Antibiotic Resistance in Seawater Samples from East Coast of Spain
Abstract
:1. Introduction
2. Materials and Methods
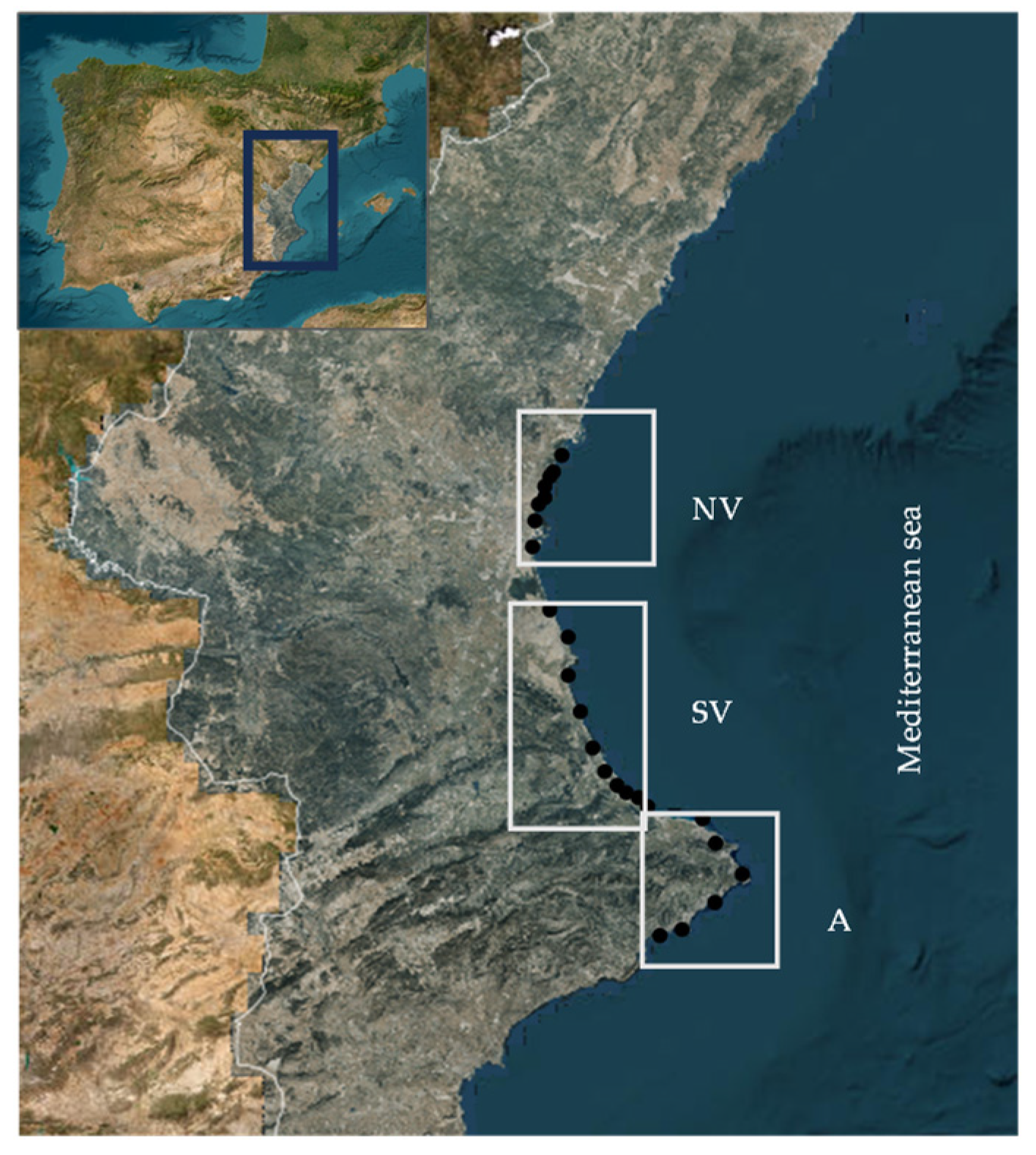
2.1. Samples
2.2. Bacterial Analysis
2.3. Determination of Antibiotic Resistance
2.4. Detection of ARG
2.5. Statistical Analysis
3. Results
3.1. Microbiological Determination of E. coli and Enterococcus sp. in Marine Water Samples
3.2. Antibiotic Resistance Determination of Bacterial Isolates
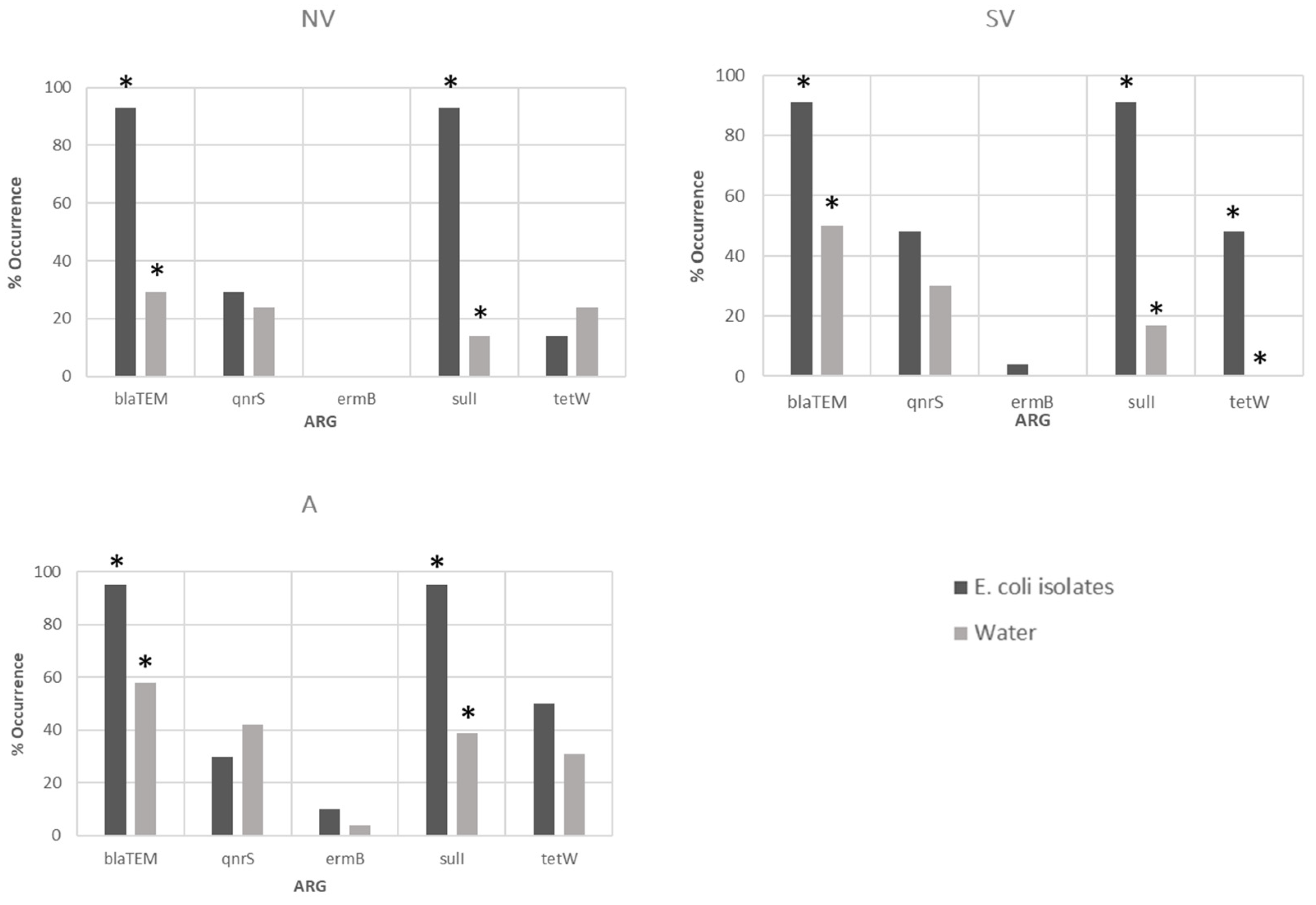
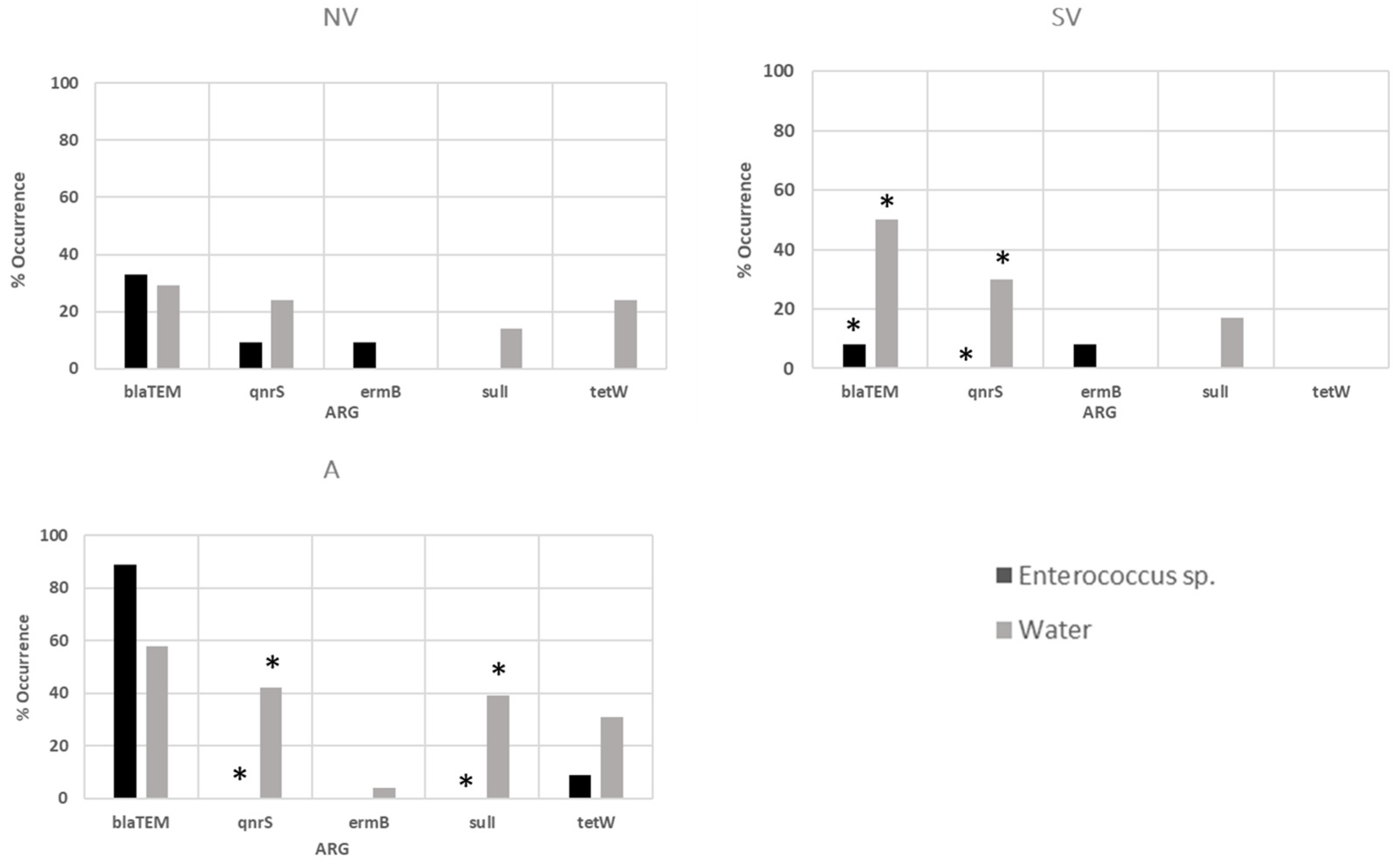
3.3. Antibiotic-Resistant Gene Determination in Water Samples and Bacterial Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Waseem, H.; Williams, M.R.; Jameel, S.; Hashsham, S.A. Antimicrobial Resistance in the Environment. Water Environ. Res. 2018, 90, 865–884. [Google Scholar] [CrossRef]
- Bondarczuk, K.; Piotrowska-Seget, Z. Microbial diversity and antibiotic resistance in a final effluent-receiving lake. Sci. Total Environ. 2019, 650, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 2015, 9, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Alduina, R. Antibiotics and environment. Antibiotics 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Gillings, M.; Gaze, W.; Pruden, A.; Samalla, K.; Tiedje, J.M.; Zhu, Y.-G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Cycón, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Joji, R.M.; Shahid, M. Evolution and implementation of One Health to control the dissemination of antibiotic-resistant bacteria and resistance genes: A review. Front. Cell Infect. Microbiol. 2023, 12, 1065796. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.S.; Jones, D.L.; Zou, J. Antibiotics and antibiotic resistance genes in agricultural soils: A systematic analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 847–864. [Google Scholar] [CrossRef]
