Green Solvent Extraction of Antioxidants from Herbs and Agro-Food Wastes: Optimization and Capacity Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Preparation of Deep Eutectic Solvents
2.3. Ultrasound-Assisted Extraction of Antioxidants
2.4. Analytical Methods for Antioxidant Capacity Determination
2.4.1. CUPRAC Method
2.4.2. ABTS Method
2.4.3. DPPH Method
2.5. Box–Behnken Optimization
2.6. Statistical and Chemometric Analyses
3. Results and Discussion
3.1. Comparative Performance of Different DESs for Antioxidant Extraction
3.2. Chemometrics of the DES-Based Extracts
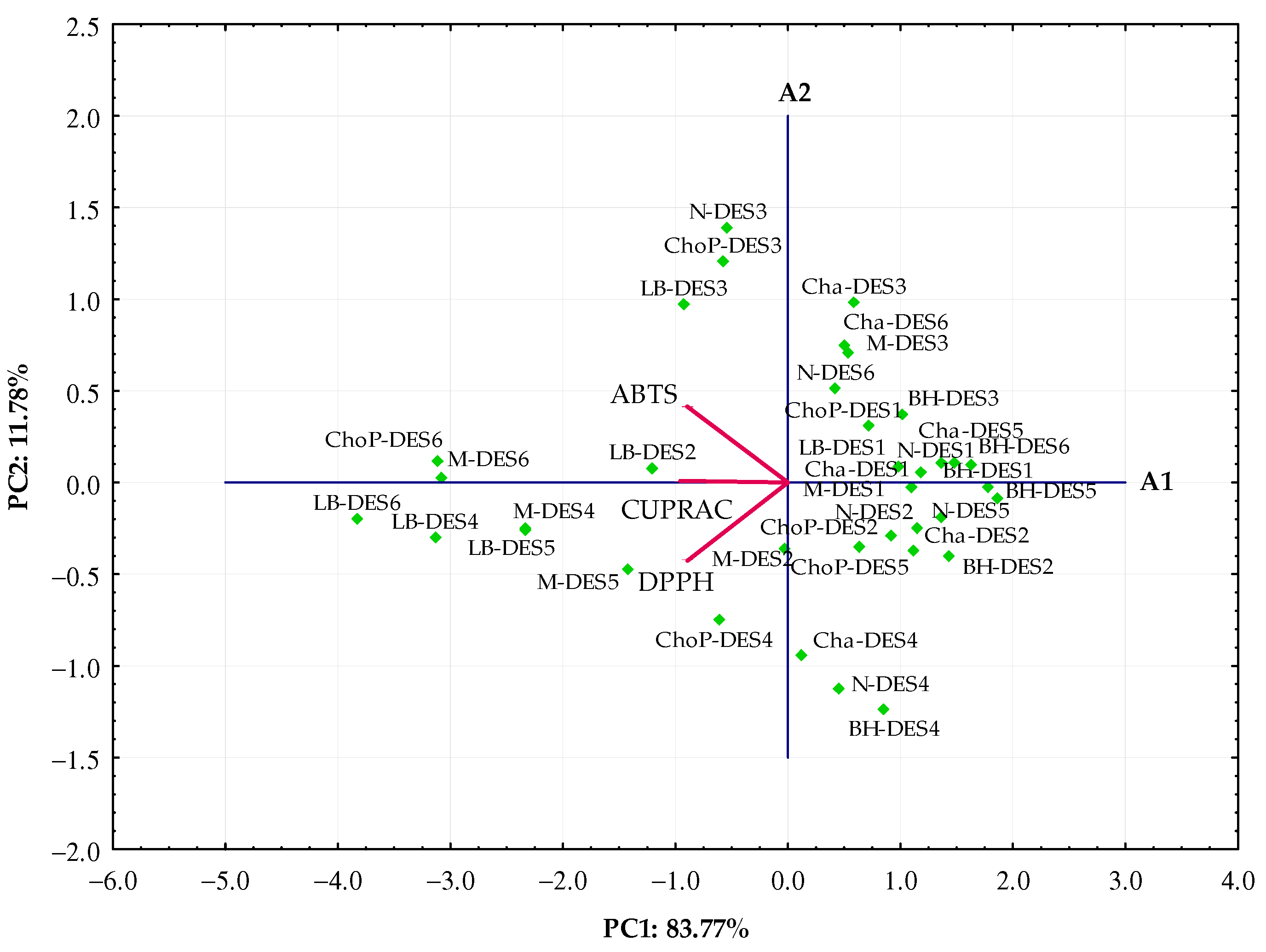
3.2.1. Principal Component Analysis
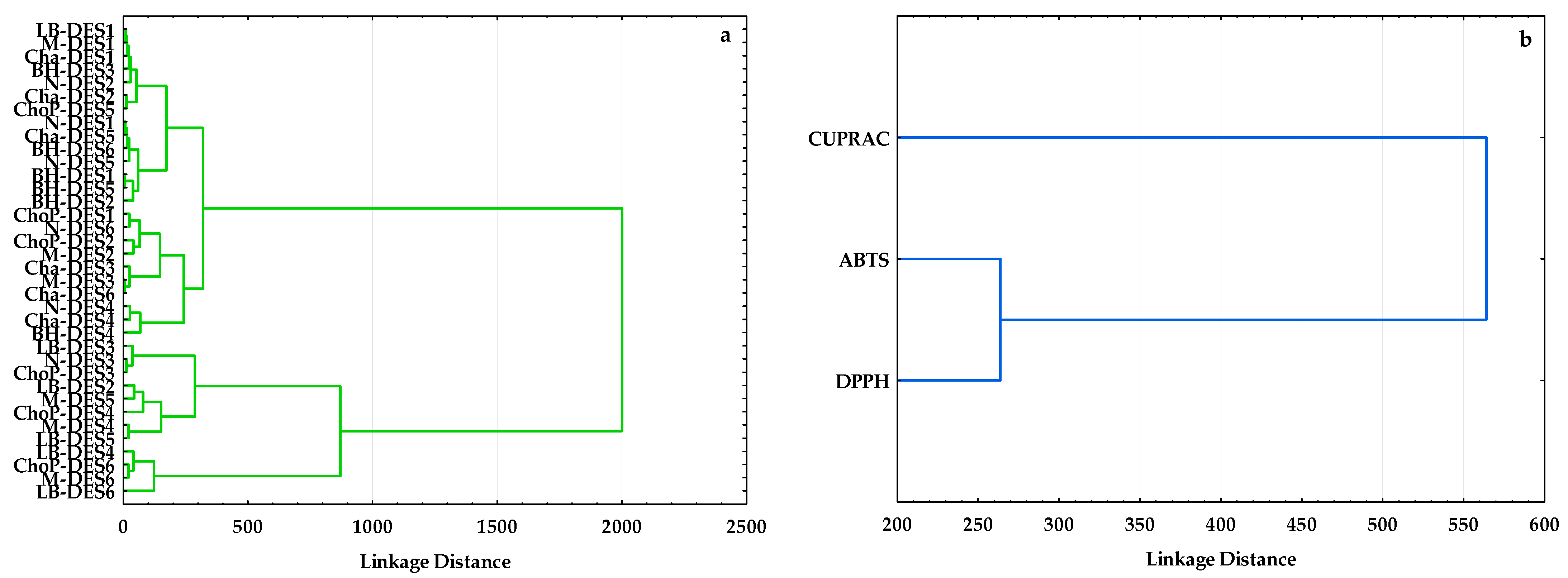
3.2.2. Hierarchical Cluster Analysis (HCA)
3.2.3. Correlations between Antioxidant Capacity Methods
3.3. Optimal Green Solvent Design for Antioxidant Extraction from Lemon Balm
3.3.1. Fitting the Models for Predicting Antioxidant Capacity
3.3.2. Analysis of Response Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Ulewicz-Magulska, B.; Wesolowski, M. Antioxidant activity of medicinal herbs and spices from plants of the Lamiaceae, Apiaceae and Asteraceae families: Chemometric interpretation of the data. Antioxidants 2023, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Singh, P.; Pandey, V.K.; Singh, R.; Dar, A.H. A literature review on bioactive properties of betel leaf (Piper betel L.) and its applications in food industry. Food Chem. Adv. 2023, 3, 100536. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Hatzikamari, M.; Christaki, S.; Lazaridou, A.; Chatzopoulou, P.; Biliaderis, C.G.; Irakli, M. Assessment of antioxidant and antibacterial potential of phenolic extracts from post-distillation solid residues of oregano, rosemary, sage, lemon balm, and spearmint. Processes 2024, 12, 140. [Google Scholar] [CrossRef]
- Kocer, S.; Copur, O.U.; Tamer, C.E.; Suna, S.; Kayahan, S.; Uysal, E.; Cavus, S.; Akman, O. Optimization and characterization of chestnut shell pigment extract obtained microwave assisted extraction by response surface methodology. Food Chem. 2024, 443, 138424. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Matos, L.C.; Santos, L. Harnessing the potential of chestnut shell extract to enhance fresh cheese: A sustainable approach for nutritional enrichment and shelf life extension. J. Food Meas. Charact. 2024, 18, 1559–1573. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Granados, M.; Sentellas, S.; Saurina, J. Microwave-assisted extraction with natural deep eutectic solvents for polyphenol recovery from agrifood waste: Mature for scaling-up? Sci. Total Environ. 