Feasibility of Non-Remanufactured Waste Bottle Glass as Supplementary Cementitious Material
Abstract
:1. Introduction
Research Significance and Objectives
2. Methodology
2.1. Non-Remanufactured Waste Bottle Glass
2.1.1. Origin
2.1.2. Laboratory Waste Glass Milling
2.1.3. Laboratory Waste Glass Incineration
2.1.4. Waste Glass Properties
2.2. Mortar Study
2.2.1. Mortar Specimen Production
2.2.2. Tests on Mortars
Mechanical Strength
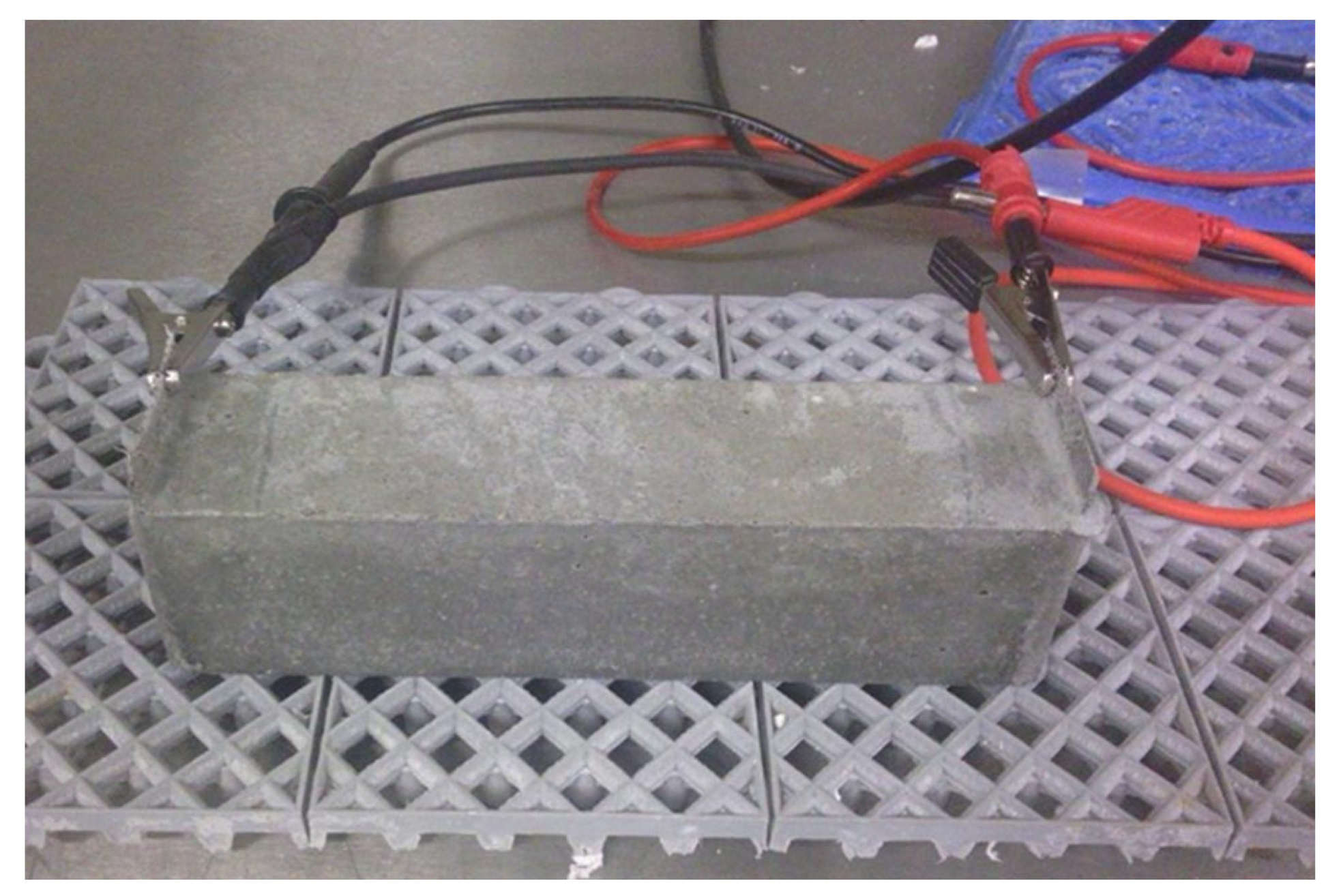
Electrical Resistivity
Water Absorption by Capillarity
Rapid Chloride Migration Test
Accelerated Carbonation Test
Expansion Due to ASR