- Jiménez-Belenguer, A.I.; Ferrús, M.A.; Hernández, M.; García-Hernández, J.; Moreno, Y.; Castillo, M.A. Prevalence and characterization of beta-lactam and carbapenem-resistant bacteria isolated from organic fresh produce retailed in Eastern Spain. Antibiotics 2023, 12, 387. [Google Scholar] [CrossRef]
- Hölzel, C.S.; Tetens, J.L.; Schwaiger, K. Unraveling the role of vegetables in spreading antimicrobial-resistant bacteria: A need for quantitative risk assessment. Foodborne Pathog. Dis. 2018, 15, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai, China. J. Hazard. Mater. 2012, 235, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Musiyiwa, K.; Mangori, L. Sources, behaviour and health risks of antimicrobial resistance genes in wastewaters: A hotspot reservoir. J. Environ. Chem. Eng. 2020, 8, 102220. [Google Scholar] [CrossRef]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.; Powell, J.; O’Connell, N.H.; Murphy, L.; Dunne, C.P. Antimicrobial resistance is prevalent in E. coli and other Enterobacterales isolated from public and private drinking water supplies in the Republic of Ireland. Microorganisms 2023, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Odonkor, S.T.; Addo, K.K. Prevalence of multidrug-resistant Escherichia coli isolated from drinking water sources. Int. J. Microbiol. 2018, 2018, 7204013. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.M.; Tun, H.M.; Poole, J.; Patidar, R.; Li, R.; Mi, R.; Amarawansha, G.E.A.; Fernando, W.G.D.; Khafipour, E.; Farenhorst, A.; et al. Detection of antibiotic resistance genes in source and drinking water samples from a First Nations community in Canada. Appl. Environ. Microbiol. 2016, 82, 4767–4775. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.L.; Salvadori, M.I.; McGeer, A.J.; Sibley, K.A.; Neumann, N.F.; Bondy, S.J.; Gutmanis, I.A.; McEwen, S.A.; Lavoie, M.; Strong, D.; et al. The role of drinking water in the transmission of antimicrobial-resistant E. coli. Epidemiol. Infect. 2012, 140, 633–642. [Google Scholar] [CrossRef]
- Wang, R.-N.; Zhang, Y.; Cao, Z.-H.; Wang, X.-Y.; Ma, B.; Wu, W.-B.; Hu, N.; Huo, Z.-Y.; Yuan, Q.-B. Occurrence of super antibiotic resistance genes in the downstream of the Yangtze River in China: Prevalence and antibiotic resistance profiles. Sci. Total Environ. 2019, 651, 1946–1957. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sanchez-Melsio, A.; Borrego, C.M.; Barcelo, D.; Balcazar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Sidhu, J.P.S.; Tiehm, A.; Toze, S. Prevalence of clinically relevant antibiotic resistance genes in surface water samples collected from Germany and Australia. Environ. Sci. Technol. 2012, 46, 9716–9726. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Dasí, D.; González, A.; Ferrús, M.A.; Castillo, M.A. Occurrence of antibiotic resistant bacteria and resistance genes in agricultural irrigation waters from Valencia city (Spain). Agric. Water Manag. 2021, 256, 107097. [Google Scholar] [CrossRef]
