Comprehensive Analysis of the Acute Toxicity of Ionic Liquids Using Microtox® Bioassays
Abstract
:1. Introduction
2. Materials and Methods
- EC50 > 1000 mg/L means that the compound is relatively harmless;
- 1000 mg/L > EC50 > 100 mg/L, practically harmless;
- 100 mg/L > EC50 > 1 mg/L toxic;
- 1mg/L > EC50 > 0.1 mg/L highly toxic;
- 0.1 mg/L > EC50 > 0.01 mg/L extremely toxic;
- EC50 < 0.01 mg/L supertoxic.
3. Results and Discussion
4. Conclusions
- -
- This study found that 16 of the 30 compounds were either non-toxic or practically harmless, and none were at the highest levels of the two classifications considered. No preferential effect of cation or anion on toxicity have been found, although the effect on bacteria is determined by the combination of both.
- -
- Protic ILs exhibit lower toxicity compared to aprotic ones at the shorter alkyl chain length, and non-aromatic ILs generally demonstrate lower toxicity than aromatic ones. Additionally, water solubility plays a significant role, with lower toxicity associated with higher hydrophilicity within each group.
- -
- Ionic liquids with ammonium cations presented the lower toxicity, while the imidazolium-based ILs are more harmful for the shortest alkyl chain length ILs, although the toxicity increases with this alkyl chain, with OAN and C8C1Im TFSI being the most toxic ILs, both with C = 8, the longest chain considered in this work.
- -
- The toxicity of similar cations ranged from the TFSI anion, the most toxic, to nitrate-based ILs, the least toxic.
- -
- FAP-based ILs, which present the opposite behavior regarding the chain length to the other anion ILs; i.e., the more toxic ILs correspond to the ILs with the shorter alkyl chain.
- -
- The identification of a critical alkyl size (CAS) at C = 6 was documented in this study, defining a tipping point in toxicity behavior related to the length of the chain. The presence of six or more carbons in the alkyl chain results in a significant increase in toxicity levels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 218–225. ISBN 978-0-08-101984-9. [Google Scholar]
- Parajó, J.J.; Villanueva, M.; Sánchez, P.B.; Salgado, J. Liquid Window of Some Biologically-Active Ionic Liquids. J. Chem. Thermodyn. 2018, 126, 1–10. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. Progress in Electrodeposition of Zinc and Zinc Nickel Alloys Using Ionic Liquids. Appl. Sci. 2020, 10, 5321. [Google Scholar] [CrossRef]
- Parajó, J.J.; Vallet, P.; Villanueva, M.; Cabeza, O.; Fernández-Carretero, F.; García Luis, A.; Di Pietro, M.E.; Mele, A.; Castiglione, F.; Salgado, J.; et al. Ionogels Based on Protic Ionic Liquid—Lithium Salt Mixtures. J. Mol. Liq. 2024, 397, 124093. [Google Scholar] [CrossRef]
