Computational Modelling and Biomechanical Analysis of Age-Related Craniocerebral Injuries: Insights into Bridging Veins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Studies
2.2. Development of the Human Head Numerical Model
2.3. Model Geometry
2.4. Mechanical Properties of Brain Tissues
2.5. Validation Boundary Conditions
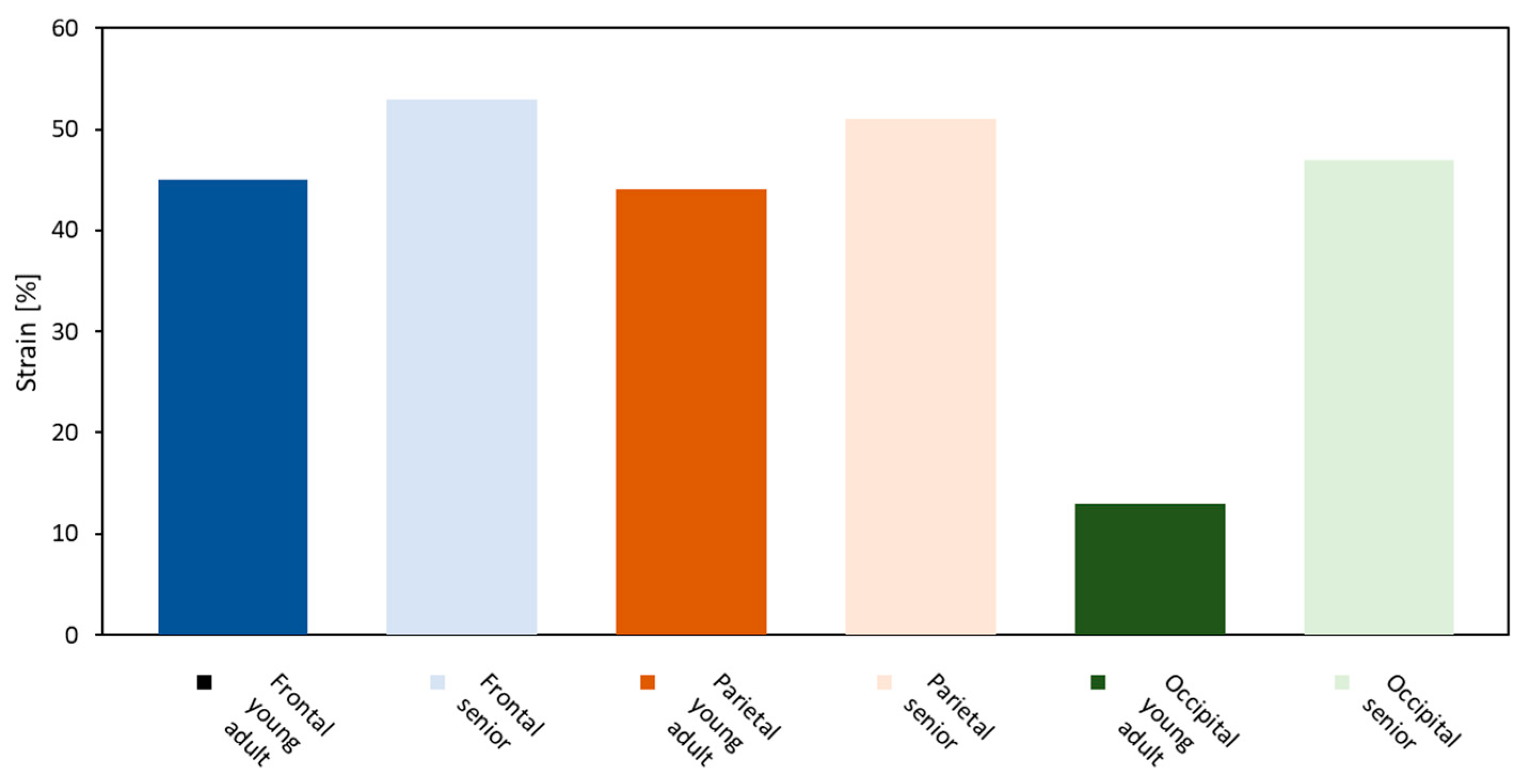
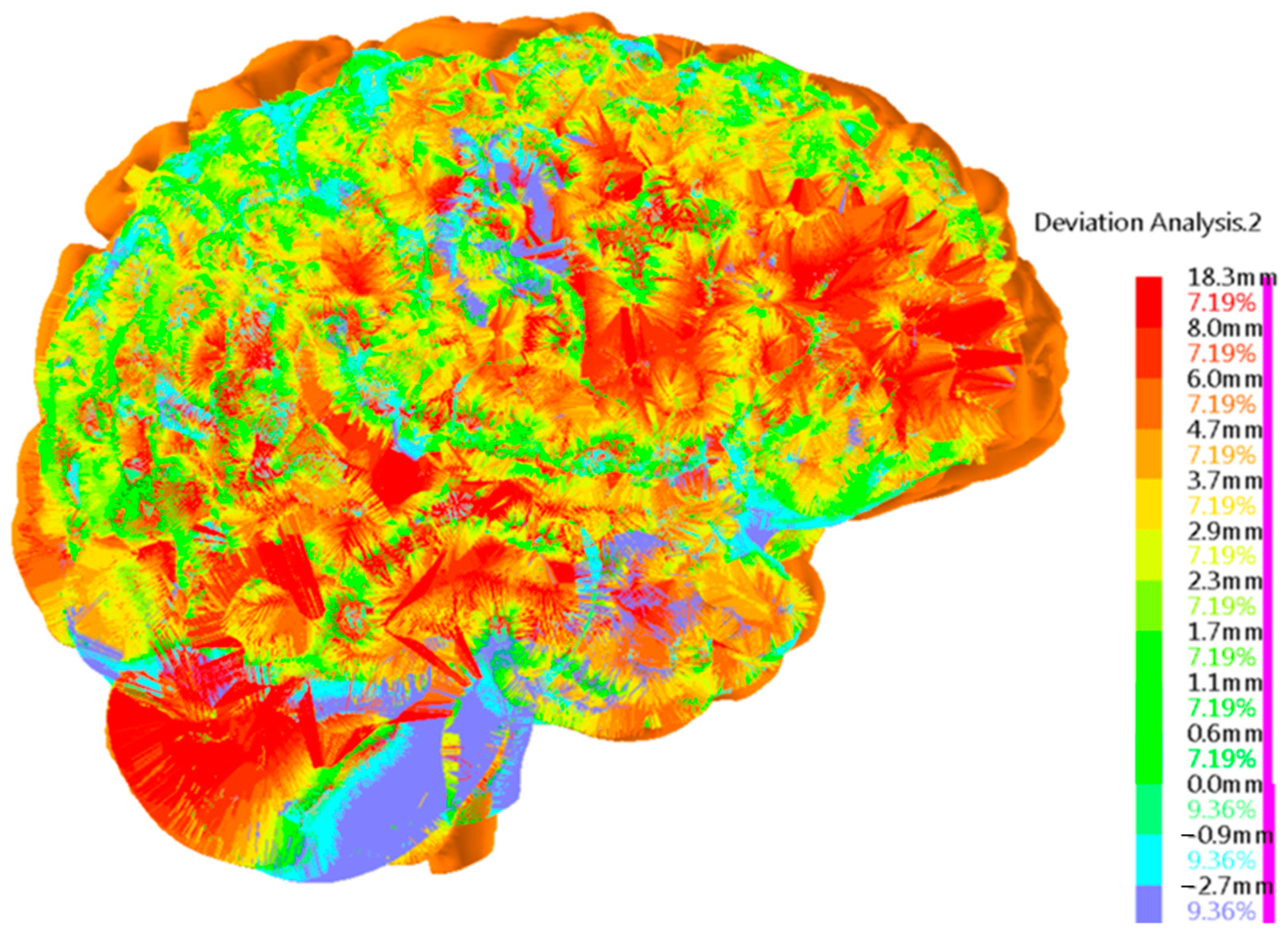
3. Results
Brain Physical Differences
4. Discussion and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dymek, M.; Ptak, M.; Ratajczak, M.; Fernandes, F.A.O.; Kwiatkowski, A.; Wilhelm, J. Analysis of HIC and Hydrostatic Pressure in the Human Head during NOCSAE Tests of American Football Helmets. Brain Sci. 2021, 11, 287. [Google Scholar] [CrossRef]
- Hawley, C.; Sakr, M.; Scapinello, S.; Bjorndalen, H. Head Injury among Older Adults and Their Clinical Management: One Year of Emergency Department Attendances at a UK Trauma Center. Brain Inj. 2022, 36, 868–875. [Google Scholar] [CrossRef]
- Florczak, A.; Skierkowska, N.A.; Wąsicki, M. Assessing the Risk of Falls of Older People Using Specialized Diagnostic Tests. Artic. J. Educ. Health Sport 2019, 9, 397–405. [Google Scholar] [CrossRef]
- Abhilash, K.; Tephilah, R.; Pradeeptha, S.; Gunasekaran, K.; Chandy, G. Injury Patterns and Outcomes of Trauma in the Geriatric Population Presenting to the Emergency Department in a Tertiary Care Hospital of South India. J. Emerg. Trauma. Shock. 2019, 12, 198–202. [Google Scholar] [CrossRef]
- Alter, S.M.; Gonzalez, M.R.; Solano, J.J.; Clayton, L.M.; Hughes, P.G.; Shih, R.D. Comparing Rates of Skull Fractures in Female versus Male Geriatric Patients Who Sustain Head Injuries. Am. J. Emerg. Med. 2023, 65, 168–171. [Google Scholar] [CrossRef]
- Ptak, M.; Ratajczak, M.; Kwiatkowski, A.; Sawicki, M.; Wilhelm, J.; Fernandes, F.A.O.; Druszcz, A. Investigation of Biomechanics of Skull Structures Damages Caused by Dynamic Loads. Acta Bioeng. Biomech. 2018, 20, 143–150. [Google Scholar]
