Calibrated Hydraulic Resistance Adjuncts for Carbon Dioxide Angiography Optimization
Abstract
1. Introduction
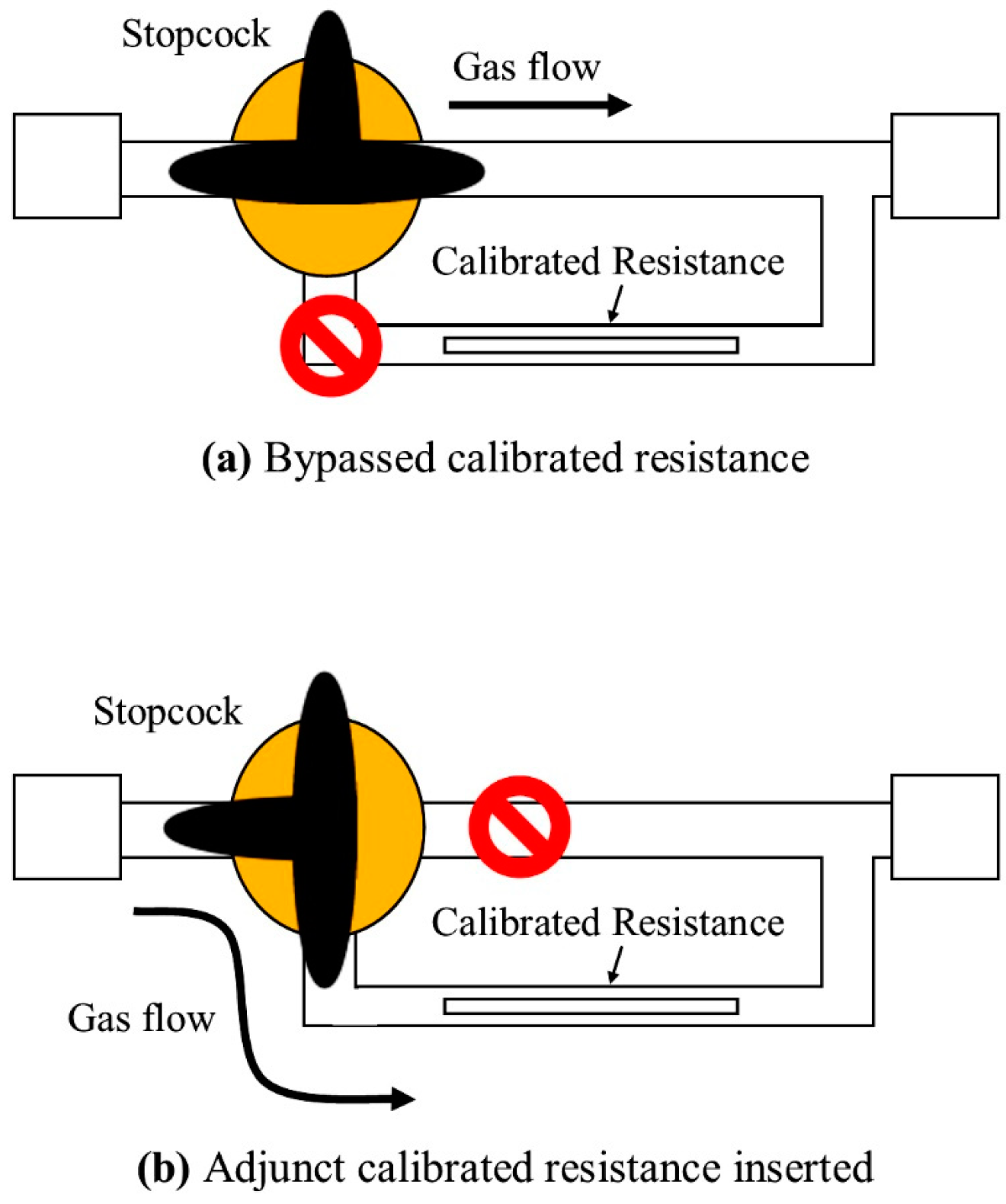
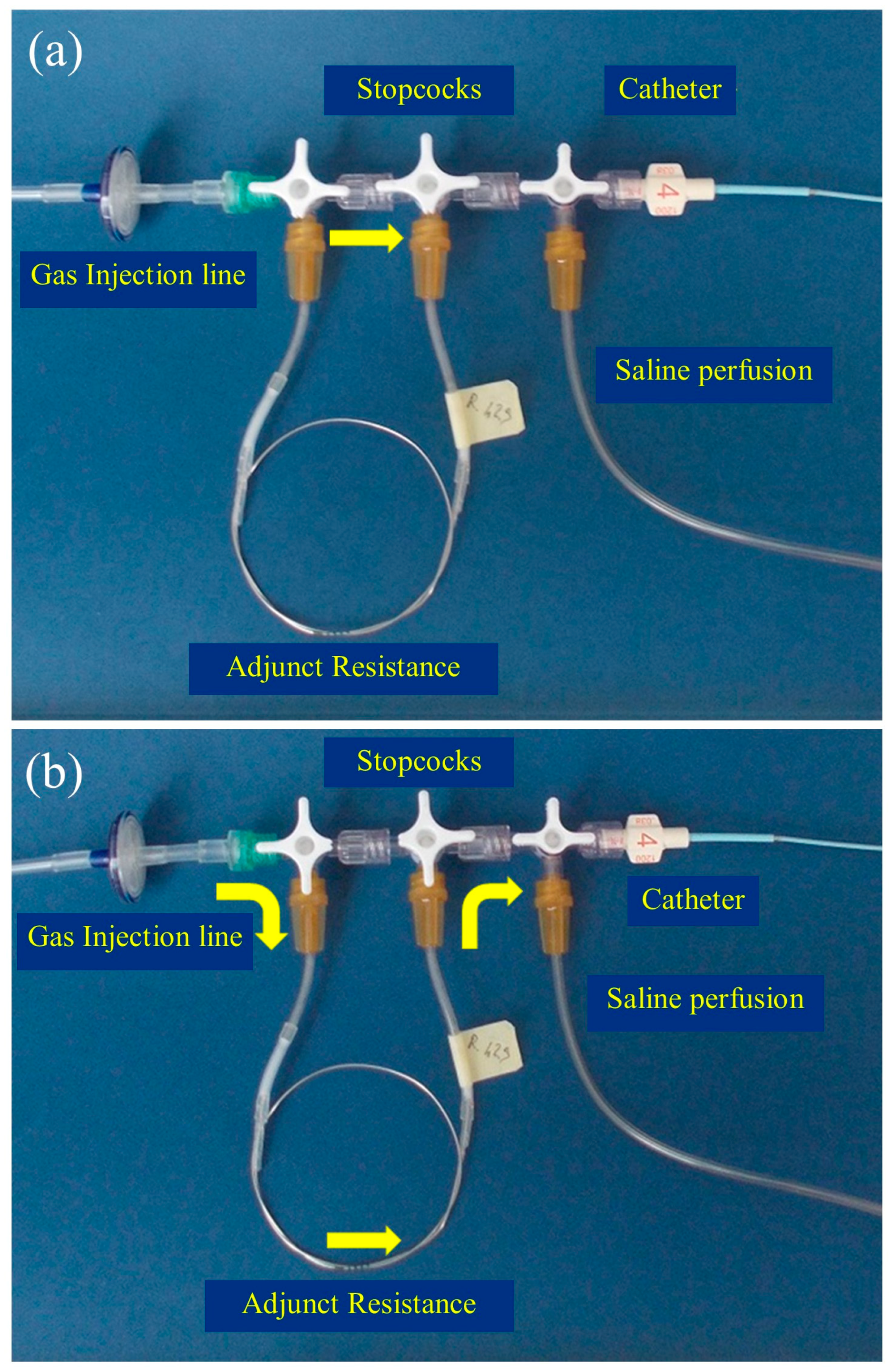
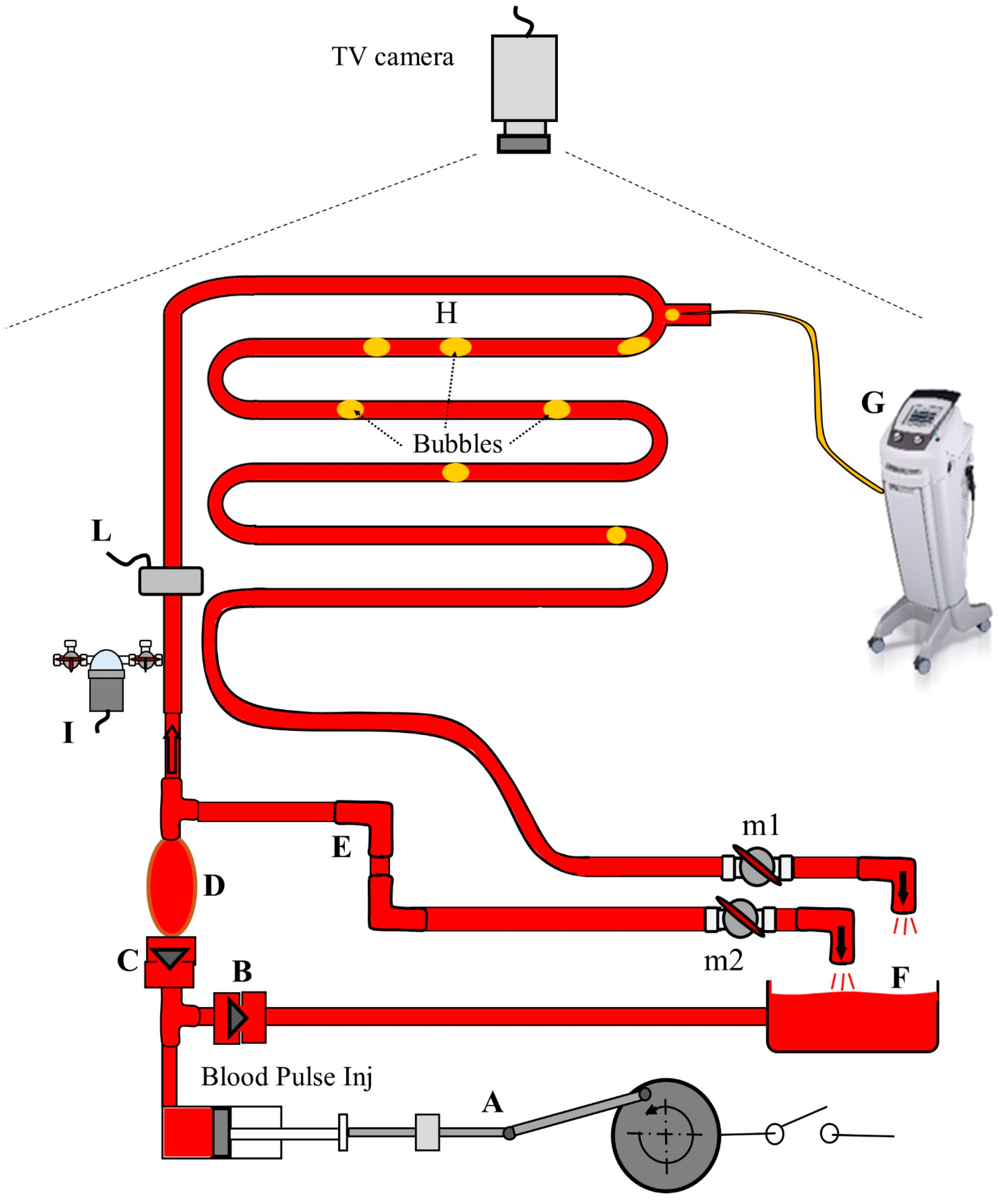
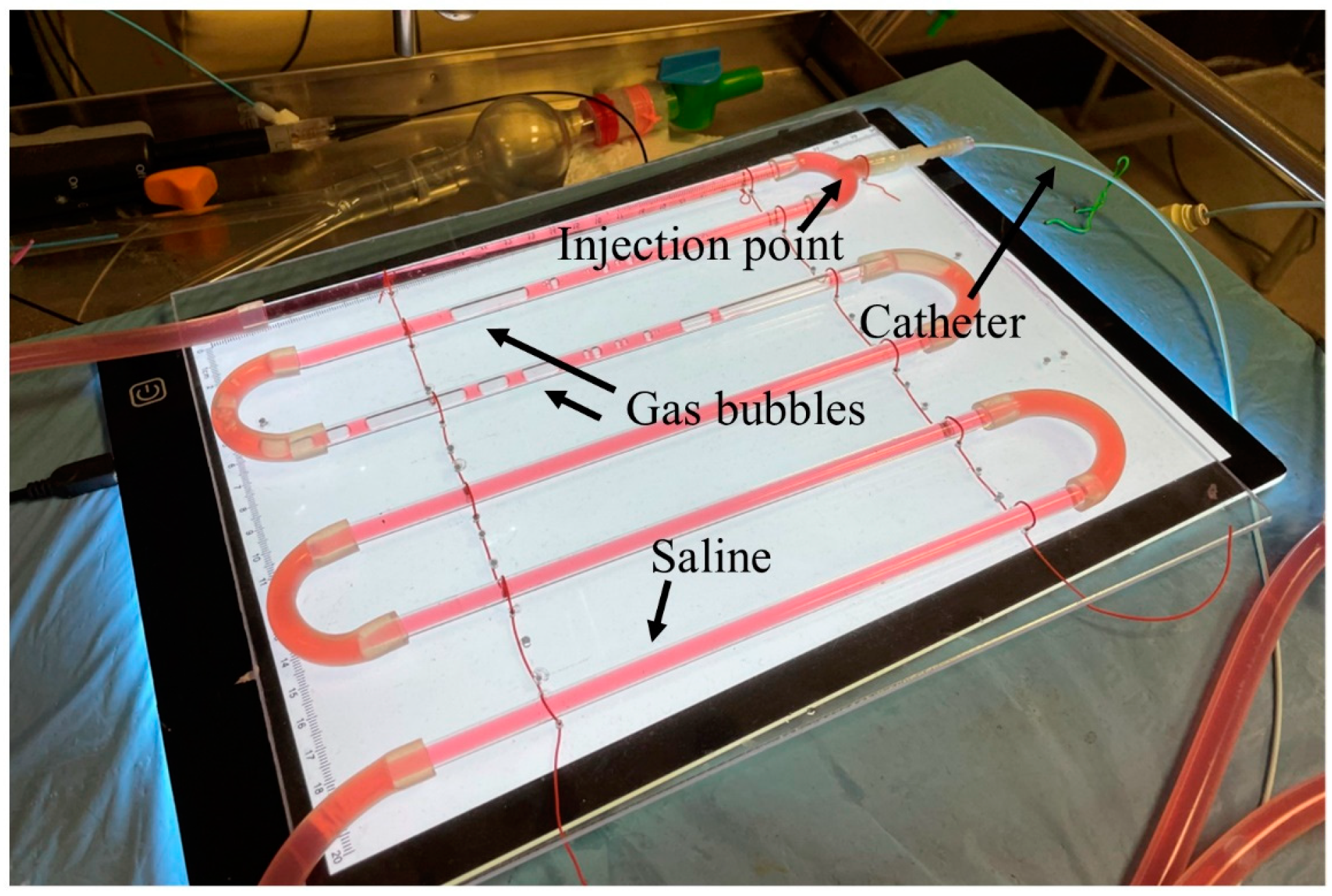
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Procedures
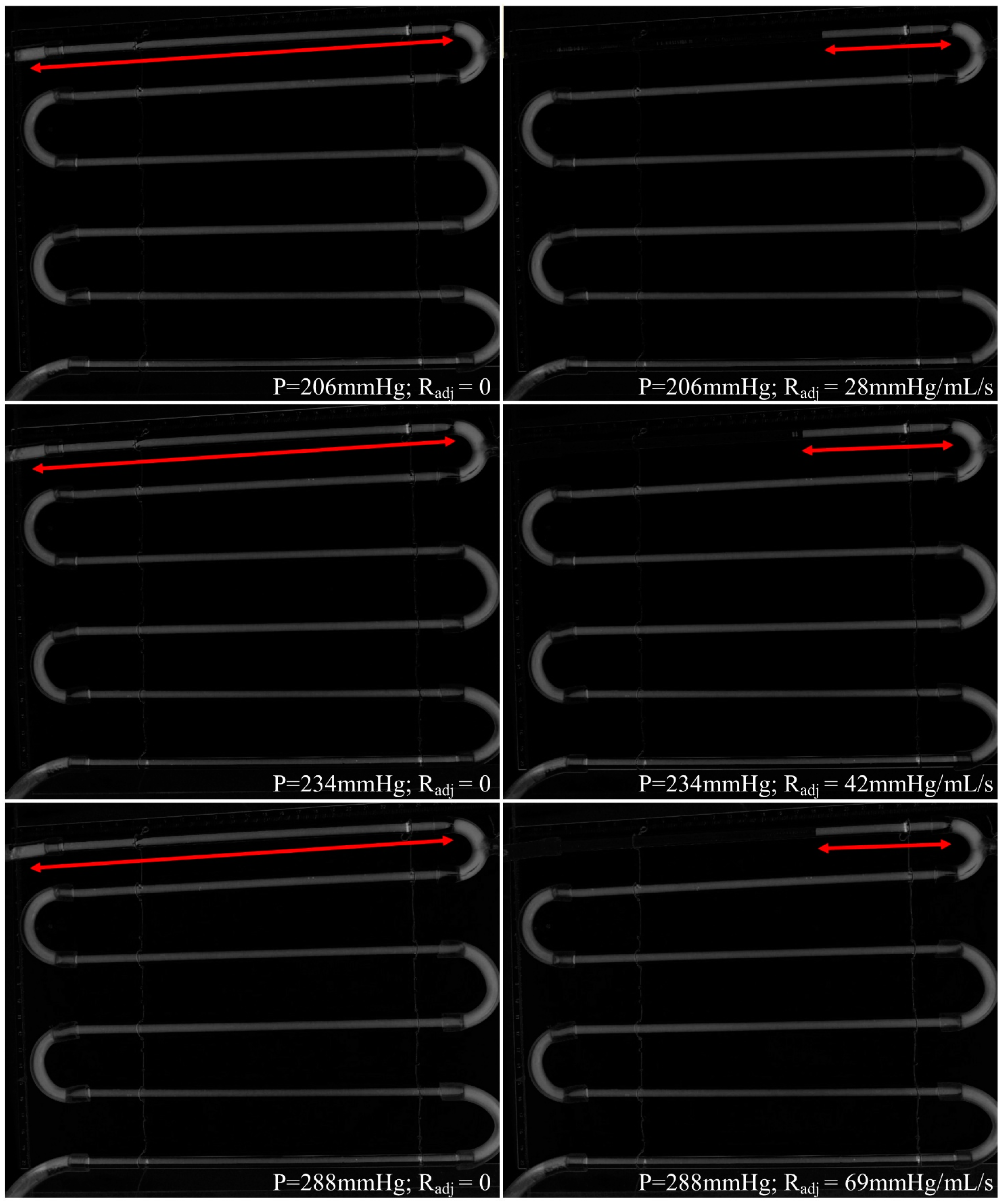
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hawkins, I.F.; Caridi, J.G. Carbon Dioxide (CO2) Digital Subtraction Angiography: 26-Year Experience at the University of Florida. Eur. Radiol. 1998, 8, 391–402. [Google Scholar] [CrossRef]
- Palena, L.M.; Diaz-Sandoval, L.J.; Candeo, A.; Brigato, C.; Sultato, E.; Manzi, M. Automated Carbon Dioxide Angiography for the Evaluation and Endovascular Treatment of Diabetic Patients with Critical Limb Ischemia. J. Endovasc. Ther. 2016, 23, 40–48. [Google Scholar] [CrossRef]
- Fontana, F.; Piacentino, F.; Ossola, C.; Curti, M.; Coppola, A.; Carcano, G.; Piffaretti, G.; Tozzi, M.; Venturini, M. Successful Endovascular Management with a Covered Stent of an External Iliac Pseudoaneurysm Following Allograft Nephrectomy Using CO2 as Contrast Medium: A Case Report. Radiol. Case Rep. 2021, 16, 3821–3823. