Porous Mg–Hydroxyapatite Composite Incorporated with Aloe barbadensis Miller for Scaphoid Fracture Fixation: A Natural Drug Loaded Orthopedic Implant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
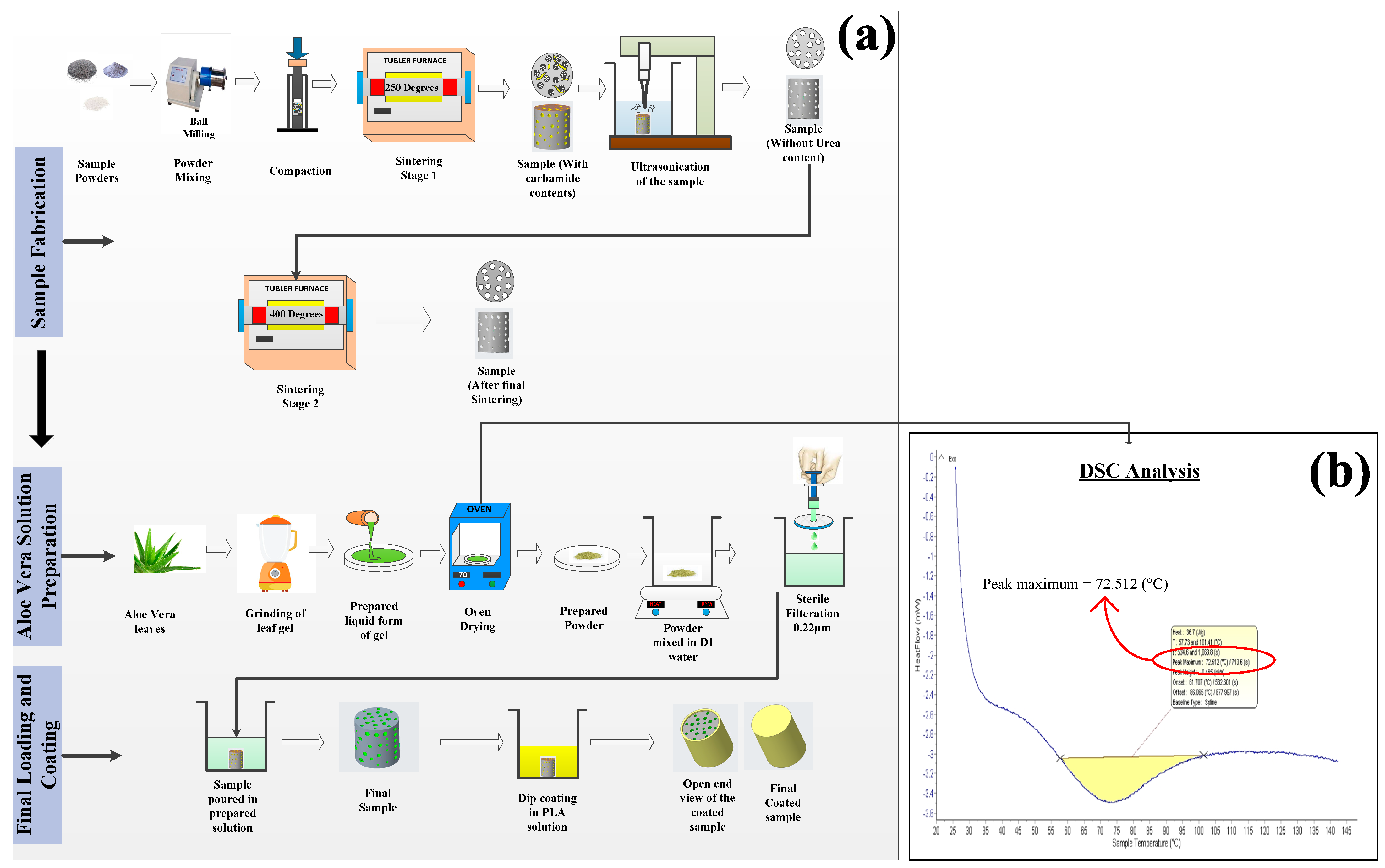
2.2. Sample Preparation
2.3. Aloe vera Solution Preparation
2.4. Coating Preparation
2.5. Characterization
2.6. Mechanical and Surface Behavior
2.7. Immersion Studies
2.8. Antibacterial Behavior
2.9. Hemocompatibility