- Araújo, S.; Silva, I.A.T.; Tacao, M.; Patinha, C.; Alves, A.; Henriques, I. Characterization of antibiotic resistant and pathogenic Escherichia coli in irrigation water and vegetables in household farms. Int. J. Food Microbiol. 2017, 257, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Amos, G.C.A.; Hawkey, P.M.; Gaze, W.H.; Wellington, E.M. Wastewater effluent contributes to the dissemination of CTX-M-15 in the natural environment. J. Antimicrob. Chemother. 2014, 69, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A review of antibiotics, antibiotic resistant bacteria, and resistance genes in aquaculture: Occurrence, contamination, and transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Su, Z.; Li, A.; Chen, J.; Huang, B.; Mu, Q.; Chen, L.; Wen, D. Wastewater discharge drives ARGs spread in the coastal area: A case study in Hangzhou Bay, China. Mar. Pollut. Bull. 2020, 151, 110856. [Google Scholar] [CrossRef]
- Carney, R.L.; Labbate, M.; Siboni, N.; Tagg, K.A.; Mitrovic, S.M.; Seymour, J.R. Urban beaches are environmental hotspots for antibiotic resistance following rainfall. Water Res. 2019, 167, 11508. [Google Scholar] [CrossRef]
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Gaze, W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef]
- Makkaew, P.; Kongprajug, A.; Chyerochana, N.; Sresung, M.; Precha, N.; Mongkolsuk, S.; Sirikanchana, K. Persisting antibiotic resistance gene pollution and its association with human sewage sources in tropical marine beach waters. Int. J. Hyg. Environ. Health 2021, 238, 113859. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763eng.pdf?ua=1 (accessed on 1 September 2023).
