Simulation of the VOC Adsorption Mechanism on Activated Carbon Surface by Nitrogen-Containing Functional Groups
Abstract
:1. Introduction
2. Models and Methods
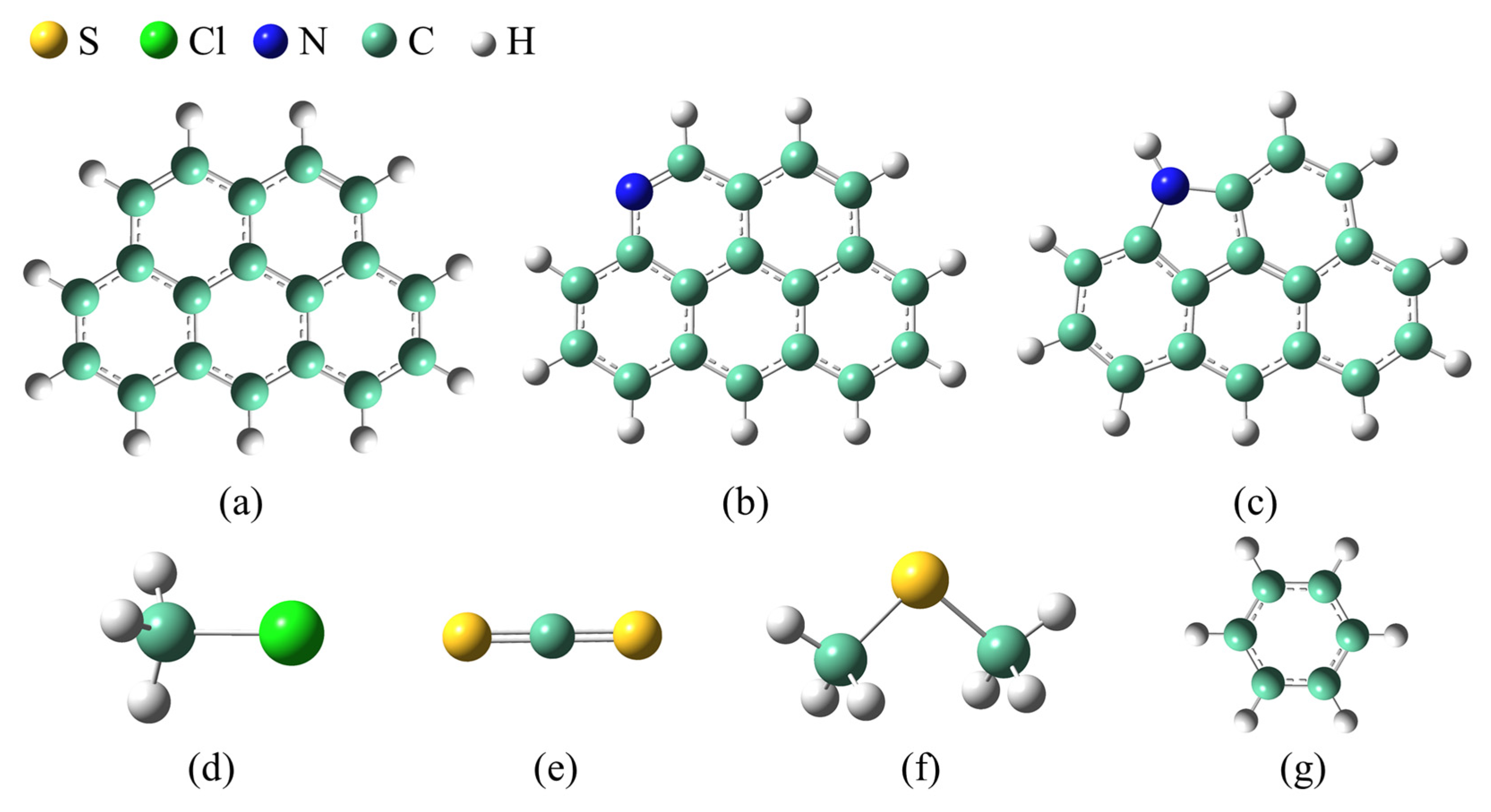
2.1. Calculation Models
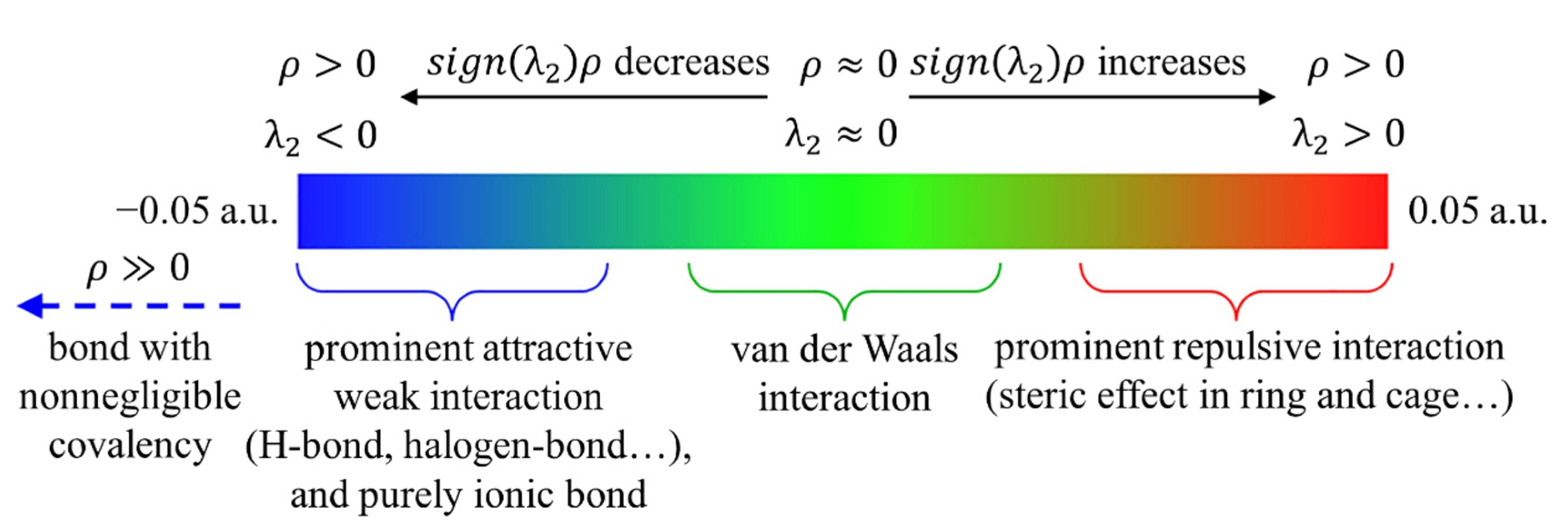
