Abstract
This study aimed to analyse the effect of chlorhexidine digluconate (CHX DG) mouthwash on the adhesion of oral bacteria to orthodontic appliances. The interactions of four bacteria (S. mutans, A. actinomycetemcomitans, S. oralis, and V. parvula) with two alloys (stainless steel [SS] and nickel-titanium [NiTi]) and three CHX DG solutions (commercial products Curasept and Perio Plus, and pure CHX DG, all with 0.12% active substance) were tested. The adhesive effect on the orthodontic wires was evaluated after 24 h for S. oralis and after 72 h for the other bacteria. The minimum bactericidal concentration of the solution for each bacterial strain was determined using the dilution method to test the antibacterial action. Salivary-pretreated orthodontic archwires were exposed to minimal bactericidal concentrations of solution and bacteria. Commercial antiseptic products, especially Perio Plus, showed a better inhibition of bacterial adhesion to both alloys than pure CHX DG solution (p < 0.05). A. actinomycetemcomitans was most inhibited in the adhesion of all bacteria by the CHX DG products. A greater inhibition of streptococci adherence was observed on SS, while that of A. actinomycetemcomitans was observed on NiTi. V. parvula inhibition was product-dependent. Although there were differences between the strains and the tested agents, it can be concluded that Perio Plus most effectively inhibited the adhesion of all tested bacteria to the SS and NiTi alloys. A. actinomycetemcomitans was most sensitive to all tested agents, while S. mutans showed the highest resistance. The effectiveness of the tested agents was better on NiTi alloys.
1. Introduction
A biofilm is a sessile community of microbial cells immersed in a matrix composed of extracellular polymeric substances (EPSs) attached to the surface. EPSs consist of exopolysaccharides, lipids, proteins, and nucleic acids (eDNA and eRNA) and offer a functional environment and structural stability. Tolerance to immune cells and antimicrobials is enhanced by EPSs [1]. Dental plaque is a complex biofilm whose formation begins with the formation of the salivary pellicle and changes progressively; therefore, we can differentiate early and mature plaque, as well as subgingival and supragingival plaque [2]. Supragingival biofilm bacteria like S. mutans, Actinomyces spp., and Lactobacillus have the greatest influence on enamel and dentin demineralisation and ultimately caries formation [3]. Some subgingival biofilm bacteria include Actinomyces spp., Tannerella forsythia, and Fusobacterium nucleatum, which are associated with gingivitis and periodontitis [4].
Malocclusion therapy in adolescents and adults commonly includes fixed orthodontic appliances containing wires, brackets, and ligatures [5]. Materials in these appliances are alloys such as stainless steel (SS), nickel–titanium (NiTi), titanium–molybdenum, and cobalt–chromium [6]. The difficulty in maintaining good oral hygiene is related to the additional retention places of the fixed appliance for plaque accumulation. Some studies suggest that plaque accumulation is especially increased in metal brackets with elastic ligatures in the maxilla, the maxillary canines, and lateral incisors [7], unlike in non-orthodontic patients, where the mandibula and maxillary molars are the primary sites of accumulation [8]. Areas around the brackets (mesial, distal, and gingival) accumulate more plaque than occlusal areas because they interfere most with archwires and ligatures; thus, natural self-cleaning is not possible [9]. Difficult and inadequate oral hygiene by brushing and a lower ability to use dental floss lead to plaque accumulation and oral flora changes [10]. It can cause gingivitis, enamel white spot lesions (WSL), and caries [11]. The progression of gingivitis can lead to periodontitis and ultimately cause irreversible damage, such as the loss of supporting tissue. Studies claim that some orthodontic patients do not complete treatment, because of poor oral hygiene. Orthodontic patients need to have proper oral hygiene because it is very challenging, and patient motivation is crucial [12]. Therefore, patients should be aware of the consequences of improper hygiene. The clinician may use tools other than informed consent to motivate the patient, such as animations, leaflets, and other verbal information [13]. Appliance placement alters the oral microbiota, and we can observe high concentrations of acidogenic bacteria such as Streptococcus and Lactobacillus which result in WSL progression and caries development in patients with fixed appliances within a month after placement [14], unlike caries development in the mouth without braces that can take up to 6 months [15]. Aetiological factors associated with periodontitis include an increase in Gram-positive supragingival bacteria, such as Streptococcus and Actinomyces. Regarding gingivitis, aetiologies include an increase in Gram-negative Fusobacterium and Bacterioides [16]. In addition to mechanical plaque control, such as brushing and flossing, chemical plaque control can be performed. The gold standard is chlorhexidine digluconate (CHX DG), which has side-effects such as staining and altered taste after long-term use [17,18]. Short-term usage is recommended for people with difficulty in maintaining oral hygiene, such as those with fixed orthodontic appliances, mental and physical disabilities, intermaxillary fixations, and postsurgical or systematic diseases with oral manifestations [19]. Orthodontic wires provide a suitable environment for the growth of oral microorganisms because they are constantly present throughout orthodontic treatment and can play a significant role in enamel demineralisation [20]. The contact areas between the wires and brackets make cleaning difficult by preventing proper access to the tooth surface. Some researchers claim that SS wires increase plaque retention capacity because of their high surface tension and energy [21].
This study aimed to analyse the anti-adhesion effect of CHX DG as a pure substance, and commercially available CHX DG-based mouthwashes, on oral bacteria to SS and NiTi alloys, which are often used in orthodontic treatment.
2. Materials and Methods
2.1. Cultivation of Bacteria
The bacteria used were Streptococcus mutans ATCC 25175, Streptococcus oralis ATCC 6249, Aggregatibacter actinomycetemcomitans ATCC 29522, and Veillonella parvula ATCC 10790 (Microbiology, St Cloud, MN, USA). Streptococci were grown on Mutans–Sanguis agar (HIMEDIA, Mumbai, India) and incubated at 37 °C for 24 h with a candle to simulate capnophilic conditions. A. actinomycetemcomitans and V. parvula were grown on blood agar (Biolife, Milan, Italy) supplemented with 5% sheep blood (Biognost, Zagreb, Croatia) and incubated at 37 °C for 3–5 days in anaerobic conditions using AnaeroGen bags (GENbox anaerobe, bioMerieux SA, Marcy-l’Etolie, France). Brain–Heart Infusion Broth (BHI—Brain–Heart Infusion, Becton, Dickinson and Company, Sparks, MD, USA) was used to resuspend bacterial cultures supplemented with 1.0 mg/mL Vitamin K and 5.0 mg/mL hemin (Sigma-Aldrich, Burlington, MA, USA). Preparation of suspensions: the optical density at 600 nm (OD600) was determined using a spectrophotometer and was set to OD600 = 1, which was interpreted to mean that there were 1 × 109 colonies formed per millilitre (colony forming unit, CFU) in the suspension. Previously prepared suspensions were diluted and a suspension with a concentration of 1 × 107 CFU/mL was used in the experiments [22].
2.2. Adhesion of Bacteria to Orthodontic Archwires
Bacterial suspensions (200 µL) were transferred to the wells and the microtitre plate was placed in the incubator for 4 h with stirring. Sterile orthodontic archwires of 0.019 × 0.025" SS (Stainless steel, American Orthodontics, Sheboygan, WI, USA) and 0.018 × 0.025” NiTi (Neo Sentalloy, Dentsply Sirona, Charlotte, NC, USA) were used. Data on surface characterisation are reported in our previous paper [22]. Before being placed in 500 μL of sterile saline and exposed to sonication for 1 min (BathoSonic ultrasonic bath-Badelin, Berlin, Germany) at an intensity of 40 kHz, the wires were rinsed twice with saline. After homogenisation, tenfold dilutions were made on the microtiter plates, and after planting on the substrate, the bacteria were incubated under anaerobic conditions or with a candle. After 24 h, S. oralis was counted, and after 72 h, A. actinomycetemcomitans, S. mutans, and V. parvula were counted [22,23,24].
