Kinetic Modeling of the Photocatalytic Degradation of Chlorinated Aromatic Volatile Organic Compounds: Mass Transfer Enhancement
Abstract
1. Introduction
2. Experimental Setup
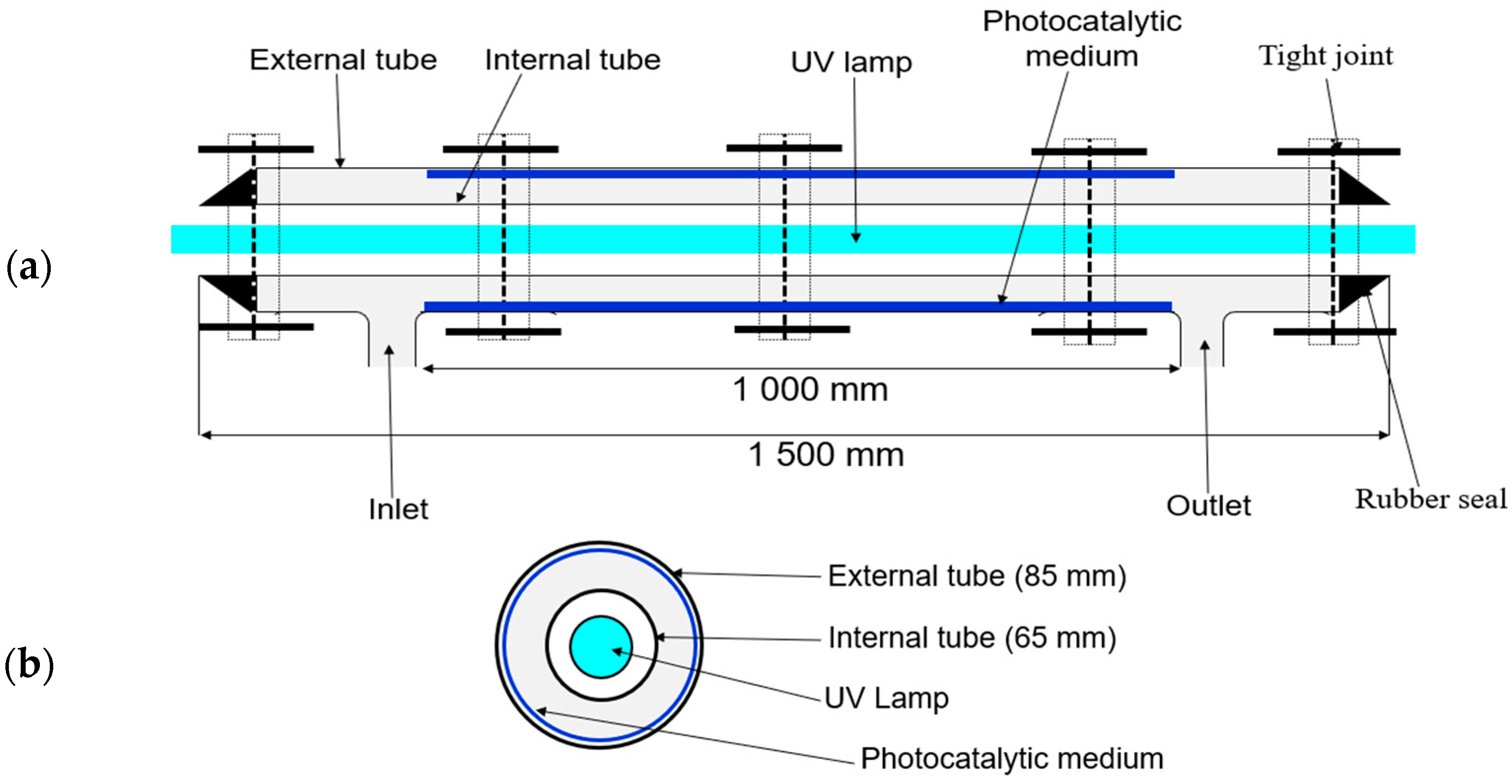
2.1. Photocatalytic Reactor Configuration
2.2. Gas Phase Analysis
3. Results and Discussion
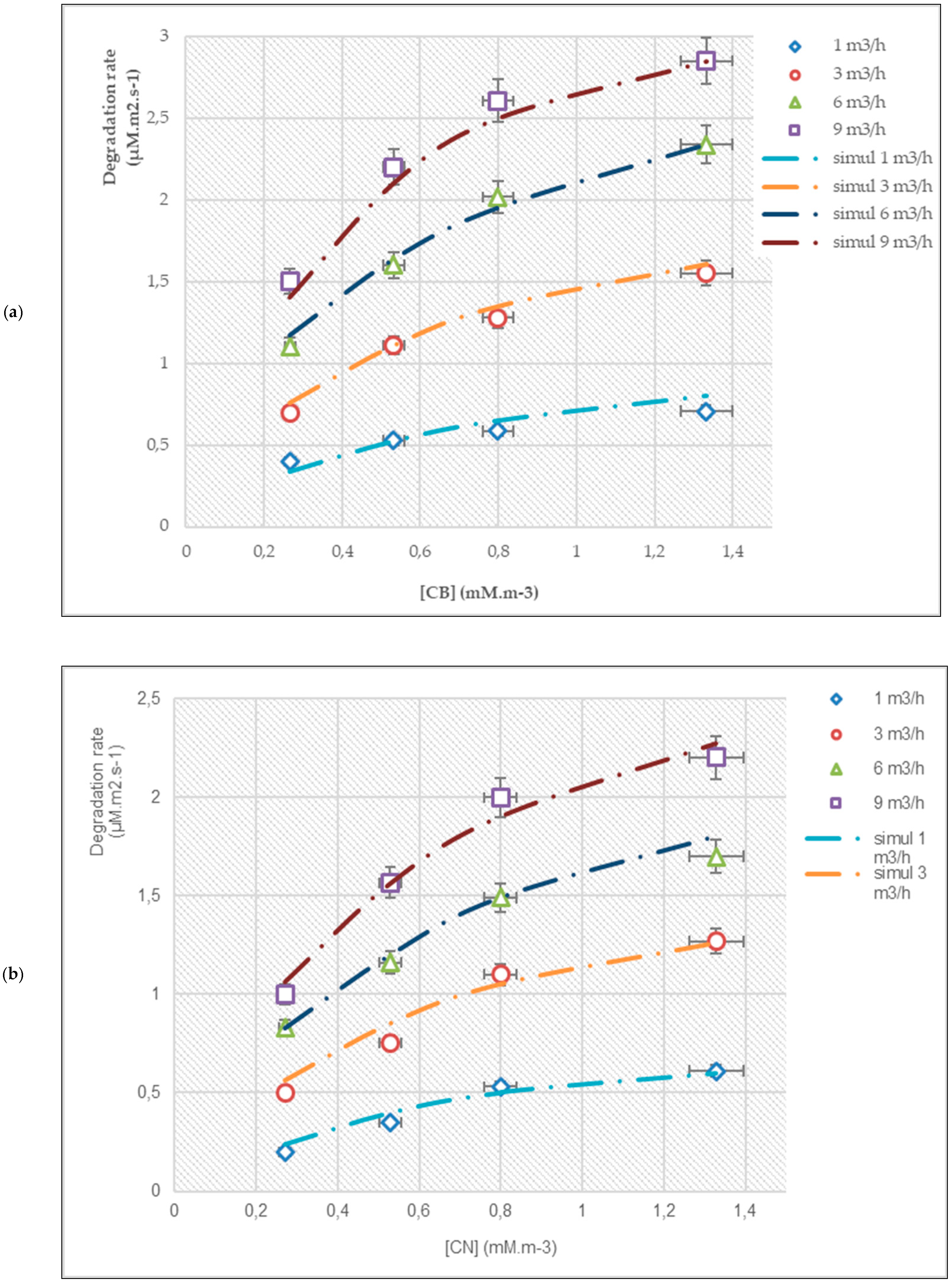
3.1. Effect of Flow Rate and Inlet Concentration
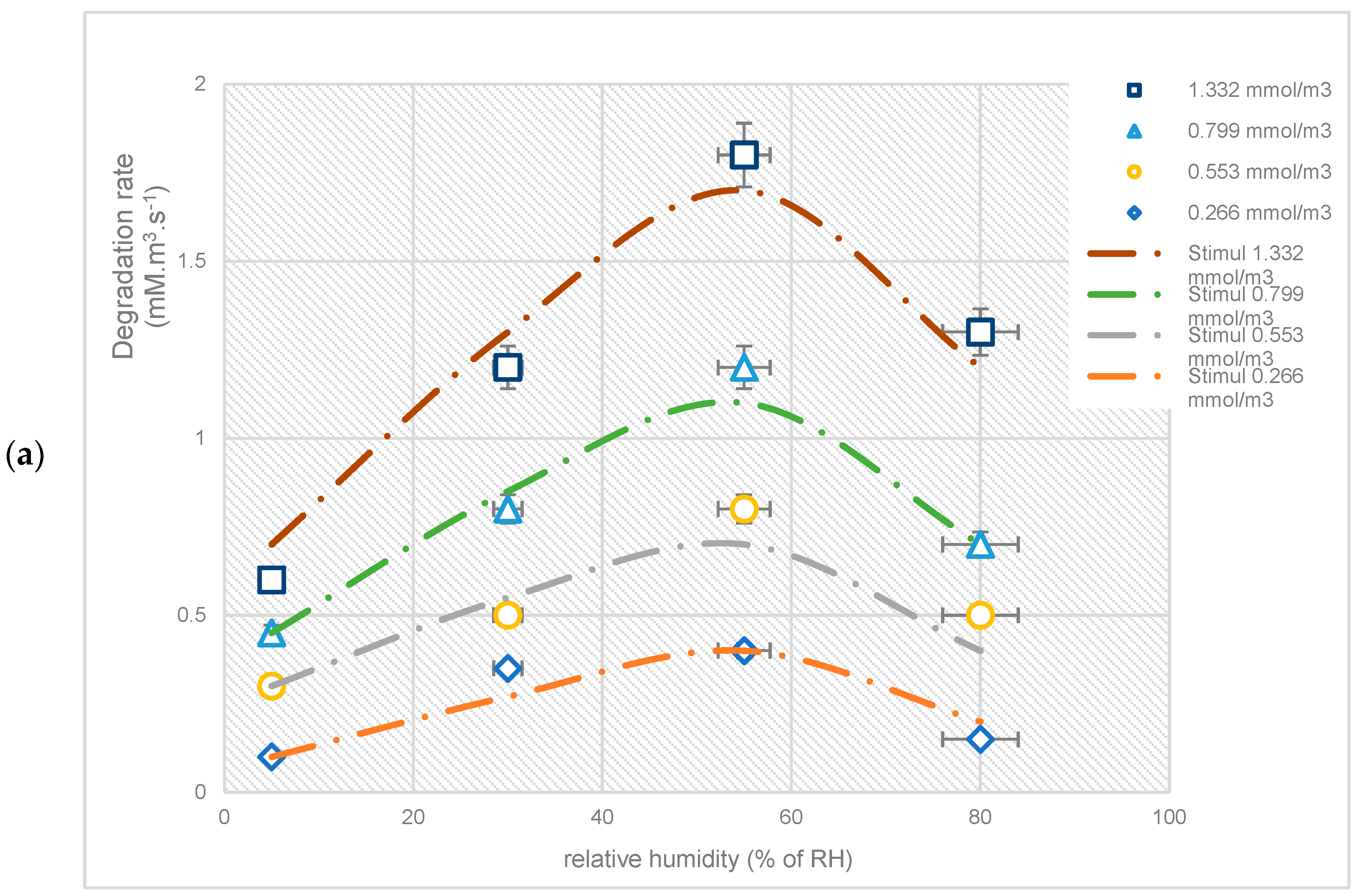
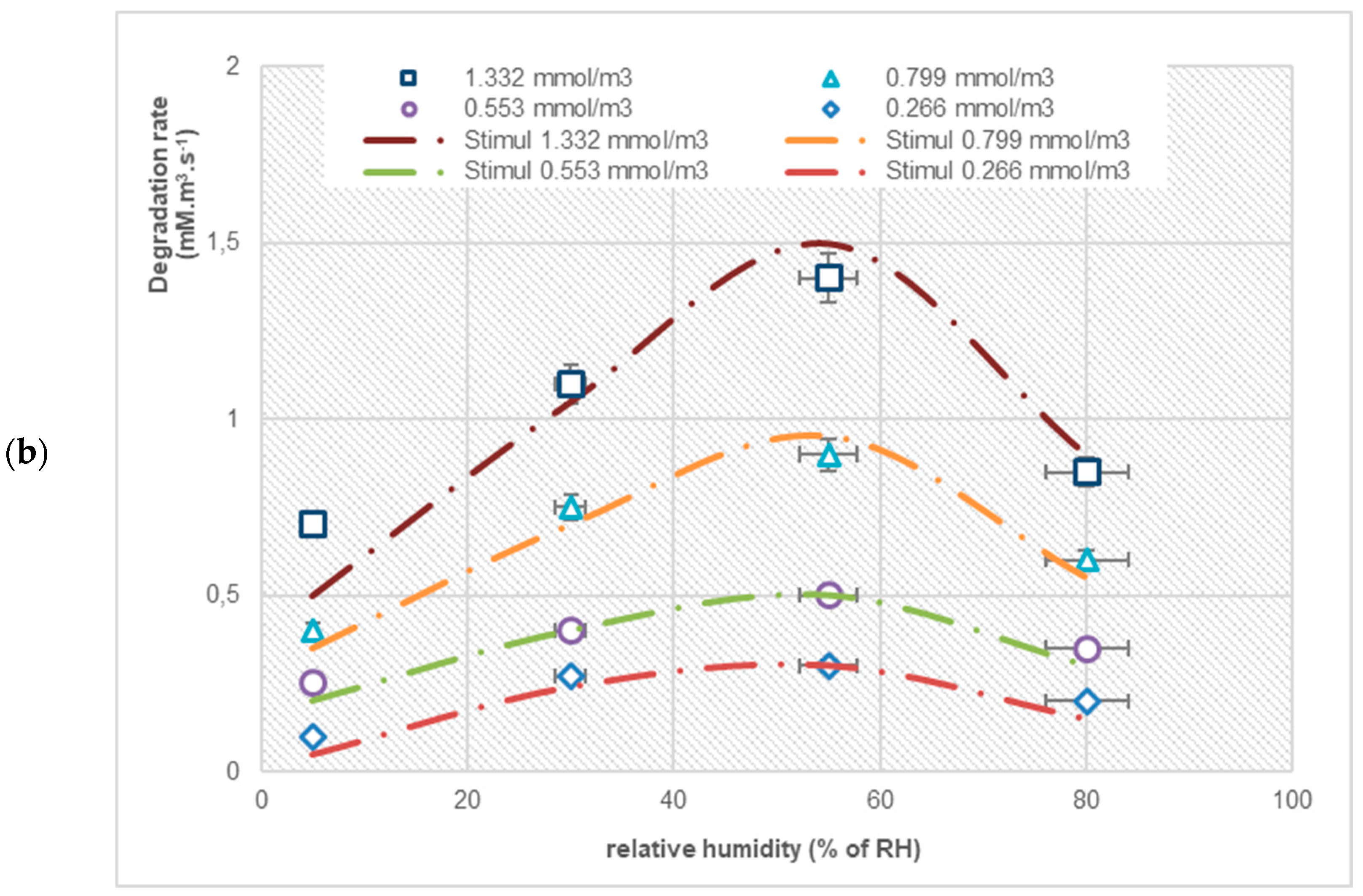
3.2. The Effect of Relative Humidity on the Degradation Rate
3.3. Selectivity of CO2
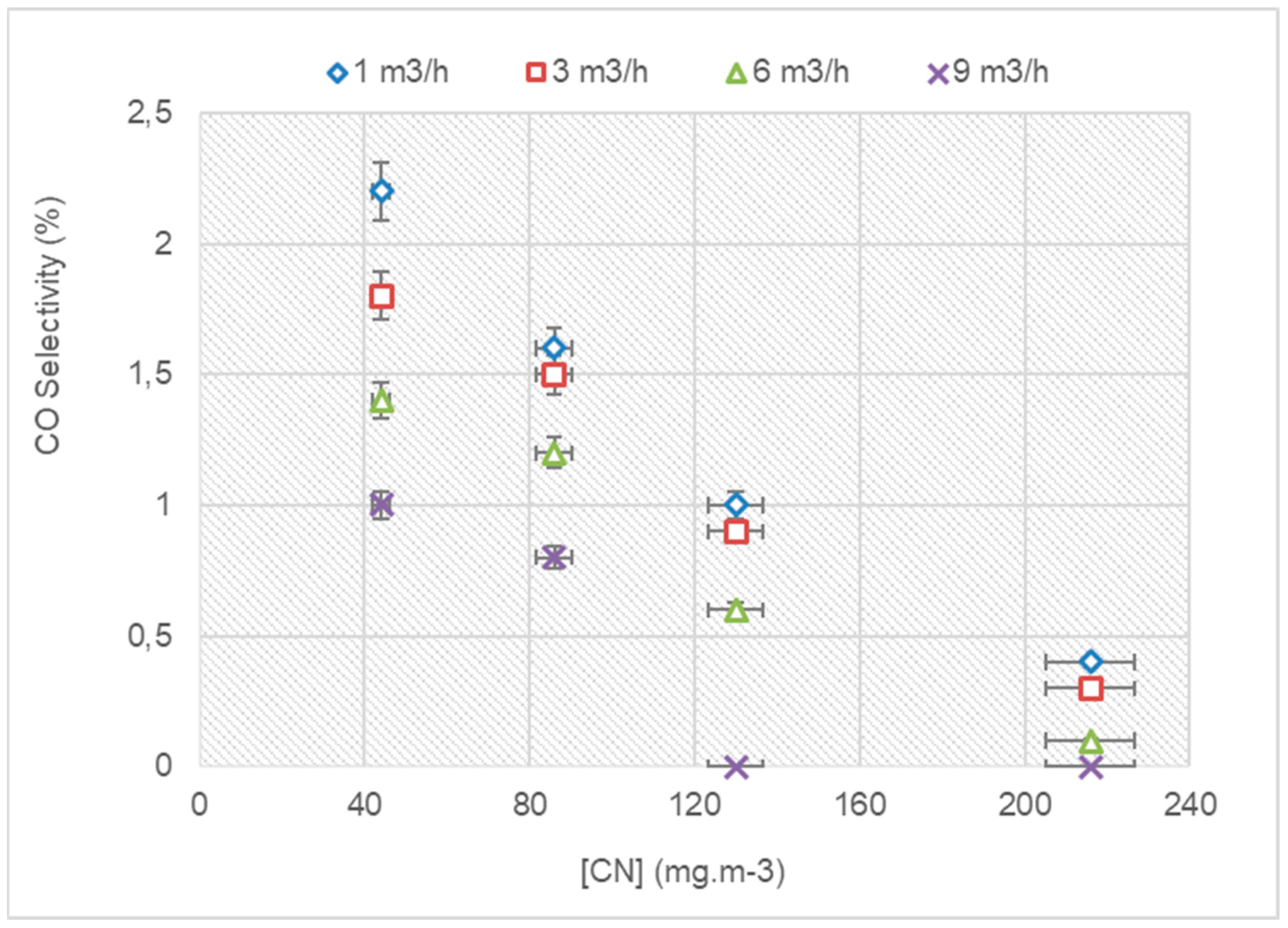
3.3.1. The COX Selectivity with a Variation in the Flow Rate and VOC Concentration
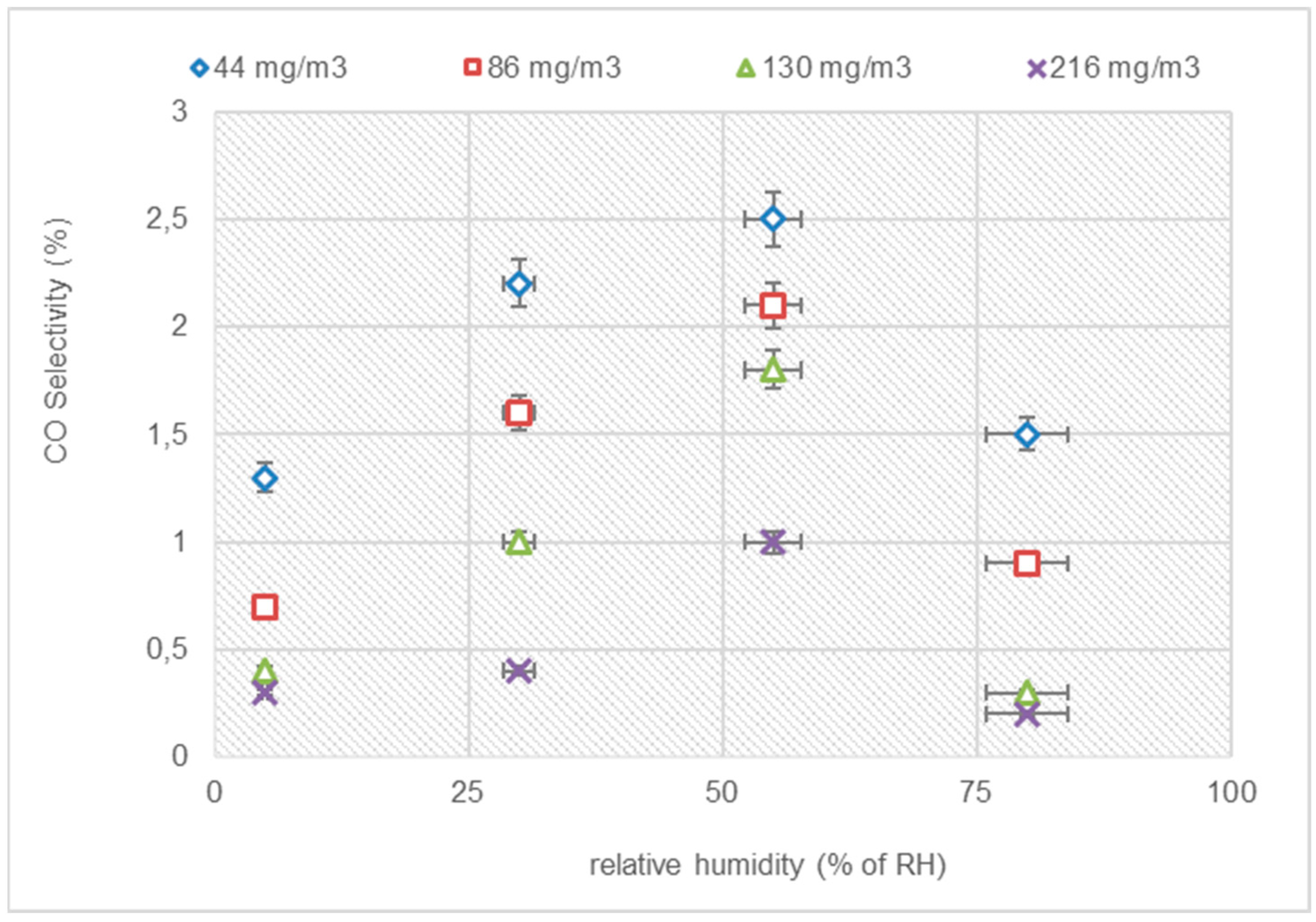
3.3.2. COX Selectivity with Variation in Humidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Rtimi, S.; Wolbert, D.; Bessais, B.; Assadi, A.A. Modeling of indoor air treatment using an innovative photocatalytic luminous textile: Reactor compactness and mass transfer enhancement. Chem. Eng. J. 2022, 430, 132636. [Google Scholar] [CrossRef]
- Abiev, R.S.; Sladkovskiy, D.A.; Semikin, K.V.; Murzin, D.Y.; Rebrov, E.V. Non-thermal plasma for process and energy intensification in dry reforming of methane. Catalysts 2020, 10, 1358. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kim, K.H. Modification strategies for visible-light photocatalysts and their performance-enhancing effects on photocatalytic degradation of volatile organic compounds. Renew. Sustain. Energy Rev. 2024, 189, 113948. [Google Scholar] [CrossRef]
- Alcázar-Medina, T.L.; Chairez-Hernández, I.; Lemus-Santana, A.A.; Núñez-Núñez, C.M.; Proal-Nájera, J.B. Amoxicillin Degradation by TiO2 P25 Solar Heterogeneous Photocatalysis: Influence of pH and Oxidizing Agent H2O2 Addition. Appl. Sci. 2023, 13, 7857. [Google Scholar] [CrossRef]