- Amaral, M.; Pereiro, A.B.; Gaspar, M.M.; Reis, C.P. Recent Advances in Ionic Liquids and Nanotechnology for Drug Delivery. Nanomedicine 2020, 16, 63–80. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and Future Perspectives for Biocides and Antifouling Products for Stone-Built Cultural Heritage: Ionic Liquids as a Challenging Alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Reid, J.E.S.J.; Prydderch, H.; Spulak, M.; Shimizu, S.; Walker, A.J.; Gathergood, N. Green Profiling of Aprotic versus Protic Ionic Liquids: Synthesis and Microbial Toxicity of Analogous Structures. Sustain. Chem. Pharm. 2018, 7, 17–26. [Google Scholar] [CrossRef]
- Thamke, V.; Singh, P.; Pal, S.; Chaudhary, M.; Kumari, K.; Bahadur, I.; Varma, R.S. Current Toxicological Insights of Ionic Liquids on Various Environmental Living Forms. J. Environ. Chem. Eng. 2022, 10, 107303. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Weyhing-Zerrer, N.; Kalb, R.; Oßmer, R.; Rossmanith, P.; Mester, P. Evidence of a Reverse Side-Chain Effect of Tris(Pentafluoroethyl)Trifluorophosphate [FAP]-Based Ionic Liquids against Pathogenic Bacteria. Ecotoxicol. Environ. Saf. 2018, 148, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.J.; Vallet, P.; Varela, L.M.; Villanueva, M.; Salgado, J. Ecotoxicity of Binary Mixtures of ILs and Inorganic Salts of Electrochemical Interest. Environ. Sci. Pollut. Res. 2021, 29, 24983–24994. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.; Coutinho, J.A.P.; Ventura, S.P.M. Aquatic Toxicology of Ionic Liquids (ILs) in Encyclopedia of Ionic Liquids, 1st ed.; Springer Nature: Singapore, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Herrero, E.; Tobajas, M.; Polo, A.; Rodriguez, J.J.; Mohedano, A.F. Toxicity and Inhibition Assessment of Ionic Liquids by Activated Sludge. Ecotoxicol. Environ. Saf. 2020, 187, 109836. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.; Parajó, J.J.; Teijeira, T.; Cruz, O.; Proupín, J.; Villanueva, M.; Rodríguez-Añón, J.A.; Verdes, P.V.; Reyes, O. New Insight into the Environmental Impact of Two Imidazolium Ionic Liquids. Effects on Seed Germination and Soil Microbial Activity. Chemosphere 2017, 185, 665–672. [Google Scholar] [CrossRef]
- Sanches, M.V.; Freitas, R.; Oliva, M.; Cuccaro, A.; Monni, G.; Mezzetta, A.; Guazzelli, L.; Pretti, C. Toxicity of Ionic Liquids in Marine and Freshwater Microorganisms and Invertebrates: State of the Art. Environ. Sci. Pollut. Res. 2023, 30, 39288–39318. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M.; Iqbal, M. Vibrio Fischeri Bioluminescence Inhibition Assay for Ecotoxicity Assessment: A Review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Borodin, O.; Smith, G.D.; Henderson, W. Li+ Cation Environment, Transport, and Mechanical Properties of the LiTFSI Doped N-Methyl-N-Alkylpyrrolidinium+TFSI- Ionic Liquids. J. Phys. Chem. B 2006, 110, 16879–16886. [Google Scholar] [CrossRef]
- Minami, I.; Kita, M.; Kubo, T.; Nanao, H.; Mori, S. The Tribological Properties of Ionic Liquids Composed of Trifluorotris(Pentafluoroethyl) Phosphate as a Hydrophobic Anion. Tribol. Lett. 2008, 30, 215–223. [Google Scholar] [CrossRef]
- Parajó, J.J.; Otero-Mato, J.M.; Lobo Ferreira, A.I.M.C.; Varela, L.M.; Santos, L.M.N.B.F. Enthalpy of Solvation of Alkali Metal Salts in a Protic Ionic Liquid: Effect of Cation Charge and Size. J. Mol. Liq. 2022, 360, 119228. [Google Scholar] [CrossRef]
