The Impact of High-Intensity Interval Exercise Including Acceleration/Deceleration Patterns on Redox Status of Healthy Male Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
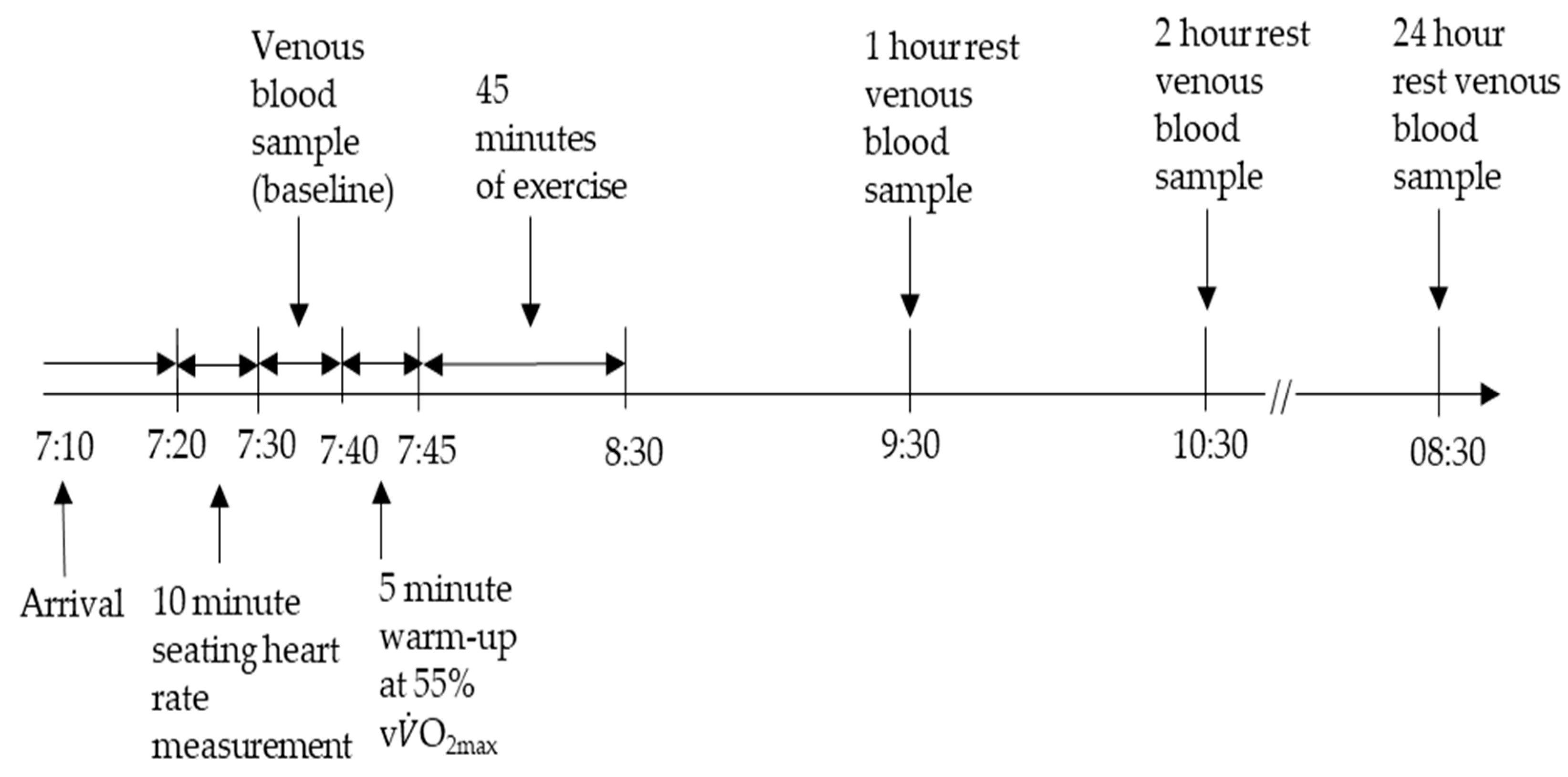
2.2. Experimental Design
2.3. Exercise Protocols
2.4. Haematology
2.5. Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Impact of Intermittency
3.2. Impact of Acceleration/Deceleration
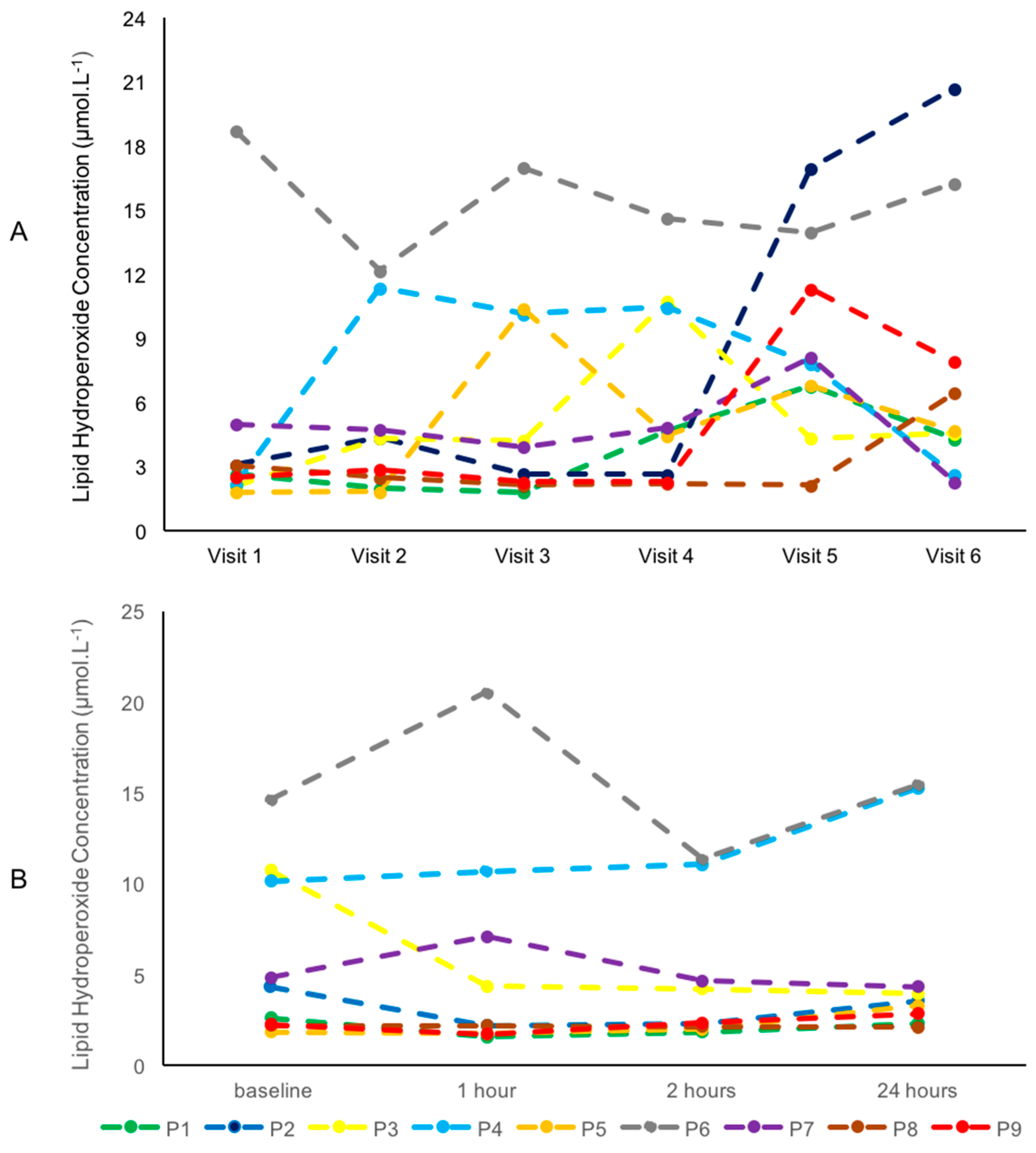
3.3. Intra-Individual Variability of Blood Metabolites
4. Discussion
4.1. Impact of Intermittency
4.2. Impact of Acceleration/Deceleration
4.3. Intra-Individual Variability in Blood Metabolites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Interplay of oxidants and antioxidants during exercise: Implications for muscle health. Phys. Sportsmed. 2009, 37, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007, 102, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A.; Ugbolue, U.C.; He, Y.; Meng, Y.; Gu, Y. Effect of Running Exercise on Oxidative Stress Biomarkers: A Systematic Review. Front. Physiol. 2020, 11, 610112. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bell, H.K.; Bloomer, R.J. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid. Med. Cell. Longev. 2009, 2, 43–51. [Google Scholar] [CrossRef]
- Powers, S.K.; Goldstein, E.; Schrager, M.; Ji, L.L. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants 2022, 12, 39. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Borras, C.; Pallardo, F.V.; Sastre, J.; Ji, L.L.; Vina, J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005, 567, 113–120. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Staib, J.L.; Phillips, T.; Hess, A.; Lennon, S.L.; Powers, S.K. Exercise, antioxidants, and HSP72: Protection against myocardial ischemia/reperfusion. Free Radic. Biol. Med. 2003, 34, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallen, J.; Ronnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Ostgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef]
- Powers, S.K.; Schrager, M. Redox signaling regulates skeletal muscle remodeling in response to exercise and prolonged inactivity. Redox Biol. 2022, 54, 102374. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Andrade, F.H.; Reid, M.B.; Westerblad, H. Contractile response of skeletal muscle to low peroxide concentrations: Myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001, 15, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Mollica, J.P.; Dutka, T.L.; Merry, T.L.; Lamboley, C.R.; McConell, G.K.