- Assadi, A.A. Efficient Photocatalytic Luminous Textile for Simulated Real Water Purification: Advancing Economical and Compact Reactors. Materials 2024, 17, 296. [Google Scholar] [CrossRef]
- Ashley, N.A.; McBride, N.; Krumholt, J.; Baker, B.; Valsaraj, K.T. Photocatalytic Reaction of Gas-Phase Naphthalene on Paint- and Sunscreen-Coated Surfaces. ISRN Chem. Eng. 2012, 2012, 72479. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Photocatalytic oxidation of trimethylamine and isovaleraldehyde in an annular reactor: Influence of the mass transfer and the relative humidity. J. Photochem. Photobiol. A Chem. 2012, 236, 61–69. [Google Scholar] [CrossRef]
- Bai, S.; Qiu, H.; Song, M.; He, G.; Wang, F.; Liu, Y.; Guo, L. Porous fixed-bed photoreactor for boosting C–C coupling in photocatalytic CO2 reduction. eScience 2022, 2, 428–437. [Google Scholar] [CrossRef]
- Boulamanti, A.K.; Korologos, C.A.; Philippopoulos, C.J. The rate of photocatalytic oxidation of aromatic volatile organic compounds in the gas-phase. Atmos. Environ. 2008, 42, 7844–7850. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, C.; Zhang, G. Progress in degradation of volatile organic compounds based on low-temperature plasma technology. Plasma Process. Polym. 2020, 17, 1900131. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Zhu, W.; Li, G.; An, T. Atomic-level insight into effect of substrate concentration and relative humidity on photocatalytic degradation mechanism of gaseous styrene. Chemosphere 2022, 291, 133074. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Sun, Y.; Yi, Y.; Xu, F.; Zhang, Q.; Wang, W. A theoretical study of OH radical-initiated atmospheric oxidation of 1-chloronaphthalene. Chem. Phys. Lett. 2018, 699, 40–47. [Google Scholar] [CrossRef]