- Shotwell, J.B.; Flowers II, R.A. Electrochemical Investigation of the Solvolytic Properties of Ethylammonium Nitrate (EAN) and Propylammonium Nitrate (PAN). Electroanalysis 2000, 12, 223–226. [Google Scholar] [CrossRef]
- Villanueva, M.; Vallet, P.; Teijeira, T.; Santiago, A.; Amigo, A.; Tojo, E.; Varela, L.M.; Parajó, J.J.; Salgado, J. Effect of the Alkyl Chain Length on Thermal Properties and Toxicity of N-Alkyl-Ammonium Nitrate Ionic Liquids (N = 2, 3, 4, 5, 6, 8) for Energy Applications. J. Therm. Anal. Calorim. 2024; submitted. [Google Scholar]
- Microbics, M. Microtox Manual—A Toxicity Handbook; Microbics Corporation. Inc.: Carlsbad, CA, USA, 1992; Volume I–IV. [Google Scholar]
- Environmental, A. Azur Environmental, Microtox Manual. Available online: https://www.modernwater.com/assets/Technical%20Support/Toxicity/Manuals/ACUTE%20User%27s%20Manual.pdf (accessed on 11 March 2024).
- Passino, D.R.M.; Smith, S.B. Smith Acute Bioassays and Hazard Evaluation of Representative Contaminants Detected in Great Lakes Fish. Environ. Toxicol. Chem. 1987, 6, 901–907. [Google Scholar] [CrossRef]
- Chang, S.C.; Wang, Y.F.; You, S.J.; Kuo, Y.M.; Tsai, C.H.; Wang, L.C.; Hsu, P.Y. Toxicity Evaluation of Fly Ash by Microtox®. Aerosol Air Qual. Res. 2013, 13, 1002–1008. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Gonçalves, A.M.M.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Ecotoxicity Analysis of Cholinium-Based Ionic Liquids to Vibrio Fischeri Marine Bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Perales, E.; García, C.B.; Lomba, L.; Aldea, L.; García, J.I.; Giner, B. Comparative Ecotoxicology Study of Two Neoteric Solvents: Imidazolium Ionic Liquid vs. Glycerol Derivative. Ecotoxicol. Environ. Saf. 2016, 132, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Mellado, N.; Ayuso, M.; Villar-Chavero, M.M.; García, J.; Rodríguez, F. Ecotoxicity Evaluation towards Vibrio Fischeri of Imidazolium- and Pyridinium-Based Ionic Liquids for Their Use in Separation Processes. SN Appl. Sci. 2019, 1, 896. [Google Scholar] [CrossRef]
- Ibrahim, M.; Mutalib, A.; Mutalib, A. Ecotoxicity of and Ionic Liquids Towards Vibrio Fischeri: Experimental and QSAR Studies. In Progress and Developments in Ionic Liquids; Handy, S., Ed.; Intech: London, UK, 2017; pp. 429–449. ISBN 978-953-51-2902-8. [Google Scholar]
- Ventura, S.P.M.; Gonçalves, A.M.M.; Sintra, T.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Designing Ionic Liquids: The Chemical Structure Role in the Toxicity. Ecotoxicology 2013, 22, 1–12. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Hidalgo, J.M.; Collado-González, M.; Díaz Baños, F.G.; Víllora, G. Assessing Chemical Toxicity of Ionic Liquids on Vibrio Fischeri: Correlation with Structure and Composition. Chemosphere 2016, 155, 405–414. [Google Scholar] [CrossRef]