2024, 912, 168716. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Finimundy, T.C.; Pinela, J.; Pires, T.C.S.P.; Calhelha, R.C.; Vaz, J.; Corrêa, R.C.G.; Oliveira, M.B.P.P.; Barros, L. Exploring the bioactive compounds from berry biowaste: Eugenia involucrata DC. as a study case. Food Biosci. 2024, 58, 103532. [Google Scholar] [CrossRef]
- Kavela, E.T.A.; Szalóki-Dorkó, L.; Máté, M. The efficiency of selected green solvents and parameters for polyphenol extraction from chokeberry (Aronia melanocarpa (Michx)) pomace. Foods 2023, 12, 3639. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Ultrasound-assisted extraction of antioxidant bioactive compounds from wastes of rapeseed industry and their application in delaying rapeseed oil oxidation. Environ. Technol. Innov. 2023, 30, 103081. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Amarowicz, R.; Szłyk, E. Antioxidant capacity of rapeseed meal and rapeseed oils enriched with meal extract. Eur. J. Lipid Sci. Technol. 2010, 112, 750–760. [Google Scholar] [CrossRef]
- Coscarella, M.; Nardi, M.; Alipieva, K.; Bonacci, S.; Popova, M.; Procopio, A.; Scarpelli, R.; Simeonov, S. Alternative assisted extraction methods of phenolic compounds using NaDESs. Antioxidants 2024, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Lauberts, M.; Mierina, I.; Pals, M.; Latheef, M.A.A.; Shishkin, A. Spent coffee grounds valorization in biorefinery context to obtain valuable products using different extraction approaches and solvents. Plants 2023, 12, 30. [Google Scholar] [CrossRef]
- Lauberts, M.; Telysheva, G.; Venskutonis, P.R.; Lauberte, L.; Dizhbite, T.; Kazernavičiūte, R.; Pukalskas, A. Diarylheptanoid-rich extract of grey and black alder barks: An effective dietary antioxidant in mayonnaise. Chem. Pap. 2017, 71, 1007–1012. [Google Scholar] [CrossRef]
- Park, B.I.; Kim, J.; Lee, K.; Lim, T.; Hwang, K.T. Flavonoids in common and tartary buckwheat hull extracts and antioxidant activity of the extracts against lipids in mayonnaise. J. Food Sci. Technol. 2019, 56, 2712–2720. [Google Scholar] [CrossRef]
- Hęś, M.; Górecka, D.; Dziedzic, K. Antioxidant properties of extracts from buckwheat by-products. Acta Sci. Pol. Technol. Aliment. 2012, 11, 167–174. [Google Scholar]
- Xiong, Z.; Wang, M.; Guo, H.; Xu, J.; Ye, J.; Zhao, J.; Zhao, L. Ultrasound-assisted deep eutectic solvent as green and efficient media for the extraction of flavonoids from Radix scutellariae. New J. Chem. 2019, 43, 644–650. [Google Scholar] [CrossRef]