2.3. Antibacterial Action of Antiseptics
The mouthwashes used were Curasept ADS 212 (Curasept, Saronno, Italy), Perio Plus (Curasept, Flawil, Switzerland), and a solution of pure 0.12% CHX DG in distilled water (Sigma-Aldrich, USA), all containing 0.12% CHX DG. The composition of Curasept ADS 212 was Aqua, Xylitol, PEG-40 Hydrogenated Castor Oil, Propylene Glycol, Sodium Citrate, PVP-VA 1%, chlorhexidine digluconate 0.12%, ascorbic acid, Sodium Metabisulfite, Sodium Benzoate, Poloxamer 407, Aroma, C.I. 42090. The composition of PerioPlus was Aqua, Xylitol, Polysorbate 20, chlorhexidine digluconate, Aroma, Phenoxyethanol, Vp/Va Copolymer, Sucralose, Cetylpyridinium Chloride, Polylysine, citric acid, Citrus Aurantium Amara Fruit Extract, Glycerin, Sodium Hydroxide, Sodium Chloride. A two-fold dilution of mouthwash solution was applied to a microtitre plate. Bacterial suspension (106 CFU/mL) was added and the microtitre plate was incubated for 24 h at 37 °C. V. parvula was incubated on a shaker for 48 h. A suspension of 100 µL of bacteria was applied to the wells. Sediment or turbidity is a sign of bacterial growth on microtitre plates. Because of the sample’s turbidity, it was impossible to determine the minimum inhibitory concentration (MIC). Therefore, the minimum bactericidal concentration (MBC) was determined. Samples from all wells were plated on Mutans–Sanguis and blood agar and incubated at 37 °C for 24–72 h. The MBC was indicated as the sample without bacterial growth from the sample with the lowest concentration of the active component [25].
2.4. Anti-Adhesive Effects of Antiseptics
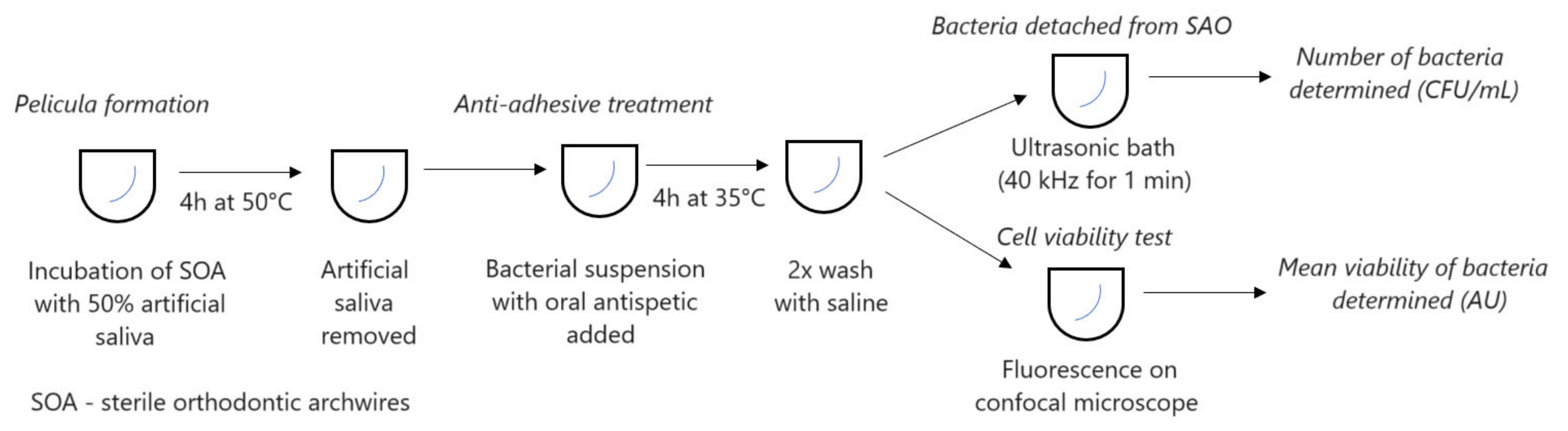
Orthodontic arches were treated for 4 h with 50% artificial saliva at 30 °C. The saliva was removed and the arches were exposed to a 200 μL half-subinhibitory concentration of oral antiseptic and bacterial suspension. The mixture was then stirred under anaerobic conditions for 4 h. After the incubation, the wires were washed twice with saline and centrifuged for 1 min (Figure 1). Bacteria were grown and incubated under anaerobic conditions (Velionella) or by candles (Streptococcus) from dilutions on microtitre plates. After 24 h, an increase was observed in the substrate for S. oralis, and after 72 h for A. actinomycetemcomitans, V. parvula, and S. mutans. Each experiment was performed in triplicate and repeated three times.
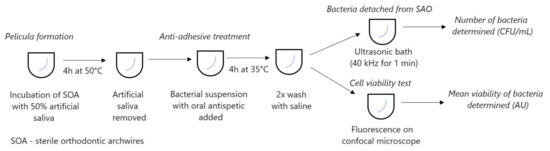
Figure 1.
Set-up of experiment.
2.5. Visualisation of Adherent Bacteria on Archwires by Fluorescence Microscopy
The LIVE/DEAD BacLight Bacterial Viability Kit L-7012 (Invitrogen, Waltham, MA, USA) was used to stain the adherent bacteria on the archwires according to the manufacturer’s instructions. SYTO 9 stained all cells in a green fluorescent colour, whereas propidium iodide (PI) stained cells with a damaged red membrane. Epifluorescence microscopy was used to visualise the adhered bacteria with GFP/FITC (excitation: 480 nm and emission: 500 nm) and rhodamine (excitation: 490 nm and emission: 635 nm) filters. Simultaneous dual-channel imaging was used to visualise green and red fluorescence. The obtained images were saved in the TIFF format and further processed using ImageJ 1.47. A minimum of three images per term were analysed [24].
2.6. Statistical Methods
A log 10 value of CFU/mL was used for the statistical analysis. Factorial analysis of variance (ANOVA) was used to analyse the interaction of alloy and bacterial types on adhesion. ANOVA with the Student–Newman–Keuls post hoc test was used to compare the adhesion between the four bacteria. The proportion of inhibition of adhesion by preparations with CHX DG compared to the control was calculated according to formula 1 (number of treatments/number of controls). ANOVA was used to analyse the interaction of the agents, alloys, and bacteria on adhesion inhibition. An independent-samples t-test was used to compare the adhesion inhibition and adhesion between the alloys. ANOVA with Student–Newman–Keuls post hoc test was used to compare the types of oral antiseptics and bacteria. The t-test was calculated using the formula r = √(t2/(t2 + df)), and the effect size was quantified via η2 for ANOVA. Cohen’s r criteria were used in the interpretation: 0.1–0.3 = small effect size, 0.3–0.5 = moderate, 0.5–0.7 = large, >0.7 very large. The squared values of r were used for the interpretation of η2. p < 0.05 was considered statistically significant. All analyses were performed using the commercial software SPSS 22 (IBM SPSS, Version 22.0, IBM, Armonk, NY, USA).
