Positional Isomeric Effects on the Physicochemical Properties of Polymeric Matrix and Polymer@TiO2 Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Chemical Synthesis of Hybrid Materials
2.3. Physicochemical Characterization
2.4. Electrochemical Analyses
2.5. Electrical Conductivity Characterization
3. Results and Discussion
3.1. FTIR Analysis
3.2. XRD Analysis
3.3. Electrical Conductivity (EC)
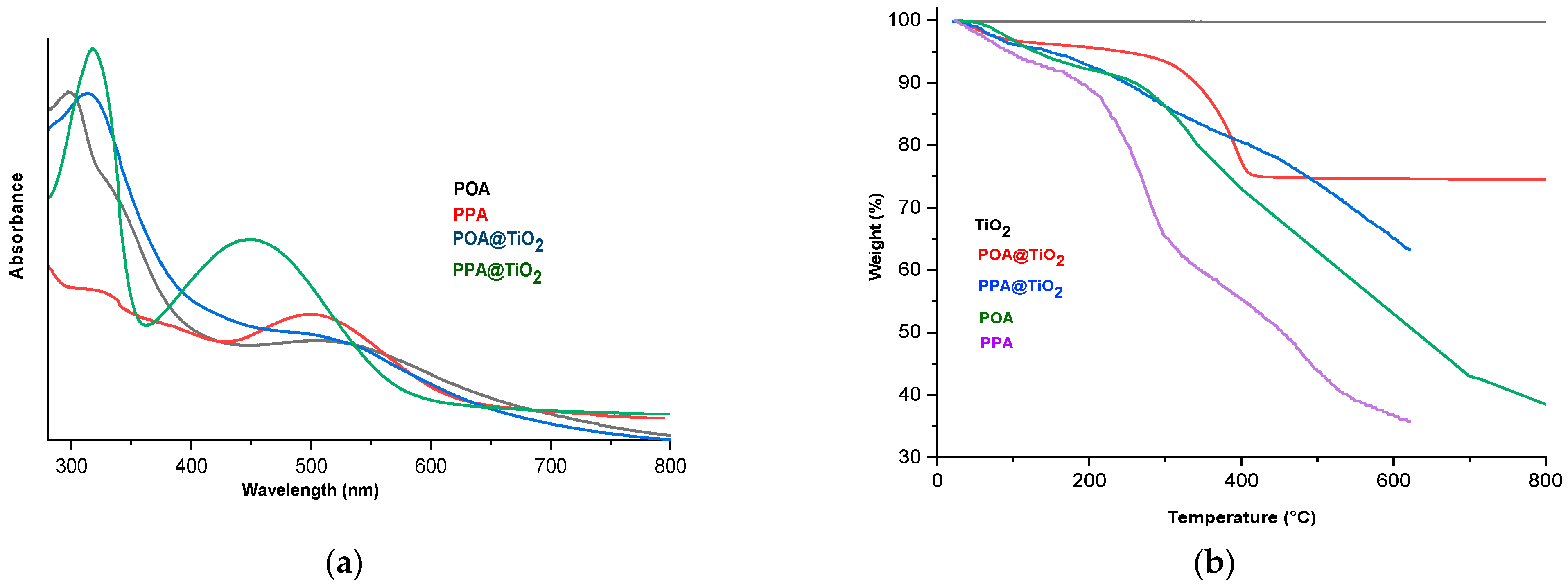
3.4. UV–Visible Analysis
3.5. TGA Analysis
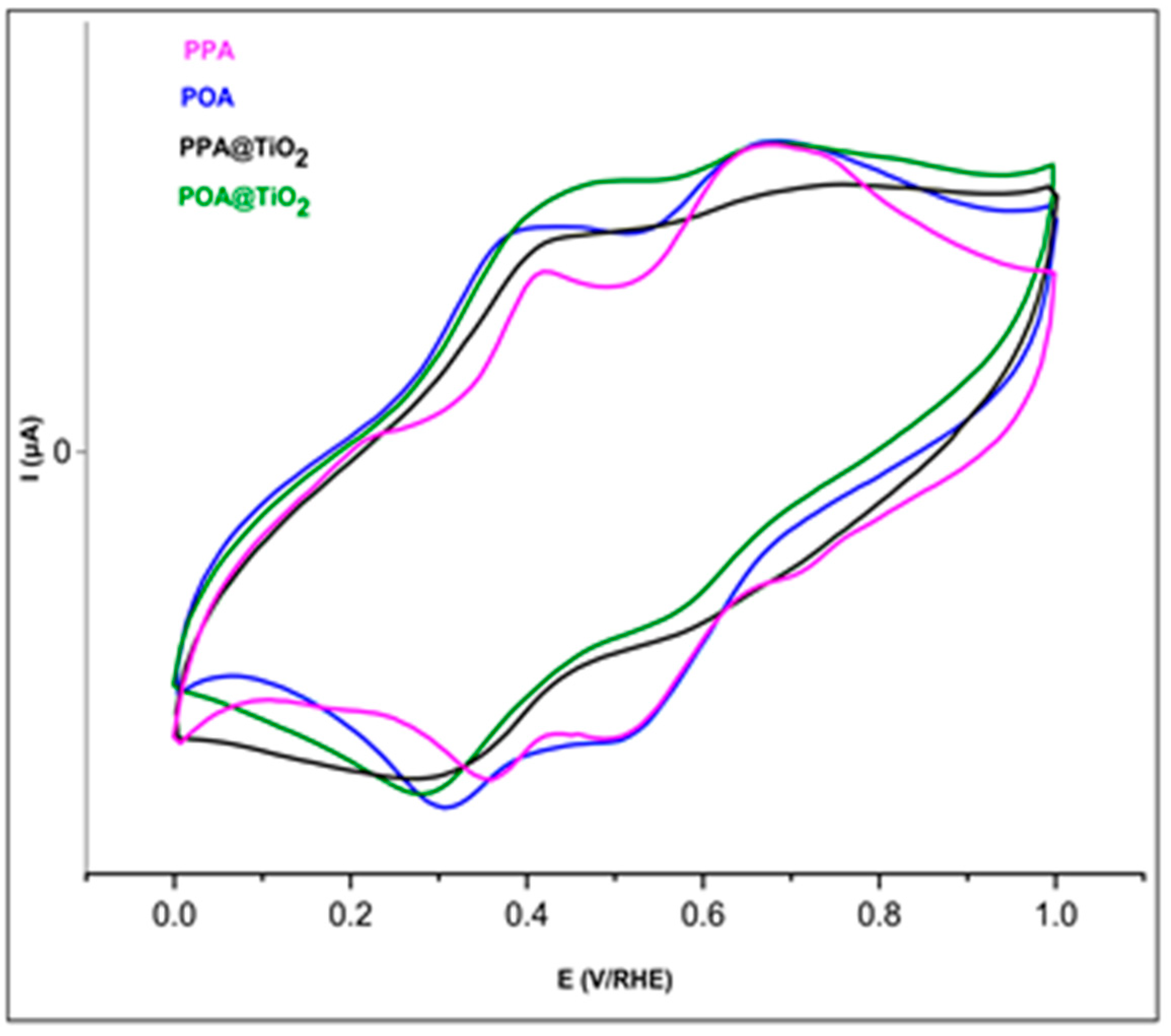
3.6. Electrochemical Study
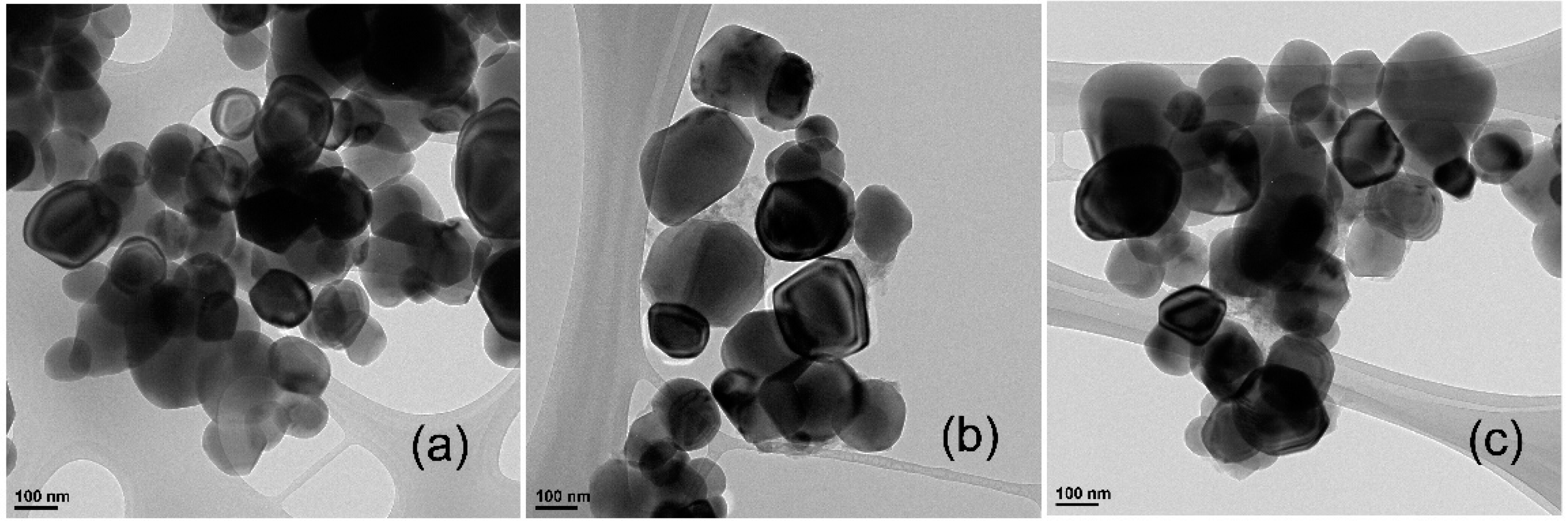
3.7. TEM Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathidevi, K.; Velmani, N.; Tamilselvi, D. Electrical conductivity study of poly(p-anisidine) doped and undoped ZnO nanocomposite. Mediterr. J. Chem. 2019, 9, 403–410. [Google Scholar] [CrossRef]
- MacDiarmid, A.; Yang, L.; Huang, W.; Humphrey, B. Polyaniline: Electrochemistry and application to rechargeable batteries. Synth. Met. 1987, 18, 393–398. [Google Scholar] [CrossRef]
- Martins, J.; Reis, T.; Bazzaoui, M.; Bazzaoui, E.; Martins, L. Polypyrrole coatings as a treatment for zinc-coated steel surfaces against corrosion. Corros. Sci. 2004, 46, 2361–2381. [Google Scholar] [CrossRef]
- Sukeerthi, S.; Contractor, A. Applications of conducting polymers as sensors. Indian J. Chem.-Sect. A 1994, 33, 565–571. [Google Scholar]
- Paul, E.W.; Ricco, A.J.; Wrighton, M.S. Resistance of polyaniline films as a function of electrochemical potential and the fabrication of polyaniline-based microelectronic devices. J. Phys. Chem. 1985, 89, 1441–1447. [Google Scholar] [CrossRef]
- Sharma, R.; Dave, S. Electrochemical Studies of o- and p-Anisidine. Int. J. Appl. Sci. Biotechnol. 2015, 3, 267–271. [Google Scholar] [CrossRef]