- Viboud, S.; Papaiconomou, N.; Cortesi, A.; Chatel, G.; Draye, M.; Fontvieille, D. Correlating the Structure and Composition of Ionic Liquids with Their Toxicity on Vibrio Fischeri: A Systematic Study. J. Hazard. Mater. 2012, 215–216, 40–48. [Google Scholar] [CrossRef]
- Mena, I.F.; Diaz, E.; Palomar, J.; Rodriguez, J.J.; Mohedano, A.F. Cation and Anion Effect on the Biodegradability and Toxicity of Imidazolium- and Choline-Based Ionic Liquids. Chemosphere 2020, 240, 124947. [Google Scholar] [CrossRef]
- Grzonkowska, M.; Sosnowska, A.; Barycki, M.; Rybinska, A.; Puzyn, T. How the Structure of Ionic Liquid Affects Its Toxicity to Vibrio Fischeri? Chemosphere 2016, 159, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; White, B.T.; Kasprzak, C.R.; Long, T.E. Advances in Phosphonium-Based Ionic Liquids and Poly(Ionic Liquid)s as Conductive Materials. Eur. Polym. J. 2018, 108, 28–37. [Google Scholar] [CrossRef]
- Hernández Battez, A.; Bartolomé, M.; Blanco, D.; Viesca, J.L.; Fernández-González, A.; González, R. Phosphonium Cation-Based Ionic Liquids as Neat Lubricants: Physicochemical and Tribological Performance. Tribol. Int. 2016, 95, 118–131. [Google Scholar] [CrossRef]
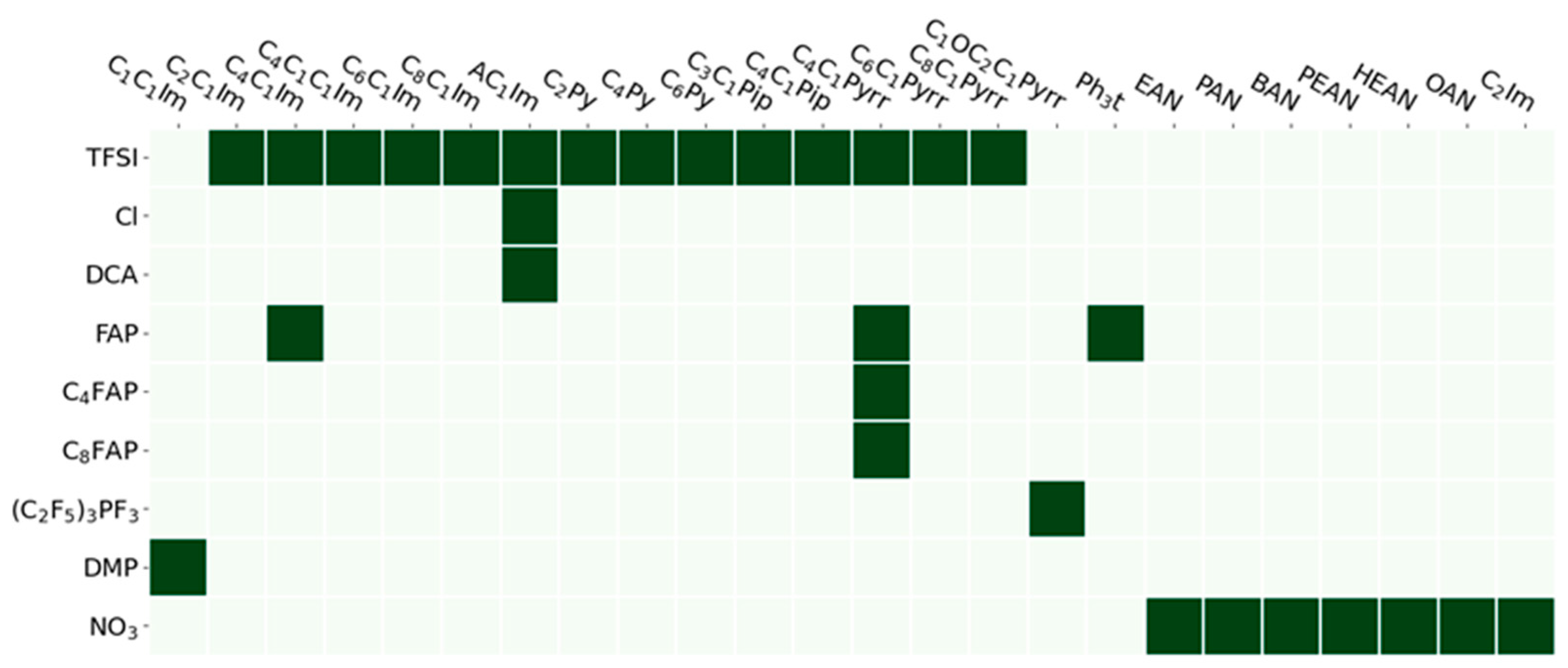
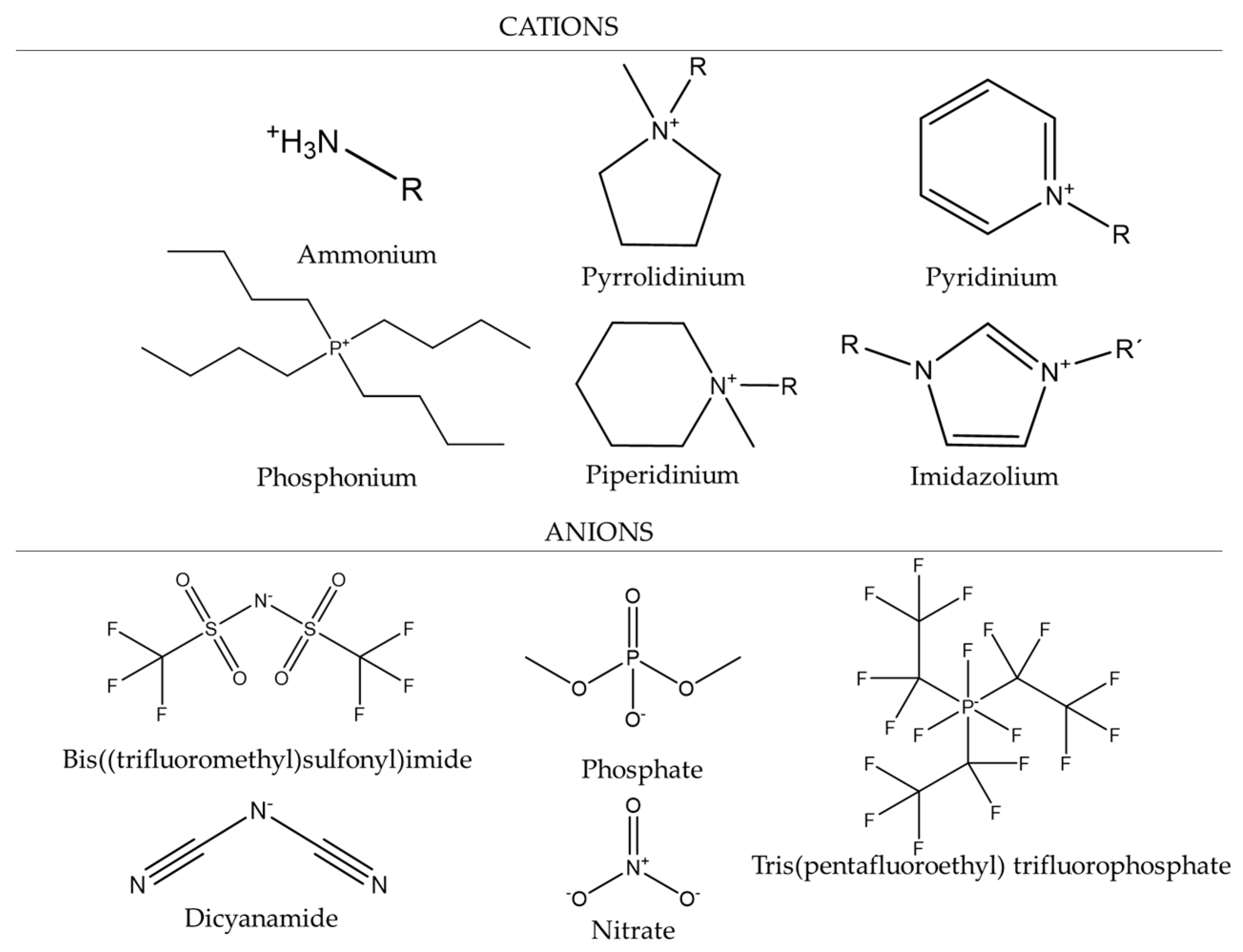

| Name Molecular Mass (g/mol) | Abbreviation CAS Number | Purity Molecular Mass (g/mol) |
|---|---|---|
| 1-ethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide | C2C1Im TFSI 174899-82-2 | >0.99 1 391.3 |
| 1-butyl-3- methylimidazolium bis((trifluoromethyl)sulfonyl)imide | C4C1Im TFSI 174899-83-3 | >0.99 1 419.4 |
| 1-butyl-2,3-dimethyl imidazolium bis(trifluoromethylsulfonyl)imide | C4C1C1Im TFSI 350493-08-2 | >0.99 1 433.39 |
| 1-hexyl-3- methylimidazolium bis((trifluoromethyl)sulfonyl)imide | C6C1Im TFSI 382150-50-7 | >0.99 1 447.4 |
| 1-octyl-3- methylimidazolium bis((trifluoromethyl)sulfonyl)imide | C8C1Im TFSI 178631-04-4 | >0.99 1 475.5 |
| 1-allyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)amide | AC1Im TFSI 655249-87-9 | >0.99 1 403.3 |
| 1-allyl-3-methylimidazolium chloride | AC1Im Cl 65039-10-3 | >0.98 1 158.6 |
| 1-allyl-3-methylimidazolium dicyanamide | AC1Im DCA 917956-73-1 | >0.99 1 190.2 |
| 1-butyl-3-methyl imidazolium tris(pentafluoroethyl) trifluorophosphate | C4C1Im FAP 917762-91-5 | >0.99 2 584.23 |
| 1,3-dimethylimidazolium dimethylphosphate | C1C1Im DMP 945611-27-8 | >0.99 1 222.18 |
| 1-ethylpyridinium bis((trifluoromethyl)sulfonyl)imide | C2Py TFSI 712354-97-7 | >0.99 1 388.3 |
| 1-butylpyridinium bis((trifluoromethyl)sulfonyl)imide | C4Py TFSI 187863-42-9 | >0.99 1 416.4 |
| 1-hexylpyridinium bis((trifluoromethyl)sulfonyl)imide | C6Py TFSI 460983-97-5 | >0.99 1 444.4 |
| 1-methyl-1-propylpiperidinium bis((trifluoromethyl)sulfonyl)imide | C3C1Pip TFSI 608140-12-1 | >0.99 1 422.4 |
| 1-methyl-1-butylpiperidinium bis((trifluoromethyl)sulfonyl)imide | C4C1Pip TFSI 623580-02-9 | >0.99 1 436.4 |
| 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide | C4C1Pyrr TFSI 223437-11-4 | >0.99 1 422.41 |
