural determination. Chem. Biol. Interact. 2016, 249, 46–55. [Google Scholar] [CrossRef]
- Tripathi, A.; Misra, K. Inhibition of P-glycoprotein mediated efflux of Paclitaxel by coumarin derivatives in cancer stem cells: An in silico approach. Comb. Chem. High Throughput Screen. 2016, 19, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Tagde, P.; Ahmed, Z.; Khan, F.S.; et al. Multidrug resistance of cancer cells and the vital role of P-glycoprotein. Life 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Hyohdoh, I.; Furuichi, N.; Ozawa, S.; Watanabe, F.; Matsushita, M.; Sakaitani, M.; Ori, K.; Takanashi, K.; Harada, N.; et al. The sulfamide moiety affords higher inhibitory activity and oral bioavailability to a series of coumarin dual selective RAF/MEK inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 6223–6227. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, W. Coumarins. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 293–297. [Google Scholar] [CrossRef]
- Clark, G.S. Coumarin. An aroma chemical profile. Perfum. Flavor. 1995, 20, 23–34. Available online: https://img.perfumerflavorist.com/files/base/allured/all/document/2016/02/pf.9545.pdf (accessed on 10 December 2023).
- Hazleton, L.W.; Murer, H.K.; Thiessen, R., Jr.; Tusing, T.W.; Zeitlin, B.R. Toxicity of coumarin. J. Pharmacol. Exp. Ther. 1956, 118, 348–358. Available online: https://jpet.aspetjournals.org/content/118/3/348.long (accessed on 10 December 2023). [PubMed]
- Carlton, B.D.; Aubrun, J.-C.; Simon, G.S. Effects of coumarin following perinatal and chronic exposure in Sprague-Dawley rats and CD-1 mice. Fundam. Appl. Toxicol. 1996, 30, 145–151. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Monographs on fragrance raw materials: Coumarin. Food Chem. Toxicol. 1974, 12, 385–388. [Google Scholar] [CrossRef]
- Kitagawa, H.; Iwaki, R. Coumarin derivatives for medicinal purposes. XVII Yakugaku Zasshi 1963, 83, 1124–1128. [Google Scholar] [CrossRef]
- Shimamoto, K.; Takaori, S. Pharmakologische untersuchungen mit einem Melilotus-extrakt [Pharmacologic studies with a Melilotus extract]. Arzneimittelforschung 1965, 15, 897–899. [Google Scholar]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Cook, E.L.; Fitzhugh, O.G. Food flavourings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol. 1964, 2, 327–343. [Google Scholar] [CrossRef]
- Nieschulz, O.; Schmersahl, P. Uber choleretische wirkstoffe aus Artemisia abrotanum L [On choleretic agents from Artemisia abrotanum L]. Arzneimittelforschung 1968, 18, 1330–1336. [Google Scholar] [PubMed]
- Mishkinsky, J.S.; Goldschmied, A.; Joseph, B.; Ahronson, Z.; Sulman, F.G. Hypoglycaemic effect of Trigonella foenum graecum and Lupinus termis (leguminosae) seeds and their major alkaloids in alloxan-diabetic and normal rats. Arch. Int. Pharmacodyn. Ther. 1974, 210, 27–37. [Google Scholar] [PubMed]
- Kiso to Rinsho. Clinical Report; Yubunsha Co., Ltd.: Tokyo, Japan, 1971; Volume 5, p. 1619. [Google Scholar]