3. Results
3.1. Minimum Bactericidal Concentrations (MBC)
The MBCs are listed in Table 1. The CHX DG solution as a control (active substance), and Perio Plus and Curasept as commercially available mouthwash are shown in percentages (w/v).

Table 1.
Minimum bactericidal concentrations for individual bacteria (% (w/v)).
3.2. Adhesion to Alloys
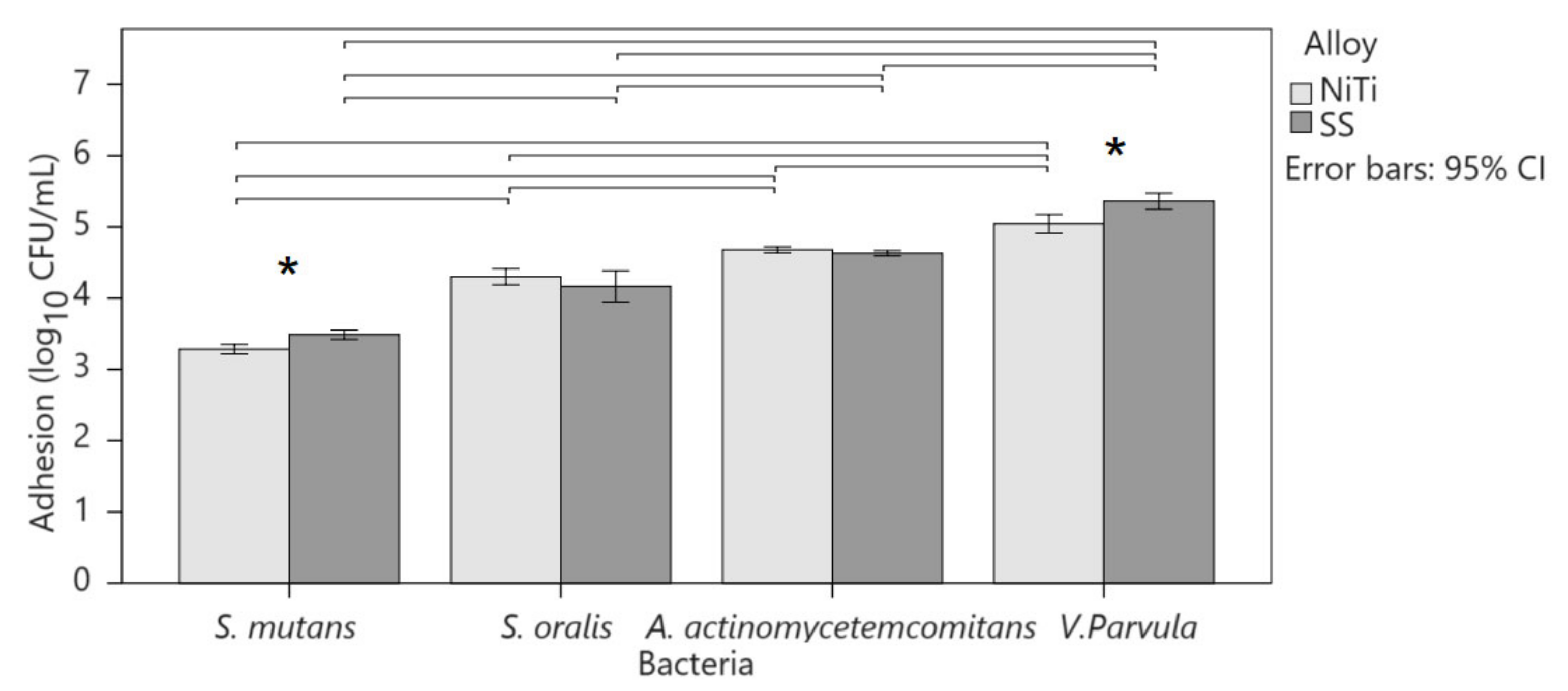
Adhesion to alloys was highest in V. parvula, followed by A. actinomycetemcomitans, S. oralis, and S. mutans (Figure 2). The magnitude of the effect was greater for SS than for NiTi (p < 0.001; η2 = 0.930 and 0.909, respectively). Two-factor ANOVA pointed to the interaction of the alloy and type of bacteria in adhesion (p < 0.001; η2 = 0.184). S. mutans showed greater adhesion to SS than to NiTi (3.5 ± 0.1 vs. 3.2 ± 0.1; p < 0.001; r = 0.678) as well as V. parvula (5.4 ± 0.3 vs. 5.0 ± 0.3; p < 0.001; r = 0.519), although the effect was greater on S. mutans. A. actinomycetemcomitans (NiTi 4.7 ± 0.1 and SS 4.6 ± 01) and S. oralis (NiTi 4.3 ± 0.2 and SS 4.2 ± 0.3) showed equal adhesion to both alloys.
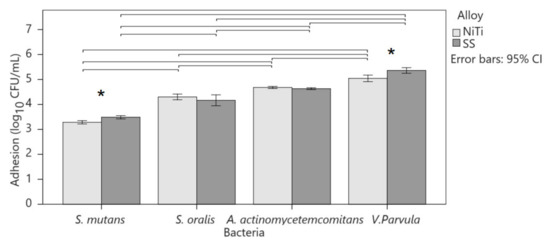
Figure 2.
Comparison of bacterial adhesion to alloys. Columns show mean values with 95% confidence intervals. Asterisks indicate significant differences between alloys for the same bacterium, and horizontal lines connect bacteria with a significant difference within the same alloy.