- Dahiru, U.H.; Saleem, F.; Zhang, K.; Harvey, A. Plasma-assisted removal of methanol in N2, dry and humidified air using a dielectric barrier discharge (DBD) reactor. RSC Adv. 2022, 12, 10997–11007. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Shahna, F.G.; Bahrami, A.; Jaleh, B.; Abedi, K.A.D. Photocatalytic degradation of volatile chlorinated organic compounds with ozone addition. Arch. Environ. Prot. 2017, 43, 65–72. [Google Scholar] [CrossRef]
- Khezami, L.; Assadi, A.A. Treatment of Mixture Pollutants with Combined Plasma Photocatalysis in Continuous Tubular Reactors with Atmospheric-Pressure Environment: Understanding Synergetic Effect Sources. Materials 2023, 16, 6857. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Li, X.; Zeng, L.; Zhu, M. Heterogeneous Photocatalytic Activation of Persulfate for the Removal of Organic Contaminants in Water: A Critical Review. ACS ES&T Eng. 2022, 2, 527–546. [Google Scholar] [CrossRef]
- Jaison, A.; Mohan, A.; Lee, Y.C. Recent Developments in Photocatalytic Nanotechnology for Purifying Air Polluted with Volatile Organic Compounds: Effect of Operating Parameters and Catalyst Deactivation. Catalysts 2023, 13, 407. [Google Scholar] [CrossRef]
- Qi, J.; Wang, C.; Sun, J.; Li, S. TiO2 Assisted Photocatalytic Decomposition of 2-Chloronaphthalene on Iron Nanoparticles in Aqueous Systems: Synergistic Effect and Intermediate Products. Russ. J. Phys. Chem. A 2019, 93, 1620–1626. [Google Scholar] [CrossRef]
- Jo, W.K.; Park, K.H. Heterogeneous photocatalysis of aromatic and chlorinated volatile organic compounds (VOCs) for non-occupational indoor air application. Chemosphere 2004, 57, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. B Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Kenfoud, H.; Baaloudj, O.; Nasrallah, N. Structural and electrochemical characterizations of Bi12CoO20 sillenite crystals: Degradation and reduction of organic and inorganic pollutants. J. Mater. Sci. Mater. Electron. 2021, 32, 16411–16420. [Google Scholar] [CrossRef]