| 1-(2-methoxyethyl)-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate | C1OC2C1PyrrFAP 1195983-48-2 | >0.98 2 589.24 |
| 1-hexyl-1-methylpyrrolidinium bis((trifluoromethyl)sulfonyl)imide | C6C1Pyrr TFSI 380497-19-8 | >0.99 1 450.5 |
| 1-octyl-1-methylpyrrolidinium bis((trifluoromethyl)sulfonyl)imide | C8C1Pyrr TFSI 927021-43-0 | >0.99 1 478.5 |
| 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl) trifluorophosphate | C4C1Pyrr FAP 851856-47-8 | >0.98 2 587.28 |
| 1-butyl-1-methylpyrrolidinium tris(nonafluorobutyl) trifluorophosphate | C4C1Pyrr C4FAP 851856-47-8 | >0.99 2 830.32 |
| 1-butyl-1-methylpyrrolidinium tris(perfluorooctyl) trifluorophosphate | C4C1Pyrr C8FAP --- | >0.98 2 1430.41 |
| Tetrabutylphosphonium tris(pentafluoroethyl) trifluorophosphate | Ph3t FAP 482635-81-4 | >0.98 2 704.36 |
| Propylammonium Nitrate | EAN 22113-86-6 | >0.97 1 108.10 |
| Butylammonium Nitrate | PAN 22113-88-8 | >0.97 1 122.12 |
| Ethylammonium Nitrate | BAN 58888-50-9 | >0.97 1 136.15 |
| Penthylammonium nitrate | PEAN --- | ≥99 3 150.18 |
| Hexylammonium nitrate | HEAN --- | ≥99 3 164.20 |
| Octylammonium nitrate | OAN --- | ≥99 3 192.25 |
| Ethylimidazolium nitrate | C2Im NO3 501693-38-5 | >0.98 1 159.14 |
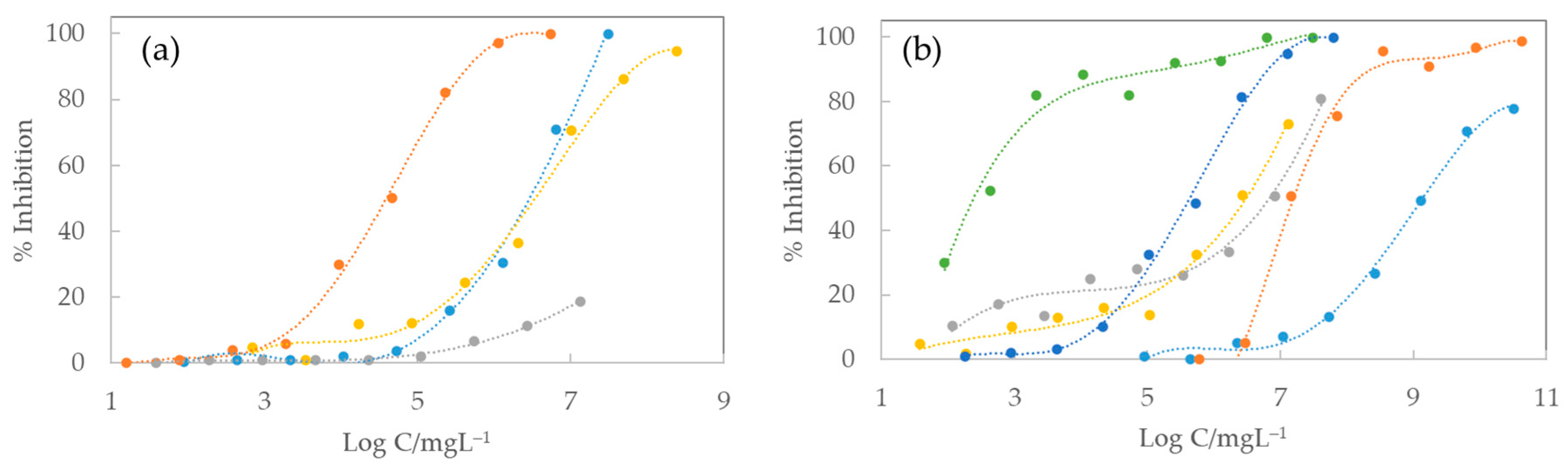
| IL | EC50 5 min/mg/L (Lower; Upper) Limits | EC50 15 min/mg/L (Lower; Upper) Limits | EC50 30 min/mg/L (Lower; Upper) Limits |
|---|---|---|---|
| C2C1Im TFSI | 367.43 (247.06; 487.80) | 189.97 (104.68; 275.27) | 113.08 (41.74; 184.42) |
| C4C1Im TFSI | 78.48 (36.48; 120.48) | 54.85 (24.91; 84.79) | 46.58 (14.68; 78.48) |
| C4C1C1Im TFSI [12] | 150.44 (72.43; 228.49) | 113.32 (82.29; 144.35) | 98.70 (82.39; 115.01) |
| C6C1Im TFSI | 26.29 (24.00; 28.57) | 23.64 (21.83; 25.45) | 29.60 (27.66; 31.53) |
| C8C1Im TFSI | 3.57 (3.43; 3.71) | 4.23 (3.86; 4.59) | 5.97 (4.85; 7.10) |
| AC1Im TFSI | 655.63 (417.77; 893.49) | 337.68 (235.35; 440.01) | 249.30 (175.09; 323.52) |
| AC1Im Cl | 1399.39 (463.05; 2335.73) | 842.87 (531.77; 1153.97) | 715.21 (479.39; 951.03) |
| AC1Im DCA | 1181.58 (866.64; 1496.51) | 639.62 (538.85; 740.39) | 534.19 (454.01; 614.38) |
| C4C1Im FAP | 97.64 (70.21; 125.1) | 77.43 (59.59; 95.27) | 74.37 (58.05; 90.69) |
| C1C1Im DMP | 1186.31 (1071.08; 1300.92) | 1198.33 (1111.72; 1285.07) | 1254.24 (1172.95; 1337.75) |
| C2Py TFSI | 314.24 (175.08; 453.40) | 133.72 (54.34; 213.11) | 74.31 (0.00; 150.51) |
| C4Py TFSI | 150.21 (133.75; 166.66) | 106.84 (93.51; 120.17) | 92.90 (78.26; 107.54) |
| C6Py TFSI | 44.16 (40.40; 47.92) | 40.30 (36.29; 44.30) | 45.84 (41.92; 49.76) |
| C3C1Pip TFSI | 215.24 (161.47; 269.01) | 138.13 (104.77; 171.49) | 117.07 (82.16; 151.98) |
| C4C1Pip TFSI | 150.63 (140.30; 160.95) | 119.23 (110.40; 128.07) | 107.37 (101.19; 113.56) |
| C4C1Pyrr TFSI [12] | 1463.91 (1162.13; 1765.69) | 964.58(791.32; 1137.88) | 714.43 (577.92; 851.21) |
| C6C1Pyrr TFSI | 88.95 (79.22; 98.69) | 70.29 (62.57; 78.00) | 75.26 (66.25; 84.27) |
| C8C1Pyrr TFSI | 15.71 (13.10; 18.31) | 15.80 (13.03; 18.57) | 23.30 (16.95; 30.25) |