2.10. In Vitro Cytotoxicity Analysis
2.10.1. Cell Viability
2.10.2. Cell Apoptosis/Necrosis
2.11. Statistical Analysis
3. Results
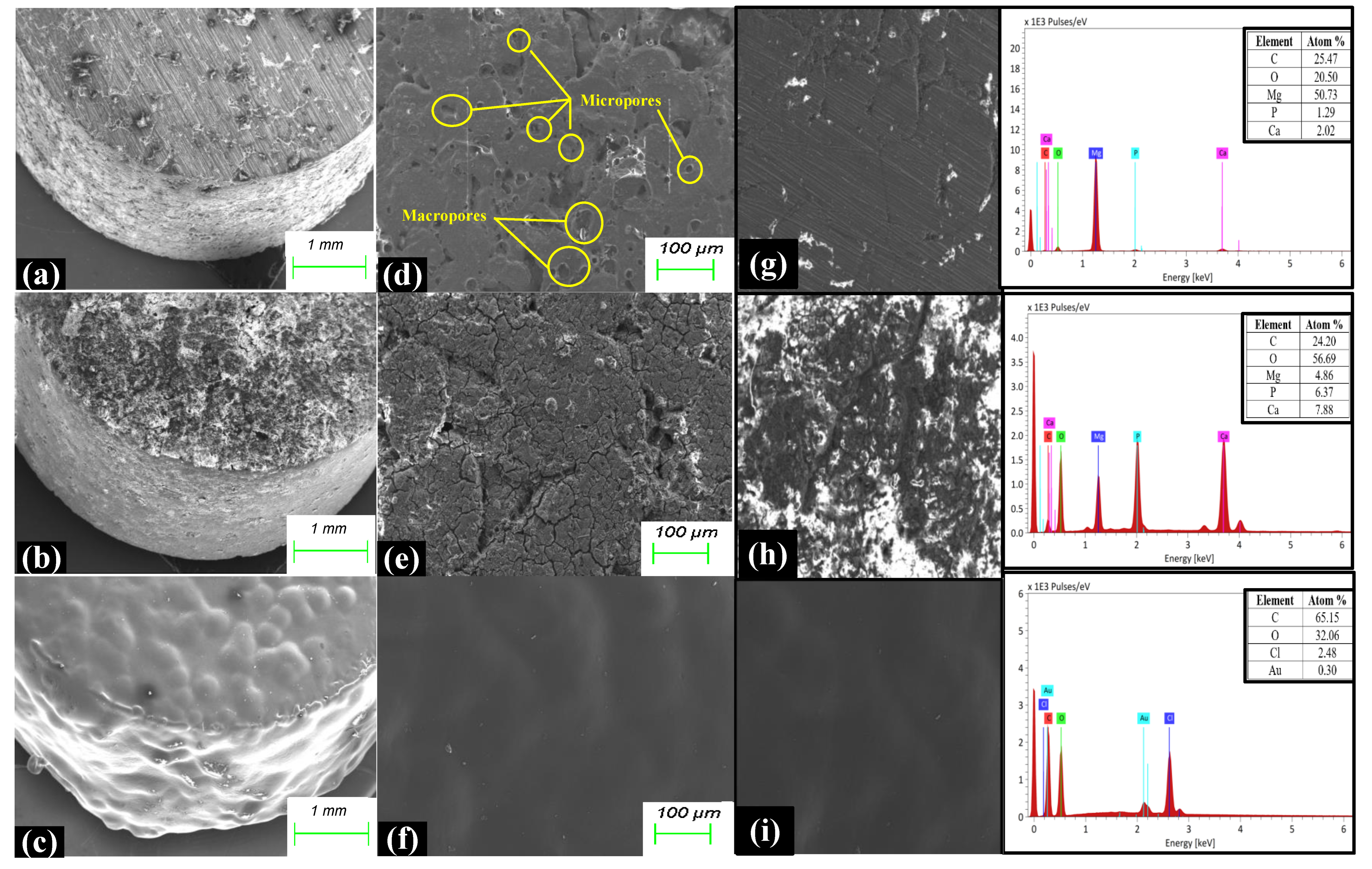
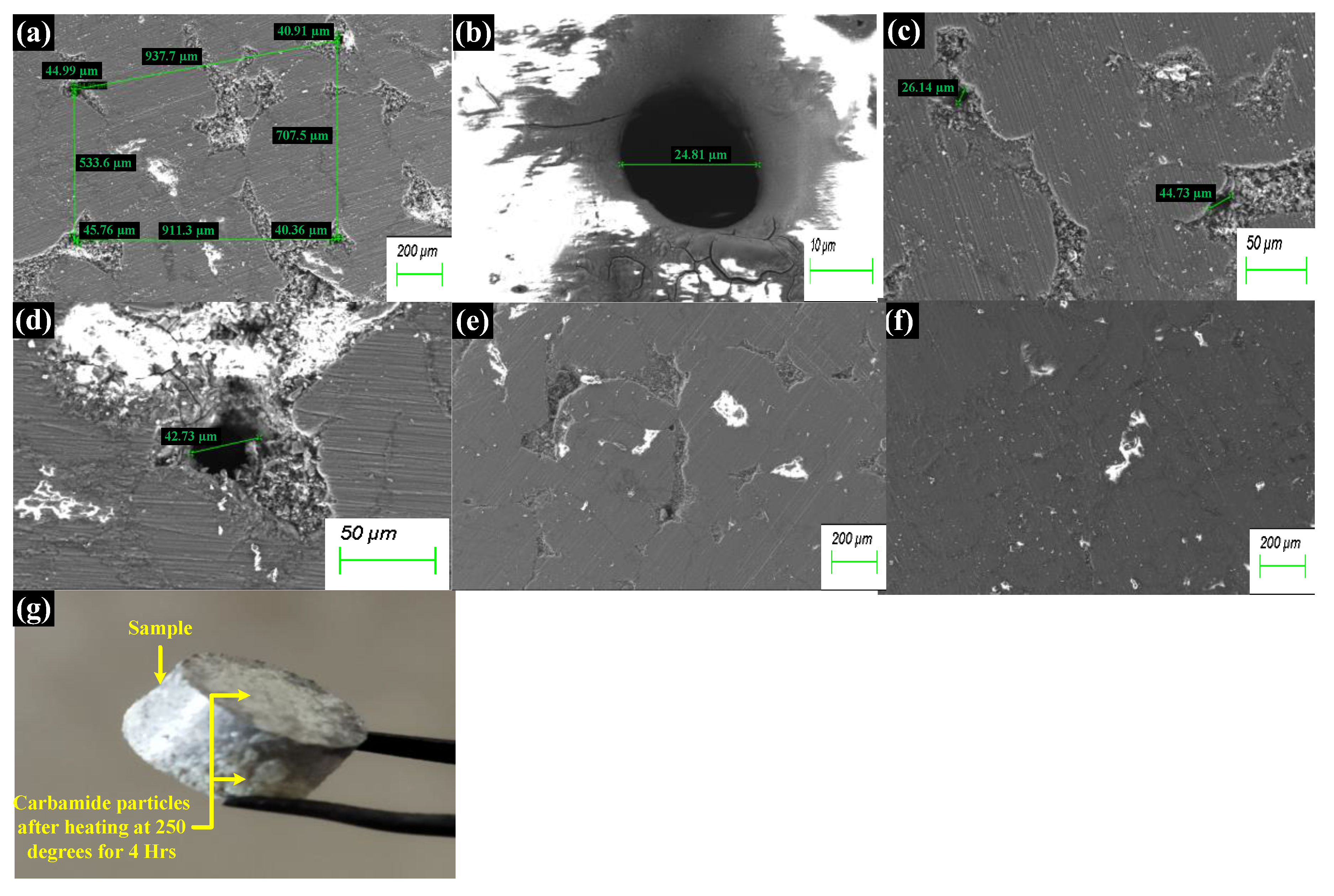
3.1. Characterization
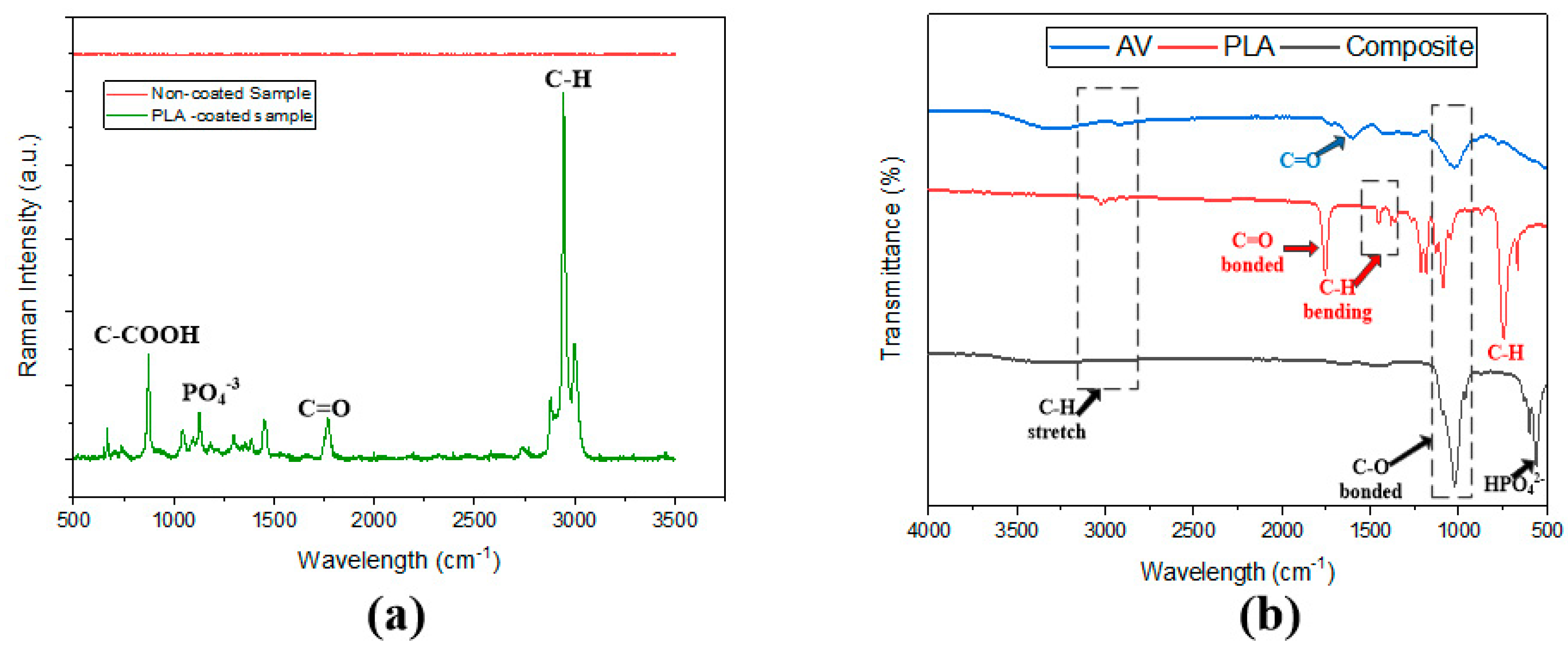
3.2. FTIR and Raman Spectroscopy
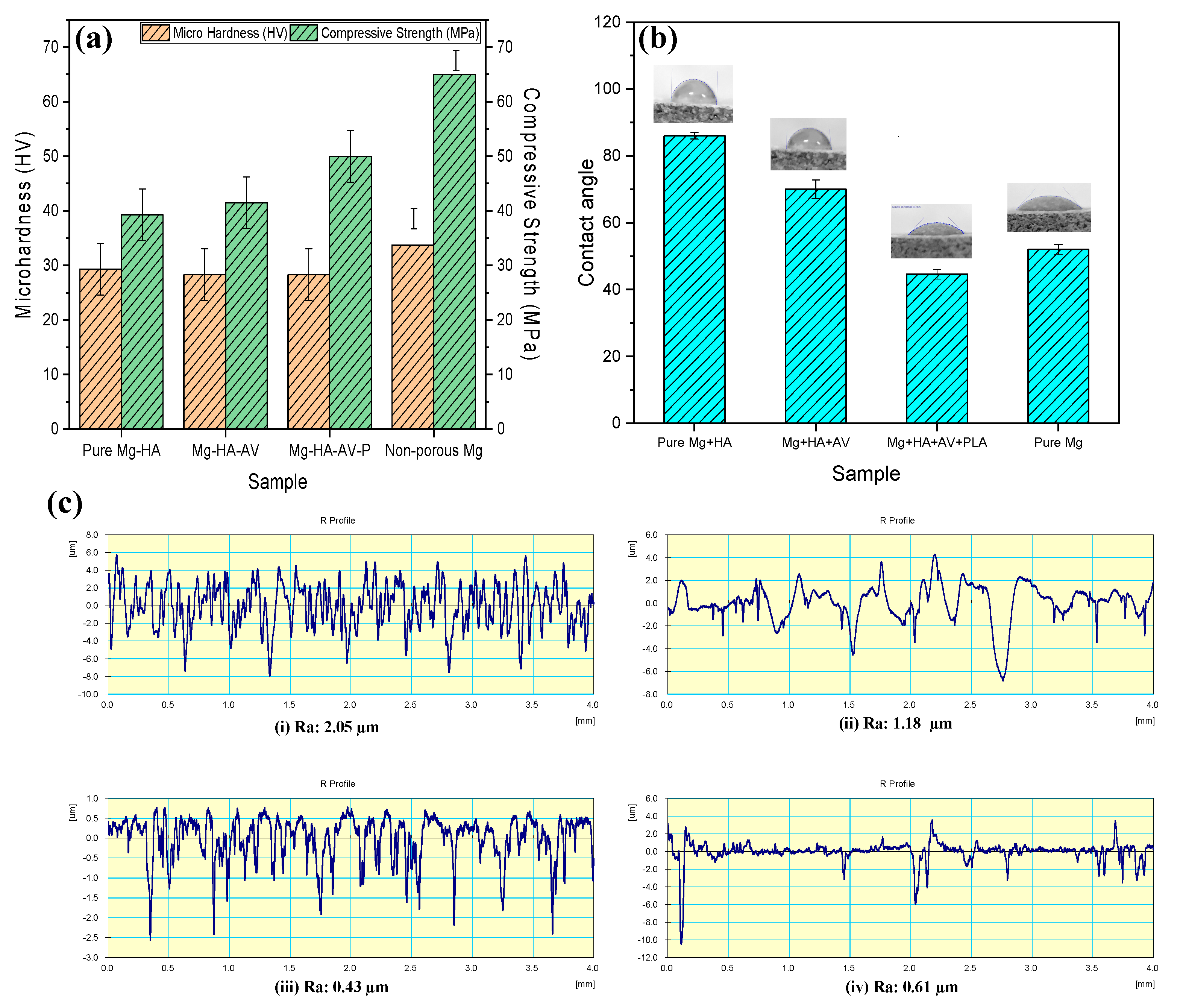
3.3. Mechanical and Surface Properties
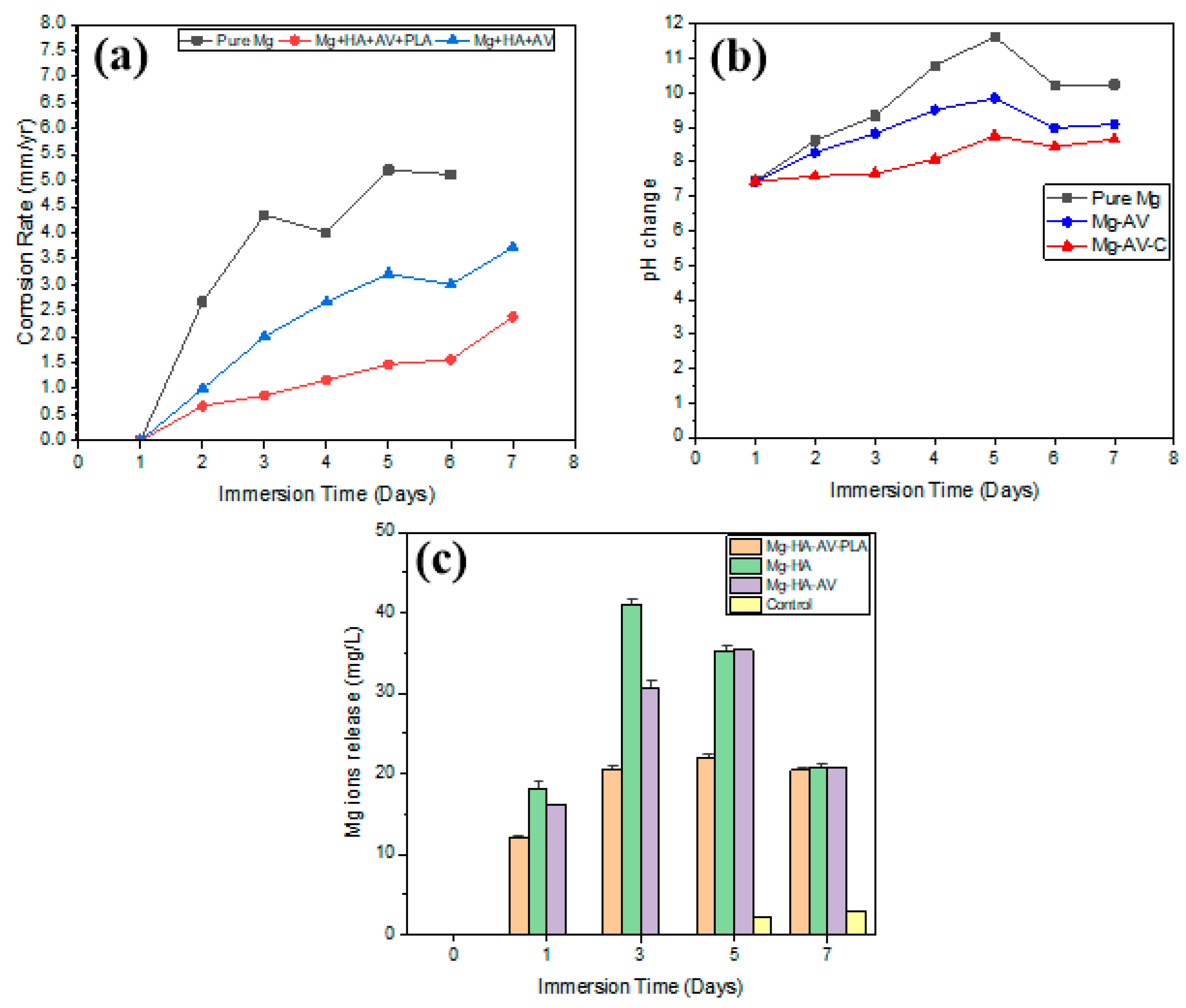
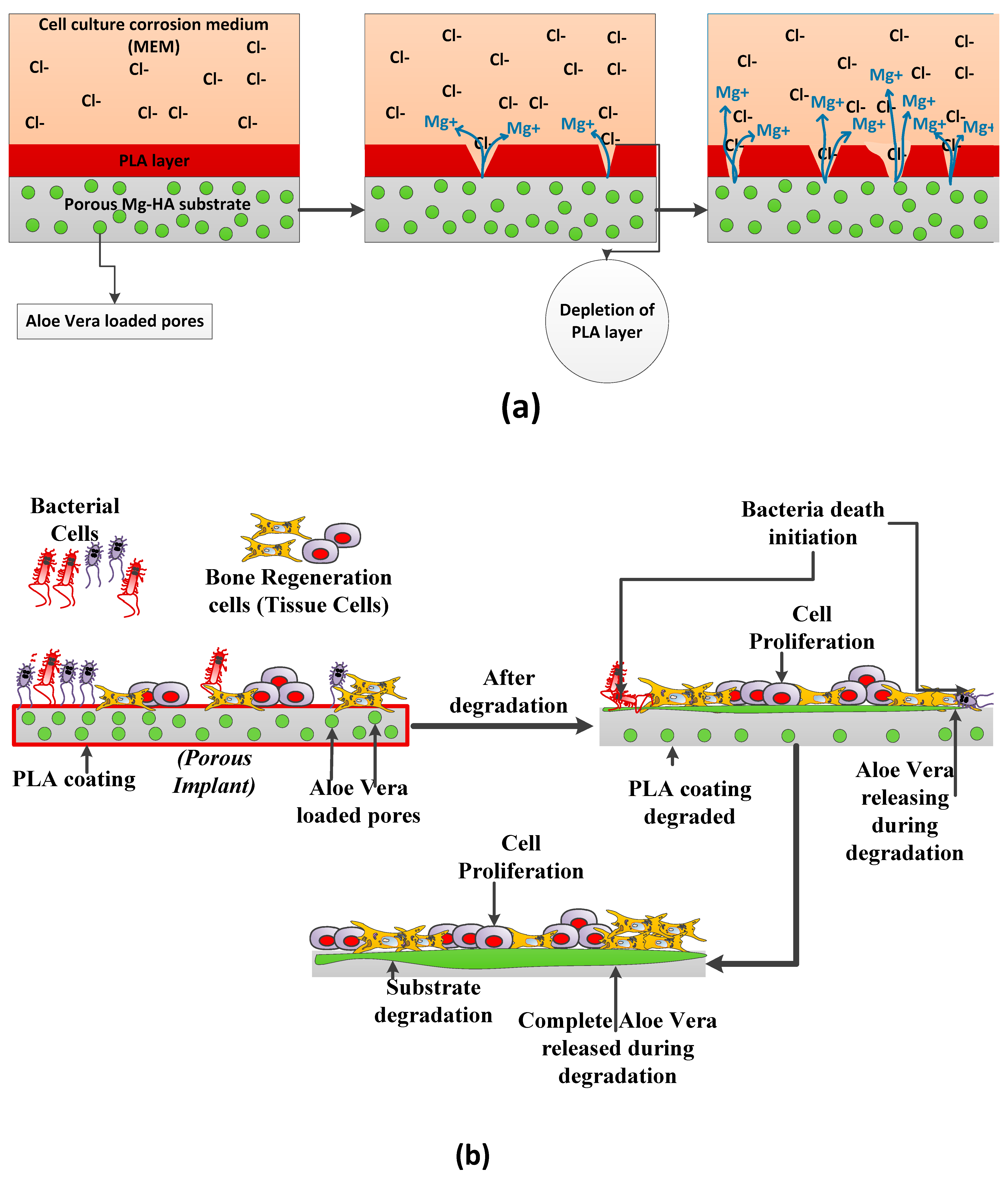