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Noureldin, M.; Kashef, O.E.; Zaghlol, H. Safety and Effectiveness of Carbon Dioxide Contrast Medium in Infra-Inguinal Endovascular Interventions for Patients With Chronic Threatening Lower Limb Ischemia and Renal Impairment: A Multicentric Trial. J. Endovasc. Ther. 2023, 16, 15266028231159241. [Google Scholar] [CrossRef] [PubMed]
- Corazza, I.; Sapignoli, S.; Cercenelli, L.; Marcelli, E.; Faggioli, G.; Gargiulo, M.; Stella, A.; Diemberger, I.; Rossi, P.L.; Zannoli, R. Automated CO2 Angiography: Injection Pressure and Volume Settings. Med. Eng. Phys. 2020, 80, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Vacirca, A.; Faggioli, G.; Mascoli, C.; Gallitto, E.; Pini, R.; Spath, P.; Logiacco, A.; Palermo, S.; Gargiulo, M. CO2 Automated Angiography in Endovascular Aortic Repair Preserves Renal Function to a Greater Extent Compared with Iodinated Contrast Medium. Analysis of Technical and Anatomical Details. Ann. Vasc. Surg. 2022, 81, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kroneberger, C.; Enzweiler, C.N.; Schmidt-Lucke, A.; Rückert, R.-I.; Teichgräber, U.; Franiel, T. Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease and Peripheral Arterial Disease. Acta Radiol. Open 2015, 4, 2058460115583034. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Nikolsky, E. Contrast-Induced Nephropathy: Definition, Epidemiology, and Patients at Risk. Kidney Int. Suppl. 2006, 100, S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Messina, S.; Polimeno, M.; Corcione, N.; Ferraro, P.; Biondi-Zoccai, G.; Giordano, G. Peripheral Diagnostic and Interventional Procedures Using an Automated Injection System for Carbon Dioxide (CO2): Case Series and Learning Curve. Heart Lung Vessel. 2015, 7, 18–26. [Google Scholar] [PubMed]
- Bürckenmeyer, F.; Schmidt, A.; Diamantis, I.; Lehmann, T.; Malouhi, A.; Franiel, T.; Zanow, J.; Teichgräber, U.K.M.; Aschenbach, R. Image Quality and Safety of Automated Carbon Dioxide Digital Subtraction Angiography in Femoropopliteal Lesions: Results from a Randomized Single-Center Study. Eur. J. Radiol. 2021, 135, 109476. [Google Scholar] [CrossRef]
- Gallitto, E.; Faggioli, G.; Vacirca, A.; Pini, R.; Mascoli, C.; Fenelli, C.; Logiacco, A.; Abualhin, M.; Gargiulo, M. The Benefit of Combined Carbon Dioxide Automated Angiography and Fusion Imaging in Preserving Perioperative Renal Function in Fenestrated Endografting. J. Vasc. Surg. 2020, 72, 1906–1916. [Google Scholar] [CrossRef]
