Effects of Potential Prebiotics from Codium fragile on Intestinal Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CFE
2.2. Potential Prebiotics for Improving Intestinal Diseases
2.2.1. Mouse Model
2.2.2. Real-Time PCR Quantification
2.2.3. Short Chain Fatty Acid (SCFA) Analysis
2.2.4. β-Glucuronidase Activity Analysis
2.3. Potential Prebiotics for Improving Intestinal Diseases
2.3.1. Mouse Model
2.3.2. Evaluation of the Severity of Colitis
2.3.3. Myeloperoxidase (MPO) Activity
2.3.4. Histological Evaluation
2.3.5. Statistical Analysis
3. Results
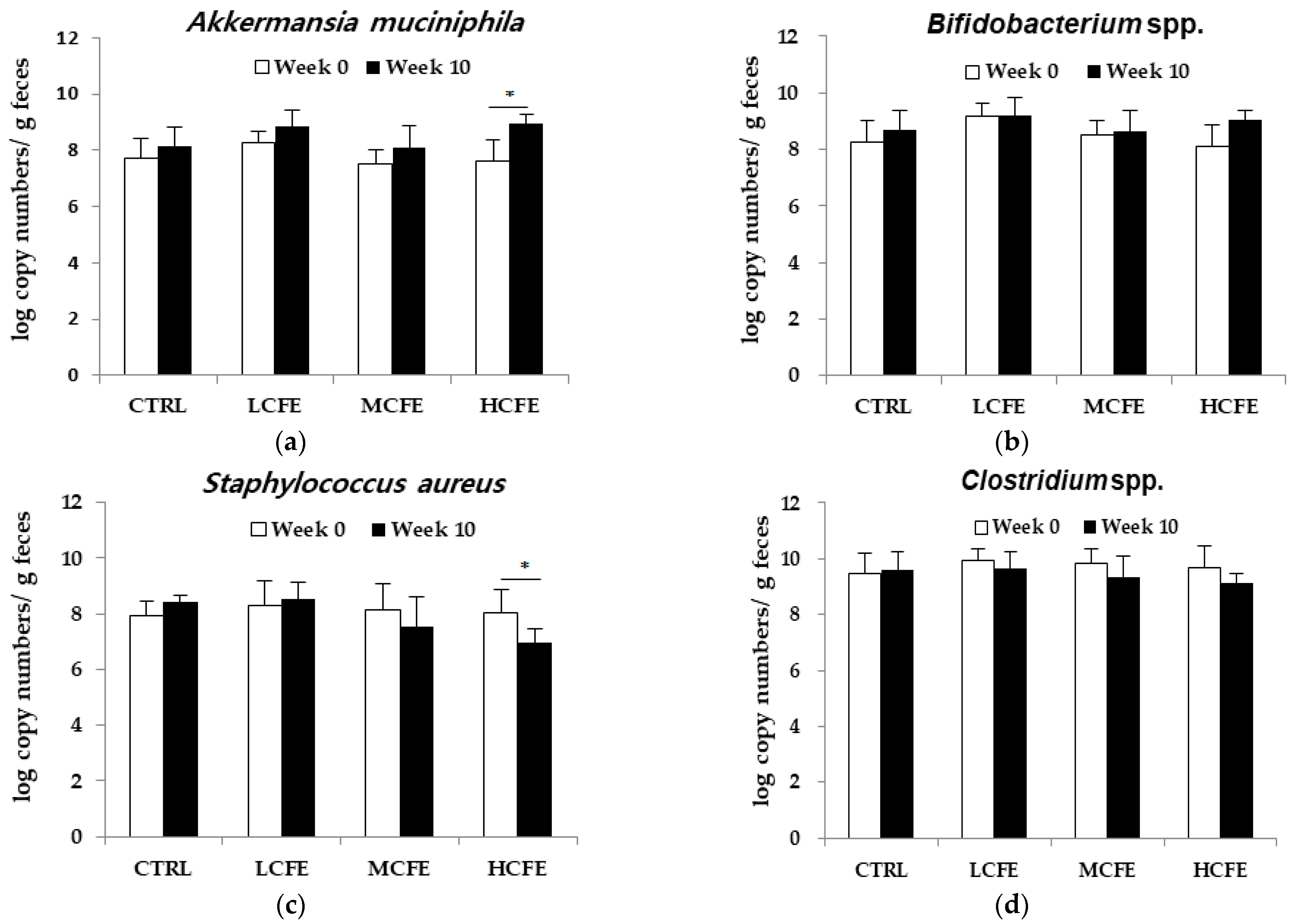
3.1. Effect of CFE on the Growth of Individual Bacteria
3.2. Effect of CFE on Cecum SCFA Production
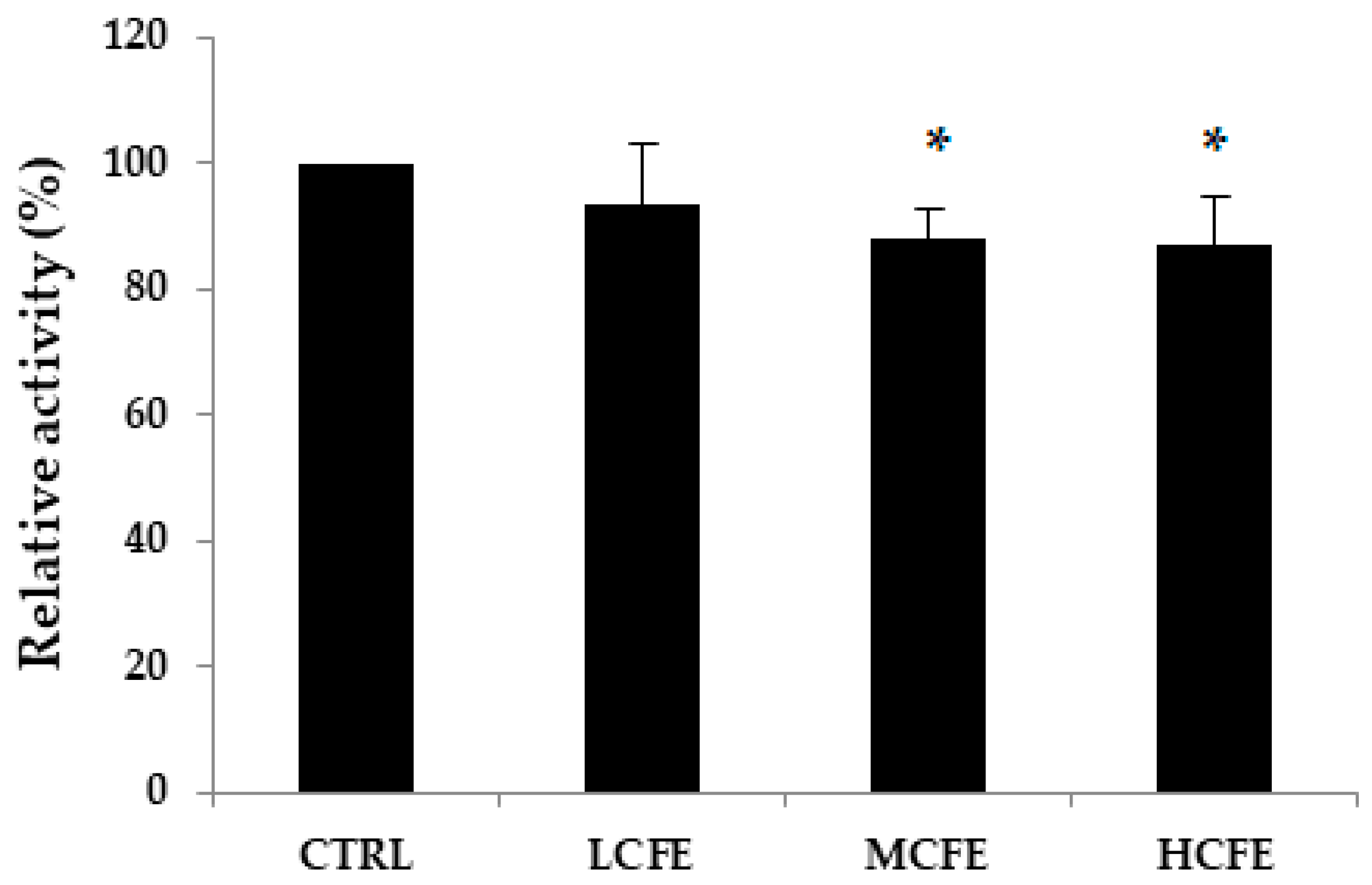
3.3. Effect of CFE on Fecal β-Glucuronidase Activities
3.4. Effect of CFE on Mouse Colitis
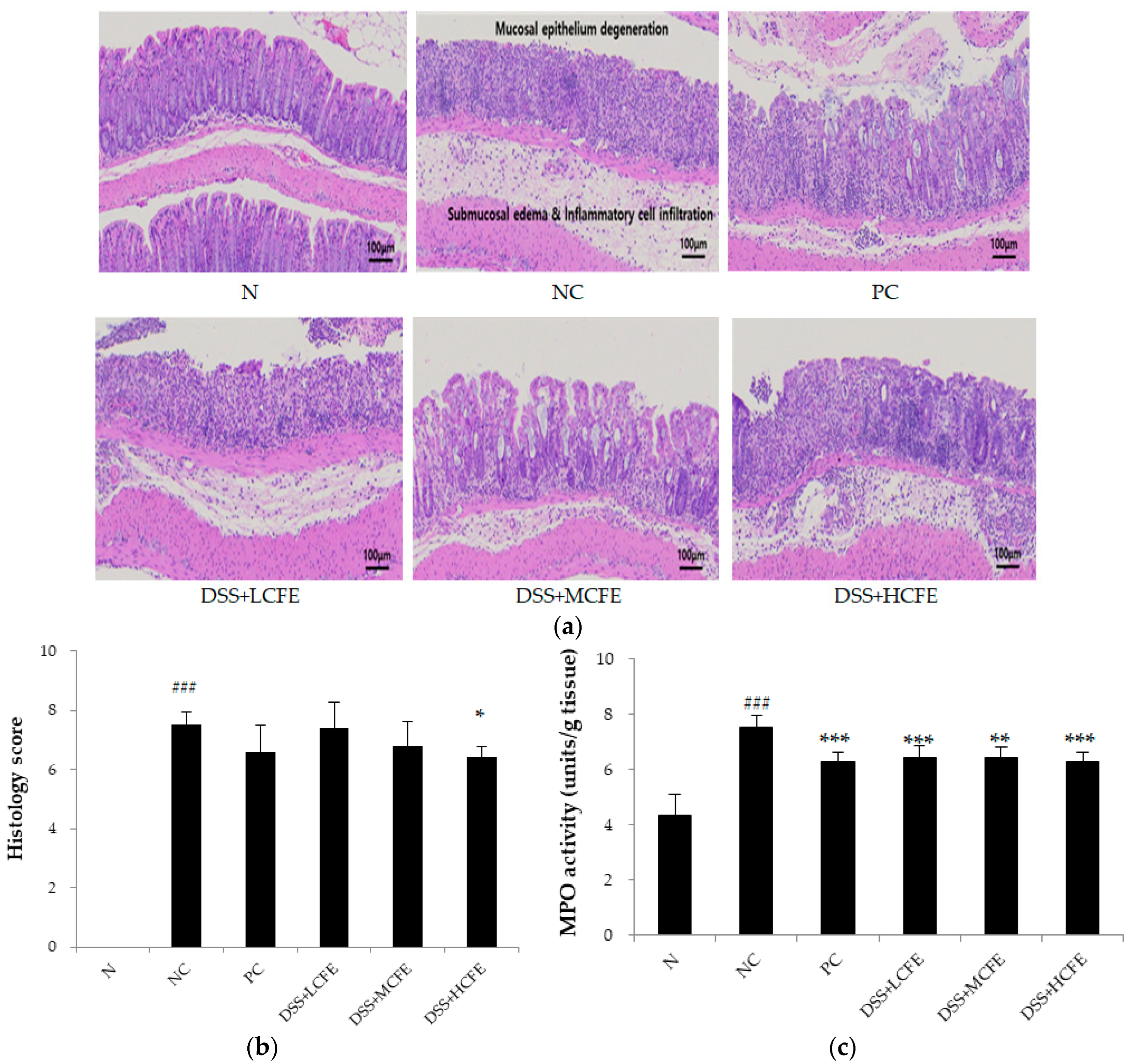
3.5. Effect of CFE on the Histological Injury of Colonic Epithelium Caused by DSS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez, M.J.; Falque, E.; Dominguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.L.; Wang, G.; Chen, L. Fucoxanthin, a Marine Xanthophyll Isolated From Conticribra weissflogii ND-8: Preventive Anti-Inflammatory Effect in a Mouse Model of Sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Yayeh, T.; Im, E.J.; Kwon, T.H.; Roh, S.S.; Kim, S.; Kim, J.H.; Hong, S.B.; Cho, J.Y.; Park, N.H.; Rhee, M.H. Hemeoxygenase 1 partly mediates the anti-inflammatory effect of dieckol in lipopolysaccharide stimulated murine macrophages. Int. Immunopharmacol. 2014, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Rod-in, W.; Jang, A.-Y.; Lee, S.-M.; Jung, S.