- Koné, N.Z.A.; Belkessa, N.; Serhane, Y.; Coulibaly, S.L.; Kamagate, M. Chlorobenzene Mineralization Using Plasma/Photocatalysis Hybrid Reactor: Exploiting the Synergistic Effect. Catalysts 2023, 13, 431. [Google Scholar] [CrossRef]
- Kong, C.P.Y.; Suhaimi, N.A.A.; Shahri, N.N.M.; Lim, J.W.; Nur, M.; Hobley, J.; Usman, A. Auramine O UV Photocatalytic Degradation on TiO2 Nanoparticles in a Heterogeneous Aqueous Solution. Catalysts 2022, 12, 975. [Google Scholar] [CrossRef]
- Li, J.; Xie, X.; Li, L.; Wang, X.; Wang, H.; Jing, S.; Ying, Q.; Qin, M.; Hu, J. Fate of Oxygenated Volatile Organic Compounds in the Yangtze River Delta Region: Source Contributions and Impacts on the Atmospheric Oxidation Capacity. Environ. Sci. Technol. 2022, 56, 11212–11224. [Google Scholar] [CrossRef] [PubMed]
- Drhimer, F.; Rahmani, M.; Regraguy, B.; El Hajjaji, S.; Mabrouki, J.; Amrane, A.; Fourcade, F.; Assadi, A.A. Treatment of a Food Industry Dye, Brilliant Blue, at Low Concentration Using a New Photocatalytic Configuration. Sustainability 2023, 15, 5788. [Google Scholar] [CrossRef]
- Masresha, G.; Jabasingh, S.A.; Kebede, S.; Doo-Arhin, D.; Assefa, M. A review of prospects and challenges of photocatalytic decomposition of volatile organic compounds (VOCs) under humid environment. Can. J. Chem. Eng. 2023, 101, 6905–6918. [Google Scholar] [CrossRef]
- Maung, T.Z.; Bishop, J.E.; Holt, E.; Turner, A.M.; Pfrang, C. Indoor Air Pollution and the Health of Vulnerable Groups: A Systematic Review Focused on Particulate Matter (PM), Volatile Organic Compounds (VOCs) and Their Effects on Children and People with Pre-Existing Lung Disease. Int. J. Environ. Res. Public Health 2022, 19, 8752. [Google Scholar] [CrossRef]
