Innovative Methods of Encapsulation and Enrichment of Cereal-Based Pasta Products with Biofunctional Compounds
Abstract
:1. Introduction
2. Methods of Encapsulation
2.1. Emulsion-Based Encapsulation and Hydrogels
2.2. Spray-Drying
2.3. Freeze-Drying
2.4. Liposomes
2.5. Selected Other Methods
3. Application of Microencapsulated Ingredients in Pasta Products
3.1. Oils and Fatty Acids
3.2. Fruit and Vegetable Biocompounds
3.3. Probiotic Bacteria and Other Microorganisms
3.4. Vitamins and Minerals
4. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Biological, chemical, and microbiological | |
| AI | Acid index |
| CL | Cooking loss |
| DHA | Docosahexaenoic acid (C22:6 n − 3) |
| DWPI | Denatured whey protein isolate |
| EPA | Eicosapentaenoic acid (C20:5 n − 3) |
| FA | Fatty acids |
| FOS | Fructo oligosaccharide |
| HPV | Hydroperoxide value |
| HTD | High-temperature dried (55 °C) |
| K232 | Conjugated dienes |
| K270 | Conjugated trienes |
| L-5-MTHF | L-5-methyltetrahydro-folate |
| LC n − 3 PUFA | Long-chain omega-3 polyunsaturated fatty acids |
| n − 3 | Omega-3 fatty acids |
| n-OSA | Octenyl succinic anhydride |
| OFA | Oxygenated fatty acid |
| OSI | Oxidative stability index |
| PV | Peroxide value |
| RTD | Room-temperature dried (28 °C) |
| SC-CO2 | Supercritical carbon dioxide |
| SPI | Soy protein isolate |
| WA | Water absorption |
| WPI | Whey protein isolate |
| Instrumental techniques | |
| CC | Coacervation |
| FD | Freeze-drying |
| GP | Gel particle encapsulation |
| SD | Spray-drying |
References
- Contor, L. Functional Food Science in Europe. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 20–23. [Google Scholar]
- Raczyk, M.; Kruszewski, B.; Zachariasz, E. Effect of Tomato, Beetroot and Carrot Juice Addition on Physicochemical, Antioxidant and Texture Properties of Wheat Bread. Antioxidants 2022, 11, 2178. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat Bread Fortification by Grape Pomace Powder: Nutritional, Technological, Antioxidant, and Sensory Properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An Overview on Concepts, Methods, Properties and Applications in Foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Figueiredo, J.A.; Silva, C.R.P.; Oliveira, M.F.S.; Norcino, L.B.; Campelo, P.H.; Botrel, D.A.; Borges, S.V. Microencapsulation by Spray Chilling in the Food Industry: Opportunities, Challenges, and Innovations. Trends Food Sci. Technol. 2022, 120, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An Overview of Microencapsulation in the Food Industry: Opportunities, Challenges, and Innovations. Eur. Food Res. Technol. 2020, 246, 1371–1382. [Google Scholar] [CrossRef]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Bigović, D.; Janković, T. Application of Gum Arabic in the Production of Spray-Dried Chokeberry Polyphenols, Microparticles Characterisation and in Vitro Digestion Method. Lek. Sirovine 2018, 2020, 9–16. [Google Scholar] [CrossRef]