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total Environ. 2021, 777, 146009. [Google Scholar] [CrossRef]
- ISO 9308-2:2012; Water quality —Enumeration of Escherichia coli and coliform bacteria. Part 2: Most probable number method. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 7899-1:1998/Cor 1:2000; Water quality Detection and enumeration of intestinal enterococci. Part 1: Miniaturized method (Most Probable Number) for surface and wastewater. International Organization for Standardization: Geneva, Switzerland, 2000.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. In Twenty-Fifth Informational Supplement; CLSI DocumentM100-S25; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015. [Google Scholar]
- ISO 20776-1:2019; Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1: Broth micro-dilution reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. International Organization for Standardization: Geneva, Switzerland, 2019.
- Xi, C.; Zhang, Y.; Marrs, C.F.; Ye, W.; Simon, C.; Foxman, B.; Nriagu, J. Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl. Environ. Microbiol. 2009, 75, 5714–5718. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Z.T.; Michel, F.C., Jr.; Wittum, T.; Morrison, M. Development and application of real-time PCR assays for quantification of erm genes conferring resistance to macrolides-lincosamides-streptogramin B in livestock manure and manure management systems. Appl. Environ. Microbiol. 2007, 73, 4407–4416. [Google Scholar] [CrossRef]
- Pei, R.; Kim, S.C.; Carlson, K.H.; Pruden, A. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef]
- Aminov, R.I.; Garrigues-Jeanjean, N.; Mackie, R.I. Molecular ecology of tetracycline resistance: Development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl. Environ. Microbiol. 2001, 67, 22–32. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Belding, C.; Boopathy, R. Presence of antibiotic-resistant bacteria and antibiotic resistance genes in coastal recreational waters of southeast Louisiana, USA. J. Water Supply Res. Technol. AQUA 2018, 67, 800–809. [Google Scholar] [CrossRef]
- Korajkic, A.; McMinn, B.R.; Harwood, V.J. Relationships between microbial indicators and pathogens in recreational water settings. Int. J. Environ. Res. Public Health 2018, 15, 2842. [Google Scholar] [CrossRef]
- Aragonés, L.; López, I.; Palazón, A.; López-Úbeda, R.; García, C. Evaluation of the quality of coastal bathing waters in Spain through fecal bacteria Escherichia coli and Enterococcus. Sci. Total Environ. 2016, 566–567, 288–297. [Google Scholar] [CrossRef]
- Ariza, E.; Jimenez, J.A.; Sarda, R.; Villares, M.; Pinto, J.; Fraguell, R.; Roca, E.; Marti, C.; Valdemoro, H.; Ballester, R. Proposal for an integral quality index for urban and urbanized beaches. Environ. Manag. 2010, 45, 998–1013. [Google Scholar] [CrossRef]
- McLellan, S.L. Genetic diversity of Escherichia coli isolated from urban rivers and beach water. Appl. Environ. Microbiol. 2004, 70, 4658–4665. [Google Scholar] [CrossRef]
- May, C.W.; Horner, R.R.; Karr, J.R.; Mar, B.W.; Welch, E.B. Effects of urbanization on small streams in the Puget Sound ecoregion. Watershed Prot. Tech. 1999, 2, 79–84. [Google Scholar]
- Winter, J.G.; Duthie, H.C. Effects of urbanization on water quality, periphyton and invertebrate communities in a southern Ontario stream. Can. Water Resour. J. 1998, 23, 245–257. [Google Scholar] [CrossRef]