3. Results and Discussion
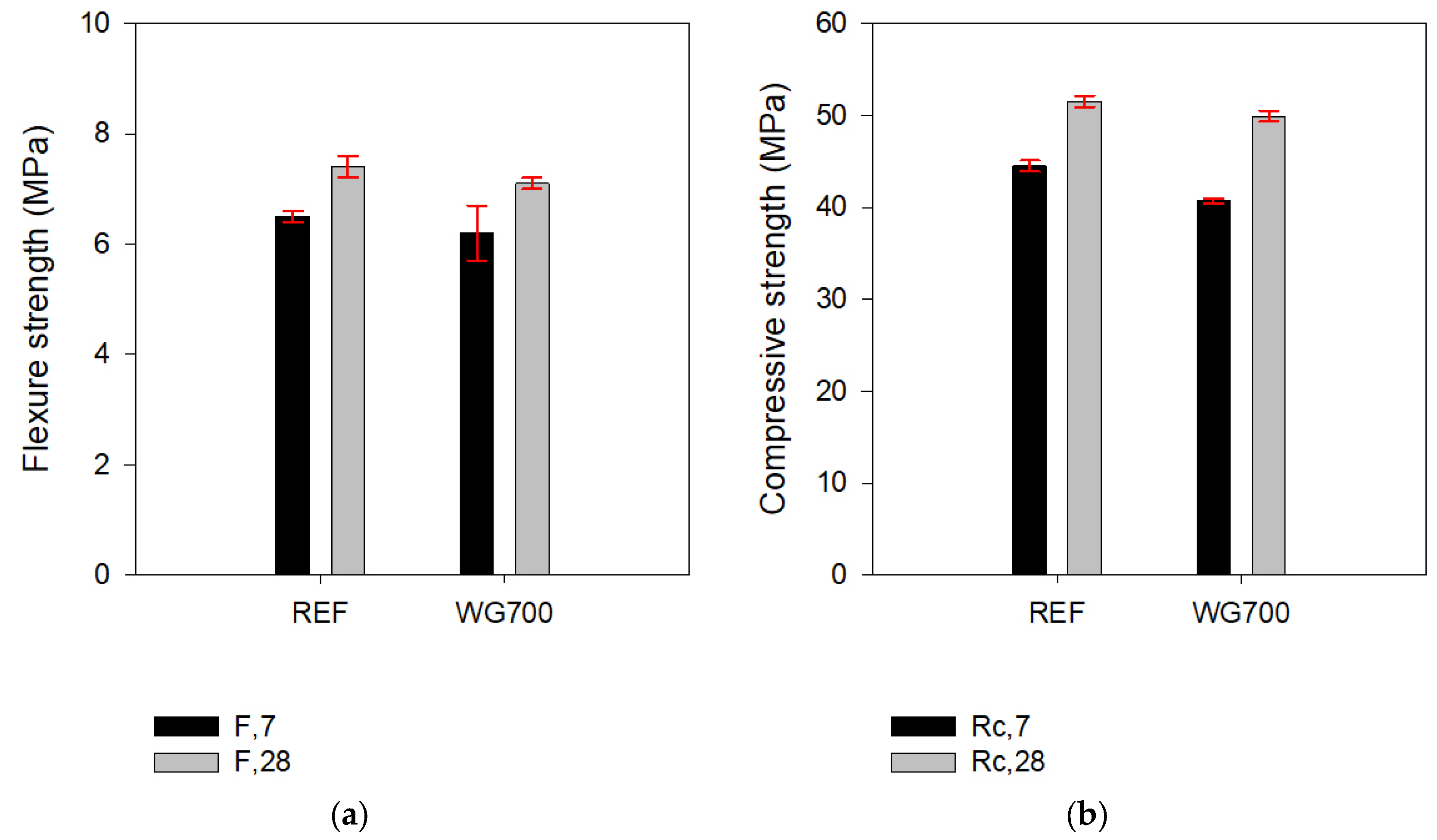
3.1. Mechanical Strength and Strength Activity Index
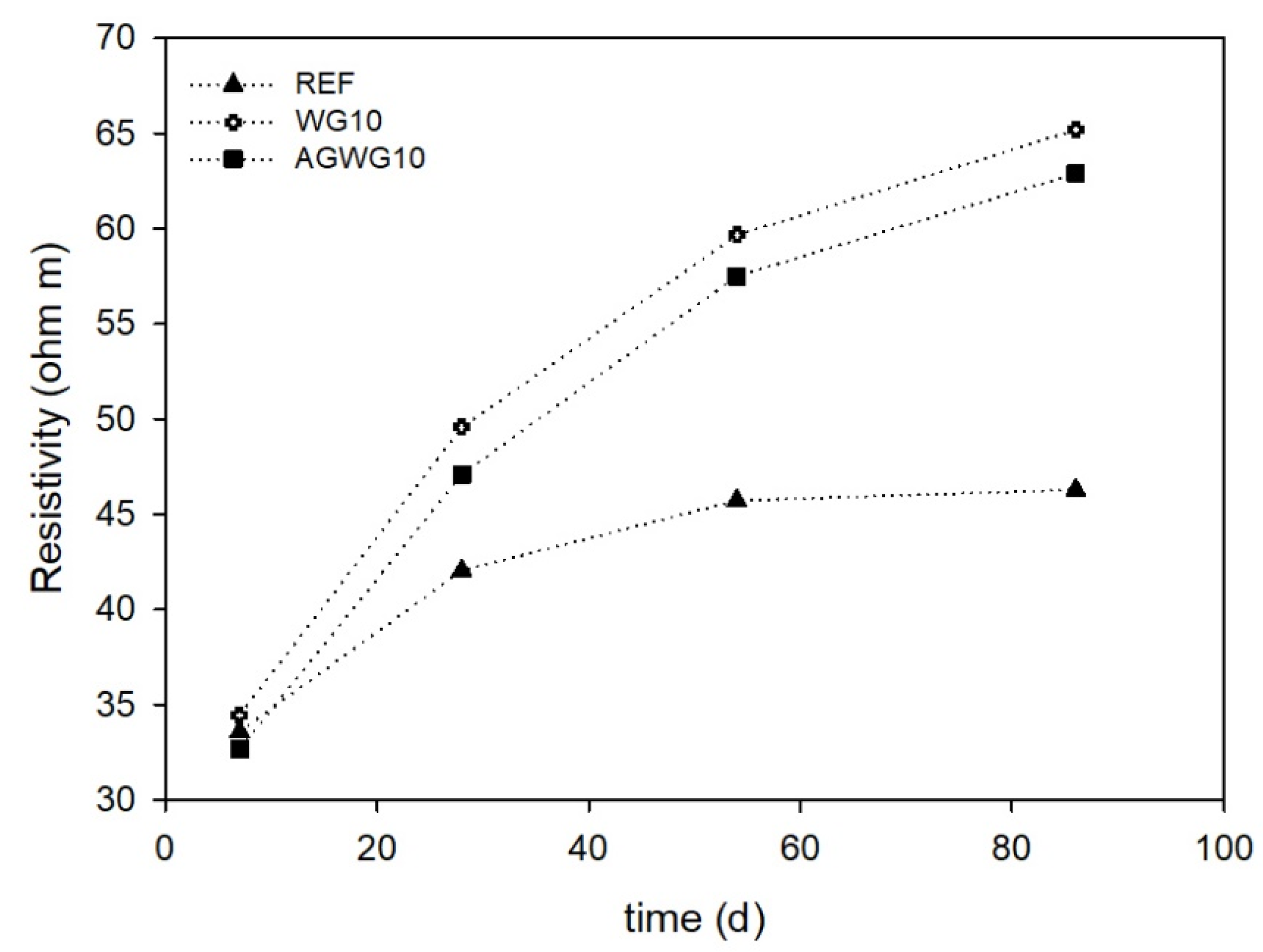
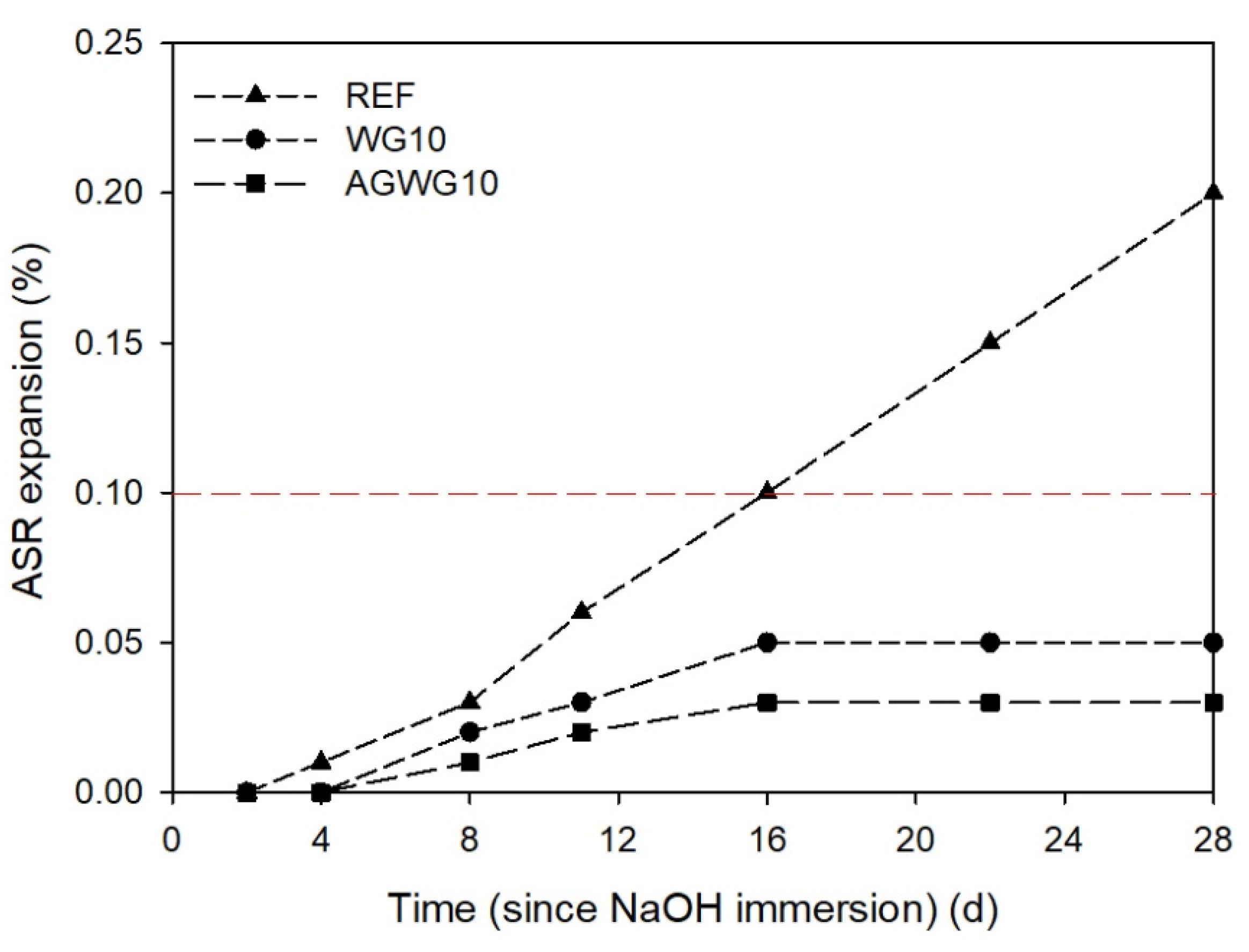
3.2. Durability Indicators
4. Conclusions and Final Remarks
- The main oxide composition revealed that WG consists mainly of SiO2, CaO, and Na2O.
- A substantial decrease in mortar mechanical strength occurred when WG or AGWG were used as a 10% mass cement replacement.
- The mechanical strength of mortar, including 10% of incinerated waste glass powder, (WG700) achieved the REF mortar levels.