- Herman; Ibrahim, A.; Rahay, B.P.; Arifuddin, M.; Nur, Y.; Prabowo, W.C.; Maryono; Ambarwati, N.S.S.; Rijai, L.; Ahmad, I. Single factor effect of natural deep eutectic solvent citric acid-glucose based microwave-assisted extraction on total polyphenols content from Mitragyna speciosa Korth. Havil leaves. Pharmacogn. J. 2021, 13, 1109–1115. [Google Scholar] [CrossRef]
- Tsvetov, N.; Pasichnik, E.; Korovkina, A.; Gosteva, A. Extraction of bioactive components from Chamaenerion angustifolium (L.) Scop. with choline chloride and organic acids natural deep eutectic solvents. Molecules 2022, 27, 4216. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.; Nguyen, T.P.D.; Ho, D.C.; Phung, H.T.; Nguyen, H.T. Green solvents-based rutin extraction from Sophora japonica L. J. Appl. Res. Med. Aromat. Plants 2023, 36, 100508. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Antioxidant extracts of nettle (Urtica dioica) leaves: Evaluation of extraction techniques and solvents. Molecules 2022, 27, 6015. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, C.; Jing, H.; Du, X.; Fan, X.; Zhang, Y.; Wang, H. Comparison of different extraction methods on yield, purity, antioxidant, and antibacterial activities of proanthocyanidins from chokeberry (Aronia melanocarpa). J Food Meas. Charact. 2022, 16, 2049–2059. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Tymczewska, A.; Klebba, J.; Szydłowska-Czerniak, A. Antioxidant capacity and total phenolic content of spice extracts obtained by ultrasound-assisted extraction using deep eutectic and conventional solvents. Appl. Sci. 2023, 13, 6987. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A.; Momot, M.; Stawicka, B. Physicochemical, antioxidative, and sensory properties of refined rapeseed oils. J. Am. Oil Chem. Soc. 2019, 96, 405–419. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, F.; Jian, Y.; Chernyshev, V.M.; Zhang, Y.; Wang, Z.; Yuan, Z.; Chen, X. Extraction of flavonoids from Glycyrrhiza residues using deep eutectic solvents and its molecular mechanism. J. Mol. Liq. 2022, 363, 119848. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of water addition on choline chloride/glycol deep eutectic solvents: Characterization of their structural and physicochemical properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, X.; Liu, W. Application of deep eutectic solvents in food analysis: A review. Molecules 2019, 24, 4594. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dai, L.; Chu, D.; Pang, R.; Deng, J.; Wang, S.; Li, J.; Chen, H.; Zheng, X. Deep eutectic solvents as efficient extractants of caffeoylquinic acids from Blumea aromatica: A comparative analysis of content and antioxidant potential. Arab. J. Chem. 2024, 17, 105596. [Google Scholar] [CrossRef]