- Chaudhari, S.; Gaikwad, A.; Patil, P. Poly(o-anisidine) coatings on brass: Synthesis, characterization and corrosion protection. Curr. Appl. Phys. 2009, 9, 206–218. [Google Scholar] [CrossRef]
- Coromelci, C.G.; Turcu, E.; Doroftei, F.; Palamaru, M.N.; Ignat, M. Conjugated Polymer Modifying TiO2 Performance for Visible-Light Photodegradation of Organics. Polymers 2023, 15, 2805. [Google Scholar] [CrossRef]
- Katančić, Z.; Chen, W.-T.; Waterhouse, G.I.; Kušić, H.; Božić, A.L.; Hrnjak-Murgić, Z.; Travas-Sejdic, J. Solar-active photocatalysts based on TiO2 and conductive polymer PEDOT for the removal of bisphenol A. J. Photochem. Photobiol. A Chem. 2020, 396, 112546. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A. Effect of band gap engineering in anionic-doped TiO2 photocatalyst. Appl. Surf. Sci. 2017, 391, 326–336. [Google Scholar] [CrossRef]
- Ignat, M.; Rotaru, R.; Samoila, P.; Sacarescu, L.; Timpu, D.; Harabagiu, V. Relationship between the component synthesis order of zinc ferrite–titania nanocomposites and their performances as visible light-driven photocatalysts for relevant organic pollutant degradation. Comptes Rendus Chim. 2018, 21, 263–269. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef]
- Dimitrijevic, N.M.; Tepavcevic, S.; Liu, Y.; Rajh, T.; Silver, S.C.; Tiede, D.M. Nanostructured TiO2/Polypyrrole for Visible Light Photocatalysis. J. Phys. Chem. C 2013, 117, 15540–15544. [Google Scholar] [CrossRef]
- Kailasa, S.; Reddy, M.S.B.; Rani, B.G.; Rao, K.V.; Deshmukh, K.; Sadasivuni, K.K. Highly sensitive electrochemical volatile organic compound (Acetone) sensing based on TiO2 Nanocubes @Polyaniline nanostructure. Inorg. Chem. Commun. 2023, 157. [Google Scholar] [CrossRef]
- Turkten, N.; Karatas, Y.; Bekbolet, M. Preparation of PANI Modified ZnO Composites via Different Methods: Structural, Morphological and Photocatalytic Properties. Water 2021, 13, 1025. [Google Scholar] [CrossRef]