- Holtzhausen, L.J.; Souissi, S.; Al Sayrafi, O.; May, A.; Farooq, A.; Grant, C.C.; Korakakis, V.; Rabia, S.; Segers, S.; Chamari, K. Arabic Translation and Cross-Cultural Adaptation of Sport Concussion Assessment Tool 5 (SCAT5). Biol. Sport 2021, 38, 129–144. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lang, J.; Zhang, Z.; Bi, Y.; Liu, R.; Jiang, S.; Hou, L. Effect of Different Motor Skills Training on Motor Control Network in the Frontal Lobe and Basal Ganglia. Biol. Sport 2020, 37, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Waltzman, D.; Haarbauer-Krupa, J.; Womack, L.S. Traumatic Brain Injury in Older Adults—A Public Health Perspective. JAMA Neurol. 2022, 79, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Santelli, L.; Tomassini, V.; Bosnell, R.; Smith, S.; De Stefano, N.; Johansen-Berg, H. Age-Related Changes in Grey and White Matter Structure throughout Adulthood. Neuroimage 2010, 51, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Ageing and the Brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Demsas, F.; Joiner, M.M.; Telma, K.; Flores, A.M.; Teklu, S.; Ross, E.G. Disparities in Peripheral Artery Disease Care: A Review and Call for Action. Semin. Vasc. Surg. 2022, 35, 141–154. [Google Scholar] [CrossRef]
- Kleiven, S.; von Holst, H. Consequences of Head Size Following Trauma to the Human Head. J. Biomech. 2002, 35, 153–160. [Google Scholar] [CrossRef]
- Horanin-Dusza, M. The Analysis of the Biomechanical and Histological Properties of Cerebral Bridging Veins in Alcoholics and Nonalcoholics-the Importance in the Subdural Hematomas Etiology; Medical University: Wrocław, Poland, 2009. (In Polish) [Google Scholar]
- Famaey, N.; Ying Cui, Z.; Umuhire Musigazi, G.; Ivens, J.; Depreitere, B.; Verbeken, E.; Vander Sloten, J. Structural and Mechanical Characterisation of Bridging Veins: A Review. J. Mech. Behav. Biomed. Mater. 2015, 41, 222–240. [Google Scholar] [CrossRef]
- Migueis, G.F.J.; Fernandes, F.A.O.; Ptak, M.; Ratajczak, M.; Alves de Sousa, R.J. Detection of Bridging Veins Rupture and Subdural Haematoma Onset Using a Finite Element Head Model. Clin. Biomech. 2019, 63, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kurowiak, J.; Mackiewicz, A.; Klekiel, T.; Będziński, R. Evaluation of Selected Properties of Sodium Alginate-Based Hydrogel Material—Mechanical Strength, ΜDIC Analysis and Degradation. Materials 2022, 15, 1225. [Google Scholar] [CrossRef]