- Popova, A.; Dalemska, Z.; Mihaylova, D.; Hristova, I.; Alexieva, I. Melissa officinalis L.—GC profile and antioxidant activity. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 634–638. [Google Scholar]





| Solvent | Sample | Extraction Technique | Analytical Method | Key Findings | Ref. | |
|---|---|---|---|---|---|---|
| SpectroM | ChromM | |||||
| Extraction with natural deep eutectic solvents (NADESs) | ||||||
| ChCl:Gl (1:4) ChCl:Ge (1:4) ChCl:Pe (1:4) ChCl:Be (1:4) ChCl:LA (1:4) ChCl:MA:H2O (1:1:3) ChCl:Gu (1:1:2) Pr:Ge (1:4) Pr:Gu:H2O (5:3:8) Pr:Fr:H2O (1:1:5) CitA:Fr:H2O (1:1:3) CitA:Gu:H2O (1:1:5) | Radix scutellariae—perennial herb | Ultrasonic irradiation at Troom for 42 min | RP-HPLC-UV | Different DESs were investigated as tunable and superior extraction media for extraction of flavonoids from Radix scutellariae. | [20] | |
| CitA:Gu (4:1; 5:1; 6:1) | Mitragyna speciosa Korth. Havil (Rubiaceae family)—herb | MAE | TPC | The proposed NADES is very suitable for extracting polyphenol compounds from herb leaves. | [21] | |
| ChCl:MalA (1:1) ChCl:MA (1:1) ChCl:CitA (1:1) ChCl:TarA (2:1) | Chamaenerion angustifolium (L.) Scop. (fireweed)—perennial herbaceous plant of the Onagraceae family | UAE | TPC TFC DPPH | ChCl:CitA is the most effective solvent for the extraction of biologically active compounds. | [22] | |
| ChCl:CitA (1:1) ChCl:LA (1:2) ChCl:AceA (1:2) ChCl:ForA (1:2) ChCl:OxaA (1:1) ChCl:TarA:H2O (1:1:1) ChCl:Ge (1:2) ChCl:Pro (1:2) ChCl:Eth (1:2) ChCl: Sor (1:1) ChCl:Gu (1:1) ChCl:Fr (1:1) ChCl:Xyl:H2O (1:1:1) ChCl:Suc:H2O (1:2:2) ChCl:Mal:H2O (1:2:2) ChCl:Act (1:2) MeOH MeOH:H2O (60:40) EtOH EtOH:H2O (60:40) | Sophora japonica L. (S. japonica)—flowering herb belonging to the Fabaceae family | TSE at Troom for 60 min | DPPH | HPLC-UV | ChCl:Eth showed the best extraction. It can be productively recovered and reused at least three times for rutin extraction from S. japonica without notably changing the extraction yield, the target compound recovery efficiency, and the purity of the obtained rutin. ChCl:Eth had no significant effect on the antioxidant activity of rutin, and recovered rutin demonstrated more antioxidant activity than MeOH extract. | [23] |