- PHTXA6 Pharmacology and Toxicology. In English translation of FATOAO; Academic Press: New York, NY, USA,, 1958; Volume 21, p. 555.
- Yamagami, I.; Suzuki, Y.; Ito, K. Pharmacological studies on the components of Fraxinus japonica Blume. Nihon Yakurigaku Zasshi. 1968, 64, 714–729. [Google Scholar] [PubMed]
- Pencheva, I.; Kostovs, I.; Konstantinov, S.; Naidenova, E.; Karaivanova, M.; Manolov, I. Cardioprotective efficacy of new esculin metal complexes. Acta Pharm. 1998, 48, 127–131. [Google Scholar]
- United Nations (ONU). United Nations Purple Book—Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 8th ed.; ONU: New York, NY, USA; Geneva, Switzerland, 2019; p. 117. Available online: https://unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev08/ST-SG-AC10-30-Rev8e.pdf (accessed on 22 November 2023).
- Lindberg, R.L.; Negishi, M. Alteration of mouse cytochrome P450coh substrate specificity by mutation of a single amino-acid residue. Nature 1989, 339, 632–634. [Google Scholar] [CrossRef]
- Raunio, H.; Rahnasto-Rilla, M. CYP2A6: Genetics, structure, regulation, and function: Review. Drug Metab. Drug Interact. 2012, 27, 73–88. [Google Scholar] [CrossRef]
- Pelkonen, O.; Rautio, A.; Raunio, H.; Pasanen, M. CYP2A6: A human coumarin 7-hydroxylase. Toxicology 2000, 144, 139–147. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the European Commission on Coumarin in flavourings and other food ingredients with flavouring properties. EFSA J. 2008, 793, 1–15. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/793 (accessed on 22 November 2023).
- Abraham, K.; Wöhrlin, F.; Lindtner, O.; Heinemeyer, G.; Lampen, A. Toxicology and risk assessment of coumarin: Focus on human data. Mol. Nutr. Food Res. 2010, 54, 228–239. [Google Scholar] [CrossRef]
- Ehlers, D.; Hilmer, S.; Bartholomae, S. Hochdruckflüssigchromatographische Untersuchung von Zimt-CO2-Hochdruckextrakten im Vergleich zu Zimtölen. Z. Für Lebensm. -Unters. Und-Forsch. 1995, 200, 282–288. [Google Scholar] [CrossRef]
- Nykänen, L.; Nykänen, I.; Moring, M. Aroma compounds dissolved from oak chips by alcohol. In Progress in Flavour Research; Adda, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 339–346. [Google Scholar]
- Reg. (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJEU L354 European Union. 2008, pp. 34–50. Available online: http://data.europa.eu/eli/reg/2008/1334/oj (accessed on 22 November 2023).
- Popping, B.; Buck, N.; Bánáti, D.; Brereton, P.; Gendel, S.; Hristozova, N.; Chaves, S.M.; Saner, S.; Spink, J.; Willis, C.; et al. Food inauthenticity: Authority activities, guidance for food operators, and mitigation tools. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4776–4811. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Martins, A.R.; Talhavini, M.; Vieira, M.L.; Zacca, J.J.; Braga, J.W.B. Discrimination of whisky brands and counterfeit dentification by UV–Vis spectroscopy and multivariate data analysis. Food Chem. 2017, 229, 142–151. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Boqué, R.; Borràs, E.; Busto, O.; Wardencki, W.; Namieśnik, J.; Dymerski, T. Authentication of whisky due to its botanical origin and way of production by instrumental analysis and multivariate classification methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 849–853. [Google Scholar] [CrossRef]