- Mohammed, S.S.; Shnain, Z.Y.; Abid, M.F. Use of TiO2 in Photocatalysis for Air Purification and Wastewater Treatment: A Review. Eng. Technol. J. 2022, 3, 1131–1143. [Google Scholar] [CrossRef]
- Petit, N.; Bouzaza, A.; Wolbert, D.; Petit, P.; Dussaud, J. Photocatalytic degradation of gaseous perchloroethylene in continuous flow reactors: Rate enhancement by chlorine radicals. Catal. Today 2007, 124, 266–272. [Google Scholar] [CrossRef]
- Assadi, A.A.; Baaloudj, O.; Khezami, L.; Ben Hamadi, N.; Mouni, L.; Assadi, A.A.; Ghorbal, A. An Overview of Recent Developments in Improving the Photocatalytic Activity of TiO2-Based Materials for the Treatment of Indoor Air and Bacterial Inactivation. Materials 2023, 16, 2246. [Google Scholar] [CrossRef]
- Rissanen, K.; Aalto, J.; Gessler, A.; Hölttä, T.; Rigling, A.; Schaub, M.; Bäck, J. Drought effects on volatile organic compound emissions from Scots pine stems. Plant. Cell Environ. 2022, 45, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Rouf, Z.; Dar, I.Y.; Javaid, M.; Dar, M.Y.; Jehangir, A. Volatile Organic Compounds Emission from Building Sector and Its Adverse Effects on Human Health. In Ecological and Health Effects of Building Materials; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 67–86. [Google Scholar] [CrossRef]
- Serhane, Y.; Belkessa, N.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Continuous air purification by front flow photocatalytic reactor: Modelling of the influence of mass transfer step under simulated real conditions. Chemosphere 2022, 295, 133809. [Google Scholar] [CrossRef]