3.3. Inhibition of Adhesion
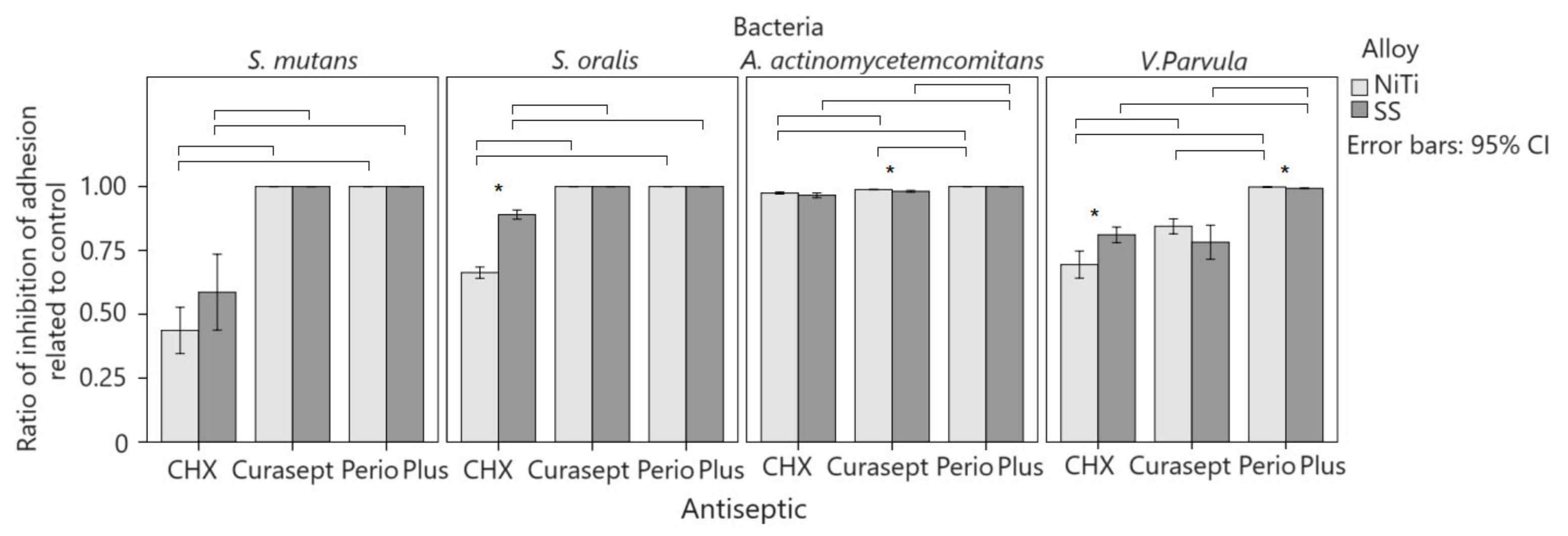
Three-factor ANOVA indicated a significant interaction of the alloy, bacteria, and agent on adhesion reduction with a smaller effect size (p = 0.001; η2 = 0.084). Two-factor ANOVA showed that the interaction of the bacteria and alloy was significant and with a greater effect with Perio Plus (p < 0.001; η2 = 0.452) as well as with pure CHX DG solution (p < 0.001; η2 = 0.194). Curasept showed no significant interactions. Compared to other bacteria, A. actinomycetemcomitans showed the greatest inhibition of adhesion to CHX DG solutions (Figure 3). The inhibition of adhesion was lowest on S. mutans and highest on A. actinomycetemcomitans on both alloys. The effect of CHX DG solution on the inhibition of adhesion was greater with NiTi than with SS (η2 = 0.829 and 0.626) and was significantly different for bacteria. On SS, there was less inhibition of adhesion for V. parvula than for S. oralis, and after the application of Curasept, there was less inhibition of adhesion of V. parvula on NiTi (84% vs. 99–100%) (p < 0.001; η2 = 0.757) and SS (78 vs. 98–100%; p < 0.001; η2 = 0.713) than other bacteria. Perio Plus had a weaker effect on the inhibition of V. parvula on NiTi (99.8% 100%) (p < 0.001; η2 = 0.654) with a greater effect size on SS (99.3% vs. 100%; p < 0.001; η2 = 0.779) than it acted on other bacteria with both alloys.
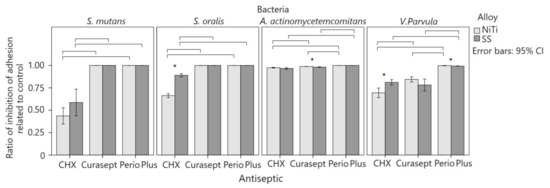
Figure 3.
Comparison of control adhesion and antiseptic treatment. Average column values are with 95% confidence intervals. Significant differences between alloys for the same bacteria and antiseptic are marked with an asterisk. Horizontal lines connect antiseptics that show a significant difference between the same alloy and bacteria.
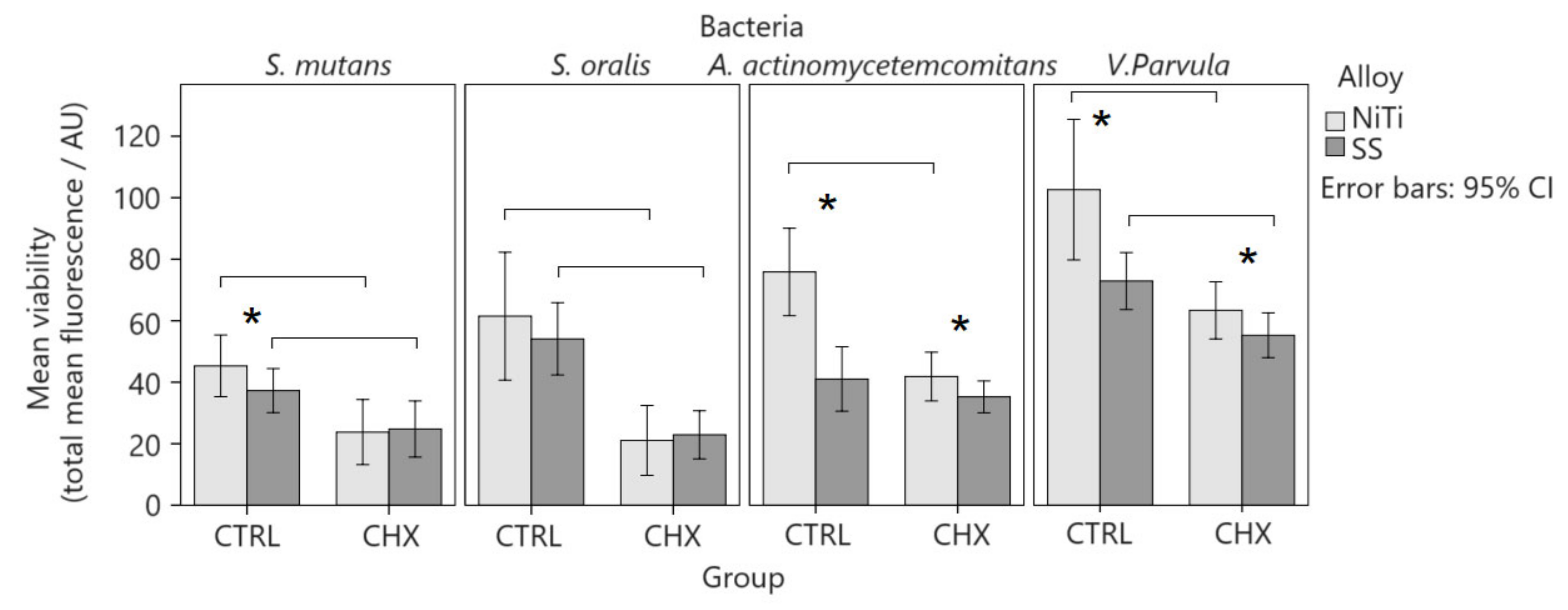
Viability depended on the type of bacteria, exposure, and alloy combination (p = 0.047; η2 = 0.217; Figure 4 and Figure 5). It was smaller with SS wires than with NiTi wires and after CHX DG therapy. Viability was highest in V. parvula and lowest in S. mutans. CHX DG induced lower viability on both alloys in V. parvula and S. mutans, whereas in A. actinomycetemcomitans, it was only on NiTi, with a large effect size (p ≤ 0.010; r = 0.841–0.958). There were significant differences in the viability of the control on SS in the order V. parvula > S. oralis > A. actinomycetemcomitans = S. mutans (p < 0.001). In the NiTi control, there were significant differences in viability in the order of V. parvula > A. actinomycetemcomitans > S. oralis > S. mutans (p < 0.001). In both alloys, significant differences in the viability of the CHX DG were observed in the following order: V. parvula >A. actinomycetemcomitans > S. mutans = S. oralis (p < 0.001).