- Kolias, A.G.; Chari, A.; Santarius, T.; Hutchinson, P.J. Chronic Subdural Haematoma: Modern Management and Emerging Therapies. Nat. Rev. Neurol. 2014, 10, 570–578. [Google Scholar] [CrossRef]
- Dyniewicz, B.; Bajkowski, J.M.; Bajer, C.I. Effective Viscoplastic-Softening Model Suitable for Brain Impact Modelling. Materials 2022, 15, 2270. [Google Scholar] [CrossRef] [PubMed]
- Glowinski, S.; Obst, M.; Majdanik, S.; Potocka-Banaś, B. Dynamic Model of a Humanoid Exoskeleton of a Lower Limb with Hydraulic Actuators. Sensors 2021, 21, 3432. [Google Scholar] [CrossRef]
- Pietroń, K.; Mazurkiewicz, Ł.; Sybilski, K.; Małachowski, J. Correlation of Bone Material Model Using Voxel Mesh and Parametric Optimization. Materials 2022, 15, 5163. [Google Scholar] [CrossRef] [PubMed]
- Klekiel, T.; Arkusz, K.; Sławiński, G.; Malesa, P.; Będziński, R. Numerical Analyses of Fracture Mechanism of the Pelvic Ring during Side-Impact Load. Materials 2022, 15, 5734. [Google Scholar] [CrossRef]
- Sybilski, K.; Małachowski, J. Sensitivity Study on Seat Belt System Key Factors in Terms of Disabled Driver Behavior during Frontal Crash. Acta Bioeng. Biomech. 2019, 21, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Joszko, K.; Wolański, W.; Burkacki, M.; Suchoń, S.; Zielonka, K.; Muszyński, A.; Gzik, M. Biomechanical Analysis of Injuries of Rally Driver with Head Supporting Device. Acta Bioeng. Biomech. 2016, 18, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Franklyn, M.; Fildes, B.; Zhang, L.; Yang, K.; Sparke, L. Analysis of Finite Element Models for Head Injury Investigation: Reconstruction of Four Real-World Impacts. Stapp Car Crash J. 2005, 49, 1–32. [Google Scholar] [PubMed]
- Horgan, T.J.; Gilchrist, M.D. The Creation of Three-Dimensional Finite Element Models for Simulating Head Impact Biomechanics. Int. J. Crashworthiness 2003, 8, 353–366. [Google Scholar] [CrossRef]
- Kleiven, S. Predictors for Traumatic Brain Injuries Evaluated through Accident Reconstructions. Stapp Car Crash J. 2007, 51, 81–114. [Google Scholar] [PubMed]