- Tulebekov, Y.; Orazov, Z.; Satybaldiyev, B.; Snow, D.D.; Schneider, R.; Uralbekov, B. Reaction Steps in Heterogeneous Photocatalytic Oxidation of Toluene in Gas Phase—A Review. Molecules 2023, 28, 6451. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Shi, Q.; Liu, S.; Qian, L.; Yu, Z.; Wang, H.; Lei, J.; Gao, Z.; Long, H.; et al. Volatile organic compounds (VOC) emissions control in iron ore sintering process: Recent progress and future development. Chem. Eng. J. 2022, 448, 137601. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Chien, Y.; Kurioka, T.; Sone, M.; Chen, C.; Chang, T.M.; Hsu, Y. Photocatalytic Fibers for Environmental Puri fi cation: Challenges and Opportunities in the Post-Pandemic Era. Adv. Energy Sustain. Res. 2023, 5, 2300212. [Google Scholar] [CrossRef]
- Xu, P.; Ding, C.; Li, Z.; Yu, R.; Cui, H.; Gao, S. Photocatalytic degradation of air pollutant by modified nano titanium oxide (TiO2) in a fluidized bed photoreactor: Optimizing and kinetic modeling. Chemosphere 2023, 319, 137995. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Tsukamoto, H.; Araki, K.; Tanimura, T.; Tejedor-Tejedor, I.; Anderson, M.A. Photocatalytic degradation of gaseous tetrachloroethylene on porous TiO2 pellets. Appl. Catal. B Environ. 2001, 33, 109–117. [Google Scholar] [CrossRef]
- Zadi, T.; Assadi, A.A.; Nasrallah, N.; Bouallouche, R.; Tri, P.N.; Bouzaza, A.; Azizi, M.M.; Maachi, R.; Wolbert, D. Treatment of hospital indoor air by a hybrid system of combined plasma with photocatalysis: Case of trichloromethane. Chem. Eng. J. 2018, 349, 276–286. [Google Scholar] [CrossRef]
- Zatta, D.; Segata, M.; Biasioli, F.; Allegretti, O.; Bochicchio, G.; Verucchi, R.; Chiavarini, F.; Cappellin, L. Comparative Analysis of Volatile Organic Compound Purification Techniques in Complex Cooking Emissions: Adsorption, Photocatalysis and Combined Systems. Molecules 2023, 28, 7658. [Google Scholar] [CrossRef]