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012, 590, 1443–1463. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. Effects of High Intensity Exercise on Oxidative Stress and Antioxidant Status in Untrained Humans: A Systematic Review. Biology 2021, 10, 1272. [Google Scholar] [CrossRef]
- Draeger, C.L.; Naves, A.; Marques, N.; Baptistella, A.B.; Carnauba, R.A.; Paschoal, V.; Nicastro, H. Controversies of antioxidant vitamins supplementation in exercise: Ergogenic or ergolytic effects in humans? J. Int. Soc. Sports Nutr. 2014, 11, 4. [Google Scholar] [CrossRef]
- Atakan, M.M.; Li, Y.; Kosar, S.N.; Turnagol, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. [Google Scholar] [CrossRef]
- Thompson, W.R. WORLDWIDE SURVEY OF FITNESS TRENDS FOR 2016: 10th Anniversary Edition. ACSM’s Health Fit. J. 2015, 19, 9–18. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kessler, H.S.; Sisson, S.B.; Short, K.R. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012, 42, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Tschakert, G.; Hofmann, P. High-intensity intermittent exercise: Methodological and physiological aspects. Int. J. Sports Physiol. Perform. 2013, 8, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.; Bagatini, M.D.; Roth, M.A.; Martins, C.C.; Rezer, J.F.; Mello, F.F.; Lopes, L.F.; Morsch, V.M.; Schetinger, M.R. Acute effects of resistance exercise and intermittent intense aerobic exercise on blood cell count and oxidative stress in trained middle-aged women. Braz. J. Med. Biol. Res. 2012, 45, 1172–1182. [Google Scholar] [CrossRef]
- Kingsley, M.I.; Wadsworth, D.; Kilduff, L.P.; McEneny, J.; Benton, D. Effects of phosphatidylserine on oxidative stress following intermittent running. Med. Sci. Sports Exerc. 2005, 37, 1300–1306. [Google Scholar] [CrossRef]
- Thompson, D.; Williams, C.; Kingsley, M.; Nicholas, C.W.; Lakomy, H.K.; McArdle, F.; Jackson, M.J. Muscle soreness and damage parameters after prolonged intermittent shuttle-running following acute vitamin C supplementation. Int. J. Sports Med. 2001, 22, 68–75. [Google Scholar] [CrossRef]