- Dakshayini, B.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Zenasni, M.; Quintero-Jaime, A.; Benyoucef, A.; Benghalem, A. Synthesis and characterization of polymer/V2O5 composites based on poly(2-aminodiphenylamine). Polym. Compos. 2021, 42, 1064–1074. [Google Scholar] [CrossRef]
- Babel, V.; Hiran, B.L. A review on polyaniline composites: Synthesis, characterization, and applications. Polym. Compos. 2021, 42, 3142–3157. [Google Scholar] [CrossRef]
- Zak, A.K.; Abrishami, M.E.; Majid, W.A.; Yousefi, R.; Hosseini, S. Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Benykhlef, S.; Bekhoukh, A.; Berenguer, R.; Benyoucef, A.; Morallon, E. PANI-derived polymer/Al2O3 nanocomposites: Synthesis, characterization, and electrochemical studies. Colloid Polym. Sci. 2016, 294, 1877–1885. [Google Scholar] [CrossRef]
- Gong, C.-L.; Li, Y.-F.; Yang, H.-X.; Wang, X.-L.; Zhang, S.-J.; Yang, S.-Y. Characterization and thermal stability of PMR polyimides using 7-oxa-bicyclo[2,2,1]hept-5-ene-2, 3-dicarboxylic anhydride as end caps. Chin. J. Polym. Sci. 2011, 29, 741–749. [Google Scholar] [CrossRef]
- Khamngoen, K.; Paradee, N.; Sirivat, A. Chemical oxidation polymerization and characterization of poly ortho-anisidine nanoparticles. J. Polym. Res. 2016, 23, 172. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA-modified PANI/SWNTs nanocomposite for differential pulse voltammetry based determination of Cu(II) ions. Sens. Actuators B Chem. 2018, 260, 331–338. [Google Scholar] [CrossRef]
- Dhanalakshmi, J.P.; Raj, M.A.; Vijayakumar, C.T. Thermal degradation kinetics of structurally diverse poly(bispropargyl ethers-bismaleimide) blends. Chin. J. Polym. Sci. 2016, 34, 253–267. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and structural analysis of polyaniline and poly(o-anisidine) layers generated in a DC glow discharge plasma by using an oblique angle electrode deposition configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef]