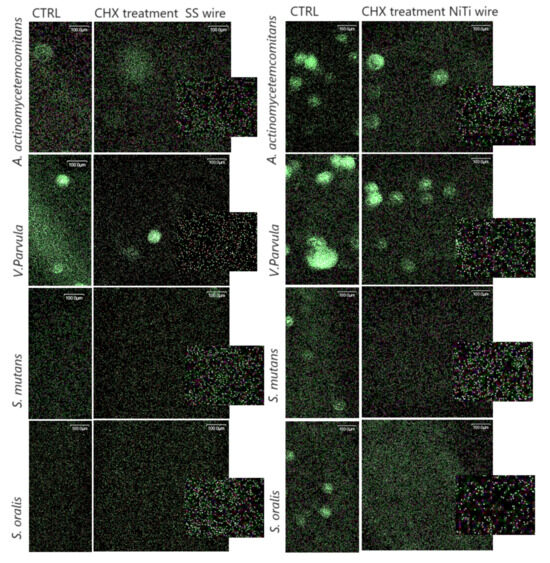
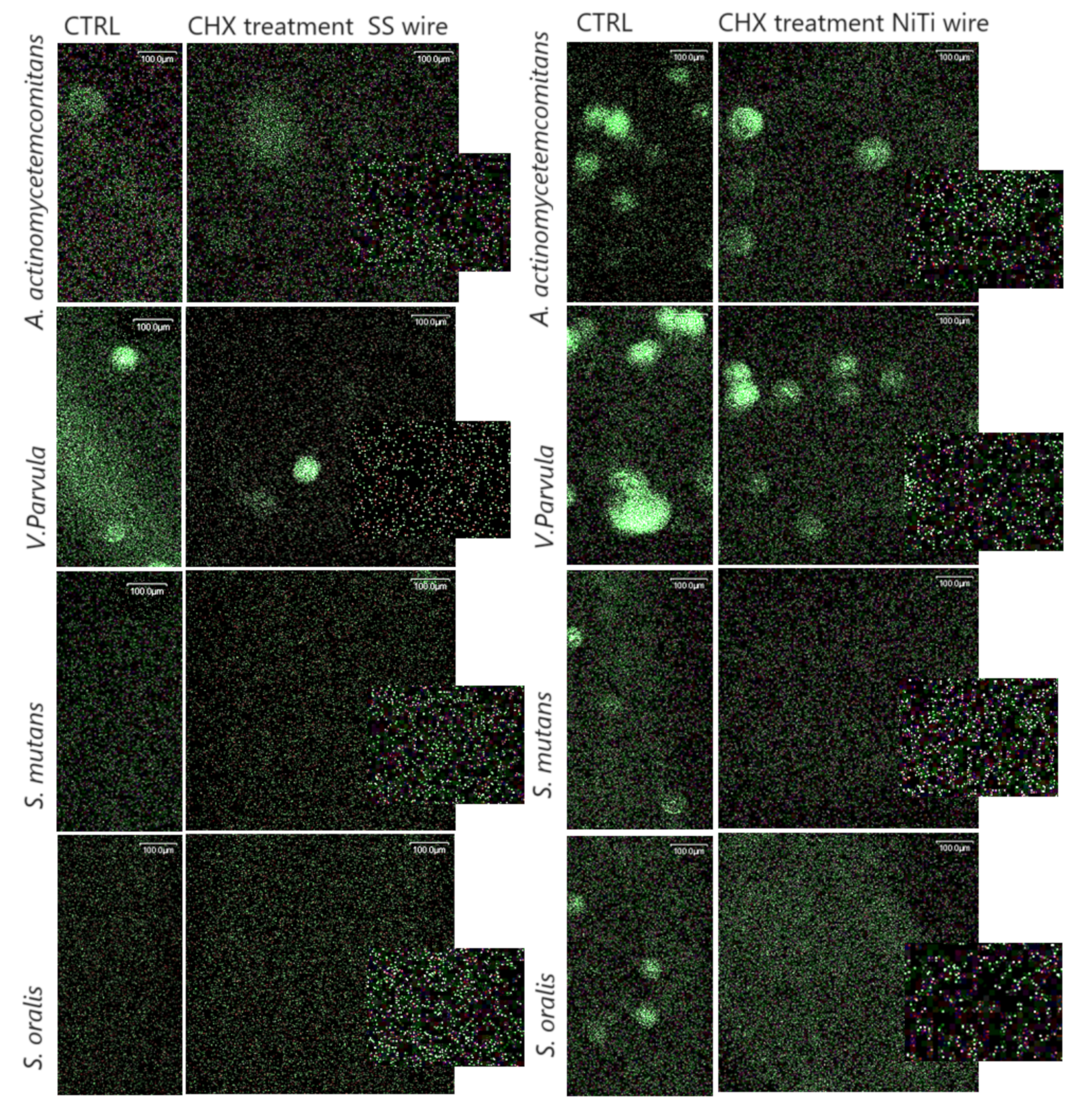
Figure 4.
Representative images of bacteria adhering within 4 h to NiTi and SS wires with and without CHX treatment. Biofilms were stained with the LIVE/DEAD Sustainability Kit (FilmTracer™ LIVE/DEAD® Biofilm Viability Kit, Invitrogen). Green cells represent viable, live bacteria, while red cells represent dead bacteria.
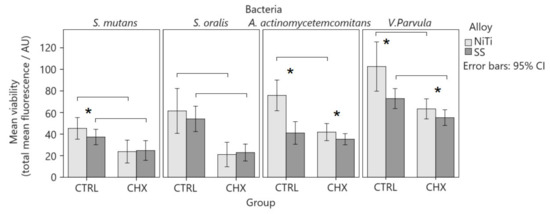
Figure 5.
Comparison of viability by fluorescence on confocal microscope. Total mean fluorescence is measured for propidium iodide and SYTO® 9 stained adherent bacteria before and after treatment with CHX DG. The experiment is repeated 2×, a minimum of three images are analysed, and the mean fluorescence with 95% confidence interval is shown. Significant differences between alloys for the same bacteria and condition are marked with an asterisk.
3.4. Differences between Alloys
Two-factor ANOVA showed that the interaction of bacteria and agent was significant and with a greater effect for NiTi (p < 0.001; η2 = 0.609) than for SS (p < 0.001; η2 = 0.380). The inhibition of V. parvula depended on the solution. On SS, there was a greater inhibition of adhesion in streptococci, whereas, on NiTi, there was a greater inhibition of adhesion in A. actinomycetemcomitans. After CHX DG therapy, the inhibition of adhesion to SS was greater than that to NiTi in S. oralis (96% vs. 89%; p < 0.001; r = 0.303) and V. parvula (81% vs. 69%; p < 0.001; r = 0.532). The inhibition of adhesion to SS after CHX DG in S. mutans was higher, but not significant, owing to the large scattering of the data. The inhibition of A. actinomycetemcomitans was equal for both metals after the application of CHX DG. An excellent inhibition of adhesion was observed for both metals after Curasept therapy in A. actinomycetemcomitans (99% vs. 98%; p = 0.001; r = 0.486), although it was higher for NiTi than for SS. A greater inhibition was observed for V. parvula (84% vs. 78%), although the difference was not statistically significant, because of the scattering of the data. After therapy with Curasept for streptococci, no differences were observed between the alloys. Perio Plus therapy was the least dependent on the type of alloy used. V. parvula showed a greater inhibition of adhesion to NiTi than to SS alloy after Perio Plus therapy (99.8% vs. 99.3%; p < 0.001; r = 0.811), and there was no difference between the alloys for other bacteria (100% inhibition).