- Gheonea (Dima), I.; Aprodu, I.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Microencapsulation of Lycopene from Tomatoes Peels by Complex Coacervation and Freeze-Drying: Evidences on Phytochemical Profile, Stability and Food Applications. J. Food Eng. 2020, 288, 110166. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Microencapsulation of Betacyanin from Dragon Fruit Peel by Complex Coacervation: Physicochemical Characteristics, Thermal Stability, and Release Profile of Microcapsules. Food Biosci. 2022, 49, 101882. [Google Scholar] [CrossRef]
- Tylkowski, B.; Tsibranska, I. Polyphenols Encapsulation—Application of Innovation Technologies to Improve Stability of Natural Products. Phys. Sci. Rev. 2019, 1, 20150005. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-Based Encapsulation and Delivery Systems for Polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Hanula, M.; Szpicer, A.; Górska-Horczyczak, E.; Khachatryan, G.; Pogorzelska-Nowicka, E.; Poltorak, A. Quality of Beef Burgers Formulated with Fat Substitute in a Form of Freeze-Dried Hydrogel Enriched with Açai Oil. Molecules 2022, 27, 3700. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Antipina, M.N. Emulsion-Based Techniques for Encapsulation in Biomedicine, Food and Personal Care. Curr. Opin. Pharmacol. 2014, 18, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, J.; Zeng, Y.; Dai, J.; Li, S.; Yan, J.; Liu, Y. Effect of Water-in-Oil-in-Water (W/O/W) Double Emulsions to Encapsulate Nisin on the Quality and Storage Stability of Fresh Noodles. Food Chem. X 2022, 15, 100378. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.N.; Khaled, B.M.; Liu, F.; Zhong, F. Hydrogel Beads for Designing Future Foods: Structures, Mechanisms, Applications, and Challenges. Food Hydrocoll. Health 2022, 2, 100073. [Google Scholar] [CrossRef]
- Di Girolamo, S.; Puorger, C.; Lipps, G. Stable and Selective Permeable Hydrogel Microcapsules for High-Throughput Cell Cultivation and Enzymatic Analysis. Microb. Cell Fact. 2020, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Hanula, M.; Szpicer, A.; Górska-Horczyczak, E.; Khachatryan, G.; Pogorzelski, G.; Pogorzelska-Nowicka, E.; Poltorak, A. Hydrogel Emulsion with Encapsulated Safflower Oil Enriched with Açai Extract as a Novel Fat Substitute in Beef Burgers Subjected to Storage in Cold Conditions. Molecules 2022, 27, 2397. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods 2023, 12, 350. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Zhao, R.; Shi, Q.; Nian, Y.; Hu, B. Hydrogels as Promising Carriers for the Delivery of Food Bioactive Ingredients. Front. Nutr. 2022, 9, 1006520. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil Encapsulation Techniques Using Alginate as Encapsulating Agent: Applications and Drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef]