-K.; You, S.; Park, W.J. Immune-enhancing effects of anionic macromolecules extracted from Codium fragile coupled with arachidonic acid in RAW264.7 cells. PLoS ONE 2020, 15, e0239422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, R.P.; Kumar, I.; Yadav, P.; Singh, S.K.; Kaushalendra; Singh, P.K.; Gupta, R.K.; Singh, S.M.; Kesawat, M.S.; et al. Algal Metabolites Can Be an Immune Booster against COVID-19 Pandemic. Antioxidants 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Hayashi, T. Isolation of sulfated galactan from Codium fragile and its antiviral effect. Biol. Pharm. Bull. 2009, 32, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- O’ Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Yang, Y.; Park, J.; You, S.G.; Hong, S. Immuno-stimulatory effects of sulfated polysaccharides isolated from Codium fragile in olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2019, 87, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.L.; Jeong, S.; Kim, B.R.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; Kim, D.Y.; Kim, B.G. Codium fragile F2 sensitize colorectal cancer cells to TRAIL-induced apoptosis via c-FLIP ubiquitination. Biochem. Biophys. Res. Commun. 2019, 508, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carmony, R.N.; Gerber, G.K.; Luevano, J.M.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbes 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; de Vos, E.A. Role of the intestinal microbiome in health and disease: From correlation to causation. Nutr. Rev. 2012, 70, S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.R.; Brown, I.L.; Topping, D.L. Starches, resistant starches, the gut ant starches, the gut microflora and human health. Curr. Issues Intest. Microbiol. 2000, 1, 25–37. [Google Scholar]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2006, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietila, T.E.; Kainulainen, V.; Klievink, J. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Ottman, N.; Davids, M.; Suarez-Diez, M.; Boeren, S.; Schaap, P.J.; Martins Dos Santos, V.A.P. Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl. Environ. Microbiol. 2017, 83, 01014–01017. [Google Scholar] [CrossRef]
- Png, C.W.; Linden, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Rajilic-Stojanovic, M.; Shanahan, F.; Guarner, F.; de Vos, W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel. Dis. 2013, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Laura, R.G.; Adam, S.C.; Lauren, F. Ulcerative colitis in adults. JAMA 2020, 324, 1205–1206. [Google Scholar]
- Bergemalm, D.; Andersson, E.; Hultdin, J.; Eriksson, C.; Rush, S.T.; D’Amato, M.; Gomollon, F.; Jahnsen, J.; Ricanek, P.; Satsangi, J.; et al. Systemic inflammation in preclinical ulcerative colitis. Gastroenterology 2021, 161, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Catalan-Serra, I.; Brenna, Ø. Immunotherapy in inflammatory bowel disease: Novel and emerging treatments. Hum. Vaccines Immunother. 2018, 14, 2597–2611. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Siegel, C.A. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol. Clin. 2014, 43, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; CK Rajendran, S.R.; Udenigwe, C.C.; Aryee, A.N.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, e12392. [Google Scholar] [CrossRef]
- Xie, S.-Z.; Liu, B.; Ye, H.-Y.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Liu, J.; Duan, J.; Luo, J.-P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Oh, S.; Kim, S.; Jung, K.; Pharm, T.N.A.; Yang, S.; Ahn, B. Potential Prebiotic and Anti-Obesity Effects of Codium fragile Extract. Appl. Sci. 2022, 12, 959. [Google Scholar] [CrossRef]