- Wilhelm, J.; Ptak, M.; Fernandes, F.A.O.; Kubicki, K.; Kwiatkowski, A.; Ratajczak, M.; Sawicki, M.; Szarek, D. Injury Biomechanics of a Child’s Head: Problems, Challenges and Possibilities with a New AHEAD Finite Element Model. Appl. Sci. 2020, 10, 4467. [Google Scholar] [CrossRef]
- Ptak, M.; Dymek, M.; Sawicki, M.; Fernandes, F.A.O.; Wnuk, M.; Wilhelm, J.; Ratajczak, M.; Witkowska, D.; Kwiatkowski, A.; Poźniak, B.; et al. Experimental and Computational Approach to Human Brain Modelling—AHEAD. Arch. Civil. Mech. Eng. 2023, 23, 218. [Google Scholar] [CrossRef]
- Fernandes, F.A.O.; Tchepel, D.; Alves de Sousa, R.J.; Ptak, M. Development and Validation of a New Finite Element Human Head Model. Eng. Comput. 2018, 35, 477–496. [Google Scholar] [CrossRef]
- Monea, A.G.; Baeck, K.; Verbeken, E.; Verpoest, I.; Sloten, J.V.; Goffin, J.; Depreitere, B. The Biomechanical Behaviour of the Bridging Vein–Superior Sagittal Sinus Complex with Implications for the Mechanopathology of Acute Subdural Haematoma. J. Mech. Behav. Biomed. Mater. 2014, 32, 155–165. [Google Scholar] [CrossRef]
- DYNAmore GmbH. LS-DYNA Examples, Wave-Structure Interaction; DYNAmore GmbH: Dresden, Germany, 2012. [Google Scholar]
- Giordano, C.; Kleiven, S. Development of a 3-Year-Old Child FE Head Model, Continuously Scalable from 1.5-to 6-Year-Old. In Proceedings of the IRCOBI Conference, Malaga, Spain, 14–16 September 2016; pp. 288–302. [Google Scholar]
- Ratajczak, M.; Ptak, M.; Chybowski, L.; Gawdzińska, K.; Będziński, R. Material and Structural Modeling Aspects of Brain Tissue Deformation under Dynamic Loads. Materials 2019, 12, 271. [Google Scholar] [CrossRef]
- DynaMore Human Model—Total HUman Model for Safety THUMS v 4.0. Available online: https://www.dynamore.se/en/products/models/human (accessed on 16 December 2023).
- Gomez-Gesteira, M.; Rogers, B.D.; Crespo, A.J.C.; Dalrymple, R.A.; Narayanaswamy, M.; Dominguez, J.M. SPHysics—Development of a Free-Surface Fluid Solver—Part 1: Theory and Formulations. Comput. Geosci. 2012, 48, 289–299. [Google Scholar] [CrossRef]
- Hardy, W.N.; Mason, M.J.; Foster, C.D.; Shah, C.S.; Kopacz, J.M.; Yang, K.H.; King, A.I.; Bishop, J.; Bey, M.; Anderst, W.; et al. A Study of the Response of the Human Cadaver Head to Impact. Stapp Car Crash J. 2007, 51, 17–80. [Google Scholar] [CrossRef] [PubMed]