- Abdelzaher, A.M.; Wright, M.E.; Ortega, C.; Solo-Gabriele, H.M.; Miller, G.; Elmir, S.; Newman, S.; Shih, P.; Bonilla, J.A.; Bonilla, T.D.; et al. Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Appl. Environ. Microbiol. 2010, 76, 724–732. [Google Scholar] [CrossRef]
- Haugland, R.A.; Siefring, S.C.; Wymer, L.J.; Brenner, K.P.; Dufour, A.P. Comparison of Enterococcus measurements in freshwater at two recreational beaches by quantitative polymerase chain reaction and membrane filter culture analysis. Water Res. 2005, 39, 559–568. [Google Scholar] [CrossRef]
- Whitman, R.L.; Nevers, M.B.; Korinek, G.C.; Byappanahalli, M.N. Solar and temporal effects on Escherichia coli concentration at a Lake Michigan swimming beach. Appl. Environ. Microbiol. 2004, 70, 4276–4285. [Google Scholar] [CrossRef]
- Alves, M.S.; Pereira, A.; Araújo, S.M.; Castro, B.B.; Correia, A.C.M.; Henriques, I. Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Front. Microbiol. 2014, 5, 426. [Google Scholar] [CrossRef]
- Bennani, M.; Amarouch, H.; Oubrim, N.; Cohen, N. Identification and antimicrobial resistance of fecal Enterococci isolated in coastal Mediterranean environments of Morocco. Eur. J. Sci. Res. 2012, 70, 266–275. [Google Scholar]
- da Costa Andrade, V.; Zampieri Bdel, B.; Ballesteros, E.R.; Pinto, A.B.; de Oliveira, A.J. Densities and antimicrobial resistance of Escherichia coli isolated from marine waters and beach sands. Environ. Monit. Assess. 2015, 187, 342. [Google Scholar] [CrossRef]
- Na, G.; Zhang, W.; Zhou, S.; Gao, H.; Lu, Z.; Wu, X.; Li, R.; Qiu, L.; Cai, Y.; Yao, Z. Sulfonamide antibiotics in the Northern Yellow Sea are related to resistant bacteria: Implications for antibiotic resistance genes. Mar. Poll. Bull. 2014, 84, 70–75. [Google Scholar] [CrossRef]
- Letchumanan, V.; Yin, W.F.; Lee, L.H.; Chan, K.G. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Şanlıtürk, G.; Güran, M. Monitoring of microbiological dynamics in beach sand and seawater samples from recreational and non-recreational beaches over a two-year period. Int. J. Environ. Health Res. 2022, 32, 1973–1985. [Google Scholar] [CrossRef]
- Hernández, F.; Calısto-Ulloa, N.; Gómez-Fuentes, C.; Gómez, M.; Ferrer, J.; González-Rocha, G.; Bello-Toledo, H.; Botero-Coy, A.M.; Boıx, C.; Ibáñez, M.; et al. Occurrence of antibiotics and bacterial resistance in wastewater and sea water from the Antarctic. J. Hazard. Mater. 2019, 363, 447–456. [Google Scholar] [CrossRef]
- Gambino, D.; Savoca, D.; Sucato, A.; Gargano, V.; Gentile, A.; Pantano, L.; Vicari, D.; Alduina, R. Occurrence of antibiotic resistance in the Mediterranean Sea. Antibiotics 2022, 11, 332. [Google Scholar] [CrossRef]
- Fernandes Cardoso de Oliveira, A.J.; Ranzani de França, P.T.; Pinto, A.B. Antimicrobial resistance of heterotrophic marine bacteria isolated from seawater and sands of recreational beaches with different organic pollution levels in southeastern Brazil: Evidences of resistance dissemination. Environ. Monit. Assess. 2010, 169, 375–384. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2021; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Vignaroli, C.; Pasquaroli, S.; Citterio, B.; Di Cesare, A.; Mangiaterra, G.; Fattorini, D.; Biavasco, F. Antibiotic and heavy metal resistance in enterococci from coastal marine sediment. Environ. Pollut. 2018, 237, 406–413. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-derivatives small molecules with antibacterial activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef]