3.4. Corrosion Properties
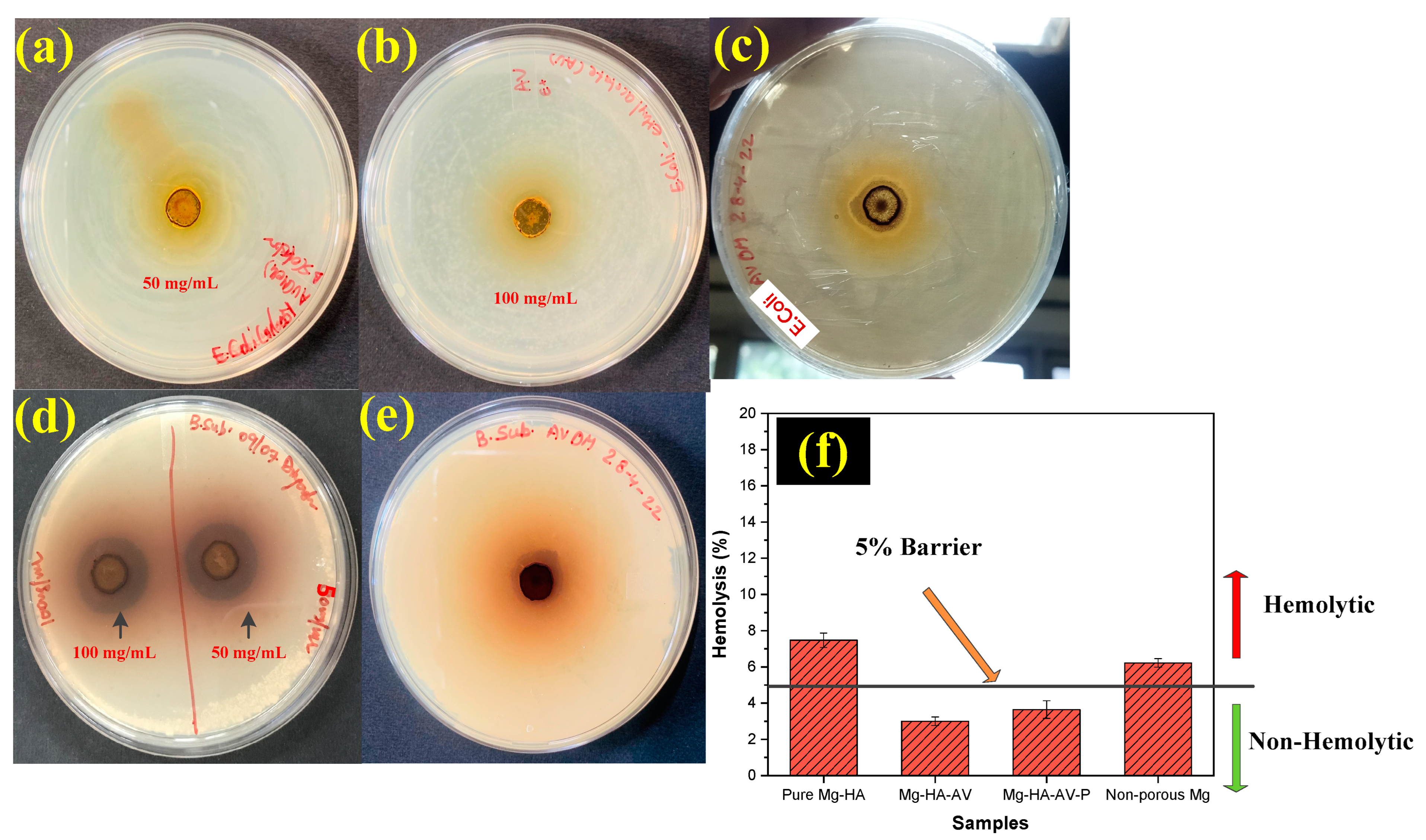
3.5. Antibacterial and Hemocompatibility Analysis
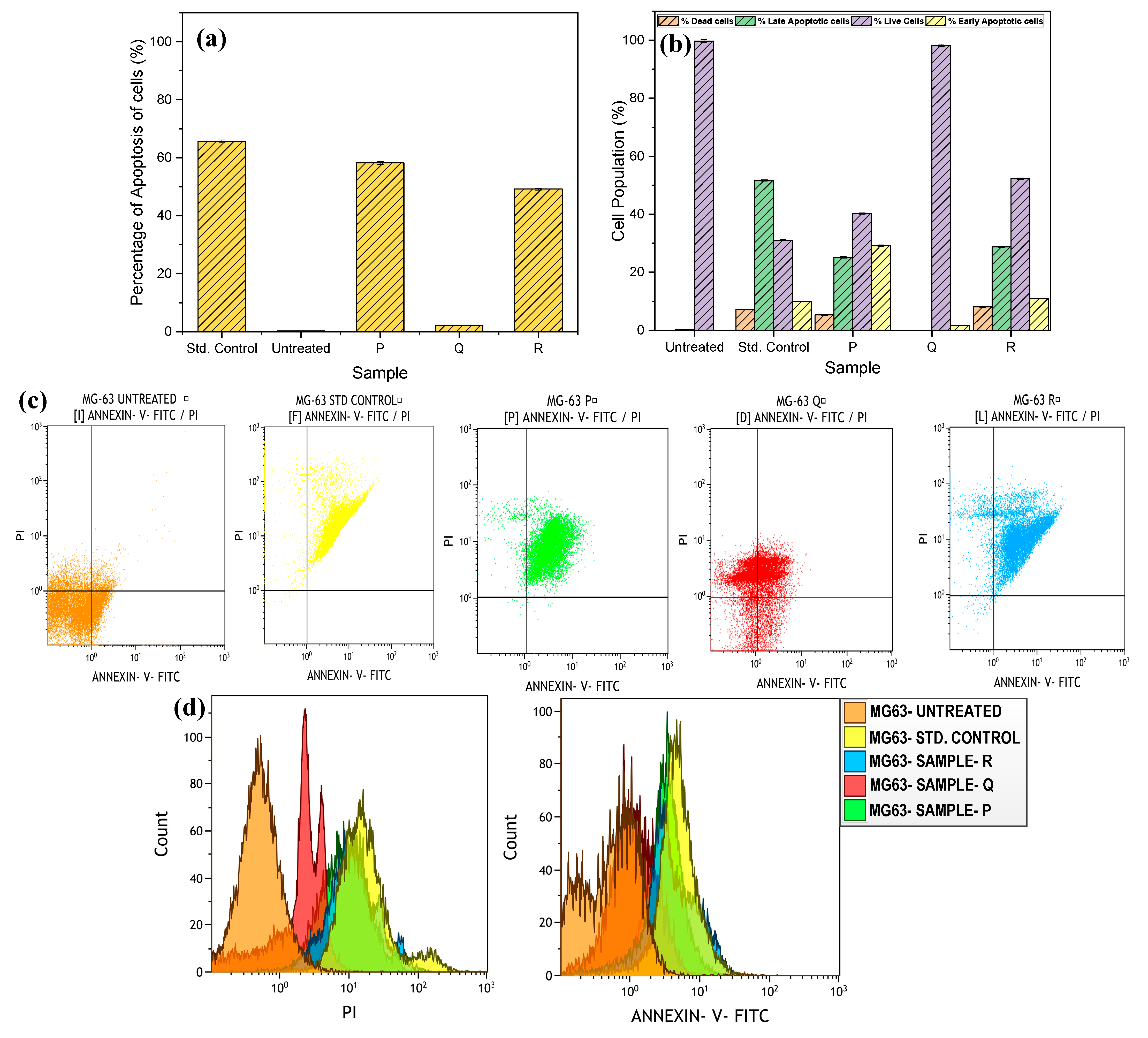
3.6. Cytotoxicity Analysis
4. Discussion
4.1. Microstructure, Mechanical Properties, and Corrosion Behavior of the Developed Porous Composite
4.2. In-Vitro Biocompatibility Analysis
5. Conclusions
- The microstructure images revealed two different pore sizes generated in the matrix using a spacer holder technique. The suitable porosity of 72.25% was optimized with 20% carbamide for aloe vera incorporation with an adequate strength.
- The successful PLA coating and aloe vera incorporation led to an excellent microhardness of 28.37 HV and UCS value of 50.1 Mpa, with an impressive surface hydrophobicity and reduced surface roughness.
- A reduction in corrosion rates was observed after PLA coating, leading to a decline in the release of Mg ions, hence increasing the longevity of the composite in the cell culture medium.
- The minimum %age of aloe vera solution (in mg/mL) was formulated to be 50% (50 mg/mL) for successful antibacterial and hemocompatibility properties.
- The in vitro cell cytotoxicity experiments depicted magnificent cell viability of 98% after the addition of aloe vera to the porous matrix. In addition, a decline in the cell apoptosis rates was observed with aloe vera samples as compared to the pure Mg–HA substrate.
6. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA/HAP | Hydroxyapatite |
| Mg | Magnesium |
| AV | Aloe vera |
| PLA | Polylactic acid |
| PLGA | Poly-(l-lactic)- glycolic acid |
| S. aureus | Staphylococcus aureus |
| E. coli | Escherichia coli |
| B. subtilis | Bacillus subtilis |
| AB | Antibacterial |
| CFU | Colony forming units |
| UCS | Ultimate compressive strength |
| HR | Hemolysis rate |
| EDTA | Ethylenediamine tetraacetic acid |
| PBS | Phosphate buffered saline |
| DMEM | Dulbecco’s modified Eagle medium |
| IZD | Inhibition zone diameter |
| C | Carbamide particle |
| NA | Nutrient agar |
References
- Rancy, S.; Zelken, J.; Lipman, J.; Wolfe, S. Scaphoid Proximal Pole Fracture Following Headless Screw Fixation. J. Wrist Surg. 2015, 5, 71–76. [Google Scholar] [CrossRef]
- Rossello, M.I. A case of total scaphoid titanium custom-made 3D-printed prostheses with one-year follow-up. Case Rep. Plast. Surg. Hand Surg. 2020, 7, 7–12. [Google Scholar] [CrossRef]
- Qin, J.; Ma, J.; Liang, Q.; Li, J.; Tang, B. Tribological, cytotoxicity and antibacterial properties of graphene oxide/carbon fibers/polyetheretherketone composite coatings on Ti–6Al–4V alloy as orthopedic/dental implants. J. Mech. Behav. Biomed. Mater. 2021, 122, 104659. [Google Scholar] [CrossRef]
- Soro, N.; Brodie, E.G.; Abdal-hay, A.; Alali, A.Q.; Kent, D.; Dargusch, M.S. Additive manufacturing of biomimetic Titanium-Tantalum lattices for biomedical implant applications. Mater. Des. 2022, 218, 110688. [Google Scholar] [CrossRef]
- Findik, F. Recent developments of metallic implants for biomedical applications. Gels 2020, 8, 323. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqbal, S.; Iqbal, T.; Bakhsheshi-Rad, H.R.; Alsakkaf, A.; Kamil, A.; Abdul Kadir, M.R.; Idris, M.H.; Raghav, H.B. Zinc-doped hydroxyapatite—Zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2020, 30, 123–133. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]
- Xue, K.; Liang, L.X.; Cheng, S.C.; Liu, H.P.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Wang, Z.L. Corrosion resistance. antibacterial activity and drug release of ciprofloxacin-loaded micro-arc oxidation/silane coating on magnesium alloy AZ31. Prog. Org. Coat. 2021, 158, 106357. [Google Scholar] [CrossRef]
- Brown, A.; Zaky, S.; Ray, H.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553. [Google Scholar] [CrossRef]
- Dutta, S.; Bavya Devi, K.; Roy, M. Processing and degradation behavior of porous magnesium scaffold for biomedical applications. Adv. Powder Technol. 2017, 28, 3204–3212. [Google Scholar] [CrossRef]
- Jia, G.; Huang, H.; Niu, J.; Chen, C.; Weng, J.; Yu, F.; Wang, D.; Kang, B.; Wang, T.; Yuan, G.; et al. Exploring the interconnectivity of biomimetic hierarchical porous Mg scaffolds for bone tissue engineering: Effects of pore size distribution on mechanical properties, degradation behavior and cell migration ability. J. Magnes. Alloys 2021, 9, 1954–1966. [Google Scholar] [CrossRef]
- Li, H.F.; Shi, Z.Z.; Wang, L.N. Opportunities and challenges of biodegradable Zn-based alloys. J. Mater. Sci. Technol. 2020, 46, 136–138. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, Y. Study on Properties of New Mg-Y-Nd-(La+Ce)-Zr Degradable Magnesium Alloy. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- Seitz, J.M.; Durisin, M.; Goldman, J.; Drelich, J.W. Recent Advances in Biodegradable Metals for Medical Sutures: A Critical Review. Adv. Healthc. Mater. 2015, 4, 1915–1936. [Google Scholar] [CrossRef]
- Rzychoń, T.; Michalska, J.; Kielbus, A. Corrosion Resistance of Mg-RE-Zr Alloys. J. Achiev. Mater. Manuf. Eng. 2007, 21, 304–308. [Google Scholar]
- Cifuentes, S.C.; Lieblich, M.; Saldaña, L.; González-Carrasco, J.L.; Benavente, R. In vitro degradation of biodegradable polylactic acid/Mg composites: Influence of nature and crystalline degree of the polymeric matrix. Materialia 2019, 6, 100270. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, B.; Cai, Z. Recent Progress on Mg- And Zn-Based Alloys for Biodegradable Vascular Stent Applications. J. Nanomater. 2019, 2019, 1310792. [Google Scholar] [CrossRef]
- Glöckel, F.; Uggowitzer, P.J.; Felfer, P.; Pogatscher, S.; Höppel, H.W. Influence of Zn and Sn on the precipitation behavior of new Al-Mg-Si alloys. Materials 2019, 12, 2547. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Das Lala, S.; Deb, P.; Barua, E.; Deoghare, A.B.; Chatterjee, S. ScienceDirect Characterization of Hydroxyapatite Derived from Eggshells for Medical Implants. Mater. Today: Proc. 2019, 15, 323–327. [Google Scholar] [CrossRef]
- El-Mahallawy, N.; Palkowski, H.; Klingner, A.; Diaa, A.; Shoeib, M. Effect of 1.0 wt.% Zn addition on the microstructure, mechanical properties, and bio-corrosion behaviour of micro alloyed Mg-0.24Sn-0.04Mn alloy as biodegradable material. Mater. Today Commun. 2020, 24, 100999. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Sharma Kopparthy, S.D.; Gupta, M. A study on the effect of low-cost eggshell reinforcement on the immersion, damping and mechanical properties of magnesium–zinc alloy. Compos. B Eng. 2020, 182, 107650. [Google Scholar] [CrossRef]
- Aida, S.F.; Zuhailawati, H.; Anasyida, A.S. The Effect of Space Holder Content and Sintering Temperature of Magnesium Foam on Microstructural and Properties Prepared by Sintering Dissolution Process (SDP) Using Carbamide Space Holder. Procedia Eng. 2017, 184, 290–297. [Google Scholar] [CrossRef]
- Julmi, S.; Krüger, A.K.; Waselau, A.C.; Meyer-Lindenberg, A.; Wriggers, P.; Klose, C.; Maier, H.J. Processing and coating of open-pored absorbable magnesium-based bone implants. Mater. Sci. Eng. C 2019, 98, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Li, S.; Zeng, R.; Zhang, F.; Wang, Z.; Guan, S. Corrosion resistance and drug release profile of gentamicin-loaded polyelectrolyte multilayers on magnesium alloys: Effects of heat treatment. J. Colloid. Interface Sci. 2019, 547, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Functional PEO layers on magnesium alloys: Innovative polymer-free drug-eluting stents. Surf. Innov. 2018, 6, 237–243. [Google Scholar] [CrossRef]
- Aggarwal, D.; Kumar, V.; Sharma, S. Drug-loaded biomaterials for orthopedic applications: A review. J. Control. Release 2022, 344, 113–133. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shie, M.Y.; Wu, Y.H.A.; Lee, K.X.A.; Wei, L.J.; Shen, Y.F. Anti-inflammation performance of curcumin-loaded mesoporous calcium silicate cement. J. Formos. Med. Assoc. 2017, 116, 679–688. [Google Scholar] [CrossRef]
- Pengjam, Y.; Panichayupakaranant, P.; Tanrattanakul, V. Curcuminoid (CRE-Ter)/Liposome as delivery platform for anti-osteoclastogenesis via NF-κB/ERK pathways in RANKL-induced RAW 264.7 cells through PLA foams. Heliyon 2021, 7, e07823. [Google Scholar] [CrossRef]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef]