- da Silva, L.C.; Castelo, R.M.; Cheng, H.N.; Biswas, A.; Furtado, R.F.; Alves, C.R. Methods of Microencapsulation of Vegetable Oil: Principles, Stability and Applications—A Minireview. Food Technol. Biotechnol. 2022, 60, 308–320. [Google Scholar] [CrossRef]
- Rigon, R.T.; Zapata Noreña, C.P. Microencapsulation by Spray-Drying of Bioactive Compounds Extracted from Blackberry (Rubus Fruticosus). J. Food Sci. Technol. 2016, 53, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Szpicer, A.; Onopiuk, A.; Barczak, M.; Kurek, M. The Optimization of a Gluten-Free and Soy-Free Plant-Based Meat Analogue Recipe Enriched with Anthocyanins Microcapsules. LWT 2022, 168, 113849. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Microencapsulation of Food Bioactive Components by Spray Drying: A Review. Dry. Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A Review on the Encapsulation of Bioactive Components Using Spray-Drying and Freeze-Drying Techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tanska, M.; Brandt, W.; Czaplicki, S. Impact of the Encapsulation Process by Spray- And Freeze-Drying on the Properties and Composition of Powders Obtained from Cold-Pressed Seed Oils with Various Unsaturated Fatty Acids. Pol. J. Food Nutr. Sci. 2020, 70, 241–252. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Spray Drying, Freeze Drying and Related Processes for Food Ingredient and Nutraceutical Encapsulation; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Zhao, Y.; Khalid, N.; Shu, G.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Complex Coacervates from Gelatin and Octenyl Succinic Anhydride Modified Kudzu Starch: Insights of Formulation and Characterization. Food Hydrocoll. 2019, 86, 70–77. [Google Scholar] [CrossRef]
- Tatasciore, S.; Santarelli, V.; Neri, L.; González Ortega, R.; Faieta, M.; Di Mattia, C.D.; Di Michele, A.; Pittia, P. Freeze-Drying Microencapsulation of Hop Extract: Effect of Carrier Composition on Physical, Techno-Functional, and Stability Properties. Antioxidants 2023, 12, 442. [Google Scholar] [CrossRef]
- Abdul Mudalip, S.K.; Khatiman, M.N.; Hashim, N.A.; Che Man, R.; Arshad, Z.I.M. A Short Review on Encapsulation of Bioactive Compounds Using Different Drying Techniques. Mater. Today Proc. 2019, 42, 288–296. [Google Scholar] [CrossRef]