- Du, X.; Xu, Y.; Xiong, L.; Bai, Y.; Zhu, J.; Mao, S. Polyaniline with high crystallinity degree: Synthesis, structure, and electrochemical properties. J Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Rajakani, P.; Vedhi, C. Electrocatalytic properties of polyaniline–TiO2 nanocomposites. Int. J. Ind. Chem. 2015, 6, 247–259. [Google Scholar] [CrossRef]
- Han, J.-C.; Wang, S.-F.; Deng, R.; Wu, Q.-Y. Polydopamine/Imogolite Nanotubes (PDA/INTs) Interlayer Modulated Thin Film Composite Forward Osmosis Membrane For Minimizing Internal Concentration Polarization. Chin. J. Polym. Sci. 2022, 40, 1233–1241. [Google Scholar] [CrossRef]
- Norouzian, R.-S.; Lakouraj, M.M.; Zare, E.N. Novel conductive PANI/hydrophilic thiacalix[4]arene nanocomposites: Synthesis, characterization and investigation of properties. Chin. J. Polym. Sci. 2014, 32, 218–229. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X. Formation of Polyaniline Nanofibers: A Morphological Study. J. Phys. Chem. B 2008, 112, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Olivares, F.R.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Synthesis and characterization of polyaniline-based polymer nanocomposites as anti-corrosion coatings. Coatings 2021, 11, 653. [Google Scholar] [CrossRef]
- Sozeri, H.; Kurtan, U.; Topkaya, R.; Baykal, A.; Toprak, M. Polyaniline (PANI)–Co0.5Mn0.5Fe2O4 nanocomposite: Synthesis, characterization and magnetic properties evaluation. Ceram. Int. 2013, 39, 5137–5143. [Google Scholar] [CrossRef]
- Salih, R.A. Synthesis, identification and study of electrical conductivity of the doped poly aniline. Arab. J. Chem. 2010, 3, 155–158. [Google Scholar] [CrossRef]