3.5. Differences between Solutions
V. parvula (p < 0.001; η2 = 0.208) and S. oralis (p < 0.001; η2 = 0.675) showed a significant interaction between the agents and alloys, with a larger effect in S. oralis, as shown by two-factor ANOVA. S. mutans and A. actinomycetemcomitans did not show significant interactions. CHX DG produced the lowest inhibition of adhesion and Perio Plus produced the highest inhibition among all bacteria. CHX DG caused less adhesion inhibition than Perio Plus and Curasept (100%; p < 0.001) on both alloys (66–89%) in S. oralis, with a larger effect size on NiTi than SS (η2 = 0.939 and 0.708), and no difference between the last two solutions. A smaller inhibition of adhesion on both alloys (44–59%) was shown by CHX DG compared to Perio Plus and Curasept (100%) in S. mutans, with a larger effect size on NiTi (p < 0.001; η2 = 0.871) than SS (p = 0.003; η2 = 0.532) and with no difference between the two last solutions. Adhesion inhibition was high with all three agents (97–100%) in A. actinomycetemcomitans. Perio Plus had the greatest inhibition, and CHX DG had the smallest effect size on SS (p = 0.003; η2 = 0.311) than NiTi (p < 0.001; η2 = 0.618). After Perio Plus therapy, V. parvula had the highest inhibition on NiTi alloy, and the lowest after using CHX DG (100% and 69%; p < 0.001; η2 = 0.671). On SS, Perio Plus showed the highest inhibition of adherence but the lowest inhibition after Curasept therapy (99% and 78%; p < 0.001; η2 = 0.604).
4. Discussion
This study has shown that the adhesion of oral bacteria to orthodontic appliances depends on complex interactions between the type of alloy, antiseptic product used, and bacteria. The MIC could not be determined in the present study, because of the presence of sediment in the wells. Other studies report that MIC in different strains of S. mutans treated with CHX DG varied from 0.03 to 0.2 µg/mL, and that of MBC varied from 0.24 to 0.9 µg/mL. Sub-inhibitory concentrations of CHX DG have also been reported to influence the growth of planktonic S. mutans cells and biofilm [26].
Bacterial adhesion is the initial step in biofilm formation. Orthodontic appliances create new adhesion surfaces, and multiple bacterial species colonise the oral cavity and adhere to these surfaces to prevent swallowing. According to the present study, adhesion to alloys was highest in V. parvula and lowest in S. mutans with A. actinomycetemcomitans and S. oralis. S. mutans and V. parvula showed greater adhesion to the SS wires, whereas A. actinomycetemcomitans and S. oralis had similar effects on both wires.
Bacterial hydrophobicity is one of the factors influencing the adhesion of oral bacteria to orthodontic alloys during the first 4 h, while alloy surface characteristics have little influence. In the same study, V. parvula was found to have extremely hydrophobic properties, S. oralis and S. mutans were moderately hydrophobic, and A. actinomycetemcomitans exhibited hydrophilic properties. Both NiTi and SS exhibit hydrophilic characteristics; however, NiTi is more hydrophilic [22]. In addition, NiTi has a higher roughness, polarity, and surface-free energy than SS, which makes NiTi more prone to bacterial adhesion. However, the difference between the surface-free energies of the alloy and bacteria is inversely proportional to bacterial adhesion [22,27]. In addition, some biological processes regulate bacterial adhesion, such as the production of extracellular polysaccharides, cell viability, charge and wall stiffness, bacterial surface protuberances, metabolic activity, hydrophobicity, and adhesin-mediated receptor–ligand binding [28].
The process of tooth preparation for bracket bonding by enamel etching alters the surface properties, and this initial step influences adhesion and biofilm formation [29]. Rough areas increase the space for biofilm accumulation and adhesion, whereas higher wettability makes it easier for biofilms to adhere to dental materials [30]. The correlation between the bonding procedure and the accumulation of S. mutans and P. gingivalis has been confirmed [31].
The market offers aesthetic archwires, in addition to traditional archwires made of iron, titanium, or cobalt alloys [32]. The wires are coated with epoxy resin and polytetrafluoroethylene [33]. In vitro research with S. mutans showed that there was no major difference in biofilm formation or bacterial accumulation between the coated and uncoated archwires when colony counting [34]. When comparing Cu-NiTi archwires and NiTi wires, it appears that S. mutans adheres better to the Cu-NiTi wires [35].
The main method of oral hygiene is the mechanical removal of plaque by tooth brushing (manual or electric). In addition to manual techniques, the gold standard for chemical plaque control is bisbiguanide CHX mouthwash. It was developed in the UK in the 1940s as a general disinfectant. In the 1970s, it was being used as a mouthwash because its anti-plaque effects were discovered [36]. In dentistry, 0.12–0.2% CHX can be used preoperatively, postoperatively (if brushing is enabled), or long-term (CHX chips). The recommended dosage for maximum effect per use is 18–20 mg [37].
The bacterial wall is negatively charged and the CHX wall is positively charged; therefore, they are attracted to each other. On the surface of the bacterial wall, phosphate-containing molecules strongly adsorb onto CHX. CHX penetrates the bacterial wall, damaging the bacterial cell and inhibiting enzymes related to the cytoplasmic membrane, and causes an overflow of low-molecular-weight cytoplasmic components, such as potassium ions [38]. This is the bacteriostatic stage which can be reversed if CHX is removed, but if it persists, the antimicrobial action continues to the bactericidal stage, where cytoplasmic coagulation and precipitation occur, and complexes such as adenosine triphosphate and nucleic acids are formed. Oral surfaces are negatively charged; therefore, CHX, a cationic molecule, adheres well to these surfaces and exhibits good interference with bacterial adhesion [39]. Good binding to the oral surface allows it to last longer in the mouth. CHX exhibits a prolonged action and can be released through a period of 8–12 h in the mouth [19,40]. CHX has antifungal, antimicrobial (anaerobes and aerobes), and antiviral (RNA, DNA, and lipophilic-enveloped viruses) activities [41].
This study showed that the anti-adhesive effect is mostly related to the type of mouthwash used. CHX DG showed the lowest inhibition of adhesion, whereas Perio Plus showed the highest inhibition. The Curasept acted similarly to Perio Plus. A. actinomycetemcomitans was most inhibited, whereas V. parvula was least inhibited. Some differences were observed but not with commercial products. There is unclear evidence of whether mouthwash with CHX DG concentrations below 0.1% has an antiplaque effect. Some authors claim that CHX DG 0.05% with herbal extract has a similar antiplaque effect as CHX DG at concentrations of 0.1%, while others claim that CHX DG at concentrations below 0.1% has an unclear antiplaque effect [42,43]. It is generally recommended to rinse twice daily with 20 mg of CHX DG mouthwash at concentrations of 0.12–0.2% [44]. In addition to the DG concentrations of CHX, it is necessary to be aware of toothpaste components. The effect of CHX DG can be reduced with ingredients such as sodium lauryl sulfate, cocamidopropyl betaine (CAPB), calcium and anionic surfactants, and sodium dodecyl sulfate in toothpaste. To avoid a reduction in the effect, the recommendation is to wait 30 min after brushing before using the mouthwash [45]. Fluoride toothpaste should be switched with CAPB paste because it provides better enamel remineralisation as a surfactant with a 30 min delayed rinse [46]. In addition to mouthwash, CHX DG can be used as a gel or dentifrice, although mouthwash should be the first choice, especially in non-brushing models. Researchers have shown that CHX mouthwash reduces S. mutans better than CHX gels and varnishes, even at higher concentrations, but when used alone, it does not have a clinical impact on significant caries reduction [47]. This study found that the cell viability was dependent on the bacteria, alloys, and exposure. The viability of the wires was greatest in V. parvula and smallest in S. mutans. CHX DG decreased the viability of S. mutans and V. parvula on SS and NiTi and decreased the viability of A. actinomycetemcomitans only on NiTi.