- Kiefer, J.; Cromwell, A.L. Analysis of single malt Scotch whisky using Raman spectroscopy. Anal. Methods 2017, 9, 511–518. [Google Scholar] [CrossRef]
- Žiak, Ľ.; Sádecká, J.; Májek, P.; Hroboňová, K. Simultaneous determination of phenolic acids and scopoletin in brandies using synchronous fluorescence spectrometry coupled with partial least squares. Food Anal. Methods 2014, 7, 563–570. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Skorobogatova, N.; Chibisova, M.; Budnikov, H. New electrochemistry-based approaches to brandy quality evaluation using antioxidant parameters. Food Anal. Methods 2015, 8, 1794–1803. [Google Scholar] [CrossRef]
- Stupak, M.; Goodall, I.; Tomaniova, M.; Pulkrabova, J.; Hajslova, J. A novel approach to assess the quality and authenticity of Scotch whisky based on gas chromatography coupled to high resolution mass spectrometry. Anal. Chim. Acta 2018, 1042, 60–70. [Google Scholar] [CrossRef]
- Heinz, H.A.; Elkins, J.T. Comparison of unaged and barrel aged whiskies from the same Mash Bill using gas chromatography/mass spectrometry. J. Brew. Distilling 2019, 8, 1–6. [Google Scholar] [CrossRef]
- Yang, K.; Somogyi, A.; Thomas, C.; Zhang, H.; Cheng, Z.; Xu, S.; Miller, C.; Spivey, D.; Blake, C.; Smith, C.; et al. Analysis of barrel-aged Kentucky Bourbon whiskey by ultrahigh resolution mass spectrometry. Food Anal. Methods 2020, 13, 2301–2311. [Google Scholar] [CrossRef]
- Heller, M.; Vitali, L.; Oliveira, M.A.L.; Costa, A.C.O.; Micke, G.A. A rapid sample screening method for authenticity control of whiskey using capillary electrophoresis with online preconcentration. J. Agric. Food Chem. 2011, 59, 6882–6888. [Google Scholar] [CrossRef]
- Fotakis, C.; Zervou, M. NMR metabolic fingerprinting and chemometrics driven authentication of Greek grape marc spirits. Food Chem. 2016, 196, 760–768. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Kuballa, T.; Lachenmeier, D.W. Nontargeted NMR analysis to rapidly detect hazardous substances in alcoholic beverages. Appl. Magn. Reson. 2012, 42, 343–352. [Google Scholar] [CrossRef]
- Kuballa, T.; Hausler, T.; Okaru, A.O.; Neufeld, M.; Abuga, K.O.; Kibwage, I.O.; Rehm, J.; Luy, B.; Walch, S.G.; Lachenmeier, D.W. Detection of counterfeit brand spirits using 1H NMR fingerprints in comparison to sensory analysis. Food Chem. 2018, 245, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tosato, F.; Correia, R.M.; Oliveira, B.G.; Fontes, A.M.; Franca, H.S.; Coltro, W.K.; Romao, W. Paper spray ionization mass spectrometry allied to chemometric tools for quantification of whisky adulteration with additions of sugarcane spirit. Anal. Methods 2018, 10, 1952–1960. [Google Scholar] [CrossRef]