- Electrical resistivity was higher on glass-modified mortars, indicating the possibility of glass powder pozzolanic reactivity.
- The resistance against chlorides and capillary water absorption was similar among all mortars under study.
- ASR was reduced by over 30% in mortar specimens with 10% WG or AGWG as a cement replacement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Z.; Masanet, E.; Tiwari, A.; Akolawala, S. Decarbonizing Concrete: Deep Decarbonization Pathways for the Cement and Concrete Cycle in the United States, India, and China; Industrial Sustainability Analysis Laboratory, Northwestern University: Evanston, IL, USA, 2021. [Google Scholar]
- Belaïd, F. How does concrete and cement industry transformation contribute to mitigating climate change challenges? Resour. Conserv. Recycl. Adv. 2022, 15, 200084. [Google Scholar] [CrossRef]
- International Energy Agency. Technology Roadmap—Low-Carbon Transition in the Cement Industry. Available online: www.wbcsdcement.org (accessed on 20 November 2023).
- OCDE. Global Material Resources Outlook to 2060; OECD: Paris, France, 2019. [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon dioxide reduction potential in the global cement industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Schmidt, W.; Alexander, M.; John, V. Education for sustainable use of cement based materials. Cem. Concr. Res. 2018, 114, 103–114. [Google Scholar] [CrossRef]
- Miller, S.A.; Habert, G.; Myers, R.J.; Harvey, J.T. Achieving net zero greenhouse gas emissions in the cement industry via value chain mitigation strategies. One Earth 2021, 4, 1398–1411. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterisation, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Jensen, M.; Kovler, K.; de Belie, N. Concrete with Supplementary Cementitious Materials. In Proceedings of the International RILEM Conference on Materials, Systems and Structures in Civil Engineering 2016-Segment on Concrete with Supplementary Cementitious Materials, Lyngby, Denmark, 15–19 August 2016. [Google Scholar]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Malhotra, V.M.; Mehta, P.K. Pozzolanic and Cementitious Materials. In Pozzolanic and Cementitious Materials; CRC Press LLC: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- View of Assessing, Understanding and Unlocking Supplementary Cementitious Materials. Available online: https://letters.rilem.net/index.php/rilem/article/view/12/15 (accessed on 4 October 2022).
- Paul, S.C.; Šavija, B.; Babafemi, A.J. A comprehensive review on mechanical and durability properties of cement-based materials containing waste recycled glass. J. Clean. Prod. 2018, 198, 891–906. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Mo, K.H.; Shi, C. A critical review of waste glass powder—Multiple roles of utilisation in cement-based materials and construction products. J. Environ. Manag. 2019, 242, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, J.V.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Value-added utilisation of waste glass in concrete. Cem. Concr. Res. 2004, 34, 81–89. [Google Scholar] [CrossRef]