- Li, G.; Walker, M.J.; De Oliveira, D.M.P. Vancomycin resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Vignaroli, C.; Luna, G.M.; Pasquaroli, S.; Di Cesare, A.; Petruzzella, R.; Paroncini, P.; Biavasco, F. Epidemic Escherichia coli ST131 and Enterococcus faecium ST17 in coastal marine sediments from an Italian beach. Environ. Sci. Technol. 2013, 47, 13772–13780. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Luna, G.M.; Vignaroli, C.; Pasquaroli, S.; Tota, S.; Paroncini, P.; Biavasco, F. Aquaculture can promote the presence and spread of antibiotic-resistant Enterococci in marine sediments. PLoS ONE 2013, 8, e62838. [Google Scholar] [CrossRef]
- Sucato, A.; Vecchioni, L.; Savoca, D.; Presentato, A.; Arculeo, M.; Alduina, R. A Comparative Analysis of Aquatic and Polyethylene-Associated Antibiotic-Resistant Microbiota in the Mediterranean Sea. Biology 2021, 10, 200. [Google Scholar] [CrossRef]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is Caretta Caretta a Carrier of Antibiotic Resistance in the Mediterranean Sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef]
- Xu, N.; Qiu, D.; Zhang, Z.; Wang, Y.; Chen, B.; Zhang, Q.; Wang, T.; Hong, W.; Zhou, N.-Y.; Penuelas, J.; et al. A global atlas of marine antibiotic resistance genes and their expression. Water Res. 2023, 244, 120488. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on Surveillance of Antibiotic consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Pepi, M.; Focardi, S. Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the Mediterranean area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.-G.; Zhang, K.; Zhang, Y. Occurrence and distribution of antibiotic resistance genes in the coastal area of the Bohai Bay, China. Mar. Pollut. Bull. 2016, 107, 245–250. [Google Scholar] [CrossRef]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegard, B.; Söderstrom, H.; Joakim Larsson, D.G. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE 2011, 6, 1–7. [Google Scholar] [CrossRef]
- Li, L.G.; Huang, Q.; Yin, X.; Zhang, T. Source tracking of antibiotic resistance genes in the environment—Challenges, progress, and prospects. Water Res. 2020, 185, 116127. [Google Scholar] [CrossRef]
- Divya, S.P.; Hatha, A.A.M. Screening of tropical estuarine water in south-west coast of India reveals emergence of ARGs-harboring hypervirulent Escherichia coli of global significance. Int. J. Hyg Environ. Health 2019, 222, 235–248. [Google Scholar] [CrossRef]
- Henriques, I.S.; Fonseca, F.; Alves, A.; Saavedra, M.J.; Correia, A. Occurrence and diversity of integrons and β-lactamase genes among ampicillin-resistant isolates from estuarine waters. Res. Microbiol. 2006, 157, 938–947. [Google Scholar] [CrossRef]
- Schwartz, T.; Kohnen, W.; Janses, B.; Obst, U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef]
| Target Gene | Sequence | Conditions | Product (bp) | Reference |
|---|---|---|---|---|
| blaTEM | 5′-GCKGCCAACTTACTTCTGACAACG-3′ 5′-CTTTATCCGCCTCCATCCAGTCTA-3′ | 95 °C 3 min (1 cycle); 95 °C 15 s and 60 °C 20 s (40 cycles); 72 °C 1 min | 247 | [41] |
| ermB | 5′-GATACCGTTTACGAAATTGG-3′ 5′-GAATCGAGACTTGAGTGTGC-3′ | 95 °C 3 min (1 cycle); 95 °C 15 s and 58 °C 20 s (40 cycles); 72 °C 1 min | 364 | [42] |