- Moller, J.K.; Catharino, R.R.; Eberlin, M.N. Electrospray ionization mass spectrometry fingerprinting of whisky: Immediate proof of origin and authenticity. Analyst 2005, 130, 890–897. [Google Scholar] [CrossRef][Green Version]
- Kew, W.; Mackay, C.L.; Goodall, I.; Clarke, D.; Uhrín, D. Complementary ionization techniques for the analysis of Scotch whisky by high resolution mass spectrometry. Anal. Chem. 2018, 90, 11265–11272. [Google Scholar] [CrossRef]
- Stupak, M.; Kocourek, V.; Kolouchova, I.; Hajslova, J. Rapid approach for the determination of alcoholic strength and overall quality check of various spirit drinks and wines using GC e MS. Food Control 2017, 80, 307–313. [Google Scholar] [CrossRef]
- Kelly, T.J.; O’Connor, C.; Kilcawley, K.N. Sources of Volatile Aromatic Congeners in whiskey. Beverages 2023, 9, 64. [Google Scholar] [CrossRef]
- Panossian, A.; Mamikonyan, G.; Torosyan, M.; Gabrielyan, E.; Mkhitaryan, S. Analysis of Aromatic Aldehydes in brandy and wine by high-performance capillary electrophoresis. Anal. Chem. 2001, 73, 4379–4383. [Google Scholar] [CrossRef]
- Rentzsch, M.; Wilkens, A.; Winterhalter, P. Non-flavonoid phenolic compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 509–527. [Google Scholar] [CrossRef]

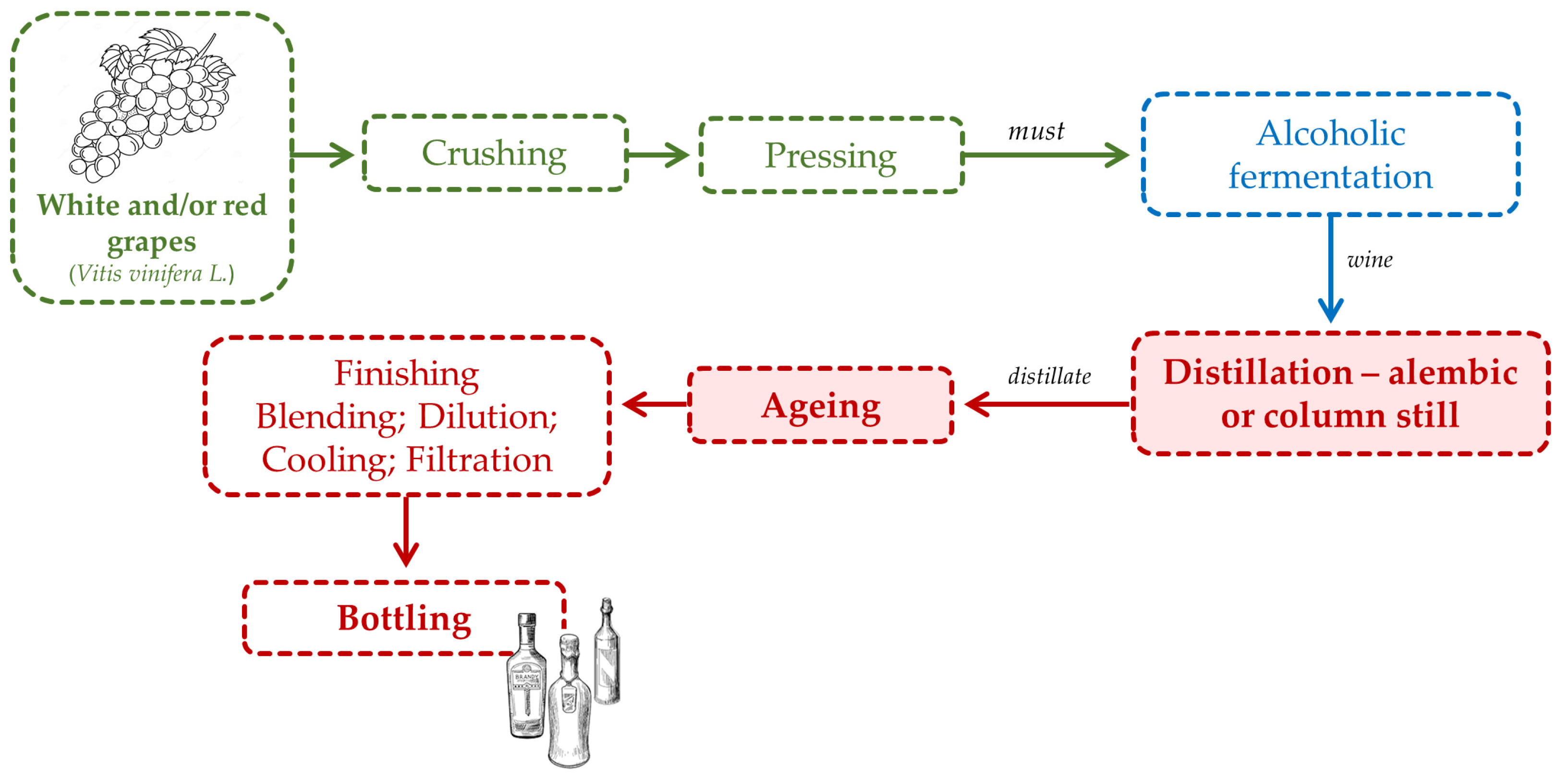
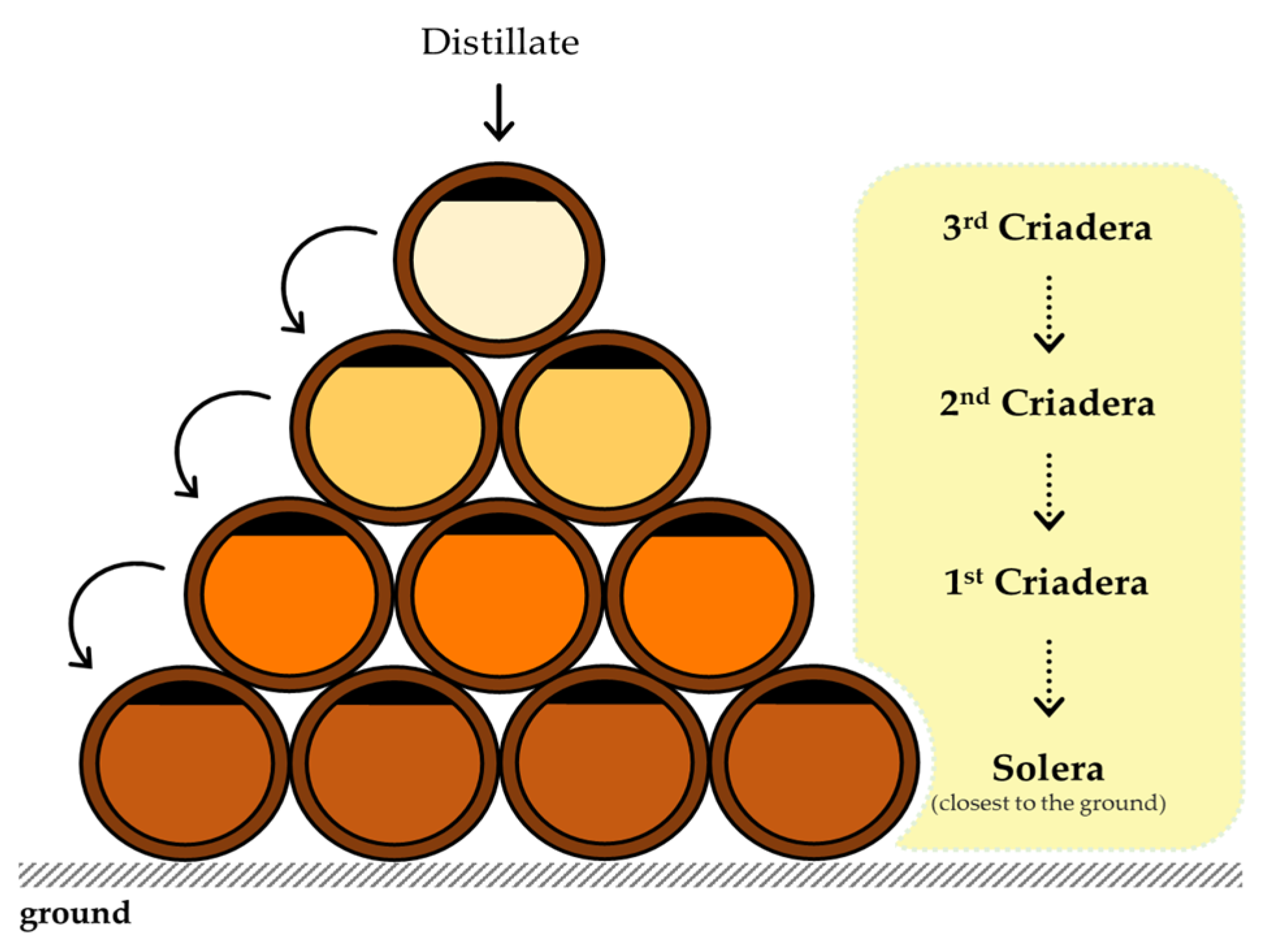
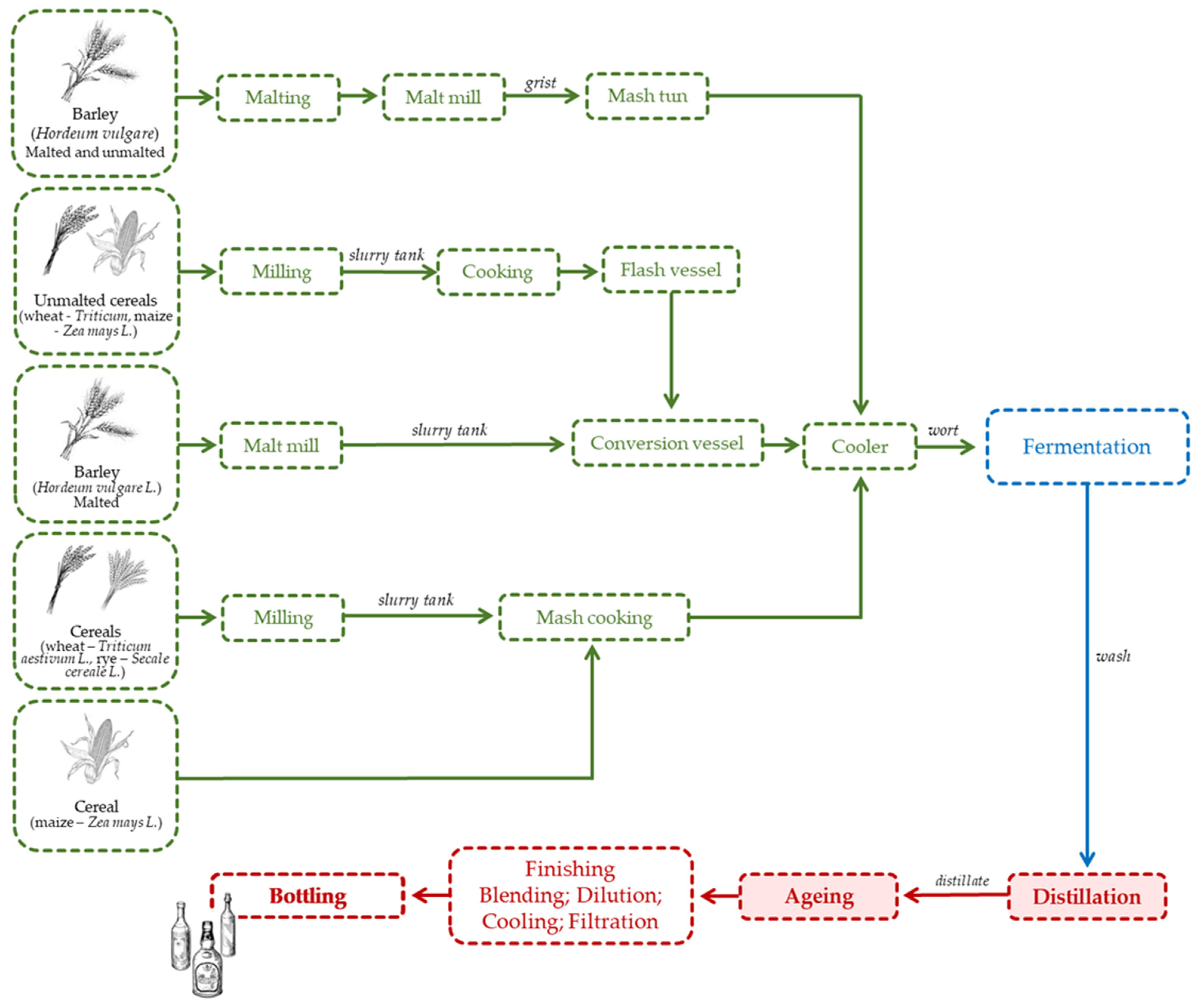
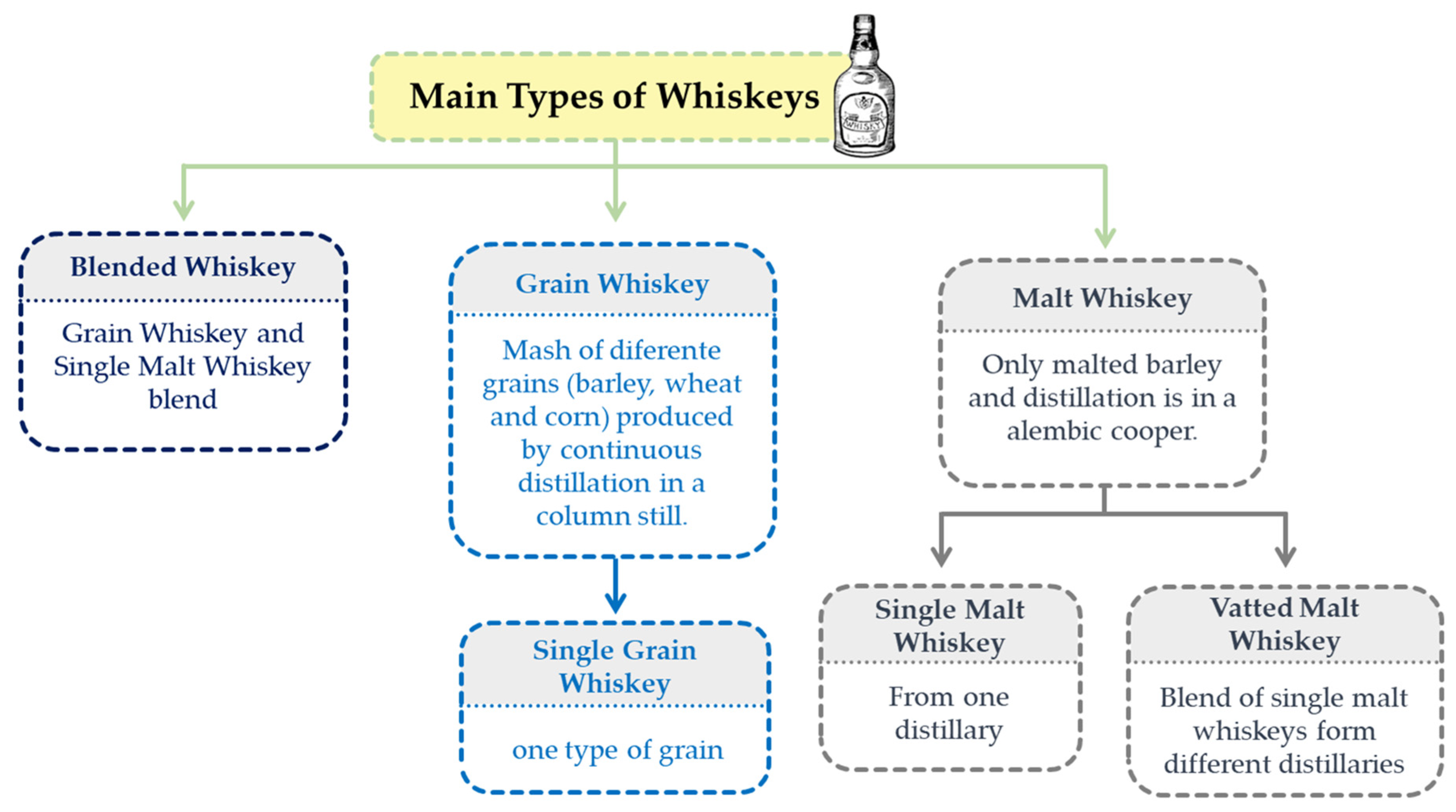
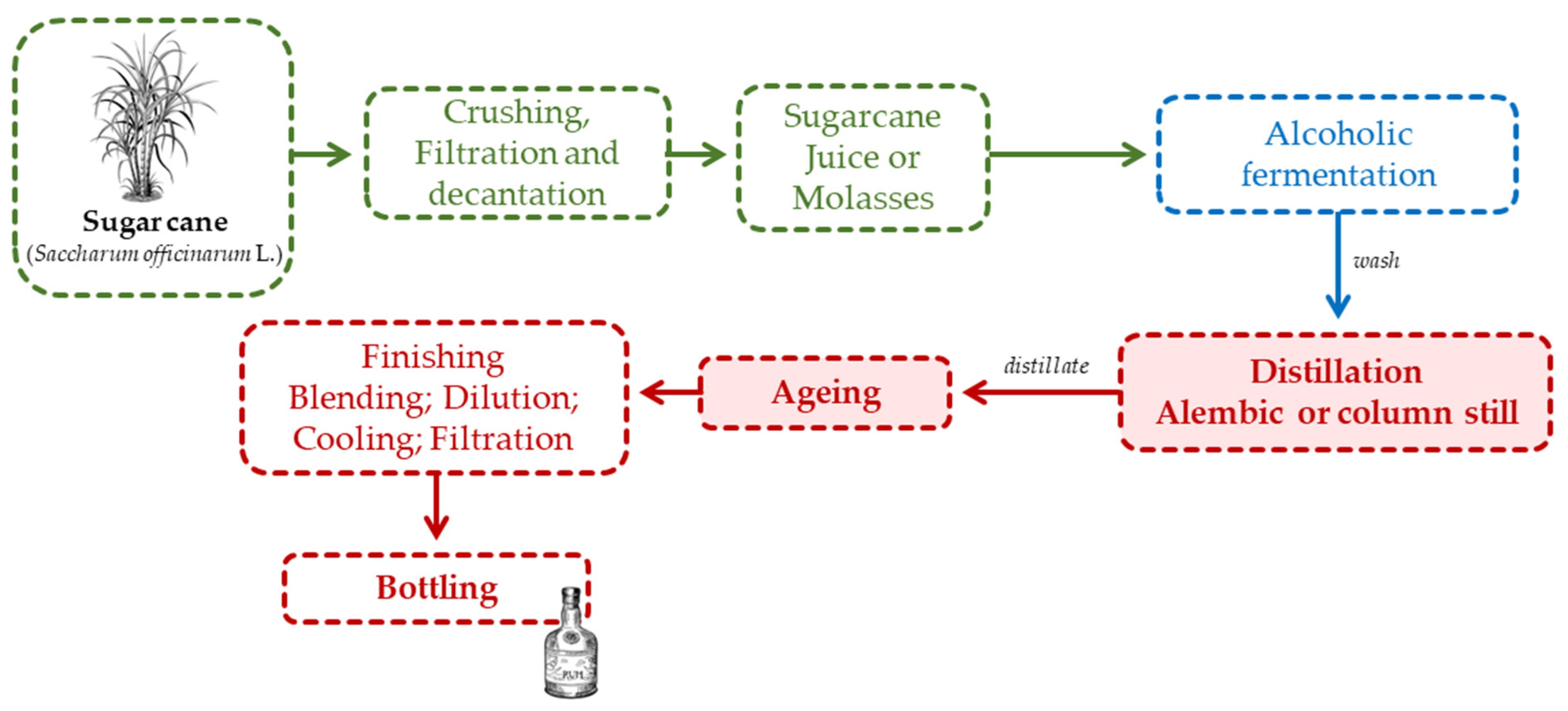
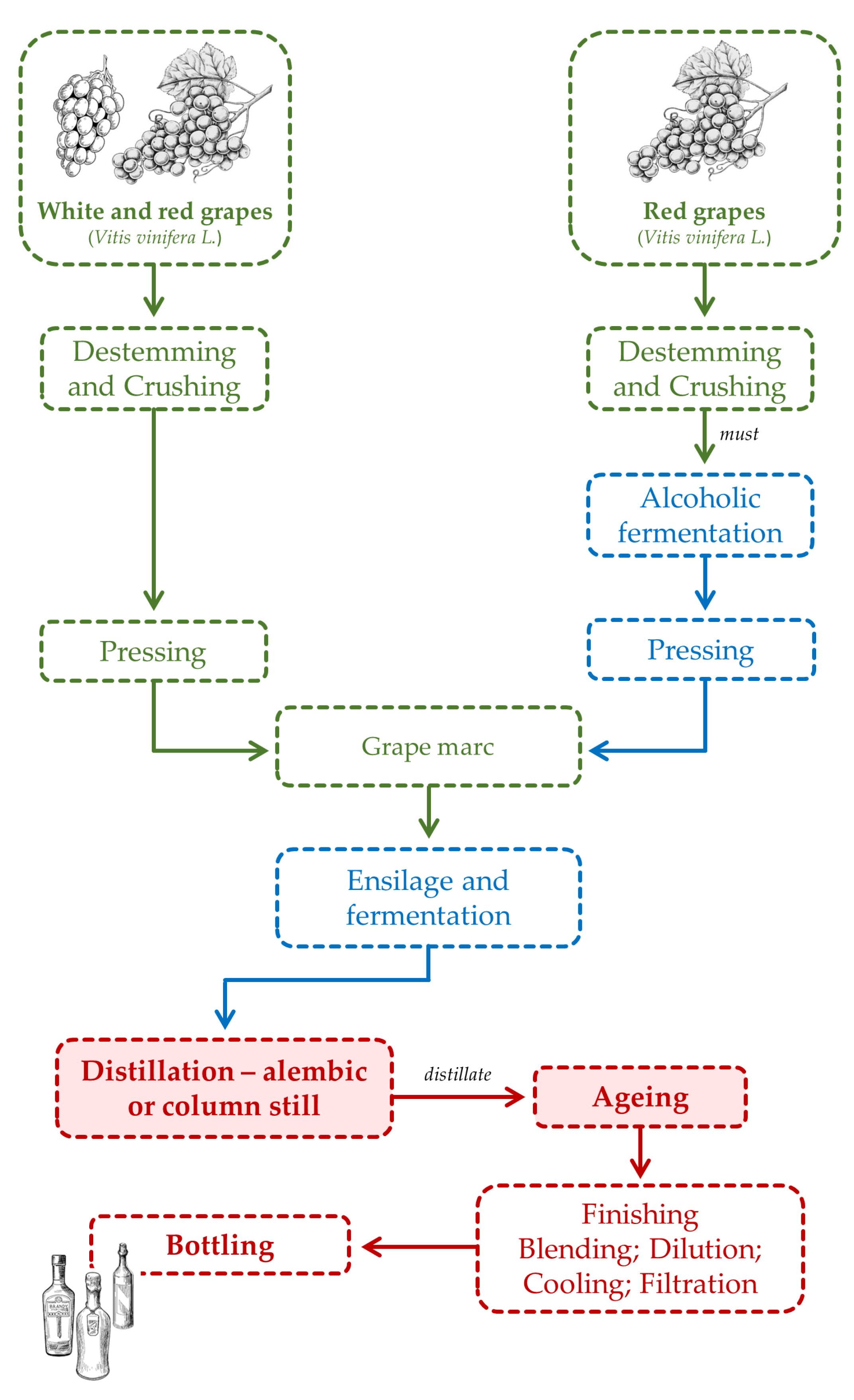
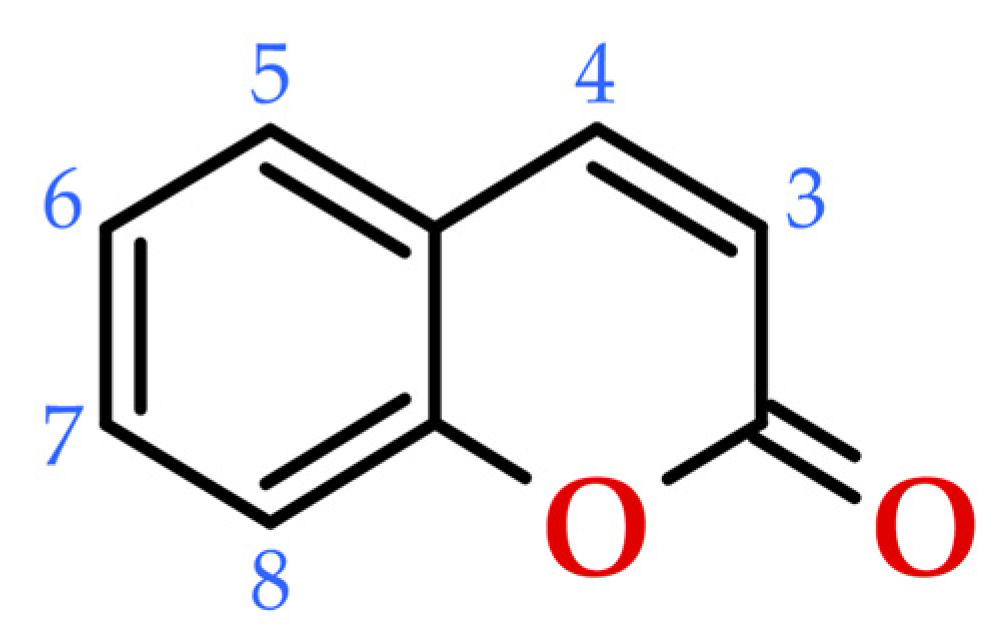
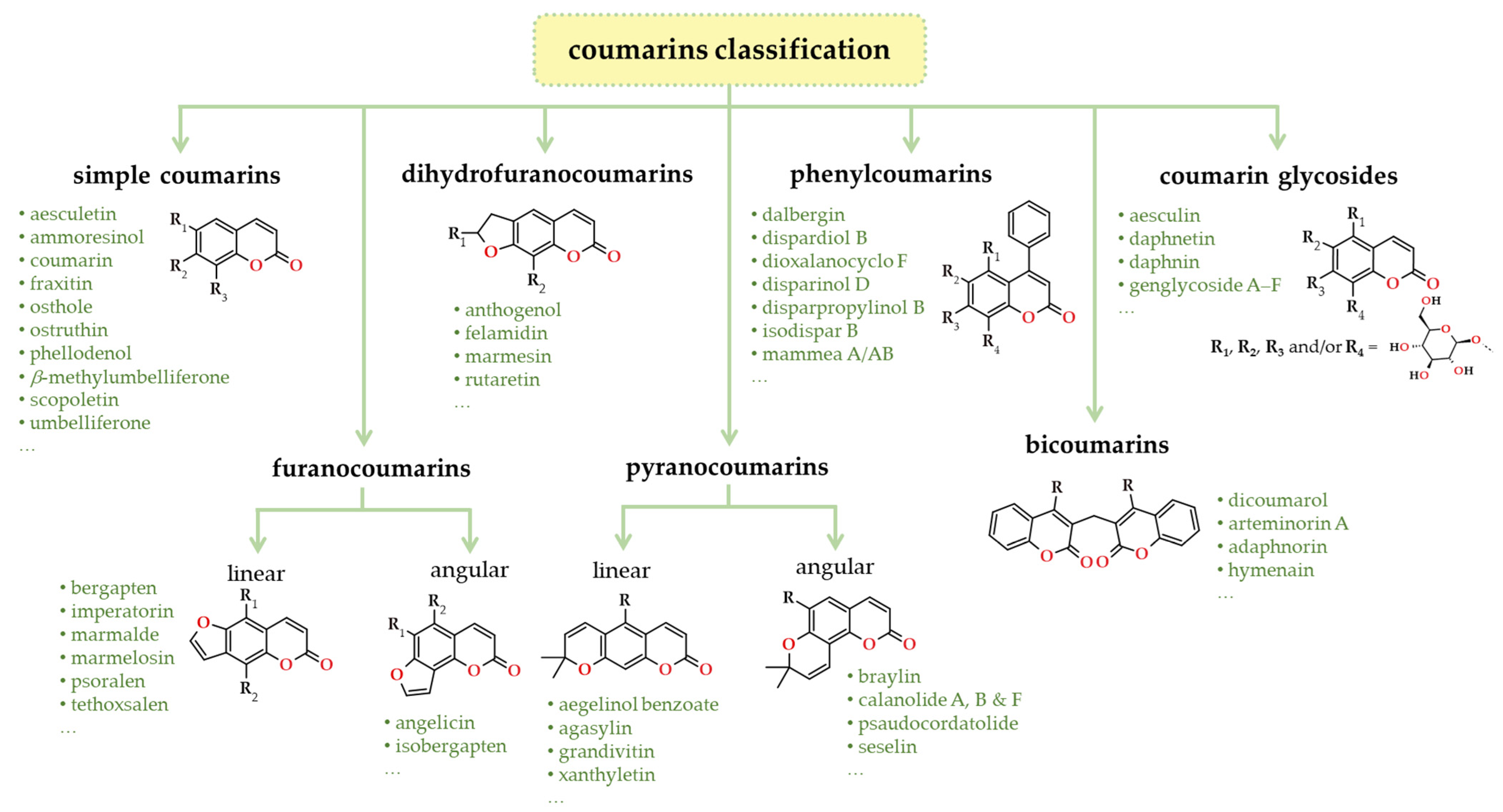

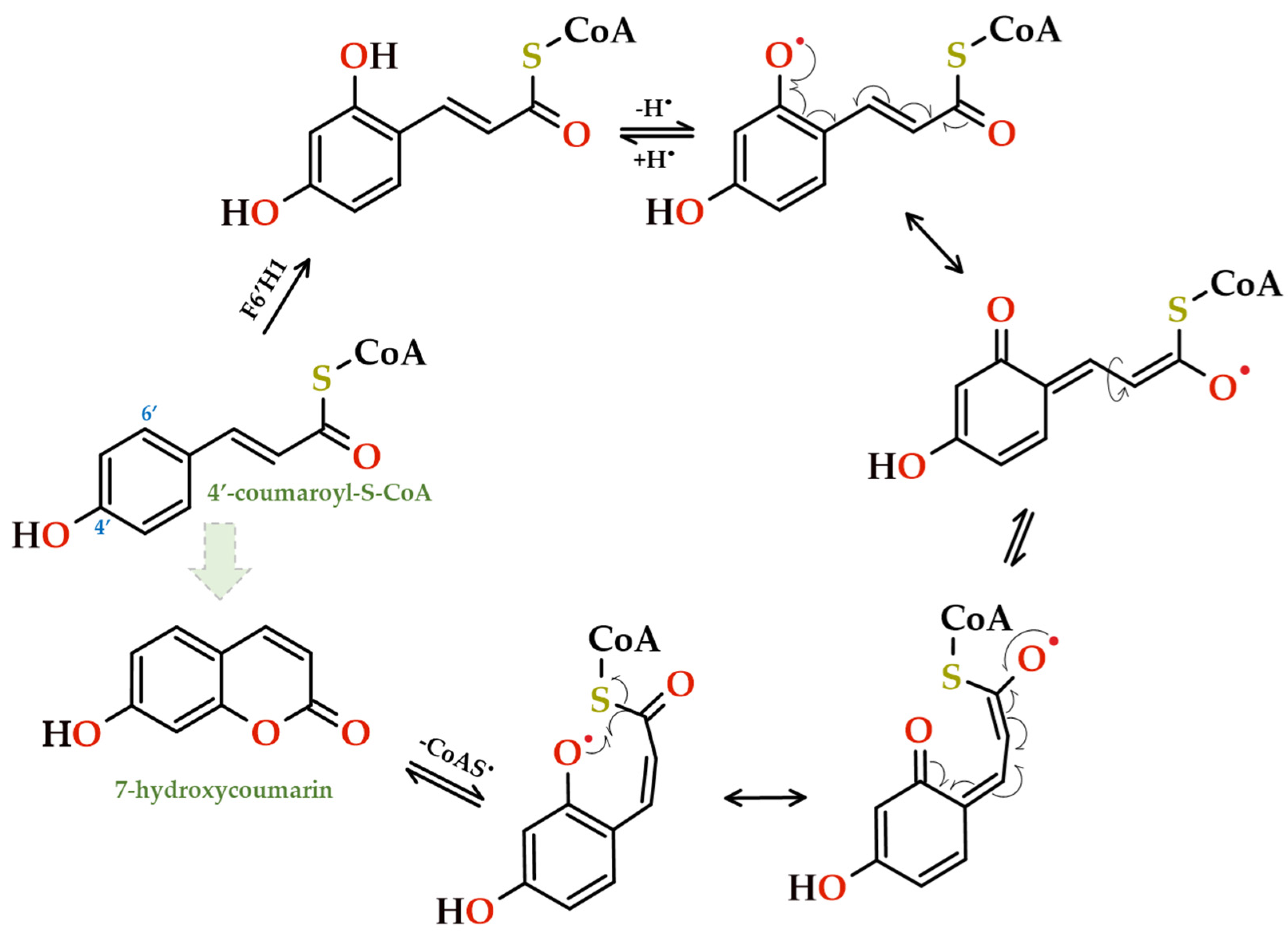
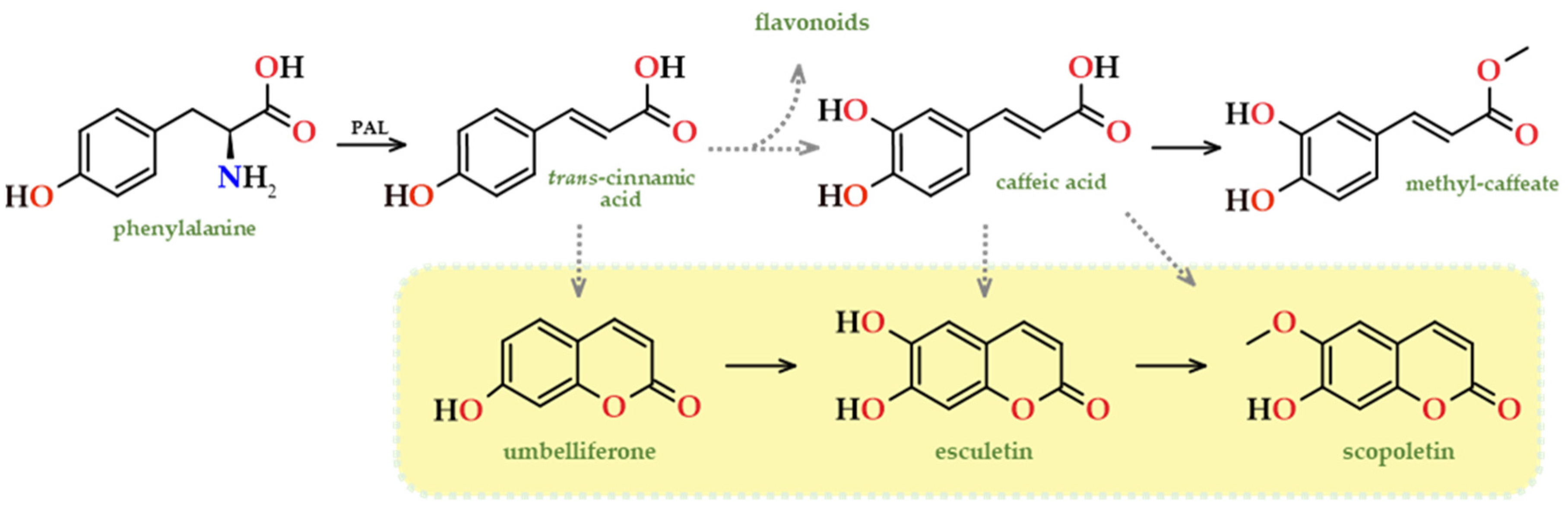

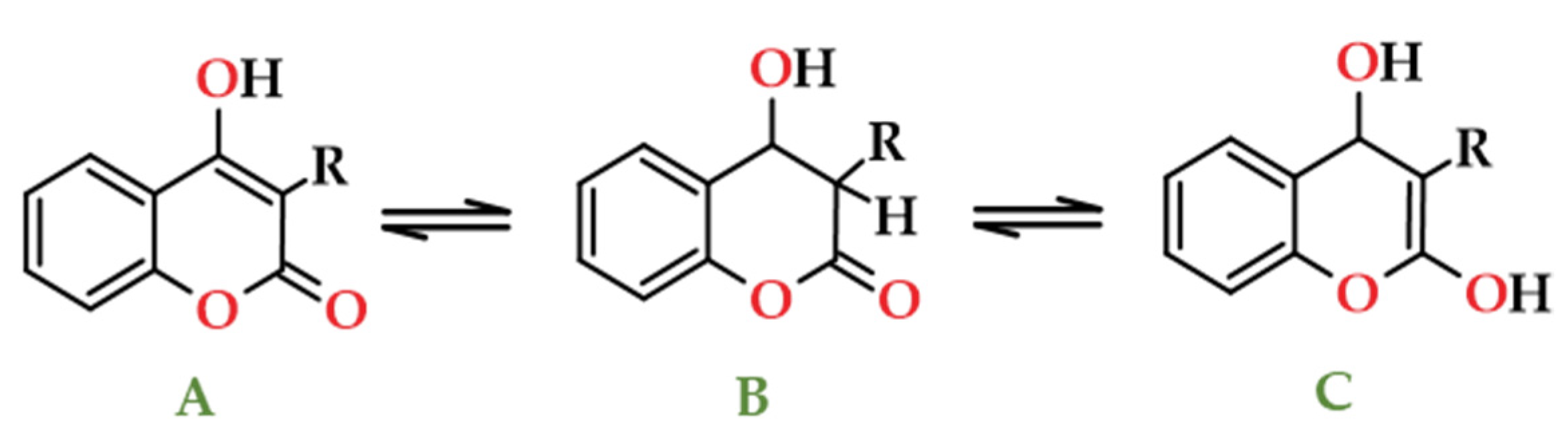
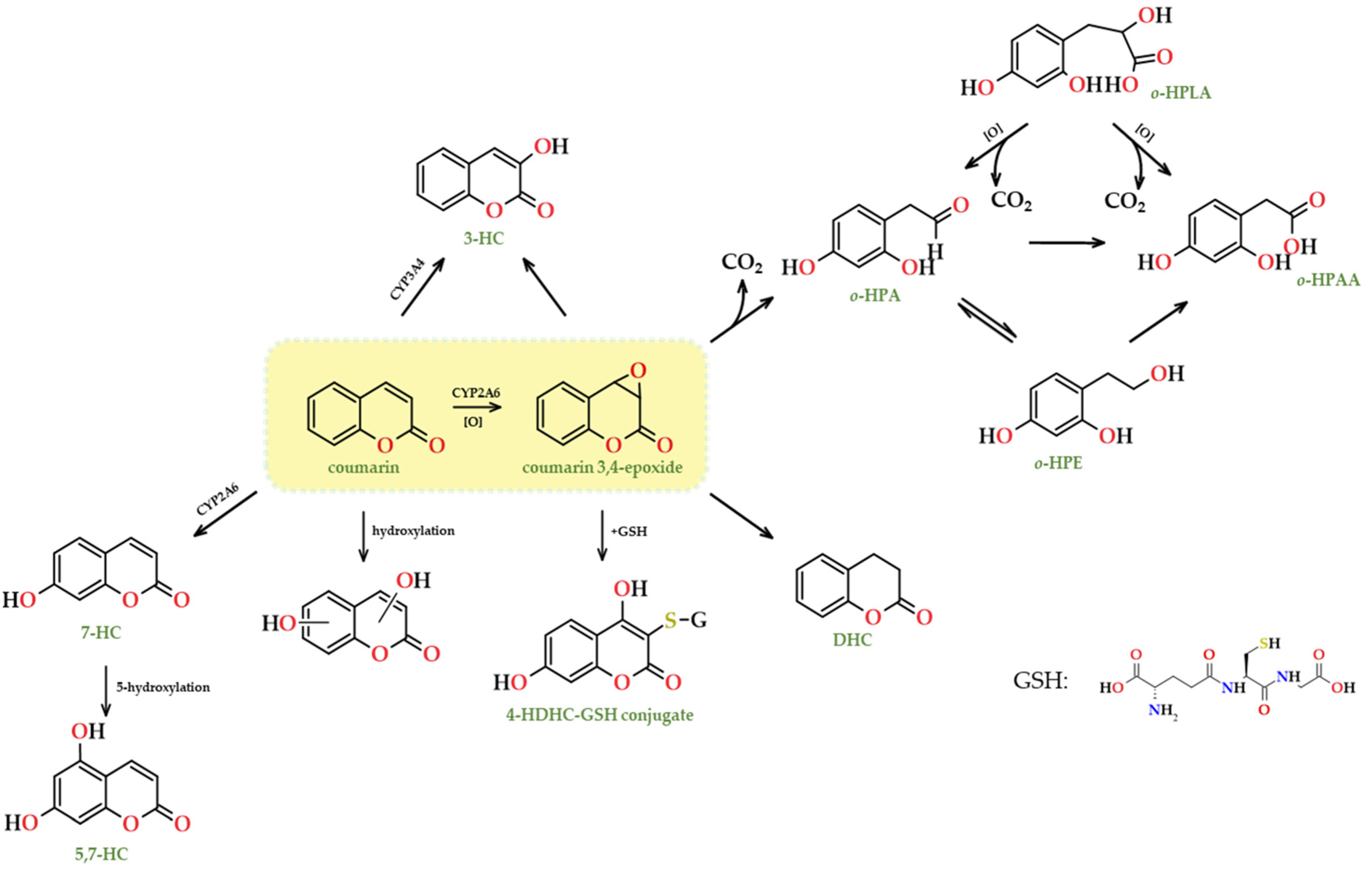
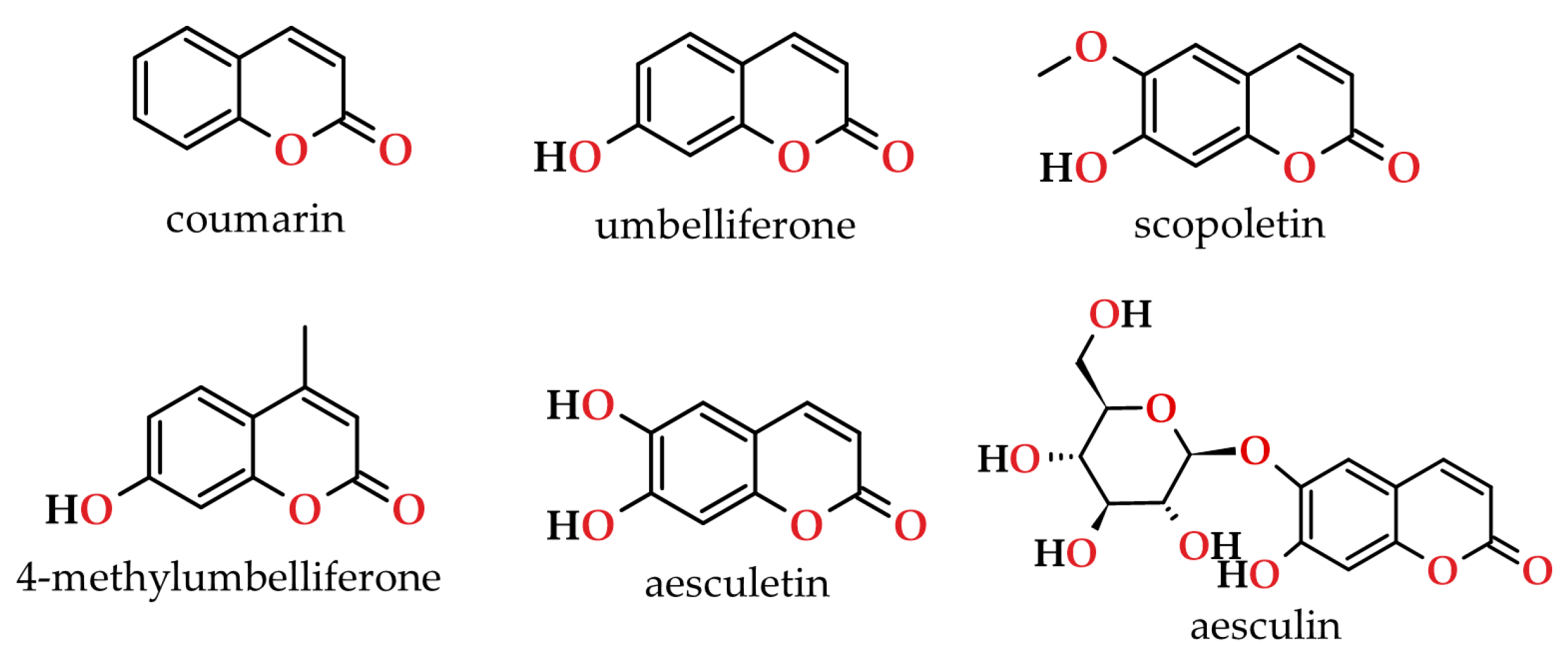
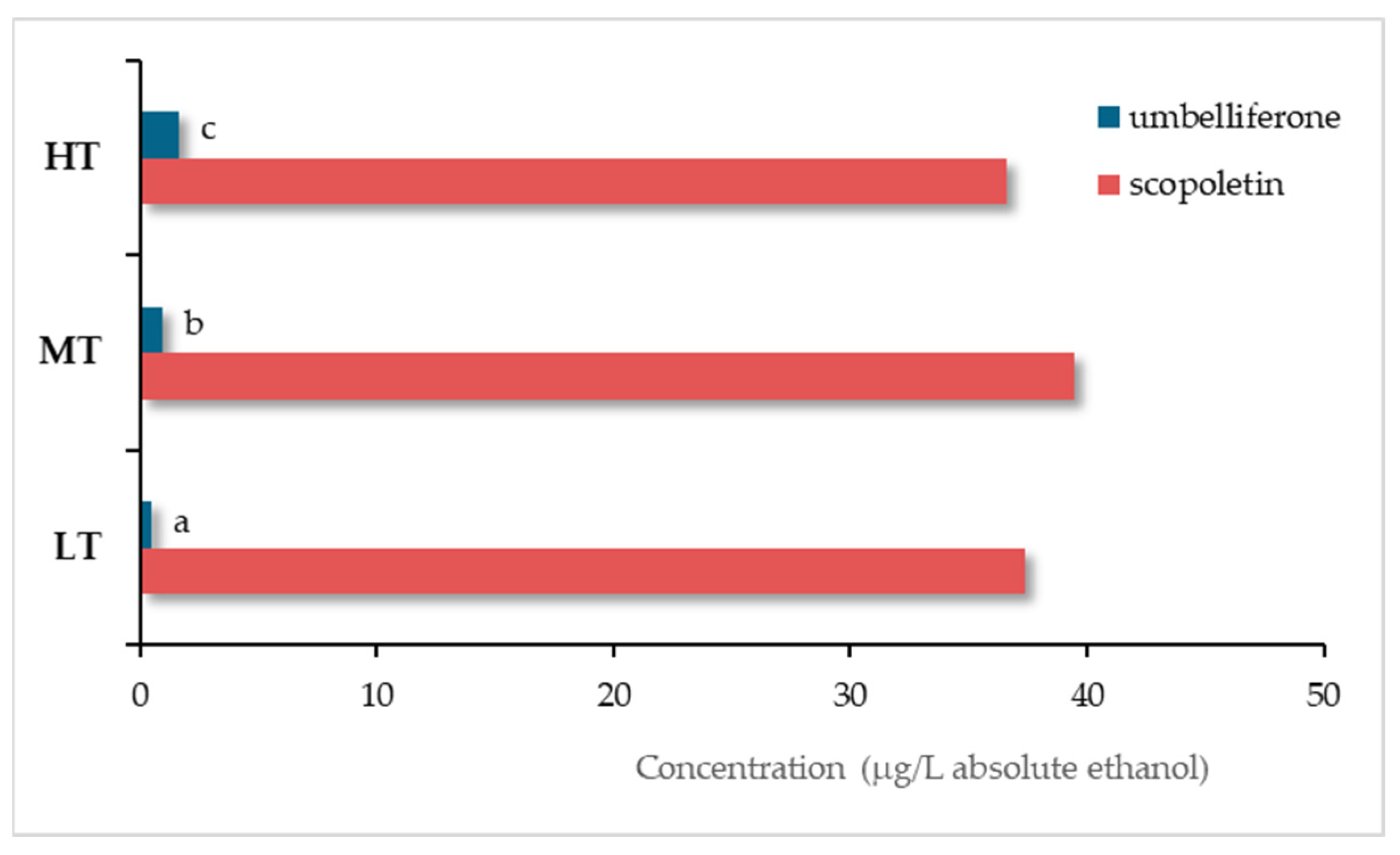
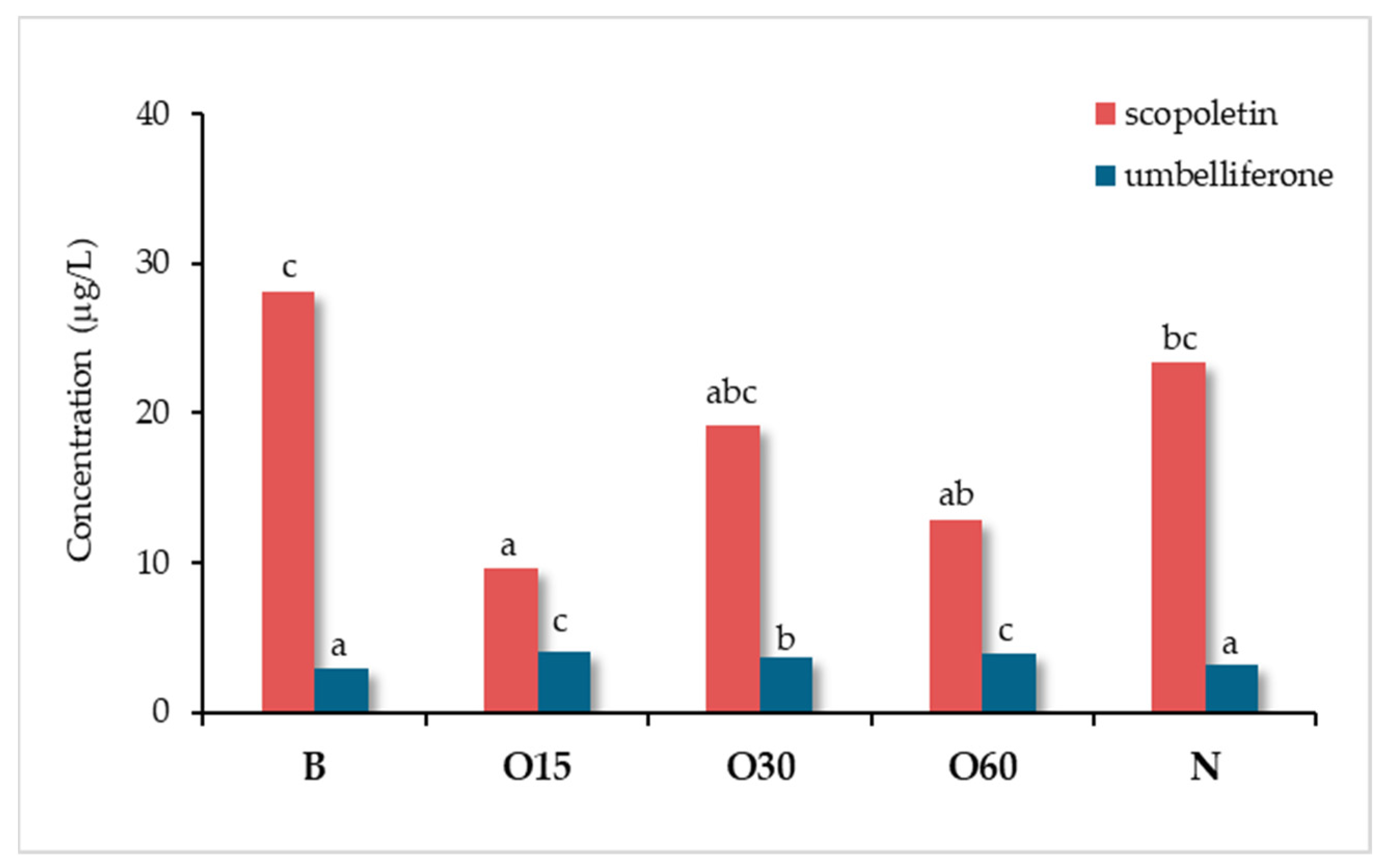

| Source | Species’ Country of Origin | Compound | Mean Content | References |
|---|---|---|---|---|
| Oak Quercus robur L. | France Limousin | scopoletin | 65 μg/L | [38] |
| scopoletin | 4.7 μg/g FW | [123] | ||
| scopoletin | 5.28 μg/g DW | [124] | ||
| umbelliferone | 0.006 μg/g DW | [124] | ||
| aesculetin | 1.68 μg/g FW | [138] | ||
| Bulgaria | scopoletin | 125 μg/L | [38] | |
| Spain | aesculetin | 2.1 μg/g FW | [138] | |
| Croatia | aesculin | 0.44 μg/g FW | [139] | |
| Serbia | aesculin | 0.22 μg/g FW | [139] | |
| Bosnia and Herzegovina | aesculin | 0.11 μg/g FW | [139] | |
| Oak Quercus sessiliflora Salisb. | France Allier | scopoletin | 55 μg/L | [38] |
| scopoletin | 3.6 μg/g FW | [123] | ||
| scopoletin | 3.45 μg/g DW | [124] | ||
| umbelliferone | 0.006 μg/g DW | [124] | ||
| aesculetin | 1.97 μg/g FW | [138] | ||
| Spain | aesculetin | 4.2 μg/g FW | [138] | |
| Serbia | aesculin | 0.44 μg/g FW | [139] | |
| Oak | Spain | scopoletin | 1.08–5.61 μg/g FW | [140] |
| Quercus pyrenaica | aesculetin | 1.60 μg/g FW | [138] | |
| Willd. | Portugal | scopoletin | 1.37 μg/g DW | [124] |
| umbelliferone | 0.006 μg/g DW | [124] | ||