- Campbell, W.W.; Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L.; et al. High-Intensity Interval Training for Cardiometabolic Disease Prevention. Med. Sci. Sports Exerc. 2019, 51, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Currie, K.D.; Dizonno, V.; Oh, P.I.; Goodman, J.M. Acute physiological responses to high-intensity interval exercise in patients with coronary artery disease. Eur. J. Appl. Physiol. 2023, 123, 737–747. [Google Scholar] [CrossRef]
- Levinger, I.; Shaw, C.S.; Stepto, N.K.; Cassar, S.; McAinch, A.J.; Cheetham, C.; Maiorana, A.J. What Doesn’t Kill You Makes You Fitter: A Systematic Review of High-Intensity Interval Exercise for Patients with Cardiovascular and Metabolic Diseases. Clin. Med. Insights Cardiol. 2015, 9, 53–63. [Google Scholar] [CrossRef]
- Forster, H.V.; Dempsey, J.A.; Thomson, J.; Vidruk, E.; DoPico, G.A. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J. Appl. Physiol. 1972, 32, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Midgley, A.W.; McNaughton, L.R.; Wilkinson, M. Is there an Optimal Training Intensity for Enhancing the Maximal Oxygen Uptake of Distance Runners? Sports Med. 2006, 36, 117–132. [Google Scholar] [CrossRef]
- Vieira-Souza, L.M.; Aidar, F.J.; Matos, D.G.d.; Silva, A.N.d.; Miguel-dos-Santos, R.; Santos, J.L.d.; Costa, R.d.A.; Marçal, A.C.; Lauton-Santos, S.; Cabral, B.G.d.A.T.; et al. Short-term hiit does not promote oxidative stress or muscle damage. Rev. Bras. Med. Esporte 2021, 27, 138–141. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Goldfarb, A.H.; Wideman, L.; McKenzie, M.J.; Consitt, L.A. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 2005, 19, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Theodorou, A.A.; Panayiotou, G.; Zafeiridis, A.; Dipla, K.; Nikolaidis, M.G.; Vrabas, I.S. Reductive stress after exercise: The issue of redox individuality. Redox Biol. 2014, 2, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Camus, G.; Felekidis, A.; Pincemail, J.; Deby-Dupont, G.; Deby, C.; Juchmes-Ferir, A.; Lejeune, R.; Lamy, M. Blood levels of reduced/oxidized glutathione and plasma concentration of ascorbic acid during eccentric and concentric exercises of similar energy cost. Arch. Int. Physiol. Biochim. Biophys. 1994, 102, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Falvo, M.J.; Fry, A.C.; Schilling, B.K.; Smith, W.A.; Moore, C.A. Oxidative stress response in trained men following repeated squats or sprints. Med. Sci. Sports Exerc. 2006, 38, 1436–1442. [Google Scholar] [CrossRef]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Morrow, J.D.; Utter, A.C.; Dumke, C.L. Effect of resistance exercise and carbohydrate ingestion on oxidative stress. Free Radic. Res. 2005, 39, 1219–1224. [Google Scholar] [CrossRef]