| C4C1Pyrr FAP | 805.87 (554.86; 1056.83) | 707.70 (562.28; 853.11) | 604.90 (516.64; 693.16) |
| C1OC2C1Pyrr FAP | 62.37 (31.43; 93.31) | 37.94 (23.69; 52.19) | 31.59 (22.00; 41.19) |
| C4C1Pyrr C4FAP | 96.75 (71.83; 121.66) | 62.28 (36.83; 87.74) | 50.54 (22.51; 78.57) |
| C4C1Pyrr C8FAP | -- | -- | 5430.07 (1845.01; 8224.73) |
| Ph3t FAP | 3555.25 (2429.44; 4605.18) | 1096.36 (569.37; 1623.35) | 805.63 (417.84; 1193.42) |
| EAN [12] | 12,582.07 (8186.64; 16977.50) | 10,665.47 (6650.14; 14680.80) | 9711.63 (6561.46; 12860.79) |
| PAN [23] | 8314.99 (7268.61; 9361.37) | 5932.88 (5043.45; 6822.30) | 5827.78 (4998.72; 6656.84) |
| BAN [23] | 1491.99 (636.69; 2347.04) | 1066.71 (551.52; 1581.90) | 1017.14 (478.49; 1555.78) |
| PEAN [23] | 1116.9 (945.1; 1288.8) | 1073.6 (836.3; 1311.0) | 1029.8 (792.5; 1267.1) |
| HEAN [23] | 85.69 (77.71; 93.68) | 57.54 (52.98; 62.10) | 50.12 (44.85; 55.39) |
| OAN [23] | 9.70 (6.37; 13.03) | 7.33 (5.23; 9.43) | 7.38 (5.51; 9.25) |
| C2Im NO3 [12] | 612.55 (395.90; 828.01) | 573.77 (372.29; 774.55) | 597.89 (408.00; 785.08) |
| IL | EC20 5 min/mg/L (Lower; Upper) Limits | EC20 15 min/mg/L (Lower; Upper) Limits | EC20 30 min/mg/L (Lower; Upper) Limits |
|---|---|---|---|
| C2C1Im TFSI | 97.11 (37.29; 156.94) | 43.33 (7.85; 78.81) | 22.82 (0.00; 48.22) |
| C4C1Im TFSI | 19.51 (1.67; 37.35) | 13.45 (1.05; 25.86) | 10.50 (0.00; 22.63) |
| C4C1C1Im TFSI [12] | 46.34 (5.74; 86.95) | 39.09 (20.78; 57.40) | 36.45 (26.05; 46.85) |
| C6C1Im TFSI | 12.60 (10.59; 14.60) | 12.43 (10.69; 14.17) | 17.15 (15.22; 19.08) |
| C8C1Im TFSI | 1.49 (1.37; 1.60) | 1.77 (1.48; 2.06) | 2.50 (1.68; 3.33) |
| AC1Im TFSI | 110.00 (46.34; 173.66) | 68.95 (27.41; 110.49) | 61.21 (25.35; 97.07) |
| AC1Im Cl | 376.21 (0.00; 797.78) | 310.13 (106.25; 514.02) | 268.03 (108.68; 427.38) |
| AC1Im DCA | 436.58 (262.10; 611.06) | 309.55 (225.00;394.10) | 291.63 (217.70; 365.56) |
| C4C1Im FAP | 24.57 (12.19; 36.94) | 21.24 (13.01; 29.47) | 19.05 (11.28; 28.62) |
| C1C1Im DMP | 917.35 (839.85; 994.63) | 970.14 (914.78; 1026.19) | 997.02 (945.86; 1050.47) |
| C2Py TFSI | 54.17 (9.12; 99.22) | 26.67 (0.00; 54.39) | 12.37 (0.00; 34.03) |
| C4Py TFSI | 64.42 (52.13; 76.71) | 45.45 (35.55; 55.35) | 39.90 (28.93; 50.87) |
| C6Py TFSI | 21.69 (18.49; 24.88) | 20.00 (16.57; 23.44) | 24.66 (21.03; 28.29) |
| C3C1Pip TFSI | 66.74 (36.70; 96.77) | 46.42 (26.57; 66.26) | 40.91 (19.42; 62.41) |
| C4C1Pip TFSI | 67.57 (59.56; 75.58) | 57.01 (49.72; 64.29) | 55.22 (49.76; 60.69) |
| C4C1Pyrr TFSI [12] | 684.04 (441.90; 926.09) | 416.73 (286.18; 545.93) | 289.18 (192.91; 386.85) |
| C6C1Pyrr TFSI | 39.71 (32.17; 47.25) | 33.50 (27.14; 39.87) | 41.44 (32.94;49.95) |
| C8C1Pyrr TFSI | 6.84 (4.87; 8.81) | 7.51 (5.22; 9.80) | 13.36 (7.02; 19.70) |
| C4C1Pyrr FAP | 428.33 (201.78; 654.88) | 385.65 (252.92; 518.38) | 337.60 (256.32; 418.89) |
| C1OC2C1Pyrr FAP | 20.47 (2.84; 38.10) | 13.00 (4.67; 21.33) | 11.36 (5.71; 17.01) |
| C4C1Pyrr C4FAP | 49.88 (28.06; 71.70) | 28.72 (8.44; 49.09) | 21.95 (1.27; 42.63) |