| Oak Quercus faginea Lam. | Spain | aesculetin | 4.9 μg/g FW | [138] |
| Oak Quercus alba L. Quercus lyrata Walt. Quercus stellata | North America | scopoletin | 425 ug/L | [38] |
| scopoletin | 25.15 ug/g DW | [124] | ||
| umbelliferone | 0.007–0.025 μg/g DW | [124] | ||
| aesculetin | 1.45 μg/g FW | [138] | ||
| Wanghen | ||||
| Chestnut Castanea sativa Mill. | Portugal | scopoletin | 1.07 μg/g DW | [124] |
| umbelliferone | nd | |||
| France | scopoletin | 6.73–16.7 μg/g FW | [141] | |
| Cherry Prunus avium L. | Spain | scopoletin | 18.8 μg/g FW | [142] |
| Serbia | aesculetin | 102.30 μg/g FW | [139] | |
| Myrobalan plum Prunus cerasifera Ehrh. | Serbia | aesculin | 1.87 μg/g FW | [139] |
| Mulberry Morus alba L. | Serbia | aesculin | 2.31 μg/g FW | [139] |
| aesculetin | 29.15 μg/g FW | [139] | ||
| Amendoim Pterogyne sp. | Brazil | scopoletin | 0.007 mg/L | [43] |
| coumarin | 0.067 mg/L | [43] | ||
| Cabreúva-parda Myrocarpus frondosus Allem. | Brazil | scopoletin | 0.018 mg/L | [43] |
| coumarin | 0.050 mg/L | [43] | ||
| Canela-Sassafrás Ocotea pretiosa (Vell.) Rohwer | Brazil | scopoletin | 0.028 mg/L | [43] |
| coumarin | 0.011 mg/L | [43] | ||
| Jatobá Hymenaea courbaril L. | Brazil | scopoletin | nd | |
| coumarin | 9.15 mg/L | [43] | ||
| Pequi Caryocar brasiliense Cambess. | Brazil | scopoletin | nd | |
| coumarin | 0.071 mg/L | [43] | ||
| Castanheira Bertholletia excelsa Humb. & Bonpl. | Brazil | scopoletin | nd | |
| coumarin | 0.007 mg/L | [43] |
| Compound | λexcitation (nm) | λemission (nm) |
|---|---|---|
| 4-methylumbelliferone | 218 | 454 |
| coumarin | 280 | 393 |
| aesculin | 335–337 | 409 |
| umbelliferone | 340, 380 | 467 |
| scopoletin | 345–347 | 430, 460 |
| aesculetin | 347–353, 340 | 467 |
| Spirit Beverage | Sample Preparation | References |
|---|---|---|
| Wine Spirit | Coumarins should be extracted, since this matrix is complex and rich in phenolic compounds. An ether extraction was therefore carried out | [36] |
| Samples directly analysed (without preparation) | [38] | |
| Adding an internal standard to the samples, filtering them through a 0.45 µm membrane and analysing them by direct injection | [39,40,152,164,165] | |
| De-alcoholised samples (to 8% alcohol) and filtered through a 0.45 µm membrane | [22] | |
| Brandy | Samples directly analysed (without preparation) | [38] |
| Samples filtered at 0.20 µm membrane and directly injected | [123] | |
| De-alcoholised samples (to 8% alcohol) and filtered through a 0.45 µm membrane | [22] | |
| Whiskey | Samples extracted with ethyl acetate | [35] |
| Samples directly analysed (without preparation) | [38] | |
| De-alcoholised samples (to 8% alcohol), filtered through a 0.45 µm membrane | [22] | |
| Sugar cane spirit | Samples extracted with ethyl acetate (Rum) | [35] |
| SPE extraction (Cachaça) | [43] | |
| Samples filtered at 0.45 µm polyethylene membrane and directly injected | [166] | |
| For fluorescence detection, the samples were not prepared; for UV-Vis detection, the samples were diluted 30 times with an ethanol/water solution (40:60% v/v) (Cachaça) | [167] | |