- Zhang, L.; Moralejo, C.; Anderson, W.A. A review of the influence of humidity on photocatalytic decomposition of gaseous pollutants on TiO2-based catalysts. Can. J. Chem. Eng. 2020, 98, 263–273. [Google Scholar] [CrossRef]
- Zhang, L.; Sawell, S.; Moralejo, C.; Anderson, W.A. Heterogeneous photocatalytic decomposition of gas-phase chlorobenzene. Appl. Catal. B Environ. 2007, 71, 135–142. [Google Scholar] [CrossRef]
- Zhang, M.; An, T.; Fu, J.; Sheng, G.; Wang, X.; Hu, X.; Ding, X. Photocatalytic degradation of mixed gaseous carbonyl compounds at low level on adsorptive TiO2/SiO2 photocatalyst using a fluidized bed reactor. Chemosphere 2006, 64, 423–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B.; Chen, H.; Yuan, R. Heterogeneous photocatalytic oxidation for the removal of organophosphorus pollutants from aqueous solutions: A review. Sci. Total Environ. 2023, 856, 159048. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Hadadi, A.; Manseri, A.; Bouzaza, A.; Assadi, A.; Amrane, A.; Zamouche, M.; El Jery, A.; et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts 2023, 13, 336. [Google Scholar] [CrossRef]



| Reactors | Formulas | Conditions |
|---|---|---|
| Tangential flow reactor | Si | |
| Si |
| Q (m3·h−1) | Re | Dmol (cm2·s−1) | Kf (×103 m·s−1) | ||
|---|---|---|---|---|---|
| CB | CN | CB | CN | ||
| 1 | 213 | 0.0494 | 0.0478 | 0.40 | 0.39 |
| 3 | 639 | 1.02 | 1.00 | ||
| 6 | 1279 | 1.85 | 1.81 | ||
| 9 | 1919 | 2.60 | 2.55 | ||
| VOCs | k (mM/m3·s) | K (m3/mM) | R2 (%) |
|---|---|---|---|
| CB | 0.92 | 8.4 | 99 |
| CN | 0.10 | 9.1 | 99 |
| VOCs | kA (mM·m−3·s−1) | KA (m3·mM−1) | KW (m3·mmol−1) | K’A (m3·mmol−1) | K’W (m3·mmol−1) |
|---|---|---|---|---|---|
| CB | 0.920 | 8.400 | 0.029 | 1.500 | 0.456 |
| CN | 0.100 | 9.100 | 0.02178 | 1.756 | 0.200 |
| References | Reactors | Pollutants | k (mM·m−3·s−1) | K (m3·mM−1) |
|---|---|---|---|---|