- Mascoli, C.; Faggioli, G.; Gallitto, E.; Vento, V.; Indelicato, G.; Pini, R.; Vacirca, A.; Stella, A.; Gargiulo, M. The Assessment of Carbon Dioxide Automated Angiography in Type II Endoleaks Detection: Comparison with Contrast-Enhanced Ultrasound. Contrast Media Mol. Imaging 2018, 2018, 7647165. [Google Scholar] [CrossRef] [PubMed]
- Mascoli, C.; Faggioli, G.; Gallitto, E.; Vento, V.; Pini, R.; Vacirca, A.; Indelicato, G.; Gargiulo, M.; Stella, A. Standardization of a Carbon Dioxide Automated System for Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2018, 51, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Criado, E.; Upchurch, G.R.; Young, K.; Rectenwald, J.E.; Coleman, D.M.; Eliason, J.L.; Escobar, G.A. Endovascular Aortic Aneurysm Repair with Carbon Dioxide-Guided Angiography in Patients with Renal Insufficiency. J. Vasc. Surg. 2012, 55, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Kawasaki, D.; Shintani, Y.; Fukunaga, M.; Nakama, T.; Koshida, R.; Higashimori, A.; Yokoi, Y.; CO2 Angiography Registry Investigators. Endovascular Therapy by CO2 Angiography to Prevent Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease: A Prospective Multicenter Trial of CO2 Angiography Registry. Catheter. Cardiovasc. Interv. 