- Tsui, E.Y.K.; Fai Ma, K.; Cheung, Y.K.; Chan, J.H.M.; Yuen, M.K. Rapid Spontaneous Resolution and Redistribution of Acute Subdural Hematoma in a Patient with Chronic Alcoholism: A Case Report. Eur. J. Radiol. 2000, 36, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Oh, Y.J.; Cho, Y.N.; Choi, Y.-C. Subdural Hemorrhage Mimicking Peripheral Neuropathy. J. Korean Neurosurg. Soc. 2014, 56, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Wolański, W.; Kawlewska, E.; Larysz, D.; Gzik, M.; Gorwa, J.; Michnik, R. Prediction of the Child’s Head Growth in the First Year of Life. In Lecture Notes in Computational Vision and Biomechanics; Spinger: Berlin/Heidelberg, Germany, 2019; pp. 267–275. [Google Scholar]
- DeCarli, C.; Massaro, J.; Harvey, D.; Hald, J.; Tullberg, M.; Au, R.; Beiser, A.; D’Agostino, R.; Wolf, P.A. Measures of Brain Morphology and Infarction in the Framingham Heart Study: Establishing What Is Normal. Neurobiol. Aging 2005, 26, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Zanto, T.P.; Gazzaley, A. Aging of the Frontal Lobe. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–389. [Google Scholar]
- Toma, M.; Nguyen, P.D.H. Fluid–Structure Interaction Analysis of Cerebrospinal Fluid with a Comprehensive Head Model Subject to a Rapid Acceleration and Deceleration. Brain Inj. 2018, 32, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Madhukar, A.; Chen, Y.; Ostoja-Starzewski, M. Effect of Cerebrospinal Fluid Modeling on Spherically Convergent Shear Waves during Blunt Head Trauma. Int. J. Numer. Method. Biomed. Eng. 2017, 33, e2881. [Google Scholar] [CrossRef] [PubMed]
- Ptak, M.; Wilhelm, J.; Sawicki, M.; Dymek, M.; Fernandes, F.A.O.; Kristen, H.; Garatea, E. Assessment of Child Safety on Bicycles in Baby Carriers—The Importance of Evaluating Both Head and Neck Injuries. J. Safety Res. 2023, 85, 254–265. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Z.; Zhang, Y.; Li, Q.M. Impact Analysis of a Honeycomb-Filled Motorcycle Helmet Based on Coupled Head-Helmet Modelling. Int. J. Mech. Sci. 2021, 199, 106406. [Google Scholar] [CrossRef]