| [29] | Front-flow continuous reactor | Cyclohexane | 0.025 | 7.12 |
| [28] | Annular reactor | Isovaleraldehyde | 0.49 | 1.62 |
| Trimethylamine | 0.69 | 1.19 | ||
| [27] | Continuous reactor | Chloroform | 0.04 | 2.74 |
| Glutaraldehyde | 0.09 | 1.16 | ||
| This study | Cylindric reactor | Chlorobenzene | 0.92 | 8.4 |
| Chloronaphthalene | 0.10 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koné, N.A.; Assadi, A.A.; Belkessa, N.; Khezami, L.; Coulibaly, S.L.; Serhane, Y.; Elfalleh, W.; Coulibaly, L.; Bouzaza, A.; Amrane, A. Kinetic Modeling of the Photocatalytic Degradation of Chlorinated Aromatic Volatile Organic Compounds: Mass Transfer Enhancement. Appl. Sci. 2024, 14, 1507. https://doi.org/10.3390/app14041507
Koné NA, Assadi AA, Belkessa N, Khezami L, Coulibaly SL, Serhane Y, Elfalleh W, Coulibaly L, Bouzaza A, Amrane A. Kinetic Modeling of the Photocatalytic Degradation of Chlorinated Aromatic Volatile Organic Compounds: Mass Transfer Enhancement. Applied Sciences. 2024; 14(4):1507. https://doi.org/10.3390/app14041507
Chicago/Turabian StyleKoné, N’Zanon Aly, Amine Aymen Assadi, Nacer Belkessa, Lotfi Khezami, Sandotin Lassina Coulibaly, Youcef Serhane, Walid Elfalleh, Lacina Coulibaly, Abdelkrim Bouzaza, and Abdeltif Amrane. 2024. "Kinetic Modeling of the Photocatalytic Degradation of Chlorinated Aromatic Volatile Organic Compounds: Mass Transfer Enhancement" Applied Sciences 14, no. 4: 1507. https://doi.org/10.3390/app14041507
APA StyleKoné, N. A., Assadi, A. A., Belkessa, N., Khezami, L., Coulibaly, S. L., Serhane, Y., Elfalleh, W., Coulibaly, L., Bouzaza, A., & Amrane, A. (2024). Kinetic Modeling of the Photocatalytic Degradation of Chlorinated Aromatic Volatile Organic Compounds: Mass Transfer Enhancement. Applied Sciences, 14(4), 1507. https://doi.org/10.3390/app14041507