| LA:ChCl (3:1) LA:AceS (3:1) LA:AceAm (3:1) LA:Gc:H2O (3:1:3) LA:Gc (3:1) EtOH (60%) | Mint (Mentha spicata) Sage (Salvia officinalis) Dittany (Origanum dictamnus) Fennel (Foeniculum vulgare) Marjoram (Origanum Majorana)—Greek medicinal plants | UAE | TPC TFC DPPH TRP | NADES composed of LA:Gc displayed significantly higher capacity than the highly efficient EtOH regarding the extraction of polyphenols. NADES composed of LA:AceS and LA:AceAm showed relatively lower efficiency. Extracts with high polyphenol concentration may also possess higher antiradical activity and reducing power. | [24] | |
| Ge:H2O (50:50%) (1% CitA) Ge:H2O (50:50%) (1% ForA) EtOH:H2O (50:50%) (1% CitA) EtOH:H2O (50:50%) (1% ForA) H2O (1% CitA) H2O (1% ForA) | Chokeberry (Aronia melanocarpa (Michx)) pomace | TSE UAE |
TPC TAC | HPLC | The 50% Ge acidified with 1% ForA was identified as optimal for extracting TPC, while 50% Ge acidified with 1% CitA can be used for extraction of polyphenols in replacement of EtOH. However, 50% EtOH +1% CitA yielded significantly higher total anthocyanin content obtained by spectrometric and HPLC measurements. | [11] |
| Extraction with conventional solvents | ||||||
| EtOH (50%) | Oregano (Origanum vulgare) Rosemary (Rosmarinus officinalis L.) Spearmint (Mentha spicata) Lemon balm (Melissa officinalis L.) Greek sage (Salvia fruticosa Miller)—post-distillation solid residues of medicinal and aromatic plants | UAE | TPC TFC ABTS DPPH FRAP | HPLC-DAD-MS | The lemon balm extract had the highest phenolic concentration and moderate antioxidant activity, along with spearmint. In the rosemary and Greek sage extracts, the primary recognized compounds were rosmarinic acid, carnosol, and carnosic acid, whereas in the Greek oregano, spearmint, and lemon balm extracts, there were salvianolic acid isomers and rosmarinic acid. | [6] |
| MeOH EtOH H2O | Nettle (Urtica dioica) | UAE TSE TSE without stirring | TPC DPPH ABTS | The high antioxidant activity had nettle extracts prepared by UAE using water as a solvent in a shorter time. | [25] | |
| EtOH (60%) | Chokeberry (Aronia melanocarpa) | UMAE UAE MAE TSE | ABTS DPPH SASC TRP | HPLC-MS | The UMAE was an effective, simple, and rapid method for extracting proanthocyanidins from chokeberry with excellent antioxidant activity compared with the other extraction techniques. The main proanthocyanidins in purified chokeberry proanthocyanidins were B-type procyanidins, including procyanidin B2, B5 dimer, and procyanidin C1 trimer, with a degree of polymerization of 14. | [26] |