- Yanaoka, T.; Dokko, Y. A Parametric Study of Age-Related Factors Affecting Intracranial Responses under Impact Loading Using a Human Head/Brain FE Model. In Proceedings of the IRCOBI Conference, Gothenburg, Sweden, 11–13 September 2013. [Google Scholar]
- Abdi, H.; Sanchez-Molina, D.; Garcia-Vilana, S.; Rahimi-Movaghar, V. Quantifying the Effect of Cerebral Atrophy on Head Injury Risk in Elderly Individuals: Insights from Computational Biomechanics and Experimental Analysis of Bridging Veins. Injury 2023, 54, 111125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Kleiven, S. Biomechanics of Acute Subdural Hematoma in the Elderly: A Fluid-Structure Interaction Study. J. Neurotrauma 2019, 36, 2099–2108. [Google Scholar] [CrossRef] [PubMed]

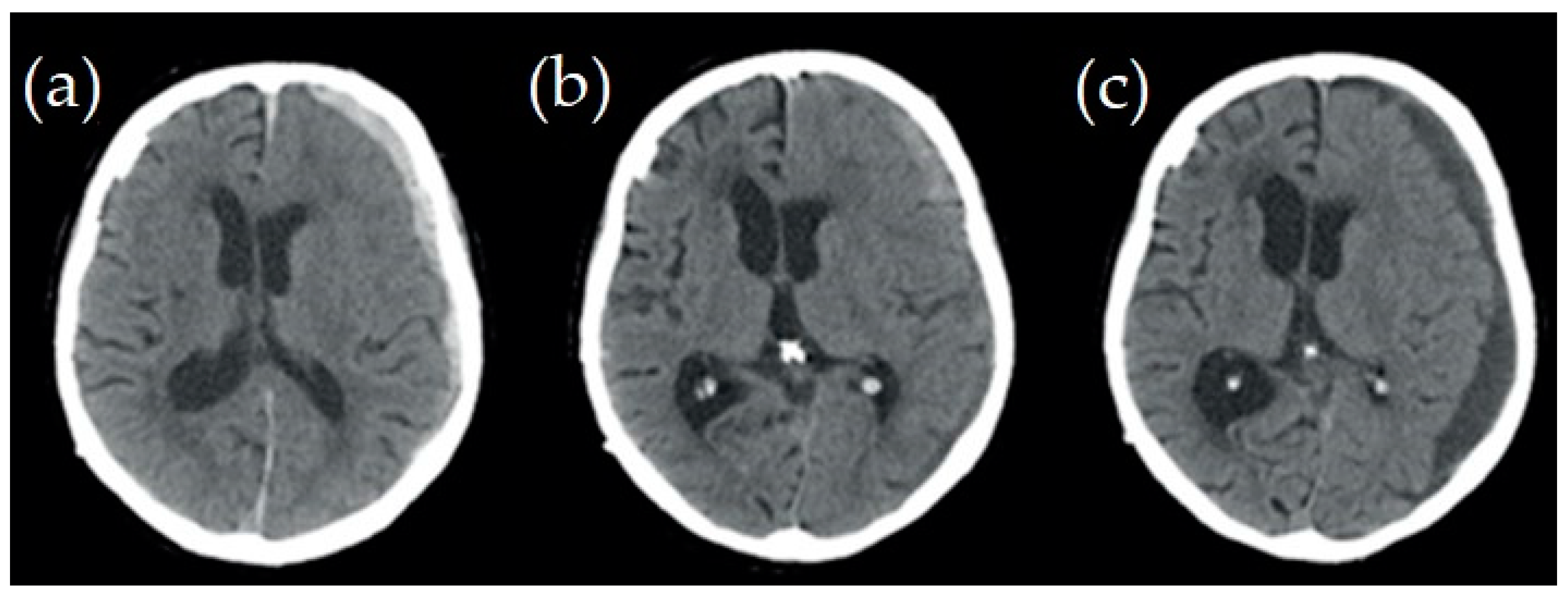
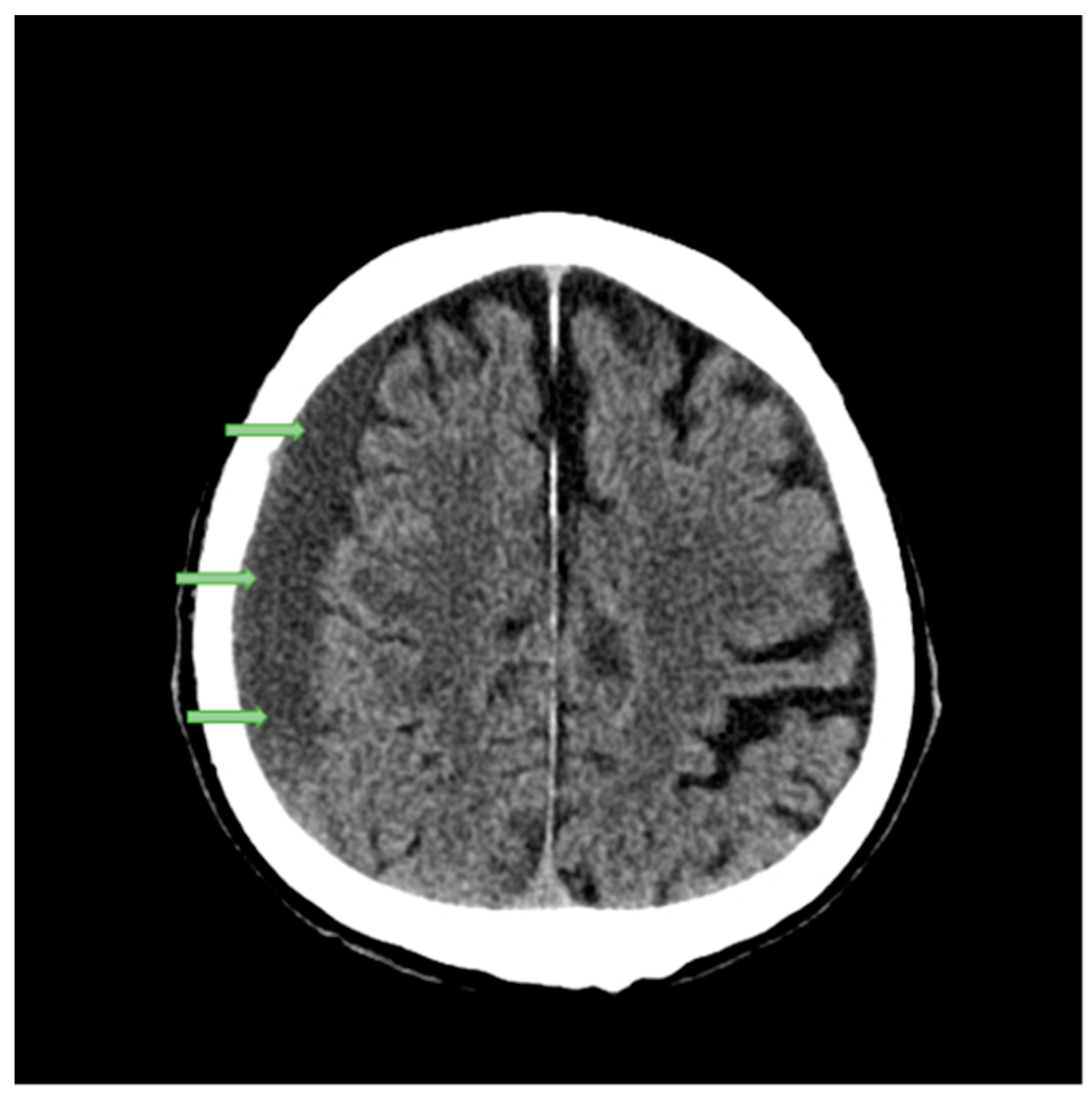
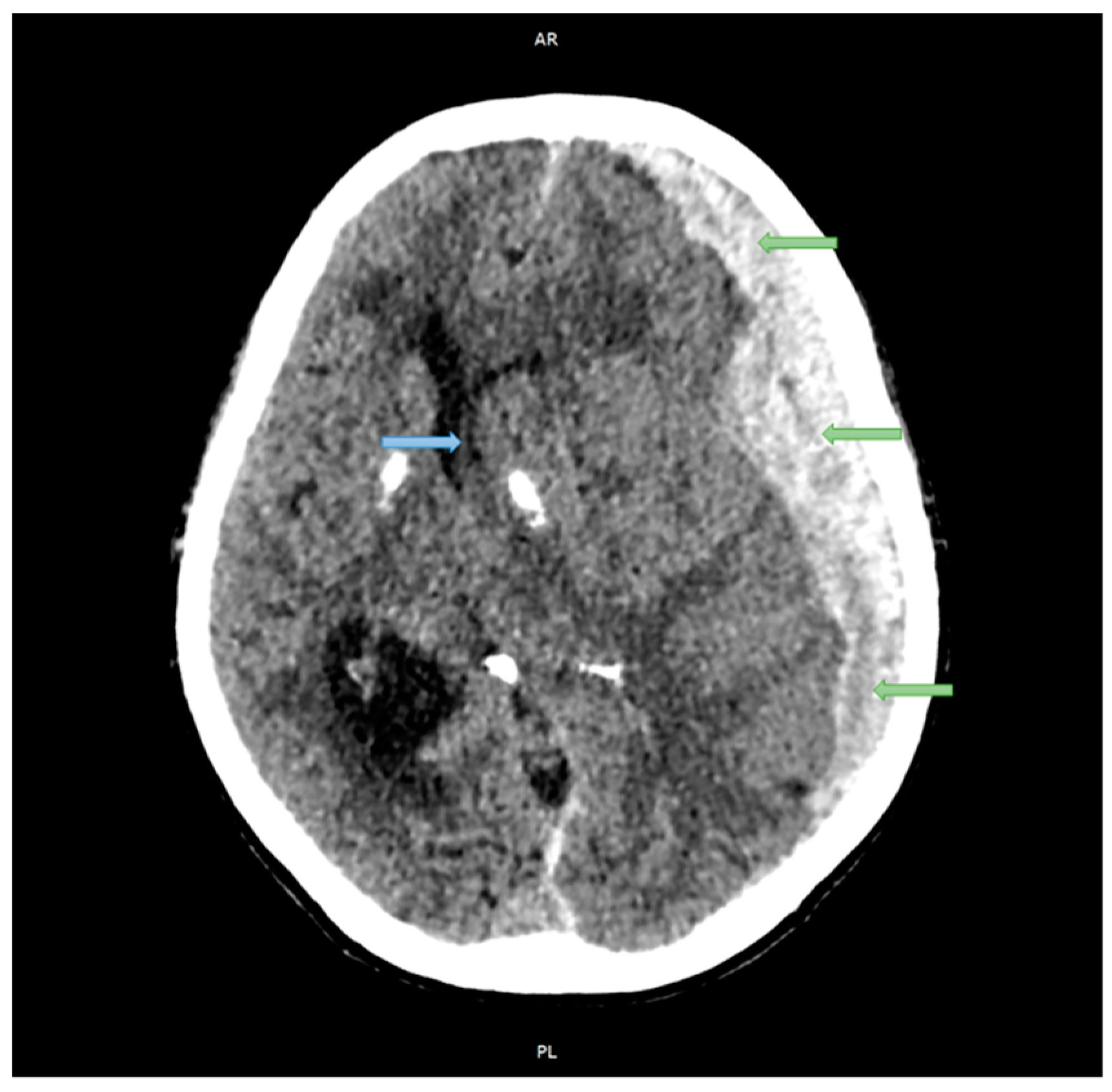
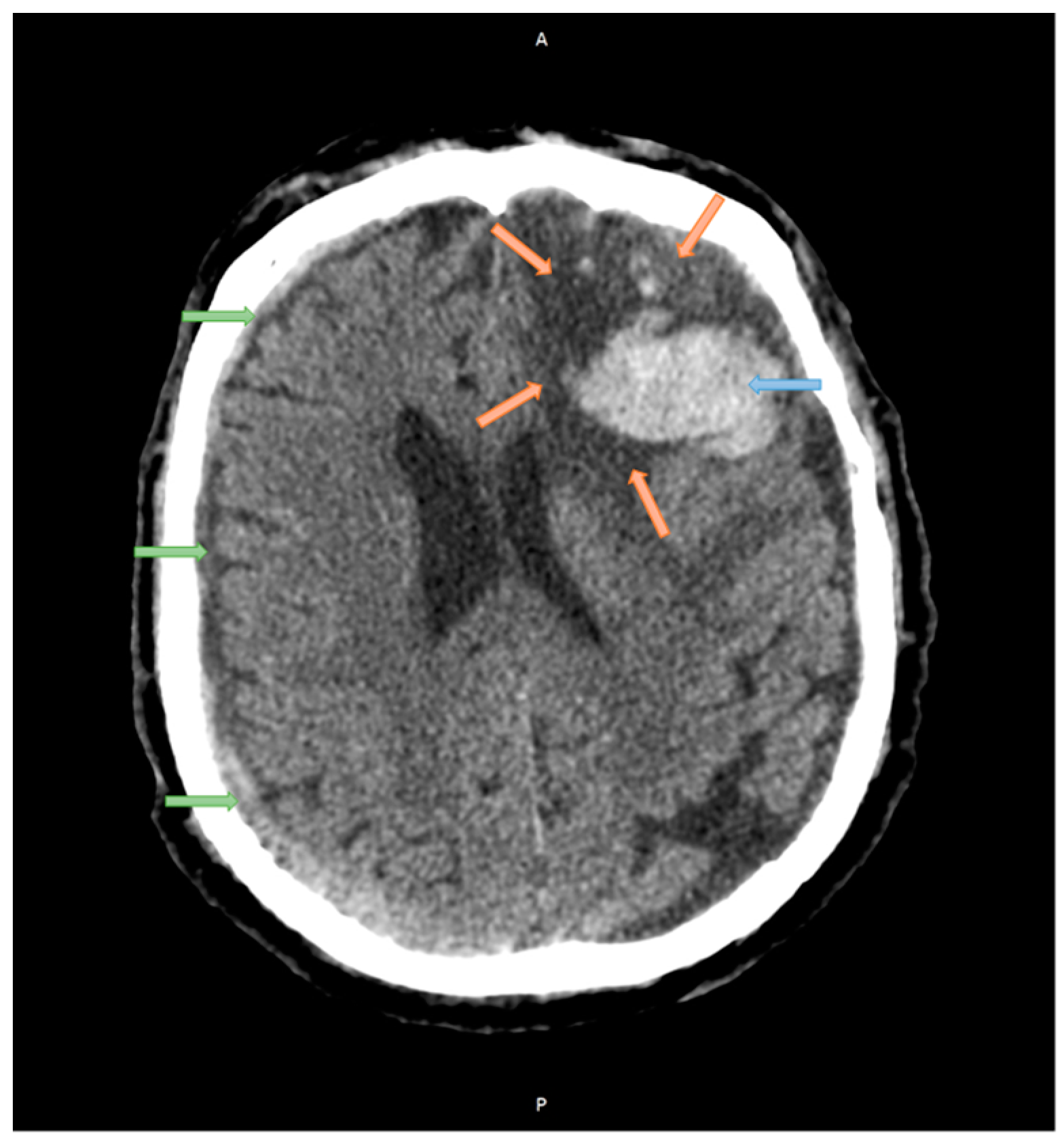
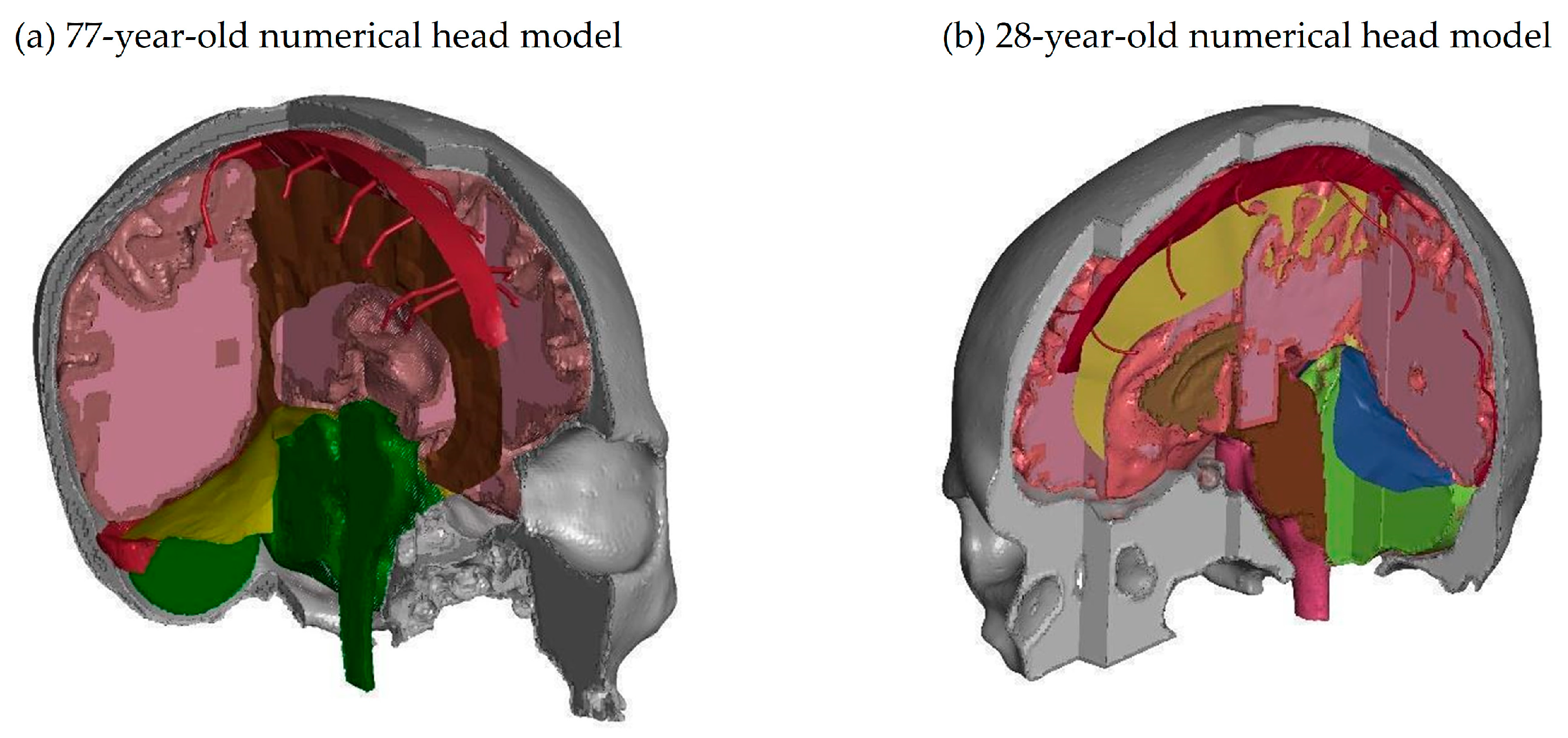
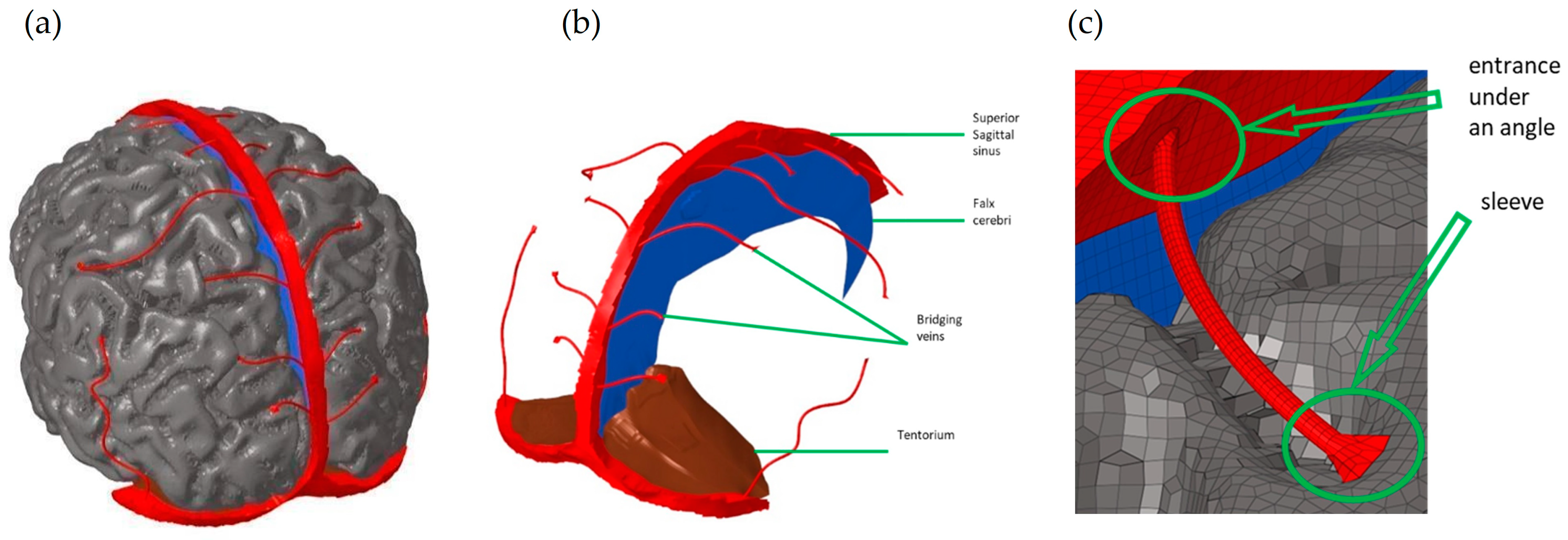
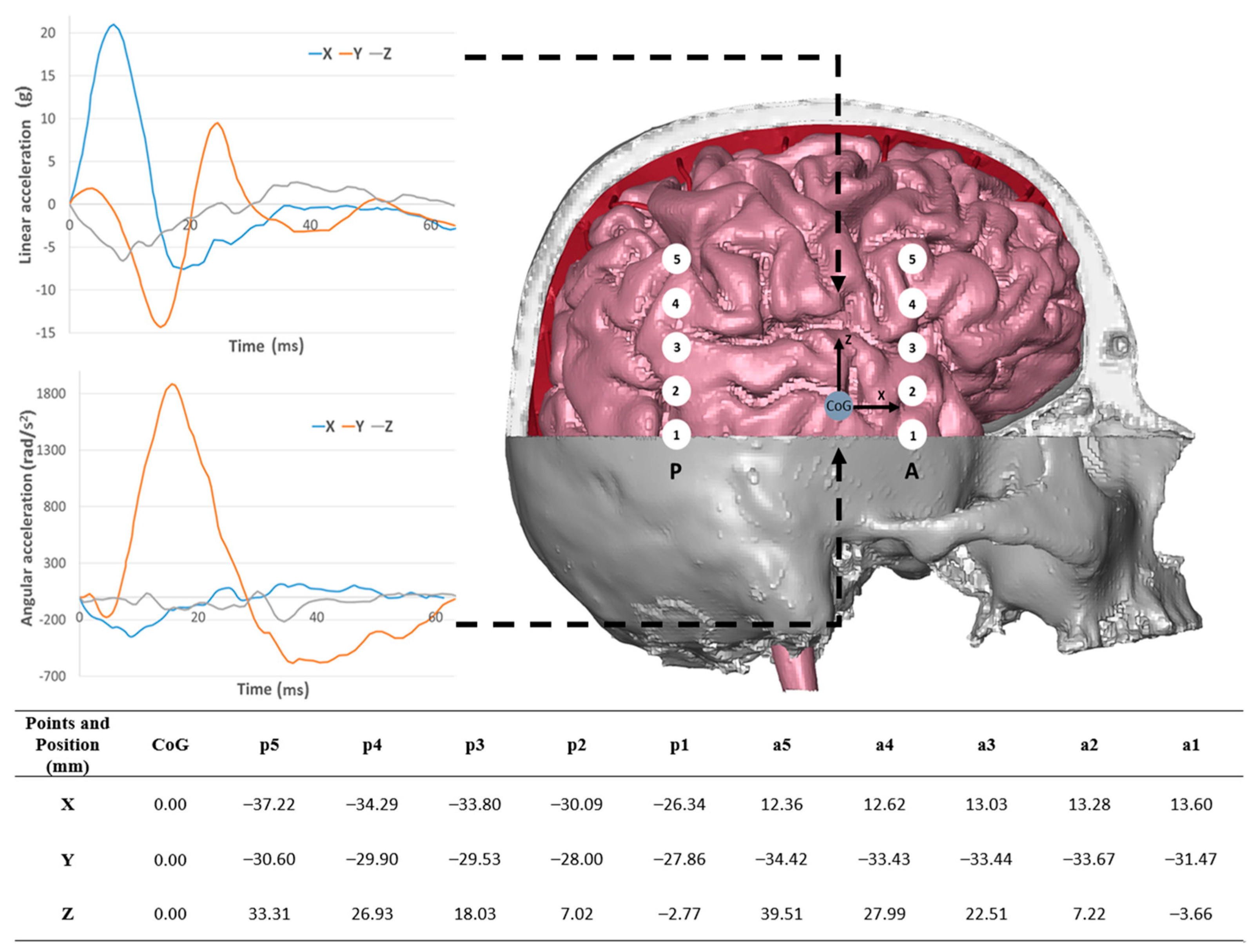

| Structure | Reference | Density [t/m3] | Young’s Modulus or Bulk Modulus [MPa] | Other Material Parameters | Type No. of FEs | Image of Structure |
|---|---|---|---|---|---|---|
| White matter—left/right hemisphere | THUMS MAT_VISCOELASTIC 2 [35] | 1.00 × 10−9 | Bulk modulus 2.16 × 10−3 | G0 = 12.5 × 10−3 G1 = 6.125 × 10−3 | hexa 231,146/ 237,494 |   |
| Grey matter—left/right hemisphere | THUMS MAT_VISCOELASTIC 2 [35] | 1.00 × 10−9 | Bulk modulus 2.16 × 10−3 | G0 = 10 × 10−3 G1 = 5 × 10−3 | hexa 313,176/ 311,954 |   |
| Cerebellum brainstem | F.A.O. Fernandes et al., 2018 [30] | 1.04 × 10−9 | – | Ν = 0.49999 Mu1 = 0.0012 Alpha1 = 5.05007 | hexa 204,567 |  |
| Pia mater | LLC Elemance—GHBMC Model 2014; M. Ratajczak et al., 2019 [32,34] | 1.13 × 10−9 | 31.5 | ν = 0.45000 | shell 245,632 |  |
| Dura mater | LLC Elemance—GHBMC Model 2014; M. Ratajczak et al., 2019 [32,34] | 1.13 × 10−9 | 31.5 | ν = 0.45000 | shell 92,152 |  |
| Falx cerebri | LLC Elemance—GHBMC Model 2014; M. Ratajczak et al., 2019 [32,34] | 1.13 × 10−9 | 31.5 | ν = 0.45000 | shell 1197 |  |
| Tentorium cerebelli | LLC Elemance—GHBMC Model 2014; M. Ratajczak et al., 2019 [32,34] | 1.13 × 10−9 | 31.5 | ν = 0.45000 | shell 1669 |  |