2015, 85, 870–877. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, C.; Sardanelli, F.; Perego, M.; Alì, M.; Casilli, F.; Inglese, L.; Mauri, G. Carbon Dioxide (CO2) Angiography as an Option for Endovascular Abdominal Aortic Aneurysm Repair (EVAR) in Patients with Chronic Kidney Disease (CKD). Int. J. Cardiovasc. Imaging 2017, 33, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Chanson, H. 13—Summary of Basic Hydraulic Principles. In Hydraulics of Open Channel Flow, 2nd; Chanson, H., Ed.; Butterworth-Heinemann: Oxford, UK, 2004; pp. 249–252. ISBN 978-0-7506-5978-9. [Google Scholar]
- Pfitzner, J. Poiseuille and His Law. Anaesthesia 1976, 31, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Corazza, I.; Taglieri, N.; Pirazzini, E.; Rossi, P.L.; Lombi, A.; Scalise, F.; Caridi, J.G.; Zannoli, R. Carbon Dioxide Coronary Angiography: A Mechanical Feasibility Study with a Cardiovascular Simulator. AIP Adv. 2018, 8, 015225. [Google Scholar] [CrossRef]
- Zannoli, R.; Corazza, I.; Branzi, A. Mechanical Simulator of the Cardiovascular System. Phys. Medica 2009, 25, 94–100. [Google Scholar] [CrossRef]