- Kamali Rousta, L.; Bodbodak, S.; Nejatian, M.; Ghandehari Yazdi, A.P.; Rafiee, Z.; Xiao, J.; Jafari, S.M. Use of Encapsulation Technology to Enrich and Fortify Bakery, Pasta, and Cereal-Based Products. Trends Food Sci. Technol. 2021, 118, 688–710. [Google Scholar] [CrossRef]
- Trilokia, M.; Campus Chatha, M.; Julie Bandral, I.D.; Ankita Chib, I.; Preeti Choudhary Agromet Observer, I.; Corresponding Author, I.; Bandral, J.D.; Chib, A.; Choudhary, P. Microencapsulation for Food: An Overview. Pharma. Innov. J. 2022, 11, 1174–1180. [Google Scholar]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of Anthocyanins—Critical Review of Techniques and Wall Materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Soumya, S.B.S.; Sawant, A.A.; Khandetod, Y.P. Microencapsulation Techniques Used for Underutilized Fruits of Western Ghat: An Overview. Pharma Innov. J. 2021, 10, 826–833. [Google Scholar]
- Chen, H.W.; Chang, Y.W.; Fang, W.P. A New Approach for the Microencapsulation of Clitoria Ternatea Petal Extracts by a High-Pressure Processing Method. Pharmaceutics 2021, 13, 23. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.; Desale, R.J.; Fulpagare, Y.G. Microencapsulation: Applications in the Different Dairy Products. Int. J. Pharm. Biomed. Eng. 2019, 6, 7–11. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Santos, P.D.F.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of Carotenoid-Rich Materials: A Review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef] [PubMed]
- Mirzamani, S.S.; Bassiri, A.; Tavakolipour, H.; Azizi, M.H.; Kargozari, M. Fluidized Bed Microencapsulation of Lactobacillus Sporogenes with Some Selected Hydrocolloids for Probiotic Bread Production. J. Food Biosci. Technol. 2021, 11, 23–34. [Google Scholar]
- Pattnaik, M.; Pandey, P.; Martin, G.J.O.; Mishra, H.N.; Ashokkumar, M. Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development. Foods 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P.; Gaiani, C.; Edorh, J.-M.; Borges, F.; Beaupeux, E.; Maudhuit, A.; Desobry, S. Comparison of Electrostatic Spray Drying, Spray Drying, and Freeze Drying for Lacticaseibacillus Rhamnosus GG Dehydration. Foods 2023, 12, 3117. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of Bioactive Compounds Using Competitive Emerging Techniques: Electrospraying, Nano Spray Drying, and Electrostatic Spray Drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Macías-Cortés, E.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Medina-Torres, L.; González-Laredo, R.F. Microencapsulation of Phenolic Compounds: Technologies and Novel Polymers. Rev. Mex. Ing. Quim. 2020, 19, 491–521. [Google Scholar] [CrossRef]