2.2. Calculation Methods
3. Results and Discussion
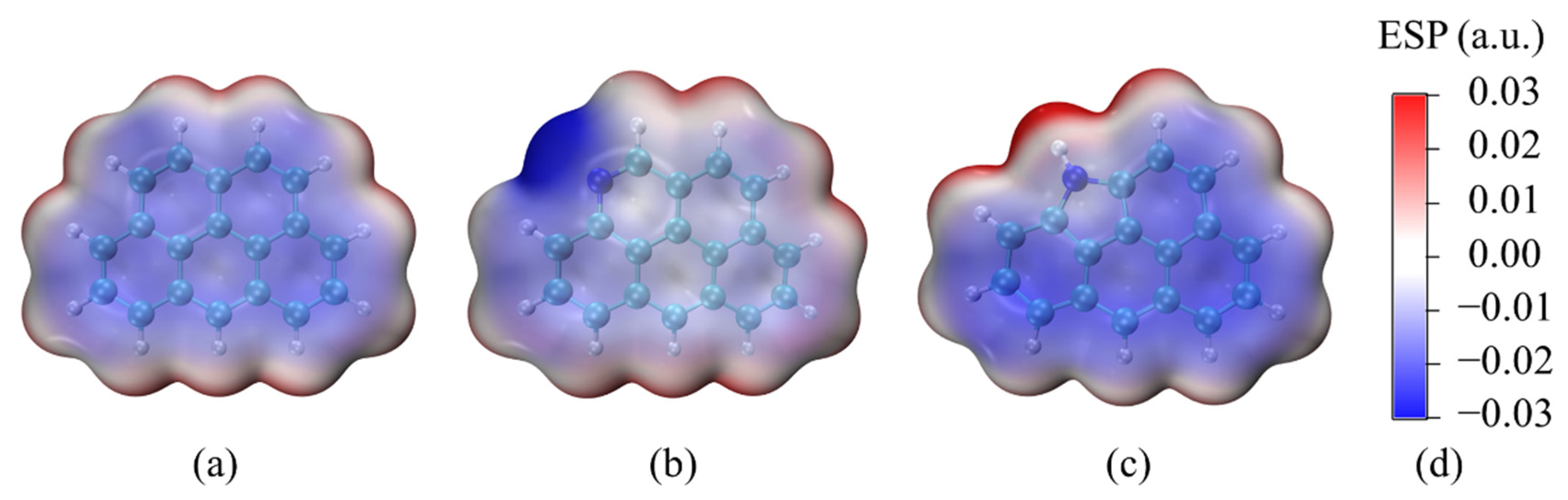
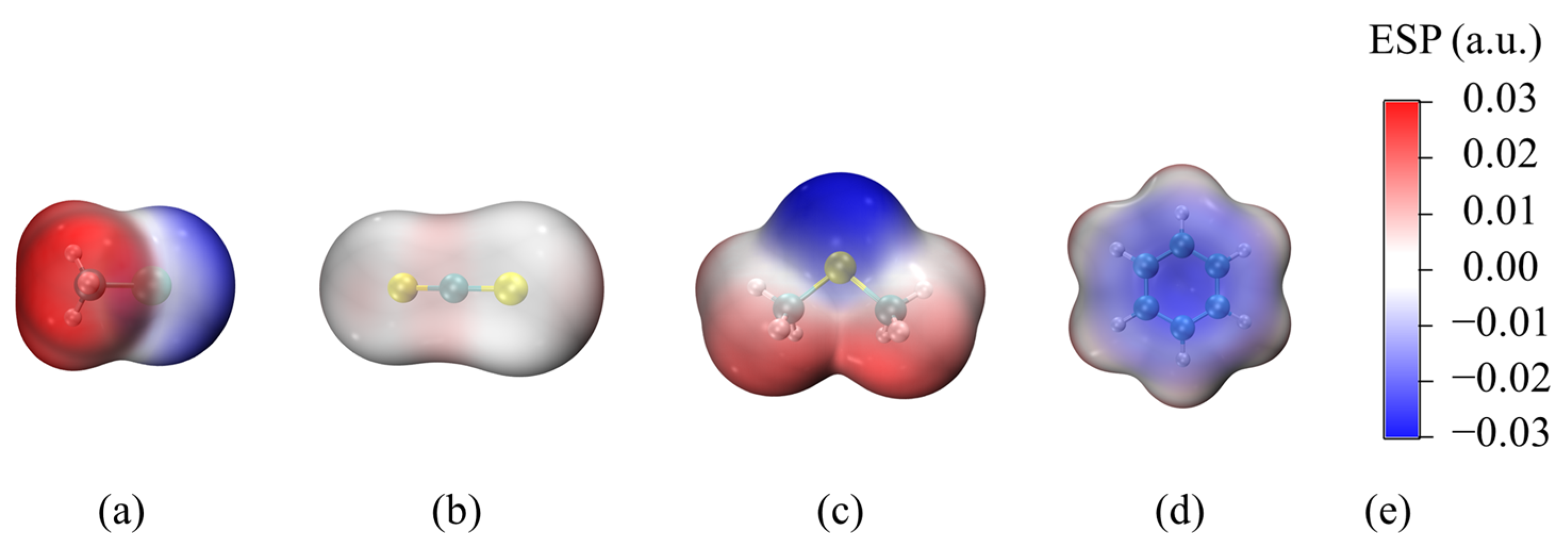
3.1. ESP Analysis
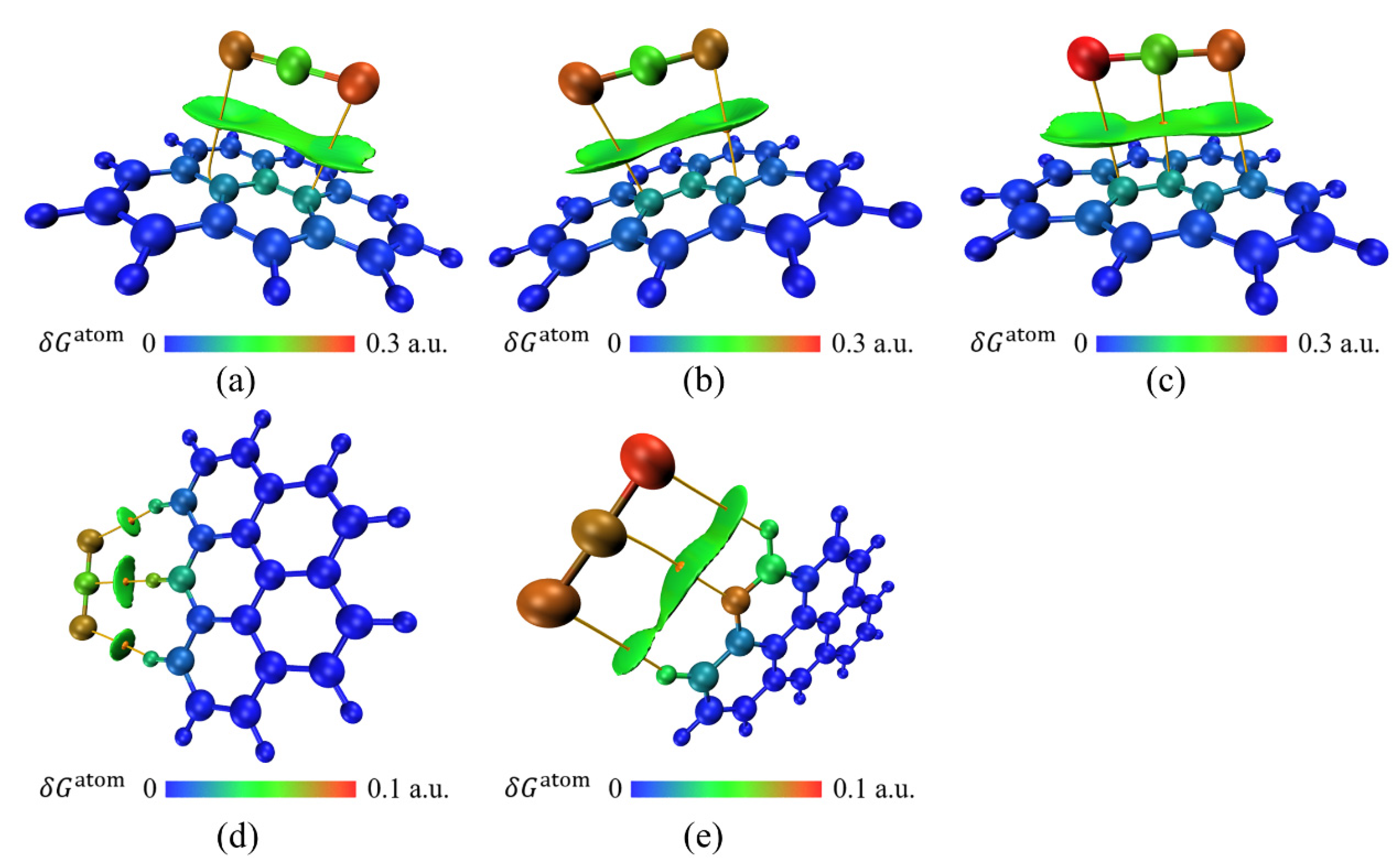
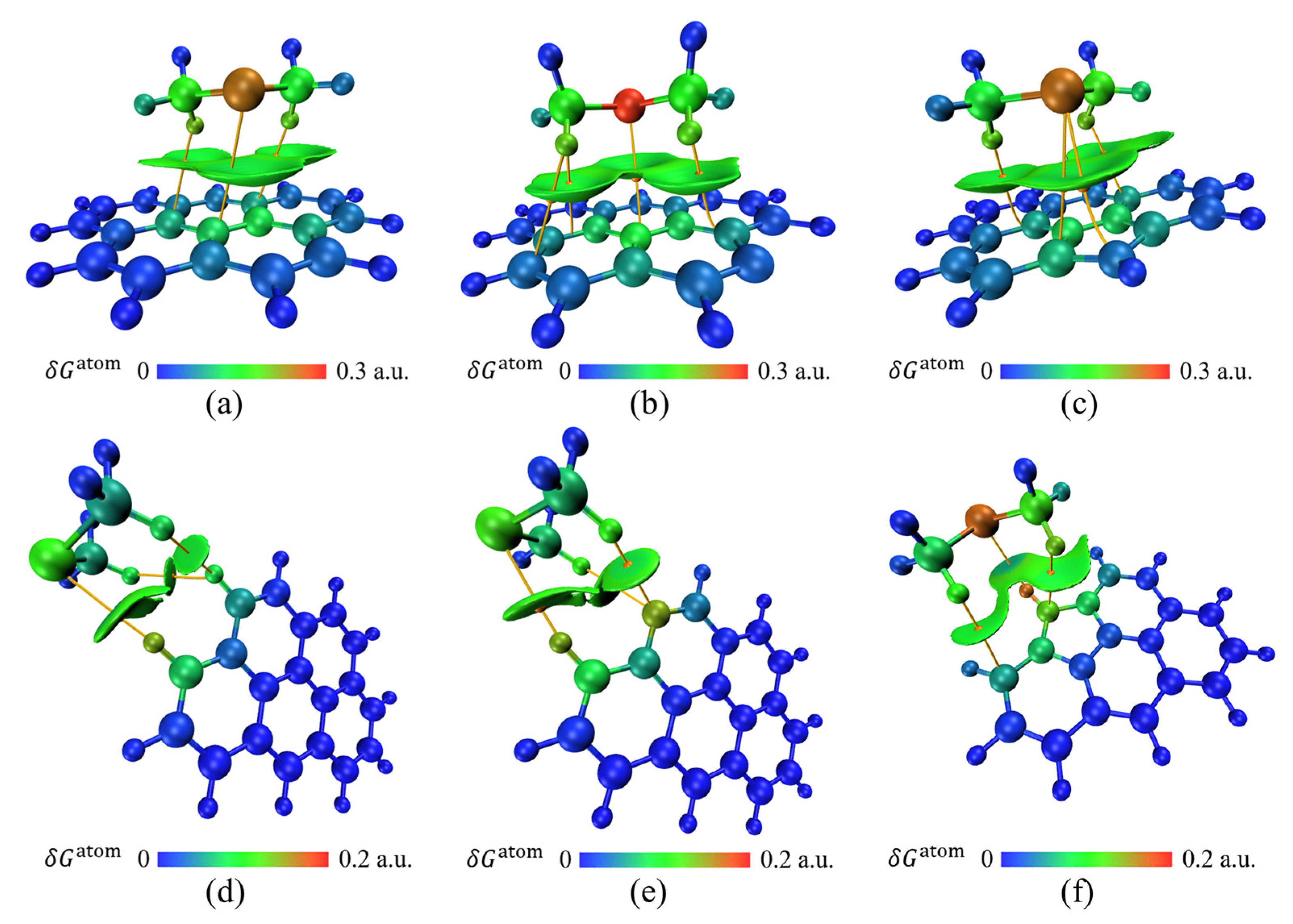
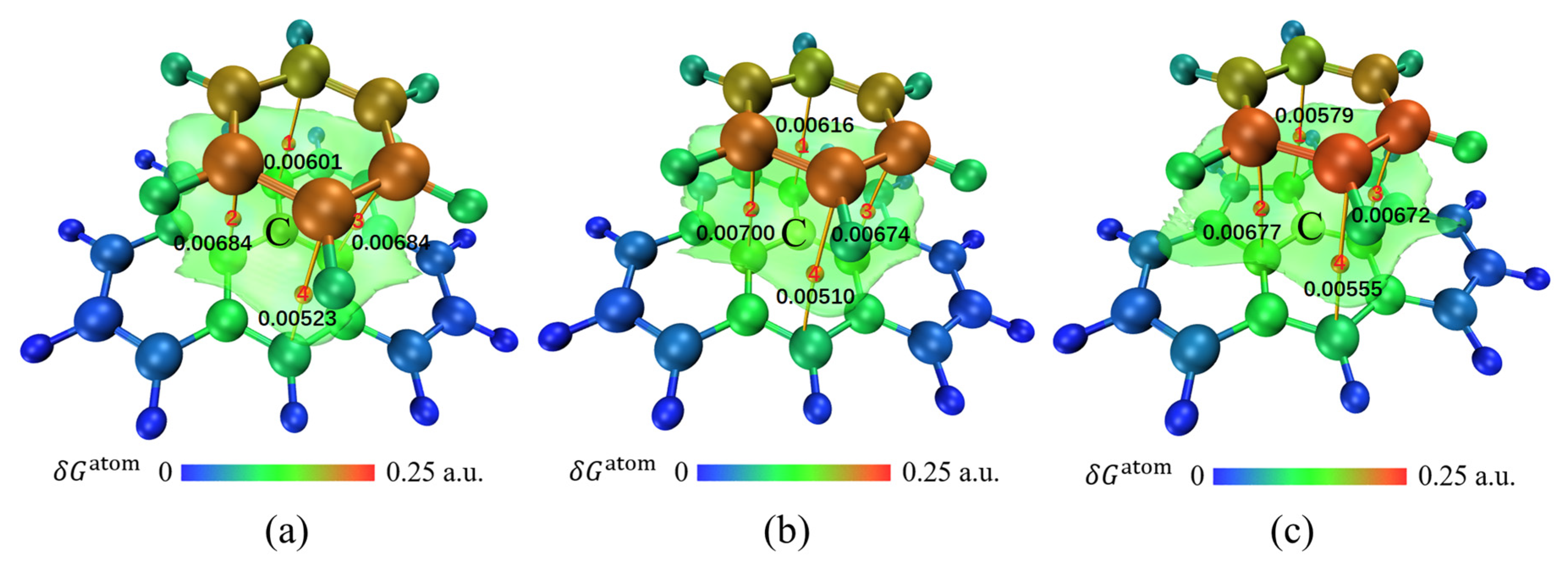
3.2. Physical Adsorption of CH3Cl by Activated Carbon
3.3. Physical Adsorption of CS2 by Activated Carbon
3.4. Physical Adsorption of C2H6S by Activated Carbon
3.5. Physical Adsorption of C6H6 by Activated Carbon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Yao, B.; Fang, X. Anthropogenic emissions of ozone-depleting substance CH3Cl during 2000–2020 in China. Environ. Pollut. 2022, 310, 119903. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xin, J.; Hu, B.; Song, T.; Wang, L.; Wu, F.; Wang, Y. The spatial-temporal distribution characteristics of atmospheric chloromethane according to data from the CARE-China network. Atmos. Environ. 2021, 260, 118484. [Google Scholar] [CrossRef]
- Chloromethane Market Size, Share & Trends Analysis Report by Product (Chloroform, Methylene Chloride, Methyl Chloride), by Application, by End Use (Pharma, Personal Care Products), and Segment Forecasts, 2020–2027. Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/chloromethane-market (accessed on 23 November 2023).