| De-alcoholised samples (to 8% alcohol), filtered through a 0.45 µm membrane (Rum) | [22] |
| Spirit Beverage | Analytical Methods | References | |
|---|---|---|---|
| Separation | Detection | ||
| Wine Spirit | HPLC | FLD | [36,38,39,40,152,164,165] |
| HMRS with ESI/HESI II | [22] | ||
| Brandy | HPLC | FLD | [38,123] |
| HMRS with ESI/HESI II | [22] | ||
| Fluorescence Spectrometry | [48] | ||
| Whiskey | TLC | Fluorometer | [35] |
| HPLC | FLD | [38] | |
| HMRS with ESI/HESI II | [22] | ||
| Sugar cane spirit | TLC | Fluorometer | [35] |
| HPLC | FLD | [43] | |
| HPLC | DAD | [166] | |
| UV-Vis spectrophotometry and spectrofluorimetry | [167] | ||
| HPLC | HMRS with ESI/HESI II | [22] | |
| Origin | Wood Species | Compound | Mean Content (μg/L) | References |
|---|---|---|---|---|
| Armagnac | Oak | scopoletin umbelliferone 4-methylumbelliferone aesculetin | 301.1 2.8 2.8 1.9 | [36] [36] [36] [36] |
| Lourinhã | Oak Q. robur | scopoletin scopoletin umbelliferone umbelliferone | 88.0 37.12 1.0 0.95 | [39] [19] [39] [19] |
| Oak Q. sessiliflora | scopoletin umbelliferone | 19.74 0.78 | [19] [19] | |
| Oak Q. pyrenaica | scopoletin umbelliferone | 10.33 0.98 | [19] [19] | |
| Oak Q. alba Q. bicolor Q. lyrata Q. stellata | scopoletin umbelliferone | 164.77 1.48 | [19] [19] | |
| Chestnut C. sativa | scopolein scopoletin umbelliferone umbelliferone | 9.0 8.63 5.0 0.92 | [39] [19] [39] [19] |
| Origin | Wood Species | Compound | Mean Content (μg/L) | References |
|---|---|---|---|---|
| Italy | Oak Q. robur | scopoletin | 102.1 | [123] |
| Oak Q. sessiliflora | scopoletin | 101.5 | [123] |
| Origin | Wood Species | Compound | Mean Content (μg/L) | References |
|---|---|---|---|---|
| Brazil | European oak | 4-methylumbelliferone coumarin | nd nd | [44] |
| Amburana Amburana cearensis (Allemão) A.C.Sm. | 4-methylumbelliferone coumarin | 0.014 0.049 | [44] | |
| Balsam Myroxylon peruiferum L. f. | 4-methylumbelliferone coumarin | nd 0.002 | [44] | |
| Jatoba Hymenaeae carbouril L. | 4-methylumbelliferone coumarin | nd nd | [44] | |
| Peroba Paratecoma peroba (Record) Kuhlm. | 4-methylumbelliferone coumarin | nd nd | [44] |
| Compound | Pharmacological Activity | Research Method | Experimental | Doses | References |
|---|---|---|---|---|---|
| Umbelliferone | Neuroprotective effects | Chronic unpredictable mild stress (CUMS) | Male Sprague Dawley rats. | 15 mg/kg 30 mg/kg | [236] [236] |
| Anti-inflammatory | Inflammatory cytokines in hippocampus | Male Sprague Dawley rats | 15 mg/kg 30 mg/kg | [236] [236] | |
| Lipopolysaccharide (LPS)-induced acute lung injury (ALI) | BALB/c mice | 40 mg/kg | [237] | ||
| Antidepressant | Post-traumatic stress disorder model (PTSD) | Male Sprague Dawley rats | 60 mg/kg | [238] | |
| Antidiabetic | Streptozotocin (STZ)-induced diabetes | Male Wistar rats | 30 mg/kg | [239,240] | |
| Anticancer | Cell cycle analysis and apoptosis detection | HepG2 HCC cells | 50 μM | [241] | |