| H2O (distilled) | Rapeseed meal | UAE TSE | TPC FRAP | HPLC-DAD | Rapeseed meal extracts prepared by UAE revealed higher TPC and FRAP values than those obtained with the TSE. Phenylacetic acid and ferulic acid were the most predominant phenolic compounds in these extracts. | [13] |
| Ac MeOH H2O | Buckwheat by-products (grain, hull, and bran) | TSE at Troom for 24 h | TPC DPPH MChA | The capacity of scavenging DPPH radicals by buckwheat hull extracts was higher than that of extracts from unhulled and hulled buckwheat grains. However, bran extracts had a lower activity than grains. MeOH and Ac bran extracts formed complexes with iron ions to a higher degree than hull extracts. An opposite dependence was observed in the case of water extracts. | [19] | |
| No. | Combination of HBA and HBD | Molar Ratio (mol/mol) | Water Content (%) | Code |
|---|---|---|---|---|
| DES1 | Choline chloride/Citric acid | 1:1 | 30 | ChCl:CitA |
| DES2 | Glucose/Citric acid | 1:1 | 50 | Gu:CitA |
| DES3 | Glucose/Urea | 1:1 | 30 | Gu:U-30 |
| DES4 | Glucose/Urea | 1:1 | 50 | Gu:U-50 |
| DES5 | Betaine/Citric acid | 1:1 | 30 | B:CitA |
| DES6 | Betaine/Urea | 1:1 | 40 | B:U |
| Independent Variable | Levels with the Codes | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Choline chloride/Urea ratio (ChCl:U) (mol/mol) | 1:2 (0.5) | 1:1 (1) | 3:2 (1.5) |
| Water content (WC) (%) | 20 | 30 | 40 |
| Extraction time (t) (min) | 5 | 10 | 15 |
| Exp. | Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ChCl:U (mol/mol) | WC (%) | t (min) | CUPRAC * (µmol TE/g) | ABTS * (µmol TE/g) | DPPH * (µmol TE/g) | ||||
| Exp. ± SD | Pred. | Exp. ± SD | Pred. | Exp. ± SD | Pred. | ||||
| 1 | 0.5 (−1) | 20 (−1) | 10 (0) | 234.8 ± 8.4 c | 231.9 | 104.5 ± 4.3 d,e | 104.5 | 121.5 ± 13.3 c | 121.7 |
| 2 | 1.5 (1) | 20 (−1) | 10 (0) | 159.5 ± 10.3 a,b | 162.4 | 73.8 ± 3.8 b | 73.7 | 102.1 ± 6.8 b | 101.9 |
| 3 | 0.5 (−1) | 40 (1) | 10 (0) | 413.9 ± 15.7 g | 411.0 | 164.0 ± 4.0 g | 164.0 | 186.6 ± 2.2 e | 186.8 |
| 4 | 1.5 (1) | 40 (1) | 10 (0) | 394.4 ± 5.0 g | 397.3 | 201.0 ± 7.5 h | 201.0 | 180.5 ± 0.3 e | 180.3 |
| 5 | 0.5 (−1) | 30 (0) | 5 (−1) | 341.8 ± 16.1 e | 344.7 | 99.1 ± 11.7 c,d | 99.1 | 180.4 ± 8.1 e | 180.2 |
| 6 | 1.5 (1) | 30 (0) | 5 (−1) | 291.6 ± 22.3 d | 288.7 | 115.4 ± 5.4 e | 115.4 | 155.1 ± 10.4 d | 155.2 |
| 7 | 0.5 (−1) | 30 (0) | 15 (1) | 374.1 ± 28.2 f,g | 377.1 | 168.1 ± 5.7 g | 168.0 | 184.4 ± 4.7 e | 184.3 |