Perio Plus is the only alcohol solution used in this study. When comparing mouthwash 0.2% CHX DG with a non-alcohol base and essential oils containing alcohol, both showed similar results on gingival health; however, because of the side-effects of alcohol-based mouthwash, such as reduced salivation and burning mouth, it is recommended to use the non-alcohol-based one [48]. In contrast, a 7-day study using CHX DG showed a decrease in the pH of the saliva which is associated with enamel demineralisation and caries. CHX DG showed a shift to an acidic environment; therefore, mouthwash application should be cautiously considered [49]. Alcohol is probably not the only reason for the greater effectiveness of Perio Plus, but rather a combination of different substances with a potential antimicrobial effect. In addition to CHX DG, Perio Plus also contains several other components with an antimicrobial effect, such as xylitol, citric acid, and Polysorbate 20. In Curasept, several substances that could also help with antimicrobial activity can be found, such as Sodium Citrate and ascorbic acid. Mixtures of substances in lower concentrations show a better antimicrobial and anti-adhesion effect, and since it is a short-term exposure, they also show a better effect. Perio Plus has proven to be the most effective probably for this reason.
The advantage of this research was the controlled environment for experiments in a microbiological laboratory, so it was possible to analyse each bacterium and product separately. The disadvantage is that bacteria work together in the oral cavity and coexist in dynamic media, making it difficult to imitate the oral cavity under laboratory conditions. The pH of the mouth was not considered in this study. Various factors influence biofilm formation on orthodontic appliances in the oral cavity; however, this connection is not fully understood. Additional research is required to clarify these gaps. The difference between the results of clinical and laboratory tests is large, which shows the complexity of dental biofilms.
5. Conclusions
Although there were differences between the strains and the tested agents, it can be concluded that Perio Plus most effectively inhibited the adhesion of all tested bacteria to the SS and NiTi alloys. A. actinomycetemcomitans was the most sensitive to all tested agents, while S. mutans showed the highest resistance. The effectiveness of the tested agents was better on NiTi alloys.
Author Contributions
Conceptualization, I.G., D.G. and S.S.; methodology, S.S.; validation, L.K., Z.M., I.G. and S.S., formal analysis, S.S.; investigation, D.G.; resources, S.S.; data curation, S.S.; writing—original draft preparation, M.B. and D.G.; writing—review and editing, L.K., Z.M., I.G., S.S., M.B. and D.G; visualization, S.S. and D.G.; supervision, S.S. and I.G.; project administration, S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
Croatian Science Foundation IP-2020-02-4027 (Environmental factors and microbial interactions in the structure of the dental biofilm) and DOK-2021-02-6438 (Career development of young researchers—training of new PhDs).
Data Availability Statement
The datasets used and/or analysed during the current study are available on the Digital Academic Repository of the University of Rijeka, Faculty of Dental Medicine, from the corresponding author on reasonable request. (Špalj S. Okolišni čimbenici i mikrobiološke interakcije u strukturi dentalnog biofilma: istraživački podaci [Internet]. Fakultet dentalne medicine; 2023, Available from: https://urn.nsk.hr/urn:nbn:hr:271:843444 (accessed on 4 January 2024)).
Acknowledgments
We thank Gabrijela Begic for help in laboratory procedures. Preliminary data were presented as a poster at Italian Orthodontic Society International Spring Meeting in Genova (18–19 March 2022).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, A.O.A.; Marquezan, M.; Nojima, M.D.C.G.; Alviano, D.S.; Maia, L.C. The influence of orthodontic fixed appliances on the oral microbiota: A systematic review. Dent. Press J. Orthod. 2014, 19, 46–55. [Google Scholar] [CrossRef]
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral biofilm architecture on natural teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, J.; Dong, Y.; Lu, H.; Zhou, H.; Hansen, B.F.; Song, X. Periodontal health and relative quantity of subgingival porphyromonas gingivalis during orthodontic treatment. Angle Orthod. 2011, 81, 609–615. [Google Scholar] [CrossRef]
- Kapila, S.; Sachdeva, R. Mechanical properties and clinical applications of orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 1989, 96, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chieng, J.; Wong, C.; Benic, G.; Farella, M. Factors affecting dental biofilm in patients wearing fixed orthodontic appliances. Prog. Orthod. 2017, 18, 4. [Google Scholar] [CrossRef]
- Furuichi, Y.; Lindhe, J.; Ramberg, P.; Volpe, A.R. Patterns of de novo plaque formation in the human dentition. J. Clin. Periodontol. 1992, 19, 423–433. [Google Scholar] [CrossRef]
- Garcez, A.S.; Suzuki, S.S.; Ribeiro, M.S.; Mada, E.Y.; Freitas, A.Z.; Suzuki, H. Biofilm retention by 3 methods of ligation on orthodontic brackets: A microbiologic and optical coherence tomography analysis. Am. J. Orthod. Dentofac. Orthop. 2011, 140, e193-8. [Google Scholar] [CrossRef]
- Goes, P.; Dutra, C.S.; Lisboa, M.R.P.; Gondim, D.V.; Leitão, R.; Brito, G.A.C.; Rego, R.O. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J. Oral Sci. 2016, 58, 569–574. [Google Scholar] [CrossRef]
- Kleter, G.A. Discoloration of dental carious lesions (a review) Arch. Oral Biol. 1998, 43, 629–632. [Google Scholar] [CrossRef]
- Huang, J.; Yunxia, Y.; Jiuhui, J.; Cuiying, L. Effects of motivational methods on oral hygiene of orthodontic patients A systematic review and meta-analysis. Medicine 2018, 97, e13182. [Google Scholar] [CrossRef]
- Shqaidef, A.J.; Saleh, M.Y.N.; Kussad, J.; Khambay, B.S. Do parents of adolescent patients undergoing fixed appliance treatment recall more information using written material or an animated video? A randomized controlled trial. Saudi Dent. J. 2023, 35, 95–102. [Google Scholar] [CrossRef]
- Lundström, F.; Krasse, B. Streptococcus mutans and lactobacilli frequency in orthodontic patients; the effect of chlorhexidine treatments. Eur. J. Orthod. 1987, 9, 109–116. [Google Scholar] [CrossRef]
- Mattousch, T.J.; van der Veen, M.H.; Zentner, A. Caries lesions after orthodontic treatment followed by quantitative light-induced fluorescence: A 2-year follow-up. Eur. J. Orthod. 2007, 29, 294–298. [Google Scholar] [CrossRef]
- Sinclair, M.; Berry, C.W.; Bennet, C.L.; Israelson, H. Changes in gingival and gingival flora with bonding and banding. Angle Orthod. 1987, 57, 271–278. [Google Scholar]