- Corazza, I.; Barletta, G.; Guaraldi, P.; Cecere, A.; Calandra-Buonaura, G.; Altini, E.; Zannoli, R.; Cortelli, P. A New Integrated Instrumental Approach to Autonomic Nervous System Assessment. Comput. Methods Programs Biomed. 2014, 117, 267–276. [Google Scholar] [CrossRef]
- Thomas, R.P.; Viniol, S.; König, A.M.; Portig, I.; Swaid, Z.; Mahnken, A.H. Feasibility and Safety of Automated CO2 Angiography in Peripheral Arterial Interventions. Medicine 2021, 100, e24254. [Google Scholar] [CrossRef] [PubMed]
- Caridi, J.G.; Hawkins, I.F.; Klioze, S.D.; LeVeen, R.F. Carbon Dioxide Digital Subtraction Angiography: The Practical Approach. Tech. Vasc. Interv. Radiol. 2001, 4, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Hawkins, I.F. Carbon Dioxide Angiography Principles, Techniques, and Practices; Informa Healthcare: New York, NY, USA, 2007; ISBN 978-1-4200-1626-0. [Google Scholar]
- Massaini, G.; Lazzarotto, T.; Masciello, F.; Panci, S.; Michelagnoli, S.; Chisci, E. A Pilot Study of Endovascular Repair for Ruptured Aortic Aneurysms with the Use of Carbon Dioxide Angiography Alone. J. Endovasc. Ther. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Glechner, A.; Persad, E.; Klerings, I.; Gartlehner, G.; Moertl, D.; Steiner, S. Risk of Contrast-Associated Acute Kidney Injury in Patients Undergoing Peripheral Angiography with Carbon Dioxide Compared to Iodine-Containing Contrast Agents: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7203. [Google Scholar] [CrossRef]
- Liss, P.; Eklöf, H.; Hellberg, O.; Hägg, A.; Boström-Ardin, A.; Löfberg, A.-M.; Olsson, U.; Örndahl, P.; Nilsson, H.; Hansell, P.; et al. Renal Effects of CO2 and Iodinated Contrast Media in Patients Undergoing Renovascular Intervention: A Prospective, Randomized Study. J. Vasc. Interv. Radiol. 2005, 16, 57–65. [Google Scholar] [CrossRef]
- Sharafuddin, M.J.; Marjan, A.E. Current Status of Carbon Dioxide Angiography. J. Vasc. Surg. 2017, 66, 618–637. [Google Scholar] [CrossRef]

| Injection Pressure (mmHg) | Radj (mmHg/mL/s) | Duration ± 0.4 (s) | Time to Obtain the Complete Radiological Image ± 0.4 (s) | Tube Length Covered by Reflux (cm) |
|---|---|---|---|---|
| 150 | 0 | 10.5 | 8.0 | 2 |
| 206 | 0 | 6.0 | 7.8 | >24 |
| 28 | 10.4 | 7.8 | 6 | |
| 234 | 0 | 4.1 | 7.8 | >24 |
| 42 | 7.5 | 8.0 | 8 | |
| 288 | 0 | 4.0 | 7.7 | >24 |
| 69 | 7.5 | 8.3 | 7 |
| Injection Pressure (mmHg) | Radj (mmHg/mL/s) | Systolic Increase after Injection ± 2 (mmHg) | Diastolic Increase after Injection ± 2 (mmHg) |