| Antihypertensive | Vascular activity assay | Male spontaneously hypertensive rats (SHR) | 300 μM | [242] | |
| 4-Methylumbelliferone | Anticancer | Human pancreatic cancer cells (KP1-NL) transplanted into the hypodermis of nude mice | Male nude mice | 3 mg/g | [243] |
| Anticancer | Breast cancer xenograft models | BALB/C nu/nu mice | 0.5 mg/g | [244] | |
| Antitumor | Inhibition of fibroblast and melanoma cells | Human. Melanoma cell line, C8161 and MV3 | 0.5 mM | [245] | |
| Anti-inflammatory | Inflammatory cytokines in astrocyte cultures | Rat astrocytes (glial cells) | 400 μM | [246] | |
| Antidiabetic | Glucose level and glucose-tolerance test in mice fed a high-fat diet | Male C57BL/6J mice | 0.2 g/kg | [247] | |
| Neuroprotective | Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition | in vitro assay | 1 mg/mL | [248] | |
| Aesculetin | Antiadipogenic | PPARg Expression | 3T3-L1 Preadipocyte cells | 100 μM | [249] |
| Antidiabetic | Streptozotocin (STZ)-induced diabetes | Male albino rats (Wistar strain) | 40 mg/kg | [250] | |
| Antithrombotic | Platelet aggregation | Human assay | 50 μM | [251] | |
| Antitumor | Mitogen-activated protein kinase (MAPK) assay | HepG2 cells | 100 μM | [252] | |
| Anticancer | Trail-induced apoptosis | Oral cancer SAS cells | 10 μg/mL | [253] | |
| Anti-inflammatory | Colitis induced by trinitro-benzenesulphonic acid (TNBS) | Male Wistar rats | 5 mg/kg | [254] | |
| Aesculin | Antidiabetic | Dexamethasone-induced insulin resistance | Male Institute of Cancer Research (ICR) mice | 40 mg/kg | [255] |
| Streptozotocin (STZ)-induced diabetes | Male C57BL/6J | 20 mg/kg | [256] | ||
| Renoprotective | STZ-induced diabetic renal damage | Male ICR mice | 20 mg/kg | [257] | |
| Gastroprotective | Gastric mucosal injury index | Male Kunming mice | 20 mg/kg | [258] | |
| Antioxidant | AAPH-induced erythrocyte hemolysis assay | Erythrocytes | 50 μM | [259] | |
| Anti-inflammatory | Carrageenan-induced paw oedema | Male and female Kunming mice | 20 mg/kg | [260] | |
| Endotoxin shock induced by lipopolysaccharide (LPS) | Male and female Kunming mice | 20 mg/kg | [261] | ||
| Liver injury induced by lipopolysaccharide/D-Galactosamine (LPS/D-Gal) | BALB/c mice | 40 mg/kg | [262] | ||
| Coumarin | Antidiabetic | Animal model of induced type 2 diabetes mellitus (NIDDM) | Male albino Wistar rats | 100 mg/kg | [263] |
| α-Glucosidase inhibition assay | in vitro assay | 20 mM | [264] | ||
| Anticancer | Inhibition of proliferation and induction of apoptosis | Human cervical cancer HeLa cells | 100 μM | [265] | |
| Inhibition of proliferation | Human lung cancer cell lines, A427 and Calu-1 | 1.0 mM | [266] | ||
| Inhibition of proliferation | Human lung cancer cell lines, A427 and SK-LU-1 | 100 μg/mL | [267] | ||
| Anti-inflammatory | Colonic damage induced by trinitrobenzenesulfonic acid (TNBS) | Male Wistar rats | 25 mg/kg | [268] | |
| Scopoletin | Antibacterial | Minimum inhibitory concentrations (MIC) | Pseudomonas aeruginosa | 0.66 μg/mL | [269] |