- Martin, B.J.; Campbell, P.M.; Rees, T.D.; Buschang, P.H. A randomized controlled trial evaluating antioxidant-essential oil gel as a treatment for gingivitis in orthodontic patients. Angle Orthod. 2016, 86, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Yeturu, S.K.; Acharya, S.; Urala, A.S.; Pentapati, K.C. Effect of Aloe vera, chlorine dioxide, and chlorhexidine mouth rinses on plaque and gingivitis: A randomized controlled trial. J. Oral Biol. Craniofac. Res. 2016, 6, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Addy, M. Chlorhexidine compared with other locally delivered anti-microbials. A short review. J. Clin. Periodontol. 1986, 13, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Balenseifen, J.W.; Madonia, J.V. Study of dental plaque in orthodontic patients. J. Dent. Res. 1970, 49, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Eliades, G.; Brantley, W.A. Microbial attachment on orthodontic appliances. I. Wettability and early pellicle formation on bracket materials. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 351–360. [Google Scholar] [CrossRef]
- Begić, G.; Didović, M.P.; Blagojević, S.L.; Badovinac, I.J.; Žigon, J.; Perčić, M.; Peloza, O.C.; Gobin, I. Adhesion of oral bacteria to commercial d-PTFE membranes: Polymer microstructure makes a difference. Int. J. Mol. Sci. 2022, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Radovic, R.; Begic, G.; Lucic Blagojevic, S.; Karleusa, L.J.; Spalj, S.; Gobin, I. Temporal dynamics of adhesion of oral bacteria to orthodontic appliances. Dent. Mater. J. 2023, 42, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Begić, G.; Badovinac, I.J.; Karleuša, L.; Kralik, K.; Peloza, O.C.; Kuiš, D.; Gobin, I. Streptococcus salivarius as an important factor in dental biofilm homeostasis: Influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in mixed biofilm. Int. J. Mol. Sci. 2023, 24, 7249. [Google Scholar] [CrossRef] [PubMed]
- Akca, E.; Akca, G.; Toksoy Topçu, F.; Macit, E.; Pikdöken, L.; Özgen, S. The comparative evaluation of the antimicrobial effect of propolis with chlorhexidine against oral pathogens: An in vitro study. Biomed. Res. Int. 2016, 2016, 3627463. [Google Scholar] [CrossRef] [PubMed]
- Gazola Filho, J.; Leal Vizoto, N.; de Aguiar Loesch, M.L.; Dias de Sena, M.; Mendes da Camara, D.; Sampaio Caiaffa, K.; de Oliveira Mattos-Graner, R.; Duque, C. Genetic and physiological effects of subinhibitory concentrations of oral antimicrobial agents on Streptococcus mutans biofilms. Microb. Pathog. 2021, 150, 104669. [Google Scholar] [CrossRef] [PubMed]
- Nespoli, A.; Passaretti, F.; Szentmiklósi, L.; Maróti, B.; Placidi, E.; Cassetta, M.; Yada, R.Y.; Farrar, D.H.; Tian, K.V. Biomedical NiTi and β-Ti alloys: From composition, microstructure and thermo-mechanics to application. Metals 2022, 12, 406. [Google Scholar] [CrossRef]
- Alam, F.; Balani, K. Adhesion force of Staphylococcus aureus on various biomaterial surfaces. J. Mech. Behav. Biomed. Mater. 2017, 65, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Nandhra, S.S.; Littlewood, S.J.; Houghton, N.; Luther, F.; Prabhu, J.; Munyombwe, T.; Wood, S.R. Do we need primer for orthodontic bonding? A randomized controlled trial. Eur. J. Orthod. 2015, 37, 147–155. [Google Scholar] [CrossRef]
- An, J.S.; Kim, K.; Cho, S.; Lim, B.S.; Ahn, S.J. Compositional differences in multi-species biofilms formed on various orthodontic adhesives. Eur. J. Orthod. 2017, 39, 528–533. [Google Scholar] [CrossRef]
- Jeon, D.M.; An, J.S.; Lim, B.S.; Ahn, S.J. Orthodontic bonding procedure influence biofilm composition. Prog. Orthod. 2020, 21, 14. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Gronostajski, Z.; Wielgus, A.; Chojnacka, K. Transparent orthodontic archwires: A systematic literature review. Arch. Civ. Mech. Eng. 2017, 17, 651–657. [Google Scholar] [CrossRef]
- Dittmer, M.P.; Hellemann, C.F.; Grade, S.; Heuer, W.; Stiesch, M.; Schwestka-Polly, R.; Demling, A.P. Comparative three-dimensional analysis of initial biofilm formation on three orthodontic bracket materials. Head Face Med. 2015, 11, 1–6. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Thomson, J.J.; Alhabeil, J.A.; Toma, J.M.; Plecha, S.C.; Pacheco, R.R.; Cuevas-Suárez, C.E.; Piva, E.; Lund, R.G. In vitro Streptococcus mutans adhesion and biofilm formation on different esthetic orthodontic archwires. Angle Orthod. 2021, 91, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.S.; Jagdish, N.; Kailasam, V.; Padmanabhan, S. Streptococcus mutans adhesion on nickel titanium (NiTi) and copper-NiTi archwires: A comparative prospective clinical study. Angle Orthod. 2017, 87, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Nowakowska-Toporowska, A.; Wezgowiec, J.; Nowakowska, D. Design and characteristics of new experimental chlorhexidine dental gels with anti-staining properties. Adv. Clin. Exp. Med. 2019, 28, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in dentistry: Pharmacology, uses, and adverse effect. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Łukomska-Szymańska, M.; Sokołowski, J.; Łapińska, B. Chlorhexidine—Mechanism of action and its application to dentistry. J. Stomatol. 2017, 70, 405–417. [Google Scholar]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of chlorhexidine rinses after periodontal or implant surgery: A systematic review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bonesvoll, P.; Lökken, P.; Rölla, G.; Paus, P.N. Retention of chlorhexidine in the human oral cavity after mouth rinses. Arch. Oral Biol. 1974, 19, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Poppolo Deus, F.; Ouanounou, A. Mouthwashes and their use in dentistry: A review. Oral Heal. 2021. Available online: https://www.oralhealthgroup.com/features/mouthwashes-and-their-use-in-dentistry-a-review/ (accessed on 14 January 2024).
- Laugisch, O.; Ramseier, C.A.; Salvi, G.E.; Hägi, T.T.; Bürgin, W.; Eick, S.; Sculean, A. Effects of two different post-surgical protocols including either 0.05% chlorhexidine herbal extract or 0.1% chlorhexidine on post-surgical plaque control, early wound healing and patient acceptance following standard periodontal surgery and implant placement. Clin. Oral Investig. 2016, 20, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Duss, C.; Lang, N.P.; Cosyn, J.; Persson, G.R. A randomized, controlled clinical trial on the clinical, microbiological, and staining effects of a novel 0.05% chlorhexidine/herbal extract and a 0.1% chlorhexidine mouthrinse adjunct to periodontal surgery. J. Clin. Periodontol. 2010, 37, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Labriola, A.; Zambelli, R.; Prato, G.P.; Nieri, M.; Tonetti, M.S. Chlorhexidine with an anti-discoloration system after periodontal flap surgery: A cross-over, randomized, triple-blind clinical trial. J. Clin. Periodontol. 2008, 35, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, A.; Kaspar, S.; Kathirvelu, R.; Srinivasan, B.; Srinivasan, S.; Sundram, R. Chlorhexidine: An elixir for periodontics. J. Pharm. Bioallied. Sci. 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Almohefer, S.A.; Levon, J.A.; Gregory, R.L.; Eckert, G.J.; Lippert, F. Caries lesion remineralization with fluoride toothpastes and chlorhexidine—Effects of application timing and toothpaste surfactant. J. Appl. Oral Sci. 2018, 26, e20170499. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Kulkarni, P.J.; Singh, D.K.; Jalaluddin, M.; Mandal, A. Comparative evaluation of antiplaque efficacy between essential oils with alcohol-based and chlorhexidine with nonalcohol-based mouthrinses. Int. Soc. Prev. Community Dent. 2017, 7 (Suppl. S1), S36–S41. [Google Scholar] [CrossRef]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).