|---|---|---|---|
| 150 | 0 | 7 | 5 |
| 206 | 0 | 10 | 25 |
| 28 | 6 | 10 | |
| 234 | 0 | 13 | 25 |
| 42 | 6 | 10 | |
| 288 | 0 | 15 | 25 |
| 69 | 10 | 10 |
| Catheter with Saline | ||
|---|---|---|
| Pinj (mmHg) | Radj (mmHg/mL/s) | Delay ± 0.4 (s) |
| 150 | 0 | 6.0 |
| 288 | 69 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corazza, I.; Rossi, P.L.; Zannoli, R. Calibrated Hydraulic Resistance Adjuncts for Carbon Dioxide Angiography Optimization. Appl. Sci. 2024, 14, 1276. https://doi.org/10.3390/app14031276
Corazza I, Rossi PL, Zannoli R. Calibrated Hydraulic Resistance Adjuncts for Carbon Dioxide Angiography Optimization. Applied Sciences. 2024; 14(3):1276. https://doi.org/10.3390/app14031276
Chicago/Turabian StyleCorazza, Ivan, Pier Luca Rossi, and Romano Zannoli. 2024. "Calibrated Hydraulic Resistance Adjuncts for Carbon Dioxide Angiography Optimization" Applied Sciences 14, no. 3: 1276. https://doi.org/10.3390/app14031276
APA StyleCorazza, I., Rossi, P. L., & Zannoli, R. (2024). Calibrated Hydraulic Resistance Adjuncts for Carbon Dioxide Angiography Optimization. Applied Sciences, 14(3), 1276. https://doi.org/10.3390/app14031276