- Xia, Y.; Wiesinger, J.M.; MacDiarmid, A.G.; Epstein, A.J. Camphorsulfonic Acid Fully Doped Polyaniline Emeraldine Salt: Conformations in Different Solvents Studied by an Ultraviolet/Visible/Near-Infrared Spectroscopic Method. Chem. Mater. 1995, 7, 443–445. [Google Scholar] [CrossRef]
- Neetika, M.; Rajni, J.; Singh, P.K.; Bhattacharya, B.; Singh, V.; Tomar, S. Synthesis and properties of polyaniline, poly(o-anisidine), and poly[aniline-co-(o-anisidine)] using potassium iodate oxidizing agent. High Perform. Polym. 2016, 29, 266–271. [Google Scholar] [CrossRef]
- Rannou, P.; Gawlicka, A.; Berner, D.; Pron, A.; Nechtschein, M.; Djurado, D. Spectroscopic, Structural and Transport Properties of Conductive Polyaniline Processed from Fluorinated Alcohols. Macromolecules 1998, 31, 3007–3015. [Google Scholar] [CrossRef]
- Alam, M.; Alandis, N.M.; Ansari, A.A.; Shaik, M.R. Optical and Electrical Studies of Polyaniline/ZnO Nanocomposite. J. Nanomater. 2013, 2013, 157810. [Google Scholar] [CrossRef]
- Raotole, M.L.; Vaidya, A.M.; Raotole, P.M. Systematic Study of Synthesis of Poly(O-Anisidine-Co-O-Toluidine) Coatings and Its Performance Against Corrosion of Copper. IJARIIT 2018, 104, 252–260. [Google Scholar]
- Basnayaka, P.A.; Ram, M.K.; Stefanakos, E.K.; Kumar, A. Supercapacitors based on graphene–polyaniline derivative nanocomposite electrode materials. Electrochim. Acta 2013, 92, 376–382. [Google Scholar] [CrossRef]
- Begum, B.; Bilal, S.; Shah, A.U.H.A.; Röse, P. Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage. Polymers 2021, 13, 2883. [Google Scholar] [CrossRef]
- Radja, I.; Djelad, H.; Morallon, E.; Benyoucef, A. Characterization and electrochemical properties of conducting nanocomposites synthesized from p-anisidine and aniline with titanium carbide by chemical oxidative method. Synth. Met. 2015, 202, 25–32. [Google Scholar] [CrossRef]