| Antihypertensive | Multiple model of hypertension (blood pressure) | Male Wistar-Kyoto rats | 10 mg/kg | [270] | |
| Antidiabetic | Streptozotocin (STZ)-induced diabetes | Male ICR mice | 10 mg/kg | [271] | |
| α-Glucosidase/α-amylase inhibition assay | in vitro assay | 100 μM | [271] | ||
| Glucose uptake assay | 3T3-L1 preadipocyte cells | 20 μM | [272] | ||
| Anti-inflammatory | Carrageenan-induced paw oedema | Female Sprague Dawley rats | 10 mg/kg | [273] | |
| Anti-ageing | Autophagy assay | Human lung fibroblast cells (IMR 90) | 16 μM | [274] |
| Compound | n-Octanol/Water (log KOW) a | Test Type/Route b | Organism | Dose | References | Remarks |
|---|---|---|---|---|---|---|
| coumarin | 1.39 | LD50/oral | Rat | 293 mg/kg | [287] | May cause irritation. Harmful if swallowed: causes liver injury and somnolence |
| LD50/oral | Mouse | 196 mg/kg | [288] | |||
| LD50/intraperitoneal | Mouse | 220 mg/kg | [289] | |||
| LD50/subcutaneous | Mouse | 242 mg/kg | [288] | |||
| LD50/oral | Guinea pig | 202 mg/kg | [290] | |||
| umbelliferone | 1.03 (TOXNET) | LD50/intravenous | Mouse | 450 mg/kg | [291] | Causes liver effects, effects on plasma proteins, hypoglycemia, and changes in clotting factors in 45-day intermittent intraperitoneal studies of rats |
| scopoletin | nd | LD50/oral | Rat | 3800 mg/kg | [292] | Irritant. |
| 4-methylumbelliferone | nd | LD50/intravenous | Mouse | 350 mg/kg | [291] | Irritant |
| LD50/oral | Rat | 3850 mg/kg | [293] | |||
| LD50/intraperitoneal | Rat | 2550 mg/kg | [293] | |||
| LD50/subcutaneous | Rat | 7200 mg/kg | [293] | |||
| LD50/oral | Mouse | 2850 mg/kg | [293] | |||
| LD50/intraperitoneal | Mouse | 1250 mg/kg | [294] | |||
| aesculetin | 0.55 | LD50/intraperitoneal | Mouse | 1500 mg/kg | [295] | Irritant |
| aesculin | nd | LD50/intraperitoneal | Mouse | 1900 mg/kg | [296] | Irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Alves, S.; Lourenço, S.; Fernandes, T.A.; Canas, S. Coumarins in Spirit Beverages: Sources, Quantification, and Their Involvement in Quality, Authenticity and Food Safety. Appl. Sci. 2024, 14, 1010. https://doi.org/10.3390/app14031010
Oliveira-Alves S, Lourenço S, Fernandes TA, Canas S. Coumarins in Spirit Beverages: Sources, Quantification, and Their Involvement in Quality, Authenticity and Food Safety. Applied Sciences. 2024; 14(3):1010. https://doi.org/10.3390/app14031010
Chicago/Turabian StyleOliveira-Alves, Sheila, Sílvia Lourenço, Tiago A. Fernandes, and Sara Canas. 2024. "Coumarins in Spirit Beverages: Sources, Quantification, and Their Involvement in Quality, Authenticity and Food Safety" Applied Sciences 14, no. 3: 1010. https://doi.org/10.3390/app14031010
APA StyleOliveira-Alves, S., Lourenço, S., Fernandes, T. A., & Canas, S. (2024). Coumarins in Spirit Beverages: Sources, Quantification, and Their Involvement in Quality, Authenticity and Food Safety. Applied Sciences, 14(3), 1010. https://doi.org/10.3390/app14031010