| Superior sagittal sinus and transversal sinus | LLC Elemance—GHBMC Model 2014; M. Ratajczak et al., 2019 [32,34] | 1.04 × 10−9 | 28.2 | ν = 0.45000 | shell 10,627 |  |
| Bridging veins | Monea et al., 2014 [31] | 1.13 × 10−9 | 30 | 0.48000 | shell 10,627 |  |
| Lamina interna | Giordano and Kleiven 2016; M. Ratajczak et al., 2019 [33,34] | 2.1 × 10−9 | 4 × 10+3 | 0.25000 | hexa 196,734 |  |
| Diploe | Giordano and Kleiven 2016; M. Ratajczak et al., 2019 [33,34] | 1.0 × 10−9 | 1 × 10+3 | 0.30000 | hexa 326,812 |  |
| Lamina externa | Giordano and Kleiven 2016; M. Ratajczak et al., 2019 [33,34] | 2.1 × 10−9 | 4 × 10+3 | 0.25000 | hexa 326,812 |  |
| Cerebrospinal fluid (CSF) | DYNAmore GmbH 2018; Gomez-Gesteira et al., 2012 [32,36] | 1 × 10−9 | – | viscosity coefficient 7 × 10−10 | SPH 191,406 |  |
| Corpus callosum | THUMS MAT_VISCOELASTIC 2 [35] | 1.04 × 10−9 | same as for WM | same as for WM | hexa 3190 |  |
| 28-Year-Old Model | 77-Year-Old Model | Effective Strain Criterion [-] | |
|---|---|---|---|
| Frontal veins |  | 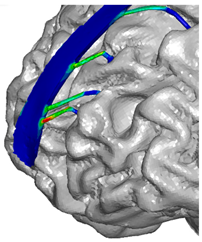 |  |
| Parietal veins | 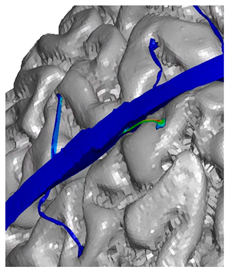 | 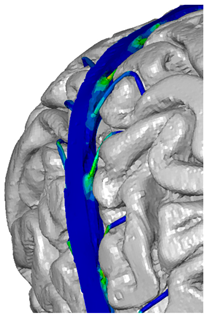 | |
| Occipital veins | 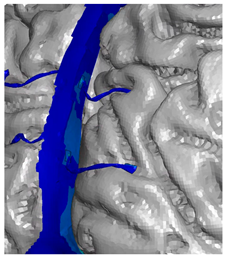 | 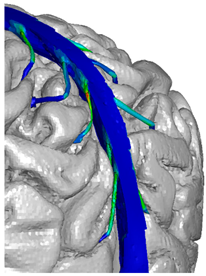 |
| Brain Parameters (White and Grey Matter) | Young Adults (28 y)  | Senior (77 y) | Difference (Young Adult vs. Senior) | Comments |
|---|---|---|---|---|
| Brain Volume | 1.0406 [dm3] | 0.98088 [dm3] | 5.7% | Young Adults: On average, young adults tend to have larger brain volumes compared to older adults, with a ~6% difference. This is largely due to ongoing brain development and growth during childhood and adolescence [40]. Senior Adults: Brain volume typically decreases with age. This reduction can be attributed to factors such as loss of neurons and their connections, as well as changes in brain structural integrity. This decrease in volume can affect various cognitive functions. |