| C4C1Pyrr C8FAP | -- | -- | 1267.78 (446.89; 2088.66) |
| Ph3t FAP | 959.49 (513.08; 1045.91) | 402.74(151.78; 653.70) | 324.91 (37.84; 611.99) |
| EAN [12] | 4314.31 (1548.95; 7081.66) | 3236.68 (951.77; 5522.60) | 3012.33 (1264.99; 4761.67) |
| PAN [23] | 4309.57 (3391.12; 5228.02) | 3116.85 (2332.68; 3901.02) | 3301.43 (2521.37; 4081.48) |
| BAN [23] | 326.04 (0.00; 669.80) | 318.35 (30.59; 606.11) | 287.02 (0.00; 575.25) |
| PEAN [23] | 381.72 (274.82; 488.63) | 351.09 (208.36; 493.83) | 346.65 (200.17; 493.12) |
| HEAN [23] | 49.72 (42.04; 57.40) | 34.67 (29.90; 39.43) | 32.30 (26.60; 38.01) |
| OAN [23] | 4.24 (1.70; 6.78) | 3.85 (1.93; 5.78) | 5.02 (2.61; 7.43) |
| C2Im NO3 [12] | 195.44 (79.12; 312.90) | 194.19 (79.98; 310.53) | 223.45 (105.10; 342.82) |
| IL | EC10 5 min/mg/L (Lower; Upper) Limits | EC10 15 min/mg/L (Lower; Upper) Limits | EC10 30 min/mg/L (Lower; Upper) Limits |
|---|---|---|---|
| C2C1Im TFSI | 44.54 (7.06; 82.03) | 18.24 (0.07; 38.18) | 8.94 (0.02; 22.05) |
| C4C1Im TFSI | 8.63 (0.00; 18.97) | 5.91 (0.00; 13.03) | 4.39 (0.00; 11.01) |
| C4C1C1Im TFSI [12] | 23.25 0.00; 50.07) | 20.96 (7.96; 33.95) | 20.34(12.63; 28.05) |
| C6C1Im TFSI | 8.19 (6.42; 9.96) | 8.53 (6.91;10.16) | 12.46 (10.54; 14.37) |
| C8C1Im TFSI | 0.88 (0.80; 0.98) | 1.07 (0.83; 1.30) | 1.50 (0.84; 2.17) |
| AC1Im TFSI | 38.67 (2.85; 74.48) | 27.19 (3.07; 51.31) | 26.89 (4.41; 49.37) |
| AC1Im Cl | 174.31 (0.00; 435.31) | 172.68 (17.45; 327.90) | 150.85 (28.69; 273.00) |
| AC1Im DCA | 243.68 (91.39; 395.97) | 202.36 (124.95; 279.78) | 204.59 (133.20; 275.98) |
| C4C1Im FAP | 10.95 (3.62; 18.28) | 10.19 (4.96; 15.43) | 8.38 (3.86; 12.89) |
| C1C1Im DMP | 763.66 (679.73; 846.46) | 847.12 (794.31; 921.86) | 901.85 (845.03; 958.37) |
| C2Py TFSI | 39.23 (29.18; 49.29) | 27.55 (19.52; 35.59) | 24.32 (15.38; 33.26) |
| C4Py TFSI | 19.35 (0.00; 40.98) | 10.38 (0.00; 24.55) | 4.33 (0.00; 14.24) |
| C6Py TFSI | 14.30 (11.49; 17.11) | 13.27 (10.23; 16.31) | 17.15 (13.77; 20.53) |
| C3C1Pip TFSI | 42.26 (35.50; 49.01) | 37.00 (30.62; 43.38) | 37.41 (32.40; 42.41) |
| C4C1Pip TFSI | 33.61 (13.56; 53.97) | 24.51 (10.51;38.50) | 22.10 (6.61; 37.60) |
| C4C1Pyrr TFSI [12] | 438.08 (225.18; 650.98) | 254.32 (146.51; 362.18) | 170.23 (93.44; 247.12)) |
| C6C1Pyrr TFSI | 24.76 (18.44; 31.09) | 21.71 (16.15; 27.26) | 29.22 (21.09; 37.36) |
| C8C1Pyrr TFSI | 4.20 (2.59; 5.82) | 4.86 (2.88; 6.84) | 9.57 (3.44; 15.71) |
| C4C1Pyrr FAP | 295.81 (79.77; 511.84) | 270.28 (142.70; 397.86) | 23.992 (161.01; 318.83) |
| C1OC2C1Pyrr FAP | 10.66 (0.81; 19.79) | 6.94 (1.09; 12.79) | 6.24 (2.26; 10.22) |
| C4C1Pyrr C4FAP | 33.83 (13.75; 53.92) | 18.26 (2.88; 33.62) | 13.47 (1.04; 24.25) |
| C4C1Pyrr C8FAP | -- | -- | 540.79 (114.86; 1000.97) |
| Ph3t FAP | 445.54 (162.01; 729.08) | 224.03 (15.24; 457.16) | 190.91 (3.87; 305.99) |
| EAN [12] | 2304.89 (248.43; 4361.05) | 1609.79 (560.06; 3163.56) | 1517.65 (332.07; 2703.22) |