- Lu, Q.; Lee, J.-H.; Lee, J.H.; Choi, H.J. Magnetite/Poly(ortho-anisidine) Composite Particles and Their Electrorheological Response. Materials 2021, 14, 2900. [Google Scholar] [CrossRef] [PubMed]
- Ghann, W.; Kang, H.; Sheikh, T.; Yadav, S.; Chavez-Gil, T.; Nesbitt, F.; Uddin, J. Fabrication, Optimization and Characterization of Natural Dye Sensitized Solar Cell. Sci. Rep. 2017, 7, 41470. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, H.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Effects of Incorporating Titanium Dioxide with Titanium Carbide on Hybrid Materials Reinforced with Polyaniline: Synthesis, Characterization, Electrochemical and Supercapacitive Properties. Fibers 2022, 10, 46. [Google Scholar] [CrossRef]
- Benz, D.; Felter, K.M.; Köser, J.; Thöming, J.; Mul, G.; Grozema, F.C.; Hintzen, H.T.; Kreutzer, M.T.; Ommen, J.R.V. Assessing the Role of Pt Clusters on TiO2 (P25) on the Photocatalytic Degradation of Acid Blue 9 and Rhodamine B. J. Phys. Chem. C 2020, 124, 8269–8278. [Google Scholar] [CrossRef]
- Bhanvase, B.; Kamath, S.; Patil, U.; Patil, H.; Pandit, A.; Sonawane, S. Intensification of heat transfer using PANI nanoparticles and PANI-CuO nanocomposite based nanofluids. Chem. Eng. Process.-Process. Intensif. 2016, 104, 172–180. [Google Scholar] [CrossRef]

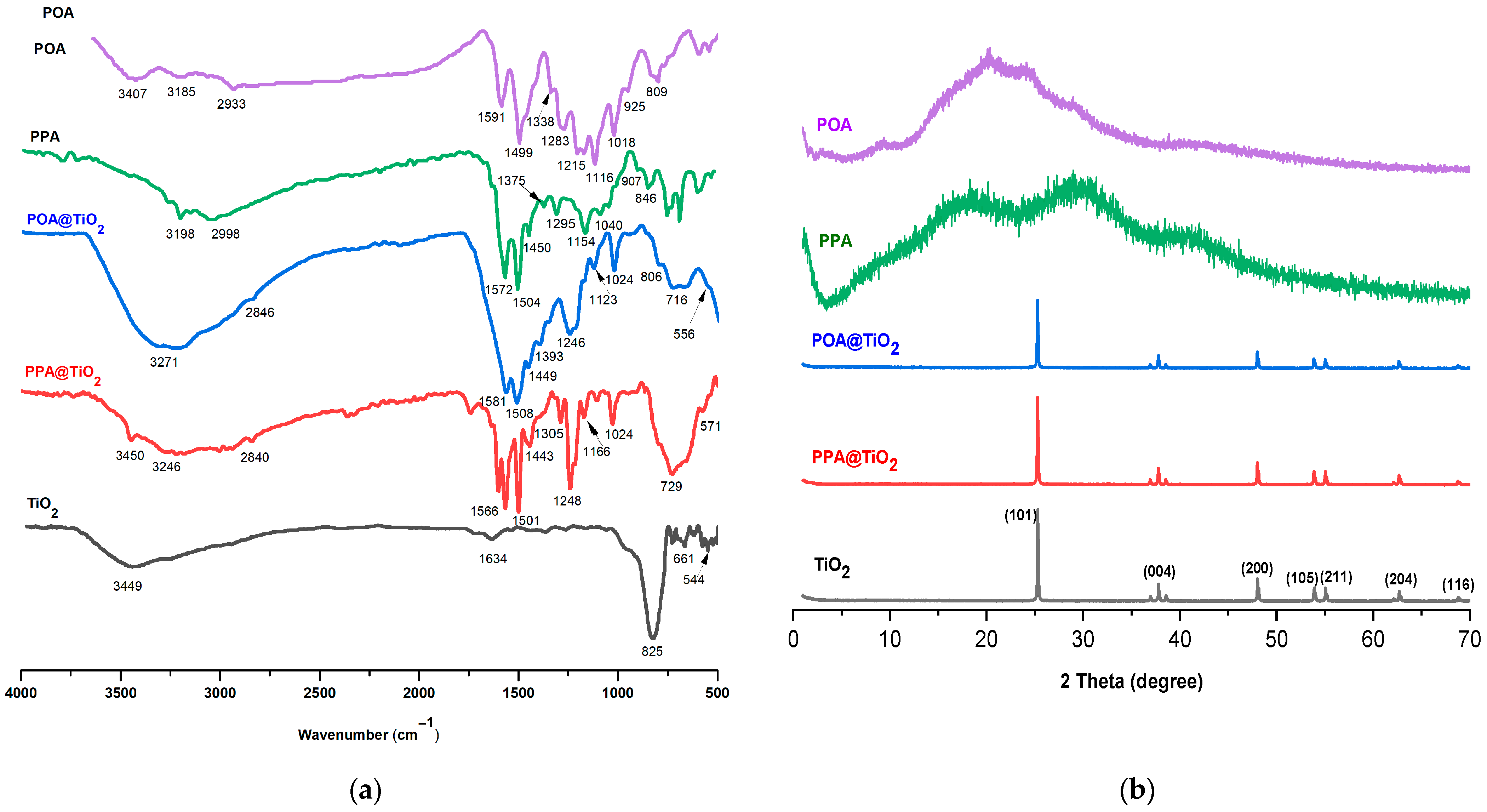
| Peak Assignment | POA | PPA | POA@TiO2 | PPA@TiO2 | TiO2 |
|---|---|---|---|---|---|
| –NH Stretching vibrations | 3185 | 3198 | 3271 | 3246 | // |
| –CH Stretching vibrations | 2933 | 2998 | 2846 | 2840 | // |
| Quinonoid | 1591 | 1572 | 1581 | 1566 | // |
| Benzenoid | 1499 | 1504 | 1508 | 1501 | // |
| –C–N | 1338 | 1375 | 1393 | 1305 | // |