- Goldin, B.R.; Swenson, L.; Dwyer, J.; Sexton, M.; Gorbach, S.L. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzyme. J. Natl. Cancer. Inst. 1980, 64, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Ye, Z.Y.; Zhang, X.M.; Chen, H.; Ye, M. Protective effect of Lachnum polysaccharide on dextran sulfate sodium-induced colitis in mice. Food Funct. 2020, 11, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Lee, J.; Lee, Y.; Kin, D. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Li, X.; Wu, Z.; Zhang, F.; Wang, N.; Wu, N.; Yang, X.; Liu, Y.L. Fungal-bacterial interactions in mice with dextran sulfate sodium (DSS)-induced acute and chronic colitis. RSC Adv. 2016, 6, 65995–66006. [Google Scholar] [CrossRef]
- Kim, D.; Lee, M.; Yoo, J.; Park, K.; Ma, J. Fermented herbal formula KIOM-MA-128 protects against acute colitis induced by dextran sodium sulfate in mice. BMC Complement Altern. Med. 2017, 17, 354. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; Keshavarzian, A.; Patterson, J.A.; Venkatachalam, M.; Gillevet, P.; Hamaker, B.R. Starch-entrapped microspheres extend in vitro fecal fermentation, increase butyrate production, and influence microbiota pattern. Mol. Nutr. Food Res. 2009, 53, S121–S130. [Google Scholar] [CrossRef] [PubMed]
- Timm, D.A.; Stewart, M.L.; Hospattankar, A.; Slavin, J.L. Wheat dextrin, psyllium, and inulin produce distinct fermentation patterns, gas volumes, and short-chain fatty acid profiles in vitro. J. Med. Food 2010, 13, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside–from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.L.; Onnerfalt, J.; Xu, J.; Molin, G.; Ahrne, S.; Thorngren-Jerneck, K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity 2012, 20, 2257–2261. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; Garcia-Valdes, L.; Segura, M.T.; Martin-Lagos, J.A.; Anjos, T. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Zhitao, L.; Guoao, H.; Li, Z.; Zhenglong, S.; Yun, J.; Min-jie, G.; Xiaobei, Z. Study of growth, metabolism, and morphology of Akkermansia muciniphila with an in vitro advanced bionic intestinal reactor. BMC Microbiol. 2021, 21, 61. [Google Scholar]
- Ouwerkerk, J.P.; van der Ark, K.C.; Davids, M.; Claassens, N.J.; Robert Finestra, T.; de Vos, W.M.; Belzer, C. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl. Environ. Microbiol. 2016, 82, 6983–6993. [Google Scholar] [CrossRef]
- Kim, D.H.; Jin, Y.H. Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch. Pharm. Res. 2021, 24, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Wei, W.C.; Ding, M.L.; Zhou, K.; Xie, H.F.; Zhang, M.A.; Zhang, C.F. Protective effects of widelolactone on dextran sodium sulfate induced murine colitis partly through inhibiting the NLRP3 inflammasome activation via AMPK signaling. Biomed. Pharmacother. 2017, 94, 27–36. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | g/kg |
|---|---|
| Casein | 200.00 |
| Cornstarch | 397.486 |
| Dextrose | 132.00 |
| Sucrose | 100.00 |
| Cellulose | 50.00 |
| Soybean oil | 0.014 |
| t-Butylhydroquinone | 0.014 |
| Salt Mix | 35.00 |
| Vitamin mix | 10.00 |
| L-Cystine | 3.00 |
| Choline Bitartrate | 2.50 |
| Target | Primer | Primer Sequence (5′–3′) | |
|---|---|---|---|
| Probiotics | Bifidobacterium spp. | Forward Reverse | CTCCTGGAAACGGGTGG GGTGTTCTTCCCGATATCTAC |
| Akkermansia muciniphila | Forward Reverse | CAGCACGTGAAGGTGGGGAC CCTTGCGGTTGGCTTCAGAT | |