- Logan, J.A.; McElroy, M.B.; Wofsy, S.C.; Prather, M.J. Oxidation of CS2 and COS: Sources for atmospheric SO2. Nature 1979, 281, 185–188. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Goldberg, L.; McKenna, M.J. A critical review of the literature on carbon disulfide toxicity. CRC Crit. Rev. Toxicol. 1983, 11, 169–278. [Google Scholar] [CrossRef] [PubMed]
- Gelbke, H.P.; Göen, T.; Mäurer, M.; Sulsky, S.I. A review of health effects of carbon disulfide in viscose industry and a proposal for an occupational exposure limit. Crit. Rev. Toxicol. 2009, 39, 1–126. [Google Scholar] [CrossRef]
- Sieja, K.; Mach-Szczypiński, J.V. Health effect of chronic exposure to carbon disulfide (CS2) on women employed in viscose industry. Med. Pr. 2018, 69, 329–335. [Google Scholar] [CrossRef]
- Bortnikova, S.B.; Devyatova, A.Y.; Yurkevich, N.V.; Grakhova, S.P.; Ogudov, A.S.; Zubtsovskaya, N.A.; Edelev, A.V.; Volynkin, S.S. Gas anomalies in the air above the sulfide tailings and adjacent soils in Komsomolsk Settlement (Kemerovo Region, Russia). Water Air Soil Pollut. 2021, 232, 412. [Google Scholar] [CrossRef]
- Murrieta-Rico, F.N.; Antúnez-García, J.; Yocupicio-Gaxiola, R.I.; Galván, D.H.; González, J.C.; Petranovskii, V. Zeolites as initial structures for the preparation of functional materials. J. Appl. Res. Technol. 2022, 20, 92–116. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.; Zhang, S.; Zhuang, L.; Hu, B.; Wang, S.; Chen, J.; Wang, X. Application of biochar-based photocatalysts for adsorption-(photo)degradation/reduction of environmental contaminants: Mechanism, challenges and perspective. Biochar 2022, 4, 45. [Google Scholar] [CrossRef]
- Goel, N.; Kunal, K.; Kushwaha, A.; Kumar, M. Metal oxide semiconductors for gas sensing. Eng. Rep. 2023, 5, e12604. [Google Scholar] [CrossRef]
- Hong, Y.; Jin, H.-J.; Kwak, H.W. Nitrogen-rich magnetic bio-activated carbon from sericin: A fast removable and easily separable superadsorbent for anionic dye removal. Macromol. Res. 2020, 28, 986–996. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Zheng, Q.R. Methane adsorption on the graphene sheets, activated carbon and carbon black. Appl. Therm. Eng. 2016, 108, 605–613. [Google Scholar] [CrossRef]
- Liu, X. The Effect of Surface Structure of Carbon Materials Surface on SO2 Adsorption and Oxidation: A DFT Calculation Study; Harbin Institute of Technology: Harbin, China, 2017. [Google Scholar]
- Wang, L.; Sha, L.; Zhang, S.; Cao, F.; Ren, X.; Levendis, Y.A. Preparation of activated coke by carbonization, activation, ammonization and thermal treatment of sewage sludge and waste biomass for SO2 absorption applications. Fuel Process. Technol. 2022, 231, 107233. [Google Scholar] [CrossRef]
- Kiranakumar, H.V.; Thejas, R.; Naveen, C.S.; Khan, M.I.; Prasanna, G.D.; Reddy, S.; Oreijah, M.; Guedri, K.; Bafakeeh, O.T.; Jameel, M. A review on electrical and gas-sensing properties of reduced graphene oxide-metal oxide nanocomposites. Biomass Convers. Bior. 2022. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Wei, M.; Marrakchi, F.; Yuan, C.; Cheng, X.; Jiang, D.; Zafar, F.F.; Fu, Y.; Wang, S. Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. J. Hazard. Mater. 2022, 425, 127887. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Johnson, E.R.; Mackie, I.D.; DiLabio, G.A. Dispersion interactions in density-functional theory. J. Phys. Org. Chem. 2009, 22, 1127–1135. [Google Scholar] [CrossRef]
- Gao, H.; Wang, S.; Hao, M.; Shao, W.; Zhang, S.; Zhang, L.; Ren, X. CO2 adsorption performance of activated coke prepared from biomass and coal. Energies 2023, 16, 3872. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Xu, S.; Ren, X.; Zhang, Y.; Cao, F.; Sun, Q.; Wennersten, R.; Yang, L. The effect of nitrogen- and oxygen-containing functional groups on C2H6/SO2/NO adsorption: A density functional theory study. Energies 2023, 16, 7537. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Hao, M.; Zhang, Y.; Ren, X.; Cao, F.; Zhang, L.; Sun, Q.; Wennersten, R. Effect of functional groups on VOCs adsorption by activated carbon: DFT study. Surf. Sci. 2023, 736, 122352. [Google Scholar] [CrossRef]
- Guo, S.; Zou, Z.; Chen, Y.; Long, X.; Liu, M.; Li, X.; Tan, J.; Chen, R. Synergistic effect of hydrogen bonding and π-π interaction for enhanced adsorption of rhodamine B from water using corn straw biochar. Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhu, F.; Jiang, T.; Wei, F.; Gao, Y.; Xiang, C.; Duan, Y.; Hua, Y.; Zhou, S.; Wang, Y. Oxygen-containing functional groups enhance uranium adsorption by aged polystyrene microplastics: Experimental and theoretical perspectives. Chem. Eng. J. 2023, 465, 142730. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Petersson, G.A.; Nakatsuji, H.J.R.A.; et al. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D.G. Perspectives on basis sets beautiful: Seasonal plantings of diffuse basis functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T. Multiwfn_3.8_dev.pdf. Beijing Kein Research Center for Natural Sciences. Available online: http://sobereva.com/multiwfn/ (accessed on 23 November 2023).