| B–N+H–B & Q=N+H–B | 1018 | 1040 | 1024 | 1024 | // |
| C–O aromatic | 1116 | 1154 | 1123 | 1166 | // |
| CH3 group | 1418 | 1450 | 1449 | 1443 | // |
| C–O–C | 1283 | 1295 | 1246 | 1248 | // |
| TiO | // | // | 716–556 | 729–571 | 825–544 |
| Samples | PPA@TiO2 | POA@TiO2 | PPA | POA |
|---|---|---|---|---|
| EC (S.cm−1) | 0.08 | 0.09 | 0.22 | 0.34 |
| Materials | Redox Peak (V) | Absorption Band (nm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Epa1 | Epc1 | Epa2 | Epc2 | |||||
| PPA | 0.40 | 0.36 | 0.04 | 0.74 | 0.71 | 0.03 | 328 | 503 |
| POA | 0.41 | 0.30 | 0.11 | 0.67 | 0.51 | 0.16 | 298 | 530 |
| POA@TiO2 | 0.45 | 0.28 | 0.17 | 0.67 | 0.56 | 0.11 | 314 | 512 |
| PPA@TiO2 | 0.43 | 0.33 | 0.10 | / | / | / | 318 | 452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shehri, B.M.; Bekhoukh, A.; Benkhatou, S.; Moulefera, I.; Khormi, A.Y.; Hakami, R.A.; Alelyani, M.; Abdelkader, J.; Benyoucef, A.; Bakkour, Y. Positional Isomeric Effects on the Physicochemical Properties of Polymeric Matrix and Polymer@TiO2 Nanocomposites. Appl. Sci. 2024, 14, 2106. https://doi.org/10.3390/app14052106
Al-Shehri BM, Bekhoukh A, Benkhatou S, Moulefera I, Khormi AY, Hakami RA, Alelyani M, Abdelkader J, Benyoucef A, Bakkour Y. Positional Isomeric Effects on the Physicochemical Properties of Polymeric Matrix and Polymer@TiO2 Nanocomposites. Applied Sciences. 2024; 14(5):2106. https://doi.org/10.3390/app14052106
Chicago/Turabian StyleAl-Shehri, Badria M., Amina Bekhoukh, Soumia Benkhatou, Imane Moulefera, Afaf Y. Khormi, Rabab A. Hakami, Magbool Alelyani, Jinan Abdelkader, Abdelghani Benyoucef, and Youssef Bakkour. 2024. "Positional Isomeric Effects on the Physicochemical Properties of Polymeric Matrix and Polymer@TiO2 Nanocomposites" Applied Sciences 14, no. 5: 2106. https://doi.org/10.3390/app14052106