| Pathogens | Staphylococcus aureus | Forward Reverse | GCCCCTTAGTGCTGCAGCTA AGTTTCAACCTTGCGGTCGTA |
| Clostridium spp. | Forward Reverse | TTGAGCGATTTACTTCGGT CCATCCTGTACTGGCTCAC | |
| Grade | Infiltration Lesion | Epithelial Lesion |
|---|---|---|
| 0 | None | None |
| 1 | Infiltration around crypt bases | Some loss of goblet cells |
| 2 | Infiltration spreading to muscularis mucosa | Extensive loss of goblet cells |
| 3 | Extensive infiltration in the muscularis Mucosa with abundant edema | Some loss of crypt |
| 4 | Infiltration spreading to submucosa | Extensive loss of crypt |
| Groups | SCFAs (μmol/g) | |
|---|---|---|
| Acetic Acid | Butyric Acid | |
| CTRL | 9.05 ± 2.03 | 2.54 ± 0.48 |
| LCFE | 13.83 ± 1.12 ** | 3.27 ± 0.98 |
| MCFE | 18.91 ± 2.70 *** | 3.70 ± 0.53 * |
| HCFE | 20.24 ± 1.60 *** | 3.94 ± 0.69 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.W.; Kim, S.K.; Ahn, B.J.; Yim, S.K.; Yang, S.H. Effects of Potential Prebiotics from Codium fragile on Intestinal Diseases. Appl. Sci. 2024, 14, 3037. https://doi.org/10.3390/app14073037
Oh SW, Kim SK, Ahn BJ, Yim SK, Yang SH. Effects of Potential Prebiotics from Codium fragile on Intestinal Diseases. Applied Sciences. 2024; 14(7):3037. https://doi.org/10.3390/app14073037
Chicago/Turabian StyleOh, Su Won, Sung Keun Kim, Byung Jae Ahn, Sung Kun Yim, and Seung Hwan Yang. 2024. "Effects of Potential Prebiotics from Codium fragile on Intestinal Diseases" Applied Sciences 14, no. 7: 3037. https://doi.org/10.3390/app14073037
APA StyleOh, S. W., Kim, S. K., Ahn, B. J., Yim, S. K., & Yang, S. H. (2024). Effects of Potential Prebiotics from Codium fragile on Intestinal Diseases. Applied Sciences, 14(7), 3037. https://doi.org/10.3390/app14073037