| PAN [23] | 2932.68 (2072.90; 3792.46) | 2138.10 (1402.95; 2873.26) | 2366.64 (1600.94; 3132.34) |
| BAN [23] | 136.59 (0.00; 354.65) | 160.30 (0.00; 369.51) | 139.74 (0.00; 341.90) |
| PEAN [23] | 203.54 (121.38; 285.71) | 182.43 (75.64; 289.23) | 183.21 (72.35; 294.07) |
| HEAN [23] | 36.14 (28.64; 43.65) | 25.76 (20.96; 30.57) | 24.98 (19.07; 30.89) |
| OAN [23] | 2.62 (0.53; 4.71) | 2.64 (0.87; 4.42) | 4.00 (1.36; 0.66) |
| C2Im NO3 [12] | 100.10 (21.33; 179.99) | 103.80 (22.16; 184.19) | 127.39 (37.59; 214.25) |
| IL | Passino and Smith [26] | Chang et al. [27] |
|---|---|---|
| C2C1Im TFSI | Practically harmless | Non-toxic |
| C4C1Im TFSI | Toxic | Toxic |
| C4C1C1Im TFSI [12] | Practically harmless | Non-toxic |
| C6C1Im TFSI | Toxic | Toxic |
| C8C1Im TFSI | Toxic | Very toxic |
| AC1Im TFSI | Practically harmless | Non-toxic |
| AC1Im Cl | Relatively harmless | Non-toxic |
| AC1Im DCA | Relatively harmless | Non-toxic |
| C4C1Im FAP | Toxic | Toxic |
| C1C1Im DMP | Relatively harmless | Non-toxic |
| C2Py TFSI | Practically harmless | Non-toxic |
| C4Py TFSI | Practically harmless | Non-toxic |
| C6Py TFSI | Toxic | Toxic |
| C3C1Pip TFSI | Practically harmless | Non-toxic |
| C4C1Pip TFSI | Practically harmless | Non-toxic |
| C4C1Pyrr TFSI [12] | Relatively harmless | Non-toxic |
| C6C1Pyrr TFSI | Toxic | Toxic |
| C8C1Pyrr TFSI | Toxic | Toxic |
| C4C1Pyrr FAP | Practically harmless | Non-toxic |
| C1OC2C1Pyrr FAP | Toxic | Toxic |
| C4C1Pyrr C4FAP | Toxic | Toxic |
| C4C1Pyrr C8FAP | -- | -- |
| Ph3t FAP | Relatively harmless | Non-toxic |
| EAN [12] | Relatively harmless | Non-toxic |
| PAN [23] | Practically harmless | Toxic |
| BAN [23] | Practically harmless | Toxic |
| PEAN [23] | Relatively harmless | Non-toxic |
| HEAN [23] | Toxic | Toxic |
| OAN [23] | Toxic | Very toxic |
| C2Im NO3 [12] | Practically harmless | Non-toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parajó, J.J.; Santiago-Alonso, A.; Vallet, P.; Teijeira, T.; Emeterio, R.S.; Villanueva, M.; Salgado, J. Comprehensive Analysis of the Acute Toxicity of Ionic Liquids Using Microtox® Bioassays. Appl. Sci. 2024, 14, 2480. https://doi.org/10.3390/app14062480
Parajó JJ, Santiago-Alonso A, Vallet P, Teijeira T, Emeterio RS, Villanueva M, Salgado J. Comprehensive Analysis of the Acute Toxicity of Ionic Liquids Using Microtox® Bioassays. Applied Sciences. 2024; 14(6):2480. https://doi.org/10.3390/app14062480
Chicago/Turabian StyleParajó, Juan José, Antía Santiago-Alonso, Pablo Vallet, Tamara Teijeira, Raquel San Emeterio, María Villanueva, and Josefa Salgado. 2024. "Comprehensive Analysis of the Acute Toxicity of Ionic Liquids Using Microtox® Bioassays" Applied Sciences 14, no. 6: 2480. https://doi.org/10.3390/app14062480
APA StyleParajó, J. J., Santiago-Alonso, A., Vallet, P., Teijeira, T., Emeterio, R. S., Villanueva, M., & Salgado, J. (2024). Comprehensive Analysis of the Acute Toxicity of Ionic Liquids Using Microtox® Bioassays. Applied Sciences, 14(6), 2480. https://doi.org/10.3390/app14062480