- Quindry, J.; Miller, L.; McGinnis, G.; Irwin, M.; Dumke, C.; Magal, M.; Triplett, N.T.; McBride, J.; Urbiztondo, Z. Muscle-fiber type and blood oxidative stress after eccentric exercise. Int. J. Sport. Nutr. Exerc. Metab. 2011, 21, 462–470. [Google Scholar] [CrossRef]
- Quindry, J.C.; Stone, W.L.; King, J.; Broeder, C.E. The effects of acute exercise on neutrophils and plasma oxidative stress. Med. Sci. Sports Exerc. 2003, 35, 1139–1145. [Google Scholar] [CrossRef]
- Bailey, D.M.; Young, I.S.; McEneny, J.; Lawrenson, L.; Kim, J.; Barden, J.; Richardson, R.S. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1689–H1699. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, F.; Jelinek, H.F.; Perkins, S.; Al-Aubaidy, H.A.; de Jong, B.; Butkowski, E. Acute-Phase Inflammatory Response to Single-Bout HIIT and Endurance Training: A Comparative Study. Mediat. Inflamm. 2016, 2016, 5474837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Budkowska, M.; Cecerska-Heryc, E.; Marcinowska, Z.; Siennicka, A.; Dolegowska, B. The Influence of Circadian Rhythm on the Activity of Oxidative Stress Enzymes. Int. J. Mol. Sci. 2022, 23, 14275. [Google Scholar] [CrossRef] [PubMed]
- Kliszczewicz, B.; Quindry, C.J.; Blessing, L.D.; Oliver, D.G.; Esco, R.M.; Taylor, J.K. Acute Exercise and Oxidative Stress: CrossFit vs. Treadmill Bout. J. Hum. Kinet. 2015, 47, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, I.A.; Vuorimaa, T.; Ahotupa, M.; Vasankari, T.J. Strenuous physical exercise accelerates the lipid peroxide clearing transport by HDL. Eur. J. Appl. Physiol. 2016, 116, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Chen, Y.W.; Lip, G.Y.; Fisher, J.P.; Aldred, S. Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J. Sports Sci. 2016, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal 2013, 19, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Reactive oxygen species: Destroyers or messengers? Biochem. Pharmacol. 2009, 77, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.W.; Ashton, T.; McEneny, J.; Young, I.S.; Davies, B.; Bailey, D.M. Critical difference applied to exercise-induced oxidative stress: The dilemma of distinguishing biological from statistical change. J. Physiol. Biochem. 2012, 68, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Experimental verification of regression to the mean in redox biology: Differential responses to exercise. Free Radic. Res. 2016, 50, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics | Value (±SD) |