- Butler, J.H.; Hooper, P.D. Glass Waste. In Waste: A Handbook for Management; Academic Press: Cambridge, MA, USA, 2019; pp. 307–322. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Saha, A.K.; Sarker, P.K. Reuse of waste glass as a supplementary binder and aggregate for sustainable cement-based construction materials: A review. J. Build. Eng. 2020, 28, 101052. [Google Scholar] [CrossRef]
- Matos, M.; Sousa-Coutinho, J. Durability of mortar using waste glass powder as cement replacement. Constr. Build. Mater. 2012, 36, 205–215. [Google Scholar] [CrossRef]
- Matos, M.; Coutinho, J.S. Waste glass powder in cement: Macro and micro scale study. Adv. Cem. Res. 2016, 28, 423–432. [Google Scholar] [CrossRef]
- Paul, D.; Bindhu, K.R.; Matos, A.M.; Delgado, J. Eco-friendly concrete with waste glass powder: A sustainable and circular solution. Constr. Build. Mater. 2022, 355, 129217. [Google Scholar] [CrossRef]
- Matos, M.; Ramos, T.; Nunes, S.; Sousa-Coutinho, J. Durability Enhancement of Scc with Waste Glass Powder. Mater. Res. 2016, 19, 67–74. [Google Scholar] [CrossRef]
- Nunes, S.; Matos, A.M.; Duarte, T.; Figueiras, H.; Sousa-Coutinho, J. Mixture design of self-compacting glass mortar. Cem. Concr. Compos. 2013, 43, 1–11. [Google Scholar] [CrossRef]
- Parghi, A.; Alam, M.S. Physical and mechanical properties of cementitious composites containing recycled glass powder (RGP) and styrene butadiene rubber (SBR). Constr. Build. Mater. 2016, 104, 34–43. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Nassar, R.U.D.; Soroushian, P. Strength and durability of recycled aggregate concrete containing milled glass as partial replacement for cement. Constr. Build. Mater. 2012, 29, 368–377. [Google Scholar] [CrossRef]
- Kamali, M.; Ghahremaninezhad, A. An investigation into the hydration and microstructure of cement pastes modified with glass powders. Constr. Build. Mater. 2016, 112, 915–924. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Pozzolanic properties of fine and coarse color-mixed glass cullet. Cem. Concr. Compos. 2011, 33, 19–29. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Liu, S.; Xie, G.; Wang, S. Effect of glass powder on microstructure of cement pastes. Adv. Cem. Res. 2015, 27, 259–267. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Properties of high volume glass powder concrete. Cem. Concr. Compos. 2017, 75, 22–29. [Google Scholar] [CrossRef]
- Aliabdo, A.; Elmoaty, A.E.M.A.; Aboshama, A.Y. Utilisation of waste glass powder in the production of cement and concrete. Constr. Build. Mater. 2016, 124, 866–877. [Google Scholar] [CrossRef]
- Patel, R.; Tiwari, R.; Shrivastava, R.; Yadav, R.K. Effective utilisation of waste glass powder as the substitution of cement in making paste and mortar. Constr. Build. Mater. 2019, 199, 406–415. [Google Scholar] [CrossRef]