- Thangavelu, M.; Adithan, A.; John Peter, J.S.; Hossain, M.A.; Kim, N.S.; Hwang, K.C.; Khang, G.; Kim, J.H. Ginseng compound K incorporated porous Chitosan/biphasic calcium phosphate composite microsphere for bone regeneration. Int. J. Biol. Macromol. 2020, 146, 1024–1029. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Chen, K.Y.; Lin, K.C.; Chen, Y.S.; Yao, C.H. A novel porous gelatin composite containing naringin for bone repair. Evid.-Based Complement. Altern. Med. 2013, 2013, 283941. [Google Scholar] [CrossRef] [PubMed]
- Amirghofran, Z.; Ahmadi, H.; Karimi, M.H.; Kalantar, F.; Gholijani, N.; Malek-Hosseini, Z. In vitro inhibitory effects of thymol and carvacrol on dendritic cell activation and function. Pharm. Biol. 2016, 54, 1125–1132. [Google Scholar] [CrossRef]
- Mahmoud, R.; Safwat, N.; Fathy, M.; Mohamed, N.A.; El-Dek, S.; El-Banna, H.A.; Farghali, A.; Abo El-Ela, F.I. Novel anti-inflammatory and wound healing controlled released LDH-Curcumin nanocomposite via intramuscular implantation, in-vivo study. Arab. J. Chem. 2022, 15, 103646. [Google Scholar] [CrossRef]
- Banerjee, D.; Bose, S. Effects of aloe vera gel extract in doped hydroxyapatite-coated titanium implants on in vivo and in vitro biological properties. ACS Appl. Bio. Mater. 2019, 2, 3194–3202. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.M.; Duraisamy, N.; Raj, V. Drug loaded chitosan/aloe vera nanocomposite on Ti for orthopedic applications. Mater. Today Proc. 2021, 51, 1714–1719. [Google Scholar] [CrossRef]
- Srividya, S.; Sastry, T.P.; Jeevitha, D.; Samiksha, N. Synthesis and Characterization of a Novel Bone Graft Material Containing Biphasic Calcium Phosphate and Chitosan Fortified with Aloe Vera. Int. J. Pharm. Pharm. Sci. 2014, 2, 358–361. [Google Scholar] [CrossRef]
- Singh, V. Medicinal plants and bone healing. Natl. J. Maxillofac. Surg. 2017, 8, 4. [Google Scholar] [CrossRef]
- Li, H.; Wu, R.; Yu, H.; Zheng, Q.; Chen, Y. Bioactive Herbal Extracts of Traditional Chinese Medicine Applied with the Biomaterials: For the Current Applications and Advances in the Musculoskeletal System. Front. Pharmacol. 2021, 12, 778041. [Google Scholar] [CrossRef]
- Kannan, M.B.; Liyanaarachchi, S. Hybrid coating on a magnesium alloy for minimizing the localized degradation for load-bearing biodegradable mini-implant applications. Mater. Chem. Phys. 2013, 142, 350–354. [Google Scholar] [CrossRef]
- Badv, M.; Bayat, F.; Weitz, J.I.; Didar, T.F. Single and multi-functional coating strategies for enhancing the biocompatibility and tissue integration of blood-contacting medical implants. Biomaterials 2020, 258, 120291. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Thompson, M.; Zhao, N.; Zhu, D. Similarities and differences in coatings for magnesium-based stents and orthopaedic implants. J. Orthop. Translat. 2014, 2, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, M.; Mazaheri, M.; Balak, Z.; Kafashan, H.; Kim, H.S. Fabrication of Mg/Al12Mg17 in-situ surface nanocomposite via friction stir processing. Mater. Sci. Eng. A 2018, 712, 655–662. [Google Scholar] [CrossRef]
- Ikpi, M.; Dong, J.; Wei, J.; Ke, W.; Xu, S.; Ikpi, M.E.; Chen, N. Effects of Cadmium alloying on the Corrosion and Mechanical Properties of Magnesium. Int. J. Electrochem. Sci. 2012, 7, 4735–4755. [Google Scholar] [CrossRef]
- Luo, X.; Song, X.; Cao, Y.; Song, L.; Bu, X. Investigation of calcium carbonate synthesized by steamed ammonia liquid waste without use of additives. RSC Adv. 2020, 10, 7976–7986. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/poly(ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef]
- Chauhan, P.; Kumar, A. Development of a microbial coating for cellulosic surface using aloe vera and silane. Carbohydr. Polym. Technol. Appl. 2020, 1, 100015. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 3rd ed.; Thomson Brooks/Cole: Pacific Grove, CA, USA, 2000. [Google Scholar]
- Fardsadegh, B.; Jafarizadeh-Malmiri, H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process. Synth. 2019, 8, 399–407. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Suresh, S. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique Synthesis and Characterization of Yttrium Stabilized Zirconia Nanoparticles View project Biodegradable superabsorbent material for application in personal disposable hygiene products View project. Artic. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite -Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Voicu, S.I.; Ciocan, L.T. Synthesis and characterization of PLA-micro-structured hydroxyapatite composite films. Materials 2020, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Relinque, J.J.; de León, A.S.; Hernández-Saz, J.; García-Romero, M.G.; Navas-Martos, F.J.; Morales-Cid, G.; Molina, S.I. Development of surface-coated Polylactic Acid/Polyhydroxyalkanoate (PLA/PHA) nanocomposites. Polymers 2019, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Luo, Y.; Yu, J.; Liu, B.; Hu, M.; Chai, L.; Wang, C. Effects of high-repetition-rate femtosecond laser micromachining on the physical and chemical properties of polylactide (PLA). Opt. Express 2015, 23, 26932. [Google Scholar] [CrossRef]
- Seyedraoufi, Z.S.; Mirdamadi, S. Synthesis, microstructure and mechanical properties of porous Mg-Zn scaffolds. J. Mech. Behav. Biomed. Mater. 2013, 21, 1–8. [Google Scholar] [CrossRef]
- Walsh, W.R.; Pelletier, M.H.; Bertollo, N.; Lovric, V.; Wang, T.; Morberg, P.; Parr, W.C.H.; Bergadano, D. Bone ongrowth and mechanical fixation of implants in cortical and cancellous bone. J. Orthop. Surg. Res. 2020, 15, 177. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, P.; Zhang, B.; Tan, L.; Yang, K.; Lin, J. Research on the corrosion resistance and formation of double-layer calcium phosphate coating on AZ31 obtained at varied temperatures. Mater. Sci. Eng. C 2014, 43, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, X.; Tang, H.; Sun, H.; Qin, L.; Zheng, Y.; Gu, X.; Fan, Y. In vitro and in vivo degradation behavior of Mg–2Sr–Ca and Mg–2Sr–Zn alloys. Bioact. Mater. 2020, 5, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Johari, M.; Tabaian, S.H.; Saeedi, S. Microstructural Characterization and Investigation on Corrosion Properties of Mg-Zn-RE-Ca Alloy as a Possible Biomedical Implant. Met. Mater. Int. 2022, 28, 1386–1400. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, L.; Tang, J.; Li, Z.; Zhao, G.; Zhang, C. Investigation on corrosion behavior and mechanical properties of an extruded Mg-Zn-Al-Sn-Mn alloy. Mater. Charact. 2021, 180, 111439. [Google Scholar] [CrossRef]
- Polat, O.; Toy, S.; Kibar, B. Surgical outcomes of scaphoid fracture osteosynthesis with magnesium screws. Jt. Dis. Relat. Surg. 2021, 32, 721–728. [Google Scholar] [CrossRef]
- Allemann, F.; Halvachizadeh, S.; Rauer, T.; Pape, H.C. Clinical outcomes after carbon-plate osteosynthesis in patients with distal radius fractures. Patient Saf. Surg. 2019, 13, 30. [Google Scholar] [CrossRef]
- Liu, H.Y.; Du, L.; Zhao, Y.T.; Tian, W.Q. In vitro hemocompatibility and cytotoxicity evaluation of halloysite nanotubes for biomedical application. J. Nanomater. 2015, 2015, 384. [Google Scholar] [CrossRef]