|---|---|
| Age (y) | 21 ± 3 |
| Height (cm) | 180 ± 4 |
| Body mass (kg) | 79.4 ± 7.9 |
| Body fat (%) | 12 ± 3 |
| Systolic BP (mmHg) | 133 ± 10 |
| Diastolic BP (mmHg) | 71 ± 6 |
| Resting HR (beats·min−1) | 58 ± 13 |
| Estimated HRmax (beats·min−1) | 192 ± 2 |
| Peak HR (beats·min−1) | 183 ± 9 |
| vO2max (km·hr−1) | 14 ± 6 |
| O2max (mL·kg−1·min−1) | 52.0 ± 2 |
| (A) | ||||
|---|---|---|---|---|
| Lipid Hydroperoxides (μmol·L−1) | ||||
| Exercise Protocol | Baseline | 1 h | 2 h | 24 h |
| Control | 4.35 (2.28–10.16) | 2.20 (1.77–7.09) | 2.36 (2.4–4.67) | 3.55 (2.85–4.33) |
| Low intermittent | 4.22 (2.88–4.68) | 4.68 (2.41–13.00) | 4.56 (3.16–10.98) | 4.39 (2.49–9.74) |
| Moderately Intermittent | 2.51 (2.08–4.73) | 2.57 (2.30–7.62) | 2.69 (2.53–8.88) | 2.23 (2.00–7.79) |
| Highly Intermittent | 3.92 (2.01–10.39) | 3.79 (2.01–8.97) | 3.94 (2.30–8.78) | 3.72 (2.23–4.00) |
| (B) | ||||
| GSH (μΜ) | ||||
| Exercise Protocol | Baseline | 1 h | 2 h | 24 h |
| Control | 1.44 (0.78–1.91) | 1.49 (0.95–2.17) | 1.80 (1.37–2.87) | 1.64 (1.16–1.98) |
| Low intermittent | 0.96 (0.73–1.04) | 1.76 (1.03–2.61) | 1.54 (1.37–2.68) | 1.69 (1.58–1.72) |
| Moderately Intermittent | 1.51 (1.11–2.39) | 1.80 (1.38–2.35) | 2.04 (1.59–2.89) | 1.66 (1.38–2.36) |
| Highly Intermittent | 1.26 (0.84–1.71) | 1.79 (1.06–1.94) | 1.54 (1.35–1.65) | 0.99 (0.90–1.86) |
| (C) | ||||
| Superoxide Dismutase (U/mL) | ||||
| Exercise Protocol | Baseline | 1 h | 2 h | 24 h |
| Control | 1.15 (0.94–2.45) | 1.33 (1.17–2.85) | 1.32 (1.03–2.65) | 1.22 (1.07–3.03) |
| Low intermittent | 0.95 (0.95–1.29) | 1.51 (0.83–1.73) | 1.02 (1.00–1.19) | 1.11 (0.94–1.70) |
| Moderately Intermittent | 1.01 (0.99–1.83) | 1.39 (0.99–1.89) | 1.28 (1.09–2.03) | 1.41 (0.81–1.90) |
| Highly Intermittent | 1.20 (1.07–1.38) | 1.11 (0.97–1.28) | 1.15 (0.85–1.20) | 1.19 (0.93–1.39) |
| (A) | ||||
|---|---|---|---|---|
| Lipid Hydroperoxides (μmol·L−1) | ||||
| Exercise Protocol | Baseline | 1 h | 2 h | 24 h |
| Control | 4.35 (2.28–10.16) | 2.20 (1.77–7.09) | 2.36 (2.14–4.67) | 3.55 (2.85–4.33) |