- Reis, D.R.; Ambrosi, A.; Luccio, M. Di Encapsulated Essential Oils: A Perspective in Food Preservation. Futur. Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Bodbodak, S.; Nejatian, M.; Ghandehari Yazdi, A.P.; Kamali Rousta, L.; Rafiee, Z.; Jalali-Jivan, M.; Kharazmi, M.S.; Jafari, S.M. Improving the Thermal Stability of Natural Bioactive Ingredients via Encapsulation Technology. Crit. Rev. Food Sci. Nutr. 2022, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.; Farouk, A. Encapsulation of Essential Oils and Their Use in Food Applications. In Essential Oils—Advances in Extractions and Biological Applications; IntechOpen: London, UK, 2022; pp. 1–15. [Google Scholar]
- Martins, E.; Poncelet, D.; Renard, D. A Novel Method of Oil Encapsulation in Core-Shell Alginate Microcapsules by Dispersion-Inverse Gelation Technique. React. Funct. Polym. 2017, 114, 49–57. [Google Scholar] [CrossRef]
- González, A.; Bordón, M.G.; Bustos, M.C.; Córdova Salazar, K.L.; Ribotta, P.D.; Martínez, M.L. Study of the Incorporation of Native and Microencapsulated Chia Seed Oil on Pasta Properties. Int. J. Food Sci. Technol. 2021, 56, 233–241. [Google Scholar] [CrossRef]
- Iafelice, G.; Caboni, M.F.; Cubadda, R.; Di Criscio, T.; Trivisonno, M.C.; Marconi, E. Development of Functional Spaghetti Enriched with Long Chain Omega-3 Fatty Acids. Cereal Chem. 2008, 85, 146–151. [Google Scholar] [CrossRef]
- Verardo, V.; Ferioli, F.; Riciputi, Y.; Iafelice, G.; Marconi, E.; Caboni, M.F. Evaluation of Lipid Oxidation in Spaghetti Pasta Enriched with Long Chain N − 3 Polyunsaturated Fatty Acids under Different Storage Conditions. Food Chem. 2009, 114, 472–477. [Google Scholar] [CrossRef]
- Pashaei, N.; Salehifar, M.; Fahimdanesh, M. Evaluation of Stability Omega-3 Fatty Acids Stability in Different Drying Conditions and Its Microencapsulated Retention in Enriched Spaghetti. J. Food Technol. Nutr. 2020, 17, 39–48. [Google Scholar]
- Jeyakumari, A.; Zynudheen, A.; Murthy, L.; Parvathy, U. Effect of Sodium Alginate/Carrageenan Composites on the Oxidative Stability of Microencapsulated Fish Oil and Its Application in Noodles. ICAR-Cent. Inst. Fish. Technol. 2017, Asian Fish, 368. [Google Scholar]
- Durante, M.; Lenucci, M.S.; Gazza, L.; Taddei, F.; Nocente, F.; De Benedetto, G.E.; De Caroli, M.; Piro, G.; Mita, G. Bioactive Composition and Sensory Evaluation of Innovative Spaghetti Supplemented with Free or α-Cyclodextrin Chlatrated Pumpkin Oil Extracted by Supercritical CO2. Food Chem. 2019, 294, 112–122. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High Value-Added Compounds from Fruit and Vegetable by-Products–Characterization, Bioactivities, and Application in the Development of Novel Food Products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit By-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.S.F.; Nunes, G.L.; da Silva, A.F.M.; Viegas, G.I.; Lucas, B.N.; Barin, J.S.; de Menezes, C.R.; da Rosa, C.S. Addition of Microencapsulated Soybean Molasses to Pasta Formulations. Cienc. Rural 2021, 51, e20200722. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Improvement of Thermal Stability and Antioxidant Activity of Anthocyanins of Echium Amoenum Petal Using Maltodextrin/Modified Starch Combination as Wall Material. Int. J. Biol. Macromol. 2020, 148, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Wang, Y.; Ho, C.T.; Patiguli, M.; Zhang, Y.; Yu, B.; Zhang, C.; Aadil, R.M.; Qu, W.; Xiao, R.; et al. Addition of Chlorophyll Microcapsules to Improve the Quality of Fresh Wheat Noodles. Learn. Technol. 2023, 183, 114940. [Google Scholar] [CrossRef]
- Ćetković, G.; Šeregelj, V.; Brandolini, A.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Šovljanski, O.; Četojević-Simin, D.; Škrobot, D.; Mandić, A.; et al. Composition, Texture, Sensorial Quality, and Biological Activity after in Vitro Digestion of Durum Wheat Pasta Enriched with Carrot Waste Extract Encapsulates. Int. J. Food Sci. Nutr. 2022, 73, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Zen, C.K.; Tiepo, C.B.V.; da Silva, R.V.; Reinehr, C.O.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of Functional Pasta with Microencapsulated Spirulina: Technological and Sensorial Effects. J. Sci. Food Agric. 2020, 100, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Elsebaie, E.M.; Asker, G.A.; Mousa, M.M.; Kassem, M.M.; Essa, R.Y. Technological and Sensory Aspects of Macaroni with Free or Encapsulated Azolla Fern Powder. Foods 2022, 11, 707. [Google Scholar] [CrossRef]
- Šeregelj, V.; Škrobot, D.; Kojić, J.; Pezo, L.; Šovljanski, O.; Šaponjac, V.T.; Vulić, J.; Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; et al. Quality and Sensory Profile of Durum Wheat Pasta Enriched with Carrot Waste Encapsulates. Foods 2022, 11, 1130. [Google Scholar] [CrossRef]
- Singh, P.; Bohra, A.; Sheoran, S.; Arora, S. Microencapsulation of Ferulic Acid from Vegetables Waste and Its Application in Pasta. Int. J. Res. Anal. Rev. 2019, 6, 674–686. [Google Scholar]
- Safeer Abbas, M.; Afzaal, M.; Saeed, F.; Asghar, A.; Jianfeng, L.; Ahmad, A.; Ullah, Q.; Elahi, S.; Ateeq, H.; Shah, Y.A.; et al. Probiotic Viability as Affected by Encapsulation Materials: Recent Updates and Perspectives. Int. J. Food Prop. 2023, 26, 1324–1350. [Google Scholar] [CrossRef]
- Sbehat, M.; Mauriello, G.; Altamimi, M. Microencapsulation of Probiotics for Food Functionalization: An Update on Literature Reviews. Microorganisms 2022, 10, 1948. [Google Scholar] [CrossRef]
- Tolve, R.; Galgano, F.; Caruso, M.C.; Tchuenbou-Magaia, F.L.; Condelli, N.; Favati, F.; Zhang, Z. Encapsulation of Health-Promoting Ingredients: Applications in Foodstuffs. Int. J. Food Sci. Nutr. 2016, 67, 888–918. [Google Scholar] [CrossRef] [PubMed]