- Li, J.; Zheng, W.; Zheng, Y.F.; Lou, X. Cell responses and hemocompatibility of g-HA/PLA composites. Sci. China Life Sci. 2011, 54, 366–371. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics the bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Liu, S.; Ulugun, B.; DeFlorio, W.; Arcot, Y.; Yegin, Y.; Salazar, K.S.; Castillo, A.; Taylor, T.M.; Cisneros-Zevallos, L.; Akbulut, M. Development of durable and superhydrophobic nanodiamond coating on aluminum surfaces for improved hygiene of food contact surfaces. J. Food Eng. 2021, 298, 110487. [Google Scholar] [CrossRef]
- Thompson, D.O.; Chimenti, D.E. Review of Progress in Quantitative Nondestructive Evaluation: Kingston, Rhode Island, 26–31 July 2009; American Institute of Physics: College Park, MD, USA, 2010. [Google Scholar]
- Bayat, A.; Ebrahimi, M.; Moshfegh, A.Z. Correlation between surface roughness and hydrophobicity of GLAD RF sputtered PTFE/W/Glass nanorod thin films. Vacuum 2014, 101, 279–282. [Google Scholar] [CrossRef]
- Shi, P.; Niu, B.; Shanshan, E.; Chen, Y.; Li, Q. Preparation and characterization of PLA coating and PLA/MAO composite coatings on AZ31 magnesium alloy for improvement of corrosion resistance. Surf. Coat. Technol. 2015, 262, 26–32. [Google Scholar] [CrossRef]
- Mousa, H.M.; Abdal-Hay, A.; Bartnikowski, M.; Mohamed, I.M.A.; Yasin, A.S.; Ivanovski, S.; Park, C.H.; Kim, C.S. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Asadi, H.; Suganthan, B.; Ghalei, S.; Handa, H.; Ramasamy, R.P. A multifunctional polymeric coating incorporating lawsone with corrosion resistance and antibacterial activity for biomedical Mg alloys. Prog. Org. Coat. 2021, 153, 106157. [Google Scholar] [CrossRef]
- Subramaniam, G.; Yew, X.Y.; Sivasamugham, L.A. Antibacterial activity of Cymbopogon citratus against clinically important bacteria. South Afr. J. Chem. Eng. 2020, 34, 26–30. [Google Scholar] [CrossRef]
- Issazadeh, S.A.; Hatami, S.; Yavarmanesh, M. In vitro investigation of chemical composition and antibacterial activity of alcoholic, hydroalcoholic extracts, and essential oil of Spinacia oleracea leaves from Iran. J. Food Saf. 2021, 41, e12891. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, C.; Parafati, L.; Cirvilleri, G.; Mauromicale, G. Antimicrobial activity of cultivated cardoon (Cynara cardunculus L. var. altilis DC.) leaf extracts against bacterial species of agricultural and food interest. Ind. Crops Prod. 2019, 129, 206–211. [Google Scholar] [CrossRef]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic compounds in extra virgin olive oil stimulate human osteoblastic cell proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef]
- Rethinam, S.; Vijayan, S.; Aruni, A.W.; Basaran, B.; Alagumuthu, T.; Ramamoorthy, R. Enhanced tissue regeneration using an nano- bioactive scaffold- A novel perspective. Mater. Chem. Phys. 2020, 240, 122303. [Google Scholar] [CrossRef]
- Naveen, K.V.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.H. Eco-friendly synthesis and characterization of Aloe vera/Gum Arabic/silver nanocomposites and their antibacterial, antibiofilm, and wound healing properties. Colloids Interface Sci. Commun. 2022, 46, 100566. [Google Scholar] [CrossRef]
- Mazzulla, S.; Sesti, S.; Schella, A.; Perrotta, I.; Anile, A.; Drogo, S. Protective Effect of Aloe vera (Aloe barbadensis Miller) on Erythrocytes Anion Transporter and Oxidative Change. Food Nutr. Sci. 2012, 3, 1697–1702. [Google Scholar] [CrossRef]
- Paul, S.; Modak, D.; Chattaraj, S.; Nandi, D.; Sarkar, A.; Roy, J.; Chaudhuri, T.K.; Bhattacharjee, S. Aloe vera gel homogenate shows anti-inflammatory activity through lysosomal membrane stabilization and downregulation of TNF-α and Cox-2 gene expressions in inflammatory arthritic animals. Futur. J. Pharm. Sci. 2021, 7, 12. [Google Scholar] [CrossRef]
- Carvalho, J.R.G.; Conde, G.; Antonioli, M.L.; Santana, C.H.; Littiere, T.O.; Dias, P.P.; Chinelatto, M.A.; Canola, P.A.; Zara, F.J.; Ferraz, G.C. Long-term evaluation of poly(Lactic acid) (PLA) implants in a horse: An experimental pilot study. Molecules 2021, 26, 7224. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, J.; Li, J.; Zheng, Q.; Wang, Z.; Zhang, X.; Zhang, S. Effects of biodegradable Mg-6Zn alloy extracts on apoptosis of intestinal epithelial cells. Mater. Sci. Eng. B Solid. State Mater. Adv. Technol. 2012, 177, 388–393. [Google Scholar] [CrossRef]
- Carter, P.; Rahman, S.M.; Bhattarai, N. Facile fabrication of aloe vera containing PCL nanofibers for barrier membrane application. J. Biomater. Sci. Polym. Ed. 2016, 27, 692–708. [Google Scholar] [CrossRef]
- Rahman, S.; Carter, P.; Bhattarai, N. Aloe Vera for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef]
- Sridhar, R.; Ravanan, S.; Venugopal, J.R.; Sundarrajan, S.; Pliszka, D.; Sivasubramanian, S.; Gunasekaran, P.; Prabhakaran, M.; Madhaiyan, K.; Sahayaraj, A.; et al. Curcumin-and natural extract-loaded nanofibres for potential treatment of lung and breast cancer: In vitro efficacy evaluation. J. Biomater. Sci. Polym. Ed. 2014, 25, 985–998. [Google Scholar] [CrossRef]
- Suganya, S.; Venugopal, J.; Agnes Mary, S.; Ramakrishna, S.; Lakshmi, B.S.; Giri Dev, V.R. Aloe vera incorporated biomimetic nanofibrous scaffold: A regenerative approach for skin tissue engineering. Iran. Polym. J. (Engl. Ed.) 2014, 23, 237–248. [Google Scholar] [CrossRef]


| Sample | Carbamide (C) wt% | Density (g/cm3) | Porosity (%) |
|---|---|---|---|
| Mg-HA | 0 | 1.7512 ± 0.013 | 3.12 ± 0.131 |
| Mg-HA-10C | 10 | 0.9251 ± 0.012 | 51.1 ± 0.122 |
| Mg-HA-20C | 20 | 0.6858 ± 0.043 | 72.25 ± 0.024 |
| Mg-HA-30C | 30 | 0.4557 ± 0.005 | 81.5 ± 0.154 |
| Mg-HA-20C-AV | 20 | 1.0052 ± 0.025 | 70.25 ± 0.115 |
| Mg-HA-20C-AV+PLA coating | 20 | 2.1250 ± 0.002 | 2.18 ± 0.113 |
| Sample | % Dead Cells | % Late Apoptotic Cells | % Live Cells | % Early Apoptotic Cells |
|---|---|---|---|---|
| Untreated | 0 | 0.18 | 99.74 | 0.08 |
| Std. Control | 7.22 | 51.71 | 31.07 | 10 |
| P2 | 5.35 | 25.2 | 40.29 | 29.16 |
| Q2 | 0 | 0 | 98.29 | 1.71 |
| R2 | 8.07 | 28.73 | 52.31 | 10.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, D.; Sharma, S.; Gupta, M. Porous Mg–Hydroxyapatite Composite Incorporated with Aloe barbadensis Miller for Scaphoid Fracture Fixation: A Natural Drug Loaded Orthopedic Implant. Appl. Sci. 2024, 14, 1512. https://doi.org/10.3390/app14041512
Aggarwal D, Sharma S, Gupta M. Porous Mg–Hydroxyapatite Composite Incorporated with Aloe barbadensis Miller for Scaphoid Fracture Fixation: A Natural Drug Loaded Orthopedic Implant. Applied Sciences. 2024; 14(4):1512. https://doi.org/10.3390/app14041512
Chicago/Turabian StyleAggarwal, Divyanshu, Siddharth Sharma, and Manoj Gupta. 2024. "Porous Mg–Hydroxyapatite Composite Incorporated with Aloe barbadensis Miller for Scaphoid Fracture Fixation: A Natural Drug Loaded Orthopedic Implant" Applied Sciences 14, no. 4: 1512. https://doi.org/10.3390/app14041512
APA StyleAggarwal, D., Sharma, S., & Gupta, M. (2024). Porous Mg–Hydroxyapatite Composite Incorporated with Aloe barbadensis Miller for Scaphoid Fracture Fixation: A Natural Drug Loaded Orthopedic Implant. Applied Sciences, 14(4), 1512. https://doi.org/10.3390/app14041512