- Qu, Z.; Sun, F.; Liu, X.; Gao, J.; Qie, Z.; Zhao, G. The effect of nitrogen-containing functional groups on SO2 adsorption on carbon surface: Enhanced physical adsorption interactions. Surf. Sci. 2018, 677, 78–82. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Zhang, M.; Ma, C. Effect of N-doping on NO2 adsorption and reduction over activated carbon: An experimental and computational study. Fuel 2019, 258, 116109. [Google Scholar] [CrossRef]
- Bhattarai, S.; Sutradhar, D.; Chandra, A.K.; Zeegers-Huyskens, T. A theoretical investigation of the interaction between substituted pyridines and CS2. Versatility of the CS2 molecule. J. Mol. Struct. 2020, 1202, 127309. [Google Scholar] [CrossRef]
- Guo, B.; Li, C.; Xie, K. Ftir study on behavior of adsorbing CS2 by activated carbon. Coal Convers. 2004, 27, 54–57. [Google Scholar]
- Chen, J.; Zhai, Y.; Chen, H.; Li, C.; Zeng, G.; Pang, D.; Lu, P. Effects of pretreatment on the surface chemistry and pore size properties of nitrogen functionalized and alkylated granular activated carbon. Appl. Surf. Sci. 2012, 263, 247–253. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Zheng, L.; Dang, Z.; Zhang, L. Classical theory and electron-scale view of exceptional Cd(II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chem. Eng. J. 2018, 350, 1000–1009. [Google Scholar] [CrossRef]
- Zhai, Y.; Pang, D.; Chen, H.; Xiang, B.; Chen, J.; Li, C.; Zeng, G.; Qiu, L. Effects of ammonization on the surface physico-chemical properties of sludge-based activated carbon. Appl. Surf. Sci. 2013, 280, 590–597. [Google Scholar] [CrossRef]

| NF | PD | PR | |
|---|---|---|---|
| Plane | −0.216 (−0.221) | −0.201 (−0.223) | −0.229 (−0.265) |
| Edge | — | −0.115 | — |
| NF | PD | PR | |
|---|---|---|---|
| Plane | −0.236 | −0.231 | −0.250 |
| Edge | −0.060 | −0.080 | — |
| NF | PD | PR | |
|---|---|---|---|
| Plane | −0.302 | −0.326 | −0.333 |
| Edge | −0.107 | −0.196 | −0.283 |
| NF | PD | PR | |
|---|---|---|---|
| Plane | −0.369 | −0.379 | −0.371 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, S.; Xu, S.; Cao, F.; Ren, X.; Sun, Q.; Yang, L.; Wennersten, R.; Mei, N. Simulation of the VOC Adsorption Mechanism on Activated Carbon Surface by Nitrogen-Containing Functional Groups. Appl. Sci. 2024, 14, 1793. https://doi.org/10.3390/app14051793
Zhang Y, Zhang S, Xu S, Cao F, Ren X, Sun Q, Yang L, Wennersten R, Mei N. Simulation of the VOC Adsorption Mechanism on Activated Carbon Surface by Nitrogen-Containing Functional Groups. Applied Sciences. 2024; 14(5):1793. https://doi.org/10.3390/app14051793
Chicago/Turabian StyleZhang, Yan, Shuhui Zhang, Shaofeng Xu, Fan Cao, Xiaohan Ren, Qie Sun, Li Yang, Ronald Wennersten, and Ning Mei. 2024. "Simulation of the VOC Adsorption Mechanism on Activated Carbon Surface by Nitrogen-Containing Functional Groups" Applied Sciences 14, no. 5: 1793. https://doi.org/10.3390/app14051793