| 8 | 1.5 (1) | 30 (0) | 15 (1) | 352.8 ± 14.6 f,g | 349.8 | 157.8 ± 7.8 g | 157.8 | 183.0 ± 10.9 e | 183.1 |
| 9 | 1 (0) | 20 (−1) | 5 (−1) | 133.9 ± 12.6 a,b | 133.9 | 53.9 ± 5.5 a | 53.9 | 84.9 ± 4.8 a | 84.9 |
| 10 | 1 (0) | 40 (1) | 5 (−1) | 349.0 ± 9.9 f,g | 349.0 | 89.3 ± 4.8 c | 89.3 | 132.5 ± 8.4 c | 132.5 |
| 11 | 1 (0) | 20 (−1) | 15 (1) | 168.0 ± 5.1 b | 168.0 | 72.7 ± 9.3 b | 72.7 | 96.1 ± 8.2 a,b | 96.1 |
| 12 | 1 (0) | 40 (1) | 15 (1) | 493.7 ± 29.7 h | 493.7 | 273.5 ± 6.9 i | 273.5 | 209.5 ± 3.1 f | 209.5 |
| 13 | 1 (0) | 30 (0) | 10 (0) | 284.2 ± 0.6 d | 278.4 | 140.9 ± 7.6 f | 124.0 | 152.9 ± 0.6 d | 141.4 |
| 14 | 1 (0) | 30 (0) | 10 (0) | 274.5 ± 2.8 d | 278.4 | 139.6 ± 10.3 f | 124.0 | 149.9 ± 6.5 d | 141.4 |
| 15 | 1 (0) | 30 (0) | 10 (0) | 276.4 ± 6.9 d | 278.4 | 91.5 ± 3.2 c | 124.0 | 121.4 ± 3.1 c | 141.4 |
| Model Parameters | df | SS | MS | F-Value |
|---|---|---|---|---|
| CUPRAC | ||||
| Regression | 11 | 119,139.5 | 10830.9 | 413.2 * |
| Residual | 3 | 120.5 | 40.2 | |
| Lack-of-fit | 1 | 68.1 | 68.1 | 2.6 |
| Pure error | 2 | 52.4 | 26.2 | |
| Total | 14 | 119,260.0 | ||
| R2, Adjusted R2 | 0.9992, 0.9961 | |||
| ABTS | ||||
| Regression | 11 | 37,749.5 | 3431.8 | 4.3 |
| Residual | 3 | 1586.91 | 529.0 | |
| Lack-of-fit | 1 | 0.01 | 0.01 | 1 × 10−5 |
| Pure error | 2 | 1586.9 | 793.5 | |
| Total | 14 | 39,336.41 | ||
| R2, Adjusted R2 | 0.9658, 0.8405 | |||
| DPPH | ||||
| Regression | 11 | 17,320.8 | 1574.6 | 5.2 |
| Residual | 3 | 603.4 | 201.1 | |
| Lack-of-fit | 1 | 0.2 | 0.2 | 6.6 × 10−4 |
| Pure error | 2 | 603.2 | 301.6 | |
| Total | 14 | 17,924.2 | ||
| R2, Adjusted R2 | 0.9704, 0.8620 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamieau, M.; Loulergue, P.; Szydłowska-Czerniak, A. Green Solvent Extraction of Antioxidants from Herbs and Agro-Food Wastes: Optimization and Capacity Determination. Appl. Sci. 2024, 14, 2936. https://doi.org/10.3390/app14072936
Hamieau M, Loulergue P, Szydłowska-Czerniak A. Green Solvent Extraction of Antioxidants from Herbs and Agro-Food Wastes: Optimization and Capacity Determination. Applied Sciences. 2024; 14(7):2936. https://doi.org/10.3390/app14072936
Chicago/Turabian StyleHamieau, Malo, Patrick Loulergue, and Aleksandra Szydłowska-Czerniak. 2024. "Green Solvent Extraction of Antioxidants from Herbs and Agro-Food Wastes: Optimization and Capacity Determination" Applied Sciences 14, no. 7: 2936. https://doi.org/10.3390/app14072936