| Low acceleration | 4.68 (4.31–8.13) | 4.95 (4.13–7.02) | 4.65 (4.23–7.84) | 6.69 (3.85–6.87) |
| Moderate acceleration | 6.81 (6.44–11.32) | 6.22 (4.72–13.27) | 4.85 (4.58–9.59) | 4.79 (4.25–9.80) |
| High acceleration | 2.51 (2.08–4.73) | 2.57 (2.30–7.62) | 2.69 (2.53–8.88) | 2.23 (2.00–7.79) |
| (B) | ||||
| GSH (μΜ) | ||||
| Exercise Protocol | Baseline | 1 h | 2 h | 24 h |
| Control | 1.44 (0.78–1.91) | 1.49 (0.95–2.17) | 1.80 (1.37–2.87) | 1.64 (1.16–1.98) |
| Low acceleration | 1.63 (1.43–2.20) | 1.44 (0.86–2.11) | 1.61(1.17–1.93) | 2.15 (1.30–3.43) |
| Moderate acceleration | 2.24 (2.08–2.78) | 2.01 (1.40–2.59) | 3.07 (2.16–3.24) | 1.98 (1.76–2.96) |
| High acceleration | 1.51 (1.11–2.39) | 1.80 (1.38–2.35) | 2.04 (1.59–2.89) | 1.66 (1.38–2.36) |
| (C) | ||||
| Superoxide Dismutase (U/mL) | ||||
| Exercise Protocol | Baseline | 1 h | 2 h | 24 h |
| Control | 1.15 (0.94–2.45) | 1.33 (1.17–2.85) | 1.32 (1.03–2.65) | 1.22 (1.07–3.03) |
| Low acceleration | 1.26 (1.04–3.29) | 1.27 (1.01–4.28) | 1.45 (1.14–2.85) | 1.57 (1.30–3.63) |
| Moderate acceleration | 3.05 (1.23–3.59) | 2.56 (1.44–3.53) | 2.65 (1.51–3.26) | 2.76 (1.21–3.38) |
| High acceleration | 1.04 (1.04–3.29) | 1.39 (1.01–4.28) | 1.28 (1.14–2.85) | 1.41 (1.30–3.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalari, E.; Jones, H.S.; Hadjicharalambous, M.; Fogarty, M.C. The Impact of High-Intensity Interval Exercise Including Acceleration/Deceleration Patterns on Redox Status of Healthy Male Adults. Appl. Sci. 2024, 14, 2655. https://doi.org/10.3390/app14062655
Chalari E, Jones HS, Hadjicharalambous M, Fogarty MC. The Impact of High-Intensity Interval Exercise Including Acceleration/Deceleration Patterns on Redox Status of Healthy Male Adults. Applied Sciences. 2024; 14(6):2655. https://doi.org/10.3390/app14062655
Chicago/Turabian StyleChalari, Eleanna, Huw S. Jones, Marios Hadjicharalambous, and Mark C. Fogarty. 2024. "The Impact of High-Intensity Interval Exercise Including Acceleration/Deceleration Patterns on Redox Status of Healthy Male Adults" Applied Sciences 14, no. 6: 2655. https://doi.org/10.3390/app14062655
APA StyleChalari, E., Jones, H. S., Hadjicharalambous, M., & Fogarty, M. C. (2024). The Impact of High-Intensity Interval Exercise Including Acceleration/Deceleration Patterns on Redox Status of Healthy Male Adults. Applied Sciences, 14(6), 2655. https://doi.org/10.3390/app14062655