| qnrS | 5′-GACGTGCTAACTTGCGTGAT-3′ 5′-TGGCATTGTTGGAAACTTG-3′ | 95 °C 3 min (1 cycle); 95 °C 15 s and 62 °C 20 s (40 cycles); 72 °C 1 min | 240 | [22] |
| sulI | 5′-CGCACCGGAAACATCGCTGCAC-3′ 5′-TGAAGTTCCGCCGCAAGGCTCG-3′ | 95 °C 3 min (1 cycle); 95 °C 15 s and 65 °C 20 s (40 cycles); 72 °C 1 min | 163 | [43] |
| tetW | 5′-GAGAGCCTGCTATATGCCAGC-3′ 5′-CTTTATCCGCCTCCATCCAGTCTA-3′ | 95 °C 3 min (1 cycle); 95 °C 15 s and 60 °C 20 s (40 cycles); 72 °C 1 min | 168 | [44] |
| Zone | No. Tested Isolates | Number of E. coli Resistant Isolates | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMX | TMP | CIP | TET | MER | AZI | NAL | FOT | CHL | TGC | TAZ | COL | AMP | GEN | MDR a | ||
| NV | 14 | 2 | 3 | 2 | 2 | 0 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 4 | 1 | 2 |
| SV | 23 | 4 | 4 | 2 | 2 | 1 | 3 | 4 | 2 | 0 | 0 | 4 | 1 | 12 | 0 | 2 |
| A | 20 | 3 | 2 | 4 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 |
| Total | 57 | 9 | 9 | 10 | 8 | 1 | 5 | 8 | 2 | 0 | 0 | 5 | 1 | 20 | 1 | 5 |
| % of resistant isolates | 15.8 | 15.8 | 17.5 | 10.4 | 1.8 | 8.8 | 14 | 3.5 | 0 | 0 | 8.8 | 1.8 | 35.1 | 1.8 | 14.7 | |
| Zone | No. Tested Isolates | Number of Enterococcus Resistant Isolates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | CHL | CIP | DAP | ERY | GEN | LIN | QUIN/ DAL | TEI | TET | TGC | VAN | ||
| NV | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SV | 12 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| A | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Total | 30 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 6 | 0 | 0 |
| % of resistant isolates | 0 | 0 | 0 | 0 | 3.3 | 0 | 0 | 0 | 0 | 6.6 | 0 | 0 | |
| Zone | No. Samples | Number of Positive Samples | ||||
|---|---|---|---|---|---|---|
| blaTEM | qnrS | ermB | sulI | tetW | ||
| NV | 21 | 6 | 5 | 0 | 3 | 5 |
| SV | 30 | 15 | 9 | 0 | 5 | 0 |
| A | 26 | 15 | 11 | 1 | 10 | 8 |
| Total (%) | 77 | 36 (46.7) | 25 (32.5) | 1 (1.2) | 18 (23.4) | 13 (16.9) |
| Zone | No. Isolates | Number of Positive Isolates | ||||
|---|---|---|---|---|---|---|
| blaTEM | qnrS | ermB | sulI | tetW | ||
| NV | 14 | 13 | 4 | 0 | 13 | 2 |
| SV | 23 | 21 | 11 | 1 | 21 | 11 |
| A | 20 | 19 | 6 | 2 | 19 | 10 |
| Total (%) | 57 | 53 (92.9) | 21 (36.8) | 3 (5.2) | 53 (92.9) | 23 (45.1) |
| Zone | No. Isolates | Number of Positive Isolates | ||||
|---|---|---|---|---|---|---|
| blaTEM | qnrS | ermB | sulI | tetW | ||
| NV | 9 | 3 | 1 | 1 | 0 | 0 |
| SV | 12 | 1 | 0 | 1 | 0 | 0 |
| A | 9 | 8 | 0 | 0 | 0 | 1 |
| Total (%) | 30 | 12 (40.0) | 1 (3.3) | 2 (6.6) | 0 | 1 (3.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasí, D.; Camaró-Sala, M.L.; González, A.; García-Ferrús, M.; Jiménez-Belenguer, A.I.; Castillo, M.Á. Antibiotic Resistance in Seawater Samples from East Coast of Spain. Appl. Sci. 2024, 14, 1965. https://doi.org/10.3390/app14051965
Dasí D, Camaró-Sala ML, González A, García-Ferrús M, Jiménez-Belenguer AI, Castillo MÁ. Antibiotic Resistance in Seawater Samples from East Coast of Spain. Applied Sciences. 2024; 14(5):1965. https://doi.org/10.3390/app14051965
Chicago/Turabian StyleDasí, Diego, María Luisa Camaró-Sala, Ana González, Miguel García-Ferrús, Ana Isabel Jiménez-Belenguer, and María Ángeles Castillo. 2024. "Antibiotic Resistance in Seawater Samples from East Coast of Spain" Applied Sciences 14, no. 5: 1965. https://doi.org/10.3390/app14051965