- De Prisco, A.; Mauriello, G. Probiotication of Foods: A Focus on Microencapsulation Tool. Trends Food Sci. Technol. 2016, 48, 27–39. [Google Scholar] [CrossRef]
- Rajam, R.; Subramanian, P. Encapsulation of Probiotics: Past, Present and Future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Alkan, S. Production of Functional Turkish Noodle (Erişte) Supplementary Probiotic and Determining of Some Quality Properties. Food Health 2020, 6, 140–150. [Google Scholar] [CrossRef]
- Rajam, R.; Kumar, S.B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus Plantarum MTCC 5422 in Fructooligosaccharide and Whey Protein Wall Systems and Its Impact on Noodle Quality. J. Food Sci. Technol. 2015, 52, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.P.; He, J. Microencapsulation of Vitamins in Food Applications to Prevent Losses in Processing and Storage: A Review. Food Res. Int. 2020, 137, 109326. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Ren, M.; Liang, D.Y.; Lai, S.; Hartono, B.; Sum, K.; Pratap-Singh, A. Sprayed Microcapsules of Minerals for Fortified Food. J. Funct. Foods 2023, 101, 105401. [Google Scholar] [CrossRef]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D Microencapsulation and Fortification: Trends and Technologies. J. Steroid Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, B.N.; Lazar, R.; Blaga, A.; Rocha, F. Preliminary Evaluation and Studies on the Preparation, Characterization and in Vitro Release Studies of Different Biopolymer Microparticles for Controlled Release of Folic Acid. Powder Technol. 2020, 369, 279–288. [Google Scholar] [CrossRef]
- Liu, Y.; Green, T.J.; Kitts, D.D. Stability of Microencapsulated L-5-Methyltetrahydrofolate in Fortified Noodles. Food Chem. 2015, 171, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Yingleardrattanakul, P.; Thompson, A.K.; Taprap, R.; Pinsirodom, P.; Bindu, C. Evaluation of the Efficiency of Various Folic Acid Microencapsulation Techniques for Rice Vermicelli (Khanom Jeen) Fortification. Food Humanit. 2024, 2, 100198. [Google Scholar] [CrossRef]

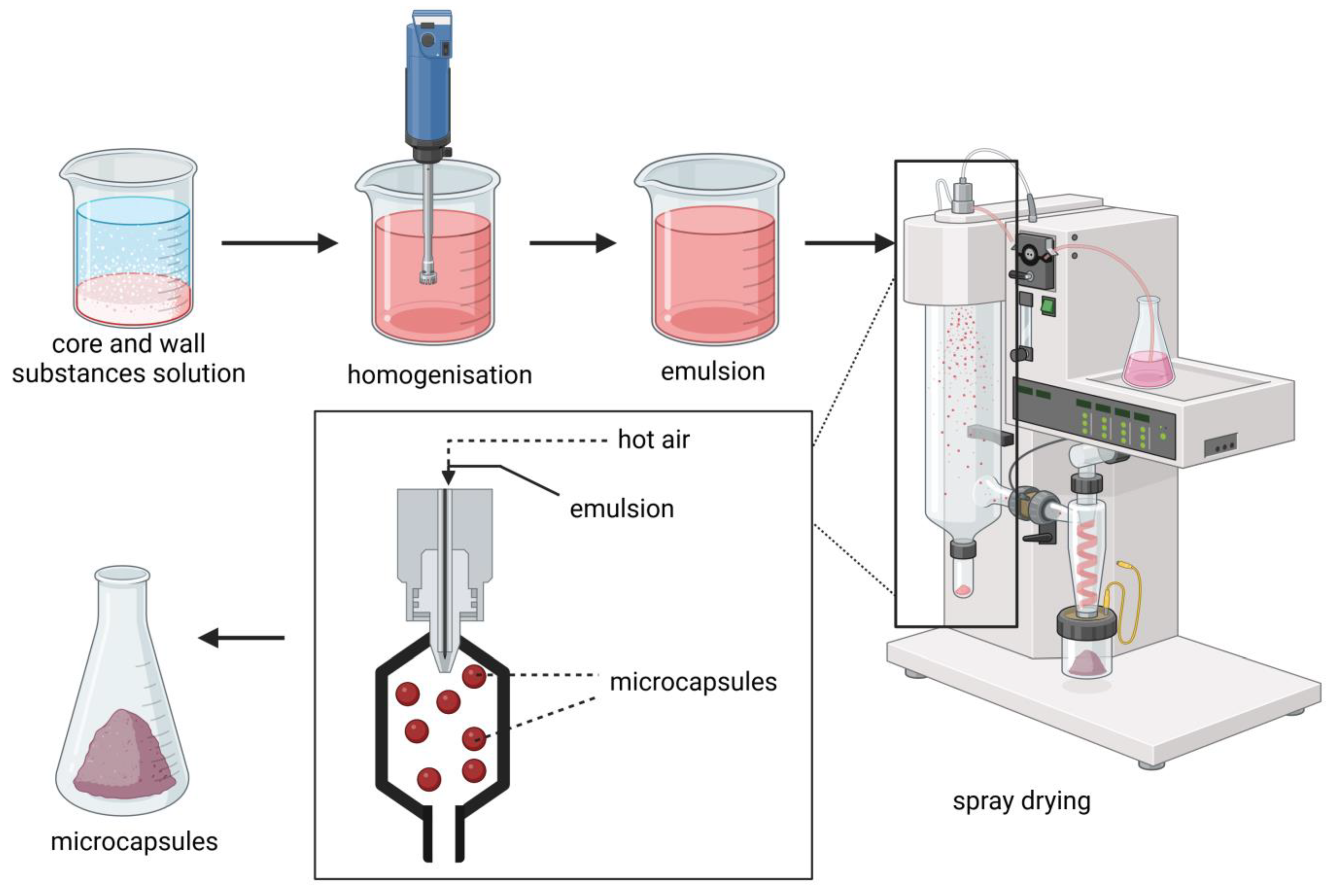
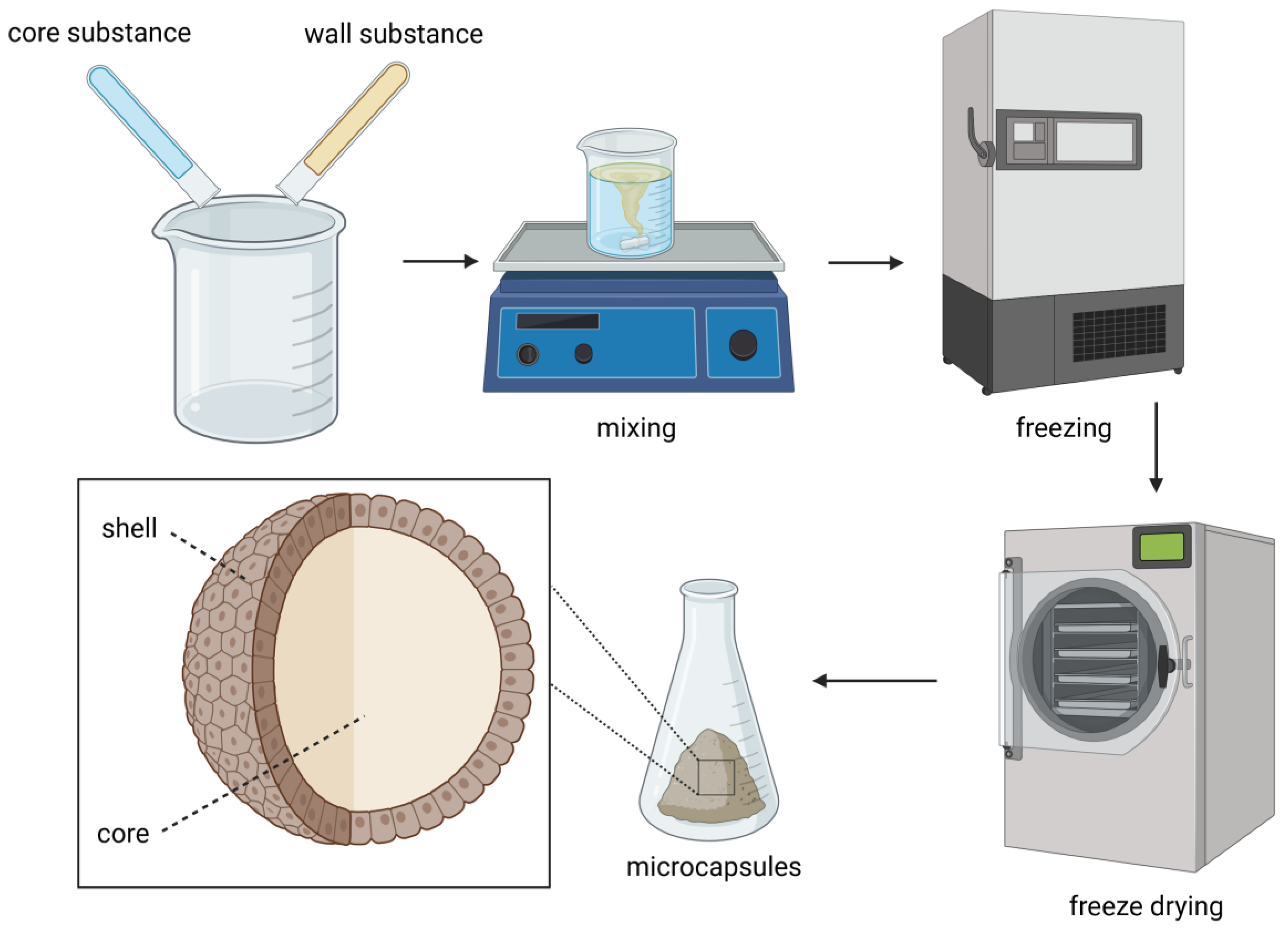
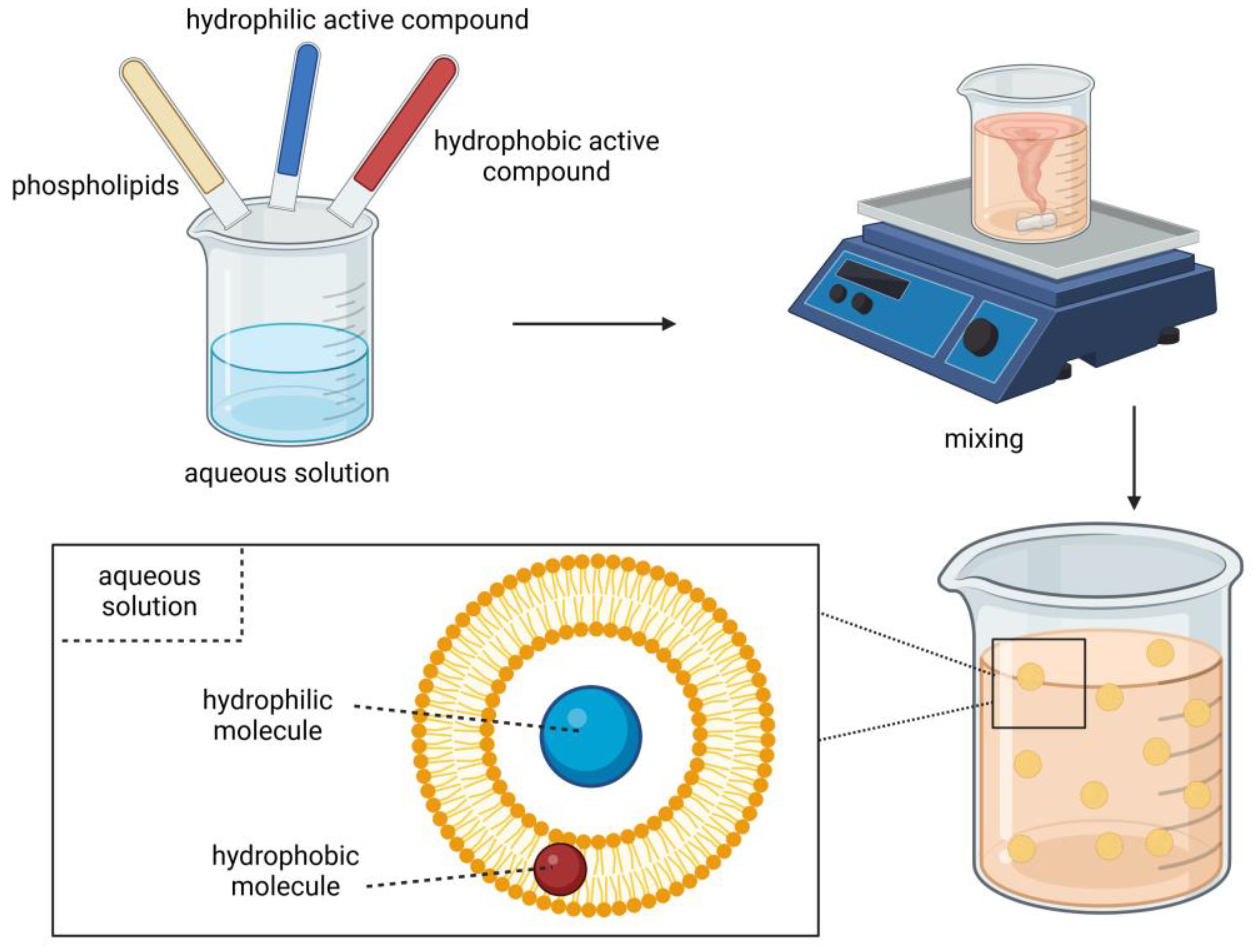
| Core Material | Wall Material | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| Chia seed oil | Soy protein isolate | FD |
| [57] |
| LC n − 3 PUFA (marine oil) | Cornstarch-coated matrix of fish gelatin and sucrose | No data. Marketable product was used—ROPUFA 10 n − 3 food powder (Roche, Basel, Switzerland) |
| [58] |
| LC n − 3 PUFA (marine oil) | Cornstarch-coated matrix of fish gelatin and sucrose | No data. Marketable product was used—ROPUFA 10 n − 3 food powder (Roche, Basel, Switzerland) |