| Brain Area | 165,893.3 [mm3] | 1,619,62.1 [mm2] | 2.4% | Young Adults: Younger individuals generally have a larger brain area compared to older adults, with a ~2% difference. The brain area encompasses the surface of the brain, which is important for processing information and facilitating communication between different brain regions. Senior Adults: Over time, there may be a slight reduction in the brain’s surface area. This could be related to the gradual decline in cognitive functions, such as memory and processing speed, experienced by some older individuals. |
| Brain Mass | 1.18 [kg] | 1.02 [kg] | 13.3% | Young Adults: Young adults typically have greater brain mass compared to older adults, with a ~13% difference. Brain mass is closely related to brain volume and is largely responsible for the organ’s overall functionality. Senior Adults: As individuals age, there is often a decline in brain mass, primarily due to a decrease in the number of neurons and synaptic connections. This mass reduction can contribute to age-related cognitive decline. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, M.; Ptak, M.; Dymek, M.; Kubacki, R.; de Sousa, R.J.A.; Sbriglio, C.; Kwiatkowski, A. Computational Modelling and Biomechanical Analysis of Age-Related Craniocerebral Injuries: Insights into Bridging Veins. Appl. Sci. 2024, 14, 2681. https://doi.org/10.3390/app14072681
Ratajczak M, Ptak M, Dymek M, Kubacki R, de Sousa RJA, Sbriglio C, Kwiatkowski A. Computational Modelling and Biomechanical Analysis of Age-Related Craniocerebral Injuries: Insights into Bridging Veins. Applied Sciences. 2024; 14(7):2681. https://doi.org/10.3390/app14072681
Chicago/Turabian StyleRatajczak, Monika, Mariusz Ptak, Mateusz Dymek, Rafał Kubacki, Ricardo J. Alves de Sousa, Claudia Sbriglio, and Artur Kwiatkowski. 2024. "Computational Modelling and Biomechanical Analysis of Age-Related Craniocerebral Injuries: Insights into Bridging Veins" Applied Sciences 14, no. 7: 2681. https://doi.org/10.3390/app14072681
APA StyleRatajczak, M., Ptak, M., Dymek, M., Kubacki, R., de Sousa, R. J. A., Sbriglio, C., & Kwiatkowski, A. (2024). Computational Modelling and Biomechanical Analysis of Age-Related Craniocerebral Injuries: Insights into Bridging Veins. Applied Sciences, 14(7), 2681. https://doi.org/10.3390/app14072681