- Lu, J.X.; Shen, P.; Zhang, Y.; Zheng, H.; Sun, Y.; Poon, C.S. Early-age and microstructural properties of glass powder blended cement paste: Improvement by seawater. Cem. Concr. Compos. 2021, 122, 104165. [Google Scholar] [CrossRef]
- Khan, Q.S.; Sheikh, M.N.; McCarthy, T.J.; Robati, M.; Allen, M. Experimental investigation on foam concrete without and with recycled glass powder: A sustainable solution for future construction. Constr. Build. Mater. 2019, 201, 369–379. [Google Scholar] [CrossRef]
- Mohammed, S.M.H.T.K. Mechanical Properties, Impact Resistance and Bond Strength of Green Concrete Incorporating Waste Glass Powder and Waste Fine Plastic Aggregate; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Bignozzi, M.C.; Saccani, A.; Barbieri, L.; Lancellotti, I. Glass waste as supplementary cementing materials: The effects of glass chemical composition. Cem. Concr. Compos. 2015, 55, 45–52. [Google Scholar] [CrossRef]
- Federico, L.M.; Chidiac, S.E. Waste glass as a supplementary cementitious material in concrete—Critical review of treatment methods. Cem. Concr. Compos. 2009, 31, 606–610. [Google Scholar] [CrossRef]
- Schwarz, N.; Cam, H.; Neithalath, N. Influence of a fine glass powder on the durability characteristics of concrete and its comparison to fly ash. Cem. Concr. Compos. 2008, 30, 486–496. [Google Scholar] [CrossRef]
- Omran, A.; Tagnit-Hamou, A. Performance of glass-powder concrete in field applications. Constr. Build. Mater. 2016, 109, 84–95. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Pugazhmani, G.; Sripragadeesh, R.; Muthu, D.; Venkatasubramanian, C. Experimental study on the mechanical and durability properties of concrete with waste glass powder and ground granulated blast furnace slag as supplementary cementitious materials. Constr. Build. Mater. 2017, 156, 739–749. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Sustainable concrete for circular economy: A review on use of waste glass. Glass Struct. Eng. 2022, 7, 3–22. [Google Scholar] [CrossRef]
- Sengul, O. Use of electrical resistivity as an indicator for durability. Constr. Build. Mater. 2014, 73, 434–441. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R. Electrical Resistivity of Concrete for Durability Evaluation: A Review. Adv. Mater. Sci. Eng. 2017, 2017, 1–30. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Using lithium nitrate and pozzolanic glass powder in concrete as ASR suppressors. Cem. Concr. Compos. 2008, 30, 497–505. [Google Scholar] [CrossRef]


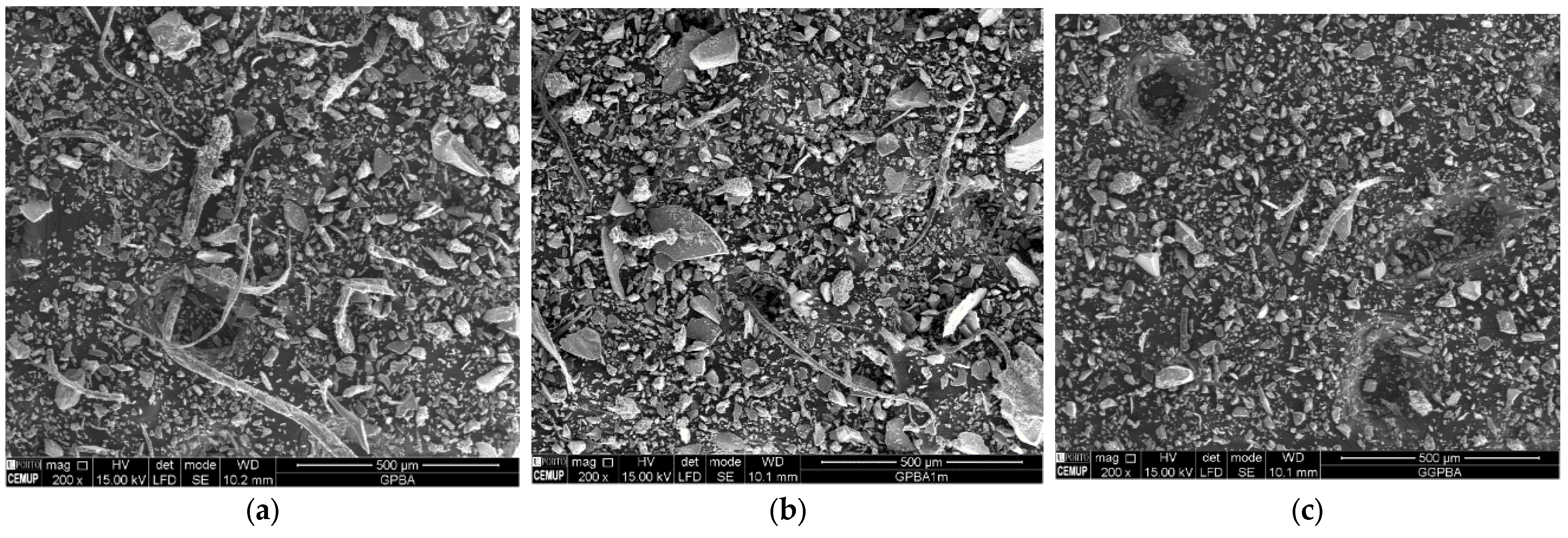




| Materials | Cement | WG | GWG | AGWG |
|---|---|---|---|---|
| Density (kg/m3) | 3110 | 2280 | 2280 | 2280 |
| Specific surface (cm2/g) | 3742 | 3462 | 3131 | 4779 |
| d10 (µm) | 1.42 | 4.45 | 4.57 | 2,92 |
| d50 (µm) | 13.50 | 25.96 | 32.96 | 18.34 |
| d90 (µm) | 40.52 | 127.00 | 197.00 | 82.83 |
| Materials | Chemical Composition (% by Mass) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Fe2O3 | Na2O | Al2O3 | MgO | K2O | TiO2 | P2O5 | Cl | SO3 | Insoluble Residue | LOI | |
| Cement | 20.33 | 63.31 | 3.02 | 0.20 | 4.83 | 1.91 | 0.73 | 0.05 | 2.85 | 1.03 | 2.28 | ||