| [59] |
| LC n − 3 PUFA | Modified starch, sucrose, sodium ascorbate, silicon dioxide | No data. Marketable product was used—ROPUFA 10 n − 3 food powder (Roche, Basel, Switzerland) |
| [60] |
| Fish oil | Sodium alginate, Carrageenan along with gelatin and maltodextrin | SD α-cyclodextrin |
| [61] |
| Pumpkin oil | α-cyclodextrin | FD |
| [62] |
| Core Material | Wall Material | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| Soybean molasses | Hi-maize® | SD |
| [67] |
| Carrot waste extract | Whey protein (FD), whey protein/inulin (SD) | FD, SD |
| [70] |
| Chlorophyll | Gum arabic/whey protein isolate | FD |
| [69] |
| Spirulina | Sodium alginate/calcium chloride | SD |
| [71] |
| Azolla | Sodium alginate/calcium chloride | FD |
| [72] |
| Carrot waste extract | Whey protein (FD), whey protein/inulin (SD) | FD, SD |
| [73] |
| Ferulic acid from tomato pomace | Whey protein isolate (1:1 and 1.5:1 wall-to-core ratio) | SD (105 °C and 90 °C drying) |
| [74] |
| Core Material | Wall Material | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| Bacillus clausii | Alginate | Extrusion |
| [80] |
| Lactobacillus plantarum (MTCC 5422) | FOS, DWPI, WPI, and their mixtures | FD |
| [81] |
| Core Material | Wall Material | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| L-5-methyltetrahydro-folate (L-5-MTHF) | Modified starch | SD |
| [86] |
| Folic acid | Pectin/sodium alginate/sodium chloride/chitosan (GP), gelatin/gum Arabic/chitin (CC), pectin/sodium alginate (SD) | Gel particle encapsulation (GP), coacervation (CC), SD |
| [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bińkowska, W.; Szpicer, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Innovative Methods of Encapsulation and Enrichment of Cereal-Based Pasta Products with Biofunctional Compounds. Appl. Sci. 2024, 14, 1442. https://doi.org/10.3390/app14041442
Bińkowska W, Szpicer A, Wojtasik-Kalinowska I, Półtorak A. Innovative Methods of Encapsulation and Enrichment of Cereal-Based Pasta Products with Biofunctional Compounds. Applied Sciences. 2024; 14(4):1442. https://doi.org/10.3390/app14041442
Chicago/Turabian StyleBińkowska, Weronika, Arkadiusz Szpicer, Iwona Wojtasik-Kalinowska, and Andrzej Półtorak. 2024. "Innovative Methods of Encapsulation and Enrichment of Cereal-Based Pasta Products with Biofunctional Compounds" Applied Sciences 14, no. 4: 1442. https://doi.org/10.3390/app14041442
APA StyleBińkowska, W., Szpicer, A., Wojtasik-Kalinowska, I., & Półtorak, A. (2024). Innovative Methods of Encapsulation and Enrichment of Cereal-Based Pasta Products with Biofunctional Compounds. Applied Sciences, 14(4), 1442. https://doi.org/10.3390/app14041442