| WG | 60.91 | 10.78 | 1.15 | 9.63 | 2.54 | 1.07 | 0.92 | 0.15 | 0.16 | 0.16 | 0.49 | 12.27 | |
| WG | NP 4220 | ASTM C 618 | |||
|---|---|---|---|---|---|
| Pozzolans in General | Fly Ash and Pozzolan | ||||
| N | F | C | |||
| SiO2 + Al2O3 + Fe2O3 (%) | 64.60 | - | >70 | >70 | >50 |
| CaO (%) | 10.78 | <10.0 | - | - | - |
| Cl (%) | 0.16 | <0.1 | - | - | - |
| SO3 (%) | 0.49 | <3.0 | <4.0 | <5.0 | <5.0 |
| (Na2O)eq (%) | <5.0 | - | - | - | |
| Loss on ignition (LOI) (%) | 12.27 | ≤5.0 | <10 | <6.0 | <6.0 |
| Mortar ID | Cement (g) | WG (g) | AGWG (g) | WG700 | Water (g) | Sand (g) |
|---|---|---|---|---|---|---|
| REF | 450 | - | - | - | 225 | 1350 |
| WG10 | 405 | 45 | - | - | 225 | 1350 |
| AGWG10 | 405 | - | 45 | - | 225 | 1350 |
| WG700 | 405 | - | - | 45 | 225 | 1350 |
| Test Standard | Curing Regime | Testing Age | Number of Samples for Each Age and Each Mortar Type | Sample Geometry and Size | |
|---|---|---|---|---|---|
| Mechanical strength | NP EN 196-1 | Water curing at 20 °C | 7, 28, 56, and 84 days | 3 | Prismatic 40 × 40 × 160 mm3 |
| Electrical resistivity | Two-electrode method | 7, 28, 56, and 84 days | 3 | Prismatic 40 × 40 × 160 mm3 | |
| Water absorption by capillarity | RILEM TC 116-PCD | 42 days (28 days wet curing + 14 days at 40 °C, until constant mass achieved) | 3 | Prismatic 40 × 40 × 45 mm3 | |
| Resistance to chloride migration | NT Build 492 | 28 days | 3 | Cylindrical h = 50 mm, Ø = 100 mm | |
| Carbonation resistance | RILEM CPC-18 | 63 days (14 wet curing + 14 days at 20 °C and HR 60% + 35 days CO2 exposure) | 3 | Prismatic 40 × 40 × 160 mm3 | |
| Risk of alkali–silica reaction | ASTM C 1567 adapted | 1-day water curing at 80 °C (according to ASTM C 1567) | Up to 28 days | 3 | Prismatic 25 × 25 × 250 mm3 |
| Age (Days) | WG10 | AGWG10 |
|---|---|---|
| 7 | 42.2% | 35.2% |
| 28 | 43.1% | 35.2% |
| 54 | 41.7% | 37.5% |
| 86 | 41.9% | 38.5% |
| Mortar ID | Sorptivity (mg/(mm2 × min0.5)) |
|---|---|
| REF | 0.0490 ± 0.003 |
| WG10 | 0.0370 ± 0.003 |
| AGWG10 | 0.0440 ± 0.008 |
| Mortar ID | xd (mm) | U (V) | Dnssm (10−12 m2/s) |
|---|---|---|---|
| REF | 22.07 | 25.27 | 14.26 |
| WG10 | 25.23 | 25.36 | 15.98 |
| AGWG10 | 20.85 | 25.00 | 13.50 |
| Mortar ID | Carbonation Depth (mm) |
|---|---|
| REF | 3.58 ± 1.81 |
| WG10 | 9.30 ± 1.65 |
| AGWG10 | 11.30 ± 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, A.M.; Sousa-Coutinho, J. Feasibility of Non-Remanufactured Waste Bottle Glass as Supplementary Cementitious Material. Appl. Sci. 2024, 14, 2004. https://doi.org/10.3390/app14052004
Matos AM, Sousa-Coutinho J. Feasibility of Non-Remanufactured Waste Bottle Glass as Supplementary Cementitious Material. Applied Sciences. 2024; 14(5):2004. https://doi.org/10.3390/app14052004
Chicago/Turabian StyleMatos, Ana Mafalda, and Joana Sousa-Coutinho. 2024. "Feasibility of Non-Remanufactured Waste Bottle Glass as Supplementary Cementitious Material" Applied Sciences 14, no. 5: 2004. https://doi.org/10.3390/app14052004




